Abstract
Objectives:
Hippocampal avoidance during whole-brain radiotherapy is performed to prevent neural stem cell injury causing neurocognitive dysfunction. Nevertheless, the estimated risk of metastases in hippocampal avoidance area in small-cell lung cancer is unknown. The current study aimed to characterize the metastatic distribution within the brain relative to the hippocampus, estimate the incidence of hippocampal metastasis in patients with small-cell lung cancer, and identify clinical and radiographic variables that may be associated with the risk of hippocampal avoidance area metastasis.
Materials and Methods:
Patients with small-cell lung cancer treated with therapeutic whole-brain radiotherapy between January 2010 and December 2015 were reviewed. T1-weighted, postcontrast axial magnetic resonance images obtained just before therapeutic cranial irradiation were retrieved and reviewed for each patient. The hippocampal avoidance area was defined as hippocampus and 5-mm ring area adjacent to the hippocampus to account for necessary dose falloff between the hippocampus and the whole-brain planning target volume. Metastatic lesions within hippocampal avoidance area were defined as hippocampal metastasis. Hippocampal metastasis rate and characteristics of patients with hippocampal metastasis were analyzed and compared to patients without hippocampal metastasis.
Results:
Fifty-four patients evaluated with cranial magnetic resonance imaging were enrolled. Hippocampal metastasis rate was 32% (17 patients). A total of 4.4% of all metastases involved the hippocampal avoidance area. The most common location was frontal lobe. Being younger than 65 years of age was found to be an independent risk factor for HM (odds ratio: 4.8, 95% confidence interval: 1-23.2, P = .049). The number of brain metastases was significantly higher in patients with hippocampal metastasis (P = .027), and hippocampal metastasis rate was also higher in patients having larger hippocampus (P = .026) and larger brain volumes (P = .02).
Conclusion:
Hippocampal metastasis might be more common in small-cell lung cancer. Reducing the dose to the hippocampus by hippocampal avoiding whole-brain radiotherapy plan in small-cell lung cancer may be risky for the development of HM compared with other malignant solid tumors.
Keywords: small-cell lung cancer, hippocampal avoidance, whole-brain radiotherapy, brain metastasis, hippocampal metastasis
Introduction
New memory is composed of neural stem cells located in the subgranular zone of the hippocampus (HP), which have lifelong mitotic activity and are radiosensitive. Neural stem cell compartment injury has been suggested to be the cause of radiation-induced early cognitive decline.1–4 Recent clinical studies have shown a dose–response-related risk of postradiotherapy decline in neurocognitive functions due to radiation dose received by the HP that may reduce patients’ quality of life.5 Similar morbidity has been observed after prophylactic cranial irradiation (PCI) in patients with lung cancer also.6–9
The rationale for avoiding the hippocampal neural stem cell niche during whole-brain radiotherapy (WBRT) in patients with brain metastases (BM) or for prophylactic purposes is based on the theory that this approach may delay or reduce the onset, frequency, and/or severity of neurocognitive dysfunction without compromising intracranial disease control, thereby improving therapeutic ratio. Multiple Linear accelerator (LINAC)-based intensity-modulated radiotherapy (IMRT) and tomotherapy treatment planning techniques have been identified for hippocampal avoiding WBRT (HA-WBRT) so far.10–12 These techniques have demonstrated the ability to reduce mean dose to neural stem cell compartment by at least 80%, while providing acceptable coverage and dose homogeneity to the remaining whole-brain parenchyma.13 Nevertheless, the risk of disease progression within the HA area due to the risk of missing micrometastasis is a major challenge.14 Therefore, the issue of whether or not to spare the hippocampal region is an area still debated. Regarding this challenge, current data are lacking on the risk of hippocampal metastasis (HM), especially in small-cell lung cancer (SCLC).
The objectives of this study were as follows: (1) to characterize the distribution of metastatic lesions within the brain relative to the HP and designate the baseline incidence of metastasis in HA area in patients with SCLC presenting with BM both de novo (patients who were found to have BM at the time of diagnosis or who were found to have BM while receiving or immediately after chemotherapy and who did not receive prophylactic WBRT [P-WBRT] or therapeutic WBRT [T-WBRT] before) or after central nervous system (CNS) progression (patients who experienced CNS progression after P-WBRT or T-WBRT), (2) to estimate the risk of progression of disease in HA region for patients with SCLC after HA-WBRT technique by the assumption that BM development risk in HA area is in the same scale as at presentation with BM, and (3) to identify clinical and radiographic variables that correlate with the risk of metastasis in HA area.
Materials and Methods
Between January 2010 and December 2015, all consecutive patients with SCLC presented with BM and treated by T-WBRT were reviewed retrospectively. All patients irrespective of the number of BM were included. Patients who had BM diagnosed by cranial computed tomography (CT) or non-contrast-enhanced (CE) magnetic resonance imaging (MRI), patients with leptomeningeal metastases, and patients who had accompanying pathologically non-small-cell lung cancer component were excluded.
The initial pretreatment T1-weighted, postcontrast axial MRI (1.5 or 3 T) images showing intracranial metastatic disease were retrieved from hospital records and reviewed for each patient. Image sets were imported to the Monaco treatment planning software (version 5.0; Electa Business Area Software Systems, Maryland Heights, MO) for contouring. Hippocampus and each metastatic lesions were contoured on T1-weighted MRI axial sequences by a neuroradiologist (Figure 1). Anatomic boundaries of HP were delineated according to radiation therapy oncology group (RTOG)-0933 protocol.8 The HA area (including the HP) was generated by expanding the hippocampal contour by 5 mm volumetrically to simulate planning at risk volume that accounts for systematic setup error and necessary dose falloff between the HP and the whole-brain clinical target volume (CTV).
Figure 1.
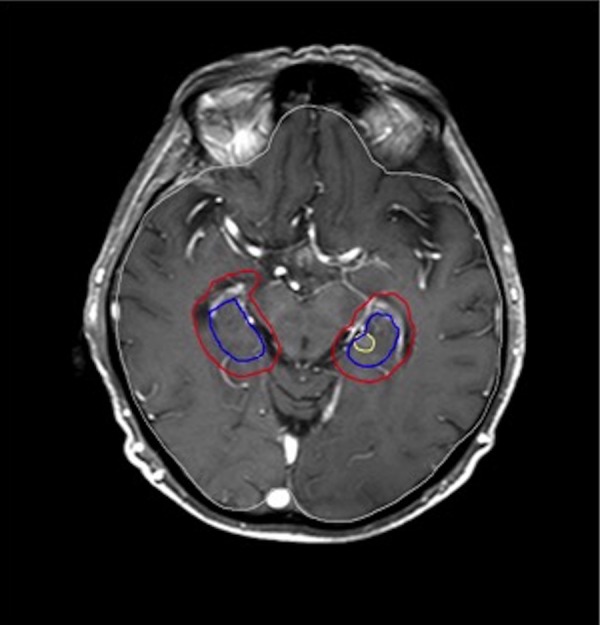
Contrast-enhanced axial T1 magnetic resonance imaging (MRI) of a patient who had 6 brain metastases (BM), one of them (1.7 cm3) was located just in the center of hippocampus (HP) proper (shown in yellow). Hippocampus is shown in blue, HP + 5 mm in red.
The percentage of whole-brain volume occupied by the volume of HA area is calculated by volume of the HA area divided by whole-brain CTV. The volume of each metastasis as well as its location in brain parenchyma and the distance from the HP were recorded. Metastatic lesions were grouped as within HP proper, in HA area, and in the rest of the brain if they were outside the expansion volume.
Definitions
Patients who were found to have BM at the time of diagnosis or who were found to BM while receiving or immediately after chemotherapy and who did not receive P-WBRT or T-WBRT before were defined as “de novo” metastatic group. Patients who experienced CNS progression after T-WBRT or P-WBRT were defined as “CNS progressing group.” Central nervous system progression was defined as the development of new metastatic lesion or 30% increase in size of a target lesion, according to the Response Evaluation Criteria In Solid Tumors.15
Patients were also classified as oligometastatic (1-3 metastases) or nonoligometastatic (≥4 metastases).
Hippocampal metastasis was defined as the metastatic lesion located in the HA area (HP proper and/or HP + 5 mm volume). “Rest of brain” was used to define the lesions located 5 mm further than HP.
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software (SPSS Inc, Chicago, Illinois). Continuous variables were expressed as median (25th-75th percentiles), and categorical variables were expressed as n (%). Comparisons were done with Mann-Whitney U test or Fisher exact test where appropriate. A P value of <.05 was considered significant.
Results
Fifty-four patients with SCLC presented with BM who were evaluated with CE cranial MRI and treated with T-WBRT were enrolled. The HM rate was 32% (17/54 patients).
All demographic data including age, gender, number of metastases, HP and brain volumes, and the comparisons of these variables between patients who have HM and who do not are summarized in Table 1. There was a significant hippocampal and brain volume difference between patients who had HM and who did not. Nevertheless, the HP to brain volume ratio was comparable. On average, the volume of the HA area occupied 3.2% of the whole brain.
Table 1.
Patient Characteristics and Comparisons Between Patients With and Without Hippocampus Metastasis.a
| Patient Characteristics | Total | HP Metastasis (−) | HP Metastasis (+) | P |
|---|---|---|---|---|
| Male gender, n (%) | 47 (87) | 33 | 14 | .66 |
| Age, years | 62 (56-68) | 63 (56-70) | 61 (56-64) | .34 |
| HP volume, cm3 | 12.5 (10.7-15.1) | 11.4 (10.4-14.3) | 14.1 (12.4-15.2) | .026 |
| HA area volume, cm3 | 43.7 (37.9-49.4) | 41.4 (37.1-49.6) | 46.6 (43.3-48.8) | .052 |
| Brain volume, cm3 | 1403.1 (1294.1-1511.1) | 1386.4 (1268.9-1494.4) | 1464.3 (1401.6-1529.5) | .02 |
| HP/brain volume (%) | 0.89 (0.77-1.04) | 0.87 (0.74-1.03) | 0.95 (0.87-1.01) | .17 |
| HA area/brain volume (%) | 3.2 (2.9-3.4) | 3.1 (2.8-3.4) | 3.2 (2.9-3.4) | .59 |
| Total number of metastases, n | 3 (1-6) | 2 (1-4) | 6 (3-10) | .027 |
| Total volume of metastases, cm3 | 3.8 (1.1-10.5) | 2.9 (1.1-9.8) | 6.7 (2.2-15.6) | .10 |
| Oligometastatic/nonoligometastatic patients, n | 31/23 | 23/14 | 8/9 | .38 |
Abbreviations: HA, hippocampal avoidance; HP, hippocampus.
aData are presented as n (%) or median (25th-75th percentiles).
Hippocampal metastasis to brain volume ratio, total volume of metastases, having multimetastases, being younger than 65 years of age, and having extensive disease were included into a logistic regression model to evaluate their effect on having HM. Only being younger than 65 years of age was found to be an independent risk factor for HM (Table 2). In 17 patients having HM, 13 were under the age of 65.
Table 2.
Logistic Regression Analysis for Incidence of Hippocampus and/or Hippocampal Avoidance Area Metastasis.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| HP/brain volume | 4.1 | 0.2-72.7 | .34 |
| Total volume of metastases | 1.1 | 0.9-1.1 | .16 |
| Being <65 years of age | 4.8 | 1-23.2 | .049 |
| Having extensive disease | 2 | 0.4-9.1 | .36 |
| Having multimetastases | 2.9 | 0.6-12.7 | .14 |
Abbreviations: CI, confidence interval; HP, hippocampus; OR, odds ratio.
Of 54 patients with BM, 50 (92.5%) patients were in the de novo group. There were 4 patients in the central progressing group who received cranial irradiation previously. Two of them developed BM after P-WBRT and 2 had CNS progression after T-WBRT. Two patients progressing after P-WBRT developed 5 deposits, 2 of them were in HP proper and 1 was in HP + 5 mm area, resulting in 3 of 5 of lesions in the HA area. Of 2 patients progressing after T-WBRT, none of them had lesion in the HA area.
A total of 446 metastases were analyzed in 54 patients. This yielded a median 3 (1-6) metastases per patient, with a median volume of 3.8 cm3 (1.1-10.4 cm3) per metastasis. The number of patients with oligometastasis was 15 (28%). Although the HM rate was 25% in patients with oligometastasis, it was 39% in patients without oligometastasis (P = .38).
The total number of metastases was significantly higher in patients who had HM (P = .027). But the total volume of metastatic lesions was comparable (P = .10; Table 1).
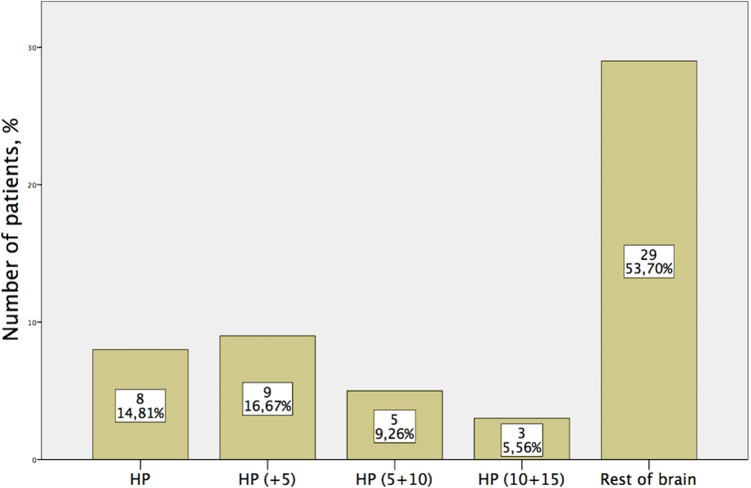
We found that 4.4% of all metastases involved the HA area; 2.2% of metastases were located within the HP proper and 2.2% were in HP + 5 mm volume. Most of the metastases were located in the rest of brain area. Eight patients had metastases in HP proper and 9 had in HP + 5 mm area (Figure 2).
Figure 2.
Distribution of patients with brain metastasis according to the distance from the hippocampus (HP).
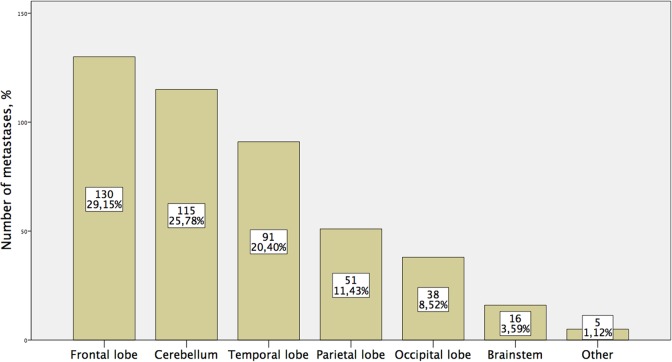
Thirty-four (63%) patients had extracranial metastases at the time of evaluation, bone being the most common site (16/34), followed by adrenal (12/34) and liver (10/34). Twenty-two percent of patients had solitary, 19% had 2, and 58% had 3 or more extracranial metastases. Patients with extracranial metastases tend to have more HM, but this increase was not significant (35% vs 25%, P = .54). The most common location of metastasis was frontal lobe followed by cerebellum and temporal lobe (Figure 3).
Figure 3.
Distribution of all metastases according to the location in the central nervous system.
Discussion
The main finding of this study is the high percentage of HM in patients with SCLC having brain metastasis. We found that 8 patients had metastases in HP proper and 9 patients in HP + 5 mm volume, which make a total of 17 (32%) patients having metastases in the HA area. Hippocampal metastasis risk increases significantly in patients with ≤65 years of age. The number of BM was significantly correlated with HM risk, and the risk is higher in nonoligometastatic patients. Hippocampal metastasis rate was higher in patients having larger HP and larger brain volumes, which can be stated as the first finding in the literature.
It has to be stated that all patients irrespective of the number of BM were included in the current analysis in contrast to most other studies.13,16,17 Therefore, the high rate of HM in our data might be the consequence of relatively higher incidence of nonoligometastatic disease seen in our patient cohort, which is very common finding in SCLC histologic subgroup.13,17,18 Also Marsh et al described that HM was significantly higher in nonoligometastatic patients compared to oligometastatic ones, 93.8% versus 6.2%, respectively.17 In contrast, in a cohort of Wan et al, nonoligometastatic patients’ rate was only 22.5%.19
In the current study, the number of BM was significantly correlated with HM risk in agreement with Sun et al.2 In RTOG-0933, also there was a trend between the number of CNS metastatic deposits and the risk of metastasis in the HA area.13
Although there wasn’t any correlation between age and risk of HM in many studies,2,16,20 Wu et al reported that age ≤60 years was an independent risk factor for HM,21 similarly being younger than 65 years of age seemed to be independent risk factor in our study.22 Also HM was higher in patients with larger HP and larger brain volumes, which is a unique finding so far. By combining these 2 findings, it can be suggested that younger patients having higher volume of HP and brain23,24 may have higher risk of HM, in whom neurocognitive morbidity is a much more important challenge to deal with.
Contrast-enhanced MRI has higher sensitivity and specificity relative to non-CE-MRI or CE-CT for the detection of BM in solid tumors and lung cancer.18,25–28 National Comprehensive Cancer Network (NCCN) 2017 guideline states that cranial MRI is more sensitive than cranial CT and is preferred over CT for initial evaluation of patients with SCLC.29 Seute et al revealed that, in contrast to CT era, estimated prevalence of BM, silent metastases, solitary metastases, and multiple metastases increased and non- or undetectable micrometastases decreased in the MRI era.30 Ramlov et al evaluated intracranial relapse pattern after PCI and revealed that 5 of 21 relapsing patients had metastases in the limbic system. Six MRIs and 18 CT scans were available for these relapsing patients and they claimed these numbers might had been even higher if all patients had MRI, and they claimed that reducing the radiation dose to HP in patients with SCLC during PCI might not be safe.18 Therefore, patients who had BM diagnosed by cranial CT or noncontrast MRI were excluded from our study as this may underestimate the real incidence of HM.
To our knowledge, there has been only 1 study evaluating HM specifically in SCLC. In this retrospective study, Kundapur et al evaluated 67 patients with SCLC with BM by cranial CT and/or MRI. Hippocampal metastasis rate was only 5.7%, and 0.9% of lesions was in the HA area.20 But neither the percentage of patients who were evaluated only by CT nor the use of contrast agent was mentioned in detail, so this radiological underevaluation might be the cause of such lower rates of the HA area metastasis when compared with the current study.
Data regarding the relapse risk in the HA area in SCLC after HA P-WBRT or T-WBRT are unclear because of the lack of clinical data. There are only 2 prospective data available regarding HA T-WBRT and both of them excluded patients with SCLC. The first one is single-armed phase II RTOG-0933 trial, in which 67 of 113 patients progressed after HA T-WBRT and only 4.5% progressed in the HA area, which was much lower than the prior estimated risk (8.5%) in a safety profile study by the same group.1,13,16 They excluded patients with SCLC because of general assumption that these patients would have more diffuse distribution of metastases in brain, thus an increased risk of HM in contrast to other malignancies.20 The second data are from early results by Oehlke et al: the progression in the HA area was 2 (10%) of 20 in 40 weeks in different primaries other than SCLC.31
Currently, there are 6 retrospective series evaluating HM risk in patients with brain metastasis with different histological primaries. In these studies, patients with SCLC occupied only a small size of patient populations (10-44 patients), and HM rate in SCLC has a wide range between 2.1% and 45%.14,16,17,19,32
Among these studies, Harth et al reported in subgroup analysis that the metastasis rate was 45.5% (5/11 patients with SCLC) in the HA area, which is even higher than ours and might be the result of proper radiological evaluation done by CE-MRI in all patients and recruitment of patients having more than 10 metastases, which has a similar study design with the current study.14
In a review of 107 patients, Marsh et al reported higher percentage of limbic metastases among 11 patients with SCLC (2.1% HP and 4.8% other limbic sites) compared to other histologies. They explained this by higher presentation rates of nonoligometastatic patients (64%) among patients with SCLC.17
It is very difficult to interpret the results to make a conclusion about the safety profile of HA P-WBRT. The major challenge is the wide range of primary cancer types, different imaging modalities, inclusion criteria in terms of burden of metastatic deposits, definitions of HM (HP proper, HA area, limbic circuit) and patients with oligometastasis, and absence of contouring details, all might have significant impact on outcomes.
In summary, except Harth et al, the studies might have underestimated the risk of metastases in the HA area in SCLC, which might be because of the following explanations.
We included patients who had BM diagnosed by CE-MRI. This could explain the underestimated risk of HP metastases in some of the other studies using CT for metastasis detection and HP contouring.20
We included all patients irrespective of their number of cranial metastases because of the disseminated nature of BM seen in patients with SCLC. Exclusion of miliary metastases and patients having more than 10 metastases or recruitment of patients with oligometastasis in some studies may be the other cause of underestimation of the risk of metastases to the HA area,1,16,32 because most of the metastases in the HA area were detected more commonly in nonoligometastatic patients, which in line with the literature.2,17
Sample sizes of patients with SCLC in recent studies were relatively small (from 10 to 44 patients)14,16,17,19,32 even some of them excluded patients with SCLC from their cohort.13,31
Interobserver variability in delineating HP and using different contouring techniques might be another reason.
In our study, HP was delineated according to RTOG HP contouring atlas by a dedicated neuroradiologist. Figure 1 shows an example of our HP contouring and of a metastasis lying in HP proper. Because of the retrospective nature of the study, MRIs were not done for the purpose of HP delineation so they did not meet the criteria of RTOG, in which 1.25-mm slice is recommended to contour the HP accurately. This might result in overestimation of actual volume of the HA area. For this reason to test ourselves, we evaluated the percentage of whole brain volume occupied by the HA area, and the results were in agreement with the findings of Gondi et al (2.1%) and Kundapur et al (2.7%).10,20 Whether this correlation done or the RTOG HP contouring atlas was considered is not clear in most of the studies.14–16 The slice thickness of MRI images was not described in these studies either, except by Wan et al (6 mm), which is an inaccurate thickness for HP delineation.17,19 If we also consider the effect of contouring the HP on CT images in certain number of patients in some of the studies, the higher risk of HM in our results would be easier to interpret.
Our final result implies that frontal lobe was the most commonly involved location, followed by cerebellum and temporal lobe, which is in line with the literature.32 Our study has inevitable limitations. First, it is a retrospective, single-centre study probably leading to a negative impact on the external validity of the outcomes. Second, findings are based on a small number of patients and an occurrence of a selection bias is possible because of the fact that only patients available with CE-MRI imaging were included. Third, HP contouring was performed by a single neuroradiologist who was only available for our center, which makes the κ statistics (interobserver agreement) unavailable for this study. Last but not least, the assumption of BM development risk in the HA area after HA-WBRT is in the same scale as at presentation with BM was based on an analysis of baseline incidence of HM, which is similar to previous studies.14,16,20 The best way to evaluate the risk of HM is to randomize patients into HA-WBRT or WBRT and follow them with sequential imaging, but to our knowledge, no such data exist in the literature.
Conclusions
Reducing the dose to the HP by HA-WBRT plan, especially for PCI in SCLC, might create an additional risk for the emergence of new BM within the HA area. As far as we know, this is the first study estimating HM risk specifically in patients with SCLC who were evaluated thoroughly by CE-MRI, which should be the standard imaging tool for the diagnosis of BM to be able to rule out underdiagnosis. As we know, almost 60% of patients with SCLC eventually develop BM in their disease course,17 and these metastases have a tendency of being disseminated in nature. We claim that HP is not a region with lower risk of metastases for SCLC, with an estimated risk of 32%; therefore, sparing of HP would be risky in SCLC in contrast to other solid tumors.
Nevertheless in SCLC, HA-WBRT during either T-WBRT or P-WBRT warrants further evaluation as part of planned clinical trials. NRG CC003 is a National Cancer Institute–approved randomized phase II/III trial of HP avoidance during PCI for SCLC, which started recruiting patients by December 2015.33 This study might explain the real risk to the HA area metastases in SCLC in near future. Until then, it is quite likely that the trade would be going on between doctors and patients, considering risk of neurocognitive dysfunction and risk of progression in the HA area which could adversely worsen cognitive function even more than conventional P-WBRT does.
Abbreviations
- BM
brain metastases
- CNS
central nervous system
- CTV
clinical target volume
- CE
contrast-enhanced
- CT
computed tomography
- HA
hippocampal avoidance
- HP
hippocampus
- HM
hippocampal metastasis
- IMRT
intensity-modulated radiotherapy
- MRI
magnetic resonance imaging
- P-WBRT
prophylactic WBRT
- PCI
prophylactic cranial irradiation
- SCLC
small-cell lung cancer
- T-WBRT
therapeutic WBRT
- WBRT
whole-brain radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun B, Huang Z, Wu S, et al. Incidence and relapse risk of intracranial metastases within the perihippocampal region in 314 patients with breast cancer. Radiother Oncol. 2016;118(1):181–186. [DOI] [PubMed] [Google Scholar]
- 3. Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 4. Bonaguidi MA, Wheeler MA, Shapiro JS, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gondi V, Hermann BP, Mehta MP, Tome WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. [DOI] [PubMed] [Google Scholar]
- 6. Le Pechoux C, Laplanche A, Faivre-Finn C, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann Oncol. 2011;22(5):1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marsh JC, Godbole RH, Herskovic AM, Gielda BT, Turian JV. Sparing of the neural stem cell compartment during whole-brain radiation therapy: a dosimetric study using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2010;78(3):946–954. [DOI] [PubMed] [Google Scholar]
- 12. Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive. and neurologic end points. J Clin Oncol. 2002;20(16):3445–3453. [DOI] [PubMed] [Google Scholar]
- 13. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harth S, Abo-Madyan Y, Zheng L, et al. Estimation of intracranial failure risk following hippocampal-sparing whole brain radiotherapy. Radiother Oncol. 2013;109(1):152–158. [DOI] [PubMed] [Google Scholar]
- 15. Chalian H, Tore HG, Horowitz JM, Salem R, Miller FH, Yaghmai V. Radiologic assessment of response to therapy: comparison of RECIST Versions 1.1 and 1.0. Radiographics. 2011;31(7):2093–2105. [DOI] [PubMed] [Google Scholar]
- 16. Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 2010;95(3):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marsh JC, Herskovic AM, Gielda BT, et al. Intracranial metastatic disease spares the limbic circuit: a review of 697 metastatic lesions in 107 patients. Int J Radiat Oncol Biol Phys. 2010;76(2):504–512. [DOI] [PubMed] [Google Scholar]
- 18. Ramlov A, Tietze A, Khalil AA, Knap MM. Prophylactic cranial irradiation in patients with small cell lung cancer. A retrospective study of recurrence, survival and morbidity. Lung Cancer. 2012;77(3):561–566. [DOI] [PubMed] [Google Scholar]
- 19. Wan JF, Zhang SJ, Wang L, Zhao KL. Implications for preserving neural stem cells in whole brain radiotherapy and prophylactic cranial irradiation: a review of 2270 metastases in 488 patients. J Radiat Res. 2013;54(2):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kundapur V, Ellchuk T, Ahmed S, Gondi V. Risk of hippocampal metastases in small cell lung cancer patients at presentation and after cranial irradiation: a safety profile study for hippocampal sparing during prophylactic or therapeutic cranial irradiation. Int J Radiat Oncol Biol Phys. 2015;91(4):781–786. [DOI] [PubMed] [Google Scholar]
- 21. Wu SG, Rao MY, Zhou J, et al. Distribution of metastatic disease in the brain in relation to the hippocampus: a retrospective single-center analysis of 6064 metastases in 632 patients. Oncotarget. 2015;6(41):44030–44036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balducci L. Management of cancer in the elderly. Oncology (Williston Park). 2006;20(2):135–43; discussion 144, 146, 151-2. [PubMed] [Google Scholar]
- 23. Frisoni GB, Ganzola R, Canu E, et al. Mapping local hippocampal changes in Alzheimer’s disease and normal ageing with MRI at 3 Tesla. Brain. 2008;131(pt 12):3266–3276. [DOI] [PubMed] [Google Scholar]
- 24. Apostolova LG, Green AE, Babakchanian S, et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemiere C, Peretti-Viton P, Thomas P, Gaubert JY, di Stefano-Louineau D, Kleisbauer JP. Spreading evaluation in primitive bronchogenic carcinoma: benefit of cerebral MRI compared to CT scan. Eur J Cancer. 1995;31A(10):1715. [DOI] [PubMed] [Google Scholar]
- 26. Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki K, Yamamoto M, Hasegawa Y, et al. Magnetic resonance imaging and computed tomography in the diagnoses of brain metastases of lung cancer. Lung Cancer. 2004;46(3):357–360. [DOI] [PubMed] [Google Scholar]
- 28. Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44(3):275–281. [DOI] [PubMed] [Google Scholar]
- 29. NCCN Guidelines Version 3. 2017 Small Cell Lung Cancer. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Updated November 16, 2016. Accessed June, 2017.
- 30. Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. 2008;112(8):1827–1834. [DOI] [PubMed] [Google Scholar]
- 31. Oehlke O, Wucherpfennig D, Fels F, et al. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: local tumour control and survival. Strahlenther Onkol. 2015;191(6):461–469. [DOI] [PubMed] [Google Scholar]
- 32. Ghia A, Tome WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68(4):971–977. [DOI] [PubMed] [Google Scholar]
- 33. Whole-brain radiation therapy with or without hippocampal avoidance in treating patients with limited stage or extensive stage small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02635009. Updated June 21, 2016, Accessed June, 2017.