Abstract
The effects of temperature, pH and long-term storage on the secondary structure and conformation changes of bovine haemoglobin (bHb) were studied using circular dichroism (CD) and ultraviolet--visible (UV–vis) spectroscopies. Neural network software was used to deconvolute the CD data to obtain the fractional content of the five secondary structures. The storage stability of bHb solutions in pH 6, 7 and 8 buffers was significantly higher at 4 °C than at 23 °C for the first 3 days. A complete denaturation of bHb was observed after 40 days irrespective of storage temperature or pH. The bHb solutions were also exposed to heating and cooling cycles between 25 and 65 °C and structural changes were followed by UV–vis and CD spectroscopies. These experiments demonstrated that α-helix content of bHb decreased steadily with the increasing temperature above 35 °C at all pH values. The loss in α-helicity and gain in random coil conformations was pH-dependent and the greatest under alkaline conditions. Furthermore, there was minimal recovery of the secondary structure content upon cooling to 25 °C. The use of bHb as a model drug is very common and this study elucidates the significance of storage and processing conditions on its stability.
Keywords: Bovine haemoglobin, Circular dichroism, Thermal stability, Storage stability, Ultraviolet--visible spectroscopy
1. Introduction
The US Food and Drug Administration (FDA) describes biologics as any therapeutic serum, toxin, antitoxin, virus, vaccine, blood, blood component or derivative, allergenic product, analogous product, or derivatives applicable to the prevention, treatment or cure of injuries or disease [1]. Among these biological products, proteins and peptides are steadily securing a position in the modern pharmaceutical market [2]. The advent of recombinant technology has made it possible to produce these biologics in large quantities, which renders them useful as therapeutic agents for the treatment of various diseases. However, most therapeutic proteins and peptides are prohibitively expensive for initial formulation studies where a large quantity of active compound is required. Hence, initial experiments are generally performed using readily available model proteins such as albumin and lysozyme. One such model protein is bovine haemoglobin (bHb), which is economical, easily available, well-characterised, and has been used in numerous studies [3], [4], [5]. Hb is an oxygen-carrying transport protein with a molecular weight of 64.5 kDa and a diameter of approximately 5 nm [6], [7]. It exists as a tetramer comprising two α- and two β-globin chains which are bound to each other by hydrogen bonding, salt bridges and hydrophobic interactions. Although it is a frequently used model protein, detailed data on the storage and thermal stability of bHb as functions of pH and temperature are sparse. Therefore, the thermal and storage stability of bHb in phosphate buffer solutions (PBS) of pH 6, 7 and 8 were determined in the present study by ultraviolet--visible (UV–vis) and circular dichroism (CD) spectroscopies. Similar studies on other proteins are reported by numerous authors where effect of pH and temperature was clearly evident on the conformational stability of the molecule [8], [9], [10]. Among the various techniques (Fig. 1) available to analyse the protein conformation [11], UV–vis and CD were chosen in this study as they are non-destructive and provide rapid analysis of small samples [12].
Fig. 1.
Analytical methods for protein characterisation.
Conventionally, CD results are obtained as a function of change in molar ellipticity [θ] with respect to wavelength [13]. However, various commercial software packages are available to deconvolute and interpret the spectra, including DichroWeb (Birkbeck College, University of London, UK), CONTIN-CD (European Molecular Biology Lab, Postfach, Germany), Dicroprot (Institute of Biology and Chemistry of Proteins, CNRS University, Lyon, France) and K2D (European Molecular Biology Laboratory, Heidelberg, Germany). In the present study, a neural network-based software known as CDNN (Applied Photophysics Ltd., Surrey, UK) was used to deconvolute the CD spectra into five different secondary structures using a reference database [14]. The changes in five secondary structures, namely α-helix, antiparallel, parallel, β-turn and random coil, were determined at various pHs, temperatures and storage conditions. The major advantage of CDNN is that the results obtained are easy to interpret and follow in terms of changes in secondary structure content.
2. Experimental
2.1. Materials
Bovine haemoglobin (bHb) was purchased from Sigma Aldrich (Gillingham, UK). Disodium hydrogen phosphate (Na2HPO4) and potassium dihydrogen phosphate (KH2PO4) were obtained from Alfa Aesar (Heysham, UK) and VWR (Geldenaaksebaan, Belgium), respectively. All reagents listed above were of analytical grade and used without any further purification.
2.2. Preparation of PBS and bHb solution
PBS were prepared by mixing 0.133 M Na2HPO4 and 0.133 M KH2PO4 solutions in the appropriate volume ratios to obtain required pH, i.e., pH 6 (12:88), pH 7 (50:50) and pH 8 (94.5:5.5). The buffered protein solutions of required concentrations were then prepared by dissolving bHb under gentle stirring at room temperature.
2.3. Storage stability
The storage stability studies were conducted in triplicate using 1.0 mg/mL of bHb solutions in pH 6, 7 and 8 PBS (0.133 M). The samples were stored at 23±2 and 4±1 °C prior to UV–vis or CD analysis. The UV–vis spectra were collected on a Shimadzu UV-2550 (Shimadzu, Kyoto, Japan) in 1 cm pathlength quartz cuvettes between 200 and 700 nm wavelength. The CD spectra were recorded in the far UV region between 190 and 260 nm with a bandwidth of 1 nm on a Chirascan qCD. The CD spectra obtained were then deconvoluted using CDNN software (Applied Photophysics Ltd., Surrey, UK). The UV–vis and CD data were collected after 0, 1, 2, 3 and 40 days.
2.4. Thermal stability
Each of the following thermal stability analyses was carried out in triplicate. 0.1 mg/mL bHb solutions were prepared in pH 6, 7 and 8 PBS for the thermal stability study. Each of these solutions was placed in a 1 cm pathlength quartz cuvette and subjected to a temperature ramp from 25 to 65 °C in the UV–vis spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) and cooled back to 25 °C. Temperature was regulated using a built-in Peltier with a sensitivity of ±0.5 °C. The sample was first equilibrated at 25 °C and then gradually increased or decreased by 5 °C at a time. Samples were allowed to equilibrate for 10 min before their UV–vis absorbance spectra were recorded between 200 and 700 nm.
Similarly, the 0.1 mg/mL bHb solutions prepared in pH 6, 7 and 8 PBS were subjected to heating and cooling cycles prior to CD analysis. All CD experiments were performed on a Chirascan qCD, equipped with a Peltier thermal-controlled cuvette holder. Samples were heated in 1 mm pathlength quartz cuvette from 25 to 65 °C and then re-cooled to 25 °C. CD spectra were recorded in the far UV region between 190 and 260 nm with a bandwidth of 1 nm after every 5 °C increase or decrease in temperature. The CD spectra obtained were then deconvoluted using CDNN software.
3. Results
The UV–vis absorption spectrum of bHb comprises two major peaks at 405 and 274 nm. The absorption maximum at 274 nm is characteristic peak of many proteins and arises from the phenyl group of tyrosine and tryptophan amino acids [15]. The Soret band at 405 nm results from the heme (porphyrin) moiety of the bHb protein [16]. Both of the characteristic peaks were monitored during the storage and thermal stability studies of bHb.
3.1. Storage stability of bHb
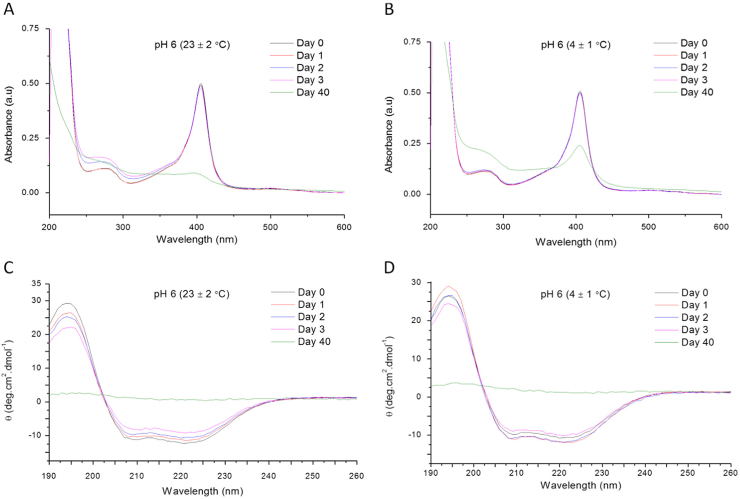
Fig. 2 contains examples of UV–vis and CD spectra for bHb solutions prepared in pH 6 PBS and stored at 23 and 4 °C.
Fig. 2.
UV–vis and CD spectra of bHb solutions prepared at pH 6 in PBS. (A) UV–vis spectrum at 23 °C; (B) UV–vis spectrum at 4 °C; (C) CD spectrum at 23 °C; (D) CD spectrum at 4 °C.
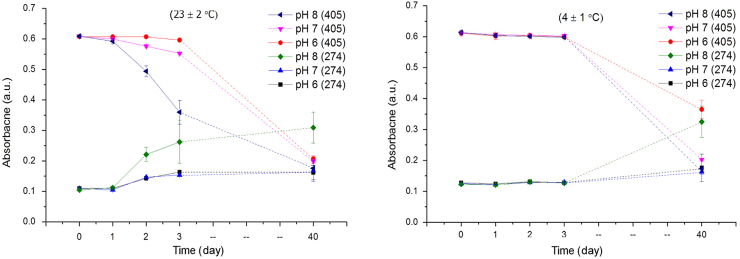
The changes in absorbance of bHb samples at 274 and 405 nm stored at 23 and 4 °C in various pH buffers are presented in Fig. 3.
Fig. 3.
Changes in UV–vis absorbance (274 and 405 nm) of bHb solutions prepared at pH 6, 7, and 8 in PBS due to storage at 23 and 4 °C (mean±SD, n=3).
The UV–vis spectra of bHb solutions prepared in pH 6 and 7 PBS and stored at 23 °C were similar, where a small decrease in absorbance of the Soret band and concomitant increase in intensity at 274 nm were observed during the first 3 days. However, protein denaturation was much faster at pH 8 as evident from the sharp decrease of absorbance at 405 nm and increase at 274 nm. Conversely, trends in sample stability at 4 °C were similar for the first 3 days irrespective of solution pH. The long-term storage (40 days) of bHb solutions resulted in a complete loss of the Soret band in all samples regardless of the storage temperature and solution pH.
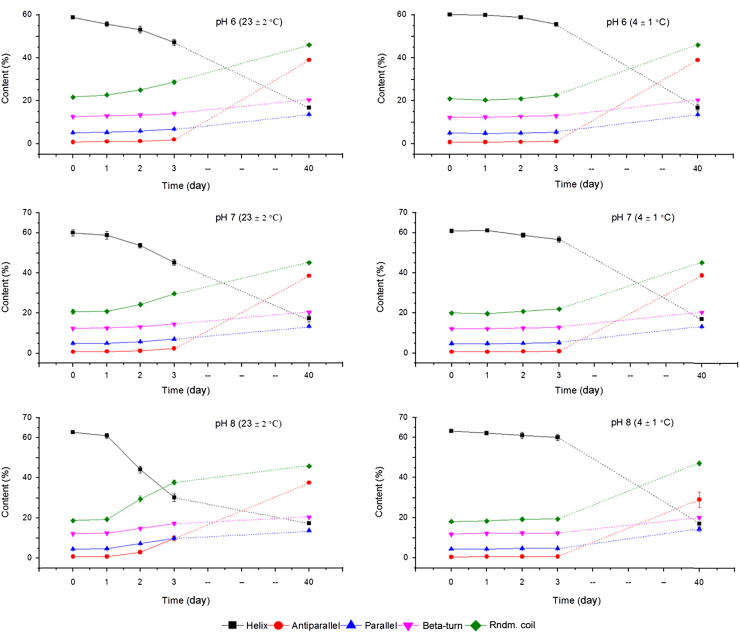
Fig. 4 presents changes in α-helix, β-turn, parallel, antiparallel and random coil contents of bHb samples prepared in pH 6, 7 and 8 buffers and stored at 23 and 4 °C for 40 days.
Fig. 4.
The change in secondary structure content of bHb at pH 6, 7, and 8 in PBS due to long-term storage at 23 and 4 °C (mean±SD, n=3).
The loss of α-helix was similar for pH 6 and 7 samples stored at 23 °C, whereas it was the greatest and most rapid at pH 8. The reduction in α-helicity resulted in an increase in random coil content which was also distinctly higher for the pH 8 sample. The bHb solution at pH 8 showed a 33% decrease in α-helix content in comparison to the minimal changes observed at lower pH. The results for samples stored at 4 °C showed modest changes in the α-helicity (between 3% and 4%) with insignificant increases in the rest of the secondary structures over the period of 3 days irrespective of solution pH. Samples analysed after 40 days of storage showed an almost complete loss of α-helicity under all selected conditions.
3.2. Thermal stability of bHb
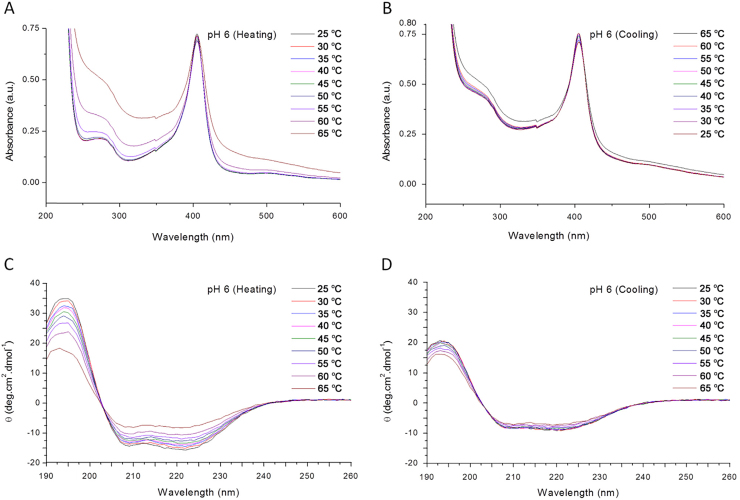
The effects of heating-cooling cycles (between 25 and 65 °C) on the UV–vis and CD spectra of bHb in pH 6 PBS are presented in Fig. 5.
Fig. 5.
UV–vis and CD spectra of bHb at pH 6 in PBS. (A) UV–vis spectra upon heating between 25 and 65 °C; (B) UV–vis spectra upon cooling between 65 and 25 °C; (C) CD spectra upon heating between 25 and 65 °C; (D) CD spectra upon cooling between 65 and 25 °C.
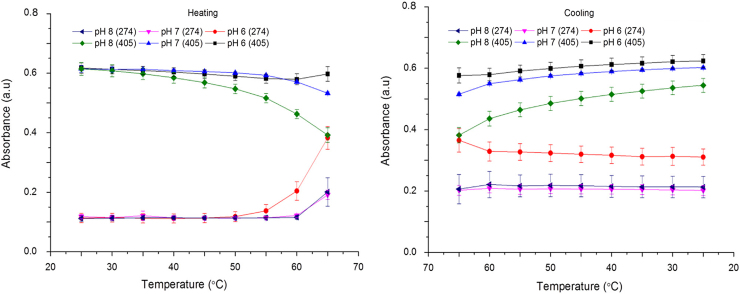
The changes in UV–vis absorbance behaviour of protein solutions at pH 6, 7 and 8 in PBS as functions of a single heating-cooling cycle are shown in Fig. 6.
Fig. 6.
The change in UV–vis absorbance of bHb at 274 and 405 nm at pH 6, 7, and 8 in PBS due to heating-cooling cycle between 25 and 65 °C (mean±SD, n=3).
A constant decrease in absorbance at 405 nm at pH 8 during the heating cycle was related to the loss of structural integrity of the heme moiety. The increase in absorbance at 274 nm was seen only after 50 °C for pH 6 and 60 °C for pH 7 and 8 solutions. Limited reduction in absorbance intensity at 274 nm for the pH 6 sample and absence of any significant changes at pH 7 and 8 during the cooling cycle imply irreversible conformational changes to the bHb structure.
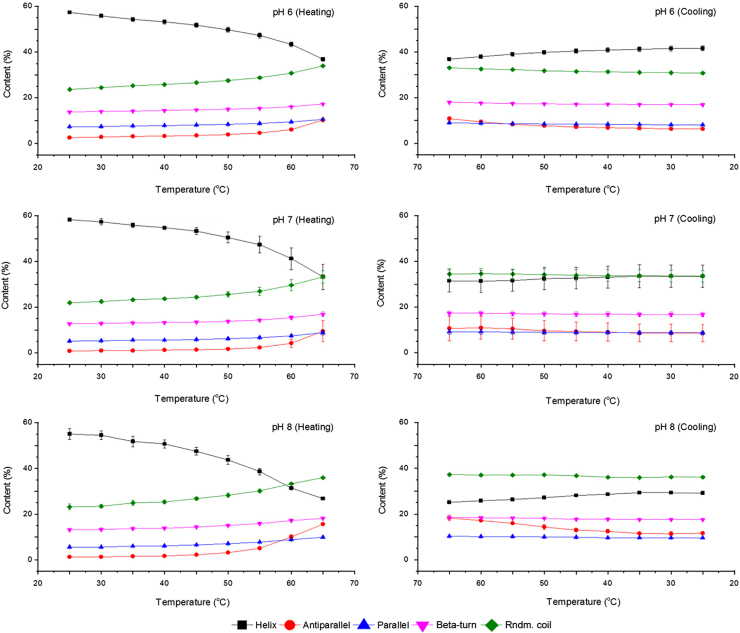
The changes in the secondary structure content of bHb under the selected conditions are presented in Fig. 7. The α-helix content decreased steadily for all samples with the highest decrease of 28% at pH 8, followed by 20% and 18% at pH 6 and 7, respectively. Interestingly, a small fraction of this change was observed to be reversible during the cooling cycle. The reversibility (i.e., recovery of α-helix content) was found to be between 1% and 5% depending on the pH.
Fig. 7.
The change in the secondary structure content of bHb at pH 6, 7, and 8 in PBS due to heating-cooling cycle between 25 and 65 °C (mean±SD, n=3).
4. Discussion
Proteins are amphoteric molecules with a net positive or negative charge below or above their isoelectric point (pI) [17]. The pI of bHb is 7.1 which implies electrostatic repulsion between the molecules below and above this value [18]. Moreover, bHb is a large molecule with intrinsically high charge density regions which also promote intra-molecular repulsions and protein unfolding in unfavourable conditions [19]. The results presented in Fig. 3 show that the rate of confirmation loss was the highest at pH 8, suggesting a close arrangement of negatively charged groups in the bHb molecule which experience strong repulsion. Conversely, lower conformational changes at pH 6 indicate that positively charged groups are more dispersed within the bHb molecule. It is known that the intra-molecular repulsive interactions between charged groups can be enhanced at higher temperatures, which is confirmed in this case by the observed accelerated loss in α-helicity at 23 °C in comparison with that at 4 °C.
According to CD data, the loss of α-helicity can be attributed to the destabilisation of hydrogen bonding between the amide hydrogen atom and carbonyl oxygen atom [20]. Results presented in Fig. 4 confirm that the stabilising hydrogen bonds in bHb are influenced by the storage temperature and pH of the solution. Furthermore, heme plays an important role in retaining the native structure of the bHb molecule; hence, its loss is a significant contributory factor in the decrease in α-helicity [21]. This phenomenon holds true in the present study, where simultaneous reductions in absorbance at 405 nm (i.e. loss of heme) and α-helicity were observed at all pH and temperatures.
The destabilisation of the native structure of a protein due to thermal energy is a well known phenomenon [20]. Hb denatures when exposed to heat and, since it consists of four sub-units, the denaturation is known to be a cooperative transition. In a previous study, differential scanning microcalorimetry showed that carboxyhemoglobin denatures at 82 °C, oxyhemoglobin at 71 °C and methemoglobin at 67 °C, at pH 7.4 in PBS [22]. Michnik et al. [23], however, showed that human methemoglobin denatures at 62 °C in pH 6.5 buffer. Artmann et al. [24] used CD spectroscopy to evaluate structural changes in human haemoglobin (HbA) and sickle cell haemoglobin (HbS) upon heating between 25 and 60 °C. He demonstrated that thermal denaturation curves of HbA were non-linear and showed that accelerated denaturation occurred between 35 and 39 °C along with reversible transitions below 39 °C which were independent of solution pH in the range 6.8–7.8.
In the present study, the thermal stability of bHb was assessed under a broad pH range, i.e., in pH 6, 7 and 8 buffer solutions. The temperatures studied in this work (Fig. 3) provide sufficient thermal energy to promote the disruption of essential stabilising forces, leading to the loss of the heme moiety and exposure of aromatic side chains to the surface. The native structure of a protein has approximately 70% of its peptide groups, 81% of its non-polar groups, 63% of its polar groups and 54% of its charged side chains buried in the interior of the molecule [25]. However, protein unfolding exposes non-polar side chains to the solvent when sufficient energy is applied to this system, as is apparent from the UV absorbance spectra in Fig. 3. The hypochromic shift observed at 405 nm indicates the loss of the heme moiety from the bHb molecule and the hyperchromic shift at 274 nm suggests exposure of aromatic side chains of the protein to the solvent [19], [26]. The observed, respective, decrease and increase in these absorbance values were also pH dependent, which suggests that the stability of the iron-containing porphyrin ring is greater at pH 6 and 7 in comparison to pH 8. The marginally higher stability under acidic and neutral conditions is likely to be attributed to factors which include the overall charge on the protein molecule, ionic strength of the media and inter-molecular protein-protein interactions.
The CD results presented in Fig. 7 showed a decrease in α-helix content with an increase in temperature, which can be explained by the breakage of stabilising hydrogen bonds. The majority of the bHb molecule (60% to 70%) exists in α-helical form and the rest (20% to 30%) consists of β-turns, parallel, antiparallel and random coils. Initial increases in temperature result in the weakening of hydrogen bonds, leading to a flexible protein molecule which exposes its side chain groups to the solvent [19]. Most proteins readily refold to their native configuration if heating is stopped at this early stage. However, continued heating breaks these intra-molecular hydrogen bonds and gives rise to new hydrogen bonds between the water, and amide nitrogen atoms and carbonyl oxygen atoms within the protein.
It is well known that proteins fold to attain a conformation that is essential for their biological activity. The native conformation is more stable than unfolded biologically inactive conformation under physiological conditions. Thermodynamically or in terms of free energy, the native configuration is only 5-20 kcal/mole more stable than the unfolded confirmation [20]. This small conformational stability of proteins is a result of large stabilizing and destabilizing forces. The major stabilizing forces are hydrogen bonding and hydrophobic effects whereas the major destabilizing force is the protein's conformational entropy [25]. Therefore, as a result of this small conformational stability of the native state, modest changes in external variables (i.e., temperature, pH, salt, concentration, etc.) lead to destabilization of the protein's structure and induce unfolding.
5. Conclusion
By analysing the UV–vis and CD spectroscopic data collected in this study, it can be concluded that bHb can withstand temperatures up to 50 °C, but at higher temperatures major irreversible changes take place in its conformation. The thermal stability was found to be independent of pH of the solution. It was also confirmed that solutions of bHb were stable for 3 days if maintained under cold conditions (i.e. 4 °C), whereas conformational changes occurred after only 1 day when stored at room temperature. Unlike the thermal stability, these conformational changes at room temperature were strongly influenced by the pH of the solution where the rate of conformation change at pH 6 and 7 was markedly slower than that at pH 8. The use of a neural network-based software (CDNN) is beneficial in following the conformational changes in a protein in comparison to raw CD data. They provide quantitative data which are easier to interpret and offer better understanding when various parameters and their effects are compared. These findings are of relevance to other researchers who intend to use bHb as a model protein under varying experimental pH and temperature regimes.
Acknowledgments
The authors are grateful to the University of Greenwich for financial support and Dr. Bruce D Alexander for his help with circular dichroism spectroscopy.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.(United States) Food and Drug Administration, Code of Federal Regulations Title 21, 21CFR600.3. 7, 2014, 〈http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3〉.
- 2.Espiritu M.J., Collier A.C., Bingham J.P. A 21st-century approach to age-old problems: the ascension of biologics in clinical therapeutics. Drug Discov. Today. 2014;19:1109–1113. doi: 10.1016/j.drudis.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Estevam L.S., Debone H.S., Yoshida C.M.P. Adsorption of bovine serum and bovine haemoglobin onto Chitosan film. Adsorpt. Sci. Technol. 2012;30:785–792. [Google Scholar]
- 4.Meng F., Ma G., Liu Y. Microencapsulation of bovine hemoglobin with high bio-activity and high entrapment efficiency using a W/O/W double emulsion technique. Colloids Surf. B Biointerfaces. 2004;33:177–183. [Google Scholar]
- 5.Liu D.M., Chen I.W. Encapsulation of protein molecules in transparent porous silica matrices via an aqueous colloidal Sol–gel process. Acta Mater. 1999;47:4535–4544. [Google Scholar]
- 6.Atyaksheva L.F., Dobryakova I.V., Ivanova I.I. Adsorption properties of hemoglobin. Russ. J. Phys. Chem. A. 2012;86:468–474. [Google Scholar]
- 7.Erickson H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online. 2009;11:32–51. doi: 10.1007/s12575-009-9008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezaei-Tavirani M.A.M., Moghaddamnia S.H., Ranjbar B. Conformational study of human serum albumin in pre-denaturation temperatures by differential scanning calorimetry, circular dichroism and UV spectroscopy. J. Biol. Biochem. Mol. 2006;39:530–536. doi: 10.5483/bmbrep.2006.39.5.530. [DOI] [PubMed] [Google Scholar]
- 9.Knubovets T., Osterhout J.J., Connolly P.J. Structure, thermostability, and conformational flexibility of hen egg-white lysozyme dissolved in glycerol. Proc. Natl. Acad. Sci. USA. 1999;96:1262–1267. doi: 10.1073/pnas.96.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuguchi M., Arai M., Ke Y. Equilibrium and kinetics of the folding of equine lysozyme studied by circular dichroism spectroscopy. J. Mol. Biol. 1998;283:265–277. doi: 10.1006/jmbi.1998.2100. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann H., Pisch-Heberle S. Protein Formulation and Delivery. 2nd ed. Informa Helthcare; New York: 2007. Analytical methods and stability testing of biopharmaceuticals; pp. 73–108. [Google Scholar]
- 12.Kelly S., Price N. The use of circular dichroism in the investigation of protein structure and function. Curr. Protein Pept. Sci. 2000;1:349–384. doi: 10.2174/1389203003381315. [DOI] [PubMed] [Google Scholar]
- 13.Kelly S.M., Jess T.J., Price N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta –Proteins Proteom. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Böhm G., Muhr R., Jaenicke R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng. 1992;5:191–195. doi: 10.1093/protein/5.3.191. [DOI] [PubMed] [Google Scholar]
- 15.Noble J.E., Bailey M.J.A. Guide to Protein Purification. 2nd ed. Academic Press; Philadelphia: 2009. Quantitation of protein; pp. 73–95. [Google Scholar]
- 16.Rimington C. Spectral-absorption coefficients of some porphyrins in the soret-band region. Biochem. J. 1960;75:620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa T., Prestrelski S.J., Kenney W.C. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001;46:307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhan G., Li C., Luo D. Electrochemical investigation of bovine hemoglobin at an acetylene black paste electrode in the presence of sodium dodecyl sulfate. Bull. Chem. Soc. 2007;28:1720–1724. [Google Scholar]
- 19.M. Mangino, Protein denaturation, Ohio state Univ, Food Sci. Lect., 2007, 〈http://class.fst.ohio-state.edu/FST822/lectures/Denat.html〉
- 20.Chi E.Y., Krishnan S., Randolph T.W. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm. Res. 2003;20:1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 21.Hargrove M.S., Olson J.S. The stability of holomyoglobin is determined by heme affinity. Biochemistry. 1996;35:11310–11318. doi: 10.1021/bi9603736. [DOI] [PubMed] [Google Scholar]
- 22.Lapshina E.A., Zavodnik I.B., Ignatenko V.A. Thermal stability and functional properties of human hemoglobin in the presence of aliphatic alcohols. Mol. Biol. 1992;26:315–320. [PubMed] [Google Scholar]
- 23.Michnik A., Drzazga Z., Kluczewska A. Differential scanning microcalorimetry study of the thermal denaturation of haemoglobin. Biophys. Chem. 2005;118:93–101. doi: 10.1016/j.bpc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Artmann G.M., Burns L., Canaves J.M. Circular dichroism spectra of human hemoglobin reveal a reversible structural transition at body temperature. Eur. Biophys. J. 2004;33:490–496. doi: 10.1007/s00249-004-0401-8. [DOI] [PubMed] [Google Scholar]
- 25.Pace C.N., Shirley B.A., McNutt M. Forces contributing to the conformational stability of proteins. FASEB J. 1996;10:75–83. doi: 10.1096/fasebj.10.1.8566551. [DOI] [PubMed] [Google Scholar]
- 26.Liong E.C., Dou Y., Scott E.E. Waterproofing the heme pocket: role of proximal amino acid side chains in preventing hemin loss from myoglobin. J. Biol. Chem. 2001;276:9093–9100. doi: 10.1074/jbc.M008593200. [DOI] [PubMed] [Google Scholar]