ABSTRACT
Damage to the lateral geniculate body by diffuse axonal injury in brain trauma is uncommon. The authors present the clinical case and in vivo fibre tractography using diffusion tensor magnetic resonance imaging of this lesion in a patient presenting with homonymous sectoranopia after a traumatic head injury.
KEYWORDS: Diffuse axonal injury, lateral geniculate body, tractography
Introduction
The lateral geniculate body (LGB) lies adjacent to the posterior commissure, pineal body, and medial geniculate body. The LGB is the main thalamic visual centre linking the retina and the visual cortical areas,1 and it is composed of three substructures: the dorsal nucleus, the pregeniculate complex, and the intergeniculate leaflet.
The dorsal nucleus has dual blood supply; consequently, visual field defects will vary depending on which artery is injured. Quadruple sectoranopia is compatible with injury to the anterior choroidal artery, which arises from the internal carotid artery. Wedge-shaped congruous horizontal sectoranopia is compatible with injury to the lateral choroidal artery, which arises from the posterior cerebral artery.2
LGB damage—usually caused by ischaemia, haemorrhage, or trauma—are uncommon, and only few case reports have been reported in the literature.3–8 Conventional magnetic resonance imaging (MRI) is usually accurate enough to confirm its damage. However, novel imaging modalities are needed to detect microstructural compromise in white matter, such as traumatic axonal injury. The present case shows the pathophysiology of the LGB damage due to axonal injury with MRI tractography.
Case description
A 38-year-old man, diagnosed with amblyopia of the right eye (RE) without other relevant medical history, presented at emergency department with severe traumatic brain injury (TBI) caused by a bicycle accident. He was oriented in person and place but he presented the following symptoms: memory impairment of recent and remote events; bradypsychia; bradylalia; agitation alternating with consciousness fades; left hemiparesis; and visual disturbances. Cranial computed tomography (CT) revealed mild bilateral frontal subarachnoid bleeding and left frontal intraparenchymal haemorrhage. Physical therapy improved his bradylalia, bradypsychia, and gait. Three months after the accident, residual left frontal necrotic intracranial lesion was found on a follow-up CT.
Six months after the accident, the patient was referred to the neuro-ophthalmology department due to a left homonymous congruous horizontal sectoranopia on a Humphrey perimetry 30-2 performed 3 months before (Figure 1A). On examination, corrected visual acuity was 20/50 in the RE and 20/20 in the left eye (LE). Biomicroscopic examination was normal, bilateral tilted optic discs were observed on funduscopy. Humphrey perimetry 30-2 revealed that the visual field defect had progressed to a left congruous homonymous incomplete hemianopia (Figure 1B), a finding that is consistent with axonal deterioration over a 6-month period.9
Figure 1.
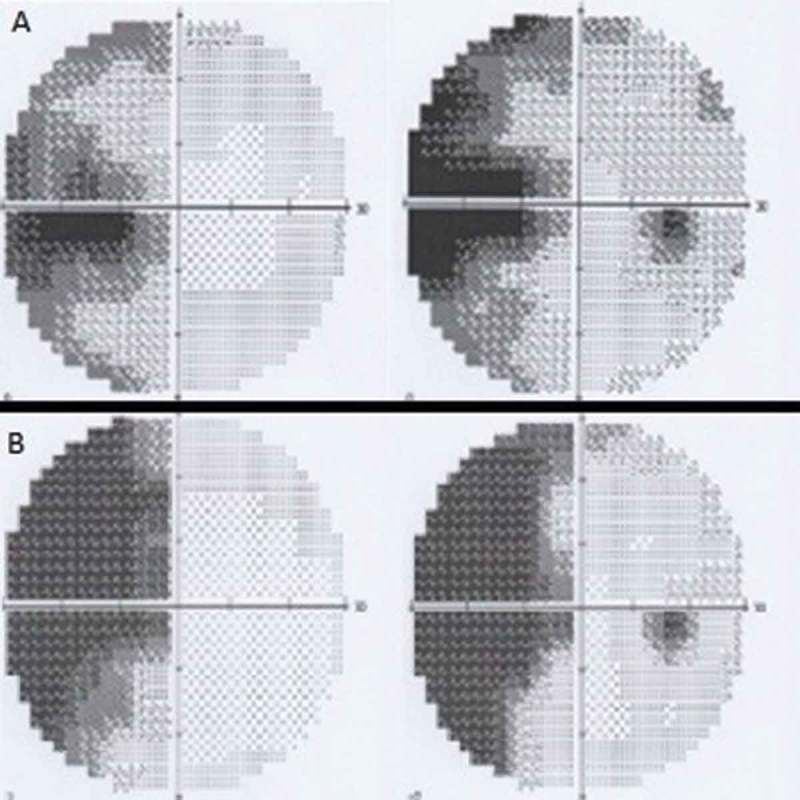
(A) Humphrey perimetry 30-2 showing left homonymous congruous horizontal sectoranopia. (B) Humphrey perimetry 30-2 showing left congruous homonymous incomplete hemianopia.
Macular optical coherence tomography (OCT) showed atrophy of the ganglion cells in the right side of both maculae, indicating anterograde degeneration of the visual pathway (Figure 2). On the other hand, OCT of the optic disc showed atrophy of nerve fibres superiorly and inferiorly in both eyes and also nasally in the LE. These findings suggested damage to the right LGB, but the pathophysiology could not be confirmed using conventional MRI.
Figure 2.
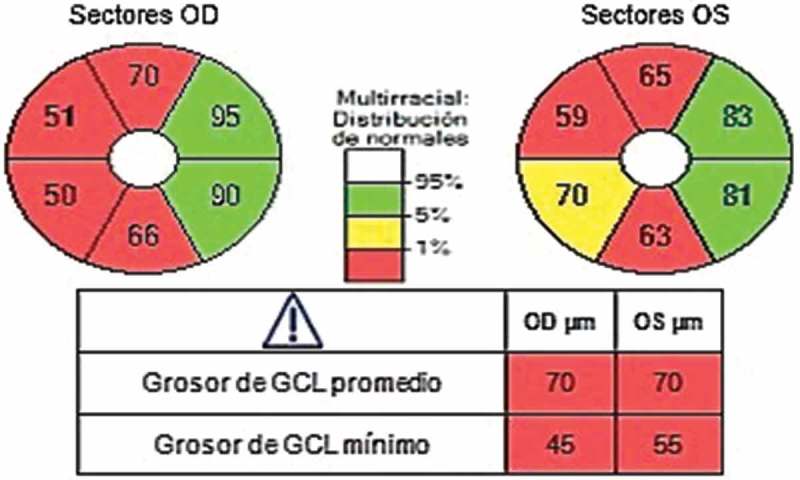
Macular optical coherence tomography. Atrophy of the ganglion cells in the right macular side of both eyes is observed.
The MRI showed a supratentorial and an infratentorial focus of haemosiderin deposition suggestive of diffuse axonal injury in the fast field echo (FFE) sequence. Asymmetries in the optic tracts appeared in the short-tau inversion recovery (STIR) sequence (Figure 3). Abnormal hypointensity in axial susceptibility-weighted imaging (SWI) was congruous with damage to the right LGB (Figure 4). The tractography pattern consisting of deteriorated structural integrity and connectivity change in the right LGB confirmed the axonal injury (Figure 5, video 1).
Figure 3.
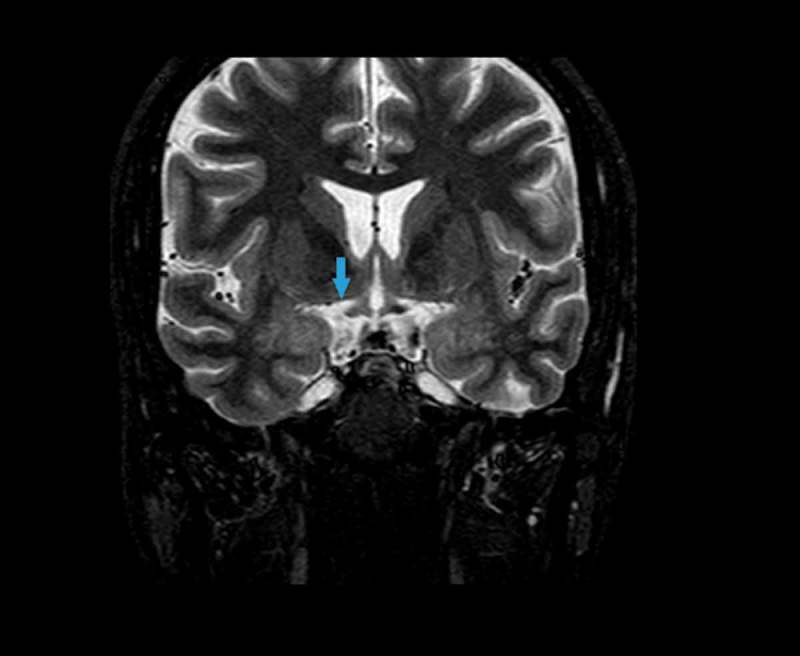
Magnetic resonance imaging. STIR sequence. Asymmetries in the optic tracts appear in the STIR sequence consisting of high signal in the right tract (blue arrow).
Figure 4.
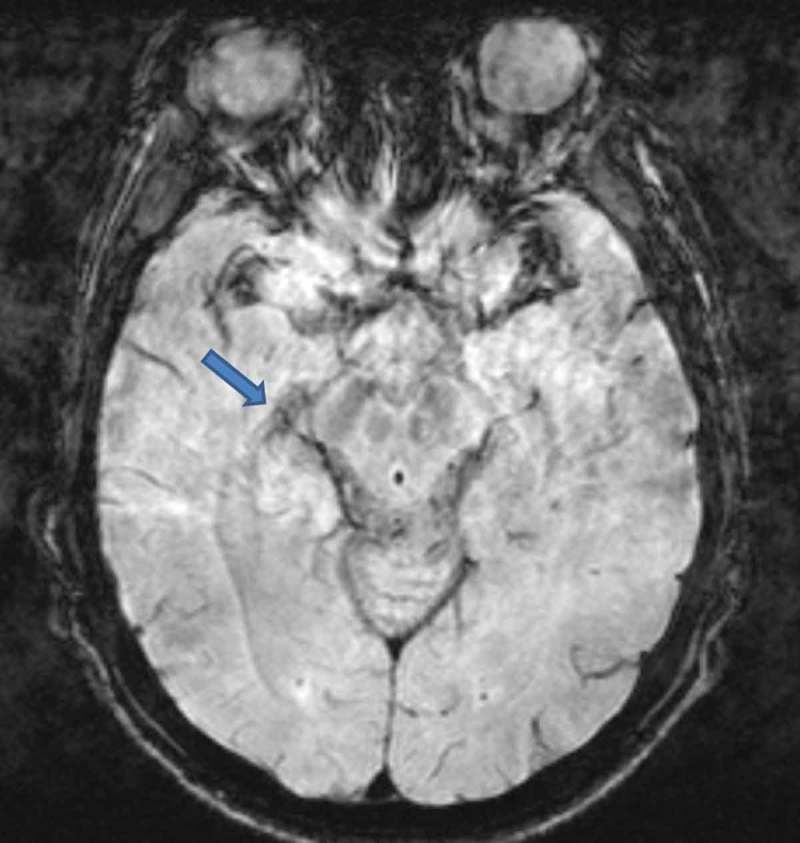
Magnetic resonance imaging. SWI sequence. Axial SWI shows abnormal hypointensity in the right lateral geniculate body (blue arrow).
Figure 5.
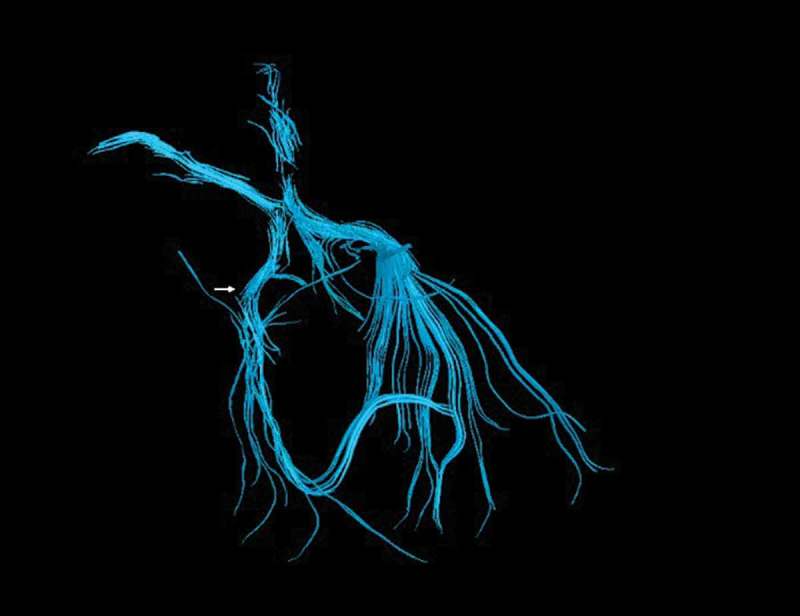
Tractography. The white arrow shows axonal disruption in the right lateral geniculate body. See Supplemental Material, Video 1.
Discussion
Although the exact mechanisms responsible for initiating secondary degeneration in diffuse axonal injury are not completely understood, in vivo and in vitro experimental models provide some insight. In response to trauma, either the axolemma undergoes primary mechanical failure, exposing the cytosol to the extracellular space, or mechanosensitive sodium channels are activated, resulting in a flux of sodium into the axoplasm. This results in a directional change in the flow of calcium, resulting in intracellular accumulation. Calcium can be sequestered in the mitochondria, generating reactive oxygen species that may disrupt oxidative metabolism and cause oxidative damage to an axon in crisis 9.
Given the indeterminate results obtained with conventional MRI, we decided to perform additional MRI sequences. The MRI study was performed using T1, T2, T2 FFE, fluid-attenuated inversion recovery (FLAIR), STIR, SWI, and diffusion tensor imaging (DTI). In addition, MRI tractography using DTI MRI was performed.
Each of these sequences provides unique data to help visualize the lesion. FFE provides a high signal intensity of the blood pool without the need to apply of an exogenous contrast agent. FLAIR is used to examine macroscopic white matter lesions and contusions on the cortical surface. STIR allows for three-dimensional imaging with excellent fat suppression.10 SWI takes advantage of susceptibility differences between tissues, resulting in an enhanced contrast that is sensitive to the paramagnetic properties of intravascular deoxyhaemoglobin and to venous blood, haemorrhage, and iron in the brain. In essence, susceptibility differences are detected as phase differences in the MRI signal. In the image processing stage, SWI superimposes these phase differences onto the conventional MRI, thereby allowing the susceptibility differences to be accentuated in the final image.11 DTI is sensitive to subtle changes in white matter fibre tracts and is capable of revealing microstructural axonal injuries.11 The last sequence was used to obtain an MRI tractography.
In conclusion, MRI tractography allows to follow fibre tracts along a diffusion direction in very small steps and to create long fibre tracts that connect distant regions in the brain. The accuracy of fibre tractography is dependent upon a number of factors, including image resolution, noise, image distortions, and partial volume effects, that result from multiple tracts crossing in a single voxel. The main advantage of DTI tractography is that the whole fibre bundle, instead of just a portion of the fibre bundle, can be evaluated.12,13 In this case report, tractography was decisive for the radiologically based diagnosis.
Acknowledgements
We would like to thank Bradley Londres for his editing work on this manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Supplemental Material
Supplemental materials for this article can be accessed on the publisher’s website.
References
- [1].Saeki N, Fujimoto N, Kubota M, Yamaura A.. MR demonstration of partial lesions of the lateral geniculate body and its functional intra-nuclear topography. Clin Neurol Neurosurg 2003;106:28–32. [DOI] [PubMed] [Google Scholar]
- [2].Rizzo JF. Embryology, anatomy, and physiology of the afferent visual pathway In: Miller NR, Newman NJ, eds. Walsh Hoyt’s Clinical Neuro-ophthalmology. 6th ed Philadelphia, PA: Lippincott Williams Wilkins; 2005:3–82. [Google Scholar]
- [3].Shibata K, Nishimura Y, Kondo H, Otuka K, Iwata M.. Isolated homonymous hemianopsia due to lateral posterior choroidal artery region infarction: a case report Clin Neurol Neurosurg 2009;11:713–716. [DOI] [PubMed] [Google Scholar]
- [4].Han YS, Lee E, Kim JS.. Horizontal nystagmus and homonymous hemianopia due to lateral geniculate body hemorrhage. Eur Neurol 2009;61:371–373. [DOI] [PubMed] [Google Scholar]
- [5].Grabe HM, Bapuraj JR, Wesolowski JR, Parmar H, Trobe JD.. Homonymous hemianopia from infarction of the optic tract and lateral geniculate nucleus in deep cerebral venous thrombosis. J Neuroophthalmol 2012;32:38–41. [DOI] [PubMed] [Google Scholar]
- [6].Borruat FX, Maeder P.. Sectoranopia after head trauma: evidence of lateral geniculate body lesion on MRI. Neurology 1995;45:590–592. [DOI] [PubMed] [Google Scholar]
- [7].Grochowicki M, Vighetto A.. Homonymous horizontal sectoranopia: report of four cases. Br J Ophthalmol 1991;75:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mulholland C, Best J, Rennie I, Mc Kenna E, Lacey B.. Bilateral sectoranopia caused by bilateral geniculate body infarction in a 14-year-old boy with inflammatory bowel disease. J AAPOS 2010;14:435–437. [DOI] [PubMed] [Google Scholar]
- [9].Siedler DG, Chuah MI, Kirkcaldie MTK, Vickers JC, King AE Diffuse axonal injury in brain trauma: insights from alterations in neurofilaments. Front Cell Neurosci 2014;8:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fleckenstein J. L, Archer BT, Barker BA, Vaughan JT, Parkey RW, Peshock RM.. Fast short-tau inversion-recovery MR imaging. Radiology 1991;179:499–504. [DOI] [PubMed] [Google Scholar]
- [11].Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM.. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics 2009;29:1433–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu M-A, M P Purohit, Helmer K, Koerte I, Lin A P, Westin C-F, Kikinis R, Kubicki M, R A Stern, Zafonte R.. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J. Y, Bakhadirov K, Abdi H, Devous MD Sr, Marquez de la Plata CD, Moore C, Madden C J, Diaz-Arrastia R. Longitudinal changes of structural connectivity in traumatic axonal injury. Neurology 2011;77:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


