Abstract
Species interaction networks are traditionally explored as discrete entities with well-defined spatial borders, an oversimplification likely impairing their applicability. Using a multilayer network approach, explicitly accounting for inter-habitat connectivity, we investigate the spatial structure of seed–dispersal networks across the Gorongosa National Park, Mozambique. We show that the overall seed–dispersal network is composed by spatially explicit communities of dispersers spanning across habitats, functionally linking the landscape mosaic. Inter-habitat connectivity determines spatial structure, which cannot be accurately described with standard monolayer approaches either splitting or merging habitats. Multilayer modularity cannot be predicted by null models randomizing either interactions within each habitat or those linking habitats; however, as habitat connectivity increases, random processes become more important for overall structure. The importance of dispersers for the overall network structure is captured by multilayer versatility but not by standard metrics. Highly versatile species disperse many plant species across multiple habitats, being critical to landscape functional cohesion.
Species interaction networks have been usually delimited by perceived habitat borders. Here, seed-dispersal is analyzed as a regional multilayer network of interconnected habitats, highlighting the key role of versatile dispersers for the functional cohesion of the whole Gorongosa landscape.
Introduction
Over the recent decades, ecological networks have proved a valuable framework to simultaneously evaluate the role of species, their interactions, and the importance of the emerging community structure for the persistence and stability of biological communities1. Such studies revealed that ignoring the complex web of interactions between plants and animals in which many vital ecosystem functions are rooted might jeopardize the long-term functioning and persistence of ecosystems2,3. To date, most studies have considered networks as entities with discrete borders defined by the experimental design, ignoring the potential across-border connections4, or alternatively as aggregations of several spatially and temporal sampling occasions into an overall network5. In nature, however, these sub-networks are linked by common species and by processes that span over several spatial and temporal scales, contributing to the functional connectivity of ecosystems6. The importance of the spatial dimension of networks of interactions is becoming increasingly recognized7–9, highlighting the key function of species that cross habitat boundaries acting as mobile links10 that connect the different habitats. Recent work has provided further evidence of the importance of the often-neglected inter-habitat links and their unequivocal ecological relevance11. Perhaps ironically, the application of such tools that proved particularly suited to tackle the intrinsic complexity of ecosystems is limited by the amount of complexity that can be sampled and analyzed, leading to a fragmentation of real networks, likely to result in oversimplifications, and eventually to incomplete or erroneous conclusions about network structure, dynamics, and stability12,13. Similarly, ignoring the role of different species as spatial couplers of ecosystems may hinder our understanding of natural processes, e.g., the flux of energy, or nutrients, between aquatic and terrestrial systems, pollen transfer by insects across the landscape, or the dispersal of seeds of invasive species by birds14. Recently, some authors have started to tackle this issue by treating different habitats, or patches of habitat as a set of layers within a larger multilayer network15–17. An expansion of the concept of beta-diversity has been proposed to measure dissimilarities between networks, by exploring species and interactions turnover between groups of independent (i.e., formally disconnected) networks16,18. In a further step, Frost et al.15 quantified the connectivity between spatial layers (habitats) of a host-parasitoid network, though they did not explore the effect of habitat connectivity to the structure of the spatial network. However, only now ecologists have started to explicitly include interlayer edges in the analysis of the actual structure of “ecological multilayer networks”12, taking advantage of recent theoretical developments and analytic tools from other research areas13,19.
A key structural pattern in networks is modularity1,20,21, measuring the extent to which species form cohesive groups (modules) where species interact more often within the same module than with species in other modules22. These modules provide insights into the phylogenetic history and trait convergence of unrelated species, resulting from local co-adaption, and ecological convergence in the use of resources23. By measuring multilayer modularity, the connectivity between layers, i.e., interlayer edge strength, is explicitly accounted for, with the advantages of detecting modules that span across layers. It also allows the identification of nodes that can belong to different modules in different layers, thus particularly relevant for maintaining the continuity of ecosystem functions in space or time12,24. However, the ideal way to quantify interlayer edge strength is still a matter of research in multilayer network research, and the investigation of the relative importance of intra- and interlayer processes is essential to understand the structure of multilayer networks12,24,25.
Contrarily to the high spatial turnover in species and interactions, the functional role of species is considered relatively stable26. Centrality measures have been largely used to assess the topological position of a species in the structure of networks27,28. In a multilayer context, such overall centrality can be estimated with Google’s PageRank29 algorithm, which has been successfully used29 to guide conservation strategies30.
Here, we investigate how a mutualistic multilayer network is structured across habitats and the importance of species to the cohesion of seed dispersal across a complex landscape. To this end, we collected seed–dispersal interactions across the Gorongosa National Park, Mozambique, to build the most complete, seed–dispersal network of the African continent to date31, including all potential guilds of seed dispersers. Gorongosa underwent a severe defaunation that affected many of the large herbivores, and its recovery is now en route32,33. In this context, seed–dispersal is particularly vital for plants to recolonize newly available patches or disturbed ground34, and is likely a key driver of long-term habitat dynamics in Gorongosa patchy landscapes35. Our objectives are twofold. First, we aim to explore the spatial distribution of seed–dispersal modules (i.e., communities of tightly interacting plants and their dispersers) spanning across the different habitats of the Gorongosa National Park. We will do so by evaluating the modularity of multilayer networks formed by discrete, yet interconnected layers representing different habitats. We used different null models to explore how the strength of the interlayer connectivity affects the overall structure of the spatial multilayer network, and to what extent this multilayer approach improves the currently used monolayer analyses of disconnected and aggregated networks. Second, we aim to assess the relative contribution of each disperser species to the cohesion of seed dispersal across habitats. We will do so by exploring dispersers multilayer versatility, which expresses their contribution to the mobile link function both within and between habitats. We discuss the potential of this new metric by comparing it to the information provided by traditional species-level descriptors.
Results
Overview of seed dispersal in Gorongosa
During this one year, we collected 1399 fecal samples (1174 mammal dung piles and 236 bird droppings) produced by 98 animal species, of which 508 (29%) had at least one undamaged seed. Overall, 12,159 undamaged seeds from 94 plant species were retrieved from the feces of 29 dispersers, comprising 508 links. Focal observations produced 85 further links (14% from the total), whereas camera traps contributed with 15 new links (2.5%). In total, we compiled 608 links between 32 animal species and 101 plant species, in four habitats (Fig. 1).
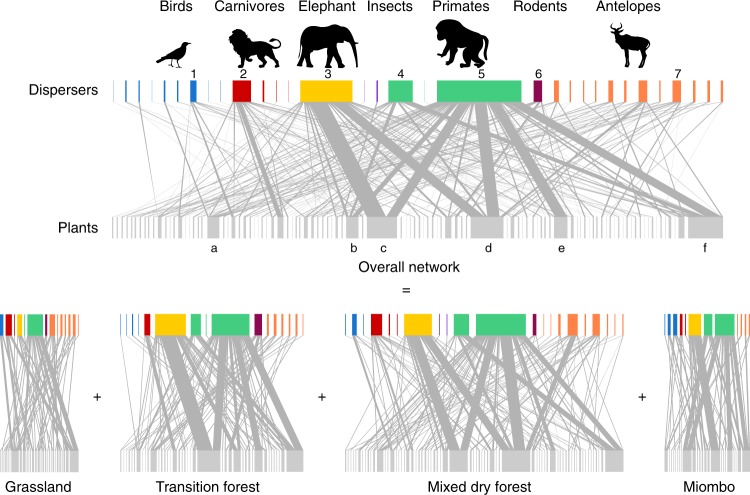
Fig. 1.
Quantitative seed–dispersal network of the Gorongosa National Park, Mozambique. Both the aggregated (top) and the individual habitat (bottom) networks are based on the same sampling effort and are represented on the same scale. The boxes in the top level represent disperser species and those on the bottom level represent the plant species dispersed. The gray lines linking the two levels represent pairwise species interactions, and their width proportional to the interaction frequency. The aggregated network was obtained by pooling all interactions across the four habitats, and summing their frequencies. Main seed dispersers: 1. Pycnonotus tricolor, 2. Civettictis civetta, 3. Loxodonta africana, 4. Cercopithecus pygerythrus, 5. Papio ursinus, 6. Hystrix africaeaustralis, and 7. Redunca arundinum. Most commonly dispersed plants: a Centaurea praecox, b Grewia inaequilatera, c Hyphaene natalensis, d Sclerocarya birrea, e Tamarindus indica, and f Ziziphus mucronata. The full list of species can be seen in Fig. 3 and Supplementary Fig. 2, for animals and plants, respectively. The silhouettes used in this figure are all sourced from Open Clipart and were made available under a CC0 1.0 licence
Overall, primates were responsible for most interactions, namely Papio ursinus (chacma baboon, 35%) and Cercopithecus pygerythrus (vervet monkey, 10%), followed by Loxodonta africana (elephant, 22%) and Civettictis civetta (African civet, 7%) (Fig. 1). The three most commonly dispersed plant species represented 41% of all recorded interactions, namely Ziziphus mucronata (Rhamnaceae, 15%), Sclerocarya birrea (Anacardiaceae, 13%), and Hyphaene natalensis (Arecaceae, 13%).
We estimated that our sampling effort captured 77% of the disperser species and 44% of the plants with similar levels of sampling completeness across the four habitats (Supplementary Fig. 1 and Supplementary Table 1).
Modular structure of the spatial multilayer network
To evaluate the extent to which the seed–dispersal interactions are sorted into distinct communities of tightly interacting species36, we calculated the multilayer modularity24,37 of the spatial network of Gorongosa (see “Methods” section and Supplementary Methods for details on the multilayer modularity algorithm). Using a multilayer formalism13, this network is defined by the animal seed–dispersal interactions (intralayer links) in each habitat (layer), with habitat connectivity (interlayer links) provided by the common species. Ultimately, interlayer links should be interpreted as the movement of matter or energy between layers, in our case the effective movement of animals and seeds between habitats, and quantified in a way that estimates the intensity of these movements (interlayer strength). Multilayer modularity was calculated across a range of interlayer strength (0–10), assuming that any co-occurring species between habitats effectively connected them with the same intensity, to test how the structure of the spatial network is affected by habitat connectivity. The multilayer modularity of the Gorongosa seed–dispersal network was very high across the whole range of values of habitat connectivity (Qmultilayer = 0.903–0.993; Fig. 2a), with an overall increasing trend toward an asymptote just below 1. We used two null models (see “Methods” section for details) to test how the structure of the spatial network is influenced by the seed–dispersal process within each habitat (intralayer null model) and by the identity of the animals connecting these habitats (interlayer null model). The structure of the empirical network, across the range of habitat connectivity values, was statistically different than that predicted by both null models, though in opposite directions: reshuffling interactions within each habitat (intralayer null model) overestimated modularity, whereas reshuffling the identity of the habitat-connecting animals in each habitat (interlayer null model) underestimated modularity (Fig. 2a). The identity of the dispersers and the intensity of movements between habitats (interlayer strength) play a more important role for the spatial structure of the seed–dispersal network than the pattern of seed dispersal within each individual habitat. Nonetheless, the modularity predicted by both null models tended to converge to that of the observed network at very high values of interlayer strength (Fig. 2a and Supplementary Data 1), indicating an increasing importance of random processes in structuring the networks. This suggests that when habitat connectivity is very high the overall network structure becomes less determined by the identity of animals connecting them, and might be more contingent on the structure of seed dispersal within habitats.
Fig. 2.
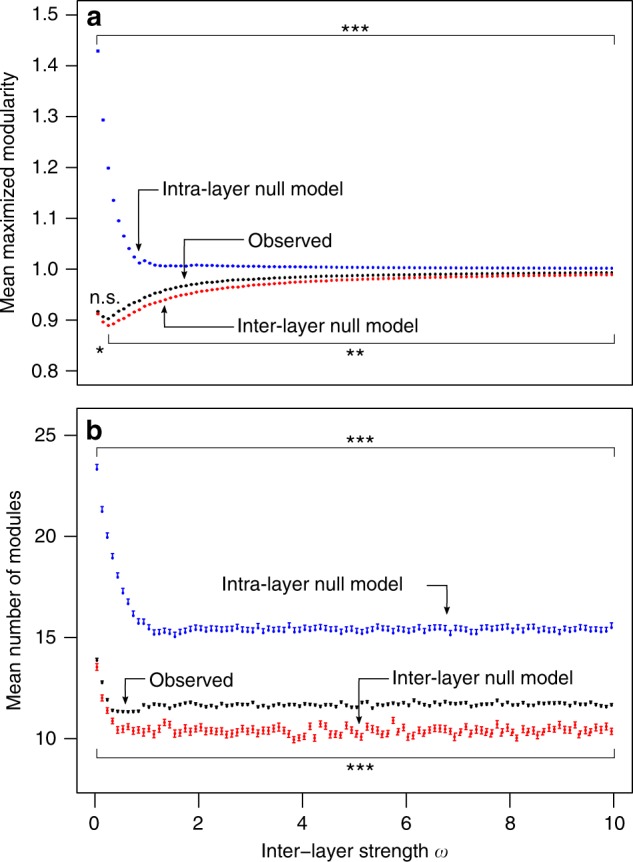
Modularity and number of modules observed in empirical networks and predicted by two different null models. Mean maximized modularity (a) and mean number of modules (b) of the observed networks (black) across the range of interlayer strength (0–10), and comparison against the two null models: intralayer null model (blue), and interlayer null model (red symbols). Values presented as the mean (±SEM) of 100 runs of the modularity function, for each interlayer strength. The significance of the observed modularity and number of modules was compared against those of the null networks with a one-sample t test. *p < 0.050, **p < 0.010, ***p < 0.001, n.s. non-significant. Full results are presented in Supplementary Data 1
To understand the added value of the multilayer approach in relation to the traditional monolayer approach, we compared the results from the multilayer analysis with those provided by the currently standard approaches of either merging all data into a single aggregated network (Qaggregated), in which interactions occurring at multiple habitats are summed across habitats, or considering each habitat as a discrete and disconnected network. The structure of the aggregated network is influenced by the distribution of the interactions among the species, with modularity being significantly lower than predicted by the intralayer null model (mean Qaggregated = 0.43 vs. Qnull models = 0.59, p < 0.001; Supplementary Fig. 3). However, it ignored habitat connectivity because it cannot incorporate such information. In the disconnected network, habitats are considered totally independent from each other, thus equivalent to calculate modularity for each of them38. Modularity was similar or slightly higher than that of the aggregated network and much lower than that of the multilayer network, ranging from 0.43, in the Mixed forest, to 0.56, in the Grassland (Fig. 2 and Supplementary Fig. 3).
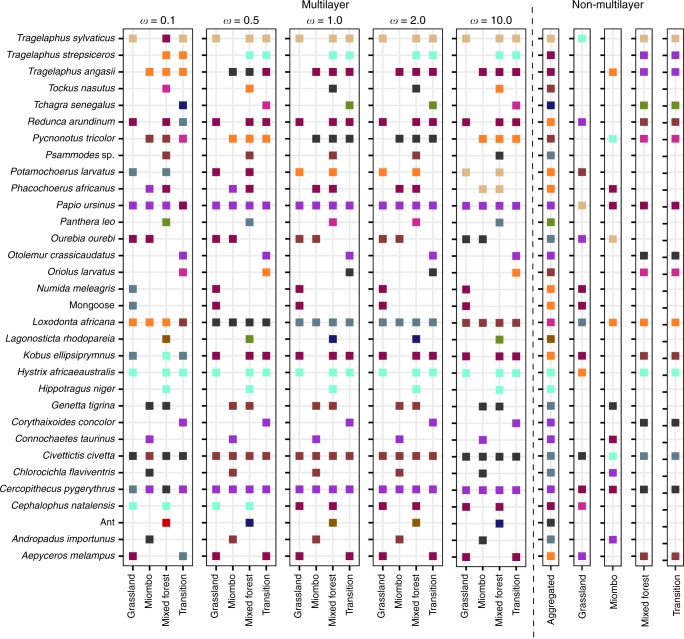
The number of modules detected in the multilayer structure is mostly constant, oscillating between 11 and 12 across most values of interlayer strength, except for very small values, where some additional modules were detected (Figs. 2b, 3, and 2). The intralayer null model consistently predicted more modules than observed, while the interlayer null model consistently predicted fewer modules than observed, across the whole range of interlayer strength (Fig. 2b and Supplementary Data 1). In the aggregated network, the average number of modules detected was 11.4, which was in line with those detected in the multilayer network (Supplementary Fig. 3 and Fig. 2), and significantly higher than those predicted by the intralayer null model (mean modules observed = 11.4 vs. null model = 9.2; t(99) = −28.60, p < 0.001). The mean number of modules in each habitat of the disconnected network was variable and ranged from 6 to 13 (Supplementary Fig. 3 and Fig. 3). In the spatial multilayer network, modules are subsets of species that strongly interact across the different layers of the network25,39. For animals, this corresponds to species that occur and disperse seeds from the same plant species in more than one habitat (Fig. 3 and Supplementary Fig. 2). For example, most primates (baboon, vervet monkey, and Otolemur crassicaudatus (bush baby)) all disperse Z. mucronata and are consistently placed in the same module in the multilayer and in the aggregated networks, but not when habitats are weakly connected or considered independent. It is worth to note that module affiliations do not necessarily group phylogenetically related species, but species that feed on similar resources, which in seed dispersal might be mostly determined by behavioral and morphological constraints (e.g., Corythaixoides concolor, the go-away bird, is consistently assigned to the same module of the bush babies, Fig. 3).
Fig. 3.
Module affiliation of animal species in the spatial multilayer network of Gorongosa. Module affiliation is shown for five different interlayer edge strengths (left block), and for the monolayer networks, considering either the aggregated network or each habitat individually (right block). In each case, the run with the highest maximized modularity was used (module affiliation for plant species is shown in Supplementary Fig. 2). Within each network, different colors represent different modules. Colors in different blocks are independent
The strength of each interaction can vary across habitats, reflecting different animal resource preferences in different contexts, and therefore, species can change their module affiliation between habitats24,38. We calculated species adjustability as the proportion of animal or plant species that switch module affiliation at least once between any pair of habitats12. Most species do not change module affiliation across habitats, exhibiting a relatively low or non-existing adjustability (Supplementary Fig. 4). When the intensity of species movement between habitats (interlayer strength) is low, animals and plants tend to interact with distinct set of species in each habitat and a higher proportion of species will change their module affiliation between habitats. As the intensity of these movements intensifies, and habitat connectivity increases, species adjustability becomes negligible and interactions tend to occur among the same species across all habitats. However, this stabilization on interaction partners happens at different levels of habitat connectivity for animals and plants (Fig. 3; Supplementary Fig. 4; and Supplementary Data 1). For both animal and plant species, adjustability was generally more affected by the identity of the animal (interlayer null model) than by the pattern of interaction within habitats (intralayer null model). For low interlayer strength, animal adjustability was significantly lower than predicted by the intralayer null model, but higher than predicted by the intralayer null model (Supplementary Fig. 4). However, both null models performed better at greater values of interlayer strength. The interaction pattern within habitats (intralayer null model) had a variable effect on plant adjustability: the observed plant adjustability was significantly higher for very low, but also for high habitat connectivity, but lower observed adjustability between these values. The interlayer null model consistently predicted significantly higher plant adjustability for lower interlayer strengths (Supplementary Fig. 4). Thus, animals are more likely to disperse the same plant species across habitats than plant species are to rely on the same dispersers, and animal movement across the landscape exerts a stronger influence in the spatial structure of the seed–dispersal network.
Contribution of disperser species to seed dispersal cohesion
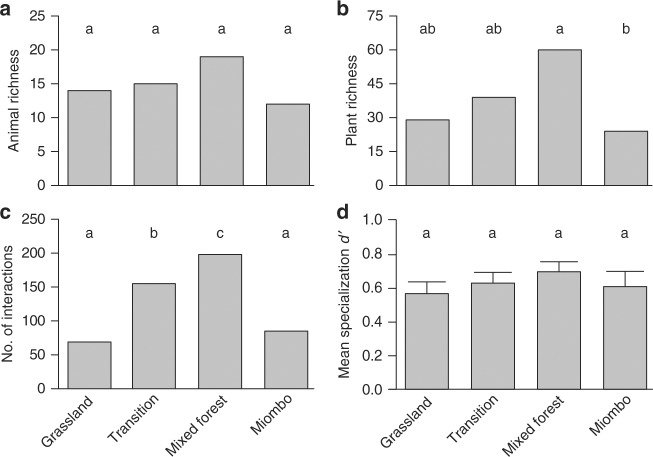
We did not detect differences on animal species richness across the four main habitats of Gorongosa (G test: G3 = 1.84, p = 0.61; Fig. 4a and Supplementary Table 2). Mixed forest holds a greater richness of plants than the other three habitats, but this was only significant in comparison to Grassland and Miombo (Fig. 4b and Supplementary Table 2). As for richness of interactions, Mixed forest had more interactions than Transition forest, and both habitats had more interactions than Grassland and Miombo (all pairwise G tests: p < 0.002; Fig. 4c and Supplementary Table 2). Dispersers’ specialization did not differ significantly among habitats (Χ2 = 2.49, df = 3, p = 0.49; Fig. 4d and Supplementary Table 3).
Fig. 4.
Network- and species-level descriptors of the interactions from each habitat of Gorongosa. Differences among the main habitats of Gorongosa in terms of a animal species richness, b plant species richness, c number of interactions, d species specialization (mean ± SEM). Different letters indicate statistical pairwise differences: a–c, pairwise G tests (see Supplementary Table 2 for full results); d, generalized linear mixed models (63 observations/occurrences of 32 animal species in four habitats; Supplementary Table 3 for full results)
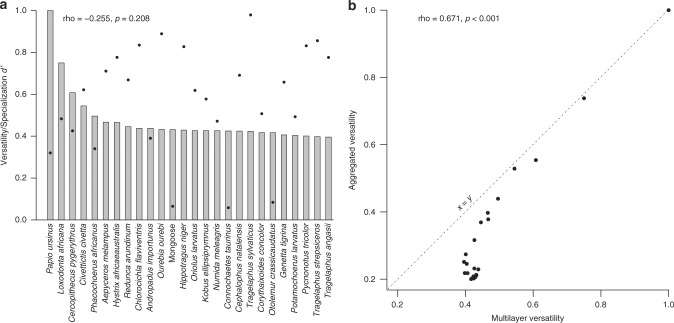
We calculated each disperser multilayer versatility, which is equivalent to an overall measure of centrality to identify those that are topologically important to the structure of the spatial network29. For this effect, we used a unimodal projection of the network, in which two animal species are connected if they disperse the same plant species40, thus providing an insight over their likely “functional redundancy”41. Links between species were quantified by weighting the number of shared interactions by the assemblage size42,43, minimizing the loss of information associated with unimodal projections44. Multilayer versatility revealed that few dispersers are disproportionately important, namely the baboon and the elephant, followed by a long tail of species with lower versatility (Fig. 5a). The importance of these species comes from being central in the structure of the seed–dispersal network because they share plant partners with many other animals, but also because they share plant species across different habitats. The versatility of dispersers in the multilayer network was correlated with their versatility in the aggregated network (rs = 0.671, p < 0.001; Fig. 5b and Supplementary Table 4), but the importance of species with low versatility is underestimated in the aggregated network (Fig. 5b). There were relatively few shared links among habitats (total edge overlap = 8.2%). However, all habitat pairs, except Miombo and Grassland, shared more than 20% of the interactions (Fig. 6).
Fig. 5.
Correlates of multilayer versatility. Correlation between animal species versatility in the multilayer network (bars) and the mean specialization d’ (dots) (a), and between multilayer versatility of the monolayer versatility of the aggregated network (b). Full data available in Supplementary Table 4
Fig. 6.
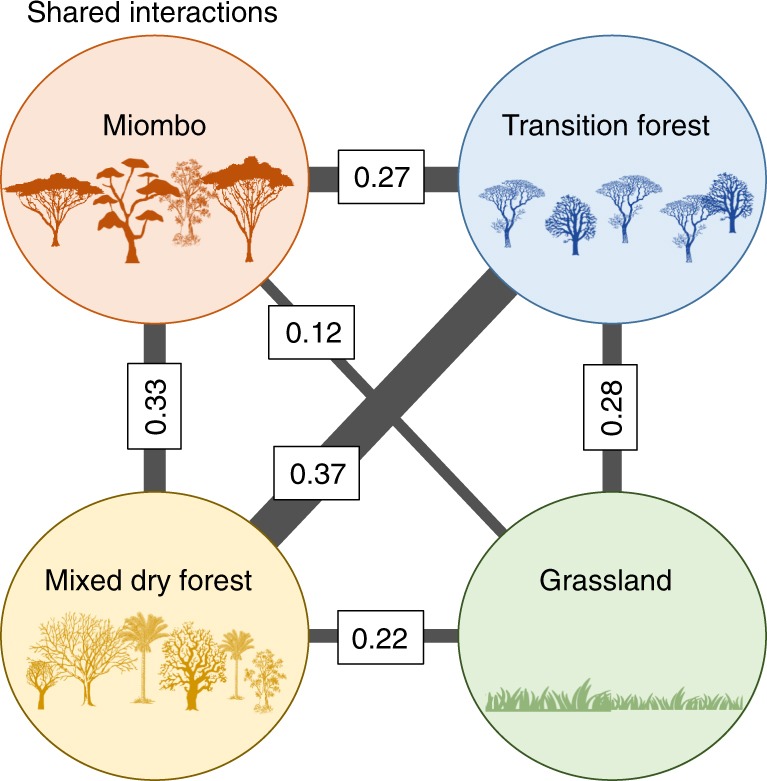
Similarity in terms of shared interactions, between the habitat pairs of Gorongosa. Similarity is assessed using the edge overlap between the habitats in the spatial multilayer network, i.e., proportion of shared links across habitats. The width of the links is proportional to the correlation between each habitat pair. The silhouettes used in this figure are all sourced from Open Clipart and were made available under a CC0 1.0 licence
We evaluated if the information condensed by multilayer versatility could be captured by other species-level metrics, namely specialization d′, number of habitats, and species multistrength. We did not find a significant correlation between multilayer versatility and both dispersers mean specialization (d′) and the number of habitats where they occur (rs = −0.255, p = 0.208, Fig. 5a; rs = 0.383, p = 0.053, respectively, Supplementary Table 4). However, dispersers multistrength was only moderately correlated with their importance (i.e., versatility) on the multilayer network (rs = 0.514, p = 0.007; Supplementary Table 4). Species multistrength extends the concept of its monolayer counterpart, expressing the total number of links of a species across all layers of the network19, i.e., the total shared interactions with all its neighboring species across the habitats. However, contrary to versatility, multistrength does not account for the distribution of these links in relation to the other species, or the number of layers in which these links occurs. Thus, although both metrics are related, multistrength will not reflect the importance of a species for the overall structure of the multilayer network as much as versatility.
Discussion
Species and communities are not randomly distributed across the planet, but they are strongly structured by spatial attributes traditionally recognized by ecologists (e.g., niches, habitats, landscapes, and biomes). Traditionally, species interaction networks have been studied as discrete entities with borders defined by the researchers based on different landscape attributes. However, species interactions do not abruptly finish at habitat borders, and therefore the decision of merging or segregating data from these spatial units is far from trivial. Nevertheless, ecologists are still faced with a paucity of tools to evaluate when such combination of data is useful, or when it might increase the noise around the patterns of interest, thus obscuring important conclusions. Although still based on the recognition of different habitats, the implementation of a multilayer approach provides a valuable tool that allows for better decisions regarding the merits of segregating or merging spatially (or temporal) explicit data.
However, interlayer connectivity has never been explicitly incorporated in the analysis of the modular structure of spatial ecological networks. In this study, we investigate the spatial structure of a seed–dispersal network spanning across multiple habitats explicitly considering interlayer connectivity. We made use of a highly comprehensive data set collected in a highly diverse African landscape including all potential disperser guilds. This adds to the sparse knowledge of seed dispersal in Africa, but has direct implications for our understanding of seed–dispersal networks across the globe.
Our results show a highly modular structure of the spatial multilayer network that is influenced by the strength of the connectivity between habitats, with about half the communities of seed dispersers detected bridging most of the habitats (Figs. 3 and 4). The network is dominated by a few highly versatile species that secure both local (habitat level) and global (landscape level) dispersal of seeds, ensuring the spatial continuity of the seed–dispersal process.
Landscapes are intrinsically dynamic, being constantly shaped by local disturbance and ecological succession45. Understanding how animals move between habitats providing key mobile links10, and how ecological interactions are distributed across habitats15,16,18, has long been recognized as critical for the dynamic of patchy habitats across complex landscapes35. Nevertheless, and despite the current interest on species interactions networks, these are yet to explicitly accommodate this interlayer dynamic when analyzing the structure of spatial networks.
Here, we implement for the first time a multilayer approach to evaluate the spatial structure of an ecological network explicitly incorporating the interlayer strength connecting networks from adjacent habitats. Our spatial multilayer seed–dispersal network exhibited a highly modular structure, i.e., species tend to interact with subsets of species (i.e., modules) within subsets of spatially coupled habitats. By explicitly including non-zero interlayer links, i.e., the habitat connectivity promoted by the common species, it is possible to account for the interdependence of the network structure across multiple habitats38, and identify modules that spread across habitat borders25.
For a more realistic module detection, interlayer strength should ideally be measured empirically to reflect the effective movement of the individual species across habitat borders15. Unfortunately, obtaining such data at the community and landscape levels, i.e., all species, across all habitats can be incredibly challenging. The alternative of assigning the same interlayer strength to all species, i.e., assuming that all species connect habitats with the same intensity, is a clearly undesirable simplification as species connect habitats with different intensities because of their differential ability to move across and establish in a given habitat46,47. Incorporating such empirical data could have important implications on modules found by the modularity function; the relative importance between intra- and interlayer process would be different for each individual species, thus affecting its probability of changing module affiliation38. Exploring the modular structure across a range of interlayer edge strengths is an alternative to obtaining empirical data, and has been often done in other fields to understand its importance for processes spanning across different layers24,25,38,48. Our analysis revealed that the modularity and the number of modules are mostly affected at extremely low levels of interlayer strength, suggesting that the spatial community structure can be maintained even if the strength of the habitat connectivity is low relative to the strength of the interactions within the habitats. The inter- to intralayer edge strength ratio is essential for the outcome of modularity estimation, affecting the probability of nodes being assigned to distinct modules38,48. As such, when interlayer coupling increases, the number of detected modules is expected to decrease24,25. Importantly, the structure of the seed–dispersal network was not fully captured by the aggregated network or by considering each habitat as an independent network. Consequently, the result obtained by using a multilayer approach is not biased by any decision regarding aggregating or disconnecting the different layers of the network. Instead, the resulting structure is a consequence of the relative importance of the processes occurring within and between layers, which is objectively defined by the relative strength of the inter- to intralayer edges.
Regarding the modules' composition, these grouped-together species that are not always phylogenetically close (e.g., primates were grouped with the go-away bird), suggesting that functional and morphological matching, such as gape-size and seed/fruit size, are more important drivers of seed–dispersal interactions40. Interestingly, we detected low adjustability for most species and module affiliations remained mostly constant across habitats. Module switching occurred only for some species (e.g., primates, elephants, or civets), and at very low values of habitat connectivity (Fig. 3). Most animals, however, tend to disperse the same plant species in different habitats, thus maintaining a similar functional role across the landscape26, even if habitat connectivity is very low. This can only be detected if the habitats are explicitly linked in the analysis of network structure. Resource availability largely determines animal movements at the landscape level17. In turn, the inter-habitat movement of dispersers is likely to affect plant regeneration dynamics, and thus resource availability. The capacity of species to adjust their interactions to specific contexts (thus increasing overall adjustability) is likely important for species persistence in changing environments, while at the same time tends to promote a greater connectivity (e.g., seed dispersal) across habitats. In Gorongosa, some of the species that changed module affiliation have generally wide-range movements and can distribute seeds between habitats, thus giving a key contribution to plant genetic diversity and spatial distribution of plant populations through seed dispersal34,49. This is particularly true for intrinsically large-scale processes, such as seed dispersal, while other processes, such as belowground mycorrhizal associations, seem to be structured at much finer scales50.
Although the number of interactions differed among habitats, and the overall edge overlap was relatively low, neither the richness of dispersers, the mean number of dispersed plants, or dispersers’ specialization varied significantly.
Multilayer versatility allowed us to identify the animal species that are most important for dispersing seeds simultaneously at the local/habitat scale and at the global/landscape scale by linking multiple habitats. In the Gorongosa landscape, primates, elephants, and African civets are central nodes in the network and likely important for its stability and cohesion51. Although the versatility of the aggregated network can correctly identify the most important dispersers, it underestimates the importance of species that are restricted to one or a few habitats29 (e.g., Genetta tigrine (genet) or Tragelaphus strepsiceros (kudu); Supplementary Fig. 5). The relatively low correlation between multilayer versatility and multistrength, and the non-significant relationship with the number of habitats where each species occurs and its specialization (d′) reflect the information gain of using multilayer versatility, which could not be captured by conventional metrics.
The most versatile seed dispersers of Gorongosa were those switching module affiliation between habitats. They are known for incorporating a high proportion of fruits into their diets52–54, having relatively long gut retention times, and traveling for long distances53–55. This allows seeds to escape the high intraspecific competition near their parent plants by diversifying the deposition site of ingested seeds, and thus increasing their chances of successful recruitment56. Baboons are ubiquitous in Gorongosa32 and assume a central role as seed dispersers across the whole park, and elephants, whose populations are still recovering in Gorongosa32, are also essential to the dispersal of plant species, particularly those with very large fruits and seeds (e.g., H. natalensis or Borassus aethiopum)57. Surprisingly, despite being locally abundant and often considered important seed dispersers in many ecosystems58, birds had a low versatility. While bird versatility might have been underestimated as a result of the different sampling method used (mist-netting), the low proportion of bird droppings with seeds (only 7 out of the 96 bird species captured where found to disperse seeds, and these were only found in 19 out of the 236 bird droppings analyzed), and the consistently low sampling completeness estimated to all sampling methods (transects: 25%, mist-netting: 13%, and focal observations: 13%) suggests that the lower bird versatility is actually structural rather than a sampling artifact. Potential biases may emerge if any particular animal or plant groups are under or oversampled due to the use of different sampling methods. This problem might be countervailed by performing analysis on rarefied or unweighted networks, though these are subjected to their own caveats. We are only aware of a single study that explored the potential consequences of merging data originated from different sampling methods in the assembly of seed–dispersal matrices59; the authors concluded that this was in fact beneficial due to the complementarity of the methodologies. Moreover, it must be noted that the consistently low estimates of sampling completeness are likely to be largely underestimated for at least two reasons: first, because species accumulation curves are based on the assumption that communities are closed, an assumption that is not met in year-round studies, where new species “enter” the community of potential interactions as a consequence of advancing phenology (i.e., fruiting season); and second, because a large proportion of the potential interactions will never be detected because they are not really possible due to spatial, temporal, and phenological mismatch between species60,61. These “forbidden links” (or “true zeros”) can amount to a large proportion of the unobserved potential links (up from 44 to 77%) in seed–dispersal networks61.
Interestingly, the significant edge overlap between habitat pairs (i.e., the proportion of shared links) confirms the a priori assumption that seed dispersal does not stop at habitat borders. Taken together with the results from multilayer modularity, showing that species tend to maintain their module affiliation throughout the landscape, these results suggest that species traits (such as mobility and size) may largely determine their multilayer versatility.
The effective conservation and restoration of natural areas requires an integrated view of how species and their interactions maintain functional ecosystems on complex landscapes, and a multilayer approach is a most valuable tool to explore these factors. Here, we took a step further in the analysis of spatial mutualistic networks, and using interlayer edges strength, we explicitly considered the interactions between plants and their dispersers across multiple habitats in the analysis of the network structure. Furthermore, we identified key spatial coupler species, which play a pivotal role in long-term vegetation dynamics in Gorongosa by ensuring the dispersal of genes across the landscape. These key spatial couplers, namely primates, elephants and African civets, should be highly regarded in the protection of this essential ecosystem service.
As many other types of networks, mutualistic networks are not temporally static, and they do not abruptly stop at habitat borders7. Therefore, forcing the analysis of biotic interactions into spatially delimited “network snapshots” and ignoring habitat connectivity will inevitably limit the insights that can be gained by this approach8. Here, we show that a multilayer approach can be used to link ecological processes that occur in different spatial layers of a network providing insights that may not be captured using traditional representations of monolayer networks. Overlooking the multiple relationships between nodes on different layers may lead to an inaccurate network structure, but also to misidentification of the real role of species in the whole-network structure19,24,29. Alternatively, the explicit incorporation of the temporal and spatial dynamics into a multilayer network approach represents an important next step in the study of animal-plant interactions.
Methods
Field site and sampling
This work was carried out in the Gorongosa National Park, Mozambique (hereafter Gorongosa), a hyperdiverse park62 covering 4067 km2 at the southern end of the Great Rift Valley (18°47′43.2d″S 34°28′09.1″E). We defined four major habitats based on the vegetation structure and flooding regime: (1) grassland, periodically inundated grassland, with few shrubs and virtually no trees; (2) Transition forest, characterized by short flooding periods and dominated by trees of Faidherbia albida, H. natalensis, or Acacia xanthophloea with a mostly open understory; (3) Mixed forest, occasionally flooded and formed by a diverse mixture of tree species with a dense and closed understory; and 4) Miombo, one of the most extensive habitats in Africa, unaffected by floods, and dominated by Brachystegia spp. trees with a dense understory (see ref. 50 for details). Throughout a year, we reconstructed seed–dispersal interactions from the four habitats by retrieving intact seeds from animal dung collected in the field. Sampling took place in 12 occasions, evenly spaced between June 2014 and May 2015, except from December to February when floods make the park mostly inaccessible. In order to sample all potential disperser guilds, we employed complementary sampling protocols. In each sampling occasion, one transect ca. 2000 m and 5 m wide was performed in each habitat and separated from any other transects by at least 350 m. Overall, 48 transects were run (ca. 96 km), corresponding to a surveyed area of 480,000 m2. A team of two observers simultaneously collected animal dung samples, corresponding to the deposition of a dung pile of a single animal, and identified the disperser species by direct observation of defecating animals, or using the expertise of local park rangers and field guides63,64, and recorded direct observations of animals ingesting fruits. Bird dispersal was evaluated by collecting droppings from birds captured during mist-netting sessions on each sampling occasion, run for 5 h after dawn. Birds were kept inside individual holding bags, and released after producing a dropping65. All samples were carefully screened under a ×40 magnifying microscope, and all undamaged seeds identified against a reference collection of seeds/fruits collected in the field. Seeds that could not be identified visually were barcoded, and their DNA sequences compared against online databases, with species identified based on the best match and on a checklist of Gorongosa plants33 (see Supplementary Information for details). Most seeds (92%) were identified to the species level, 7% to genus level, and less than 1% to the family level or grouped into morphotypes, hereafter referred to as “species” for simplicity. Sampling was further complemented with the analysis of motion-triggered camera traps, operating for five nights per habitat per sampling occasion to record interactions from non-conspicuous animals or those feeding at night. We estimated sampling completeness for animal and plant species in each habitat. The use of different methods to record interactions could result in different interaction sampling completeness, thus we also estimated completeness of interaction sampling for each method. This was done by estimating the proportion of observed richness in relation to the total asymptotic richness estimated by the non-parametric estimator Chao266 using function specpool from package vegan67, in R software68.
Multilayer network construction
We assembled a multilayer quantitative bipartite seed–dispersal network for each habitat, which were visualized using the R package bipartite69. Interaction frequency was quantified by calculating a pooled frequency of occurrence, collating the information from the different sources (fecal analysis, mist-netting, camera traps, and direct observations). This pooled frequency of occurrence, resulted from the direct sum of all fecal samples containing at least one seed of a given plant species5,33,70, the number of transects where a given focal interaction was detected, and the number of camera-trap recordings (1 night = 1 sample), where a given interaction was detected.
Using a multilayer formalism13, we assembled a multilayer network formed by: (a) a set of nodes (called “physical nodes”) representing the animal dispersers and the plant species whose seeds are dispersed, (b) a set of layers representing the different habitats; (c) a set of “state nodes” that correspond to the manifestation of each node on a given layer; and (d) a set of two types of edges connecting the nodes pairwise, namely, intralayer edges (i.e., animal seed–dispersal interactions); and interlayer edges, connecting state nodes between layers (i.e., animal or plant species between habitats). Interlayer edges encode the movement of animal and plant species between habitats. While the temporal scale of the movements is not exactly the same, both animal and plant genes frequently cross and establish in neighboring habitats71, and for that reason can be considered effective habitat connectors with the strength of the interlayer links quantifying the intensity of the habitat coupling provided by this movement. Spatial multilayer networks have a categorical (non-ordinal) coupling, i.e., interlayer edges are not constrained in any specific order and any pair of layers can be connected13. The quantification of the interconnectivity of the multilayer network is used in the calculation of some (modularity and multistrength), but not all the network diagnostic (versatility and edge overlap).
To assess differences in the richness of animals, plants, and plant–animal interactions among habitats, we used a G test. If an overall effect was present, we performed pairwise G tests to identify differences between habitat pairs. This analysis was performed with the R package RVAideMemoire72.
Modular structure of the spatial multilayer network
To evaluate the extent to which the seed–dispersal interactions of Gorongosa are sorted into distinct communities, we calculated multilayer modularity (Qmultilayer). Multilayer modularity, as its application to monolayer counterparts, quantifies to what extent nodes are organized into modules of strongly interacting nodes interacting more frequently than expected by chance21. The Q modularity function was maximized applying a “generalized Louvain” method37. This method proceeds until the network configuration that maximizes the weight of the edges inside the modules in relation to a null model is found (see Supplementary Information for details).
Following Pilosof et al. 12, the modularity function was adapted to reflect the bipartite nature of our networks. The “generalized Louvain” method requires the specification of two parameters: the resolution limit γ, and the interlayer coupling ω. The resolution limit defines the detail to which the network will be resolved into communities, and can be viewed as the importance given to the null model compared to the empirical data38, and we used the default resolution γ = 124,38. The interlayer coupling quantifies the strength of the connection between layers of a network, i.e., the effect that species have in connecting the different layers. However, measuring such interlayer strength is intrinsically challenging12 and there is no absolute method to do so. To explore the importance of interlayer strength for the detection of modules, we calculated modularity along a range of values of interlayer strength (from 0 to 10), assuming uniform interlayer strength across all species, i.e., all species connecting any two pair of habitats have the same effect in the interlayer process38. The stochastic nature of this algorithm means that a different maximum is reached on each run, thus we run the modularity function 100 times, and averaged the results obtained12,25. We compared the results obtained with a multilayer network to that of two different representations of the same network: (a) an aggregated network (Qaggregated), where all interactions across the different layers were pooled to create one overall aggregated network, with the frequency of interactions that occur in multiple habitats being summed, and (b) a disconnected network where habitats are considered fully independent from each other, i.e., interlayer strength is set to zero, and thus modularity is calculated for each of them38.
We test the modular structure of the observed network comparing it to the structure of networks built under the assumptions of two null models12,25: (1) an “intralayer null model” that keeps the number of links (i.e., connectance), while redistributing the individual interactions across the whole matrix as implemented by r00_both in vegan67, was used to assess the influence of structure within each layer/habitat in the modular structure of the multilayer network25; and (2) an “interlayer model” following the same rationale of the nodal model25, i.e., keeping the same matrix, but redistributing species identities independently in each layer, to assess if the modular structure is dependent on the identity of the species connecting the habitats. We run each null model 100 times along the same range of interlayer strength (0–10). The significance of the observed modularity was compared against the distribution of the modularity of the null networks, and presented as the proportion of networks generated by the null models with modularity lower than the observed networks. For each network, we calculated the mean number of modules and the mean adjustability12 of animal and plant species as the proportion of species in each level of the network that changed module affiliation at least once between habitats. The mean number of modules and adjustability of the observed networks was compared against that of the networks generated by the null models with a one-sample t test12,25.
Multilayer modularity calculations and the interlayer null model were performed in MATLAB73.
Contribution of disperser species to seed dispersal cohesion
First, we assessed whether the specialization of seed dispersers differed consistently between habitats by calculating animal specialization (d′), which quantifies their selectiveness for seeds within the range of resources used74. However, the number of interactions of a species is considered to reflect both resources availability and consumer activity. This metric takes into account the pattern of interaction of a species in relation to the available resources, while being robust to sampling effort, network size and asymmetry74, also see ref. 75. We used a GLMM, with Gamma errors, and included disperser species as a random factor. The model was analyzed with the R package lme476. If any level of the independent variable was significant, we assessed its overall effect with a Wald Χ2 test available in R package car77. The overall fit of the model was assessed with the Akaike’s information criterion against a reduced model, which only included the intercept. Pairwise comparisons between habitats were tested with Tukey HSD test with the R package multicomp78.
Secondly, we explored seed–dispersers’ importance in the network by calculating their overall versatility as seed dispersers. Versatility identifies species that are topologically important for the structure of the multilayer network29. Versatility was calculated using software muxViz 1.079, which adapts the Google’s PageRank algorithm80 to describe the position of a node within the structure of a network based on a random walk between adjacent nodes29 (see Supplementary Information for further details), being equivalent to a global measure of centrality. The implementation of this method requires bipartite networks to be projected onto unimodal networks. While some projection methods entail some loss of information44, we applied a weighted projection which estimates interaction weight based on the proportion of shared interactions (i.e., seed species shared by disperser species) relatively to the total network size42,43, thus minimizing the loss of information. The projection was performed with function projecting_tm from the R package tnet44. This algorithm is particularly suitable to multilayer networks29 as it condensates information on dispersers niche overlap40, based on the importance of their shared dispersed seeds29.
To understand how animals shared their links across the different habitats, we calculated edge overlap with software muxViz 1.079, which quantifies the proportion of common links between animals across habitats.
We evaluated the information condensed by multilayer versatility in respect to the species versatility of an aggregated network, and to other species-level metrics, calculating its correlation with aggregated species versatility, specialization d′, multistrength, and the number of habitats in which a species is present. The specialization index d′ measures the distribution of a species interactions with each partner over the total number of partners available74. Multistrength is an extension of its monolayer version, and sums the total weight of the links incident on a node across all layers, taking into account the links connecting nodes in the different layers19 (see Supplementary Information for details). It expresses the total number of shared interactions of a dispersers species with all its neighboring species across all habitats. Versatility and multistrength were calculated using muxViz 1.079.
Code availability
MATLAB scripts for the estimation of modularity and generating the interlayer null model are available from 10.6084/m9.figshare.4955651, and R code for generating the intralayer null model, analysis of the modular structure, and generating the files to be used with muxViz are available from 10.6084/m9.figshare.4836383.
Data availability
The seed–dispersal interaction network matrices are available on reasonable request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
This work was supported by FEDER funds from the “Programa Operacional Factores de Competitividade – COMPETE” and by national funds from the Portuguese Foundation for Science and Technology – FCT through the research project PTDC/BIA-BIC/4019/2012. S.R.-E., R.H., and M.C. were also supported by FCT (Grants IF/00462/2013, IF/00441/2013, and SFRH/BD/96050/2013, respectively). We thank Greg Carr, and the Greg Carr Foundation – Gorongosa Restoration Project, Dr Marc Stalmans and all the staff from Gorongosa National Park for their logistic assistance during fieldwork, and for sharing their knowledge and passion about Gorongosa.
Author contributions
S.T., R.H., H.F., and S.R.-E. designed the study; S.T. and M.C. collected the data; S.T. led the analysis and wrote the first draft; and all authors contributed to manuscript revisions.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/2/2018
The original version of this Article contained Figshare links in the Code availability statement that were not functional. The correct Figshare links to MATLAB scripts and R code used in this study are https://doi.org/10.6084/m9.figshare.4955651 and https://doi.org/10.6084/m9.figshare.4836383, respectively. These errors have now been corrected in both the PDF and HTML versions of the Article.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-017-02658-y.
References
- 1.Thebault E, Fontaine C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science. 2010;329:853–856. doi: 10.1126/science.1188321. [DOI] [PubMed] [Google Scholar]
- 2.Tylianakis JM, Laliberté E, Nielsen A, Bascompte J. Conservation of species interaction networks. Biol. Conserv. 2010;143:2270–2279. doi: 10.1016/j.biocon.2009.12.004. [DOI] [Google Scholar]
- 3.Jordano P. Chasing ecological interactions. PLoS Biol. 2016;14:e1002559. doi: 10.1371/journal.pbio.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timóteo S, Ramos JA, Vaughan IP, Memmott J. High resilience of seed dispersal webs highlighted by the experimental removal of the dominant disperser. Curr. Biol. 2016;26:910–915. doi: 10.1016/j.cub.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Heleno RH, Ramos JA, Memmott J. Integration of exotic seeds into an Azorean seed dispersal network. Biol. Invasions. 2013;15:1143–1154. doi: 10.1007/s10530-012-0357-z. [DOI] [Google Scholar]
- 6.Allen CR, et al. Quantifying spatial resilience. J. Appl. Ecol. 2016;53:625–635. doi: 10.1111/1365-2664.12634. [DOI] [Google Scholar]
- 7.Bascompte Jordi, Jordano Pedro. Mutualistic Networks. 2017. [Google Scholar]
- 8.Hagen M, et al. Biodiversity, species interactions and ecological networks in a fragmented world. Adv. Ecol. Res. 2012;46:89–120. doi: 10.1016/B978-0-12-396992-7.00002-2. [DOI] [Google Scholar]
- 9.García-Callejas D, Molowny-Horas R, Araújo MB. Multiple interactions networks: towards more realistic descriptions of the web of life. Oikos. 2017;125:336–342. [Google Scholar]
- 10.Lundberg J, Moberg F. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems. 2003;6:0087–0098. doi: 10.1007/s10021-002-0150-4. [DOI] [Google Scholar]
- 11.Knight TM, McCoy MW, Chase JM, McCoy KA, Holt RD. Trophic cascades across ecosystems. Nature. 2005;437:880–883. doi: 10.1038/nature03962. [DOI] [PubMed] [Google Scholar]
- 12.Pilosof S, Porter MA, Pascual M, Kéfi S. The multilayer nature of ecological networks. Nat. Ecol. Evol. 2017;1:101. doi: 10.1038/s41559-017-0101. [DOI] [PubMed] [Google Scholar]
- 13.Kivela M, et al. Multilayer networks. J. Complex Netw. 2014;2:203–271. doi: 10.1093/comnet/cnu016. [DOI] [Google Scholar]
- 14.Olesen JM, et al. From broadstone to Zackenberg: space, time and hierarchies in ecological networks. Adv. Ecol. Res. 2010;42:1–69. doi: 10.1016/B978-0-12-381363-3.00001-0. [DOI] [Google Scholar]
- 15.Frost CM, et al. Apparent competition drives community-wide parasitism rates and changes in host abundance across ecosystem boundaries. Nat. Commun. 2016;7:12644. doi: 10.1038/ncomms12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poisot T, Canard E, Mouillot D, Mouquet N, Gravel D. The dissimilarity of species interaction networks. Ecol. Lett. 2012;15:1353–1361. doi: 10.1111/ele.12002. [DOI] [PubMed] [Google Scholar]
- 17.Bodin Ouml, Norberg J. A network approach for analyzing spatially structured populations in fragmented landscape. Landsc. Ecol. 2007;22:31–44. doi: 10.1007/s10980-006-9015-0. [DOI] [Google Scholar]
- 18.Trojelsgaard K, Jordano P, Carstensen DW, Olesen JM. Geographical variation in mutualistic networks: similarity, turnover and partner fidelity. Proc. R. Soc. B Biol. Sci. 2015;282:20142925–20142925. doi: 10.1098/rspb.2014.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boccaletti S, et al. The structure and dynamics of multilayer networks. Phys. Rep. 2014;544:1–122. doi: 10.1016/j.physrep.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guimarães, P. R., Pires, M. M., Marquitti, F. M. & Raimundo, R. L. eLS 1–9 (Wiley, Hoboken, NJ, 2016).
- 21.Fontaine C, et al. The ecological and evolutionary implications of merging different types of networks. Ecol. Lett. 2011;14:1170–1181. doi: 10.1111/j.1461-0248.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- 22.Guimerà R, Amaral LAN. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donatti CI, et al. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol. Lett. 2011;14:773–781. doi: 10.1111/j.1461-0248.2011.01639.x. [DOI] [PubMed] [Google Scholar]
- 24.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela JP. Community structure in time-dependent, multiscale, and multiplex networks. Science. 2010;328:876–878. doi: 10.1126/science.1184819. [DOI] [PubMed] [Google Scholar]
- 25.Bassett DS, et al. Dynamic reconfiguration of human brain networks during learning. Proc. Natl Acad. Sci. USA. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emer C, Memmott J, Vaughan IP, Montoya D, Tylianakis JM. Species roles in plant–pollinator communities are conserved across native and alien ranges. Divers. Distrib. 2016;22:841–852. doi: 10.1111/ddi.12458. [DOI] [Google Scholar]
- 27.Jordan F. Keystone species and food webs. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:1733–1741. doi: 10.1098/rstb.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín González AM, Dalsgaard B, Olesen JM. Centrality measures and the importance of generalist species in pollination networks. Ecol. Complex. 2010;7:36–43. doi: 10.1016/j.ecocom.2009.03.008. [DOI] [Google Scholar]
- 29.De Domenico M, Sole-Ribalta A, Omodei E, Gomez S, Arenas A. Ranking in interconnected multilayer networks reveals versatile nodes. Nat. Commun. 2015;6:6868. doi: 10.1038/ncomms7868. [DOI] [PubMed] [Google Scholar]
- 30.McDonald-Madden E, et al. Using food-web theory to conserve ecosystems. Nat. Commun. 2016;7:10245. doi: 10.1038/ncomms10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleuning M, Blüthgen N, Flörchinger M, Braun J, HM Specialization and interaction strength in a tropical plant-frugivore network differ among forest strata. Ecology. 2011;92:26–36. doi: 10.1890/09-1842.1. [DOI] [PubMed] [Google Scholar]
- 32.Stalmans, M., Peel, M. & Massad, T. Aerial Wildlife Count of the Parque Nacional da Gorongosa. (2014).
- 33.Correia M, Timóteo S, Rodríguez-Echeverría S, Mazars-Simon A, Heleno R. Refaunation and the reinstatement of the seed-dispersal function in Gorongosa National Park. Conserv. Biol. 2017;31:76–85. doi: 10.1111/cobi.12782. [DOI] [PubMed] [Google Scholar]
- 34.Matlack GR. Plant species migration in a mixed-history forest landscape in Eastern North America. Ecology. 1994;75:1491–1502. doi: 10.2307/1937472. [DOI] [Google Scholar]
- 35.Hanski I. Metapopulation dynamics. Nature. 1998;396:41–49. doi: 10.1038/23876. [DOI] [Google Scholar]
- 36.Guimerà R, Amaral LAN. Cartography of complex networks: modules and universal roles. J. Stat. Mech. Theory Exp. 2005;2005:P02001. doi: 10.1088/1742-5468/2005/02/P02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jutlla, I. S., Jeub, L. G. & Mucha, P. J. A Generalized Louvain Method for Community Detection Implemented in MATLABhttp://netwiki.amath.unc.edu/GenLouvain (2014).
- 38.Bazzi M, et al. Community detection in temporal multilayer networks, with an application to correlation networks. Multiscale Model. Simul. 2016;14:1–41. doi: 10.1137/15M1009615. [DOI] [Google Scholar]
- 39.Pilosof S, Morand S, Krasnov BR, Nunn CL. Potential parasite transmission in multi-host networks based on parasite sharing. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mello MAR, et al. Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos. 2015;124:1031–1039. doi: 10.1111/oik.01613. [DOI] [Google Scholar]
- 41.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opsahl, T. Projecting Two-mode Networks Onto Weighted One-mode Networkshttps://toreopsahl.com/2009/05/01/projecting-two-mode-networks-onto-weighted-one-mode-networks/ (2009).
- 43.Newman MEJ. Scientific collaboration networks. II. Shortest paths, weighted networks, and centrality. Phys. Rev. E. 2001;64:16132. doi: 10.1103/PhysRevE.64.016132. [DOI] [PubMed] [Google Scholar]
- 44.Opsahl, T. Structure and Evolution of Weighted Networks 104–122 (University of London, Queen Mary College, London, 2009).
- 45.Turner MG. Landscape ecology: the effect of pattern on process. Annu. Rev. Ecol. Syst. 1989;20:171–197. doi: 10.1146/annurev.es.20.110189.001131. [DOI] [Google Scholar]
- 46.Hampe A. Plants on the move: the role of seed dispersal and initial population establishment for climate-driven range expansions. Acta Oecol. 2011;37:666–673. doi: 10.1016/j.actao.2011.05.001. [DOI] [Google Scholar]
- 47.Bélisle M. Measuring landscape connectivity: the challange of behavioral landscape ecology. Ecology. 2005;86:1988–1995. doi: 10.1890/04-0923. [DOI] [Google Scholar]
- 48.Bassett DS, et al. Robust detection of dynamic community structure in networks. Chaos. 2013;23:13142. doi: 10.1063/1.4790830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine J, Murrell D. The community-level consequences of seed dispersal patterns. Annu. Rev. Ecol. Evol. Syst. 2003;34:549–574. doi: 10.1146/annurev.ecolsys.34.011802.132400. [DOI] [Google Scholar]
- 50.Rodríguez-Echeverría S, et al. Arbuscular mycorrhizal fungi communities from tropical Africa reveal strong ecological structure. New Phytol. 2017;213:380–390. doi: 10.1111/nph.14122. [DOI] [PubMed] [Google Scholar]
- 51.Jordán F, Liu W, Davis AJ. Topological keystone species: measures of positional importance in food webs. Oikos. 2006;112:535–546. doi: 10.1111/j.0030-1299.2006.13724.x. [DOI] [Google Scholar]
- 52.Ray JC, Sunquist ME. Trophic relations in a community of African rainforest carnivores. Oecologia. 2001;127:395–408. doi: 10.1007/s004420000604. [DOI] [PubMed] [Google Scholar]
- 53.Slater K, du Toit JT. Seed dispersal by chacma baboons and syntopic ungulates in southern African savannas. South Afr. J. Wildl. Res. 2002;32:75–79. [Google Scholar]
- 54.Campos-Arceiz A, Blake S. Megagardeners of the forest - the role of elephants in seed dispersal. Acta Oecol. 2011;37:542–553. doi: 10.1016/j.actao.2011.01.014. [DOI] [Google Scholar]
- 55.Bunney K, Bond WJ, Henley M. Seed dispersal kernel of the largest surviving megaherbivore-the African savanna elephant. Biotropica. 2017;49:395–401. doi: 10.1111/btp.12423. [DOI] [Google Scholar]
- 56.Schupp EW, Jordano P, Gómez JM. Seed dispersal effectiveness revisited: a conceptual review. New Phytol. 2010;188:333–353. doi: 10.1111/j.1469-8137.2010.03402.x. [DOI] [PubMed] [Google Scholar]
- 57.Guimarães PR, Galetti M, Jordano P. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE. 2008;3:e1745. doi: 10.1371/journal.pone.0001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howe HF, Smallwood J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982;13:201–228. doi: 10.1146/annurev.es.13.110182.001221. [DOI] [Google Scholar]
- 59.Escribando-Avila, G., Lara-Romero, C., Heleno, R. & Traveset, A. in Seed Dispersal Networks in the Tropic (eds Datillo, W. & Rico-Gray, V.) (Springer, New York).
- 60.Bascompte Jordi, Jordano Pedro. Mutualistic Networks. 2017. [Google Scholar]
- 61.Jordano P. Sampling networks of ecological interactions. Funct. Ecol. 2016;30:1883–1893. doi: 10.1111/1365-2435.12763. [DOI] [Google Scholar]
- 62.Wilson, E. O. & Naskrecki, P. A Window on Eternity: A Biologist’s Walk Through Gorongosa National Park (Simon and Schuster, New York, 2014).
- 63.Cillie, B. Pocket Guide to Mammals of Southern Africa (Sunbird Publishers, Verlag, 2010).
- 64.Murray, K. D. Scatalog: Quick ID Guide to Southern African Animal Droppings (Struik Nature, Cape Town, 2011).
- 65.Heleno RH, Ross G, Everard A, Memmott J, Ramos JA. The role of avian ‘seed predators’ as seed dispersers. Ibis. 2011;153:199–203. doi: 10.1111/j.1474-919X.2010.01088.x. [DOI] [Google Scholar]
- 66.Chao A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics. 1987;43:783. doi: 10.2307/2531532. [DOI] [PubMed] [Google Scholar]
- 67.Oksanen, J. et al. Vegan: community ecology package R package version 2. 4–1. https://CRAN.R-project.org/package=vegan (2013).
- 68.R Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, 2015).
- 69.Dormann CF, Frund J, Bluthgen N, Gruber B. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2009;2:7–24. doi: 10.2174/1874213000902010007. [DOI] [Google Scholar]
- 70.Vázquez DP, Morris WF, Jordano P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol. Lett. 2005;8:1088–1094. doi: 10.1111/j.1461-0248.2005.00810.x. [DOI] [Google Scholar]
- 71.Shea K. How the wood moves.pdf. Science. 2007;1231:1231–1233. doi: 10.1126/science.1136096. [DOI] [PubMed] [Google Scholar]
- 72.Hervé, M. RVAideMemoire: diverse basic statistical and graphical functions R Package version 0.9-68. https://CRAN.R-project.org/package=RVAideMemoire (2016).
- 73.Bioinformatics toolbox: user’s guide R2016b. Version 9.1.0.441655 (The MathWorks Inc., Natick, Massachusetts, 2015).
- 74.Blüthgen Nico, Menzel Florian, Blüthgen Nils. BMC Ecology. 2006;6(1):9. doi: 10.1186/1472-6785-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poisot T, Canard E, Mouquet N, Hochberg ME. A comparative study of ecological specialization estimators. Methods Ecol. Evol. 2012;3:537–544. doi: 10.1111/j.2041-210X.2011.00174.x. [DOI] [Google Scholar]
- 76.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 77.Fox, J. & Weisberg, S. An R Companion to Applied Regression (Sage Publications Inc., Thousand Oaks, CA, 2011).
- 78.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 79.De Domenico M, Porter MA, Arenas A. MuxViz: a tool for multilayer analysis and visualization of networks. J. Complex Netw. 2015;3:159–176. doi: 10.1093/comnet/cnu038. [DOI] [Google Scholar]
- 80.Brin S, Page L. The anatomy of a large-scale hypertextual web search engine. Comput. Netw. 2012;56:3825–3833. doi: 10.1016/j.comnet.2012.10.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The seed–dispersal interaction network matrices are available on reasonable request.