Summary
Central regulation of food intake is a key mechanism contributing to energy homeostasis. Many neural circuits that are thought to orchestrate feeding behavior overlap with the brain’s reward circuitry both anatomically and functionally. Manipulation of numerous neural pathways can simultaneously influence food intake and reward. Two key systems underlying these processes—those controlling homeostatic and hedonic feeding—are often treated as independent. Homeostatic feeding is necessary for basic metabolic processes and survival, while hedonic feeding is driven by sensory perception or pleasure. Despite this distinction, their functional and anatomical overlap imply considerable interaction that is often overlooked. Here, we argue that the neurocircuits controlling homeostatic feeding and hedonic feeding are not completely dissociable given the current data and urge researchers to assess behaviors extending beyond food intake in investigations of the neural control of feeding.
eTOC blurb
Rossi and Stuber discuss the entangled neurocircuitry that governs feeding and reward. Using insights from recently developed molecular and genetic tools, the authors argue that homeostatic feeding and hedonic feeding are highly interrelated and urge investigators to measure more than food intake when probing neuronal control of feeding.
Tightly regulating energy intake is necessary for survival of all animals. A critical component of energy balance is the ability to obtain and consume food sufficient to meet ongoing metabolic demands. The neurocircuits controlling feeding behavior are thought to be disrupted in pathologies of hypophagia (e.g., resulting in anorexia nervosa) or hyperphagia (e.g., resulting in obesity). In other pathologies, such as substance abuse, the neural circuits traditionally thought to control feeding may be co-opted by drugs of abuse, suggesting overlapping feeding and reward circuitry within the brain. The cells most closely linked to facilitating feeding are intermingled with the cells most closely linked to reward-guided behavior. A comprehensive understanding of these systems will greatly facilitate our understanding of pathologies that rely on feeding and reward circuits. Here, we pose the question of whether such circuits are indeed dissociable and should ultimately be considered separate given the current data and accepted approaches.
For more than half a century, scientists have struggled to understand the intermingled neural basis of reward and feeding (Berridge, 1996; Hoebel and Teitelbaum, 1962; Margules and Olds, 1962; Wise, 2004). Despite early recognition that feeding and reward are intimately linked, these two topics have frequently been examined in isolation for practical reasons. For example, studies have looked at the contribution of particular brain regions to body weight regulation, energy expenditure, and food intake (for review see (Elmquist et al., 1999; Morton et al., 2006)), while others focused on the role of neuronal populations in reward-guided behavior (Corbett and Wise, 1980; Morris et al., 2006; Schultz, 2002) but relatively few have considered the two together (Castro et al., 2015; Saper et al., 2002) (Table 1). Despite much progress toward understanding how certain parts of the brain contribute to either feeding or reward, questions of motivated behavior continue to be framed in terms of homeostatic feeding—food intake that is necessary to maintain typical body weight and metabolic function—or hedonic feeding—food intake driven by sensory perception or pleasure. These distinctions can be helpful to guide first-pass efforts to define basic functional elements of integrated neural circuits; however, homeostatic and hedonic feeding systems are likely both activated during all feeding situations. The degree to which each is activated may shift depending on the type of food (i.e. palatable, aversive) and the physiological state of the animal (i.e. starvation).
Table 1. Acute manipulations of molecularly- and anatomically-defined neurons that influence food intake also affect appetitive behavior.
Appetitive behavior was limited to place preference and self-stimulation behavior. Note that manipulations are grouped by whether they putatively increase (↑) or decrease (↓) neuronal output, but different manipulations that have similar net effects on cellular activity are not necessarily equivalent to each other.
| Area | Cell type | Manipulation | Food intake | Appetitive behavior | References | |
|---|---|---|---|---|---|---|
| Arc | AgRP | ChR2, Gq, Gs, TRPV1 | ↑ | + | +/− | Aponte et al 2011; Krashes et al 2011; Atasoy et al 2012; Betley et al 2015; Dietrich et al 2015; Chen et al 2016; Nakajim et al 2016 |
| AgRP | Gi | ↓ | − | ? | Krashes et al 2011 | |
| POMC | ChR2 (extended), Gq (chronic) | ↑ | − | ? | Aponte et al 2011; Zhan et al 2013 | |
| POMC | Gi (24hr intake) | ↓ | + | ? | Atasoy et al 2012 | |
| TH | ChIEF | ↑ | + | ? | Zhang & van den Pol 2016 | |
| Vglut2 | Gq | ↑ | − | ? | Fenseleu et al 2017 | |
| Vglut2 | Gi | ↓ | + | ? | Fenseleu et al 2017 | |
| Oxtr | Gq | ↑ | − | ? | Fenseleu et al 2017 | |
| PVN | SIM1 | Gi | ↓ | + | ? | Atasoy et al 2012 |
| TRH | Gq | ↑ | + | ? | Krashes et al 2014 | |
| PACAP | Gq, Gs | ↑ | + | ? | Krashes et al 2014; Nakajima et al 2016 | |
| MC4R | Gq | ↑ | − | ? | Garfield et al 2015 | |
| MC4R | Gi | ↓ | + | ? | Garfield et al 2015 | |
| CeA | PKCδ | Gi, eNpHR3.0 | ↓ | + | ? | Cai et al 2014a |
| PKCδ | ChR2 | ↑ | − | / | Cai et al 2014a | |
| Tac2 | ChR2 | ↑ | / | ? | Cai et al 2014a | |
| Crf | ChR2 | ↑ | / | ? | Cai et al 2014a | |
| Sst | eArch3.0 | ↓ | / | ? | Kim et al 2017 | |
| Crf/Nts/Tac2 | eArch3.0 | ↓ | / | ? | Kim et al 2017 | |
| Nts | eArch3.0 | ↓ | / | ? | Kim et al 2017 | |
| Tac2 | eArch3.0 | ↓ | / | ? | Kim et al 2017 | |
| Vgat | ChR2 | ↑ | + | ? | Han et al 2017 | |
| Htr2a | ChR2 | ↑ | + | + | Douglass et al 2017 | |
| Htr2a | eNpHR3.0 | ↓ | − | / | Douglass et al 2017 | |
| PBN | CGRP | Gq, ChR2 | ↑ | − | ? | Carter et al 2013 |
| CGRP | Gi | ↓ | + | ? | Carter et al 2013 | |
| MC4R | Gi | ↓ | + | ? | Garfield et al 2015 | |
| LHA | MC4R | Gq | ↑ | / | ? | Garfield et al 2015 |
| Vglut2 | ChR2 | ↑ | − | − | Jennings et al 2013a | |
| Vglut2 | eArch3.0 | ↓ | + | + | Jennings et al 2013a | |
| Vgat | Gq, ChR2 | ↑ | + | + | Jennings et al 2015; Navarro et al 2016 | |
| Vgat | Gi, eArch3.0 | ↓ | − | − | Jennings et al 2015; Navarro et al 2016 | |
| Orexin | Gq | ↑ | + | ? | Inutsuka et al 2014 | |
| VTA | Vgat | ChR2 | ↑ | − | − | Tan et al 2012; Van Zessen et al 2012 |
| NAc | D1R | eArch3.0 | ↓ | + | ? | O’Connor et al 2015 |
| D2R | eArch3.0 | ↓ | / | ? | O’Connor et al 2015 | |
| PFC | D1R | ChR2 | ↑ | + | ? | Land et al 2014 |
| D1R | eNpHR3.0 | ↓ | − | ? | Land et al 2014 | |
+ Increase
− Decrease
/ No effect
+/− Increase or decrease depending on experimental conditions
? Effect unknown
Arc, arcuate nucleus of the hypothalamus; AgRP, agouti-related peptide; CeA, central nucleus of the hypothalamus; CGRP, calcitonin gene-related peptide; ChIEF, fast-closing mutated channelrhodopsin hybrid; ChR2, channelrhodopsin-2; Crf, corticotrophin releasing factor; D1R, d1 like dopamine receptor; D2R, d2-like dopamine receptor; eArch3.0, archaerhodopsin-3.0; eNpHR3.0, halorhodopsin-3.0; Gq, hM3Dq (Gq-coupled designer receptor exclusively activated by designer drug); Gi, hM4Dq (Gi-coupled designer receptor exclusively activated by designer drug); Htr2a, serotonin receptor 2a; Gs, Gs-coupled designer receptor exclusively activated by designer drug; LHA, lateral hypothalamic area; MC4R, melanocortin 4 receptor; NAc, nucleus accumbens; Oxtr, oxytocin receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; PBN, parabrachial nucleus; PFC, prefrontal cortex; PKCδ, protein kinase C delta type; POMC, proopiomelanocortin; PVN, paraventricular nucleus of the hypothalamus; Sim1, single minded 1; Sst, somatostatin; Tac2, tachykinin 2; TRH, thyrotropin-releasing hormone; TRPV1, transient receptor potential cation channel subfamily V member 1; Vgat, vesicular GABA transporter; Vglut2, vesicular glutamate transporter 2, VTA, ventral tegmental area;
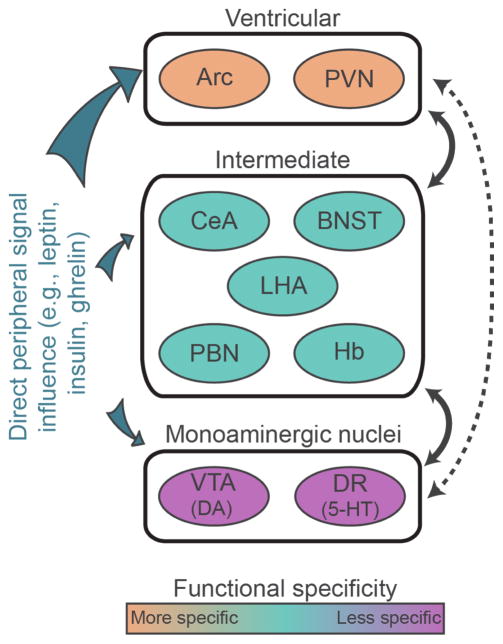
The anatomical interconnectedness as well as the functional consequences of perturbation of classical homeostatic and hedonic neurocircuits suggests that they contribute to a more complex motivational system. With a few notable exceptions discussed below, optogenetic or chemogenetic activation of appetite stimulating cells often produces rewarding phenotypes (a preference or willingness to work for stimulation), whereas activating appetite inhibiting cells tends to be aversive (avoidance of stimulation) (Jennings et al., 2013a; Jennings et al., 2015). Here, we will discuss anatomical and functional evidence to support the claim that homeostatic and hedonic feeding circuits are not presently dissociable from one another. We have divided the relevant brain regions into three broad categories: ventricular, intermediate, and monoaminergic. Ventricular neurons are those cells that are positioned adjacent to the third ventricle, regulate food intake, densely express receptors for a variety of circulating hormones, and project to downstream ‘intermediate’ targets. Intermediate neurons are those cells implicated in feeding that are positioned downstream of ventricular cells. Intermediate cell groups can provide synaptic feedback onto ventricular neurons and interact heavily with one another. Determining anatomical and physiological links between ventricular neurons and post-synaptic, molecularly-defined intermediate neurons is still an active area of research, but we have included discussion of the evidence where available. Monoaminergic neurons are found downstream and positioned to receive input from intermediate neurons, but direct input from ventricular neurons is sparse (Figure 1). The functions of given cell groups appear to be relatively specific at the level of ventricular neurons; manipulations of these cells produce pronounced effects on energy homeostasis. Intermediate neurons have more general functions, contributing to reward and aversion as well as food intake and body weight regulation. At the most general level, monoaminergic neurons (i.e. mesolimbic dopamine neurons) are involved in arousal, movement, motivation, and many other adaptive functions. We consider each of these levels and how they contribute to feeding behavior in turn.
Figure 1.
Circuits involved in feeding and reward. Schematic illustration of ventricular, intermediate, and monoaminergic nuclei. There are strong reciprocal connections between ventricular and intermediate as well as intermediate and monoaminergic nuclei (indicated by solid lines). Direct connections between ventricular neurons and monoaminergic nuclei are relatively sparse or absent in adults (indicated by the dashed line). Ventricular neurons express receptors for peripheral signaling molecules implicated in feeding are more densely than do intermediate and monoaminergic nuclei. Arc, arcuate nucleus; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DA, dopamine; DR, dorsal raphe; Hb, habenula; LHA, lateral hypothalamic area; NAc, nucleus accumbens; PBN, parabrachial nucleus; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; VTA, ventral tegmental area; 5-HT, 5-hydroxytryptamine.
The arrangement of neurons discussed here is one of many possible schemes that could be used when discussing complex neural systems. This simplified framework permits distillation of a rich literature, reaching back nearly a century, into a set of concepts that can be contained within a single manuscript. It is partially conceptual and does not imply that other connections do not exist between these brain regions. Although circulating hormones can directly affect cells throughout the brain, we have chosen hypothalamic ventricular cells as a starting point because they tend to express receptors for circulating feeding molecules such as insulin, leptin and ghrelin more densely than do intermediate or monoaminergic structures (Hill et al., 1986; Scott et al., 2009; Zigman et al., 2006) and because they function most specifically in the regulation of feeding and related behaviors, although they likely have other important functions as well (discussed below). As with any classification system, there are limitations to this approach. In addition to signaling from peripheral hormones, ventricular neurons also receive synaptic input, originating primarily from intra-hypothalamic sources (Wang et al., 2015). However, whether the synaptic input reflects feedback mechanisms or whether it can independently drive activity is unclear. Moreover, the neuronal organization described in this review is a framework for understanding a common theme: neurons most closely related to homeostatic feeding directly and indirectly interface with circuits that influence reward and aversion. Finally, the cell types and projections discussed below represent only a subset of the cells that are known to exist within these regions. Other classes of cells may have unique molecular signatures, connectivity, and functions. The present discussion is limited to neurons that are known to be involved in feeding or reward processing, and functionally unrelated cell types and connections have been omitted for clarity.
Ventricular neurons of the hypothalamus
Arcuate nucleus
Molecularly-defined cell types in the arcuate nucleus of the hypothalamus (Arc) are often targeted as an ‘entry point’ to homeostatic feeding circuits because they are strongly influenced by peripheral signals and perturbation robustly influences food intake. These neurons are located in the hypothalamus along the third ventricle and express receptors for many circulating molecules associated with homeostatic feeding, including leptin, ghrelin, and insulin (Cone, 2005; Hill et al., 1986; Scott et al., 2009; Varela and Horvath, 2012; Zigman et al., 2006). Because of their location and responsiveness to circulating hormones, much attention has been given to the cells within the Arc and their role in energy homeostasis. Two Arc neuron populations that are prominently studied in the context of homeostatic feeding exert opposing influences on food intake. They are typically characterized by their non-overlapping molecular expression profiles wherein one group expresses agouti-related peptide (AgRP) and another group expresses proopiomelanocortin (POMC). For comprehensive reviews see (Cone, 2005; Elmquist et al., 1999; Morton et al., 2006).
AgRP-expressing neurons are found exclusively in the Arc and are critically important for feeding in mice. It is generally thought that AgRP neuron output represents an orexigenic, appetite-stimulating signal, as these neurons are activated by fasting. In humans, AgRP expression is negatively correlated with body mass index (Alkemade et al., 2012). Genetic ablation of AGRP neurons causes anorexia and starvation in adult mice despite the fact that neonatally ablated mice develop normally (Gropp et al., 2005; Luquet et al., 2005). In fed mice, acute optogenetic (Betley et al., 2013) or chemogenetic stimulation induces feeding and food-directed behavior that mimics the behavior of fasted mice (Aponte et al., 2011; Krashes et al., 2011; Nakajima et al., 2016). AgRP neurons co-express genes for neuropeptide Y (Npy) (Hahn et al., 1998) and γ-aminobutyric acid (GABA) (Tong et al., 2008), and axon terminals co-release AgRP, Npy, and GABA. They send mostly non-overlapping, long-distance projections throughout the forebrain, hypothalamus, and hindbrain (discussed in detail below) (Betley et al., 2013). AgRP neurons express receptors for and are responsive to a variety of locally-produced and circulating molecules including leptin, ghrelin, Npy, and melanocortins (Cone, 2005). Interestingly, optogenetic activation of AgRP neurons promotes feeding only after a relatively long latency (on the order of minutes) despite the finding that the presentation of food rapidly inhibits their activity (Betley et al., 2015; Chen et al., 2015). The rapid inhibition in the absence of consumption may be driven by ascending midbrain and hindbrain signals or through poly-synaptic cortical sensory inputs, though the mechanism is unknown.
POMC neurons are intermingled with and have similar efferent and afferent connectivity to AgRP neurons (Cone, 2005; Wang et al., 2015). It is not known if, like AgRP neurons, individual POMC neurons send long-distance projections to only one target region or if individual neurons send axon collaterals to multiple targets. Arc POMC neurons are thought to functionally oppose AgRP neurons. They tend to co-express β-endorphin and cocaine-amphetamine-regulated transcript and are responsive to circulating hormones including leptin and insulin (Cheung et al., 1997; Cone et al., 2001; Cowley et al., 2001). Leptin, which suppresses appetite, is thought to activate POMC neurons because acute administration induces expression of the immediate early gene Fos (Elias et al., 1999) and increases Socs3 mRNA (Bjorbaek et al., 1999), suggesting activation of negative feedback intracellular pathways in these cells. Ablation of POMC neurons causes hyperphagia and obesity in mice (Gropp et al., 2005; Zhan et al., 2013). Prolonged activation of these cells suppress feeding via melanocortin receptor activation and reduces body weight (Aponte et al., 2011; Zhan et al., 2013), while prolonged inhibition potentiates food intake (Atasoy et al., 2012). The POMC product, α-melanocyte-stimulating hormone (α-MSH), agonizes melanocortin-4 receptors (MC4R). Deletion of MC4R causes obesity in mice (Huszar et al., 1997), and mutations of MC4R are thought to underlie some forms of obesity in humans (Vaisse et al., 2000). The agouti-related peptide acts as an inverse agonist of the MC4R (Nijenhuis et al., 2001), suggesting that AgRP and POMC neurons share common downstream targets. Arc POMC neurons are also locally inhibited by AgRP neurons. However, this inhibition is not necessary for AgRP-evoked feeding (Atasoy et al., 2012). Together with the observations that acute optogenetic activation of POMC somata fails to reduce feeding, while extended activation is effective, these results demonstrate that Arc AgRP and POMC neurons differentially influence feeding. However, the mechanisms underlying their functional differences are largely unclear. Since AgRP neurons synapse locally onto POMC neurons and both populations have similar long-range targets, balance between these two opposing systems is likely necessary to maintain energy homeostasis.
Although much research has focused on Arc AgRP and POMC neurons in feeding and energy homeostasis, an additional orexigenic neuron population has recently been identified in the Arc. Optogenetic activation of tyrosine hydroxylase (TH) expressing neurons in Arc drives food intake in fed mice. These cells detect the orexigenic hormone ghrelin and excite AgRP neurons while inhibiting POMC neurons (Zhang and van den Pol, 2016). Furthermore, oxytocin-receptor expressing glutamatergic (OXTR-Vglut2) neurons in the Arc rapidly promote satiety via synergistic effects with POMC neurons on post-synaptic MC4R-expressing neurons (Fenselau et al., 2017). Still, little is known about the synaptic connectivity and endogenous activity patterns of these non-canonical feeding neurons.
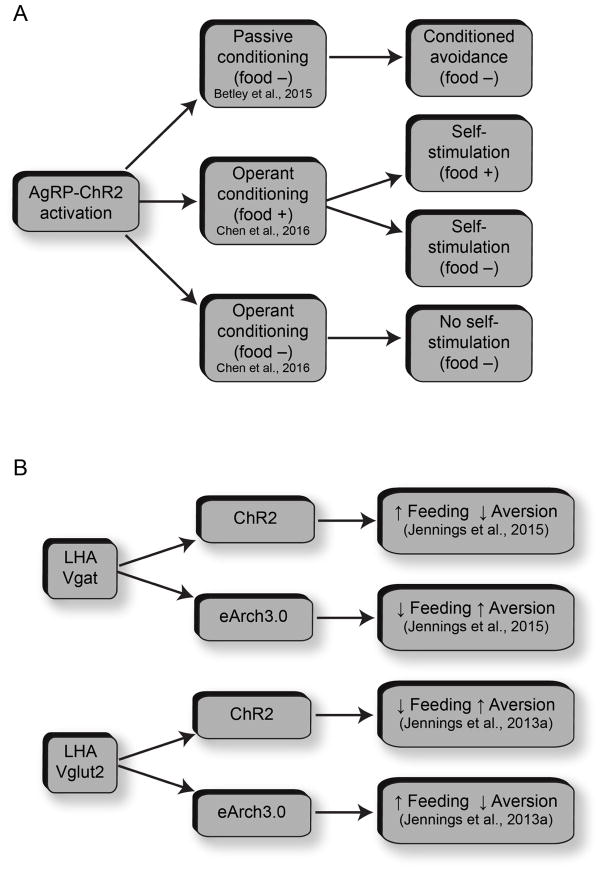
A notable exception to the phenomenon in which orexigenic neuron stimulation is rewarding and anorexogenic neuron stimulation is aversive involves the activation of AgRP neurons, which produces either aversive or rewarding phenotypes depending on the timing of stimulation with respect to food availability (Figure 2a). Following pairing of one side of a chamber with optogenetic activation of AgRP neurons in the absence of food, mice gradually learn to avoid the stimulation-paired side, displaying conditioned avoidance. One explanation for this is that AgRP neurons guide behavior via a negative-valence signal, which is used by the brain to actively avoid particular locations or environmental stimuli (Betley et al., 2015). If the AgRP neuronal signal drives the perception of hunger, it stands to reason that promoting a feeling of hunger without delivering food would be unpleasant. However, mice will lever-press to deliver AgRP stimulation if food is present and will continue self-stimulating even if food is removed. Importantly, mice fail to acquire robust self-stimulation if initial training is conducted in the absence of food (Chen et al., 2016). These paradoxical results demonstrate that the rewarding properties of AgRP neuron activation depend on the presence of food. While more work is needed to determine the mechanisms underlying this phenomenon, both phenotypes must depend on interactions with downstream targets to influence behavior. An interesting question is whether the rewarding or aversive effects of AgRP neuron stimulation depend on mesolimbic dopamine since dopaminergic circuitry mediates reward and aversion (Lammel et al., 2012).
Figure 2.
Functional heterogeneity within molecularly-defined hypothalamic neuronal populations. (A) Arc AgRP neurons promote context dependent aversion or reward. Depending on the availability of food, mice will either avoid or lever-press for AgRP stimulation. (B) Anatomically-intermingled, functionally-opposing LHA neurons influence feeding and reward.
Paraventricular nucleus
One of the many long-range output targets for Arc AgRP and POMC neurons is to the paraventricular nucleus of the hypothalamus (PVN). It sits adjacent to the third ventricle dorsal to the Arc and is implicated in a wide range of behaviors including feeding, drinking, maternal behavior, and temperature regulation via descending projections to the hindbrain and spinal cord (Insel and Harbaugh, 1989; Leibowitz, 1978; Lu et al., 2001; Stanley and Leibowitz, 1984). Microinjection of Npy into the PVN induces feeding in rats, which can be attenuated by concurrent injection of an MC4R agonist (Cowley et al., 1999). Optogenetic activation of AgRP fibers projecting to PVN recapitulates feeding induced by projection-agnostic AgRP cell body stimulation (Atasoy et al., 2012), and activation of OXTR-Vglut2 projections from Arc to PVN rapidly inhibits feeding (Fenselau et al., 2017). Chemogenetic inhibition of single minded 1 (Sim1) expressing neurons, post-synaptic targets of AgRP neurons within the PVN, promotes food intake (Atasoy et al., 2012). Similarly, targeted ablation of PVN-Sim1 neurons produces hyperphagia and obesity (Xi et al., 2013), while chemogenetic activation of MC4R-expressing neurons, a target of both AgRP and POMC neurons, suppresses feeding (Garfield et al., 2015). Optogenetic activation of MC4R-expressing neuronal projections from PVN to PBN suppresses food intake, but interestingly mice do not avoid stimulation in a conditioned place preference assay, suggesting that activation of this pathway is not aversive (Garfield et al., 2015). Paradoxically, an excitatory, orexigenic projection emanating from the PVN and projecting to AgRP neurons in the Arc has recently been described (Krashes et al., 2014), yet how this projection integrates with other cell groups is unclear.
Exactly how the interactions between ventricular neurons and downstream targets coordinate behavior is murky. However, both Arc and PVN neurons project to brain areas that influence a variety of motivated behaviors and ultimately interface with midbrain dopamine neurons. Through higher order interactions, ventricular neurons gain functions that extend far beyond homeostatic feeding.
Intermediate areas
We next consider intermediate neurons that are located downstream from ventricular neurons in discrete anatomical targets which receive axonal innervation from Arc and PVN populations. Intermediate neurons provide feedback to ventricular cells and are highly interconnected with other intermediate and monoaminergic neurons. These cell groups are implicated in both reward processing and feeding as well as other processes that have largely been studied outside the context of feeding behavior. Here, we focus the discussion on the bed nucleus of the stria terminalis (BNST), central amygdala (CeA), parabrachial nucleus (PBN), and lateral hypothalamic area (LHA), but note this is not an exhaustive list of potential intermediate targets. In general, ablation, electrical stimulation, or pharmacological perturbation of these areas can affect both feeding and reward phenotypes as well as stress and anxiety. However, these anatomically-defined regions are heterogeneous in cell type and connectivity, the details of which are largely unknown. Much work is needed to determine the functional relationships between molecularly-defined neuronal populations.
PBN
The pontine PBN is often studied for its role in gustation and taste processing. It receives gustatory input via projections from the nucleus of the solitary tract. Lesions of the PBN disrupt the ability to acquire conditioned taste aversion while sparing conditioned flavor preference (Reilly et al., 1993). Electrophysiological recordings show that PBN neurons are reciprocally connected with CeA and LHA and respond to a variety of tastants (Li et al., 2005). Together these results suggest that in addition to relaying taste information to forebrain structures, PBN is also critical for learning about taste.
The PBN receives synaptic input from both AgRP and POMC neurons as well as other intermediate populations (Betley et al., 2013; Carter et al., 2013; Stachniak et al., 2014; Williams and Elmquist, 2012; Wu et al., 2009). It is thought that the inhibitory drive from AgRP neurons onto PBN cells suppress feelings of malaise that can influence food intake and reward-directed behavior. When inhibition from ventricular neurons is removed by genetically eliminating GABA release from AgRP neurons, the resulting hyperactivity of PBN halts feeding (Wu et al., 2009; Wu et al., 2012). In agreement with these results, PBN neurons respond to malaise (Swank and Bernstein, 1994) and can alter the rewarding properties of food (Soderpalm and Berridge, 2000). Interestingly, PBN neurons that encode calcitonin gene-related peptide (CGRP) exhibit Fos induction that is inversely related to food intake. Acute activation of PBN-CGRP neurons or their projections to CeA suppresses feeding (Carter et al., 2013). Acute inhibition of somata or projections to CeA restores feeding during conditions that suppress appetite but does not induce feeding in sated mice (Campos et al., 2017; Carter et al., 2013). Of relevance to the present discussion, PBN neurons send long-distance projections to LHA, ventral tegmental area (VTA), PVN, BNST, CeA, and nucleus accumbens (NAc) (Fulwiler and Saper, 1984; Moga et al., 1990; Pritchard et al., 2000), thus positioning this area to integrate input related to energy needs from ventricular neurons and visceral signals (i.e., visceral malaise) to tune goal-oriented behavior. Indeed, optogenetic stimulation of MC4R-expressing neurons projecting from PVN to PBN (MC4RPVN→PBN) suppresses food intake. Unlike other intermediate populations, which tend to be either anorexigenic and aversive or orexigenic and appetitive, hungry mice prefer MC4RPVN→PBN stimulation (Garfield et al., 2015), though the circuit mechanisms underlying this phenomenon are unknown.
Extended amygdala
The extended amygdala comprises distinct nuclei that have historically been implicated in anxiety, stress, and fear learning (Cassell et al., 1999). Two components of the extended amygdala that are involved in both reward and feeding are the BNST and the CeA. These areas receive input from ventricular neurons and have diverse projections to intermediate and monoaminergic targets.
The BNST is a critical mediator of anxiety and fear learning that acts in coordination with other amygdaloid nuclei to regulate physiological responses to threats (Walker et al., 2003). Chemical lesions of BNST prevent rats from expressing anxiety-like behavior following aversive foot shock conditioning (Hammack et al., 2004). Electrical stimulation induces aggressive behavior in cats (Shaikh et al., 1986), potentiates anxiety-like behavior, and elevates plasma corticosterone in rats (Casada and Dafny, 1991; Dunn, 1987). Although limited attention has been given to its role in feeding, manipulations of BNST circuitry can have profound effects on food intake and reward. Optogenetic activation of AgRP fibers within the BNST evokes food intake in fed mice (Betley et al., 2013). Similarly, optogenetic activation of GABAergic BNST efferent projections to the LHA induces feeding and reward-related behaviors (Jennings et al., 2013a). It is commonly thought that inhibition of food intake by stress is mediated by corticotrophin-releasing factor (CRF). Antagonizing CRF2 receptors within the BNST potentiates feeding following restraint stress (Ohata and Shibasaki, 2011), but the role of BNST CRF is unlikely to be limited to feeding (Kash et al., 2015). Many of the details underlying the synaptic relationships between ventricular and intermediate neurons within the BNST remain to be elucidated. Presently, the post-synaptic targets of AgRP neurons within the BNST are unknown. However, feeding induced by stimulation of BNST-projecting AgRP neurons is not affected by simultaneous inhibition of putative downstream MC4R neurons within the BNST (Garfield et al., 2015).
Importantly, the BNST also receives convergent input from a variety of regions implicated in reward and anxiety phenotypes, including the amygdala, hippocampal formation, prefrontal cortex (PFC), and VTA (Weller and Smith, 1982). Chemogenetic activation of Npy1R-expressing neurons, a downstream target of AgRP neurons within the medial amygdala, which project to BNST suppresses food intake and increases territorial aggression (Padilla et al., 2016). BNST neurons project to LHA (Jennings et al., 2013a) and the VTA (Jennings et al., 2013b). These projections can influence anxiety states and reward phenotypes (reviewed by (Stamatakis et al., 2014)). The present data suggest that the BNST is a critical modulator of stress and anxiety responses and may inhibit food intake in order to bias behavior toward defensive action.
The CeA is an amygdaloid nucleus involved in regulating fear and anxiety responses. Lesions of the CeA disrupt Pavlovian learning and the expression of conditioned fear responses (e.g., freezing, elevated heart rate, elevated arterial pressure), whereas electrical stimulation produces bradycardia and reduces blood pressure (Baxter and Murray, 2002; Kapp et al., 1979; Kapp et al., 1982). Although it is primarily studied in the context of fear learning and anxiety, the CeA can also influence food intake. CeA neurons receive direct input from both AgRP and Arc POMC neurons. In contrast to the BNST, activation of AgRP projections within the CeA fails to induce feeding in sated mice (Betley et al., 2013), but administration of an MC4R antagonist directly into the CeA potentiates feeding in rats (Kask and Schioth, 2000). Together, these results suggest that Arc POMC neurons may uniquely influence feeding via CeA projections, though this remains to be tested. Recent work has shown that molecularly- and anatomically-distinct neuron populations within the CeA may selectively control unique aspects of feeding and reward (Kim et al., 2017). A subset of CeA neurons (protein kinase C-δ neurons) are activated by anorexigenic signals and optogenetic inhibition drives food intake (Cai et al., 2014a). Optogenetic activation of CeA neurons expressing serotonin receptor 2a or their axons within the PBN facilitates food intake and promotes approach (Douglass et al., 2017). Unique CeA outputs may also differentially contribute to feeding and reward-related behaviors. Indeed, descending GABAergic projections from CeA to the periaqueductal gray control prey pursuit while projections to the reticular formation control jaw movements associated with feeding in mice (Han et al., 2017). Much work is needed to understand why lesions of the CeA have relatively little effect on energy homeostasis and reward-directed behavior, yet acute manipulations influence food intake and food-directed behavior. A more detailed understanding of the synaptic connectivity and molecular profiles of CeA neurons will help to shed light on these issues.
In addition to afferents from ventricular neurons, the CeA also receives synaptic input from many cortical, thalamic, and intra-amygdala cells (Samson et al., 2005) and projects to BNST, LHA, NAc, and ascending neuromodulatory cell groups in the midbrain and hindbrain (Sah et al., 2003). As such, the CeA is well positioned to modify and coordinate fear and anxiety behaviors as well as reward seeking and feeding. Given its established role in modulating anxiety states (Tye et al., 2011), one possibility is that the CeA integrates diverse inputs to select behaviors based on the current state of the organism and ensure proper physiological arousal. When threats are perceived, CeA-mediated fear behaviors may dominate by suppressing feeding and other competing drives, likely in coordination with the BNST. This possibility remains to be directly tested.
LHA
The LHA is a critical link between feeding and reward. The cells located here receive input from ventricular neurons and have diverse efferent and afferent connections with reward circuitry. Both AgRP and Arc POMC neurons project to LHA (Wang et al., 2015) and these same projections can be activated by the satiety signal, leptin (Elias et al., 1999). Historically, the LHA was treated as a ‘feeding center’ based on the finding that ablation produces hypophagia and starvation (Anand and Brobeck, 1951a, b), whereas electrical stimulation elicits feeding (Hoebel and Teitelbaum, 1962; Margules and Olds, 1962). However, these results have been difficult to interpret because LHA manipulations also have profound effects on motivation and reward phenotypes (Carr and Simon, 1984; Hoebel and Teitelbaum, 1962; Stuber and Wise, 2016; Teitelbaum and Epstein, 1962). Rodents and primates reliably self-stimulate for electrical stimulation of the LHA at the same sites that elicit feeding (Margules and Olds, 1962; Rolls et al., 1980). Similarly, both deprivation state and the administration of circulating satiety signals such as insulin, glucagon, or leptin can modify the rate of LHA self-stimulation (Abrahamsen et al., 1995; Balagura and Hoebel, 1967; Carr and Wolinsky, 1993; Fulton et al., 2000). The mechanisms underlying electrical self-stimulation are multifaceted though, as it can have divergent effects on proximal or distal sites, is not cell-type specific, and can influence fibers of passage.
As has been discussed previously (DiLeone et al., 2003; Stuber and Wise, 2016), there is considerable molecular and anatomical heterogeneity within the LHA. This, along with the dramatic effects that coarse manipulations have on feeding and reward phenotypes, has made the LHA a prime target for applications of molecular and genetic tools. Because manipulations of many cell types have robust effects on both food intake and self-stimulation, interpreting the present data in a binary fashion as either ‘feeding’ or ‘reward’ related is fraught with problems. Recent evidence suggests that mostly non-overlapping populations of LHA neurons contribute differentially to reward and aversion as well as feeding (Figure 2b). Acute activation of LHA glutamatergic neurons suppresses feeding and drives aversion (Jennings et al., 2013a), while genetic ablation potentiates food intake and weight gain (Stamatakis et al., 2016), suggesting these cells as a negative regulator of feeding and reward. LHA glutamatergic neurons predominantly project to the lateral habenula (LHb). Though it is not traditionally thought of as a feeding center, manipulations of the LHb can influence food intake. Optogenetic inhibition of LHA glutamatergic afferents within the LHb promotes food intake and reward-related behaviors (Stamatakis et al., 2016), and optogenetic activation of LHb efferents within the ventral midbrain produces behavioral avoidance and suppresses consummatory behavior (Stamatakis and Stuber, 2012). LHA GABAergic neurons, another substantial LHA cell group, are intermingled with and seem to functionally oppose glutamatergic cells. Acute activation of LHA GABA cells enhances motivation for food, drives food consumption, and is rewarding (Jennings et al., 2015; Navarro et al., 2016), while acute inhibition or genetic ablation reduces food intake and is aversive (Jennings et al., 2015; Navarro et al., 2016). Similarly, acute activation of an adjacent GABAergic population located in the zona incerta promotes approach and food intake with a preference for palatable foods (Zhang and van den Pol, 2017). Emerging evidence suggests that activity of LHA GABA neurons may also be required for learning about cue-reward relationships in rats (Sharpe et al., 2017). LHA GABAergic neurons comprise heterogeneous subpopulations that may be functionally distinct. Chemogenetic activation of the subset of LHA GABA neurons expressing the neuropeptide galanin potentiates motivated feeding for palatable foods without affecting chow intake (Qualls-Creekmore et al., 2017). Another subset of LHA GABA neurons express pancreas duodenum homeobox 1 (Pdx1) and project to the PVH. Optogenetic activation of this pathway promotes food intake in fed mice (Wu et al., 2015). Orexin/hypocretin neurons of the LHA are also thought to contribute to reward and feeding. Their activity is reduced immediately following food consumption (Gonzalez et al., 2016). Acute activation of LHA orexin neurons, which project to the VTA, potentiates reward seeking for both drugs and food (Harris et al., 2005; Inutsuka et al., 2014). Orexin/hypocretin neurons may more generally control arousal though (Mahler et al., 2014). There are also overlapping subsets of neurons within the LHA that express melanin-concentrating hormone (MCH) (Domingos et al., 2013) or leptin receptors (Leinninger et al., 2009) and project to the VTA to influence dopamine release and intake of palatable foods.
The LHA receives input from the BNST (Jennings et al., 2013a) and VTA (Taylor et al., 2014), two regions that are heavily implicated in the control of positive and negative behavioral states. Furthermore, LHA projections to the LHb (Stamatakis et al., 2016), VTA (Nieh et al., 2015; Nieh et al., 2016), and locus coeruleus (Laque et al., 2015) can influence feeding and reward. Together, the anatomical connectivity and nuanced functions of molecularly-defined cell types within the LHA suggest that this area may contribute broadly to regulating motivation that is directed towards feeding.
The specific synaptic connectivity between ventricular and intermediate neurons (and within distinct intermediate neuron populations) is still largely unknown. Arc AgRP and POMC neurons innervate many of the same intermediate areas and likely have antagonistic effects on downstream targets. Ventricular neurons can influence—either through direct innervation or through poly-synaptic connections with intermediate neurons—the activity of canonical reward and aversion brain regions.
Monoaminergic systems
Monoaminergic systems (i.e., dopamine and serotonin) are crucial for myriad behaviors and manipulations of these cells can have profound effects on feeding and reward phenotypes. Because direct connections between ventricular neurons and monoaminergic neurons are sparse, indirect connections via intermediate cell groups likely orchestrate functional interactions.
Dopamine has long been implicated as a critical mediator of goal-directed behavior and learning. Rats and mice readily learn to selectively self-stimulate (Adamantidis et al., 2011; Ilango et al., 2014; Rossi et al., 2013; Tsai et al., 2009; Witten et al., 2011) and avoid optogenetic inhibition (Danjo et al., 2014; Ilango et al., 2014) of midbrain dopamine neurons. Optogenetic activation of VTA GABAergic neurons, which reduces dopamine release, inhibits licking for sucrose and promotes aversion (Tan et al., 2012; van Zessen et al., 2012). Destruction of dopaminergic neurons causes profound deficits in feeding, movement, motivation, and learning. In addition to its established role in reward, dopamine signaling is also a critical component of voluntary feeding and motivated behavior in general. Intracranial self-stimulation sites, which depend on dopamine release, have often been found to promote feeding and depend on the current satiety state of the subject (Abrahamsen et al., 1995; Adamantidis et al., 2011; Carr and Wolinsky, 1993; Jennings et al., 2013a; Stuber et al., 2011). Moreover, ablation of dopaminergic neurons (Ungerstedt, 1971) or selective disruption of dopamine production causes hypoactivity and aphagia in mice (Zhou and Palmiter, 1995). Circulating signals such as ghrelin and leptin can also influence the activity of dopaminergic systems (Palmiter, 2007), although hormone receptor expression is much lower than in ventricular populations. Acute administration of the orexigenic peptide, ghrelin, excites VTA dopamine neurons in rodents (Abizaid et al., 2006; Cone et al., 2014) and increases blood oxygen level dependent (BOLD) responses to food pictures in the ventral midbrain and striatum in healthy volunteers (Malik et al., 2008). In mice, the anorexigenic protein, leptin, can directly and indirectly influence the activity of VTA dopamine neurons and reduce food intake (Domingos et al., 2011; Fulton et al., 2006; Hommel et al., 2006; Leinninger et al., 2009).
The mesolimbic dopamine system is also strongly influenced by intermediate neurons. Direct connections between ventricular neurons and dopaminergic cells are quite sparse and functional interactions probably rely heavily on intermediate relays. Though direct inhibition of dopamine neurons by AgRP neurons has not been demonstrated in adult animals, AgRP neurons can modify VTA dopaminergic plasticity and dopamine-dependent behaviors during development (Dietrich et al., 2012), and acute activation of AgRP neurons in adult mice mediates a variety of non-food related behaviors, including anxiety and stereotypy (Dietrich et al., 2015). It has recently been shown that palatable food can promote feeding even in AgRP-impaired mice, an effect that is dependent on dopaminergic signaling (Denis et al., 2015) despite the sparseness of direct AgRP to VTA projections. The circuit mechanisms governing these effects are unclear, though they likely depend on at least one intermediate connection linking AgRP and mesolimbic dopaminergic signaling. Ventral tegmental dopaminergic neurons receive input from many intermediate regions including the PBN (Coizet et al., 2010). PBN afferents can inhibit putative dopaminergic neurons, which probably requires local interneurons since PBN neurons are glutamatergic (Coizet et al., 2010). In addition to the PBN, VTA dopaminergic neurons receive input from LHA, CeA, and BNST and send dense projections to the NAc and PFC (Beier et al., 2015). Both of these primary efferent targets of VTA dopamine fibers exert high-level control over food seeking and goal-directed action.
Ingestion of sugars and palatable foods are known to increase striatal dopamine release (Hajnal et al., 2004; Hernandez and Hoebel, 1988; Rada et al., 2005; Roitman et al., 2004; Roitman et al., 2008; Small et al., 2003), and the dopaminergic system is altered in obese humans and in animal models of obesity (Volkow et al., 2011). Striatal dopamine D2 receptor availability is inversely correlated with obesity in humans and rats (Johnson and Kenny, 2010; Wang et al., 2001), and lentiviral-mediated knockdown of D2 receptor expression makes rats more susceptible to weight gain when allowed to consume calorically-dense food (Johnson and Kenny, 2010). While chronic D2 receptor blockade potentiates weight gain (Cope et al., 2005), D2 receptor knockout mice are not obese (Baik et al., 1995). Similarly, microinfusions of D1- or D2-slective dopamine receptor antagonists into the NAc fail to affect food intake in rats (Baldo et al., 2002). Thus, specific functions of dopamine receptors per se in acute feeding are open to interpretation. One possibility is that altered dopamine receptor expression is an effect of obesity resulting from pathological dopamine release. The observed changes related to obesity and chronic antipsychotic administration may reflect compensatory changes of other circuit nodes and not necessarily causal involvement of dopamine receptors (Palmiter, 2007). Chronic food restriction in rats decreases extracellular dopamine in NAc (Pothos et al., 1995). Aphagia resulting from genetic disruption of dopamine production can be rescued by restoration of dopamine signaling in the dorsal or ventrolateral but not the ventromedial striatum (Darvas et al., 2014; Hnasko et al., 2006; Szczypka et al., 2001). Gastric infusion of glucose increases dopamine release in both the dorsal striatum and NAc. Intriguingly, optogenetic activation of D1 receptor expressing neurons in the dorsal striatum, but not the NAc, overrides the satiating effects of such an intra-gastric load, while genetic ablation of these neurons produces the opposite effect (Han et al., 2016). Although the mechanisms linking gastric glucose sensing with dopamine release are not fully understood, these results highlight the notion that dopamine signaling within distinct striatal compartments is functionally heterogeneous.
While dorsal striatal dopamine is critical for the ability to feed and sense glucose, NAc dopamine seems to be critical for the motivation to feed. When dopamine fibers innervating the NAc are selectively destroyed, rats become unwilling to exert effort to obtain food despite being physically capable of eating and performing instrumental actions (Aberman and Salamone, 1999). Because of this, it has been proposed that NAc dopamine invigorates and motivates behavior independently of primary effects on appetite (Salamone and Correa, 2012) and may function similarly to a gain signal. However, dopamine deficient mice that have dopamine production virally restored in the dorsal striatum show similar instrumental responding for food and comparable break points on a progressive ratio task (Robinson et al., 2007). The willingness to exert effort for food in these mice that is absent in NAc dopamine depleted mice may be due to differences in experimental timing. In viral restoration experiments, mice recover for months prior to testing, whereas dopamine depleted rats are tested within weeks of surgery. This extra time may allow the dorsal striatum to co-opt NAc functions. Alternatively, viral rescue within the dorsal striatum yields low levels of dopamine in the NAc, which may be sufficient to rescue motivational deficits. Interestingly, acute optogenetic activation of dopamine D1 receptor-expressing projections from NAc to LHA halts feeding, while inhibition potentiates consummatory behavior (O’Connor et al., 2015). In addition to dopaminergic input, neurons within the NAc receive dense glutamatergic innervation from thalamic and cortical regions that convey information related to gustation and executive function. Thus, the NAc may integrate high-level descending signals with information pertaining to homeostatic needs to direct behavior toward relevant goals (Kelley, 2004).
Another major target of VTA dopamine projections is the PFC. In humans, BOLD responses within the frontal cortex are positively correlated with the pleasantness of flavors (de Araujo et al., 2003). In rats, aspiration of medial PFC (mPFC) produces finickiness while having relatively little impact on the ability to feed (Kolb and Nonneman, 1975). Similarly, excitotoxic lesions of mPFC have little effect on energy homeostasis and body weight (Davidson et al., 2009). However, dopamine is released in the mPFC during consumption of palatable foods (Bassareo and Di Chiara, 1997; Hernandez and Hoebel, 1990). Furthermore, a subset of fasting-activated mPFC neurons expressing dopamine D1 receptors, presumed to be direct downstream targets of VTA dopamine axons, bidirectionally drive food intake (Land et al., 2014). How mPFC influences on food intake and reward guided behavior integrate with its established role in executive function, cognitive control, and learning (reviewed by (Miller and Cohen, 2001)) remain to be determined.
In addition to the mesolimbic dopaminergic system, serotonergic (5-hydroxytryptamine, 5-HT) neurons located in the dorsal raphe nucleus are highly interconnected with both intermediate and monoaminergic neurons (Muzerelle et al., 2016; Ogawa et al., 2014; Pollak Dorocic et al., 2014) and contribute to feeding. In general, pharmacological manipulations that increase 5-HT availability tend to decrease feeding, while reducing 5-HT has the opposite effect (for detailed reviews see (Blundell, 1986; Simansky, 1996). Mice lacking 5-HT2C or 5-HT1B receptors are hyperphagic and obese (Bouwknecht et al., 2001; Tecott et al., 1995), and these receptors are thought to permit suppression of feeding via interactions with Arc AgRP and POMC neurons (for discussion of this, see (Sohn et al., 2013)). However, systemically agonizing 5-HT2C receptors does not impact daily food intake or body weight in mice (Zhou et al., 2007). In vivo calcium imaging reveals that serotonergic neurons increase their activity during food consumption as well as social interactions (Li et al., 2016); however, relatively few 5-HT neurons show Fos induction following food ingestion (Takase and Nogueira, 2008), and blockade of 5-HT signaling within the nucleus of the solitary tract is associated with increased feeding following AgRP neuron ablation (Wu et al., 2012). Optogenetic activation of dorsal raphe Pet-1 neurons, which release both glutamate and 5-HT, is rewarding, promoting place preference and self-stimulation (Liu et al., 2014). Importantly, optogenetic activation of 5-HT neurons within the dorsal raphe can also increase patience (Miyazaki et al., 2014), anxiety (Ohmura et al., 2014), and pain sensitivity (Cai et al., 2014b). Given its disparate functions and wide anatomical distribution, it has been hypothesized that serotonin may underlie basic behavioral arousal or attention (Robbins, 1997). Though serotonin is able to influence feeding, affecting food intake may be one of many consequences of perturbation of this system.
Concluding remarks
The neurocircuits that may bias behavior toward either homeostatic or hedonic feeding are largely intertwined and overlapping. Assignment of specific cell types and brain regions to one category or the other is often unhelpful. Together, the systems involved in hedonic and homeostatic aspects of feeding provide a means by which the nervous system can dynamically coordinate intake of ‘rewarding’ stimuli in order to meet metabolic demands and ensure survival. Because food is essential, it is unsurprising that so many neuron populations that facilitate food intake also promote rewarding phenotypes and those that suppress appetite are aversive. Ventricular neurons of the hypothalamus represent an entry point by which peripheral signals reflecting the metabolic state of the animal can robustly and efficiently influence the nervous system to ultimately orchestrate behavior. The neurons located in the Arc and PVN integrate information from circulating hormones and instruct intermediate targets to initiate or cease the process of acquiring sustenance. Intermediate neurons located in subcortical structures function more generally, integrating input pertaining to various needs, including feeding, mating, and safety. They ultimately influence monoaminergic neurons, which are very general in function. Monoaminergic neurons (i.e., mesolimbic dopamine) are involved in motivation and can influence food intake. However, in conjunction with their downstream targets, they are also involved in numerous other processes including executive function and learning about the environment.
Recent technological advances have allowed researchers to probe functional neurocircuits in vivo with unprecedented precision. Specifically, optogenetic and chemogenetic tools have shed light onto the functional heterogeneity within anatomically-defined brain regions (Tables 1 and 2). Such tools have made it possible to begin to answer the question of whether the AgRP neuronal output represents a positive- or negative-valence signal (Figure 2a), and they have greatly facilitated disentangling the functional heterogeneity of the LHA (Figure 2b) among many other brain regions. Because of the dual outcomes (hyperphagia/reward or hypophagia/aversion) of some acute manipulations, it is important to measure both feeding and reward phenotypes. Note the frequency of question marks in Tables 1 and 2.
Table 2. Acute manipulations of molecularly- and anatomically-defined long-distance projections that influence food intake also affect appetitive behavior.
Conventions are the same as Table 1 except arrows indicate putative effects on pre-synaptic activity but do not necessarily indicate the direction of change in post-synaptic output.
| Area of origin | Projection | Manipulation | Food intake | Appetitive behavior | References | |
|---|---|---|---|---|---|---|
| Arc | AgRP→BNST | ChR2 | ↑ | + | ? | Atasoy et al 2012; Betley et al 2013; Garfield et al 2015 |
| AgRP→PVN | ChR2 | ↑ | + | ? | Betley et al 2013; Garfield et al 2015 | |
| AgRP→LHA | ChR2 | ↑ | + | ? | Betley et al 2013; Garfield et al 2015 | |
| AgRP→CeA | ChR2 | ↑ | / | ? | Betley et al 2013 | |
| AgRP→PBN | ChR2 | ↑ | / | ? | Betley et al 2013 | |
| Oxtr→PVN | ChR2 | ↑ | − | ? | Fenseleu et al 2017 | |
| PVN | Sim1→PAG | Gi | ↓ | + | ? | Stachniak et al 2014 |
| Sim1→PBN | Gi | ↓ | / | ? | Stachniak et al 2014 | |
| MC4R→PBN | ChR2 | ↑ | − | + | Garfield et al 2015 | |
| BNST | Vgat→LH | ChR2 | ↑ | + | + | Jennings et al 2013a |
| Vgat→LH | eArch3.0 | ↓ | − | − | Jennings et al 2013a | |
| Vgat→VTA | ChR2 | ↑ | / | + | Jennings et al 2013a; Jennings et al 2013b | |
| Vglut2→VTA | ChR2 | ↑ | / | − | Jennings et al 2013b | |
| CeA | Vgat→PAG | ChR2 | ↑ | + (prey pursuit) | ? | Han et al 2017 |
| Vgat→RF | ChR2 | ↑ | + (chewing) | ? | Han et al 2017 | |
| Htr2a→PBN | ChR2 | ↑ | + | + | Douglass et al 2017 | |
| PBN | CGRP→CeA | ChR2 | ↑ | − | ? | Carter et al 2013 |
| CGRP→CeA | Gi | ↓ | + | ? | Carter et al 2013 | |
| CGRP→BNST | ChR2 | ↑ | / | ? | Carter et al 2013 | |
| LHA | Vglut2→lHb | eNpHR3.0 | ↓ | + | + | Stamatakis et al 2016 |
| LHA→VTA | ChR2 | ↑ | + | ? | Nieh et al 2015 | |
| LHA→VTA | eNpHR3.0 | ↓ | / | ? | Nieh et al 2015 | |
| Vglut2→VTA | ChR2 | ↑ | / | − | Nieh et al 2015; Nieh et al 2016 | |
| Vgat→VTA | ChR2 | ↑ | + | + | Nieh et al 2015; Nieh et al 2016 | |
| Vglut2→VTA | eNpHR3.0 | ↓ | / | ? | Nieh et al 2016 | |
| Pdx1→PVH | ChR2 | ↑ | + | ? | Wu et al 2015 | |
| LHb | LHb→RMTg | ChR2 | ↑ | − | − | Stamatakis and Stuber 2012 |
| NAc | D1R→LHA | ChR2 | ↑ | + | ? | O’Connor et al 2015 |
| D2R→LHA | ChR2 | ↑ | / | ? | O’Connor et al 2015 | |
| GAD→LHA | ChR2 | ↑ | − | ? | O’Connor et al 2015 | |
Arc, arcuate nucleus of the hypothalamus; AgRP, agouti-related peptide; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the hypothalamus; CGRP, calcitonin gene-related peptide; ChR2, channelrhodopsin-2; D1R, d1 like dopamine receptor; D2R, d2-like dopamine receptor; eArch3.0, archaerhodopsin-3.0; eNpHR3.0, halorhodopsin-3.0; Gi, hM4Dq (Gi-coupled designer receptor exclusively activated by designer drug); Htr2a, serotonin receptor 2a; LHA, lateral hypothalamic area; LHb, lateral habenula; MC4R, melanocortin 4 receptor; NAc, nucleus accumbens; Oxtr, oxytocin receptor; PAG, peri-aqueductal gray; PBN, parabrachial nucleus; Pdx1, pancreas duodenum homeobox 1; PVN, paraventricular nucleus of the hypothalamus; RMTg, rostromedial tegmental nucleus; Sim1, single minded 1; Vgat, vesicular GABA transporter; Vglut2, vesicular glutamate transporter 2, VTA, ventral tegmental area;
While these tools have been invaluable in defining functional circuit nodes embedded within complex neural tissue, their limitations must be considered when interpreting results. Viral transduction, for example, can introduce unexpected variability into experiments. Seemingly small differences in injection location, virus preparation, or subject age may have a large impact on the experimental results. It is important to consider the quantitative relationship between viral transduction and behavioral outcomes (for a detailed discussion, see (Sternson et al., 2016)). Moving forward, bulk manipulations alone may be insufficient to disentangle whether or how particular cells contribute to homeostatic or hedonic feeding behavior. This is, in part, because the outcomes of bulk activation or inhibition within interconnected circuits can be difficult to predict and can create ambiguity about whether some functions attributed to particular neurons are related to unintended effects on downstream targets. Furthermore, synchronous activation of large groups of neurons is unlikely to capture the nuanced activity patterns that have been observed in vivo. It is therefore important that researchers carefully consider stimulation parameters to most closely approximate endogenous activity patterns. One potential way to address this is to record neural activity of relevant circuitry to identify and isolate subsets of neurons that selectively encode discrete aspects of behavior. Continued advancement in deep brain imaging will undoubtedly identify unique functional groups of neurons. Such populations may be defined by anatomical connections and molecular profiles. The traditionally singular molecular markers currently used to define cell types may be insufficient to specify functional units within complex, intertwined circuits. Combinatorial viral approaches allow expression of exogenous proteins in cell types that are defined by two or more features (Fenno et al., 2014). Combined with a more detailed understanding of the molecular profile of individual cells by leveraging high-throughput single-cell sequencing approaches, it may eventually be possible to identify pathways that selectively contribute to feeding.
Here, we have highlighted the major nodes involved in feeding, but our list is not exhaustive. Because so many areas and circuits may be involved in feeding and reward, some of which may not be known yet, it is important for researchers to screen for these phenotypes in a non-biased manner to determine relevant brain regions and cell types. The anatomy of the systems discussed here has been simplified for relevance to feeding, but it is important to note that intermediate and monoaminergic neurons receive input from disparate brain regions that are involved in a variety of motivated behaviors (e.g. anxiety, social behavior). The cells contributing to these behaviors may influence intermediate and monoaminergic pathways to suppress feeding in necessary situations (Burnett et al., 2016) independently of the activity of ventricular neurons. The details of such drive competition are largely unknown, but may involve selective tuning of the activity of intermediate neuron populations.
As a result of the dual role that intermediate and monoaminergic neurons play in guiding homeostatic and hedonic feeding, it is easy to imagine how they might be co-opted by drugs of abuse or unhealthy foods to the detriment of the organism. The circuits implicated in obesity and drug addiction overlap substantially with those that control typical feeding (Castro et al., 2015; Volkow et al., 2011). Elucidating the precise neurocircuits controlling unique aspects of feeding and reward seeking will be critical to understanding pathological behavior. Thus, when considering therapeutics for obesity, it is important not to immediately discount drugs that affect the reward system, as they may also have profound impact on food intake and energy homeostasis.
Acknowledgments
We thank members of the Stuber Lab for helpful discussion. The authors were supported by DA038168, DA032750, the Foundation of Hope, the Brain and Behavior Research Foundation, and the Simon’s Foundation (GDS), and DK112564 (MAR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen GC, Berman Y, Carr KD. Curve-shift analysis of self-stimulation in food-restricted rats: relationship between daily meal, plasma corticosterone and reward sensitization. Brain Res. 1995;695:186–194. doi: 10.1016/0006-8993(95)00764-h. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkemade A, Yi CX, Pei L, Harakalova M, Swaab DF, la Fleur SE, Fliers E, Kalsbeek A. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in alphaMSH are related to type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E925–933. doi: 10.1210/jc.2011-3259. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951a;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951b;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Balagura S, Hoebel BG. Self-Stimulation of Lateral Hypothalamus Modified by Insulin and Glucagon. Physiology & Behavior. 1967;2:337. [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature reviews Neuroscience. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell. 2015;162:622–634. doi: 10.1016/j.cell.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Serotonin manipulations and the structure of feeding behaviour. Appetite. 1986;(7 Suppl):39–56. doi: 10.1016/s0195-6663(86)80051-4. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5-HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 2001;74:507–516. doi: 10.1016/s0031-9384(01)00589-3. [DOI] [PubMed] [Google Scholar]
- Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Bruning JC, Krashes MJ. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014a;17:1240–1248. doi: 10.1038/nn.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Hou YY, Pan ZZ. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol Pain. 2014b;10:70. doi: 10.1186/1744-8069-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Han S, Wisse BE, Palmiter RD, Schwartz MW. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat Neurosci. 2017;20:934–942. doi: 10.1038/nn.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Simon EJ. Potentiation of reward by hunger is opioid mediated. Brain Res. 1984;297:369–373. doi: 10.1016/0006-8993(84)90578-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Wolinsky TD. Chronic food restriction and weight loss produce opioid facilitation of perifornical hypothalamic self-stimulation. Brain Res. 1993;607:141–148. doi: 10.1016/0006-8993(93)91499-i. [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Zimmerman CA, Essner RA, Knight ZA. Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife. 2016:5. doi: 10.7554/eLife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience. 2010;168:263–272. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Cope MB, Nagy TR, Fernandez JR, Geary N, Casey DE, Allison DB. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 1980;185:1–15. doi: 10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2014;111:6455–6460. doi: 10.1073/pnas.1404323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Wunsch AM, Gibbs JT, Palmiter RD. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc Natl Acad Sci U S A. 2014;111:2764–2769. doi: 10.1073/pnas.1400332111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Denis RG, Joly-Amado A, Webber E, Langlet F, Schaeffer M, Padilla SL, Cansell C, Dehouck B, Castel J, Delbes AS, et al. Palatability Can Drive Feeding Independent of AgRP Neurons. Cell Metab. 2015;22:646–657. doi: 10.1016/j.cmet.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, et al. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Zimmer MR, Bober J, Horvath TL. Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell. 2015;160:1222–1232. doi: 10.1016/j.cell.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2:e01462. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Grundemann J, Strobel C, Alcala Morales PL, Conzelmann KK, Luthi A, Klein R. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci. 2017 doi: 10.1038/nn.4623. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327–331. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenselau H, Campbell JN, Verstegen AM, Madara JC, Xu J, Shah BP, Resch JM, Yang Z, Mandelblat-Cerf Y, Livneh Y, et al. A rapidly acting glutamatergic ARC-->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nat Neurosci. 2017;20:42–51. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Iordanidou P, Strom M, Fugger L, Burdakov D. Inhibitory Interplay between Orexin Neurons and Eating. Curr Biol. 2016;26:2486–2491. doi: 10.1016/j.cub.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A, et al. Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab. 2016;23:103–112. doi: 10.1016/j.cmet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Tellez LA, Rangel MJ, Jr, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE. Integrated Control of Predatory Hunting by the Central Nucleus of the Amygdala. Cell. 2017;168:311–324. e318. doi: 10.1016/j.cell.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res Bull. 1990;25:975–979. doi: 10.1016/0361-9230(90)90197-8. [DOI] [PubMed] [Google Scholar]
- Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17:1127–1138. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J Neurosci. 2014;34:817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology. 2014;85:451–460. doi: 10.1016/j.neuropharm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013a;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013b;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiology & behavior. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain research. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA. Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells. 2015;38:1–13. doi: 10.14348/molcells.2015.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Schioth HB. Tonic inhibition of food intake during inactive phase is reversed by the injection of the melanocortin receptor antagonist into the paraventricular nucleus of the hypothalamus and central amygdala of the rat. Brain Res. 2000;887:460–464. doi: 10.1016/s0006-8993(00)03034-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron. 2017;93:1464–1479. e1465. doi: 10.1016/j.neuron.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Nonneman AJ. Prefrontal cortex and the regulation of food intake in the rat. J Comp Physiol Psychol. 1975;88:806–815. doi: 10.1037/h0076397. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laque A, Yu S, Qualls-Creekmore E, Gettys S, Schwartzenburg C, Bui K, Rhodes C, Berthoud HR, Morrison CD, Richards BK, et al. Leptin modulates nutrient reward via inhibitory galanin action on orexin neurons. Mol Metab. 2015;4:706–717. doi: 10.1016/j.molmet.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav. 1978;8:163–175. doi: 10.1016/0091-3057(78)90333-7. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol. 2005;93:1183–1196. doi: 10.1152/jn.00828.2004. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun. 2016;7:10503. doi: 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, Doya K. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol. 2014;24:2033–2040. doi: 10.1016/j.cub.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Morris G, Nevet A, Arkadir D, Vaadia E, Bergman H. Midbrain dopamine neurons encode decisions for future action. Nat Neurosci. 2006;9:1057–1063. doi: 10.1038/nn1743. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct. 2016;221:535–561. doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, et al. Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun. 2016;7:10268. doi: 10.1038/ncomms10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Olney JJ, Burnham NW, Mazzone CM, Lowery-Gionta EG, Pleil KE, Kash TL, Thiele TE. Lateral Hypothalamus GABAergic Neurons Modulate Consummatory Behaviors Regardless of the Caloric Content or Biological Relevance of the Consumed Stimuli. Neuropsychopharmacology. 2016;41:1505–1512. doi: 10.1038/npp.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160:528–541. doi: 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, Izadmehr EM, Tye KM. Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron. 2016;90:1286–1298. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA. AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Luscher C. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015;88:553–564. doi: 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, Uchida N, Watabe-Uchida M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 2014;8:1105–1118. doi: 10.1016/j.celrep.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Involvement of CRF2 receptor in the brain regions in restraint-induced anorexia. Neuroreport. 2011;22:494–498. doi: 10.1097/WNR.0b013e3283487467. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Tanaka KF, Tsunematsu T, Yamanaka A, Yoshioka M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int J Neuropsychopharmacol. 2014;17:1777–1783. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci. 2016;19:734–741. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–381. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pollak Dorocic I, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlen M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Norgren R. Projections of the parabrachial nucleus in the old world monkey. Exp Neurol. 2000;165:101–117. doi: 10.1006/exnr.2000.7450. [DOI] [PubMed] [Google Scholar]
- Qualls-Creekmore E, Yu S, Francois M, Hoang J, Huesing C, Bruce-Keller A, Burk D, Berthoud HR, Morrison CD, Munzberg H. Galanin-Expressing GABA Neurons in the Lateral Hypothalamus Modulate Food Reward and Noncompulsive Locomotion. J Neurosci. 2017;37:6053–6065. doi: 10.1523/JNEUROSCI.0155-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Robinson S, Rainwater AJ, Hnasko TS, Palmiter RD. Viral restoration of dopamine signaling to the dorsal striatum restores instrumental conditioning to dopamine-deficient mice. Psychopharmacology (Berl) 2007;191:567–578. doi: 10.1007/s00213-006-0579-9. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Burton MJ, Mora F. Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res. 1980;194:339–357. doi: 10.1016/0006-8993(80)91216-0. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH. Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One. 2013;8:e65799. doi: 10.1371/journal.pone.0065799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Duvarci S, Pare D. Synaptic plasticity in the central nucleus of the amygdala. Rev Neurosci. 2005;16:287–302. doi: 10.1515/revneuro.2005.16.4.287. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh MB, Brutus M, Siegel HE, Siegel A. Regulation of Feline Aggression by the Bed Nucleus of Stria Terminalis. Brain Research Bulletin. 1986;16:179–182. doi: 10.1016/0361-9230(86)90031-6. [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Marchant NJ, Whitaker LR, Richie CT, Zhang YJ, Campbell EJ, Koivula PP, Necarsulmer JC, Mejias-Aponte JC, Morales M, et al. Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Current Biology. 2017 doi: 10.1016/j.cub.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73:37–42. doi: 10.1016/0166-4328(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Berridge KC. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000;877:288–297. doi: 10.1016/s0006-8993(00)02691-3. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013;36:504–512. doi: 10.1016/j.tins.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus-->midbrain pathway for feeding behavior. Neuron. 2014;82:797–808. doi: 10.1016/j.neuron.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Van Swieten M, Basiri ML, Blair GA, Kantak P, Stuber GD. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An Emerging Technology Framework for the Neurobiology of Appetite. Cell Metab. 2016;23:234–253. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Takase LF, Nogueira MI. Patterns of fos activation in rat raphe nuclei during feeding behavior. Brain Res. 2008;1200:10–18. doi: 10.1016/j.brainres.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kim ER, Sun H, Xu Y, Mangieri LR, Li DP, Pan HL, Xu Y, Arenkiel BR, Tong Q. GABAergic projections from lateral hypothalamus to paraventricular hypothalamic nucleus promote feeding. J Neurosci. 2015;35:3312–3318. doi: 10.1523/JNEUROSCI.3720-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Roizen J, Lai M, Gandhi N, Kublaoui B. Paraventricular nucleus Sim1 neuron ablation mediated obesity is resistant to high fat diet. PLoS One. 2013;8:e81087. doi: 10.1371/journal.pone.0081087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, van den Pol AN. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci. 2016;19:1341–1347. doi: 10.1038/nn.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, van den Pol AN. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science. 2017;356:853–859. doi: 10.1126/science.aam7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]