Abstract
NAFLD is closely linked with hepatic insulin resistance. Accumulation of hepatic diacylglycerol activates PKC-ε, impairing insulin receptor activation and insulin-stimulated glycogen synthesis. Peripheral insulin resistance indirectly influences hepatic glucose and lipid metabolism by increasing flux of substrates that promote lipogenesis (glucose and fatty acids) and gluconeogenesis (glycerol, and fatty acid derived acetyl-CoA – an allosteric activator of pyruvate carboxylase). Weight loss with diet or bariatric surgery effectively treats NAFLD, but drugs specifically approved for NAFLD are not available. Some new pharmacological strategies act broadly to alter energy balance or influence pathways that contribute to NAFLD (e.g. agonists for PPAR γ, PPAR α/δ, FXR and analogs for FGF-21, and GLP-1). Others, specifically inhibit key enzymes involved in lipid synthesis (e.g. mitochondrial pyruvate carrier, acetyl-CoA carboxylase, stearoyl-CoA desaturase, monoacyl- and diacyl-glycerol transferases). Finally, a novel class of liver-targeted mitochondrial uncoupling agents increase hepatocellular energy expenditure, reversing the metabolic and hepatic complications of NAFLD.
Over the past century, industrialized nations have undergone an epidemiological transition. Better policies and novel therapies prevent or cure many once-fatal infectious diseases (e.g. the global eradication of smallpox and eradication of malaria and polio from many countries). But now, non-communicable diseases threaten modern societies. In many instances, the increasing burden of non-communicable disease is associated with rising rates of obesity. For the first time, life expectancy in the United States has decreased and can be partly attributed to obesity-related “metabolic diseases”, including diabetes, kidney disease, stroke and heart disease (Xu et al., 2016).
The transition from infectious to metabolic disease is apparent in the shifting epidemiology of liver diseases. Vaccines and therapies now prevent or cure many forms of viral hepatitis. However, nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as the most common chronic liver disease (Younossi and Henry, 2016). NAFLD is associated with obesity and encompasses a range of pathologies from steatosis, nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis. NAFLD also increases the risk of hepatocellular carcinoma (HCC). The incidence of HCC is rising, and NAFLD may account for up to 25% of these cases in Westernized countries, though this association may be even higher (Michelotti et al., 2013). And, the prevalence of NASH in patients awaiting liver transplant has risen dramatically over the last decade, surpassing alcoholic liver disease and second only to chronic hepatitis C (Wong et al., 2015). The liver plays key roles in glucose and lipoprotein metabolism. Unsurprisingly, NAFLD is a risk factor for many of metabolic diseases, including type 2 diabetes (T2D) (Lallukka and Yki-Jarvinen, 2016) and cardiovascular disease (Targher et al., 2010). The presence of diabetes mellitus can also increase the risk of liver diseases (Raff et al., 2015). Thus NAFLD is a common preceding condition for both clinically important liver and metabolic diseases.
Insulin regulation of hepatic glucose and lipid metabolism
A healthy liver has remarkable metabolic plasticity. Over the course of a day, hepatocytes readily shift back and forth between the disparate metabolic tasks of energy storage and supply. These transitions are seamless; multiple factors including nutrients, pancreatic and enteric hormones, and neural inputs, precisely manage substrate availability for the entire body. A complete accounting for all the pathways that regulate hepatic lipid and glucose metabolism is beyond the scope of this review and recently reviewed in greater detail elsewhere (Samuel and Shulman, 2016). We also will not discuss mechanisms governing inflammation and fibrosis in the progression of NAFLD to NASH and cirrhosis (reviewed in detail (Fuchs and Schnabl, 2016)). Instead, in this review, we focus on NAFLD as a nexus of metabolic disease and liver disease. We review how insulin normally regulates hepatic glucose and lipid metabolism, how the development of NAFLD impacts hepatic insulin action and how therapies that treat NAFLD may also impact hepatic insulin action, the development of NASH and T2D.
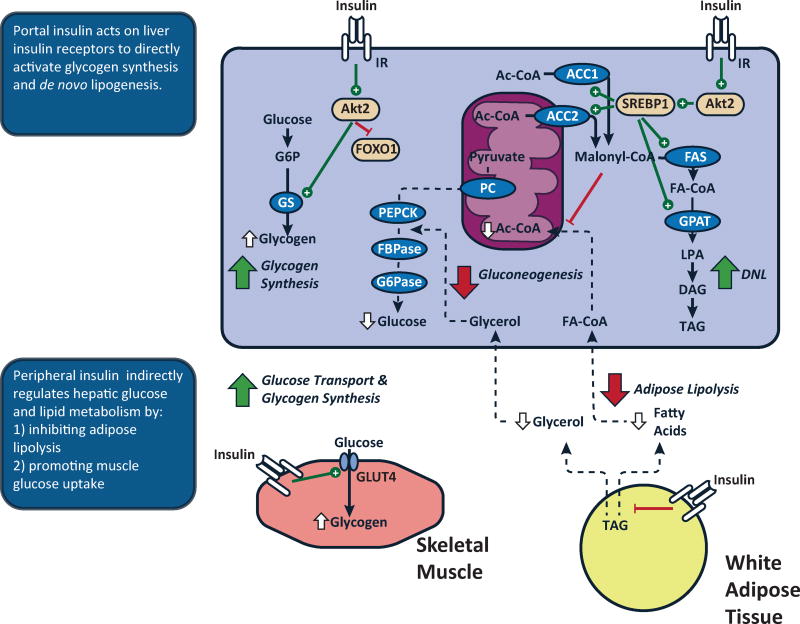
Insulin regulates hepatic glucose and lipid metabolism by both direct and indirect mechanisms. Following a meal, the relatively high concentrations of insulin within the portal vein (~3× peripheral vein) directly activates hepatic insulin receptor tyrosine kinase (IRTK) activity initiating a coordinated relay of intracellular signals (Figure 1) (Samuel and Shulman, 2012). These include 3-phosphoinositide-dependent kinase- 1 (PDK1) and mTORC2 (Guertin and Sabatini, 2007), which converge on Akt phosphorylation (Alessi et al., 1997; Guertin and Sabatini, 2007; Stephens et al., 1998). This model has been used to account for the mechanisms by which direct insulin action suppresses hepatic glucose production via: 1) activation of glycogen synthase (Petersen et al., 1998b) and 2) lowering expression of gluconeogenic enzymes via phosphorylation and nuclear exclusion of FOXO1 (Nakae et al., 2001). But, this transcriptional regulation does fully account for the changes in gluconeogenesis; the changes in protein expression lag the changes in mRNA and hepatic glucose production seen after an acute insulin stimulus.
Figure 1. Insulin action regulates hepatic glucose and lipid metabolism via direct and indirect mechanisms.
The liver is exposed to a high concentration of insulin via the portal vein. Binding of insulin to the insulin receptor tyrosine kinase (IRTK) activates Akt2 and acutely activates glycogen synthase. Direct hepatic insulin action will also decrease transcription of gluconeogenic enzymes via inactivation of FOXO1, followed later by a decrease in the protein expression of these enzymes. Insulin signaling also promotes activation and expression of SREBP1. Peripheral insulin action also indirectly impacts hepatic glucose and lipid metabolism. In skeletal muscle, insulin activates glucose transport and glycogen synthesis, limiting glucose as a substrate for hepatic metabolism. In adipose tissue, insulin acts to promote glucose uptake and inhibit lipolysis. The latter decreases fatty acid and glycerol flux. The decrease in fatty acid flux decreases hepatic mitochondrial acetyl-CoA, an allosteric activator of pyruvate carboxylase. The decrease in glycerol flux will also decrease gluconeogenesis by constraining the influx of this substrate. The decrease in fatty acid flux will also decrease esterification of adipose-derived fatty acids.
More than 50 years ago Levine and Fritz (Levine and Fritz, 1956), and subsequently Prager et al. (Prager et al., 1987), proposed that insulin inhibited hepatic glucose production indirectly, via action at peripheral sites. Subseqent studies (Lewis et al., 1996; Rebrin et al., 1996) postulated that these indirect effects of insulin to suppress hepatic glucose production were mediated by suppression of white adipose tissue (WAT) lipolysis, which Bergman hypothesized reflected transendothelial transport of insulin into the interstitium and subsequent insulin action (Bergman, 1997). More recently Perry et al. provided an alternative molecular mechanism for this indirect effect of insulin to suppress hepatic gluconeogenesis and hepatic glucose production (Perry et al., 2015a). Perry et al. showed that insulin suppression of WAT lipolysis decreases hepatic acetyl-CoA content [an allosteric activator of pyruvate carboxylase (PC)], which in turn reduces pyruvate carboxylase (PC) activity and PC flux (Figure 1) (Perry et al., 2015a). Inhibiting lipolysis also curtails glycerol delivery to the liver, reducing the conversion of glycerol to glucose by a substrate push mechanism (Perry et al., 2014; Previs et al., 1999). As a proof of this concept, atglistatin, an inhibitor of adipose triglyceride lipase, reduced rates of hepatic gluconeogenesis in normal chow-fed rats, insulin-resistant high fat-fed rats and rats treated with an antisense oligonucleotide to knockdown hepatic and adipocyte IRTK(Perry et al., 2015a) by both 1) reducing hepatic acetyl-CoA content with resulting reductions in pyruvate carboxylase acitivity/flux and 2) reducing glycerol delivery to liver and its contribution to gluconeogenesis.. Thus peripheral insulin action regulates substrate flux to the liver which influences hepatic glucose metabolism (Sindelar et al., 1997). However, the high concentration of insulin via the portal vein can still regulate hepatic glucose flux, independently of prevailing fatty acid concentrations, neural action or glucagon (Edgerton et al., 2017). This acute and direct action of insulin may be more focused on Akt2-mediated regulation of hepatic glycogen metabolism (Wan et al., 2013). These direct effects of insulin on hepatic glucose production would be expected predominate in a hepatic glycogen replete state following short-term fasting whereas the indirect effects of insulin (i.e. suppression of WAT lipolysis) would be expected to predominate the regulation of hepatic glucose production under glycogen depleted states such as long term fasting when gluconeogenesis is the major contributor to hepatic glucose production.
Insulin regulation of hepatic lipid metabolism also involves both direct –hepatic– and indirect, –extrahepatic–pathways (Figure 1) (though multiple hormonal, neural and metabolic signals modulate hepatic lipid metabolism). Direct hepatic insulin action activates SREBP1c by increasing mRNA expression and proteolytic cleavage of the SREBP1c precursor protein into a mature, nuclear transcription factor (Brown and Goldstein, 2008). Insulin signaling also increases the activity of mechanistic target of rapamycin complex 1 (mTORC1) which regulates SREBP1c mRNA expression (Li et al., 2010) and processing (Peterson et al., 2011). SREBP1c is also regulated independently of insulin, as demonstrated with induction of SREBP1c after feeding in mice lacking hepatic insulin receptors (Haas et al., 2012). Other factors also influence hepatic de novo lipogenesis (DNL). For example, monosaccharides also recruit other transcription factors, including ChREBP (Erion et al., 2013; Uyeda and Repa, 2006), PPARγ coactivator 1-β (Nagai et al., 2009) and liver X receptor (Bindesbøll et al., 2015) to activate lipogenesis.
Peripheral insulin action indirectly regulates hepatic lipid synthesis. Hepatic fatty acids may be derived from triglyceride in lipid particles (e.g. chylomicron remnants, LDL). The liver also clears fatty acids from the circulation, a major source of hepatic triglyceride (Donnelly et al., 2005). Insulin influences each of these inputs via extra-hepatic actions. For example, insulin activates lipoprotein lipase (LpL) at peripheral sites, promoting the hydrolysis of triglycerides and uptake of fatty acids into adipose and muscle. Insulin also suppresses adipose lipolysis and influences the amount of fatty acyl-CoA available for esterification into hepatic triglyceride. Vatner et al. demonstrated that hepatic triglyceride synthesis could be driven by substrate (fatty acids) availability, independent of changes in hepatic insulin signaling (Vatner et al., 2015). They tested this in awake normal rats, high fat-fed, insulin-resistant rats, and insulin receptor 2’-O-methoxyethyl chimeric antisense oligonucleotide (ASO)-treated rats infused with varying concentrations of lipid and insulin. Rates of fatty acid esterification into hepatic triglyceride were dependent on the rate of fatty acid delivery to the liver and independent of hepatocellular insulin signaling. In contrast, de novo hepatic lipogenesis was insulin-dependent and reduced in rats with defective hepatic insulin signaling.
Development of NAFLD
So, if these processes are so highly regulated and fine-tuned, why is NAFLD now emerging as a global pandemic? There is no one answer. The increase in NAFLD prevalence with modernization implicates lifestyle factors: we consume more calories that we expend. Obese patients with NAFLD consume more calories than healthy lean individuals (Wehmeyer et al., 2016). Some have implicated a specific increase in sugar consumption (Bray and Popkin, 2014). Sugars, especially fructose can activate programs of lipogenesis that further exacerbate NAFLD. Fructose is metabolized almost exclusively by the liver and therefore ingested fructose is funneled into the liver and mostly metabolized to triglycerides by DNL (Herman and Samuel). The development of hepatic insulin resistance, where insulin activation of glycogen synthase is impaired, would also be expected to redirect glucose into lipogenic pathways and further promote NAFLD. Consistent with this hypothesis, mice lacking glycogen synthase had hepatic insulin resistance but increased lipogenesis and NAFLD (Irimia et al., 2017). The presence of skeletal muscle insulin resistance can also promote increased hepatic DNL and hypertriglyceridemia by diverting ingested glucose away from skeletal muscle glycogen synthesis and into the liver for hepatic triglyceride synthesis (Flannery et al., 2012; Petersen et al., 2007). Gut microbiota have also been hypothesized to play a role in numerous disease processes and specific genera (e.g. bacteroides and ruminococcus) have been associated with steatohepatitis and fibrosis, respectively (Boursier et al., 2016).
And, of course, there are genetic factors. The polymorphisms in PNPLA3 are the best studied in this regard. A common single nucleotide polymorphism (rs738409, I148M) in the lipid droplet protein patatin-like phospholipase domain-containing protein 3 (PNLPA3, also called adiponutrin) has been associated with NAFLD and fibrosis (Romeo et al., 2008; Singal et al., 2014). PNPLA3 has dual functions as a triacylglycerol hydrolase and as an acylglycerol transacylase (Kumari et al., 2012) but the mechanism by which this polymorphism – which attenuates the hydrolase activity (Pingitore et al., 2014) – increases liver fat is still unclear. Overexpression of mutant PNPLA3 in mice had mixed effects including a decrease in hepatocyte TAG hydrolysis and an increase in enzymes regulating DNL (Li et al., 2012) [though humans with this isoform do not have increases in DNL (Mancina et al., 2015)]. Genetic deletion of this enzyme failed to induce NAFLD in mice (Basantani et al., 2011; Chen et al., 2010) while a PNPLA3 ASO decreased hepatic fatty acid esterification and protected fat-fed rats from NAFLD, PKCε activation and hepatic insulin resistance (Kumashiro et al., 2013). Thus, while the association between PNPLA3 and NAFLD is clear, the mechanism underlying the develoment of NAFLD requires further study.
Other loci have been linked to NAFLD. Polymorphisms in transmembrane 6 superfamily member 2 (rs58542926) have also been associated with NAFLD (Mahdessian et al., 2014). The protein may regulate cholesterol metabolism (Fan et al., 2016; Smagris et al., 2016) and the incorporation of polyunsaturated fatty acids into hepatic triglyceride (Luukkonen et al.) but the exact mechanism by which this polymorphism leads to NAFLD remains unclear. A polymorphism (rs641738) that decreases expression of membrane bound O-acyltransferase domain containing 7-transmembrane channel-like 4 (MBOAT7-TMC4) was associated with increased liver lipid content and worsening liver histology (Luukkonen et al., 2016a; Mancina et al., 2016). MBOAT7-TMC4 found on intracellular membranes such as ER, mitochondria and lipid droplets and is thought to function as a lysophospholipid acyltransferase, specifically regulating the incorporation of arachidonic acid into phosphatidylinositol (Gijon et al., 2008). This polymorphism is also associated with fibrosis in alcoholic liver disease (Buch et al., 2015) and chronic hepatitis C (Thabet et al., 2016), suggesting that this pathway could contain therapeutic targets for several liver diseases. Multiple polymorphisms in ApoC3 (Peter et al., 2012; Petersen et al., 2010; Zhang et al., 2016) associated with NAFLD may increase plasma ApoC3 concentrations which in turn inhibits lipoprotein lipase activity, leading to fasting and postprandial hypertriglyceridemia and prediposing lean individuals to NAFLD and insulin resistance. Less common and more subtle genetic causes have also been identified in pathways that regulate glucose metabolism [e.g. rs1260326 in glucokinase regulatory protein (Santoro et al., 2012)]. Polymorphisms that decrease expression of the monocarboxylate transporter SCL16A11 have been linked with type 2 diabetes in Mexicans and Latin Americans (Consortium, 2014) and, in vitro, inhibition of SCL16A11 in hepatocytes promotes accumulation of lipid metabolites, including diacylglycerol (Rusu et al.). Interestingly, carriers develop T2DM at lower BMI (Consortium, 2014) and future studies may help establish whether this gene regulates adipose function and/or hepatic lipid metabolism.
Adipocyte dysfunction also influences the development of ectopic lipid deposition in liver and skeletal muscle and insulin resistance in these organs (Lotta et al., 2017; Shulman, 2000). Severe NAFLD/NASH is a complication of congenital lipodystrophies (Safar Zadeh et al., 2013), where the absence of adipose tissue forces the liver to store excess fatty acids, leading to severe insulin resistance (Kim et al., 2000; Petersen et al., 2002). Recent genetic studies further support the role of adipose dysfunction in the pathogenesis of metabolic diseases. Loci linked to insulin resistance and dyslipidemia were also associated with lower adipose tissue mass, suggesting impaired adipose tissue expansion (Lotta et al., 2017). Some loci are in genes that alter Lpl activity while other loci are in genes encoding for components of the insulin signaling pathway. Specific changes in adipose tissue gene expression have also been associated with the development of NASH (du Plessis et al., 2015).
Increases in visceral adipose tissue (VAT) have been associated with dyslipidemia (Despres et al., 1990), insulin resistance (Couillard et al., 1999) and NAFLD (Kelley et al., 2003). The biology of adipose tissue expansion is complex and merits a focused discussion (as in this recent review (Cuthbertson et al., 2017)). A predominant view has been that VAT (or more specifically, mesenteric and omental fat) is itself causally related to the pathogenesis of the metabolic disturbances associated with obesity. Lipolytic rates in visceral adipose tissue increase in obesity (Nielsen et al., 2004; Rebuffe-Scrive et al., 1990) and may be driven by IL-6 (Wueest et al., 2016). The increase in fatty acid release into the portal blood supply has been hypothesized to promote hepatic steatosis, insulin resistance and dyslipidemia. However, many now consider VAT as another ectopic site for lipids when subcutaneous adipose tissue capacity is exceeded (Cuthbertson et al., 2017; Jensen, 2008; Smith, 2015). This threshold varies in individuals and influenced by numerous factors, such age, gender, genetics, fitness, and dietary patterns (Cuthbertson et al., 2017). From this vantage, VAT expansion is not a primary cause for metabolic dysregulation, but a sequala of subcutaneous adipocyte biology. This explains the close association between VAT mass and other disorders associated with ectopic lipid accumulation (insulin resistance and NAFLD).. Thus, increases in VAT may not be a true risk factor for NAFLD but instead may serve as a more visible marker of NAFLD and metabolic disease.
This changing view of VAT reflects a growing understanding of the heterogeneity of obesity itself. We consider several points to better place the specific role of VAT and more general role adipocyte function in the context of insulin resistance and NAFLD. Though rates of VAT lipolysis are increased in obesity, subcutaneous adipose tissue —especially from the upper body—, quantitively accounts for the majority of fatty acids delivered to the liver (Jensen, 2008; Nielsen et al., 2004). And, intrahepatic lipid content, not VAT mass better relates to measures of hepatic insulin sensitivity (Fabbrini et al., 2009), even in patients with marked obesity (Magkos et al., 2010). Removal of visceral adipose tissue alone (Fabbrini et al., 2010b) or in conjunction with bariatric surgery does not significantly impact key metabolic parameters (Andersson et al., 2017; Fabbrini et al., 2010b). Though, Asians have a more VAT at any given BMI than other races (Kadowaki et al., 2006; Nazare et al., 2012), the association between VAT and hepatic lipid content was similar across races(Nazare et al., 2012). The tendency for higher VAT mass in Asian populations may have genetic causes. Discrete loci may influence obesity (Wen et al., 2016) and diabetes (Imamura et al., 2016) in Asian populations and further studies could establish if these loci influence subcutaneous adipose tissue function. Aging also impairs subcutaneous adipose tissue function, possibly due to the accumulation of senescent adipocytes and impaired pre-adipocyte development, with increases in ectopic lipid accumulation in VAT, liver and skeletal muscle (Palmer and Kirkland, 2016). In a group of weight stable patients followed over 10 years, an increase in VAT mass (reflecting ectopic lipid accumulation) was associated with the development of a metabolically unhealthy phenotype (Hwang et al., 2015). Impaired expansion of subcutaneous adipose tissue may also account non-obese subjects with NAFLD. Globally, the rates of NAFLD in non-obese patients average between 10–30% in both Western and Eastern countries (Kim and Kim, 2017). There are modest gender and racial/ethnic differences in the prevalence of non-obese NAFLD: it is slightly higher in whites and Hispanics than in blacks and higher in white males than white females (Browning et al., 2004). Non-obese subjects with NAFLD are at markedly higher cardiovascular risk (Yoshitaka et al., 2017). In a Japanese cohort, the adjusted hazard ratio for cardiovascular disease was ~10-fold high in non-obese patients with NAFLD compared to those without NAFLD (Yoshitaka et al., 2017). Better strategies and tools are required to identify and treat these at-risk individuals.
Lipid-Induced Insulin Resistance
Our understanding of the mechanisms by which NAFLD causes hepatic insulin resistance is informed by the mechanisms for lipid-induced muscle insulin resistance. Several studies observed that ectopic, intramyocellular lipid (IMCL) was closely associated muscle insulin resistance in healthy young, sedentary lean individuals (Krssak et al., 1999) and non-obese, non-diabetic but insulin-resistant adults (Perseghin et al., 1999) and children (Sinha et al., 2002). Experimentally, insulin resistance is acutely induced by lipid infusions is attributed to impaired insulin-stimulated glucose transport into muscle (Dresner et al., 1999; Roden et al., 1996). Similar reductions in glucose transport were observed in insulin-resistant, obese individuals (Petersen et al., 1998a), patients with T2D (Cline et al., 1999), and lean, normoglycemic but insulin-resistant offspring of T2D parents (Perseghin et al., 1996). Numerous studies delineated the mechanistic links between accumulation of a specific lipid metabolite, diacylglycerol (DAG), and impaired insulin-stimulated muscle glucose transport. In rodents, lipid infusion and high-fat feeding increased muscle DAG content (Yu et al., 2002), activated PKCθ and impaired phosphorylation of IRS-1 by IRTK (Figure 2). Lipid infusions in healthy human volunteers similarly increased skeletal muscle DAG, nPKC activation [both PKCδ (Itani et al., 2002) and PKCθ (Szendroedi et al., 2014)] and caused muscle insulin resistance. These studies support a mechanistic model in which muscle DAG accumulation activates PKCθ, which impairs insulin signaling at a proximal step between IRS-1 tyrosine phosphorylation by IRKT, ultimately limiting the ability of insulin to promote skeletal muscle glucose transport and glycogen synthesis.
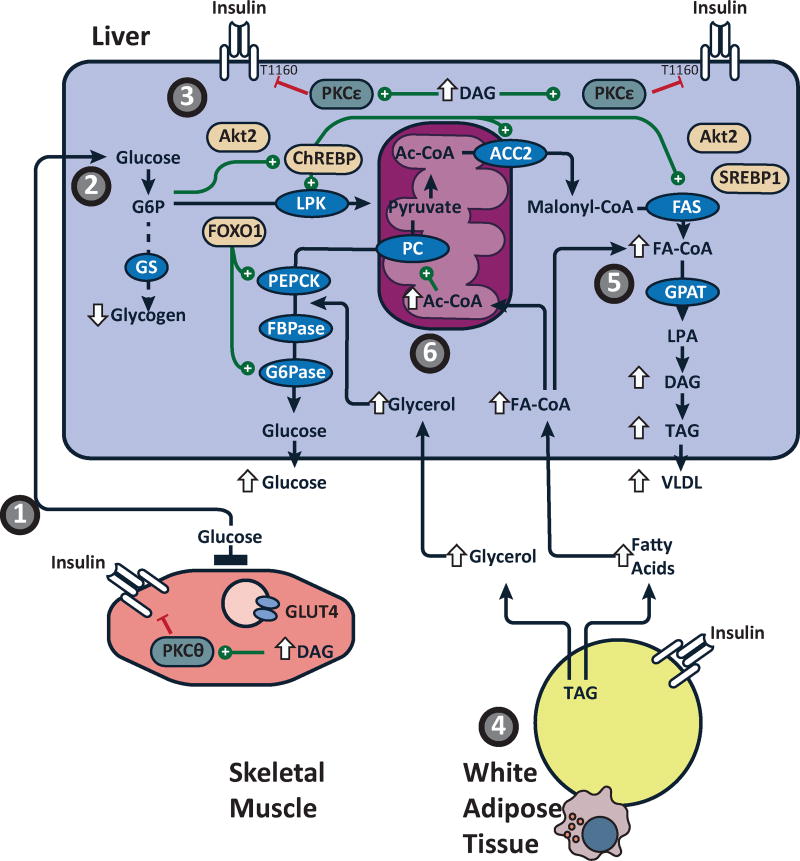
Figure 2. Mechanisms of hepatic insulin resistance in NAFLD.
(1) Skeletal muscle insulin resistance, due to increased intramyocellular ectopic lipid, impairs insulin-stimulated glucose transport activity resulting in reduced muscle glycogen synthesis, redirecting ingested carbohydrate to the liver. (2) The increase delivery of glucose to the liver provides both a substrate for and nutrient activator of (via ChREBP) hepatic de novo lipogenesis. (3) The increases in hepatic DNL and hepatic fatty acid esterification increase hepatic DAG content, activates PKCε, which impairs the direct action of insulin at hepatic IRTK, limiting the ability of insulin to acutely activate glycogen synthesis and, over a longer time period, suppress FOXO1 mediated expression of gluconeogenic enzymes. (4) Adipose insulin resistance from adipose dysfunction and inflammation impairs insulin-mediated suppression of lipolysis increasing glycerol and fatty acid release. These nutrients further impair hepatic glucose metabolism. (5) Fatty acids are esterified into DAG and TAG in an insulin independent manner. (6) Fatty acid oxidation also activates hepatic gluconeogenesis via acetyl-CoA-mediated activation of pyruvate carboxylase (PC), while glycerol delivery to live increases gluconeogenesis via a substrate push mechanism. The net results of these changes are the decrease in insulin-mediated hepatic glycogen synthesis, an increase in hepatic gluconeogenesis and an increase in hepatic lipid synthesis.
Cellular and Molecular Mechanisms of Hepatic Insulin Resistance
Though multiple models have been proposed for the development of hepatic insulin resistance in NAFLD [reviewed in (Samuel and Shulman, 2012)], a substantial body of work supports the role of DAG accumulation and PKCε activation in impairing hepatic insulin action. Briefly, studies in patients with severe generalized lipodystrophy demonstrated that ectopic lipid accumulation in liver and skeletal muscle was associated with severe hepatic and muscle insulin resistance despite the absence of adipose tissue (Petersen et al., 2002). Leptin therapy reduced calorie intake, resolved hepatic steatosis, and improved hepatic insulin action (Petersen et al., 2002). Lipodystrophic mice have a similar phenotype and fat transplantation rescued these mice by allowing a redistribution of lipids from ectopic sites to transplanted adipose tissue and normalization of insulin action (Kim et al., 2000). Similarly, mice overexpressing LpL in the liver develop hepatic steatosis and liver-specific insulin resistance (Kim et al., 2001). In rats and mice, short-term high fat diets lead to hepatic steatosis and hepatic insulin resistance without muscle lipid accumulation or peripheral insulin resistance. These studies demonstrate that ectopic lipid in the liver is specifically associated with hepatic insulin resistance and disassociate hepatic insulin resistance from obesity and visceral adiposity.
In fat-fed rats, the increase in hepatic DAG content activates PKCepsilon (PKCε), the primary PKC isoform in liver and also a novel PKC like PKCθ (Qu et al., 1999; Samuel et al., 2004). DAG mediated PKCε activation is associated with decreased insulin activation of IRTK (Samuel et al., 2007) (Figure 2). Lowering PKCε expression protected rats from lipid-induced hepatic insulin resistance and preserved IRTK activity, without altering hepatic DAG and triglyceride content (Samuel et al., 2007). Similarly, Prkce−/− mice are protected from diet-induced insulin resistance following one week of high fat feeding despite increases in liver lipid content (Raddatz et al., 2011). Recently, Petersen et al. identified Thr1160 (Thr1150 in mice) as a putative PKCε phosphorylation site adjacent to the kinase domain of the insulin receptor (Figure 2) (Petersen et al., 2016). Mice expressing a mutant form (T1150A) of IRTK were protected from high fat feeding-induced hepatic insulin resistance, despite increases in hepatic TAG, DAG content and PKCε activation. Moreover, these mice were protected from high-fat feeding induced reductions in the activation of glucokinase and hepatic glycogen synthesis, reflecting the importance of direct hepatic insulin signaling in regulating hepatic glycogen metabolism (Petersen et al., 2016). Together, these data support a model for hepatic insulin resistance where the accumulation of hepatic DAG activates PKCε and impairs IRTK activation and demonstrate that PKCε activation is necessary for lipid-induced hepatic insulin resistance.
This paradigm has been translated to humans. Kumashiro et al. studied determinants of insulin resistance in a cohort of patients undergoing bariatric surgery (Kumashiro et al., 2011). Hepatic DAG content and PKCε activation were the strongest predictors of insulin sensitivity. In contrast, there was no association between insulin sensitivity and other factors implicated in the pathogenesis of hepatic insulin resistance (e.g. JNK1 activation, ceramide content, etc.). Magkos et al. also demonstrated that hepatic DAG content (but not hepatic ceramide content) was the best predictor of hepatic insulin resistance in obese humans (Magkos et al., 2012). Luukkonen et al., performed a lipidomic analyses in liver samples of patients undergoing bariatric surgery and reported increases in specific lipid metabolites (species of fatty acids, ceramides and TAGs) including specific diacylglycerol species in association with hepatic insulin resistance (Luukkonen et al., 2016b). And, most recently, Horst et al. have also shown that cytosolic diacylglycerol content and increased PKCε activity related to hepatic insulin sensitivity in obese individuals undergoing bariatric surgery (Horst et al., 2017).
Therapies to target metabolic features of NAFLD and hepatic insulin resistance
Based on this mechanistic view of the pathogenesis of NAFLD and NAFLD-associated hepatic insulin resistance the following section summarizes possible therapies for NAFLD and NAFLD-associated hepatic insulin resistance (Figure 3). NAFLD is a common antecedent for both metabolic and liver disease and our goal is to highlight therapies that can reverse NAFLD to treat both conditions. Some of the therapies also have the potential to limit later metabolic and liver complications, such as cardiovascular disease and liver fibrosis.
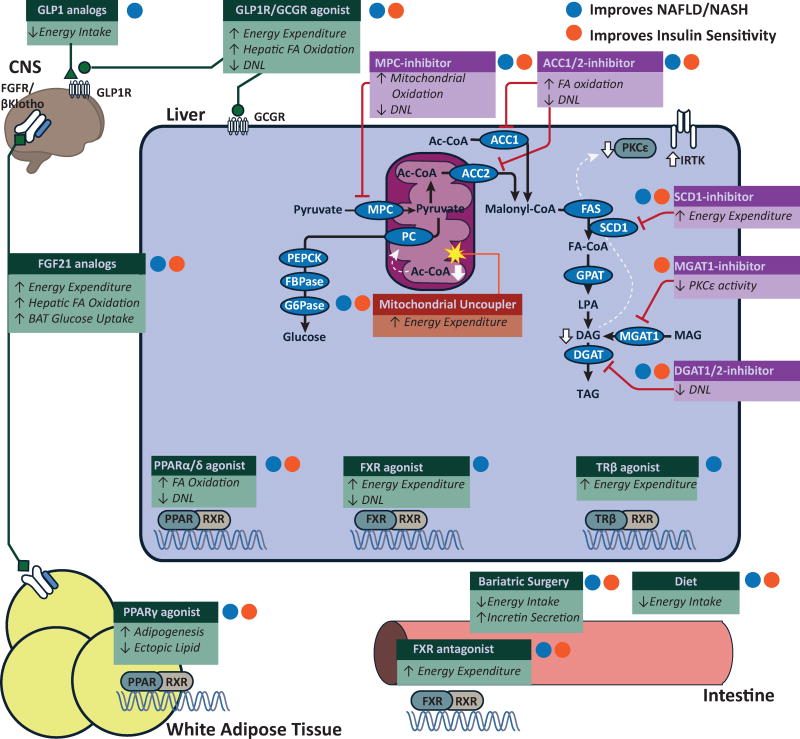
Figure 3. Potential targets to treat NAFLD and hepatic insulin resistance.
Putative targets to treat NAFLD and hepatic insulin resistance. Some therapeutic pathways (green boxes) broadly regulate metabolic pathways. Other therapeutic targets (purple boxes) are specific enzymes targets in lipid synthetic pathways. While many therapeutic targets indirectly increase energy expenditure, mitochondrial uncouplers (red boxes) directly increase cellular energy expditure decreasing cellular DAG and mitochondrial acetyl CoA which increase insulin signaling and decrease gluconeogenesis. White lines show pathways that have been reported decrease cellular DAG content and/or acetyl CoA concentration. Therapies that improve NAFLD/NASH are labeled with a blue dot. Therapies that improve insulin sensitivyt are labeled with an orange dot. Clinical trials have shown the efficacy of weight loss, bariatric surgery, GLP1 analogs (liraglutide) and PPARγ agonists (rosiglitazone, pioglitozatone). Current clinical investigation has explored the potential for PPARα/δ dual agonists (elafibranor), FXR agonists, FGF21 analogs (LY2405319, PF-052313023) and SCD1 inhibitors (Aramachol). Preclinical studies have shown possible efficacy of dual GLP1/GCGR agonists, intestinal FXR antagonists, inhibitors against MPC (MSDC-0602), ACC1/2 (ND-630), MGAT1 (MOGAT1 ASO), DGAT2 and glucagon-T3 hybrids (not depicted). Preclinical studies have also shown efficacy for liver-targeted mitochondrial uncoupling agents (CRMP) and other agents that increase cellular energy expenditure (e.g. salsalate, TTA, and niclosamide).
Clinically established therapies
Weight loss: Diet
Most patients with NAFLD, hepatic insulin resistance and T2D will benefit from weight loss. Weight loss addresses the root causes of insulin resistance, namely ectopic lipid accumulation. Petersen et al. first reported that a moderate caloric restriction (~1200 kcal/day) decreased body weight by ~10% but was sufficient to reduce hepatic lipid content, normalize hepatic insulin responsiveness and “reverse” T2D (Lim et al., 2011; Petersen et al., 2005). These results have been replicated by other hypocaloric feeding studies (Lazo et al., 2010; Petersen et al., 2005). Very low calorie diets (e.g. Optifast or LighterLife) can also quickly improve NAFLD in the span of several weeks (Baldry et al., 2017; Lewis et al., 2006), but are not sustainable indefinitely. Specific dietary programs (e.g. Mediterranean diet (Sofi and Casini, 2014)) have also been reported to be beneficial. Overall, the degree of weight loss achieved is probably the best indicator for the resolution of NAFLD/NASH(Vilar-Gomez et al., 2015). Attempts to enhance weight loss with orlistat did not specifically improve liver histology in human subjects (Harrison et al., 2009).
Aerobic exercise can have profound impacts on insulin sensitivity, but its role in treating NAFLD remains unclear. Skeletal muscle glucose transport is activated by insulin-independent mechanisms in response to exercise and aerobic conditioning. That is, myocellular mechanisms (e.g. AMPK) activated by exercise bypass the block (IRKT -> IRS-1 tyrosine phosphorylation) in insulin signaling to enhance muscle glucose uptake and potentially re-establish the normal disposal of dietary glucose into muscle glycogen. In insulin-resistant subjects, a single session of aerobic exercise augmented muscle glucose transport activity and insulin-stimulated muscle glycogen synthesis (Perseghin et al., 1996). A single bout of elliptical training increased postprandial muscle glycogen synthesis and reduced hepatic DNL without altering plasma insulin concentrations in healthy young, lean individuals with muscle insulin resistance, emphasizing the importance of altered substrate flux in driving hepatic DNL and NAFLD (Rabol et al., 2011). Even short (6 minutes) bursts of exercise before meals can significantly improve the diurnal glucose excursion (Francois et al., 2014). However, data supporting exercise to treat NAFLD is limited. Hyperphagic Otsuka Long–Evans Tokushima that are prone to developing NASH on a Western Diet only show modest improvements in inflammation and fibrosis with 12-weeks of exercise training (Linden et al., 2016). Inactive obese patients who were randomized to several aerobic exercise programs also had modest reductions in liver fat, independent of weight changes (Keating et al., 2015). Aerobic exercise improves muscle insulin sensitivity and decreases DNL, but we need more data to judge the efficacy of exercise prescriptions for NAFLD.
Weight loss: Bariatric Surgery
Bariatric surgery has emerged as the most effective treatment for obesity and related conditions, including T2D and NAFLD. Klein et al. examined liver histology in obese subjects before and 1 year after Roux-en-Y gastric bypass (RYGB) surgery. Patients had a marked loss of body weight (~30%) and metabolic improvements (~30% decrease in EGP, 40% decrease in VLDL secretion and ~50% decrease in palmitate turnover). Histological liver steatosis and expression of some genes associated with inflammation improved. However, actual histological improvements in inflammation and fibrosis were not evident (Klein et al., 2006). Mattar et al. performed a similar study in subjects undergoing (RYGB). With a greater interval between assessments, there did observe demonstrable improvement in inflammation and fibrosis. Sleeve gastrectomy has recently surpassed RYGB as the most common bariatric procedure performed in the United States (Khorgami et al., 2017) and can resolve steatosis, inflammation and fibrosis (Billeter et al., 2016; Froylich et al., 2016).
Longer term studies have demonstrated that the metabolic effects of bariatric surgery persist for an extended period. A 5-year follow up of obese patients randomly assigned to medical therapy or bariatric surgery showed that bariatric surgery improved diabetes control over the five-year period (Schauer et al., 2017). But, these results are not universal. A retrospective analysis of a larger cohort of patients undergoing sleeve gastrectomy found weight regain at 3 and 5 years after surgery and relapse of diabetes in a significant number of patients [e.g. ~50% in complete remission at 1 year vs. 20% at 5 years (Golomb et al., 2015)]. To summarize, bariatric surgery offers the greatest weight loss and best chances for resolution of T2D. Bariatric surgery is not available or appropriate for all patients with NAFLD and in some patients, may not be result in resolution NASH.
Incretin-based therapies
Enthusiasm for incretin-based therapies has been high since they were first approved in 2005. The available analogs all promote modest weight loss, but only liraglutide has been shown to be effective in treating NASH (Armstrong et al., 2013). The recent, small (~50 total subjects) Liraglutide Safety and Efficacy in Patients with Non-alcoholic Steatohepatitis (LEAN) study demonstrated that patients with NASH that were blindly randomized to 48 weeks of liraglutide were more likely to have resolution of NASH. As with many clinical trials for treating NASH, demonstrating histological remission was harder to establish. Many patients had improvement in steatosis and ballooning, but not inflammation or fibrosis. Patients treated with liraglutide were less likely to have progression of fibrosis however. Validation in larger trials is necessary.
The mechanisms by which incretins improve NAFLD and NASH are not entirely clear. Much of the beneficial effects can be attributed to the decrease in appetite which is probably regulated by CNS GLP1 action. The central action of GLP1 may also have other effects: promoting increased energy expenditure, activating brown adipose tissue activation and decreasing white adipose lipid synthesis (Lockie et al., 2012; Sisley et al., 2014). However, in the LEAN trial, among the ~25 patients treated with liraglutide, the reductions in weight and plasma glucose concentrations were similar among those with improvement in liver histology (“responders”) versus those that did not (“non-responders”), suggesting that part of the beneficial effects of GLP1 analogs may be weight independent (Armstrong et al., 2013). GLP1 analogs may also act to decrease hepatic glucose production (Seghieri et al., 2013) and possibly decrease hepatic de novo lipogenesis (Armstrong et al., 2016). Though some have proposed that GLP-1 has direct effects on hepatocytes, GLP-1 receptors have not been conclusively identified on hepatocytes and possible modes of GLP-1 action on the liver involve modulation of islet hormones and/or neural regulation of hepatocyte metabolism (Jin and Weng, 2016).
Future development of incretin-based therapies may involve dual GLP1/glucagon agonists. Initial interest in this class of molecules was fueled by the finding that oxyntomodulin, another product of preproglucagon, activated both GLP1R and glucagon receptors (GCGR) and resulted in marked weight loss. While activation of hepatic glucagon receptors would promote increases in blood sugar, hepatic glucagon action also promotes hepatic lipid oxidation and decreases DNL thereby balancing the hyperglycemic effects. In fat-fed rodents, a long-acting dual GLP1R/GCGR agonist resulted in greater weight loss than a pure GLP1R agonist, with improvements in hepatic steatosis and changes in gene expression consistent with increased hepatic lipid oxidation (Pocai et al., 2009). G49, a pegylated oxyntomodulin analog, had similar beneficial effects in mice on a methionine-choline deficient diet (MCD, a non-obese dietary model of NASH in rodents) (Valdecantos et al., 2017). MEDI0382, another peptidase-resistant oxyntomodulin derivative (Henderson et al., 2016), decreases body weight in DIO mice, with both a decrease in food intake and increase in energy expenditure. MEDI0382 also improves several lipid metrics and decreases hepatic steatosis (Henderson et al., 2016). Despite the GCGR agonism, MEDI0382 improves glucose control. MEDI0382 also reduced weight in macaques without altering plasma glucose or insulin concentrations (Henderson et al., 2016). Recently, a triple agonist peptide that targets GLP1R, GCGR and glucose-dependent insulinotropic polypeptide receptors (GIP-R) demonstrated efficacy in reducing food intake, fat mass, plasma glucose and insulin and liver fat in high-fat fed male and female mice (Jall et al., 2017). Though small clinical studies have suggested that oxyntomodulin may be effective for weight loss (Wynne et al., 2005), clinical studies of dual GLP1R/GCGR agonists or triple GLP1R/GIPR/GCGR have not been reported as of this writing.
Peroxisome proliferator-activated receptor agonists (PPAR’s)
PPARγ is critical for adipocyte differentiation and function and a key regulator of lipid metabolism. Thiazolidinediones (TZD’s) activate PPARγ and remain clinically useful in treating T2D (Sharma and Staels, 2007). TZD’s enhance peripheral insulin sensitivity (Inzucchi et al., 1998; Maggs et al., 1998) primarily by improving adipocyte function (Mayerson et al., 2002). TZD’s decrease adipocyte inflammation and increase secretion of adiponectin, enhance clearance of VLDL triglyceride (Nagashima et al., 2005), improve insulin-mediated suppression of lipolysis (Mayerson et al., 2002) and lower rates of post-prandial fatty acid turnover (Miles et al., 2003). TZD’s decrease ectopic lipid accumulation (Mayerson et al., 2002), decrease visceral adipose tissue mass (Miyazaki et al., 2002; Mori et al., 1999) and improve hepatic and muscle insulin resistance (Mayerson et al., 2002; Petersen et al., 2000; Tonelli et al., 2004).
Pioglitazone reversed NASH in a small study of patients with impaired glucose tolerance and T2D (Belfort et al., 2006). These studies were recently confirmed in a larger study of patients with pre-diabetes or T2D and biopsy proven NASH (Cusi et al., 2016). The hepatic benefits of pioglitazone may be most evident in patient with diabetes. A larger trial assessing the efficacy of pioglitazone in nondiabetic patients with NASH failed to achieve the pre-specified level of significance. Nonetheless, pioglitazone did improve several discrete metrics of hepatic inflammation (Sanyal et al., 2010). The benefits of pioglitazone (including reductions in cardiovascular risk), need to be weighed against the known side effects: weight gain, fluid retention and osteoporotic fracture (Viscoli et al., 2017).
Efforts to leverage other PPAR’s in treating metabolic diseases have had mixed results. While PPAR-α agonists do not correct NAFLD or NASH(Fabbrini et al., 2010a; Fernandez-Miranda et al., 2008), novel agonists that target both PPAR-γ and -α appeared effective in treating diabetes and hyperlipidemia but development of these drugs was halted due to adverse events (Wright et al., 2014). Recent efforts have focused on developing dual agonists against PPAR-α and -δ. The PPAR-α effects may promote fatty acid oxidation (Pawlak et al., 2015), while the PPAR-δ effects possibly decrease DNL and suppress inflammation (Bojic and Huff, 2013; Staels et al., 2013). One such agent, elafibranor (GFT505) improved diet-induced NASH in rodents (Staels et al., 2013). Human studies demonstrated some improvement in hepatic and peripheral insulin sensitivity (Cariou et al., 2013). Longer term studies have shown modest improvements in NASH, as well as modest improvements in insulin resistance and dyslipidemia (Ratziu et al., 2016). Notably, elafibranor did not cause weight gain, though there was an increase in serum creatinine. Further studies of these agents are needed.
Novel and Emerging Targets
Mitochondrial Pyruvate Carriers
Mitochondrial pyruvate transport is regulated by two key related proteins, mitochondrial pyruvate carrier 1 and 2 which form a transport complex on the inner mitochondrial membrane(Bricker et al., 2012; Herzig et al., 2012). These proteins were discovered to be inhibited by TZD compounds and putatively mediate some of the insulin sensitizing effects of these drugs; decreased mitochondrial pyruvate oxidation may promote AMPK-mediated muscle glucose uptake (Divakaruni et al., 2013). Liver-specific deletion of either MPC1 (Gray et al., 2015) or MPC2 (McCommis et al., 2015) did not alter glucose production on a C57BL/6 background where the block in MPC was bypassed with flux into the mitochondria as alanine and mitochondrial alanine transaminase. In contrast, liver-specific MPC2 deletion does lower fasting plasma glucose concentrations in diabetic models (db/db mice or streptozotocin-treated diabetic mice) supporting the development of this system as a drug target (McCommis et al., 2015).
Novel PPARγ-sparing TZD compounds have been developed that inhibit MPCs. One of these compounds, MSDC-0602 improved insulin sensitivity in 60% fat-fed mice with improvements in hepatic, adipose and muscle insulin signaling and action (Chen et al., 2012). These improvements occurred without changes in body weight or liver triglyceride content. In a separate set of studies MSDC-0602 was tested in mice fed a 40% high-fat diet supplemented with 20% fructose and 2% cholesterol, a diet that can lead to hepatic inflammation (McCommis et al., 2017). Though MSDC-0602 did not affect liver triglyceride content it did protect against some histological changes associated with NASH (ballooning and fibrosis). MSDC-0602 may impact stellate cell activation, suggesting that mitochondrial pyruvate metabolism has signaling roles. The development of MPC’s as a therapeutic target will need to carefully consider other potential functions. For example, MPC is important for glucose-stimulated insulin secretion. And, loss of MPC may be associated with oncogenesis, chemo-resistance and poor survival in patients with some solid tumors (Schell et al., 2014; Zhong et al., 2015).
Acetyl-CoA carboxylase inhibitors
Acetyl-CoA carboxylase catalyzes carboxylation of acetyl-CoA into malonyl CoA (Figure 2). ACC1, a predominantly cytoplasmic isoform highly expressed in liver and adipose, catalyzes this committed step in DNL (Savage et al., 2006). In contrast, malonyl-CoA produced by ACC2, a slightly larger (280 vs. 265 kDa), mitochondrial isoform highly expressed in skeletal and cardiac muscle, inhibits CPT1 and decreases mitochondrial lipid oxidation (Savage et al., 2006). The regulation of ACC activity is complex, involving phosphorylation (e.g. AMPK and PKA inhibit ACC), allosteric regulation (citrate promotes while palmitate inhibits ACC activity) (Hunkeler et al., 2016) and protein-protein interaction (MIG-12 mediated polymerization of ACC enhances activity) (Kim et al., 2010). However, coordinated activation of ACC promotes lipid storage by both stimulating lipogenesis and inhibiting lipid oxidation.
Thus, ACC is an intriguing therapeutic target. ACC2 knockout mice were reported as being protected from weight gain on a high-fat diet with decreases in liver triglyceride associate with improvements in hepatic insulin signaling and action (Abu-Elheiga et al., 2012). However, this phenotype was not replicated in other ACC2 deficient strains (Hoehn et al., 2012; Olson et al., 2010), possibly due to difference in background strain or other unrecognized factors (Hoehn et al., 2012). Liver-specific ACC1 knock out mice have decreased liver triglyceride but without any changes in glucose metabolism (Mao et al., 2006). In contrast, liver-specific ACC1/2 double knockout mice were prone to steatosis, a surprising phenotype attributed to a shift in fuel utilization with a decrease in lipid oxidation and increase in glycolysis (Chow et al., 2014). In rats, synthetic antisense oligonucleotides (ASO) targeting both ACC1 and ACC2 were effective in reversing high-fat diet induced hepatic insulin resistance, with evidence for both decreased hepatic lipogenesis and increased hepatic lipid oxidation(Savage et al., 2006). Consistent with the DAG-PKCε hypothesis of hepatic insulin resistance these changes were associated with decreases in liver diacylglycerol content, PKCε activation and improved hepatic insulin signaling.
Several novel small molecule inhibitors of ACC have been designed. (Harwood et al., 2003; Tong and Harwood, 2006). Chemical inhibitors of ACC are designed to: 1) compete with acetyl-CoA, 2) inhibit the carboxy-transferase reaction or 3) inhibit the biotin-carboxylation activity which also may inhibit polymer formation. ND-630 acts by this third mechanism and is a potent inhibitor of ACC1 and 2 (Harriman et al., 2016). This compound was shown to be effective in acutely inhibiting ACC activity in liver and muscle, increasing hepatic lipid oxidation. With chronic treatment, ND-630 was safe and effective in decreasing hepatic and plasma lipid concentration and improving glucose metabolism in high-sucrose and high-sucrose fed Sprague-Dawley rats as well as diabetic ZDF rats (Harriman et al., 2016). But, these results are not generalizable to ACC inhibitors as some compounds appear to worsen glucose intolerance (Bourbeau et al., 2013). Thus, the mechanism of inhibition is important to the therapeutic efficacy ACC inhibitors. Additionally, ACC inhibition could lead to accumulation of mitochondrial acetyl-CoA by decreasing DNL and increasing lipid oxidation. Increased acetyl-CoA, could allosterically activate pyruvate carboxylase and promote hepatic gluconeogenesis (Perry et al., 2015a), thereby countering the improvements in hepatic insulin sensitivity.
SCD1
Stearoyl coenzyme A desaturase 1 is responsible for converting stearoyl-CoA (18:0 fatty acyl-CoA) to oleoyl CoA (18:1 fatty acyl-CoA). SCD1 knockout mouse were protected from diet-induced obesity and glucose intolerance (Ntambi et al., 2002). However, this phenotype was attributed to altered skin integrity and increased energy expenditure (Sampath et al., 2009). Liver-specific SCD1 knockout mice still developed NAFLD on a high fat diet, but did have decreased lipogenesis on a high-carbohydrate diet and were protected from the development of NAFLD, possibly due to increased oxidation of carbohydrates to support thermogenesis (Miyazaki et al., 2007). SCD1 ASO therapy protected mice against NAFLD on a high-fat diet with several changes associated with increased energy expenditure (Jiang et al., 2005). This was associated with improvements in hepatic insulin sensitivity (Gutierrez-Juarez et al., 2006). SCD1 may be a promising target for NAFLD and mechanistically may lead to increased energy expenditure.
Several SCD1 inhibitors have been developed, though clinical data is sparse. Aramachol, a fatty acid-bile acid conjugate, is an SCD1 inhibitor that has been tested in a phase 2 trial (Safadi et al., 2014). While this drug was effective in decreasing hepatic lipid content, there was no alteration in body weight, glucose metabolism or insulin sensitivity (no changes in fasting plasma glucose or insulin concentrations). SCD1 inhibition may still a role as a potential cancer therapy though concerns over some pro-inflammatory changes will require a cautious approach to development of this class (Brown and Rudel, 2010). Moreover, the associations between SCD1 and energy metabolism merit further investigation to determine if these effects will translate to humans.
MGAT
Monoacylglycerol acyltransferase (MGAT) enzymes comprise a family of three genes that encode enzymes that catalyze the acylation of both monoacylglycerol and diacylglycerol to form DAG and TAG, respectively. This pathway complements diacylglycerol synthesis via the glycerol phosphate pathway and is thought to be very active in enterocytes but upregulated in NAFLD in rodents and humans (Hall et al., 2012). MGAT1 and 2 are active in rodents. MGAT3 is active in humans and also has diacylglycerol transferase activity when 2-monoacyglycerol concentrations are low (Shi and Cheng, 2009). High-fat feeding increases hepatic MGAT1 expression in rodents and MGAT3 expression is associated with NAFLD in obese humans. MGAT1 ASO decreased MGAT1 expression though with minimal decreases in overall MGAT activity (Hall et al., 2014). MGAT1 ASO decreased liver triglyceride content in mice fed a 40% high-fat/fructose and cholesterol diet but not in mice fed a 60% high-fat diet (Hall et al., 2014; Soufi et al., 2014). Surprisingly, diacylglycerol content was higher with MGAT1 ASO in both diet groups. Nonetheless, MGAT1 ASO normalized glucose tolerance, decreased in PKCε activation and improved hepatic insulin signaling (Hall et al., 2014). MGAT1 ASO was not effective in preventing inflammatory changes in mice fed the high-fat/fructose and cholesterol diet (Soufi et al., 2014). Chemical inhibitors of MGAT2 have been shown to prevent diet-induced obesity and hepatic steatosis, though this is likely linked to decreased food intake (Okuma et al., 2015a; Okuma et al., 2015b). Interestingly, the decrease in food intake was only evident in high-fat fed rodents and associated with increased incretin hormones, suggesting an interaction between enteric MGAT2 and feeding behavior. It is unknown whether targeting MGAT3 will be effective in treating humans. MGAT3 specific inhibitors have been designed and preliminary studies in transgenic mice expressing MGAT3 have demonstrated efficacy in vivo, though further phenotypic characterization has not been reported (Huard et al., 2015).
DGAT2
Acyl-CoA:diacylglycerol acyltransferase (DGAT), the enzyme that acylates diacylglycerols into triglycerides, is not an obvious therapeutic target but may emerge as a potentially useful one. There are two isoforms, DGAT1 and 2, encoded by discrete genes. The central action of GLP1 may also have other effects: promoting increased energy expenditure, activating brown adipose tissue activation and decreasing white adipose lipid synthesis. DGAT1 is also highly expressed in the small intestine. DGAT1 knockout mice are protected from diet-induced obesity and insulin resistance. This phenotype is associated with alterations in energy balance, which are mostly attributed to reduced enteric fat absorption. DGAT2 deficiency is embryonic lethal. However, decreasing hepatic and adipose DGAT2 expression with specific antisense oligonucleotides (ASO’s) lowers diacylglycerol content (and PKCε activity) by inhibiting lipogenesis, and reverses diet-induced hepatic insulin resistance (Choi et al., 2007). Conversely, transgenic overexpression of hepatic DGAT2 increases hepatic TAG and DAG content ~2.5–3 fold and hepatic ceramide content by ~10–15% and is associated with hepatic insulin resistance (Jornayvaz et al., 2011). Thus, DGAT2 exerts a paradoxical effect on hepatic DAG content; inhibition decreases DAG and PKCε activity while overexpression increases DAG content and PKCε activity. It is possible that transient increases in early lipid intermediates following DGAT inhibition (e.g. fatty acyl-CoA’s) may suppress the lipogenic pathway (Choi et al., 2007).
DGAT1 inhibitors are currently being investigated as potential therapies. In rodents, DGAT inhibition appears to primarily impact intestinal lipid absorption with possibly associated increases in intestinal fatty acid oxidation and GLP-1 secretion (Liu et al., 2015; Maciejewski et al., 2013; Schober et al., 2013). Preclinical studies suggest that DGAT inhibitors prevent weight gain, hepatic steatosis and insulin resistance (Cao et al., 2011; Tomimoto et al., 2015). Clinical studies show that DGAT1 inhibition may be an effective treatment in familial chylomicronemia syndrome. Though many subjects experience some gastrointestinal side effects, these were mild and tolerable (Meyers et al., 2015). DGAT2 is not thought to impact intestinal lipid handling, and thus DGAT2 inhibitors may be better tolerated in many patients with NAFLD. Development and clinical trials of specific DGAT2 inhibitors are currently underway (Futatsugi et al., 2015; Imbriglio et al., 2015). Interestingly, both MGAT and DGAT activity may also have secondary effects on energy expenditure and thus some of the beneficial effects of inhibitors of these enzyme pathways may involve altering energy balance (Shi and Cheng, 2009).
Farnesoid X receptor (FXR) Agonists
FXR is a receptor for bile acids, canonically suppressing expression of Cyp7A1, a rate-limiting step in bile acid synthesis. However, FXR also regulates the expression of numerous metabolic pathways, including gluconeogenesis and lipogenesis (Kliewer and Mangelsdorf, 2015). FXR has been developed as a novel drug target. In part, these beneficial effects of FXR agonists may be due to changes in energy balance. For example, the ability of specific bile acids to prevent obesity and glucose intolerance in fat fed mice is dependent on FXR-mediated activation of thermogenesis (Watanabe et al., 2006). A synthetic FXR agonist was able prevent hepatic inflammation and fibrosis in mice fed a MCD diet (Zhang et al., 2009). FXR agonists may also decrease hepatic DAG by activating diacylglycerol kinases, which reduce cellular diacylglycerol pools (Cai and Sewer, 2013). In a proof of concept study, six weeks of obeticholic acid, an FXR agonist, was reported to promote mild weight loss, improve insulin sensitivity and lessen markers of hepatic fibrosis in human subjects (Mudaliar et al., 2013). Longer studies have shown that obeticholic promotes mild weight loss and improved liver histology in patients with NAFLD (Neuschwander-Tetri et al.). Despite the improvement in hepatic steatosis, there was an increase in fasting serum insulin concentrations raising the possibility of worsening insulin resistance (Neuschwander-Tetri et al.). As a significant number of patients experienced pruritus as a side-effect, newer non-bile acid FXR agonists have been developed, such as BAR502 (Carino et al., 2017). In mice, BAR502 was effective in reversing obesity in fat-fed mice, with improvement in hepatic steatosis, hepatic inflammation and glucose intolerance (Carino et al., 2017).
Some of the effects of FXR agonists may be mediated by FGF19 (FGF15 is the rodent ortholog), a protein secreted by the small intestine in response to bile acids which contributes to the suppression of hepatic Cyp7A1 (Inagaki et al., 2005; Potthoff et al., 2012). But, FGF15/19 has additional effects in the liver, such as potentiating glycogen synthesis (Kir et al., 2011), decreasing expression of gluconeogenic (Potthoff et al., 2011) and lipogenic enzymes (Bhatnagar et al., 2009). Obeticholic acid has been shown to increase serum FGF19 concentrations in humans (Mudaliar et al., 2013). However, the therapeutic use of FGF19 itself has been limited by the potential for ectopically produced FGF19 to induce hepatocellular carcinoma (Nicholes et al., 2002). Recently, a novel FGF19 analog (M70 or NGM282) has been designed to selectively activate key metabolic pathways but not mitogenic pathways (Zhou et al., 2014) and is being developed as treatment for cholestatic and metabolic disorders (Degirolamo et al., 2016). A Phase 2 study of NGM282 in subjects with biopsy confirmed NASH demonstrated that NGM282 could induce a significant reduction in liver fat and inflammation, though with 30–50% increase in plasma LDL concentration (Harrison et al., 2017).
In contrast to the development of FXR agonists, there may also be a role for FXR antagonists in the treatment of metabolic disease. Ileal FXR expression was observed to positively relate to obesity in humans, raising the possibility that intestinal FXR should be inhibited (Jiang et al., 2015). Studies using glycine-β-muricholic acid, a nonhydrolyzable bile acid that antagonizes FXR, demonstrate that it increases energy expenditure in fat-fed and db/db mice, reversing hepatic steatosis and improving glucose and insulin tolerance (Jiang et al., 2015). Ileal FXR inhibition had several effects, including increases in some bile acid species and a decrease in FGF15. However, the investigators hypothesized that ileal FXR regulates synthesis of a specific ceramide species that inhibits browning of adipocytes; C16:0 ceramide “replacement” in glycine-β-muricholic acid treated mice block many of the beneficial effects (Jiang et al., 2015). Thus, inhibition of ileal FXR may increase energy expenditure via reductions of a specific ceramide molecule.
Thyroid hormone mimetics
Another proposed mechanism by which bile acids may impact hepatocellular energy metabolism is via activation of the G-protein coupled receptor TGR5 which in turn increase deiodinase-2 expression and activation of thyroid hormone. Some TGR5 agonists have been reported to have glucose lowering (Agarwal et al., 2016; Briere et al., 2015) and lipid-lowering (Zambad et al., 2013) effects in rodents, but the effects on NAFLD have not been reported. TGR5 activation promotes gallbladder filling and thus agonists against TGR5 have the potential to promote gallstone formation. Attempts have also been made to target thyroid hormone receptor-β (TRβ). TRβ-1 is the predominant form expressed in the liver and activation of TR-β has been hypothesized to have potential metabolic benefits in terms of lipid and glucose lowering. Indeed, KB-2115 has been shown to be very efficacious in treating hyperlipidemia (Ladenson et al., 2010). Moreover, KB-2115 and GC-1, another TR-β specific agonist, both prevented hepatic steatosis in rats fed a high-fat diet (Vatner et al., 2013) and in ob/ob mice (Martagon et al., 2015). However, despite these benefits, neither treatment improved insulin sensitivity due to discrete off-target effects (Vatner et al., 2013). A new approach to leverage hepatic thyroid hormone action uses a novel glucagon-T3 hybrid molecule (Finan et al., 2016). The presence of a peptidase resistant glucagon effectively targets the T3 to the liver where it is presumably released by hydrolysis of a gamma glutamic acid spacer. Glucagon-T3 has marked effects in increasing energy expenditure (without altering food intake), reduction in fat mass, reductions in plasma and hepatic lipid content and improvements in glucose tolerance. This hybrid molecule exerts these effects through multiple pathways, partly through FGF21, activation of brown adipose tissue and directly altering hepatic lipid and glucose metabolism (Finan et al., 2016). Each component of this hybrid appear to mitigate the adverse effects of the partner: by targeting the liver, glucagon-T3 does not cause cardiac of bone toxicity while the T3 component mitigates the glucose raising effects of glucagon (Finan et al., 2016).
FGF21
Therapeutic effects of PPARα agonists and thyroid hormone agonists are partly attributable to induction of fibroblast growth factor 21 (FGF21). FGF21 is a PPARα target that is primarily produced in the liver, WAT and exocrine pancreas (Inagaki et al., 2007). FGF21 coordinates the metabolic shift from fed to fasted states; FGF21 regulates hepatic ketogenesis, gluconeogenesis and adipose lipolysis (Badman et al., 2007; Inagaki et al., 2007). FGF21 action also regulates cellular energy metabolism. Mice lacking hepatic FGF21 are prone to developing glucose intolerance when placed on a high-fat diet (Markan et al., 2014) whereas FGF21 transgenic mice have enhanced glucose tolerance (Zhang et al., 2012). Rodent studies have shown that exogenous FGF21 protects high fat fed mice from insulin resistance by increasing cellular energy expenditure independently of brown adipose tissue activation (Camporez et al., 2013; Xu et al., 2009). This effect appears to be mediated centrally, via hypothalamic receptors and activation of the sympathetic nervous system (Owen et al., 2014). The increases in energy expenditure are associated with a reduction in hepatic diacylglycerol content, decreased PKCε activation and improved insulin signaling (Camporez et al., 2013). FGF21 also suppresses WAT lipolysis, another potentially therapeutic effect (Park et al., 2016).
In humans, serum FGF21 concentration is positively associated with obesity and insulin resistance (Zhang et al., 2008). NAFLD is associated with increased activity of the transcription factor cyclic AMP-responsive element-binding protein H (CREBH) which increases expression of FGF21 (Park et al., 2016). Hepatic FGF21 may even be enhanced by fructose metabolism (Dushay et al., 2015). Increased FGF21 may suppress adipose lipolysis and limit hepatic fat accumulation (Park et al., 2016). Though FGF21 is already increased in obese states, preclinical rodent studies suggest that pharmacological supplementation may be beneficial. Several analogs have been evaluated in clinical trials with mixed results. LY2405319, FGF21 variant, was tested in subjects with T2D. LY2405319 reduced body weight, plasma triglyceride and cholesterol. However, the impact on plasma glucose and insulin were less clear. PF-052313023, a long acting FGF21 analog, also decreased body weight and improved plasma lipid profile in subjects with T2D. However, there were also changes in bone turnover markers, possibly due to PPARγ mediated inhibition of osteoblastogenesis (Wei et al., 2012). A recent phase 2 study demonstrated that a once weekly pegylated FGF21 (BMS-986036) effectively decreases liver fat, serum transaminases and plasma triglyceride and LDL while increasing adiponectin without any significant adverse events, paving the way for longer phase 3 studies (Sanyal et al., 2017).
Liver-Targeted Mitochondrial Uncoupling Agents
Many of the potential agents discussed above improve hepatic insulin resistance and NAFLD partly by altering cellular energy balance. Altered cellular energy balance is a key feature of other agents that have been reported to decrease liver fat and/or improve hepatic insulin sensitivity, including salsalate, [an anti-inflammatory agent (Smith et al., 2016)], 1-triple tetradecylthioacetic acid [(TTA, a novel fatty acid analog (Lindquist et al., 2017)) and glucoraphanin [(a compound present in cruciferous vegetables that promotes browning of WAT, increases energy expenditure and protects rodents from diet-induced insulin resistance (Nagata et al., 2017)). Other novel agents have been developed to primarily alter cellular energy expenditure. Niclosamide is an antihelmintic drug that promotes mitochondrial uncoupling. It is orally bioavailable with a primary distribution to liver and kidney and was efficacious in preventing diet-induced obesity in high-fat fed mice (Tao et al., 2014). This effect was associated with alterations in cellular energy charge, activation of AMPK and reductions in hepatic DAG content.
However, it is possible to create compounds that will specifically, directly and safely increase hepatic mitochondrial energy expenditure. We previously demonstrated the efficacy of low doses of the mitochondrial protonophore, 2,4-dinitrophenol (DNP) prevent steatosis in high-fat fed rats, with decreased PKCε activation, increased hepatic insulin signaling, and hepatic insulin action (Samuel et al., 2004). Though DNP has been used clinically in the past, this compound is not safe for clinical use due to the low therapeutic index. If DNP is modified with a methyl-ester (ME), it remains inactive until activated within hepatocytes. This increases the therapeutic index of DNP-ME 50-fold over DNP. DNP-ME increased hepatic mitochondrial fat oxidation by ~60%, reversed hypertriglyceridemia, hepatic and peripheral insulin resistance and hyperglycemia in rat models of NAFLD and T2D (Perry et al., 2013). DNP-ME did not alter cellular energy charge nor did it lead to activation of AMPK; it’s actions are likely due purely to the induced energy inefficiency of the mitochondria exclusively in liver. Furthermore, correction of liver and muscle insulin resistance was associated with marked reductions in liver and skeletal muscle DAG content with decreased activation of PKCε and PKCθ, respectively. The latter improvements in muscle DAG-PKCθ activity could be attributed to DNP-ME increased in hepatic fat oxidation leading to reduced VLDL production, reduced plasma triglycerides and decrease fat delivery to skeletal muscle. Importantly, these improvements occurred independently of any changes in body weight, inflammatory mediators, FGF-21, adiponectin, or hepatic ceramide content (Perry et al., 2013).
Similar effects were seen with a second liver-targeted mitochondrial uncoupling agent, Controlled Release Mitochondrial Protonophore (CRMP) (Perry et al., 2015b). Combining DNP into a cellulose based matrix extends the time required for absorption resulting in relatively stable plasma DNP concentrations after oral administration. CRMP increases the therapeutic index by 500-fold and, as with DNP, lowers hepatic and muscle lipid (DAG) content and improves insulin hepatic and peripheral sensitivity in numerous rodent models of NAFLD/NASH (Perry et al., 2015b). While CRMP is not “liver-specific” by design, the first-pass hepatic uptake largely confine its actions to the liver. CRMP increased the rate of hepatic tricarboxylic cycle flux without changing whole body energy balance or mitochondrial activity in other tissues. Like DNP-ME, the reductions in plasma triglyceride and improvement in skeletal muscle activity were attributed to CRMP-induced increases in hepatic mitochondrial fatty acid oxidation resulting in decreased VLDL production, hypertriglyceridemia and reduced delivery and uptake of fat by the skeletal muscle. In rats fed a MCD diet, CRMP treatment reduced hepatic inflammation and reversed hepatic fibrosis. CRMP has also recently been shown to reverse hepatic steatosis and hepatic and peripheral insulin resistance in an A-ZIP/F1 mouse model of severe lipodystrophy (Abulizi et al., 2017). CRMP also decreased several markers of liver inflammation and the unfolded protein response in this lipodystrophic mouse. Furthermore, CRMP, like DNP-ME, reversed hepatic insulin resistance and diabetes with reductions in hepatic DAG-PKCε activity and hepatic acetyl-CoA content offering further support for the roles of these metabolites in causing hepatic insulin resistance and increased hepatic gluconeogenesis respectively in T2D. Taken together these studies offer proof-of-concept in support of the development of novel liver-targeted mitochondrial uncoupling agents for the treatment of NAFLD, NASH and T2D.
Conclusion
We face significant challenges in tackling the growing and global epidemic of NAFLD. The widespread prevalence of NAFLD, the attendant risks for metabolic and hepatic complications and the lack of approved therapies are significant hurdles. But, there is a solid and mechanistic foundation with which to tackle this challenge. There is ample data that shows that NAFLD is present before the overt onset of either T2D or NASH. In NAFLD, diacylglycerol mediated activation of PKCε, impairs insulin signaling and increases in hepatic acetyl-CoA content, which can mostly be attributed to rates of mitochondrial β-oxidation, exceeding rates of mitochondrial TCA flux, results in activation of pyruvate carboxylase activity/flux and stimulation of hepatic gluconeogenesis. Subsequent pathogenic changes may also trigger inflammation, hepatocyte damage and fibrosis. Challenges remain in developing better tools to predict who is at risk for NAFLD (especially in non-obese individuals), non-invasively diagnosing NAFLD, and stratifying those patients at risk for progression. Changes in health policies are needed to decrease the incidence of NAFLD and regulatory agencies may need to consider NAFLD per se as a treatment indication. But, as reviewed above, there are several promising targets for therapy. We are well poised to re-purpose or refine existing drugs to treat NAFLD and develop new agents to tip the balance of hepatic lipid metabolism to decrease NAFLD and prevent or reverse the associated metabolic and hepatic complications.
Acknowledgments
Supported by grants from the National Institutes of Health (DK-R01 DK 11398401, DK-40936 and DK-45735) and Veteran’s Administration (I01 BX000901). GIS serves on scientific advisory boards for: Merck, Novo Nordisk, Celgene, Aegerion and AstraZeneca and receives investigator-initiated support from Gilead Sciences.
Footnotes
VTS has no conflicts of interest to disclose.
References
- Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ. Acetyl-CoA carboxylase 2−/− mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. The Journal of biological chemistry. 2012;287:12578–12588. doi: 10.1074/jbc.M111.309559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulizi A, Perry RJ, Camporez JP, Jurczak MJ, Petersen KF, Aspichueta P, Shulman GI. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice. FASEB J. 2017 doi: 10.1096/fj.201700001R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Patil A, Aware U, Deshmukh P, Darji B, Sasane S, Sairam KV, Priyadarsiny P, Giri P, Patel H, et al. Discovery of a Potent and Orally Efficacious TGR5 Receptor Agonist. ACS Med Chem Lett. 2016;7:51–55. doi: 10.1021/acsmedchemlett.5b00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Current Biology. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Andersson DP, Eriksson-Hogling D, Backdahl J, Thorell A, Lofgren P, Ryden M, Arner P, Hoffstedt J. Omentectomy in Addition to Bariatric Surgery-a 5-Year Follow-up. Obes Surg. 2017;27:1115–1118. doi: 10.1007/s11695-017-2576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Barton D, Gaunt P, Hull D, Guo K, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, et al. Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open. 2013;3:e003995. doi: 10.1136/bmjopen-2013-003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, Nasiri M, Yu J, Gough SC, Newsome PN, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64:399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Baldry EL, Aithal GP, Kaye P, Idris IR, Bennett A, Leeder PC, Macdonald IA. Effects of short-term energy restriction on liver lipid content and inflammatory status in severely obese adults: Results of a randomized controlled trial using 2 dietary approaches. Diabetes, Obesity and Metabolism. 2017 doi: 10.1111/dom.12918. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW, et al. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. Journal of lipid research. 2011;52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- Bergman RN. New concepts in extracellular signaling for insulin action: the single gateway hypothesis. Recent Prog Horm Res. 1997;52:359–385. discussion 385–357. [PubMed] [Google Scholar]
- Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. The Journal of biological chemistry. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter AT, Senft J, Gotthardt D, Knefeli P, Nickel F, Schulte T, Fischer L, Nawroth PP, Buchler MW, Muller-Stich BP. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?-a Controlled Matched Pair Study of 34 Patients. Obes Surg. 2016;26:1867–1874. doi: 10.1007/s11695-015-2006-y. [DOI] [PubMed] [Google Scholar]
- Bindesbøll C, Fan Q, Nørgaard RC, MacPherson L, Ruan H-B, Wu J, Pedersen TÅ, Steffensen KR, Yang X, Matthews J, et al. Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity. Journal of lipid research. 2015;56:771–785. doi: 10.1194/jlr.M049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojic LA, Huff MW. Peroxisome proliferator-activated receptor delta: a multifaceted metabolic player. Curr Opin Lipidol. 2013;24:171–177. doi: 10.1097/MOL.0b013e32835cc949. [DOI] [PubMed] [Google Scholar]
- Bourbeau MP, Siegmund A, Allen JG, Shu H, Fotsch C, Bartberger MD, Kim KW, Komorowski R, Graham M, Busby J, et al. Piperazine oxadiazole inhibitors of acetyl-CoA carboxylase. Journal of medicinal chemistry. 2013;56:10132–10141. doi: 10.1021/jm401601s. [DOI] [PubMed] [Google Scholar]
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Popkin BM. Dietary Sugar and Body Weight: Have We Reached a Crisis in the Epidemic of Obesity and Diabetes? Health Be Damned! Pour on the Sugar. 2014;37:950–956. doi: 10.2337/dc13-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science (New York, N.Y.) 2012;337:96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere DA, Ruan X, Cheng CC, Siesky AM, Fitch TE, Dominguez C, Sanfeliciano SG, Montero C, Suen CS, Xu Y, et al. Novel Small Molecule Agonist of TGR5 Possesses Anti-Diabetic Effects but Causes Gallbladder Filling in Mice. PloS one. 2015;10:e0136873. doi: 10.1371/journal.pone.0136873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Rudel LL. Stearoyl-coenzyme A desaturase 1 inhibition and the metabolic syndrome: considerations for future drug discovery. Curr Opin Lipidol. 2010;21:192–197. doi: 10.1097/MOL.0b013e32833854ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nature genetics. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- Cai K, Sewer MB. Diacylglycerol kinase θ couples farnesoid X receptor-dependent bile acid signalling to Akt activation and glucose homoeostasis in hepatocytes. The Biochemical journal. 2013;454:267–274. doi: 10.1042/BJ20130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology. 2013;154:3099–3109. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Zhou Y, Peng H, Huang X, Stahler S, Suri V, Qadri A, Gareski T, Jones J, Hahm S, et al. Targeting Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. The Journal of biological chemistry. 2011;286:41838–41851. doi: 10.1074/jbc.M111.245456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carino A, Cipriani S, Marchianò S, Biagioli M, Santorelli C, Donini A, Zampella A, Monti MC, Fiorucci S. BAR502, a dual FXR and GPBAR1 agonist, promotes browning of white adipose tissue and reverses liver steatosis and fibrosis. Scientific Reports. 2017;7:42801. doi: 10.1038/srep42801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B, Hanf R, Lambert-Porcheron S, Zair Y, Sauvinet V, Noel B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Vigueira PA, Chambers KT, Hall AM, Mitra MS, Qi N, McDonald WG, Colca JR, Kletzien RF, Finck BN. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor gamma-sparing thiazolidinedione. The Journal of biological chemistry. 2012;287:23537–23548. doi: 10.1074/jbc.M112.363960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. The Journal of biological chemistry. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- Chow JD, Lawrence RT, Healy ME, Dominy JE, Liao JA, Breen DS, Byrne FL, Kenwood BM, Lackner C, Okutsu S, et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab. 2014;3:419–431. doi: 10.1016/j.molmet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- Consortium TSTD. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature. 2014;506:97–101. doi: 10.1038/nature12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriege P, Despres JP. Gender difference in postprandial lipemia : importance of visceral adipose tissue accumulation. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2448–2455. doi: 10.1161/01.atv.19.10.2448. [DOI] [PubMed] [Google Scholar]
- Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Annals of Internal Medicine. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes (Lond) 2017;41:853–865. doi: 10.1038/ijo.2017.4. [DOI] [PubMed] [Google Scholar]
- Degirolamo C, Sabba C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. The Journal of clinical investigation. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, et al. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:635–648 e614. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4:51–57. doi: 10.1016/j.molmet.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton DS, Kraft G, Smith M, Farmer B, Williams PE, Coate KC, Printz RL, O'Brien RM, Cherrington AD. Insulin's direct hepatic effect explains the inhibition of glucose production caused by insulin secretion. JCI Insight. 2017;2:e91863. doi: 10.1172/jci.insight.91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion DM, Popov V, Hsiao JJ, Vatner D, Mitchell K, Yonemitsu S, Nagai Y, Kahn M, Gillum MP, Dong J, et al. The role of the carbohydrate response element-binding protein in male fructose-fed rats. Endocrinology. 2013;154:36–44. doi: 10.1210/en.2012-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. The Journal of clinical endocrinology and metabolism. 2010a;95:2727–2735. doi: 10.1210/jc.2009-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010b;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Lu H, Guo Y, Zhu T, Garcia-Barrio MT, Jiang Z, Willer CJ, Zhang J, Chen YE. Hepatic Transmembrane 6 Superfamily Member 2 Regulates Cholesterol Metabolism in Mice. Gastroenterology. 2016;150:1208–1218. doi: 10.1053/j.gastro.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miranda C, Perez-Carreras M, Colina F, Lopez-Alonso G, Vargas C, Solis-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Finan B, Clemmensen C, Zhu Z, Stemmer K, Gauthier K, Muller L, De Angelis M, Moreth K, Neff F, Perez-Tilve D, et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell. 2016;167:843–857 e814. doi: 10.1016/j.cell.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Flannery C, Dufour S, Rabol R, Shulman GI, Petersen KF. Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes. 2012;61:2711–2717. doi: 10.2337/db12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois ME, Baldi JC, Manning PJ, Lucas SJ, Hawley JA, Williams MJ, Cotter JD. 'Exercise snacks' before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia. 2014;57:1437–1445. doi: 10.1007/s00125-014-3244-6. [DOI] [PubMed] [Google Scholar]
- Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016;12:127–131. doi: 10.1016/j.soard.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Schnabl B. Editors’ Introduction to the NAFLD and NASH Special Issue. Digestive Diseases and Sciences. 2016;61 doi: 10.1007/s10620-016-4152-z. [DOI] [PubMed] [Google Scholar]
- Futatsugi K, Kung DW, Orr ST, Cabral S, Hepworth D, Aspnes G, Bader S, Bian J, Boehm M, Carpino PA, et al. Discovery and Optimization of Imidazopyridine-Based Inhibitors of Diacylglycerol Acyltransferase 2 (DGAT2) Journal of medicinal chemistry. 2015;58:7173–7185. doi: 10.1021/acs.jmedchem.5b01006. [DOI] [PubMed] [Google Scholar]
- Gijon MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. The Journal of biological chemistry. 2008;283:30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term Metabolic Effects of Laparoscopic Sleeve Gastrectomy. JAMA Surg. 2015;150:1051–1057. doi: 10.1001/jamasurg.2015.2202. [DOI] [PubMed] [Google Scholar]
- Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, Fu X, Miao R, Pewa AD, Brown KS, et al. Hepatic Mitochondrial Pyruvate Carrier 1 Is Required for Efficient Regulation of Gluconeogenesis and Whole-Body Glucose Homeostasis. Cell metabolism. 2015;22:669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. The Journal of clinical investigation. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Joel T, Miao J, Chanda D, Wang Y, Zhao E, Haas Mary E, Hirschey M, Vaitheesvaran B, Farese Robert V, Jr, Kurland Irwin J, et al. Hepatic Insulin Signaling Is Required for Obesity-Dependent Expression of SREBP-1c mRNA but Not for Feeding-Dependent Expression. Cell metabolism. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Kou K, Chen Z, Pietka TA, Kumar M, Korenblat KM, Lee K, Ahn K, Fabbrini E, Klein S, et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. Journal of lipid research. 2012;53:990–999. doi: 10.1194/jlr.P025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AM, Soufi N, Chambers KT, Chen Z, Schweitzer GG, McCommis KS, Erion DM, Graham MJ, Su X, Finck BN. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes. 2014;63:2284–2296. doi: 10.2337/db13-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriman G, Greenwood J, Bhat S, Huang X, Wang R, Paul D, Tong L, Saha AK, Westlin WF, Kapeller R, et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1796–1805. doi: 10.1073/pnas.1520686113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, Kugelmas M, Bashir MR, Ling L, Rossi SJ, DePaoli AM, et al. LBO-07 - NGM282, a novel variant of FGF19, significantly reduces hepatic steatosis and key biomarkers of NASH: results of a Phase 2, multicenter, randomized, double-blinded, placebo controlled trial in biopsy-confirmed NASH patients. Journal of Hepatology. 2017;66:S92–S93. [Google Scholar]
- Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- Harwood HJ, Jr, Petras SF, Shelly LD, Zaccaro LM, Perry DA, Makowski MR, Hargrove DM, Martin KA, Tracey WR, Chapman JG, et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. The Journal of biological chemistry. 2003;278:37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- Henderson SJ, Konkar A, Hornigold DC, Trevaskis JL, Jackson R, Fritsch Fredin M, Jansson-Löfmark R, Naylor J, Rossi A, Bednarek MA, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes, Obesity and Metabolism. 2016;18:1176–1190. doi: 10.1111/dom.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Samuel VT. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends in Endocrinology & Metabolism. 27:719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science (New York, N.Y.) 2012;337:93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Cooney GJ, James DE. Phenotypic Discrepancies in Acetyl-CoA Carboxylase 2-deficient Mice. Journal of Biological Chemistry. 2012;287:15801. doi: 10.1074/jbc.O112.356915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst K, Gilijamse P, Versteeg R, Ackermans M, Nederveen A, laFelur S, Romijn J, Nieuwdorp M, Groen AK, Zhang D, et al. Hepatic Diacylglycerol-Associated Protein Kinase Cε Translocation Links Hepatic Steatosis to Hepatic Insulin Resistance in Humans. Cell Reports. 2017 doi: 10.1016/j.celrep.2017.05.035. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard K, Londregan AT, Tesz G, Bahnck KB, Magee TV, Hepworth D, Polivkova J, Coffey SB, Pabst BA, Gosset JR, et al. Discovery of Selective Small Molecule Inhibitors of Monoacylglycerol Acyltransferase 3. Journal of medicinal chemistry. 2015;58:7164–7172. doi: 10.1021/acs.jmedchem.5b01008. [DOI] [PubMed] [Google Scholar]
- Hunkeler M, Stuttfeld E, Hagmann A, Imseng S, Maier T. The dynamic organization of fungal acetyl-CoA carboxylase. Nature Communications. 2016;7:11196. doi: 10.1038/ncomms11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, Boyko EJ. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes (Lond) 2015;39:1365–1370. doi: 10.1038/ijo.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M, Takahashi A, Yamauchi T, Hara K, Yasuda K, Grarup N, Zhao W, Wang X, Huerta-Chagoya A, Hu C, et al. Genome-wide association studies in the Japanese population identify seven novel loci for type 2 diabetes. Nat Commun. 2016;7:10531. doi: 10.1038/ncomms10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbriglio JE, Shen DM, Liang R, Marby K, You M, Youm HW, Feng Z, London C, Xiong Y, Tata J, et al. Discovery and Pharmacology of a Novel Class of Diacylglycerol Acyltransferase 2 Inhibitors. Journal of medicinal chemistry. 2015;58:9345–9353. doi: 10.1021/acs.jmedchem.5b01345. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell metabolism. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell metabolism. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. New England Journal of Medicine. 1998;338:867–873. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- Irimia JM, Meyer CM, Segvich DM, Surendran S, DePaoli-Roach AA, Morral N, Roach PJ. Lack of liver glycogen causes hepatic insulin resistance and Steatosis in mice. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M117.786525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Jall S, Sachs S, Clemmensen C, Finan B, Neff F, DiMarchi RD, Tschöp MH, Müller TD, Hofmann SM. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Molecular Metabolism. 2017;6:440–446. doi: 10.1016/j.molmet.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD. Role of Body Fat Distribution and the Metabolic Complications of Obesity. The Journal of Clinical Endocrinology & Metabolism. 2008;93:s57–s63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nature Communications. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase–1. The Journal of clinical investigation. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Weng J. Hepatic functions of GLP-1 and its based drugs: current disputes and perspectives. American journal of physiology. Endocrinology and metabolism. 2016;311:E620–627. doi: 10.1152/ajpendo.00069.2016. [DOI] [PubMed] [Google Scholar]
- Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, El-Saed A, Miyamatsu N, Edmundowicz D, Kita Y, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006;30:1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. Journal of Hepatology. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. American journal of physiology. Endocrinology and metabolism. 2003;285:E906–916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- Khorgami Z, Shoar S, Andalib A, Aminian A, Brethauer SA, Schauer PR. Trends in utilization of bariatric surgery, 2010–2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017 doi: 10.1016/j.soard.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Kim CW, Moon YA, Park SW, Cheng D, Kwon HJ, Horton JD. Induced polymerization of mammalian acetyl-CoA carboxylase by MIG12 provides a tertiary level of regulation of fatty acid synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9626–9631. doi: 10.1073/pnas.1001292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:474–485. doi: 10.1016/j.cgh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. The Journal of biological chemistry. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science (New York, N.Y.) 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric Bypass Surgery Improves Metabolic and Hepatic Abnormalities Associated With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2006;130:1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Mangelsdorf DJ. Bile Acids as Hormones: The FXR-FGF15/19 Pathway. Digestive Diseases. 2015;33:327–331. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- Kumari M, Schoiswohl G, Chitraju C, Paar M, Cornaciu I, Rangrez AY, Wongsiriroj N, Nagy HM, Ivanova PT, Scott SA, et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell metabolism. 2012;15:691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumashiro N, Yoshimura T, Cantley JL, Majumdar SK, Guebre-Egziabher F, Kursawe R, Vatner DF, Fat I, Kahn M, Erion DM, et al. Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology. 2013;57:1763–1772. doi: 10.1002/hep.26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362:906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- Lallukka S, Yki-Jarvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2016;30:385–395. doi: 10.1016/j.beem.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM. Effect of a 12-Month Intensive Lifestyle Intervention on Hepatic Steatosis in Adults With Type 2 Diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R, Fritz IB. The relation of insulin to liver metabolism. Diabetes. 1956;5:209–219. doi: 10.2337/diab.5.3.209. discussion, 219–222. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Zinman B, Groenewoud Y, Vranic M, Giacca A. Hepatic glucose production is regulated both by direct hepatic and extrahepatic effects of insulin in humans. Diabetes. 1996;45:454–462. doi: 10.2337/diab.45.4.454. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Phillips ML, Slavotinek JP, Kow L, Thompson CH, Toouli J. Change in Liver Size and Fat Content after Treatment with Optifast® Very Low Calorie Diet. Obesity Surgery. 2006;16:697–701. doi: 10.1381/096089206777346682. [DOI] [PubMed] [Google Scholar]
- Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, Cohen JC, Hobbs HH. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. The Journal of clinical investigation. 2012;122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proceedings of the National Academy of Sciences. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MA, Sheldon RD, Meers GM, Ortinau LC, Morris EM, Booth FW, Kanaley JA, Vieira-Potter VJ, Sowers JR, Ibdah JA, et al. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. The Journal of Physiology. 2016;594:5271–5284. doi: 10.1113/JP272235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist C, Bjorndal B, Rossmann CR, Tusubira D, Svardal A, Rosland GV, Tronstad KJ, Hallstrom S, Berge RK. Increased Hepatic Mitochondrial FA Oxidation leads to lower TG levels in Rat Liver and Plasma, and is associated with Upregulation of Uncoupling Proteins and Downregulation of Apolipoprotein C-III. Journal of lipid research. 2017 doi: 10.1194/jlr.M074849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, McLaren DG, Chen D, Kan Y, Stout SJ, Shen X, Murphy BA, Forrest G, Karanam B, Sonatore L, et al. Potential mechanism of enhanced postprandial glucagon-like peptide-1 release following treatment with a diacylglycerol acyltransferase 1 inhibitor. Pharmacol Res Perspect. 2015;3:e00193. doi: 10.1002/prp2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, Ananthakrishnan G, Rohner-Jeanrenaud F, Drucker DJ, DiMarchi R, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61:2753–2762. doi: 10.2337/db11-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, Gaulton KJ, Eicher JD, Sharp SJ, Luan J, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nature genetics. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen PK, Zhou Y, Hyötyläinen T, Leivonen M, Arola J, Orho-Melander M, Orešič M, Yki-Järvinen H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. Journal of Hepatology. 2016a;65:1263–1265. doi: 10.1016/j.jhep.2016.07.045. [DOI] [PubMed] [Google Scholar]
- Luukkonen PK, Zhou Y, Nidhina Haridas PA, Dwivedi OP, Hyötyläinen T, Ali A, Juuti A, Leivonen M, Tukiainen T, Ahonen L, et al. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. Journal of Hepatology. doi: 10.1016/j.jhep.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, Hyötyläinen T, Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2016b;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Maciejewski BS, LaPerle JL, Chen D, Ghosh A, Zavadoski WJ, McDonald TS, Manion TB, Mather D, Patterson TA, Hanna M, et al. Pharmacological inhibition to examine the role of DGAT1 in dietary lipid absorption in rodents and humans. Am J Physiol Gastrointest Liver Physiol. 2013;304:G958–969. doi: 10.1152/ajpgi.00384.2012. [DOI] [PubMed] [Google Scholar]
- Maggs DG, Buchanan TA, Burant CF, Cline G, Gumbiner B, Hsueh WA, Inzucchi S, Kelley D, Nolan J, Olefsky JM, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased Whole-Body Adiposity Without a Concomitant Increase in Liver Fat is Not Associated With Augmented Metabolic Dysfunction. Obesity. 2010;18:1510–1515. doi: 10.1038/oby.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magkos F, Su X, Bradley D, Fabbrini E, Conte C, Eagon JC, Varela JE, Brunt EM, Patterson BW, Klein S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142:1444–1446 e1442. doi: 10.1053/j.gastro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdessian H, Taxiarchis A, Popov S, Silveira A, Franco-Cereceda A, Hamsten A, Eriksson P, van't Hooft F. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8913–8918. doi: 10.1073/pnas.1323785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e1216. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancina RM, Matikainen N, Maglio C, Soderlund S, Lundbom N, Hakkarainen A, Rametta R, Mozzi E, Fargion S, Valenti L, et al. Paradoxical dissociation between hepatic fat content and de novo lipogenesis due to PNPLA3 sequence variant. The Journal of clinical endocrinology and metabolism. 2015;100:E821–825. doi: 10.1210/jc.2014-4464. [DOI] [PubMed] [Google Scholar]
- Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martagon AJ, Lin JZ, Cimini SL, Webb P, Phillips KJ. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PloS one. 2015;10:e0122987. doi: 10.1371/journal.pone.0122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, Burgess SC, Finck BN. Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell metabolism. 2015;22:682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCommis KS, Hodges WT, Brunt EM, Nalbantoglu I, McDonald WG, Holley C, Fujiwara H, Schaffer JE, Colca JR, Finck BN. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology. 2017;65:1543–1556. doi: 10.1002/hep.29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 2015;14:8. doi: 10.1186/s12944-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- Miles JM, Wooldridge D, Grellner WJ, Windsor S, Isley WL, Klein S, Harris WS. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes. 2003;52:675–681. doi: 10.2337/diabetes.52.3.675. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic Stearoyl-CoA Desaturase-1 Deficiency Protects Mice from Carbohydrate-Induced Adiposity and Hepatic Steatosis. Cell metabolism. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. The Journal of clinical endocrinology and metabolism. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajima N, Ikeda Y. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes Care. 1999;22:908–912. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, et al. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2013;145:574–582.e571. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Loffler MG, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell metabolism. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bensadoun A, Fruchart-Najib J, Holleran S, Cohn JS, Ramakrishnan R, et al. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. The Journal of clinical investigation. 2005;115:1323–1332. doi: 10.1172/JCI23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes. 2017 doi: 10.2337/db16-0662. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. The Journal of clinical investigation. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr. 2012;96:714–726. doi: 10.3945/ajcn.112.035758. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. The Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan J-P, et al. A Mouse Model of Hepatocellular Carcinoma. The American Journal of Pathology. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. The Journal of clinical investigation. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proceedings of the National Academy of Sciences. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma C, Ohta T, Tadaki H, Hamada H, Oda T, Taniuchi H, Yamanaka K, Ishii Y, Ohe Y, Yata S, et al. JTP-103237, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity. Eur J Pharmacol. 2015a;758:72–81. doi: 10.1016/j.ejphar.2015.03.072. [DOI] [PubMed] [Google Scholar]
- Okuma C, Ohta T, Tadaki H, Ishigure T, Sakata S, Taniuchi H, Sano R, Hamada H, Kume S, Nishiu J, et al. JTP-103237, a monoacylglycerol acyltransferase inhibitor, prevents fatty liver and suppresses both triglyceride synthesis and de novo lipogenesis. J Pharmacol Sci. 2015b;128:150–157. doi: 10.1016/j.jphs.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7598–7603. doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell metabolism. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Experimental Gerontology. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JG, Xu X, Cho S, Hur KY, Lee MS, Kersten S, Lee AH. CREBH-FGF21 axis improves hepatic steatosis by suppressing adipose tissue lipolysis. Sci Rep. 2016;6:27938. doi: 10.1038/srep27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology. 2015;62:720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell. 2015a;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Kim T, Zhang XM, Lee HY, Pesta D, Popov VB, Zhang D, Rahimi Y, Jurczak MJ, Cline GW, et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell metabolism. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Zhang D, Zhang X-M, Boyer JL, Shulman GI. Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science (New York, N.Y.) 2015b;347:1253–1256. doi: 10.1126/science.aaa0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, Rothman DL, Shulman GI. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nature medicine. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- Peter A, Kantartzis K, Machicao F, Machann J, Wagner S, Templin S, Konigsrainer I, Konigsrainer A, Schick F, Fritsche A, et al. Visceral obesity modulates the impact of apolipoprotein C3 gene variants on liver fat content. Int J Obes (Lond) 2012;36:774–782. doi: 10.1038/ijo.2011.154. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Hendler R, Price T, Perseghin G, Rothman DL, Held N, Amatruda JM, Shulman GI. 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes. 1998a;47:381–386. doi: 10.2337/diabetes.47.3.381. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Krssak M, Inzucchi S, Cline GW, Dufour S, Shulman GI. Mechanism of troglitazone action in type 2 diabetes. Diabetes. 2000;49:827–831. doi: 10.2337/diabetes.49.5.827. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. The Journal of clinical investigation. 1998b;101:1203–1209. doi: 10.1172/JCI579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. The Journal of clinical investigation. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Madiraju AK, Gassaway BM, Marcel M, Nasiri AR, Butrico G, Marcucci MJ, Zhang D, Abulizi A, Zhang X-M, et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. The Journal of clinical investigation. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson Timothy R, Sengupta Shomit S, Harris Thurl E, Carmack Anne E, Kang Seong A, Balderas E, Guertin David A, Madden Katherine L, Carpenter Anne E, Finck Brian N, et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingitore P, Pirazzi C, Mancina RM, Motta BM, Indiveri C, Pujia A, Montalcini T, Hedfalk K, Romeo S. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2014;1841:574–580. doi: 10.1016/j.bbalip.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell metabolism. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager R, Wallace P, Olefsky JM. Direct and indirect effects of insulin to inhibit hepatic glucose output in obese subjects. Diabetes. 1987;36:607–611. doi: 10.2337/diab.36.5.607. [DOI] [PubMed] [Google Scholar]
- Previs SF, Cline GW, Shulman GI. A critical evaluation of mass isotopomer distribution analysis of gluconeogenesis in vivo. The American journal of physiology. 1999;277:E154–160. doi: 10.1152/ajpendo.1999.277.1.E154. [DOI] [PubMed] [Google Scholar]
- Qu X, Seale JP, Donnelly R. Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats - effects of feeding. J Endocrinol. 1999;162:207–214. doi: 10.1677/joe.0.1620207. [DOI] [PubMed] [Google Scholar]
- Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddatz K, Turner N, Frangioudakis G, Liao BM, Pedersen DJ, Cantley J, Wilks D, Preston E, Hegarty BD, Leitges M, et al. Time-dependent effects of Prkce deletion on glucose homeostasis and hepatic lipid metabolism on dietary lipid oversupply in mice. Diabetologia. 2011;54:1447–1456. doi: 10.1007/s00125-011-2073-0. [DOI] [PubMed] [Google Scholar]
- Raff EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol. 2015;3:9–16. doi: 10.14218/JCTH.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159 e1145. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. The Journal of clinical investigation. 1996;98:741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Anderson B, Olbe L, Bjorntorp P. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism: clinical and experimental. 1990;39:1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. The Journal of clinical investigation. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature genetics. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu V, Hoch E, Mercader JM, Tenen DE, Gymrek M, Hartigan CR, DeRan M, von Grotthuss M, Fontanillas P, Spooner A, et al. Type 2 Diabetes Variants Disrupt Function of SLC16A11 through Two Distinct Mechanisms. Cell. 170:199–212.e120. doi: 10.1016/j.cell.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, Oren R, Safadi R, Konikoff FM, Hershkovitz A, et al. The Fatty Acid–Bile Acid Conjugate Aramchol Reduces Liver Fat Content in Patients With Nonalcoholic Fatty Liver Disease. Clinical Gastroenterology and Hepatology. 2014;12:2085–2091.e2081. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P. The liver diseases of lipodystrophy: The long-term effect of leptin treatment. Journal of Hepatology. 2013;59:131–137. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific Deletion of Stearoyl-CoA Desaturase-1 Alters Skin Lipid Composition and Protects Mice from High Fat Diet-induced Obesity. Journal of Biological Chemistry. 2009;284:19961–19973. doi: 10.1074/jbc.M109.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of Hepatic Insulin Resistance in Non-alcoholic Fatty Liver Disease. The Journal of biological chemistry. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. The Journal of clinical investigation. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel Varman T, Shulman Gerald I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. The Journal of clinical investigation. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B, et al. A Variant in the Glucokinase Regulatory Protein (GCKR) Gene is Associated with Fatty Liver in Obese Children and Adolescents. Hepatology (Baltimore, Md.) 2012;55:781–789. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison S, Abdelmalek M, Lawitz E, Halegoua-DeMarzio D, Dong Y, Noviello S, et al. The International Liver Congress, European Association for the Study of the Liver. Amsterdam, The Netherlands: 2017. BMS-986036 (pegylated FGF21) in patients with non-alcoholic steatohepatitis: A phase 2 study. [Google Scholar]
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. The Journal of clinical investigation. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–413. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober G, Arnold M, Birtles S, Buckett LK, Pacheco-Lopez G, Turnbull AV, Langhans W, Mansouri A. Diacylglycerol acyltransferase-1 inhibition enhances intestinal fatty acid oxidation and reduces energy intake in rats. Journal of lipid research. 2013;54:1369–1384. doi: 10.1194/jlr.M035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2013;56:156–161. doi: 10.1007/s00125-012-2738-3. [DOI] [PubMed] [Google Scholar]
- Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. The Journal of clinical endocrinology and metabolism. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. American journal of physiology. Endocrinology and metabolism. 2009;297:E10–18. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. The Journal of clinical investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes. 1997;46:187–196. doi: 10.2337/diab.46.2.187. [DOI] [PubMed] [Google Scholar]
- Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The Effect of PNPLA3 on Fibrosis Progression and Development of Hepatocellular Carcinoma: A Meta-analysis. Am J Gastroenterol. 2014;109:325–334. doi: 10.1038/ajg.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma Y-Z, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of Skeletal Muscle Triglyceride Content by 1H Nuclear Magnetic Resonance Spectroscopy in Lean and Obese Adolescents: Relationships to Insulin Sensitivity, Total Body Fat, and Central Adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. The Journal of clinical investigation. 2014;124:2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. The Journal of biological chemistry. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Ford RJ, Desjardins EM, Green AE, Hughes MC, Houde VP, Day EA, Marcinko K, Crane JD, Mottillo EP, et al. Salsalate (Salicylate) Uncouples Mitochondria, Improves Glucose Homeostasis, and Reduces Liver Lipids Independent of AMPK-beta1. Diabetes. 2016;65:3352–3361. doi: 10.2337/db16-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U. Abdominal obesity: a marker of ectopic fat accumulation. The Journal of clinical investigation. 2015;125:1790–1792. doi: 10.1172/JCI81507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi F, Casini A. Mediterranean diet and non-alcoholic fatty liver disease: New therapeutic option around the corner? World Journal of Gastroenterology : WJG. 2014;20:7339–7346. doi: 10.3748/wjg.v20.i23.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi N, Hall AM, Chen Z, Yoshino J, Collier SL, Mathews JC, Brunt EM, Albert CJ, Graham MJ, Ford DA, et al. Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. Journal of Biological Chemistry. 2014;289:30177–30188. doi: 10.1074/jbc.M114.595850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PRJ, Reese CB, McCormick F, Tempst P, et al. Protein Kinase B Kinases That Mediate Phosphatidylinositol 3,4,5-Trisphosphate-Dependent Activation of Protein Kinase B. Science (New York, N.Y.)) 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Muller J, Herder C, Nowotny P, et al. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nature medicine. 2014;20:1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Day CP, Bonora E. Risk of Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease. New England Journal of Medicine. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- Thabet K, Asimakopoulos A, Shojaei M, Romero-Gomez M, Mangia A, Irving WL, Berg T, Dore GJ, Gronbaek H, Sheridan D, et al. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun. 2016;7:12757. doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimoto D, Okuma C, Ishii Y, Kobayashi A, Ohta T, Kakutani M, Imanaka T, Ogawa N. JTT-553, a novel Acyl CoA:diacylglycerol acyltransferase (DGAT) 1 inhibitor, improves glucose metabolism in diet-induced obesity and genetic T2DM mice. J Pharmacol Sci. 2015;129:51–58. doi: 10.1016/j.jphs.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- Tong L, Harwood HJ., Jr Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J Cell Biochem. 2006;99:1476–1488. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell metabolism. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Valdecantos MP, Pardo V, Ruiz L, Castro-Sanchez L, Lanzon B, Fernandez-Millan E, Garcia-Monzon C, Arroba AI, Gonzalez-Rodriguez A, Escriva F, et al. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology. 2017;65:950–968. doi: 10.1002/hep.28962. [DOI] [PubMed] [Google Scholar]
- Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez JP, Cline GW, Jurczak MJ, et al. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DF, Weismann D, Beddow SA, Kumashiro N, Erion DM, Liao XH, Grover GJ, Webb P, Phillips KJ, Weiss RE, et al. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. American journal of physiology. Endocrinology and metabolism. 2013;305:E89–100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–378.e365. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, Gorman M, Kelly MA, Lovejoy AM, Kernan WN. Pioglitazone and Risk for Bone Fracture: Safety Data From a Randomized Clinical Trial. The Journal of clinical endocrinology and metabolism. 2017;102:914–922. doi: 10.1210/jc.2016-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M, Leavens Karla F, Hunter Roger W, Koren S, von Wilamowitz-Moellendorff A, Lu M, Satapati S, Chu Q, Sakamoto K, Burgess Shawn C, et al. A Noncanonical, GSK3-Independent Pathway Controls Postprandial Hepatic Glycogen Deposition. Cell metabolism. 2013;18:99–105. doi: 10.1016/j.cmet.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Wehmeyer MH, Zyriax BC, Jagemann B, Roth E, Windler E, Schulze Zur Wiesch J, Lohse AW, Kluwe J. Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine (Baltimore) 2016;95:e3887. doi: 10.1097/MD.0000000000003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Kato N, Hwang JY, Guo X, Tabara Y, Li H, Dorajoo R, Yang X, Tsai FJ, Li S, et al. Genome-wide association studies in East Asians identify new loci for waist-hip ratio and waist circumference. Sci Rep. 2016;6:17958. doi: 10.1038/srep17958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- Wright MB, Bortolini M, Tadayyon M, Bopst M. Minireview: Challenges and Opportunities in Development of PPAR Agonists. Molecular Endocrinology. 2014;28:1756–1768. doi: 10.1210/me.2013-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wueest S, Item F, Lucchini FC, Challa TD, Muller W, Bluher M, Konrad D. Mesenteric Fat Lipolysis Mediates Obesity-Associated Hepatic Steatosis and Insulin Resistance. Diabetes. 2016;65:140–148. doi: 10.2337/db15-0941. [DOI] [PubMed] [Google Scholar]
- Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, Wren AM, Frost GS, Meeran K, Ghatei MA, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li Y-S, Lindberg RA, et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief. 2016:1–8. [PubMed] [Google Scholar]
- Yoshitaka H, Hamaguchi M, Kojima T, Fukuda T, Ohbora A, Fukui M. Nonoverweight nonalcoholic fatty liver disease and incident cardiovascular disease: A post hoc analysis of a cohort study. Medicine (Baltimore) 2017;96:e6712. doi: 10.1097/MD.0000000000006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi Z, Henry L. Contribution of Alcoholic and Nonalcoholic Fatty Liver Disease to the Burden of Liver-Related Morbidity and Mortality. Gastroenterology. 2016;150:1778–1785. doi: 10.1053/j.gastro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. The Journal of biological chemistry. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- Zambad SP, Tuli D, Mathur A, Ghalsasi SA, Chaudhary AR, Deshpande S, Gupta RC, Chauthaiwale V, Dutt C. TRC210258, a novel TGR5 agonist, reduces glycemic and dyslipidemic cardiovascular risk in animal models of diabesity. Diabetes Metab Syndr Obes. 2013;7:1–14. doi: 10.2147/DMSO.S50209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RN, Zheng RD, Mi YQ, Zhou D, Shen F, Chen GY, Zhu CY, Pan Q, Fan JG. APOC3 rs2070666 Is Associated with the Hepatic Steatosis Independently of PNPLA3 rs738409 in Chinese Han Patients with Nonalcoholic Fatty Liver Diseases. Dig Dis Sci. 2016;61:2284–2293. doi: 10.1007/s10620-016-4120-7. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. Journal of Hepatology. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yeung DCY, Karpisek M, Stejskal D, Zhou Z-G, Liu F, Wong RLC, Chow W-S, Tso AWK, Lam KSL, et al. Serum FGF21 Levels Are Increased in Obesity and Are Independently Associated With the Metabolic Syndrome in Humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Li X, Yu D, Li X, Li Y, Long Y, Yuan Y, Ji Z, Zhang M, Wen JG, et al. Application of mitochondrial pyruvate carrier blocker UK5099 creates metabolic reprogram and greater stem-like properties in LnCap prostate cancer cells in vitro. Oncotarget. 2015;6:37758–37769. doi: 10.18632/oncotarget.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, Yang H, Humphrey M, Ding X, Arora T, et al. Separating Tumorigenicity from Bile Acid Regulatory Activity for Endocrine Hormone FGF19. Cancer Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]