Abstract
Cancer immunotherapy is quickly growing to be the fourth most important cancer therapy, after surgery, radiation therapy, and chemotherapy. Immunotherapy is the most promising cancer management strategy because it orchestrates the body’s own immune system to target and eradicate cancer cells, which may result in durable antitumor responses and reduce metastasis and recurrence more than traditional treatments. Nanomaterials hold great promise in further improving the efficiency of cancer immunotherapy - in many cases, they are even necessary for effective delivery. In this review, we briefly summarize the basic principles of cancer immunotherapy and explain why and where to apply nanomaterials in cancer immunotherapy, with special emphasis on cancer vaccines and tumor microenvironment modulation.
Keywords: Nanomaterials, cancer immunotherapy, cancer vaccine, tumor microenvironment
1. Introduction
1.1 Basic concepts in cancer immunotherapy
The clinical suppression or activation of the immune system with the goal of treating a disease is referred to as immunotherapy. For example, immunosuppressive immunotherapy is used to reduce overactive inflammation in allergic reactions, chronic inflammatory bowel disease, and organ transplantation. On the other hand, cancer growth and metastasis is often mediated by immunosuppression and immune evasion, and the field of cancer immunotherapy developed to activate the immune system against malignant cells.
Since the approval of ipilimumab in 2011, cancer immunotherapy is experiencing an explosive development [1]. Ipilimumab is a monoclonal antibody that activates the immune system by targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA4), which is a protein receptor constitutively expressed on regulatory T cells (Tregs) and functions as an immune checkpoint for “off” switch of the immune responses [2]. After that, monoclonal antibodies against programmed death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) were also approved for melanoma and non-small cell lung cancer treatment [3, 4]. The most impressive feature of these therapies is their long-term control of the disease in the response population, which has never seen before in other treatments. There have been many good reviews on the basic principles of cancer immunotherapy [5–7]. Briefly, cancer immunotherapy can be classified as systematically- or locally-based, depending on whether the therapy is inducing a systemic immune activation for cancer or local immune status changes. In most occasions, the former refers to systemic cytokine administration, cancer vaccines, or adoptive cell transfer (ACT) [8–12], and the latter refers to modulation of the immunosuppressive tumor microenvironment (TME), like immune checkpoint inhibitors or some small molecular inhibitors [13, 14]. Combinations of systemic and local immunotherapies or of immunotherapies and more traditional clinical therapies have proven quite meaningful and powerful in cancer management [15–18].
1.2 Cancer-immunity cycle
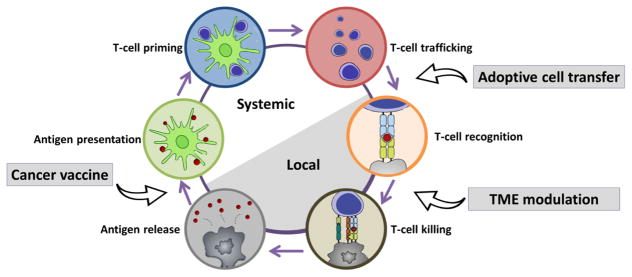
It is important to understand that the generation of an effective anti-cancer immune response necessitates a series of stepwise events which proceed and expand iteratively. This process is termed the “cancer-immunity cycle” [19] (Fig. 1). Briefly, necrotic or apoptotic tumor cells release tumor-derived antigens. These antigens are captured by dendritic cells (DCs) and presented extracellularly on major histocompatibility complex class I (MHCI) and major histocompatibility complex class II (MHCII) molecules. In the draining lymph nodes, activated DCs prime and activate immature T cells to effector T cells. These effector T cells traffic to the tumor site and specifically recognize tumor cells through T cell receptor (TCR) and MHC interactions. Upon recognition, effector T cells kill their target cancer cells by inducing apoptotic pathways. The killing of cancer cells releases additional tumor-derived antigens, and further strengthened the subsequent revolutions of the cycle.
Fig. 1. The cancer-immunity cycle.
The generation of an effective anti-cancer immune response necessitates a series of stepwise events. Cancer immunotherapy aims to initiate or re-implement the self-sustaining cancer-immunity cycle. Modified from ref [19], with permission from Elsevier.
In many occasions, the cancer-immunity cycle may be blocked at one or more of these steps, resulting in dampened anti-cancer immune responses or even immune escape. Factors leading to the immune escape may be failure to detect tumor antigens, Tregs expansion from DC priming, or suppression of T-cell function in tumors by factors in the TME [20]. Cancer immunotherapy aims to initiate or re-implement the cancer-immunity cycle (Fig. 1). For example, cancer vaccines are designed to promote cancer antigen presentation in DCs and facilitate more robust effector T-cell production [21]. TME modulation aims to release the “brake” for cytotoxic T cells (CTLs) in the immunosuppressive TME, improving the ability for killing their targeted cancer cells [22]. In ACT, antigen-specific CTLs are generated and expanded ex vivo and administered back to patients for antigen-specific tumor cell killing [23]. Still, while meeting the objective of an intact cancer-immunity cycle may require only monotherapy approaches in some patients, others may require combined therapies.
1.3 Nanomaterials for cancer immunotherapy
Nanomaterials are defined as materials with at least one dimension between 1 and 1000 nm, but in practical use may be anywhere from 1–200 nm. The past thirty years have viewed great success in the application of various nanomaterials for cancer diagnosis and therapy [24–27]. Due to rapid growth and irregular vascular structure, nanomaterials with a size of 10–200 nm avoid kidney clearance while selectively penetrating tumor tissues. Therefore, drugs loaded inside nanomaterials generally have much longer blood retention time and enhanced tumor distribution and reduced toxicity, which results in a higher tolerated dose [28–32]. In addition, nanomaterials are easily modified, and targeting ligands preloaded on the surface will help nanomaterials to be readily taken up by specific cells [33–36].
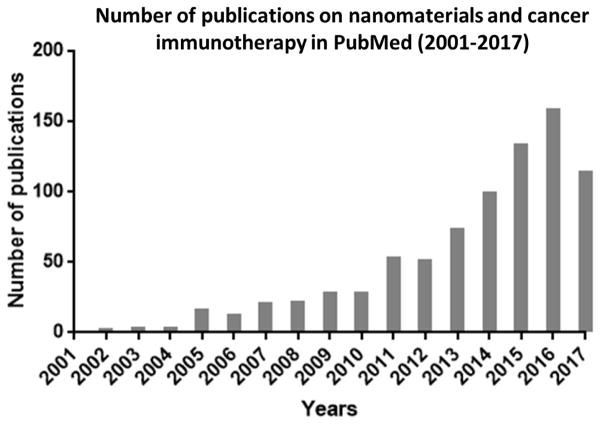
The application of nanomaterials to delivering cytotoxic drugs or imaging agents will also benefit immunotherapy. Delivery of tumor antigens is a critically important part of cancer vaccination, and it remains a clinical challenge [37–39]. For checkpoint inhibitors blocking the interactions between negative regulators and T cells, the lack of selectivity may result in significant immune-related toxicities [40–42]. Compared to delivering cytotoxic drugs to kill tumor cells, immunomodulation within the tumor may be a more efficient and thorough method of tumor eradication [43]. Additionally, some nanomaterials can inherently modulate the immune response due to some specific physiochemical characteristics [44–46]. In the past several years, a lot of pioneer works have been reported, and the number of publication is growing quickly (Fig. 2). There have been several good reviews summarizing the progresses in this field [47–53]. In this review, we will not list all of the innovative pioneer works, but explain some of the basic principles for applying nanomaterials in cancer immunotherapy. We will divide our discussion into two parts, cancer vaccination and immunosuppressive TME modulation (Fig. 3).
Fig. 2.
Number of publications on nanomaterials and cancer immunotherapy in PubMed from 2001 to 2017.
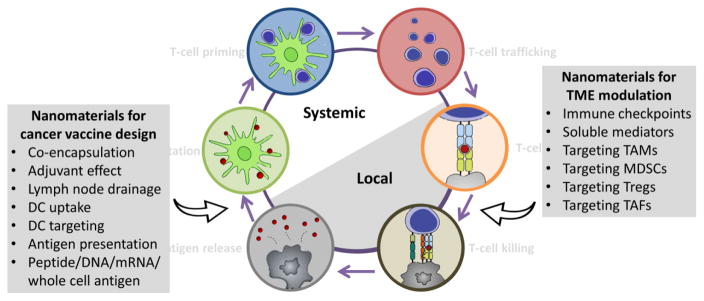
Fig. 3. Nanomaterials for balancing the cancer-immunity cycle.
Nanomaterials can be applied in cancer vaccine design, with the advantage of co-encapsulation of antigen and adjuvant, inherent adjuvant effect, lymph node drainage, DC targeting, and antigen presentation. Various antigens like peptides, DNA, mRNA and whole cell antigens can be loaded within nanomaterials. For TME modulation, nanomaterials can be designed for targeting immune checkpoints, soluble mediators, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), Tregs and tumor-associated fibroblasts (TAFs). Modified from ref [19], with permission from Elsevier.
2. Application of nanomaterials for cancer vaccine design
2.1 Basic concepts in cancer vaccine
The term “cancer vaccine” can refer either to a prophylactic vaccine, given to prevent cancer, or to a therapeutic vaccine, given to eradicate an existing tumor. Representative cancer vaccines in the clinic include Gardasil® and Cervarix® against the HPV virus for preventing cervical cancer, and Sipuleucel-T as a therapeutic vaccine for metastatic prostate cancer [54]. Normally, a cancer vaccine contains a desired tumor antigen and an adjuvant capable of generating an immune response. Adjuvants act as the “danger signals” that stimulate the maturation of DCs. DCs then present the tumor antigens from the vaccine on MHC surface molecules and subsequently stimulate an anti-cancer T cell response.
Tumor antigens may be classified as tumor-associated antigens (TAA) or tumor-specific antigens (TSA), or may be aberrantly expressed proteins known as cancer-testis antigens (CTA). TAAs are proteins or glycoproteins expressed at higher levels in tumor cells than in normal cells. The antigenicity of TAAs lies in the anomalous expression profile, which sufficiently marks the cell as “other,” thereby overcoming immune tolerance [55]. TSAs are uniquely expressed solely by tumor cells. Noncancerous host cells lack genetic material encoding for TSAs, which reduces off-target effects. These antigens may arise from somatic mutations (i.e., neoantigens) and often evade immunological tolerance. Therefore, TSAs represent promising substrates for cancer vaccine design [56–58]. CTAs are a group of proteins that are normally expressed in fetal ovaries or adult testicular germ cells, but may also be expressed in several types of cancers. Since the expression is highly tissue-restricted, CTAs are also attractive substrates for cancer vaccine design [59, 60].
Based on the components, tumor antigens can be broadly classified as whole-cell antigens and subunit antigens. Whole-cell antigens contain broad epitopes for fully preserved tumor antigens but are often poorly defined, differ from one person to another, and are difficult to manufacture [61, 62]. Subunit antigens contain fewer but more defined antigens, including oncoproteins, peptides, DNA, mRNA, polysaccharides, etc. They will induce highly focused, specific responses with low reactogenicity. Because of this, subunit antigens lack intrinsic innate and/or adaptive immune triggers, thus necessitating the inclusion of adjuvants [63, 64].
2.2 Principles for applying nanomaterials to improve cancer vaccine efficiency
2.2.1 Co-encapsulation
Overall, the therapeutic efficacy of current cancer vaccines remains suboptimal. An important reason for this is that conventional vaccine administration methods lead to rapid elimination and degradation of antigen (especially subunit antigens), as well as inefficient DC uptake and antigen-presentation [65]. In addition, giving antigen and adjuvant independently in the clinic as free, separate forms may result in immune tolerance due to lack of “danger signals” to DCs encountering antigens [66, 67]. The use of nanomaterials to co-encapsulate and co-deliver antigen and adjuvants will protect the cargos from degradation and increase DC uptake efficiency, and may result in more robust T-cell responses [38, 39, 49].
2.2.2 Adjuvant effect
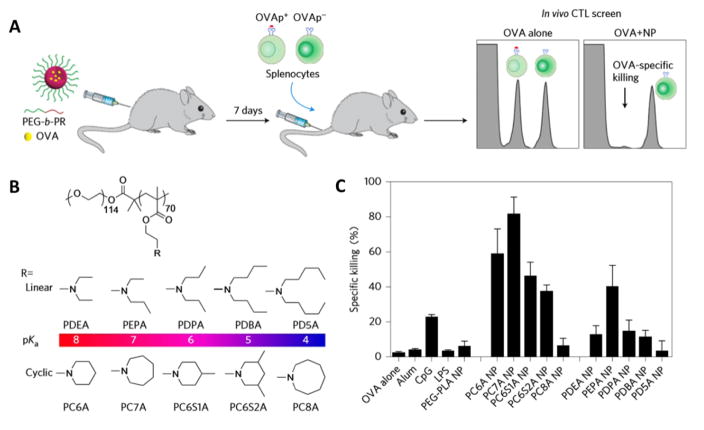
Certain nanomaterials may have inherent immune-regulatory activities in cellular and humoral immune responses, in that these nanomaterials can help to promote antigen presentation or stimulate immune responses without the incorporation of specific adjuvants [68, 69]. For example, cationic lipid (R)-DOTAP is an immunologically active enantiomer of the DOTAP racemic mixture, and could initiate a strong immune response in an exogenous antigen model [70, 71]. Specific functionalization and different lateral side dimensions of graphene and carbon-based nanomaterials showed distinct impacts on immune cells, which would induce a specific activation of dendritic cells and monocytes and serve as a nanoadjuvant for the development of an effective antitumor immune response [72–75]. In a recent study, Luo et al. performed an in vitro screening of a series of ultra-pH sensitive copolymers containing tertiary amines with linear or cyclic side chains for their ability in generating CTL responses. By using ovalbumin (OVA) as a model antigen and quantifying an in vivo CTL assay, they found that PC7A nanoparticles induced the highest OVA-specific splenocytes killings (Fig. 4). Mechanistically, the PC7A nanoparticles achieved efficient cytosolic delivery of tumor antigens while simultaneously activating the stimulator of interferon genes (STING) pathway, which offers a simple and robust strategy in boosting anti-tumor immunotherapy [76].
Fig. 4. Screening of copolymers for generating CTL responses.
(A) Schematic of the carboxyl fluorescein succinimidyl ester (CFSE) method to screen for polymer structures that generate a strong OVA-specific CTL response. (B) Ultra-pH sensitive polymer candidates for screening. (C) Quantitative comparison of OVA-specific CTL responses in different groups. Adapted by permission from Macmillan Publishers Ltd: [Nature Nanotechnology] [76], copyright (2017).
2.2.3 Lymph node drainage
Nanoparticulate vaccines are generally administered subcutaneously, and then drain to the lymph node, where they are acquired and presented by resident DCs. Migratory DCs around the injection site can also traffic the nanoparticles to the lymph node, but with relatively slower kinetics and lower efficiency. During this process, the size of nanoparticle is the primary factor determining whether it will spontaneously drain to lymph node or be taken up by the migratory DCs [77]. In one study, when polystyrene particles between 20 and 2000 nm were administered subcutaneously, 20 nm particles drained to the lymph node 2 hours post-injection, while 1000 nm particles had not drained to the lymph node even after 48 hours [78]. In another study, when antigens were covalently conjugated to solid core nano-beads ranging from 20 nm to 2000 nm, 40 nm nano-beads induced significantly higher T-cell responses than other bead sizes, suggesting 40–50 nm is an optimal size for nanovaccine to elicit a robust immune response [79].
2.2.4 DC uptake
The uptake efficiency of nanoparticulate vaccines by DCs is affected by particle size, surface charge, and hydrophobicity. Nanovaccines are taken up less efficiently by DCs when their size exceeds 500 nm [80]. Such relatively large particles are ingested mainly by macrophages [81]. In vitro studies show that particles with a positive surface charge are generally ingested more efficiently by DCs than those with a neutral or negative charge [82]. For liposomes, the lipid composition not only affects the surface charge and efficiency of uptake by APCs, but also determines whether the liposomal content is released in early or late endosomal compartments, which has a major impact on the presentation of distinct peptide epitopes [83, 84]. Hydrophobicity of polymeric nanoparticles is a key factor for opsonization, and vaccination strategies might benefit from complement activation as a signal to activate DCs [85, 86].
2.2.5 DC targeting
Ligands for DC-specific surface receptors can be grafted onto the surface of particle-based vaccines to increase DC-specific delivery. Numerous DC-specific receptors, including Fc receptors (FcRs) and a range of C-type lectin receptors (CLRs), have been harnessed for targeted delivery of vaccine components (Table 1) [87]. Nanoparticles can be targeted to FcRs using intact antibodies or Fc fragments [88, 89]. CLRs represent a family of receptors that bind to specific carbohydrate residues in a calcium-dependent manner via their carbohydrate-recognition domain. CLRs harnessed for DC targeting include mannose receptor [90, 91], DEC-205 [92, 93], dendritic cell-specific ICAM3-grabbing nonintegrin (DC-SIGN) [94, 95], DC-associated C-type lectin-1 (Dectin-1) [96], LOX-1 [97], and Clec9A [98]. The choice of specific target receptors may have a significant impact on the immunological outcome of vaccination. For example, it has been observed that antigens targeted to DEC-205 result mainly in cross-presentation of antigen to CD8+ T cells, whereas antigens targeted to Dectin-1 are preferentially presented via MHCII molecules to CD4+ T cells. The difference is explained by the fact that these CLRs are expressed on distinct DC subsets that differ in their functional properties and antigen-processing capacity [99].
Table 1.
Receptors used for DC targeting
2.2.6 Antigen presentation
Effective activation of CTL and tumor killing relies on the presentation of exogenous antigens in the context of MHCI molecules. During this process, cross-presentation is highly important. Soluble antigens internalized by macropinocytosis generally have low efficiency in cross-presentation and are poorly presented to MHCI, while nanoparticle-loaded antigens enter APCs via phagocytosis, and present antigen more efficiently by MHCI. Therefore, nanoparticulate system could induce dramatically higher humoral or cellular immune responses [100]. Cationic carriers with better endosome escape ability would help this process and further enhance CTL response [101]. Nanoparticle size also matters in antigen cross-presentation. Amorphous silica nanoparticles with diameters of 70 and 100 nm enhanced exogenous antigen entry into the cytosol from endosomes and cross-presentation, while submicron-sized silica particles (>100 nm) did not [102]. Demento et al. studied the role of sustained antigen release from nanovaccines in shaping the T cell memory phenotype. By comparing of the long-term immune effect of PLGA and liposome as the nanoparticulate platform, they found that PLGA particle induced higher frequency of effector-like memory T cell phenotype, and showed that this effect is due to sustained antigen release from the particulate platform [103].
2.3 Nanomaterial-loaded peptide vaccines for cancer immunotherapy
Peptide vaccines are composed of one or more small fragments of tumor-derived antigen proteins and a vaccine adjuvant. Typically, CD8+ T cells recognize peptides associated with MHCI molecules, which are 8 to 10 amino acids long. MHCII molecules can present much larger peptides, often 13 to 18 amino acids long, but generally stimulate CD4+ T cells [104]. Due to ease of synthesis and stable formulation, peptide vaccines are widely studied in research and clinic [65]. However, when peptide vaccines are administered subcutaneously or in other sites in free form, the short peptide fragments will endure rapid elimination and degradation [105]. Therefore, peptide vaccines in free form are not a good clinical candidate to stimulate a strong T-cell response.
Using nanomaterials for peptide vaccine delivery would greatly improve the efficiency of peptide vaccines. Peptide antigens and adjuvants can be either physically encapsulated in or chemically linked to the surface the nanoparticles. In one study, Kuai and co-workers showed that peptide antigens coupled with adjuvants could be co-delivered by conjugating them to synthetic high-density lipoprotein (sHDL) nanodiscs. This nanoparticle-mediated co-delivery resulted in accumulation within lymphoid organs and better antigen presentation. These nanovaccines generated 47-fold more neoantigen-specific CTLs when compared to soluble vaccines (Fig. 5) [106]. Poly(D,L-lactide-co-glycolide) (PLGA) has excellent safety profile in humans, therefore, PLGA particles are widely applied as peptide vaccine delivery systems. Antigens can be absorbed on or encapsulated into PLGA particles. After endocytosis by DC, PLGA particles may escape from the endosome, release antigens in the cytoplasm, and present antigen via the MHCI pathway, or antigen release happens directly inside the endosome, and present antigen via MHCII pathway [107]. In one study, PLGA nanoparticles encapsulating murine melanoma antigenic peptides, gp10025–33 and TRP2180–188, elicited stronger antigen-specific T-cell responses after intradermal injection when compared to peptide mixed with Freund’s adjuvant [108]. Efficacy of antigens loaded in other biodegradable polymers, like poly(amino acids) and polysaccharides, was also examined using various model antigens [109–112]. Using a model peptide antigen bearing four cysteines, Hao et al. reported a micelle vaccine by coupling peptides with thiopyridal derived poly(ethylene glycol)-b-poly(L-lysine) (PEG-b-PLL) block polymer. Encapsulation of immunostimulatory DNA can also be accomplished using charge based interactions, such as by condensing DNA into a cationic PLL core. These particles are engulfed by phagocytic DCs, wherein the particles are broken down through reduction of disulfide bonds within endosomes, triggering DNA and peptide release. This micellar vaccine system enhanced peptide concentration within DCs, a key factor in stimulating an immune response [113].
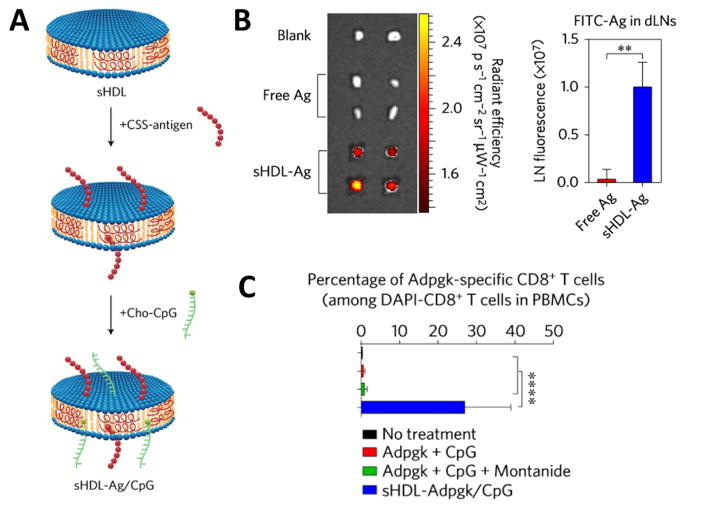
Fig. 5. sHDL nanodisc platform for cancer vaccine.
(A) sHDL nanodiscs, composed of phospholipids and apolipoprotein-1 mimetic peptides, are engineered for co-delivery of antigen (Ag) peptides and cholesterol-modified immunostimulatory molecules (Cho-CpG). (B) Fluorescence signals in the draining inguinal lymph nodes at 24 hours after subcutaneously administration of 31 nmol FITC-tagged Ag in free form or sHDL form. (C) The frequency of neoantigen Adpgk-specific CD8+ T cells in peripheral blood of C57BL/6 mice after vaccination three times with the indicated formulations (equivalent to 15.5 nmol mutated Adpgk peptide and 2.3 nmol CpG) in a biweekly interval. Adapted by permission from Macmillan Publishers Ltd: [Nature Materials] [106], copyright (2016).
Liposomes are another kind of important carrier system for cancer vaccine delivery. Liposomes are especially attractive for vaccine delivery systems due to their malleable structure and adaptability [114]. Regardless of whether antigens and adjuvants are hydrophilic or hydrophobic, liposomes can be used to encapsulate and deliver them. The inner core of liposomes is aqueous and can hold water-soluble antigens like proteins, peptides, DNA, mRNA, polysaccharide. On the other hand, hydrophobic cargo can be incorporated into the lipid bilayer. Still other systems use liposomes as a scaffold to which antigens and adjuvants are adsorbed or conjugated [115]. By changing the lipid components, size and surface charge, liposome-based vaccine systems can achieve various desired features. For example, previous work in our group has used a 1,2-dioleoyl-3-trimethylammonium-propane chloride (DOTAP)/E7 complex as an effective human papillomavirus (HPV) type 16 vaccine. In a TC-1 mouse tumor model, this liposomal vaccine used oncoprotein E7 to induce an effective antitumor response [116, 117]. Intercalating an E7-derived lipopeptide into the bilayer, rather than relying on a hydrophilic E7 peptide improved antitumor activity in this model. Compared to the previous DOTAP/E7 formulation, the DOTAP/E7-lipopeptide vaccine initiated stronger antigen-specific CD8+ T-cell responses in vivo [118]. The DOTAP/peptide formulation has been tested in a phase I clinical trial with an excellent safety profile. Based on the development of a lipid/calcium/phosphate (LCP) nanoparticle system for efficient intracellular delivery and release cargos into the cytoplasm [119], we constructed an LCP vaccine system by encapsulating a modified melanoma-specific antigen (p-TRP2 peptide) and an adjuvant (CpG oligodeoxynucleotides). The final LCP nanoparticles are 40–45 nm in diameter and have a mannose-modified surface to enhance DC uptake after draining to the lymph nodes. Using LCP as a vaccine carrier in B16F10 orthotopic models in the skin and lungs yielded significantly better tumor growth inhibition compared to administration of free antigen and adjuvant [90, 120]. This formulation also proved effective in treatment of colorectal cancer and liver metastasis when encapsulating p-AH1-A5 peptide as the antigen and 5′pppdsRNA as the adjuvant [121].
2.4 Nanomaterials for DNA and mRNA tumor antigens delivery
Nucleic acids like DNA and mRNA are being intensively studied in gene therapy of cancer or other genomic diseases. Transfecting cells with DNA encoding for oncogenic proteins or peptides is an appealing method for intracellular production and subsequent immune sampling of the target antigens [122]. Compared to traditional protein/peptide vaccine strategies, DNA-based vaccines can more closely mimic live infections. In addition, DNA vaccines eliminate the need for cold chain storage and transportation, and can be quickly altered by manipulating the transgene sequence to adapt to new and fast-emerging diseases [123, 124]. However, previous DNA cancer vaccines were mainly administered intramuscularly as naked DNA, and resulted in weak immunogenicity due to degradation by nucleases and inefficient delivery to APCs [125]. Synthetic nanomaterial-based delivery systems can be beneficial for these DNA-based therapeutics [126, 127]. Similar to DNA delivery in other biological transfection processes, DNA encoding antigens can also be delivered by various synthetic and natural nanosystems, including PLGA [128, 129], PEI/PGA [130], chitosan [131, 132], cationic lipids [133, 134], grapheme and carbon nanotubes[135], and even hybrid particles [136, 137].
Messenger RNAs (mRNAs) are another kind of non-toxic molecules for nucleic acid-based vaccination therapies. The physiological role of mRNA is to transfer genetic information from the nucleus to the cytoplasm, and then translated into the corresponding protein. The safety of mRNA-based treatments supports the use of mRNA-vaccination for therapeutic or prophylactic approaches. Due to the ease of degradation of RNA by extracellular ribonucleases, the success of RNA vaccine highly depends on a proper delivery system. Based on an established method for liposome-nucleic acids complex (lipoplex, LPX) preparation [138], a mRNA-lipoplex was constructed by mixing the reporter firefly luciferase (Luc)-encoding mRNA and cationic liposomes composed of common lipids such as N-(1-(2,3-dioleyloxy)propyl)-N,N,N-trimethylammonium chloride (DOTMA) and dioleoyl phosphatidylethanolamine (DOPE). By gradually decreasing the positive-to-negative charge ratio, i.v. injected Luc-LPX resulted in a luciferase expression shift from lungs to spleen, and even exclusively splenic signal for near-neutral and slightly negative particles. The LPX can protect RNA from degradation by extracellular ribonucleases and mediate its efficient uptake, as well as the expression of the encoded antigen by DC populations and macrophages in various lymphoid compartments [139]. This provides the possibility of designing personalized mRNA vaccines based on tumor neoantigen analysis: mutations identified by exome sequencing could be selected as vaccine targets based on their expression levels and MHC-binding capacity, and then synthetic poly-neoepitope mRNA vaccines could be rapidly manufactured on-demand. Also, several mutations could be addressed in one mRNA sequence, providing a simpler way to overcome tumor heterogeneity and immune editing problems compared to single mutation-targeted vaccines. In a CT26-Luc lung metastasis model, tumor growth was significantly inhibited after vaccination with a mixture of two RNA pentatopes. All mice survived the experiment in the RNA pentatope group, while 80% of the control mice did not [140].
2.5 Nanomaterials for whole-cell antigen delivery and immunogenic cell death
Whole-cell antigens can be found in by tumor-cell lysates or immunogenically dying tumor cells. Nanomaterials can also be applied in whole-cell antigen delivery for enhancing the immune responses. For example, 4T1 cell lysates were encapsulated into PLGA microparticles using a modified double emulsion solvent evaporation method. After subcutaneous injection, the PLGA microparticles reduced metastatic lung tumor burden by 42% without inducing autoimmunity [141]. In another study, a whole-cell cancer vaccine was constructed by an “infection-mimicking” PLGA matrix containing tumor lysate, granulocyte macrophage colony-stimulating factor (GM-CSF), and CpG-ODN. When this system was applied to animals for tumor therapy, specific and protective anti-tumor immunity was observed, and significantly prolonged animal survival was achieved [142]. In an alternative approach, a core-shell nanostructure was assembled by coating immunological adjuvant-coupling particles with cancer cell-derived membranes. These cancer-cell derived membranes coat the nanovehicles with membrane-associated tumor antigens that can be used to induce a broad antitumor immune response [143].
Immunogenic cell death (ICD) is a form of cell death that may be elicited under certain chemotherapies, radiotherapies, and photodynamic therapies [144–146]. Antitumor immune responses raised from ICD rely on the activation of DCs through exposure to tumor antigens, which then activate T-cell responses against these same tumor antigens. The identified features of ICD include exposure of calreticulin (CRT) on the surface and the release of large amounts of ATP and high-mobility box1 (HMGB1) into the extracellular milieu. Extracellular ATP release is a signal for DC recruitment and surface CRT exposure will interact with low density lipoprotein receptor-related protein 1 (LRP1) on DC to promote antigen uptake, while HMGB1 interacts with Toll-like receptor 4 (TLR4) to promote DC maturation. Therefore, ICD serves as an in situ approach for whole cell vaccination [147].
Using nanomaterials for enhancing the ICD effect or the combination of nanosystems with other ICD inducers has proven promising in inducing noticeable anti-tumor immune responses. Min et al. utilized a kind of antigen-capturing nanoparticle (AC-NPs) for improving the abscopal effect after radiotherapy of the primary tumor. By optimizing the surface properties, they showed that these AC-NPs could deliver the released antigen to APCs and significantly improve the efficacy of anti-PD-1 treatment (Fig. 6) [148]. Currently, confirmed ICD chemo-inducers include oxaliplatin, doxorubicin, mitoxantrone, bortezomib, and cyclophosphamide [149]. Co-delivery of adjuvants with an ICD inducer in a particulate delivery system has proved helpful in potentiating anti-tumor immune responses [150]. Photodynamic therapy (PDT) using nanomaterials loaded with photosensitizers will also induce ICD in tumors, and this has proven efficient in inducing an in situ antitumor vaccination and effective when combined with checkpoint blockade in elimination of tumors exposed to the NIR laser [151–155]. Moreover, antitumor immunity can inhibit the growth of distant tumors and protect mice from tumor cell re-challenge.
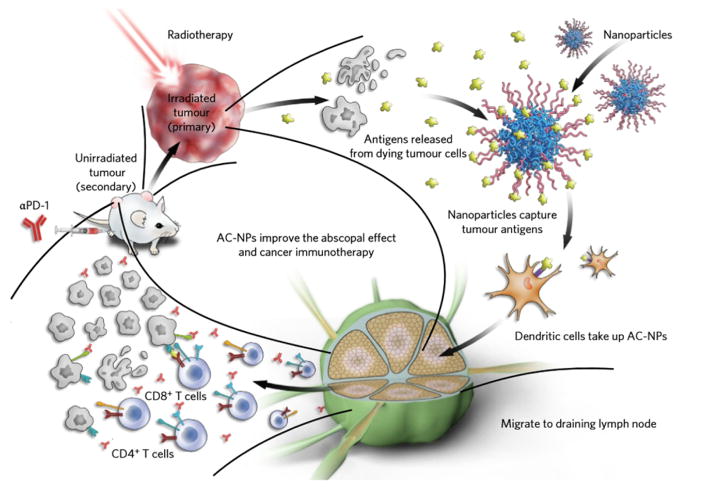
Fig. 6. Mechanism of utilizing AC-NPs for improving cancer immunotherapy.
Radiation of the primary tumor induces antigen release. Intratumorally administered AC-NPs bind with tumor antigens and improve their presentation to DCs. The improved immune activation combined with anti-PD-1 treatment eradicates the unirradiated secondary tumor. Adapted by permission from Macmillan Publishers Ltd: [Nature Nanotechnology] [148], copyright (2017).
3. Application of nanomaterials for TME modulation
3.1 Immunosuppressive TME as a barrier for successful cancer immunotherapy
The immunosuppressive TME is a significant challenge to effective cancer immunotherapy. Suppression of tumor-specific T cells is orchestrated by the activity of tumor cells, tumor-associated stromal cells such as TAFs, and tumor-infiltrating immune cells including TAMs, MDSCs, and Tregs, and as well as a variety of chemokines or cytokines such as adenosine, prostaglandin-E2 (PGE-2), transforming growth factor beta (TGF-β), indoleamine 2,3-dioxygenase (IDO), interleukin-10 (IL-10), galectins, etc. [156, 157]. Modulating the immunosuppressive TME by, for example, targeting immunosuppressive cellular modulators or inhibiting some soluble mediators will significantly promote the immune responses inside a tumor.
Since the immune system operates in a Janus fashion, any systemic inhibition on one direction will cause unexpected side effects to the other. Serious side effects and a lack of tolerance are a concern with systemic administration of inflammatory agents [158]. The side effects of immune checkpoint inhibitors become more prominent as clinical trials increase [159]. Using nanomaterials to deliver immune modulators will restrict the distribution of these agents at the tumor site and reduce systemic toxicity. Therefore, some extremely potent while highly toxic immune modulating agents may be developed in nanoformulations and applied to clinic.
3.2 Nanomaterial system targeting immune checkpoints
T-cell activation is a multiple-signal process, including TCR recognition with MHC-peptide antigen, CD28/B7 co-stimulation and necessary cytokines for stimulating T-cell expansion and differentiation. Besides, interactions between ligands and activating or inhibitory receptors play critical role in further regulating the T-cell activation or tolerance. These co-stimulation signals are frequently referred to as immune checkpoints which can be divided into co-inhibitory or co-stimulatory signals. Tumor cells can express a selected subset of co-inhibitory ligands for evading the immune surveillance, including PD-L1 which has been successfully applied as target for tumor immunotherapy. Co-stimulatory checkpoint targets, such as OX40, 4-1BB (CD137) and CD40, can also be applied for improving the anti-tumor immunity [160, 161]. Immune-related adverse effects (irAE), often in the form of major organ toxicity, may arise from immunotherapies. irAE are a new class of toxicity profiles that require dramatically different interventions than toxicities arising from cytotoxic drugs. For example, anti-CTLA4 antibodies mostly affect the skin and the gastrointestinal tract, observed in 44% and 35% of cases, respectively [162], and the main irAE profiles for PD-1/PD-L1 antibodies are asthenia (16–34%) [163].
Nanomaterials provide an ideal approach for selective delivery of immune checkpoint modulators to tumors. In a previous report, anti-CTLA-4 antibody was loaded in functionalized mesoporous silica, and after intratumoral injection, this formulation reduced tumor growth more substantially and for a longer period of time than systemic antibody injections [164]. Inspired by the accumulation of platelets to wound sites, Wang et al. conjugated PD-L1 antibodies to the surface of platelets. The platelets decorated with anti-PD-L1 resulted in 9.4-fold higher accumulation around the surgical wound and residual microtumors than those treated with free anti-PD-L1 mAbs, and significantly prolonged overall mouse survival after surgery by reducing the risk of cancer regrowth and metastatic spread [165]. Targeted delivery of siRNA against immune checkpoints is another way of inducing a tumor-specific, local immunomodulatory effect [166, 167]. For example, treatment of epithelial ovarian cancer SKOV-3 cells with PD-L1 siRNA loaded in folic acid functionalized polyethylenimine (PEI) would make these cells more sensitive to T-cell killing, and this is twofold more sensitive compared to scrambled siRNA treated controls [168].
Nanomaterial-based delivery systems can also help to improve the immune activation and reduce systemic toxicity of the immune stimulatory checkpoint targets. For example, by conjugating anti-OX40 mAb to the surface of PLGA nanoparticles, stronger CTL proliferation and cytokine production was observed when this nanoformulation was injected intratumorally compared to administration of free anti-OX40 mAb [169]. In another study, anti-CD137 mAb and engineered IL-2Fc fusion protein were anchored to the surfaces of PEGylated liposomes. While free anti-CD137 antibodies have been associated with lethal toxicity, conjugation to PEGylated liposomes reduced this inflammatory toxicity and boosted the antitumor response. The reason for the enhanced efficiency and reduced toxicity was attributed to the physical size of the PEGylated liposomes, which were small enough to distribute evenly throughout the tumor parenchyma and draining lymph nodes but too large to efficiently move from the tumor into systemic circulation following intratumoral injection [170].
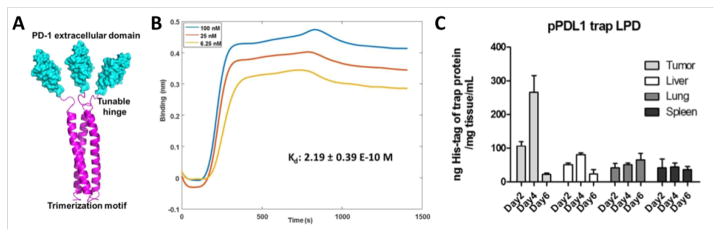
Locally expressed fusion proteins provide another option for checkpoint blockade. Take PD-L1 as an example; PD-L1 trap protein was developed by genetically fusing the extracellular domain of PD-1 with a robust trimerization domain from cartilage matrix protein through an optimized hinge linker (Fig. 7A). The trimeric form of PD-L1 trap protein binds to mouse PD-L1 with a dissociation constant (Kd) of 219 pM, a binding affinity more than a thousand times higher than that of monomeric PD-1 and PD-L1 (Fig. 7B) [171]. For local and transient expression of the PD-L1 trap protein, the optimized coding sequence for the monomeric trap was cloned into the expression vector pcDNA3.1, driven by a CMV promoter, and a strong signaling peptide from mouse serum albumin preproprotein was incorporated at the N-terminus to facilitate trap secretion from transfected cells after expression. When loading the PD-L1 trap plasmid into a lipid-protamine-DNA (LPD) nanoparticle, selective expression of PD-L1 trap protein was observed in both CT26 colon tumors and KPC pancreatic tumors. Importantly, the expression of PD-L1 trap protein is a transient process, with the highest expression from day 2 to day 4, and significantly reduced expression on day 6 (Fig. 7C). This locally expressed PD-L1 blocker is promising in enhancing the therapeutic efficiency and reducing possible side effects of free anti-PD-L1 [171].
Fig. 7. PD-L1 trap protein and local expression.
(A) The schematic of self-assembled trimeric PD-L1 trap from a fusion protein between the PD-1 extracellular domain and the trimerization domain of CMP1. (B) The binding of trimeric ligand at different concentrations to immobilized PD-L1 with an estimated Kd of 219 pM. (C) Transient and local expression of His-tag labeled trap plasmid quantified by His-tag ELISA. Adapted with permission from ref [171]. Copyright (2017) American Chemical Society.
3.3 Nanomaterial system targeting soluble mediators
Various cytokines or chemokines are important players in the immunosuppressive TME. Targeted inhibition of these key players using small molecular drugs, siRNA, or fusion proteins is an important part of TME modulation. Key cytokines like TGF-β play prominent roles in the regulation of cancer cell proliferation and migration. In the TME, TGF-β mediates the interactions between tumor cells and various stroma cells and contributes to an pro-tumoral inflammation status [172, 173]. Various kinds of TGF-β or TGF-β receptor inhibitors have been developed, and nanoparticle systems would help to improve the efficiency while reducing side effects. In a recent study, Park et al. developed nanoscale liposomal polymerial gels (nanolipogels, nLGs) for simultaneous delivery of IL-2 and small molecule TGF-β receptor-I inhibitor SB505124. The nLGs significantly increased the infiltration of CD8+ T cells, NK cells, and delayed tumor growth with prolonged mice survival [174]. Furthermore, Xu et al. found that a TGF-β siRNA-loaded liposome-protamine-hyaluronic acid (LPH) nanosystem efficiently boosted the vaccine efficiency in an advanced melanoma tumor model by increasing tumor infiltrating CD8+ T cells and decreasing Tregs [120]. IDO, an enzyme that catalyzes tryptophan degradation, is also an important regulator in tumor-mediated immune tolerance [175]. T cells will undergo proliferative arrest when exposed to IDO-provoked tryptophan shortage [176]. Many IDO inhibitors have been confirmed and applied as adjuvant therapy in cancer immunotherapy, although a need remains for greater efficiency and lower toxicity in nanoparticulate IDO inhibitors [177]. Cyclooxygenase-2 (COX-2) and its enzymatic product PGE2 are important players in cancer initiation, progression, and metastasis [178]. In a tumor immune microenvironment, PGE2 shifts the cytokine profile from pro-inflammatory to anti-inflammatory through the recruitment of Tregs and MDSCs [179, 180]. The combination of immunotherapy with COX-2 inhibitors like rofecoxib, celecoxib and aspirin has shown a significant increase in tumor inhibition [181–183]. Locally expressed fusion proteins can also be designed to block important chemokines, modulate the immunosuppressive TME, and promote anti-tumor efficiency. Aiming at understanding the important role of the CXCL12/CXCR4 axis in tumor cell migration, Goodwin et al. developed an LCP nanoparticulate system for delivering plasmid DNA encoding an engineered CXCL12 trap protein. By decorating the nanoparticle surface with galactose, the LCP nanoparticles showed rapid and extensive hepatic accumulation and predominant expression of the CXCL12 trap protein in liver, which greatly decreased the occurrence of liver metastasis in two aggressive liver metastasis models [184, 185].
3.4 Nanomaterial system targeting cellular mediators
3.4.1 Targeting TAMs
Tumor associated macrophages are categorized apart from normal macrophages because they have been hijacked by the tumor to secrete tumor growth and survival factors [186, 187]. TAMs are generally characterized by high M2/M1 ratios, producing high amount of immunosuppressive cytokines like IL-10, TGF-β and PGE2 and low levels of inflammatory cytokines like IL-12, IL-1β, TNF-α, IL-6 [188]. The ability of TAMs to present tumor-associated antigens is decreased compared to normal macrophages, as is their ability in stimulating of the functions of T cells and NK cells [189]. Therefore, targeting TAMs is an important therapeutic strategy against cancer. The current TAM-targeted approaches can be classified into four aspects (Table 2): (1) inhibiting macrophage recruitment, using inhibitors of CCL2/CCR2 [190], CCL5 [191], macrophage colony-stimulating factor 1 (CSF-1) [192], etc. (2) suppressing TAM survival, using chemical drugs (such as bisphosphonates) that deplete macrophages directly [193], recombinant immunotoxin anti-FRβ-Pseudomonas exotoxin A [194], and legumain-based DNA vaccine [195]; (3) enhancing M1 tumoricidal activity of TAMs, like agonists of nuclear factor-κB (NFκB) [196], activation of MAPK/ERK pathway [197], GM-CSF [198], etc. and (4) blocking M2 tumor-promoting activity of TAMs, like inhibitors of STAT3 [199], gamma isoform of phosphoinositide 3-kinase (PI3Kγ) [200], etc. In the application of these agents, nanoparticles are always applied to improve the delivery efficiency and enhance the therapeutic efficiency. For example, clodronate is a drug used for macrophage depletion, however, its in vivo application suffers from a short half-life and accumulation in bone. When encapsulating clodronate in liposomes and administered to melanoma-bearing mice, smaller tumors with reduced angiogenesis and tolerable toxicity was observed [201]. M2 TAMs overexpress mannose receptor (CD206), and can therefore be targeted directly by mannose-modified nanoparticles. This mannose can be conjugated to the outer surface of the nanoparticles or may be exposed in acidic environments through a sheddable PEG layer as developed by. Zhu et al. TME [202]. By phage display and high-throughput sequencing, Pun et al. identified a unique peptide sequence, M2pep, which preferentially binds to murine M2 cells, including TAMs, with low affinity for other leukocytes [203, 204]. This peptide sequence can be further applied for TAM-specific nanoparticle delivery.
Table 2.
Cellular mediators and therapeutic approaches for modulating immunosuppression in the TME
| Cellular mediator | Mechanism of intervention | Target | Strategy | Reference |
|---|---|---|---|---|
| TAMs | Inhibition of macrophage recruitment | CCL2/CCR2, CCL5/CCR5, CSF-1 | CCL2 neutralizing antibody, Maraviroc, CCL5 neutralizing antibody, CCR5 blocking antibody, CSF-1 neutralizing antibody | [190–192] |
| Suppressing TAM survival | FRβ, legumain | Bisphosphonate, clodronate, anti-FRβ-Pseudomonas exotoxin A, legumain-based DNA vaccine | [193–195, 201] | |
| Enhancing M1 macrophage activity | NFκB pathway, MAPK/ERK pathway, VEGF | TLR agonists, CuNG, GM-CSF | [196–198] | |
| Blocking M2 tumor-promoting activity | STAT3, PI3Kγ | Sunitinib, IPI-549 | [199, 200] | |
| MDSCs | Blocking development of MDSCs | JAK2/STAT3 signaling | cucurbitacin B, sunitinib | [209, 272] |
| Differentiation of MDSCs into mature cells | Flt3 | Retinoic acid, vitamins D3. | [273, 274] | |
| Inhibiting the function of MDSCs | PDE5, COX-2/PGE2, JAK1/STAT3 | Sildenafil, SC58236, acetylsalicylic acid, celecoxib, CDDO-Me | [212, 214, 275, 276] | |
| Depletion of MDSCs | Hsp90 | Gemcitabine, 17-DMAG | [277, 278] | |
| Tregs | Blocking Tregs recruitment | CCR4/CCL22 | Anti-CCR4 mAb (KW-0761) | [226] |
| Intratumoral Treg depletion | IL-2Rα/CD25 | Daclizumab | [227] | |
| Modulation of Tregs | STAT3, STAT5 | Cyclophosphamide, imatinib mesylate, sunitinib, sorafenib | [228–231] | |
| Inhibiting the function of Treg | CTLA4 | Anti-CTLA4 mAb | [232] | |
| TAFs | Inhibiton of TAF activation | FAP, PDGF-R, TGF-β, SDF-1, Hh signaling | Anti-FAP vaccination, imatinib, AMD3100, Hh antagonist, anti-Hhligand-blocking antibody | [253, 255, 256, 263] |
| Blocking interactions between TAFs and tumor cells | CXCL12/CXCR 4, TGF-β, HGF, COX-2/PGE2 | AMD3100, LY550410, celecoxib, dexamethasone | [257–261] | |
| Blocking interactions between TAFs and endothelial cells | VEGF, PDGFs, FGFs, MMPs | Avastin, sorafenib yosylate, imatinib mesylate, ponatinib, batimastat | [254, 262, 264, 265] | |
| Targeting TAF-induced inflammation | NFκB, CXCL12, CXCL13 | QNZ, AMD3100, tadalafil, sildenafil | [266–268] |
3.4.2 Targeting MDSCs
MDSCs are a suppressive class of myeloid immune cells to control disease-related inflammation, including cancer inflammation [205]. Activated MDSCs produce arginase-1 (ARG1), nitric oxide synthase 2 (iNOS2), IDO, nicotinamide adenine dinucleotide phosphate oxidase (NOX) and immunosuppressive cytokines that have the potential to inhibit CTLs, DCs, NK cells as well as expand Tregs. This leads to an immunologically permissive TME [206, 207]. Therefore, MDSCs are an important target for enhancing the anti-tumor immunity. Based on the origin and functions of MDSCs, current methods for targeting MDSCs can be placed into four categories (Table 2): (1) blocking development of MDSCs through the use of Janus kinase 2 (JAK2)/STAT3 inhibitors cucurbitacin B [208], and multi-kinase inhibitor sunitinib [209]; (2) differentiation of MDSCs into mature cells, using retinoic acid [210], vitamins D3 [211], etc. (3) inhibition of the function of MDSCs, using phosphodiesterase-5 (PDE-5) inhibitors [212], COX2/PGE2 inhibitors [213, 214], JAK1/STAT3 inhibitors like bardoxolone methyl (CDDO-Me) [215], etc. and (4) depletion of MDSCs, using gemcitabine [216] and heat shock protein 90 (Hsp90) inhibitor 17-dimethylaminoethylamino-17-demethoxylgeldanamycin (17-DMAG) [217]. Nanoparticles can help to improve the therapeutic efficiency of these inhibitors by improving the solubility, targeted delivery to tumors, and controlling release. For example, Zhao et al. showed that intravenous delivery of CDDO-Me using a PLGA nanoparticle significantly decreased both Tregs and MDSCs in a B16F10 melanoma model, and remodeled the TAFs, collagen, and vessels in the TME. When combined with an LCP nanoparticle loaded TRP2 peptide vaccine, the PLGA-CDDO-Me nanoparticle enhanced the antitumor efficacy than the TRP2 vaccine alone treatment [218]. In another study, Kourtis et al. found tumor-associated MDSCs and monocytes could be preferentially targeted by ultrasmall Pluronic-stabilized poly(propylene sulfide) nanoparticles [219].
3.4.3. Targeting Tregs
Regulatory T cells are an immunosuppressive class of T cells responsible for controlling the severity of immune responses; without Tregs, autoimmune disease and cytokine storms would be a risk in any minor infection. Tregs typically act by suppressing activation and expansion of effector T cells [220]. In cancer, however, malignant cells promote and enlarge this effect and prevent effective immunotherapy, and tumor-infiltrating Tregs reduce anti-tumor T effector cell activity and therefore correlate with poor prognosis in a number of cancers [221, 222]. Tregs can suppress effector T cells through direct mechanisms including depriving IL-2 from the surrounding via their high affinity IL-2 receptor (IL-2R), expressing CTLA-4 and down-modulating CD80/CD86 expression by APCs, and producing immune-suppressive cytokines such as IL-10 [223]. Under the deprivation of co-stimulatory signal, responder T cells with high-affinity TCRs for the presented antigen die by apoptosis. Accumulating evidence has proven that the removal of Tregs evokes and enhances anti-tumor immune responses [224, 225]. Generally, strategies targeting Tregs in tumors can be divided in four categories (Table 2): (1) blocking Tregs recruitment into tumor, for example by interfering with the CCR4/CCL22 axis [226]; (2) direct intratumoral Tregs depletion, using mAbs against IL-2Rα/CD25 [227]; (3) nonspecific modulation of Tregs by traditional chemo agents like cyclophosphamide [228], imatinib mesylate [229], sunitinib and sorafenib [230, 231]; (4) preferential inhibition of Tregs function with checkpoint blockade like anti-CTLA4 [232]. However, systemic application of agents for depleting Tregs may concurrently elicit deleterious autoimmunity. Nanoparticles provide an option for evoking effective tumor immunity without autoimmunity by specific delivery of agents to the tumor site. For example, Gamrad et al. reported cases of biofunctionalized gold nanoparticles efficiently transporting siRNA to murine Tregs [237]. To facilitate selective delivery to Tregs residing in the TME, Sacchetti et al. conjugated PEG-modified single-walled carbon nanotubes (PEG-SWCNTs) with a ligand against glucocorticoid-induced TNFR-related receptor (GITR), which is highly overexpressed on intratumoral Tregs compared to peripheral (splenic) Tregs. GITR-mediated targeting led to a greater accumulation of PEG-SWCNTs within B16 melanoma-associated Tregs than in other tumor-associated immune cells or splenic Tregs, indicating high specificity [233]. Directed targeting of TME mediators such as Tregs could lead to a breakthrough in cancer immunotherapy.
3.4.4 Targeting TAFs
Tumor-associated fibroblasts (TAFs), also called cancer-associated fibroblasts (CAFs), mediate key cancer-sustaining and proliferating pathways including angiogenesis, invasion, and metastasis [234–237]. Normal fibroblasts support the fibrous extracellular matrix (ECM) of the connective tissue. However, cancer cells activate fibroblasts into a wound-healing-like state associated with increased proliferation and cytokine production [238]. For example, about 80% of TAFs are activated in breast carcinomas [239]. Activated fibroblasts express fibroblast activation protein (FAP), α-smooth-muscle actin (α-SMA) (leading to the term “myofibroblasts”), and secrete increased amounts of ECM proteins and growth factors [240, 241]. Recent studies have also proved that TAFs promote immunosuppression and immune evasion in the TME [242]. TAFs modulate the TME through paracrine signaling pathways that induce a Th2 phenotype in neighboring immune cells, which results in tumor cell proliferation and fewer active T effectors cells. For example, in the presence of TAFs, monocytes prominently differentiate into M2 polarized TAMs [243, 244]. In pancreatic cancer, TAFs secrete thymic stromal lymophopoietin (TSLP) to activate and mature myeloid DCs into Th2 phenotype [245, 246]. In addition, Th2 cytokines induce the differentiation of myeloid cells into MDSCs [247]. Similarly, TAFs secrete factors like TGF-β and IDO to expand the Treg population [248]. TAFs also produce fibrin, increasing tumor fibrosis, which acts as physical barrier to T-cell infiltration [249, 250]. Furthermore, TAFs may inhibit T cell activity through PD-L1 or PD-L2 expression [251, 252]. Therefore, TAFs inhibit anticancer immune activity through both physical barriers and chemical signaling pathways, as well as actively promoting tumor growth and metastasis.
As we continue to study the importance of TAFs in TME, we better understand that targeting TAFs is an important component of cancer immunotherapy. Generally, immunotherapies aiming at TAFs can be classified as targeting TAFs themselves and disturbing their interactions with tumor cells or other stroma cells, including (Table 2): (1) inhibiting the expression and activity of FAP [253], platelet-derived growth factor receptor (PDGF-R) [254], TGF-β, stromal cell-derived factor-1 (SDF-1, CXCL12) [255], hedgehog (Hh) signaling [256], etc.; (2) blocking interactions between TAFs and tumor cells through blocking CXCL12/CXCR4 [257], TGF-β [258, 259], hepatocyte growth factor (HGF) [260], and COX-2/PGE2 [261]; (3) blocking interactions between TAFs and endothelial cells through VEGF [262], PDGFs [263], fibroblast growth factors (FGFs) [264], and MMPs [265]; and (4) targeting TAF-induced inflammation and immunosuppression via inhibition of NFκB [266], CXCL12 [267], CXCL13 [268], etc. High densities of fibroblasts always constitute a stromal barrier, especially in desmoplastic tumors, which hinders nanoparticles diffusion to tumor cells inside the tumor [269, 270]. However, this also indicates that targeting fibroblasts may be a much easier option for nanoparticle-based drug delivery. In one study, Miao et al. designed an LPD nanoparticle loaded with plasmid DNA encoding TNF-related apoptosis-inducing ligand (TRAIL) protein. Three injections of the LPD nanoparticles generated approximately 70% of TAFs as TRAIL-producing cells, and triggered apoptosis in the tumor cell nests adjacent to the TAFs [271]. Furthermore, when loading plasmids encoding CXCL12 fusion protein in the LPD nanoparticles, tumor-selective expression of CXCL12 trap protein resulted in subversion of the immunosuppressive TME in pancreatic cancer and effectively inhibited tumor growth [171].
4. Conclusion and Future Directions
In this review, we summarized the basic principle of cancer immunotherapy and application of nanomaterials in cancer vaccine and immunosuppressive TME modulation. Cancer vaccination focuses mainly on the systemic immune response, which can cause more effective immune cell generation in the tumor or prevention of metastasis or tumor recurrence, while TME modulation mainly focuses on the solid tumor local immune responses and helps the tumor-infiltrating immune cells to be effective inside the tumor. In most occasions, the combination of a cancer vaccine and TME modulation is necessary to restore the cancer immunity circle. Nanomaterial has been shown to be an essential piece of cancer immunotherapy, as nanomaterial-based immunotherapy produces longer-lasting and broader immune responses than free drugs alone. Due to complicated stromal signaling inside the tumor, reshaping the immune profile of the TME appears to be more effective than the use of cytotoxic drugs directly on tumor cells. Additionally, studies on cancer immunotherapy should pay more attention to tumor categories since different tumor models may have completely different immune cell infiltration statuses or TME immune signatures [279].
As the field of immune-oncology expands, we fully expect nanomaterials to develop in tandem, producing new, innovative approaches to cancer immunotherapy. For example, the effects of many chemo drugs are found to interact with host immune responses, and nanomaterials-based formulation may magnify this effect. Cancer vaccines developed using patient-derived neoantigens and more traditional subunit vaccines using model antigens are achievable goals with the versatility of nanoparticles as delivery vectors. Nanomaterials can also be harnessed to improve adoptive cell transfer via ex vivo T cell expansion through the development of synthetic antigen-presenting agents or APCs. Without a doubt, cancer immunotherapy can be improved with the use of nanomaterials, and nanomaterial-based immunotherapy is sure to be a growing area of research.
Acknowledgments
The work in the authors’ lab was supported by NIH grants CA198999 and DK100664, and by Eshelman Institute for Innovation. It was also supported by National Natural Science Foundation of China (Project 51673185) and China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nature reviews Drug discovery. 2016;15:235–47. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 6.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–90. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Vacchelli E, Bravo-San Pedro JM, Buque A, Senovilla L, Baracco EE, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nature reviews Cancer. 2016;16:131–44. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. Journal of immunology. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asmana Ningrum R. Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica. 2014;2014:970315. doi: 10.1155/2014/970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinion in immunology. 2013;25:268–76. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nature reviews Cancer. 2006;6:613–25. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews Drug discovery. 2015;14:561–84. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 16.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nature reviews Drug discovery. 2015;14:603–22. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature medicine. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–24. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 22.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nature reviews Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nano. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 25.Mitragotri S, Anderson DG, Chen X, Chow EK, Ho D, Kabanov AV, et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS nano. 2015;9:6644–54. doi: 10.1021/acsnano.5b03569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min Y, Caster JM, Eblan MJ, Wang AZ. Clinical Translation of Nanomedicine. Chemical Reviews. 2015;115:11147–90. doi: 10.1021/acs.chemrev.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. International journal of nanomedicine. 2014;9:4357–73. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of controlled release: official journal of the Controlled Release Society. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in enzyme regulation. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 30.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Molecular pharmaceutics. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I, et al. Investigating the optimal size of anticancer nanomedicine. Proceedings of the National Academy of Sciences. 2014;111:15344–9. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Tang Z, Zhang D, Song W, Zhang Y, Yang Y, et al. Pharmacokinetics, biodistribution and in vivo efficacy of cisplatin loaded poly(L-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for tumor therapy. Journal of controlled release: official journal of the Controlled Release Society. 2015;205:89–97. doi: 10.1016/j.jconrel.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, et al. Targeted nanoparticles for cancer therapy. Nano today. 2007;2:14–21. [Google Scholar]
- 34.Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM, et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proceedings of the National Academy of Sciences. 2016;113:E1142–E51. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W, Tang Z, Zhang D, Zhang Y, Yu H, Li M, et al. Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials. 2014;35:3005–14. doi: 10.1016/j.biomaterials.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu G, Zhang F, Ni Q, Niu G, Chen X. Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS nano. 2017;11:2387–92. doi: 10.1021/acsnano.7b00978. [DOI] [PubMed] [Google Scholar]
- 39.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nature materials. 2013;12:978–90. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cousin S, Italiano A. Molecular Pathways: Immune Checkpoint Antibodies and their Toxicities. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:4550–5. doi: 10.1158/1078-0432.CCR-15-2569. [DOI] [PubMed] [Google Scholar]
- 41.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Translational lung cancer research. 2015;4:560–75. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. Journal of Clinical Oncology. 2015;33:2092–9. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg Michael S. Immunoengineering: How Nanotechnology Can Enhance Cancer Immunotherapy. Cell. 161:201–4. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Smith MJ, Brown JM, Zamboni WC, Walker NJ. From Immunotoxicity to Nanotherapy: The Effects of Nanomaterials on the Immune System. Toxicological Sciences. 2014;138:249–55. doi: 10.1093/toxsci/kfu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiao Q, Li L, Mu Q, Zhang Q. Immunomodulation of Nanoparticles in Nanomedicine Applications. BioMed research international. 2014;2014:426028. doi: 10.1155/2014/426028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Getts DR, Shea LD, Miller SD, King NJC. Harnessing nanoparticles for immune modulation. Trends in Immunology. 2015;36:419–27. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-Based Immunotherapy for Cancer. ACS nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, von Roemeling CA, Chen Y, Qie Y, Liu X, Chen J, et al. Designing nanomedicine for immuno-oncology. Nature Biomedical Engineering. 2017;1:0029. [Google Scholar]
- 49.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews. 2015;115:11109–46. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung AS, Mooney DJ. Engineered Materials for Cancer Immunotherapy. Nano today. 2015;10:511–31. doi: 10.1016/j.nantod.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines. 2015;3:662–85. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontana F, Liu D, Hirvonen J, Santos HA. Delivery of therapeutics with nanoparticles: what’s new in cancer immunotherapy? Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2017:9. doi: 10.1002/wnan.1421. [DOI] [PubMed] [Google Scholar]
- 53.Liang C, Xu L, Song G, Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chemical Society reviews. 2016;45:6250–69. doi: 10.1039/c6cs00458j. [DOI] [PubMed] [Google Scholar]
- 54.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 55.Pardoll D. Does the immune system see tumors as foreign or self? Annual Review of Immunology. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 56.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desrichard A, Snyder A, Chan TA. Cancer Neoantigens and Applications for Immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:807–12. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 59.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature reviews Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 60.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunological reviews. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 61.Strugnell R, Zepp F, Cunningham A, Tantawichien T. Vaccine antigens. Perspectives in Vaccinology. 2011;1:61–88. [Google Scholar]
- 62.Vartak A, Sucheck SJ. Recent Advances in Subunit Vaccine Carriers. Vaccines. 2016:4. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garçon N, Leroux-Roels G, Cheng W-F. Vaccine adjuvants. Perspectives in Vaccinology. 2011;1:89–113. [Google Scholar]
- 64.Banday AH, Jeelani S, Hruby VJ. Cancer vaccine adjuvants--recent clinical progress and future perspectives. Immunopharmacology and immunotoxicology. 2015;37:1–11. doi: 10.3109/08923973.2014.971963. [DOI] [PubMed] [Google Scholar]
- 65.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nature reviews Clinical oncology. 2014;11:509–24. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 66.Mesa C, Fernandez LE. Challenges facing adjuvants for cancer immunotherapy. Immunology and cell biology. 2004;82:644–50. doi: 10.1111/j.0818-9641.2004.01279.x. [DOI] [PubMed] [Google Scholar]
- 67.Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Frontiers in immunology. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu M, Wang R, Nie G. Applications of nanomaterials as vaccine adjuvants. Human vaccines & immunotherapeutics. 2014;10:2761–74. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prashant CK, Bhat M, Srivastava SK, Saxena A, Kumar M, Singh A, et al. Fabrication of nanoadjuvant with poly-epsilon-caprolactone (PCL) for developing a single-shot vaccine providing prolonged immunity. International journal of nanomedicine. 2014;9:937–50. doi: 10.2147/IJN.S55892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vasievich EA, Chen W, Huang L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunology, Immunotherapy. 2011;60:629–38. doi: 10.1007/s00262-011-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasievich EA, Ramishetti S, Zhang Y, Huang L. Trp2 peptide vaccine adjuvanted with (R)-DOTAP inhibits tumor growth in an advanced melanoma model. Molecular pharmaceutics. 2012;9:261–8. doi: 10.1021/mp200350n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orecchioni M, Jasim DA, Pescatori M, Manetti R, Fozza C, Sgarrella F, et al. Molecular and Genomic Impact of Large and Small Lateral Dimension Graphene Oxide Sheets on Human Immune Cells from Healthy Donors. Advanced healthcare materials. 2016;5:276–87. doi: 10.1002/adhm.201500606. [DOI] [PubMed] [Google Scholar]
- 73.Pescatori M, Bedognetti D, Venturelli E, Menard-Moyon C, Bernardini C, Muresu E, et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013;34:4395–403. doi: 10.1016/j.biomaterials.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 74.Orecchioni M, Bedognetti D, Sgarrella F, Marincola FM, Bianco A, Delogu LG. Impact of carbon nanotubes and graphene on immune cells. Journal of translational medicine. 2014;12:138. doi: 10.1186/1479-5876-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu L, Liu Y, Chen Z, Li W, Liu Y, Wang L, et al. Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine. Advanced materials. 2013;25:5928–36. doi: 10.1002/adma.201300583. [DOI] [PubMed] [Google Scholar]
- 76.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nano. 2017;12:648–54. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature reviews Immunology. 2010;10:787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 78.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. European journal of immunology. 2008;38:1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 79.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. Journal of immunology. 2004;173:3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 80.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International journal of pharmaceutics. 2005;298:315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 81.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, et al. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 82.Wischke C, Borchert HH, Zimmermann J, Siebenbrodt I, Lorenzen DR. Stable cationic microparticles for enhanced model antigen delivery to dendritic cells. Journal of controlled release: official journal of the Controlled Release Society. 2006;114:359–68. doi: 10.1016/j.jconrel.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 83.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17463–8. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harding CV, Collins DS, Slot JW, Geuze HJ, Unanue ER. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991;64:393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- 85.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cellular and molecular life sciences: CMLS. 2009;66:2873–96. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature biotechnology. 2007;25:1159–64. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 87.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nature reviews Immunology. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 88.Cruz LJ, Rueda F, Cordobilla B, Simon L, Hosta L, Albericio F, et al. Targeting nanosystems to human DCs via Fc receptor as an effective strategy to deliver antigen for immunotherapy. Molecular pharmaceutics. 2011;8:104–16. doi: 10.1021/mp100178k. [DOI] [PubMed] [Google Scholar]
- 89.Mi W, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, et al. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. Journal of immunology. 2008;181:7550–61. doi: 10.4049/jimmunol.181.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu Z, Ramishetti S, Tseng YC, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. Journal of controlled release: official journal of the Controlled Release Society. 2013;172:259–65. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 91.He LZ, Crocker A, Lee J, Mendoza-Ramirez J, Wang XT, Vitale LA, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. Journal of immunology. 2007;178:6259–67. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 92.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. The Journal of experimental medicine. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon YJ, James E, Shastri N, Frechet JM. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18264–8. doi: 10.1073/pnas.0509541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cruz LJ, Tacken PJ, Fokkink R, Joosten B, Stuart MC, Albericio F, et al. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. Journal of controlled release: official journal of the Controlled Release Society. 2010;144:118–26. doi: 10.1016/j.jconrel.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 95.Kretz-Rommel A, Qin F, Dakappagari N, Torensma R, Faas S, Wu D, et al. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. Journal of immunotherapy. 2007;30:715–26. doi: 10.1097/CJI.0b013e318135472c. [DOI] [PubMed] [Google Scholar]
- 96.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. Journal of immunology. 2006;177:2276–84. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 97.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 98.Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Shin Teh J, Lo JC, et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–73. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 100.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song C, Noh Y-W, Lim YT. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. International journal of nanomedicine. 2016;11:3753–64. doi: 10.2147/IJN.S110796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirai T, Yoshioka Y, Takahashi H, Ichihashi K, Yoshida T, Tochigi S, et al. Amorphous silica nanoparticles enhance cross-presentation in murine dendritic cells. Biochemical and biophysical research communications. 2012;427:553–6. doi: 10.1016/j.bbrc.2012.09.095. [DOI] [PubMed] [Google Scholar]
- 103.Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–64. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. International immunology. 2016;28:319–28. doi: 10.1093/intimm/dxw027. [DOI] [PubMed] [Google Scholar]
- 105.Tsang KY, Jochems C, Schlom J. Insights on Peptide Vaccines in Cancer Immunotherapy. In: Ascierto PA, Stroncek DF, Wang E, editors. Developments in T Cell Based Cancer Immunotherapies. Cham: Springer International Publishing; 2015. pp. 1–27. [Google Scholar]
- 106.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature materials. 2017;16:489–96. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allahyari M, Mohit E. Peptide/protein vaccine delivery system based on PLGA particles. Human vaccines & immunotherapeutics. 2016;12:806–28. doi: 10.1080/21645515.2015.1102804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–78. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 109.Akagi T, Baba M, Akashi M. Biodegradable Nanoparticles as Vaccine Adjuvants and Delivery Systems: Regulation of Immune Responses by Nanoparticle-Based Vaccine. In: Kunugi S, Yamaoka T, editors. Polymers in Nanomedicine. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. pp. 31–64. [Google Scholar]
- 110.Trimaille T, Verrier B. Micelle-Based Adjuvants for Subunit Vaccine Delivery. Vaccines. 2015;3:803–13. doi: 10.3390/vaccines3040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luo Z, Li P, Deng J, Gao N, Zhang Y, Pan H, et al. Cationic polypeptide micelle-based antigen delivery system: a simple and robust adjuvant to improve vaccine efficacy. Journal of controlled release: official journal of the Controlled Release Society. 2013;170:259–67. doi: 10.1016/j.jconrel.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 112.Kim H, Uto T, Akagi T, Baba M, Akashi M. Amphiphilic Poly(Amino Acid) Nanoparticles Induce Size-Dependent Dendritic Cell Maturation. Advanced Functional Materials. 2010;20:3925–31. [Google Scholar]
- 113.Hao J, Kwissa M, Pulendran B, Murthy N. Peptide crosslinked micelles: a new strategy for the design and synthesis of peptide vaccines. International journal of nanomedicine. 2006;1:97–103. doi: 10.2147/nano.2006.1.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Therapeutic advances in vaccines. 2014;2:159–82. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30:2256–72. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen W, Yan W, Huang L. A simple but effective cancer vaccine consisting of an antigen and a cationic lipid. Cancer immunology, immunotherapy: CII. 2008;57:517–30. doi: 10.1007/s00262-007-0390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Molecular immunology. 2007;44:3672–81. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Chen W, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Molecular pharmaceutics. 2008;5:464–71. doi: 10.1021/mp700126c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. Journal of controlled release: official journal of the Controlled Release Society. 2012;158:108–14. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS nano. 2014;8:3636–45. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goodwin TJ, Huang L. Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine. 2017;35:2550–7. doi: 10.1016/j.vaccine.2017.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nature reviews Cancer. 2008;8:108–20. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 123.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunological reviews. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 124.Deering RP, Kommareddy S, Ulmer JB, Brito LA, Geall AJ. Nucleic acid vaccines: prospects for non-viral delivery of mRNA vaccines. Expert opinion on drug delivery. 2014;11:885–99. doi: 10.1517/17425247.2014.901308. [DOI] [PubMed] [Google Scholar]
- 125.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang SD, Selomulya C, Ho J, Apostolopoulos V, Plebanski M. Delivery of DNA vaccines: an overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2010;2:205–18. doi: 10.1002/wnan.88. [DOI] [PubMed] [Google Scholar]
- 127.Shah MA, Ali Z, Ahmad R, Qadri I, Fatima K, He N. DNA Mediated Vaccines Delivery Through Nanoparticles. Journal of nanoscience and nanotechnology. 2015;15:41–53. doi: 10.1166/jnn.2015.9603. [DOI] [PubMed] [Google Scholar]
- 128.Barbon CM, Baker L, Lajoie C, Ramstedt U, Hedley ML, Luby TM. In vivo electroporation enhances the potency of poly-lactide co-glycolide (PLG) plasmid DNA immunization. Vaccine. 2010;28:7852–64. doi: 10.1016/j.vaccine.2010.09.078. [DOI] [PubMed] [Google Scholar]
- 129.Matijevic M, Hedley ML, Urban RG, Chicz RM, Lajoie C, Luby TM. Immunization with a poly (lactide co-glycolide) encapsulated plasmid DNA expressing antigenic regions of HPV 16 and 18 results in an increase in the precursor frequency of T cells that respond to epitopes from HPV 16, 18, 6 and 11. Cellular immunology. 2011;270:62–9. doi: 10.1016/j.cellimm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shuaibu MN, Cherif MS, Kurosaki T, Helegbe GK, Kikuchi M, Yanagi T, et al. Effect of nanoparticle coating on the immunogenicity of plasmid DNA vaccine encoding P. yoelii MSP-1 C-terminal. Vaccine. 2011;29:3239–47. doi: 10.1016/j.vaccine.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 131.Umthong S, Buaklin A, Jacquet A, Sangjun N, Kerdkaew R, Patarakul K, et al. Immunogenicity of a DNA and Recombinant Protein Vaccine Combining LipL32 and Loa22 for Leptospirosis Using Chitosan as a Delivery System. Journal of microbiology and biotechnology. 2015;25:526–36. doi: 10.4014/jmb.1408.08007. [DOI] [PubMed] [Google Scholar]
- 132.Xu W, Shen Y, Jiang Z, Wang Y, Chu Y, Xiong S. Intranasal delivery of chitosan-DNA vaccine generates mucosal SIgA and anti-CVB3 protection. Vaccine. 2004;22:3603–12. doi: 10.1016/j.vaccine.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 133.Li P, Chen S, Jiang Y, Jiang J, Zhang Z, Sun X. Dendritic cell targeted liposomes-protamine-DNA complexes mediated by synthetic mannosylated cholesterol as a potential carrier for DNA vaccine. Nanotechnology. 2013;24:295101. doi: 10.1088/0957-4484/24/29/295101. [DOI] [PubMed] [Google Scholar]
- 134.Srinivas R, Garu A, Moku G, Agawane SB, Chaudhuri A. A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups. Biomaterials. 2012;33:6220–9. doi: 10.1016/j.biomaterials.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 135.Orecchioni M, Cabizza R, Bianco A, Delogu LG. Graphene as cancer theranostic tool: progress and future challenges. Theranostics. 2015;5:710–23. doi: 10.7150/thno.11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Farris E, Brown DM, Ramer-Tait AE, Pannier AK. Micro- and nanoparticulates for DNA vaccine delivery. Experimental biology and medicine. 2016;241:919–29. doi: 10.1177/1535370216643771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nguyen DN, Green JJ, Chan JM, Longer R, Anderson DG. Polymeric Materials for Gene Delivery and DNA Vaccination. Advanced materials. 2009;21:847–67. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barichello JM, Ishida T, Kiwada H. Complexation of siRNA and pDNA with cationic liposomes: the important aspects in lipoplex preparation. Methods in molecular biology. 2010;605:461–72. doi: 10.1007/978-1-60327-360-2_32. [DOI] [PubMed] [Google Scholar]
- 139.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 140.Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520:692–6. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gross BP, Wongrakpanich A, Francis MB, Salem AK, Norian LA. A therapeutic microparticle-based tumor lysate vaccine reduces spontaneous metastases in murine breast cancer. The AAPS journal. 2014;16:1194–203. doi: 10.1208/s12248-014-9662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nature materials. 2009;8:151–8. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano letters. 2014;14:2181–8. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annual review of immunology. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 145.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature communications. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nature reviews Immunology. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 148.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nano. 2017 doi: 10.1038/nnano.2017.113. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4:e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK, et al. Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer. The AAPS journal. 2015;17:184–93. doi: 10.1208/s12248-014-9676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, et al. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS nano. 2017;11:4463–74. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 152.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Advanced materials. 2014;26:8154–62. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 153.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communication. 2016;7:12499. doi: 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W. Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. 2016;138:16686–95. doi: 10.1021/jacs.6b09538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Current opinion in immunology. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vasievich EA, Huang L. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Molecular pharmaceutics. 2011;8:635–41. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chou SH, Shetty AV, Geng Y, Xu L, Munirathinam G, Pipathsouk A, et al. Palmitate-derivatized human IL-2: a potential anticancer immunotherapeutic of low systemic toxicity. Cancer immunology, immunotherapy: CII. 2013;62:597–603. doi: 10.1007/s00262-012-1364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of oncology: official journal of the European Society for Medical Oncology. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4–1BB, and CD40 as targets for cancer immunotherapy. Current opinion in immunology. 2013;25:230–7. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC medicine. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, et al. Local Release of Highly Loaded Antibodies from Functionalized Nanoporous Support for Cancer Immunotherapy. Journal of the American Chemical Society. 2010;132:6906–7. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering. 2017;1:0011. [Google Scholar]
- 166.Roeven MW, Hobo W, van der Voort R, Fredrix H, Norde WJ, Teijgeler K, et al. Efficient nontoxic delivery of PD-L1 and PD-L2 siRNA into dendritic cell vaccines using the cationic lipid SAINT-18. Journal of immunotherapy. 2015;38:145–54. doi: 10.1097/CJI.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 167.Iwamura K, Kato T, Miyahara Y, Naota H, Mineno J, Ikeda H, et al. siRNA-mediated silencing of PD-1 ligands enhances tumor-specific human T-cell effector functions. Gene Ther. 2012;19:959–66. doi: 10.1038/gt.2011.185. [DOI] [PubMed] [Google Scholar]
- 168.Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, George AJ, et al. Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing. Advanced healthcare materials. 2015;4:1180–9. doi: 10.1002/adhm.201500089. [DOI] [PubMed] [Google Scholar]
- 169.Chen M, Ouyang H, Zhou S, Li J, Ye Y. PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cellular immunology. 2014;287:91–9. doi: 10.1016/j.cellimm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized immunotherapy via liposome-anchored Anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer research. 2013;73:1547–58. doi: 10.1158/0008-5472.CAN-12-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miao L, Li J, Liu Q, Feng R, Das M, Lin CM, et al. Transient and Local Expression of Chemokine and Immune Checkpoint Traps to Treat Pancreatic Cancer. ACS nano. 2017 doi: 10.1021/acsnano.7b01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacology & Therapeutics. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 174.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature materials. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holtzhausen A, Zhao F, Evans KS, Hanks BA. Early Carcinogenesis Involves the Establishment of Immune Privilege via Intrinsic and Extrinsic Regulation of Indoleamine 2,3-dioxygenase-1: Translational Implications in Cancer Immunotherapy. Frontiers in immunology. 2014:5. doi: 10.3389/fimmu.2014.00438. [DOI] [PMC free article] [PubMed]
- 176.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 2003;9:1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 177.Zulfiqar B, Mahroo A, Nasir K, Farooq RK, Jalal N, Rashid MU, et al. Nanomedicine and cancer immunotherapy: focus on indoleamine 2,3-dioxygenase inhibitors. OncoTargets and therapy. 2017;10:463–76. doi: 10.2147/OTT.S119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 179.Kalinski P. Regulation of immune responses by prostaglandin E2. Journal of immunology. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Serafini P. Editorial: PGE2-producing MDSC: a role in tumor progression? Journal of leukocyte biology. 2010;88:827–9. doi: 10.1189/jlb.0510303. [DOI] [PubMed] [Google Scholar]
- 181.Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, et al. COX-2 Inhibition Potentiates Antiangiogenic Cancer Therapy and Prevents Metastasis in Preclinical Models. Science Translational Medicine. 2014;6:242ra84-ra84. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.DeLong P, Tanaka T, Kruklitis R, Henry AC, Kapoor V, Kaiser LR, et al. Use of Cyclooxygenase-2 Inhibition to Enhance the Efficacy of Immunotherapy. Cancer research. 2003;63:7845–52. [PubMed] [Google Scholar]
- 183.Zelenay S, van der Veen Annemarthe G, Böttcher Jan P, Snelgrove Kathryn J, Rogers N, Acton Sophie E, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–70. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goodwin TJ, Zhou Y, Musetti SN, Liu R, Huang L. Local and transient gene expression primes the liver to resist cancer metastasis. Sci Transl Med. 2016;8:364ra153. doi: 10.1126/scitranslmed.aag2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hu Y, Haynes MT, Wang Y, Liu F, Huang L. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS nano. 2013;7:5376–84. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 187.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. Journal of immunology. 2000;164:762–7. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 189.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Seminars in cancer biology. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 190.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Halama N, Zoernig I, Berthel A, Kahlert C, Klupp F, Suarez-Carmona M, et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer cell. 2016;29:587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 192.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 193.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. Journal of translational medicine. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K, et al. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer immunology, immunotherapy: CII. 2009;58:1577–86. doi: 10.1007/s00262-009-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. The Journal of clinical investigation. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Biswas SK, Lewis CE. NF-kappaB as a central regulator of macrophage function in tumors. Journal of leukocyte biology. 2010;88:877–84. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 197.Chakraborty P, Chatterjee S, Ganguly A, Saha P, Adhikary A, Das T, et al. Reprogramming of TAM toward proimmunogenic type through regulation of MAP kinases using a redox-active copper chelate. Journal of leukocyte biology. 2012;91:609–19. doi: 10.1189/jlb.0611287. [DOI] [PubMed] [Google Scholar]
- 198.Eubank TD, Roberts RD, Khan M, Curry JM, Nuovo GJ, Kuppusamy P, et al. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer research. 2009;69:2133–40. doi: 10.1158/0008-5472.CAN-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer research. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, et al. Targeting Tumor-Associated Macrophages and Inhibition of MCP-1 Reduce Angiogenesis and Tumor Growth in a Human Melanoma Xenograft. Journal of Investigative Dermatology. 2007;127:2031–41. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 202.Zhu S, Niu M, O’Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Molecular pharmaceutics. 2013;10:3525–30. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15919–24. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu GW, Livesay BR, Kacherovsky NA, Cieslewicz M, Lutz E, Waalkes A, et al. Efficient Identification of Murine M2 Macrophage Peptide Targeting Ligands by Phage Display and Next-Generation Sequencing. Bioconjugate chemistry. 2015;26:1811–7. doi: 10.1021/acs.bioconjchem.5b00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of immunology. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 208.Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012;27:495–503. doi: 10.1089/cbr.2012.1219. [DOI] [PubMed] [Google Scholar]
- 209.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 210.Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653–9. doi: 10.1093/jn/134.10.2653. [DOI] [PubMed] [Google Scholar]
- 211.Wiers KM, Lathers DM, Wright MA, Young MR. Vitamin D3 treatment to diminish the levels of immune suppressive CD34+ cells increases the effectiveness of adoptive immunotherapy. Journal of immunotherapy. 2000;23:115–24. doi: 10.1097/00002371-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 212.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of experimental medicine. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer research. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 214.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer research. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)-->signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer research. 2008;68:2920–6. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine Selectively Eliminates Splenic Gr-1+/CD11b+ Myeloid Suppressor Cells in Tumor-Bearing Animals and Enhances Antitumor Immune Activity. Clinical Cancer Research. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 217.Rao A, Taylor JL, Chi-Sabins N, Kawabe M, Gooding WE, Storkus WJ. Combination therapy with HSP90 inhibitor 17-DMAG reconditions the tumor microenvironment to improve recruitment of therapeutic T cells. Cancer research. 2012;72:3196–206. doi: 10.1158/0008-5472.CAN-12-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhao Y, Huo M, Xu Z, Wang Y, Huang L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials. 2015;68:54–66. doi: 10.1016/j.biomaterials.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kourtis IC, Hirosue S, de Titta A, Kontos S, Stegmann T, Hubbell JA, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PloS one. 2013;8:e61646. doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 222.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Advances in cancer research. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 223.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shatry A, Chirinos J, Gorin MA, Jones M, Levy RB. Targeting Treg cells in situ: emerging expansion strategies for (CD4(+)CD25(+)) regulatory T cells. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15:1239–43. doi: 10.1016/j.bbmt.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Finotello F, Trajanoski Z. New strategies for cancer immunotherapy: targeting regulatory T cells. Genome medicine. 2017;9:10. doi: 10.1186/s13073-017-0402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, Yoshida S, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837–42. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 227.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer immunology, immunotherapy: CII. 2012;61:353–62. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL- tumors. Journal of immunology. 2008;181:6955–63. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:6674–82. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 231.Desar IM, Jacobs JH, Hulsbergen-vandeKaa CA, Oyen WJ, Mulders PF, van der Graaf WT, et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. International journal of cancer. 2011;129:507–12. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 232.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, et al. In Vivo Targeting of Intratumor Regulatory T Cells Using PEG-Modified Single-Walled Carbon Nanotubes. Bioconjugate chemistry. 2013;24:852–8. doi: 10.1021/bc400070q. [DOI] [PubMed] [Google Scholar]
- 234.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kalluri R. The biology and function of fibroblasts in cancer. Nature reviews Cancer. 2016;16:582–98. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 236.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Experimental cell research. 2010;316:1324–31. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 237.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-Associated Fibroblasts Drive the Progression of Metastasis through both Paracrine and Mechanical Pressure on Cancer Tissue. Molecular Cancer Research. 2012;10:1403–18. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Experimental cell research. 2010;316:2713–22. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 239.Sappino AP, Skalli O, Jackson B, Schurch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. International journal of cancer. 1988;41:707–12. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 240.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 241.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 242.Takahashi H, Sakakura K, Kawabata-Iwakawa R, Rokudai S, Toyoda M, Nishiyama M, et al. Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer immunology, immunotherapy: CII. 2015;64:1407–17. doi: 10.1007/s00262-015-1742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–47. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. The Journal of Pathology. 2017;241:313–5. doi: 10.1002/path.4824. [DOI] [PubMed] [Google Scholar]
- 245.Protti MP, De Monte L. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology. 2012;1:89–91. doi: 10.4161/onci.1.1.17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, de Malefyt RW, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 247.Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36:1090–101. doi: 10.1038/onc.2016.273. [DOI] [PubMed] [Google Scholar]
- 248.Cheng Jt, Deng Yn, Yi Hm, Wang Gy, Fu Bs, Chen Wj, et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. The Journal of Cell Biology. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, et al. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene. 2017 doi: 10.1038/onc.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135:1228–37. 37.e1–2. doi: 10.1053/j.gastro.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. Journal of immunology. 2007;178:5552–62. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 253.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer research. 2005;65:11156–63. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 254.Pietras K, Sjöblom T, Rubin K, Heldin C-H, Östman A. PDGF receptors as cancer drug targets. Cancer cell. 2003;3:439–43. doi: 10.1016/s1535-6108(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 255.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 257.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 258.Hawinkels LJAC, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-[beta] signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 259.Nagaraj NS, Datta PK. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert opinion on investigational drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature reviews Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 262.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nature reviews Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS medicine. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:6130–9. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 267.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14776–81. doi: 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Miao L, Lin CM, Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:192–204. doi: 10.1016/j.jconrel.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Miao L, Newby JM, Lin CM, Zhang L, Xu F, Kim WY, et al. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS nano. 2016;10:9243–58. doi: 10.1021/acsnano.6b02776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Miao L, Liu Q, Lin CM, Luo C, Wang Y, Liu L, et al. Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012:27. doi: 10.1089/cbr.2012.1219. [DOI] [PubMed] [Google Scholar]
- 273.Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004:134. doi: 10.1093/jn/134.10.2653. [DOI] [PubMed] [Google Scholar]
- 274.Wiers KM, Lathers DM, Wright MA, Young MR. Vitamin D3 treatment to diminish the levels of immune suppressive CD34 cells increases the effectiveness of adoptive immunotherapy. Journal of immunotherapy. 2000:23. doi: 10.1097/00002371-200001000-00014. [DOI] [PubMed]
- 275.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer research. 2007:67. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 276.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)- > signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer research. 2008:68. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005:11. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 278.Rao A, Taylor JL, Chi-Sabins N, Kawabe M, Gooding WE, Storkus WJ. Combination therapy with HSP90 inhibitor 17-DMAG reconditions the tumor microenvironment to improve recruitment of therapeutic T cells. Cancer research. 2012:72. doi: 10.1158/0008-5472.CAN-12-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Klevorn LE, Teague RM. Adapting Cancer Immunotherapy Models for the Real World. Trends in Immunology. 37:354–63. doi: 10.1016/j.it.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]