Abstract
Introduction
Although end-stage renal disease (ESRD) and surrogate markers for renal dysfunction are frequently used as outcome markers for IgA nephropathy, the clinical course after reaching ESRD is not well documented. This study examined outcomes of progression to ESRD and age at death in a cohort of adults with IgA nephropathy with a long duration of follow-up.
Methods
Patient and kidney survival of 251 adult patients with IgA nephropathy from the southeastern United States diagnosed between January 1, 1976 and December 31, 2005 were analyzed.
Results
Median age at diagnosis was 36.9 years. Most patients were men (69%) and Caucasian (95%). Only 46% had an estimated glomerular filtration rate >60 ml/min per 1.73 m2 at diagnosis. Mean follow-up time from time of diagnostic biopsy to death or end of study was 19.3 years. Of 251 patients, 132 (53%) progressed to ESRD and 97 (39%) died. Life expectancy was reduced by 10.1 years, with a median observed age of death at 65.7 years and a median expected age at death of 75.8 years. Eighty-three percent of the deaths occurred after progression to ESRD.
Conclusion
Life expectancy is substantially reduced for patients diagnosed with IgA nephropathy in the southeastern United States.
Keywords: end-stage renal disease, glomerular disease, IgA nephropathy, mortality, nephropathy progression
IgA nephropathy is the most prevalent chronic glomerulonephritis in the world.1, 2 This disease was initially described in 1968, with the first clinical series reported in the mid-1970s.3, 4, 5, 6 Although end-stage renal disease (ESRD) and surrogate markers for renal dysfunction (e.g., doubling of serum creatinine) have been frequently used as outcome markers for the disease, the clinical course after reaching ESRD has not been well documented except in studies that describe recurrence of IgA nephropathy after kidney transplantation.7, 8
We investigated the clinical course of IgA nephropathy for almost 4 decades in a cohort of patients in the southeastern United States.9, 10, 11 The present study examined the outcomes of progression to ESRD and age at death for patients identified in our earlier studies. Increased mortality compared with that in the general population was described for patients with IgA nephropathy in Korea12 and Norway13, but neither study estimated the years of lost life expectancy. We found a 10-year decrease in life expectancy for the patients in our cohort.
Methods
Patients and Clinical Data
The Institutional Review Board of the University of Tennessee Health Sciences Center approved the study. Patient and kidney survival of 251 adults from central and eastern Kentucky who were diagnosed with IgA nephropathy by renal biopsy between January 1, 1976 and December 31, 2005 were analyzed. Diagnosis of IgA nephropathy was based on the presence of IgA as the dominant or co-dominant Ig in a predominantly mesangial distribution by direct immunofluorescence on renal biopsy and the lack of clinical or serological evidence for systemic lupus erythematosus, cirrhosis of the liver, vasculitis, or Henoch-Schoenlein purpura.9
Seventy-nine percent of the patients were diagnosed by private practice nephrologists at community hospitals, whereas the remaining 21% had their renal biopsy at an academic medical center (University of Kentucky Medical Center, Lexington, Kentucky). Ninety-five percent were admitted for the sole indication of a diagnostic renal biopsy. Nine patients had their biopsy during an admission in which dialysis was initiated, and 3 patients had a biopsy during an admission for another problem. Patients diagnosed in the first 2 decades of the study were included in a previous epidemiologic study.10 Patients referred from outside of the region for renal transplantation were excluded from this study.
Blood pressure, serum creatinine, and urinary protein excretion (expressed as grams per 24 hours) were documented at the time of diagnosis. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.14 Hypertension was defined by treatment with ≥1 antihypertensive medications or measured blood pressure >140/90 mm Hg. Diabetes was determined by treatment with insulin or an oral hypoglycemic agent when IgA nephropathy was diagnosed.
Survival Data
The National Death Index and United States Renal Data System were used to establish date of death and date of progression to ESRD, respectively. The average number of years of life remaining at the time of diagnostic renal biopsy was determined for each patient using U.S. Decennial Life Tables from the National Vital Statistics Report, Centers for Disease Control and Prevention. These tables provided specific survival data by state (Kentucky), race, age, gender and decade. Tables for 1979 to 1981, 1989 to 1991, and 1999 to 2001 were used for patients with biopsies performed for 1976 to 1985, 1986 to 1995, and 1996 to 2005, respectively. Expected age at death for each patient was defined as the age at biopsy plus the average years of life remaining from the time of biopsy. The observed patient survival was based on actual age at death for 97 patients and the age at study endpoint of December 31, 2015 for the 154 living patients. The median loss of life in years was defined as the median expected patient survival minus the observed patient survival.
Statistical Analysis
All data analyses were performed with SAS/STATv14.1 (SAS Institute Inc., Cary, North Carolina). Descriptive statistics, including means and SDs, and proportions were generated for the continuous and categorical variables for the overall sample by survival status. Kaplan-Meier product-limit survival curves were generated for the follow-up time and age to the overall observed and expected survival and time to death. Equality of survivor functions among these groups was compared using univariate Cox proportional hazard models. Associations between survival risk (progression to death) and clinical covariates of interest were tested using multivariate Cox proportional hazards regression models. Hazard ratios (HRs) and 95% confidence intervals were calculated for each covariate to estimate the magnitude of the risk. All P values were 2-sided and were considered significant at the α = 0.05 level.
Results
Clinical features of our cohort at the time of renal biopsy and their associations with the outcome of death are shown in Table 1. Mean age at diagnosis was 36.9 years. Most patients were men (69%) and Caucasian (95%). Hypertension was present at time of diagnostic biopsy for 130 (52%) patients, and 43 of these patients were treated at the time of diagnostic biopsy with renin angiotensin system (RAS) blockade by an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. Most of those treated with RAS blockade were diagnosed in the second half of the study (i.e., after 1995). Only 3 patients were treated with an immunosuppressive agent before diagnosis. Fifteen patients had diabetes mellitus type 1 or type 2 at the time of diagnosis. No other co-morbidity was documented at diagnosis in >2% of the patients. Less than one-half (46%) of the patients had an eGFR ≥60 ml/min per 1.73 m2 at the time of diagnosis.
Table 1.
Clinical findings at the time of diagnostic biopsy and associations with the outcome of death
| Baseline | All (N = 251) | Dead (n = 97) | Alive (n = 154) | P valuea |
|---|---|---|---|---|
| Age at diagnostic renal biopsy | 36.9 ± 11.1 | 41.1 ± 10.8 | 34.4 ± 10.6 | <0.001 |
| Male sex | 172 (68.5) | 68.0 (70.1) | 104 (67.5) | 0.669 |
| Race | ||||
| Caucasian | 239 | 93 (95.9) | 146 (94.8) | 0.928 |
| African American | 9 | 3 (3.1) | 6 (3.9) | |
| Asian | 3 | 1 (1.0) | 2 (1.3) | |
| Diabetes | 15 (6.0) | 9 (6.8) | 6 (3.9) | 0.080 |
| Hypertension | 130 (51.8) | 60 (61.9) | 70 (45.5) | 0.011 |
| Chronic kidney disease stage at diagnosis | ||||
| 1 (≥90 ml/min/1.73 m2) | 57 (22.7) | 9 (9.3) | 48 (31.4) | <0.001 |
| 2 (60 < 90 ml/min/1.73 m2) | 60 (23.9) | 16 (16.5) | 44 (28.8) | |
| 3 (30 < 60 ml/min/1.73 m2) | 61 (24.3) | 21 (21.7) | 40 (26.1) | |
| 4 (5 < 30 ml/min/1.73 m2) | 45 (17.9) | 30 (30.9) | 14 (9.2) | |
| 5 (<15 ml/min/1.73 m2) | 28 (11.2) | 21 (21.7) | 7 (4.6) | |
| Urine protein excretion (g/24 h) at diagnosis | ||||
| Mild < 1 | 57 ± 25.2 | 12 ± 14.6 | 45 ± 31.5 | <0.001 |
| Moderate ≥1 < 3 | 88 ± 38.9 | 26 ± 31.7 | 62 ± 43.4 | |
| Severe ≥3 | 81 ± 35.8 | 60 ± 51.3 | 36 ± 25.2 |
Values are presented as mean ± SD or number (%).
P values < 0.05 are considered to be significant.
Chi-square or exact chi-square test.
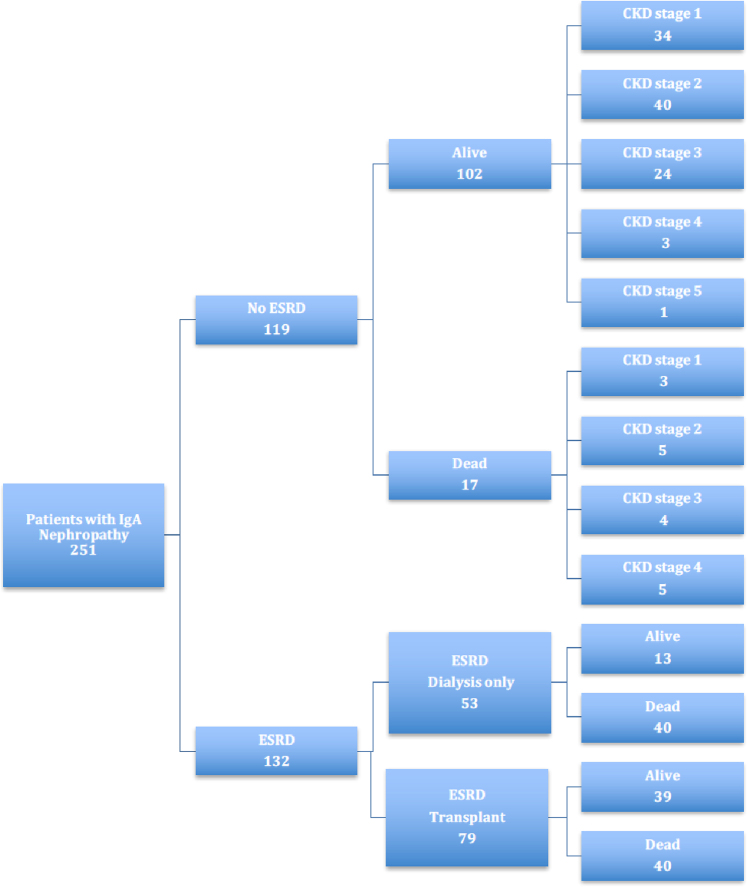
Outcomes for ESRD and death at study endpoint of December 31, 2015 are shown in Figure 1. There were 5290 person-years from time of biopsy to time of death or study endpoint for 251 patients. Mean follow-up time from biopsy to death or end of the study was 19.3 years. Of these 251 patients, 132 (53%) progressed to ESRD and 97 (39%) died. Clinical data from within 1 year of study endpoint were available for only 19% of the living patients who did not progress to ESRD. Median follow-up from diagnosis for those with incomplete clinical data was 7.6 years, and 24% had <2 years of clinical follow-up. Patients with incomplete clinical data were more likely than those with complete data to have chronic kidney disease CKD stage 4 or 5 at last visit (P = 0.045; Fisher’s exact test).
Figure 1.
Outcomes for patients from central and eastern Kentucky with IgA nephropathy at the end of the study (December 31, 2015). Chronic kidney disease (CKD) stage for those not reaching end-stage renal disease (ESRD) was at the last clinic visit that was often many years before the end of the study.
Of those who progressed to ESRD, 53 were managed with dialysis only, and 79 had ≥1 kidney transplantations. Median time from onset of dialysis to death was 5.5 years for the dialysis-only patients. The 50% allograft survival interval for first renal transplantation was 10.5 years. Twenty-two patients had 2 kidney transplants, and 1 patient had 3 kidney transplantations.
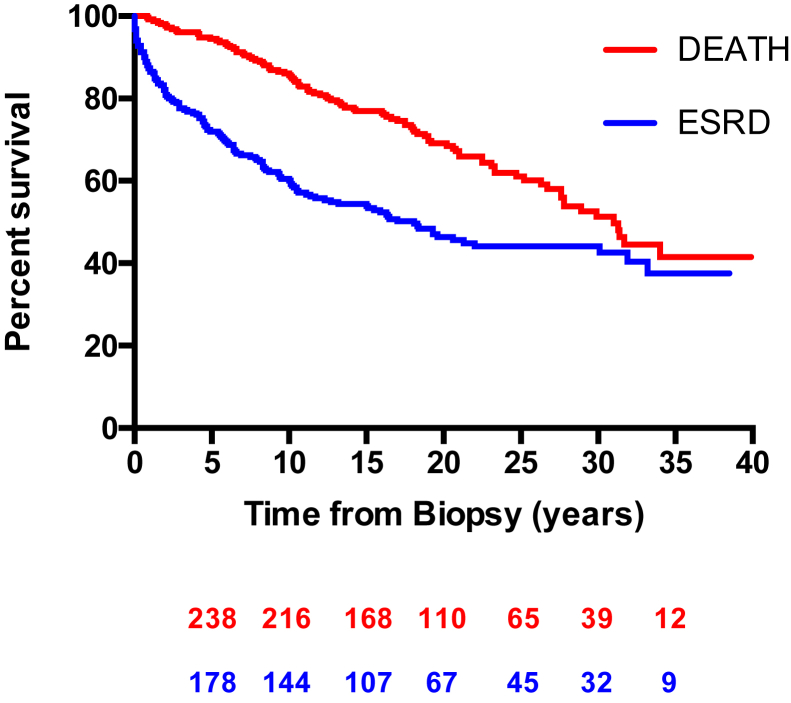
Kaplan-Meier curves for kidney survival and death for our entire cohort are shown in Figure 2. For kidney survival, the patients who died before reaching ESRD were censored at the time of death (i.e., time of death considered as last follow-up). Ten-year kidney survival was 60.1%. There was 50% kidney survival of 18.1 years, and 50% mortality occurred 31.0 years after diagnosis of IgA nephropathy.
Figure 2.
Survival in years from diagnostic kidney biopsy to date of death (red line) and to end-stage renal disease (ESRD) defined by the need for chronic dialysis or transplantation (blue line). The numbers of at-risk patients at each 5-year interval are shown below for both survival curves.
Univariate Cox proportional hazard models (Table 2) showed that time from diagnostic biopsy until death was highly associated with CKD stages 4 and 5, and 24-hour urinary protein excretion >3 g (all P < 0.001) at the time of diagnosis. Kaplan-Meier curves for CKD stage and 24-hour urinary protein excretion are shown as Supplementary Figures S1 and S2.
Table 2.
Clinical findings at the time of diagnostic biopsy and associations with time from biopsy to death
| Baseline | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age at diagnostic renal biopsy | 1.07 | 1.05−1.10 | <0.001 |
| Male sex | 1.25 | 0.78−2.02 | 0.868 |
| Race | |||
| Caucasian | 1 | ||
| African American | 1.15 | 0.36−3.66 | 0.056 |
| Asian | 1.43 | 0.19−10.33 | 0.126 |
| Diabetes | 2.22 | 0.79−6.17 | 0.126 |
| Hypertension | 2.09 | 1.33−3.29 | 0.001 |
| Chronic kidney disease stage at diagnosis | |||
| 1 (≥90 ml/min/1.73 m2) | 1 | ||
| 2 (60 < 90 ml/min/1.73 m2) | 1.97 | 0.87−4.48 | 0.103 |
| 3 (30 < 60 ml/min/1.73 m2) | 2.89 | 1.29−6.50 | 0.010 |
| 4 (15 < 30 ml/min/1.73 m2) | 9.18 | 4.17−20.23 | <0.001 |
| 5 (<15 ml/min/1.73 m2) | 10.19 | 4.41−23.57 | <0.001 |
| Urine protein excretion (g/24 h) at diagnosis | |||
| Mild <1 | 1 | ||
| Moderate > 1 < 3 | 1.82 | 0.92−3.62 | 0.087 |
| Severe >3 | 4.52 | 2.35−8.71 | <0.001 |
CI, confidence interval.
P values < 0.05 are considered to be significant.
Multivariate analysis (Table 3) showed significant associations between time from diagnostic biopsy to death for age at diagnosis (HR: 1.06; P < 0.001), CKD stage 4 (HR: 3.71; P = 0.007), CKD stage 5 (HR: 5.22; P = 0.001), and 24-hour urinary protein ≥3 g (HR: 2.18; P = 0.032).
Table 3.
Association between clinical variables at diagnostic biopsy and time from biopsy to death using a multivariable Cox regression model
| Baseline | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age at diagnosis | 1.06 | 1.03−1.08 | <0.001 |
| Male sex | 1.18 | 0.69−2.01 | 0.540 |
| Race | |||
| Ref: Caucasian | |||
| African American | 2.08 | 0.62−6.99 | 0.235 |
| Asian | 1.22 | 0.16−9.36 | 0.846 |
| Diabetes at diagnosis | 0.73 | 0.23−2.32 | 0.590 |
| Hypertension at diagnosis | 0.93 | 0.53−1.63 | 0.800 |
| Chronic kidney disease stage at diagnosis | |||
| Ref: 1 (≥90 ml/min/1.73 m2) | |||
| 2 (60 < 90 ml/min/1.73 m2) | 1.40 | 0.59−3.29 | 0.440 |
| 3 (30 < 60 ml/min/1.73 m2) | 1.57 | 0.65−3.79 | 0.318 |
| 4 (15 < 30 ml/min/1.73 m2) | 3.71 | 1.44−9.54 | 0.007 |
| 5 (<15 ml/min/1.73 m2) | 5.22 | 1.90−14.33 | 0.001 |
| Urine protein excretion (g/24 h) at diagnosis | |||
| Ref: < 1 | |||
| ≥1 < 3 | 1.36 | 0.67−2.77 | 0.397 |
| ≥3 | 2.18 | 1.07−4.44 | 0.032 |
CI, confidence interval.
P values < 0.05 are considered to be significant.
The median age at death for the patients was 65.7 years compared with age at death predicted at the time of kidney biopsy of 75.8 years. Thus, the reduction in life expectancy was 10.1 years (Table 4). None of the 97 deceased patients surpassed their predicted life expectancy from time of biopsy. The median loss of life for the deceased patients was 19.5 years (range: 3.5−47.2 years). For the entire cohort, 18.5% (47 patients) died >20 years before their predicted age of death.
Table 4.
Median observed age at death compared to predicted age at death
| Period | N | Age at biopsy | Predicted age for 50% mortality | Observed age for 50% mortality | Reduction in life expectancy (yr) |
|---|---|---|---|---|---|
| 1976−1985 | 67 | 32.3 ± 9.8 | 73.4 (72.8−74.0) | 62.5 (56.0−a) | 10.9 |
| 1986−1995 | 93 | 37.0 ± 9.6 | 75.1 (74.5−76.3) | 63.5 (61.1−a) | 11.6 |
| 1996−2005 | 91 | 40.4 ± 12.3 | 77.3 (76.7−78.5) | b | |
| 1976−2015 | 251 | 36.9 ± 11.0 | 75.8 (75.1−76.7) | 65.7 (62.3−a) | 10.1 |
Values are presented as mean ± SD and median (95% confidence limits).
Upper limit did not cross the median.
Fifty percent mortality not reached.
Data for treatment after diagnosis with RAS blockade, fish oil, corticosteroids, nonsteroidal immunosuppressive agents are included in Supplementary Tables S1, S2, and S3.
Discussion
We found that median life expectancy was reduced by 10 years in patients diagnosed with IgA nephropathy in central and eastern Kentucky. A potential limitation of this estimate was that the age for surviving patients was censored at study end on December 31, 2015. If all the surviving patients could be followed until the end of life, the difference between true age at death and predicted age for death life expectancy might differ from our prediction.
The clinical severity at diagnosis with respect to eGFR, degree of proteinuria, and presence of hypertension appeared comparable to other North American cohorts, although survival rates differed among these cohorts.15, 16, 17, 18 The age at diagnosis of 36.9 years and 10-year renal survival of 60.1% in our study was almost identical to that in the Toronto cohort (37.0 years; 61.6%).17 In contrast, a cohort from the Mayo Clinic showed no significant difference in frequency of death compared with that expected in the general population (97% vs. 97% at 5 years from diagnosis and 89% vs. 94% at 10 years).15 The better patient survival in the Mayo cohort might have been due to the shorter mean duration of follow-up of only 6.3 years.
The presence of diabetes at diagnosis was not associated with shorter time from biopsy to death with both univariate analysis and multivariate analysis. This finding was surprising and might be due to the small number of patients with diabetes in this study. A possible effect of diabetes on survival deserves further study in a larger cohort of patients with IgA nephropathy. CKD stages 4 or 5 were likely much stronger risk factors for shortening the time to death than having diabetes at diagnosis in our cohort.
Renal survival and mortality rates differed substantially between geographic regions. Geddes et al.17 documented substantial variability in the 10-year renal survival in patients with IgA nephropathy across 3 continents, ranging from 95.7% in Helsinki, to 87.0% in Sydney, 63.9% in Glasgow, and 61.6% in Toronto. When age, eGFR, urinary protein excretion, and blood pressure at diagnosis were considered, this variability appeared to relate mostly to lead-time bias because centers with better survival had more patients with mild disease.
Korean and Norwegian studies also appeared to show better overall patient survival than that in our patients.12, 13 A Norwegian cohort of 661 patients biopsied between 1988 and 2008 had 80 deaths (12.6%) and 146 cases of ESRD (23.0%) with 7464 person-years of follow-up.13 Mortality was twice the expected rate for the Norwegian population, but did not significantly increase until after patients reached ESRD. The Norwegian cohort differed markedly from ours, in that only 32% of their patients had eGFR <60 ml/min per 1.73 m2 at diagnosis compared with 54% in our Kentucky cohort. A Korean cohort of 1364 patients, biopsied between 1979 and 2008, had 71 deaths (5.3%) and 277 cases with ESRD (20.6%) during 13,916 person-years of follow-up.12 The authors concluded that the overall mortality for patients with IgA nephropathy was higher than that of the general population. Compared with our cohort, the Korean patients were younger and had a better eGFR at diagnosis, which suggested an earlier identification of disease.12 Decreased access to preventative care, which resulted in delayed diagnosis, might be important in our cohort because patients in the United States do not have access to the universal health care that is available in Korea and Norway.
Patients with early manifestations of IgA nephropathy, such as persistent microscopic hematuria or recurrent episodes of visible hematuria in concomitance with an upper respiratory tract infection, should have early referral to a nephrologist. The development of new treatments combined with an early initiation during the course of disease may be the best way to prevent or slow the progression to ESRD.19 With early diagnosis and effective therapy, life expectancy will likely be lengthened.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the renal pathologists who interpreted biopsies of the study patients: Drs. Dinyar Bhathena, Robert Horn, Fritz Lower, Lilia Mauricio, Bonnie Mitchell, and Ray Smith. The authors appreciate the medical care provided by the nephrologists for the patients: Drs. Richard Baehler, William Caudill, Thomas Ferguson, R. Greg McMorrow, Khalil Rahman, Craig Stafford, Timothy Neufeld, Clifford Matthews, Paulo Fanti, Thomas Waid, Peter Sawaya, and Robert Friedler. A generous gift from Anna and Donald Waite, a grant from the Dialysis Clinics, Inc. and National Institutes of Health Grants DK61525 and DK082753 provided funding in support of this study.
Footnotes
Supplementary Material.
Table S1. Treatment for patients after diagnostic biopsy.
Table S2. Associations between treatment after diagnosis and time to death using univariate Cox proportional hazards models.
Table S3. Association between clinical variables at diagnostic biopsy and treatments after diagnosis and time from biopsy to death using multivariable Cox proportional hazards models.
Figure S1. Kaplan-Meier curves for CKD stage at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.
Figure S2. Kaplan-Meier curves for the 24-hour urinary protein excretion group at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Treatment for patients after diagnostic biopsy.
Associations between treatment after diagnosis and time to death using univariate Cox proportional hazards models.
Association between clinical variables at diagnostic biopsy and treatments after diagnosis and time from biopsy to death using multivariable Cox proportional hazards models.
Kaplan-Meier curves for CKD stage at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.
Kaplan-Meier curves for the 24-hour urinary protein excretion group at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.
References
- 1.D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 2.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Berger J., Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–695. [in French] [PubMed] [Google Scholar]
- 4.Clarkson A.R., Seymour A.E., Thompson A.J. IgA nephropathy: a syndrome of uniform morphology, diverse clinical features and uncertain prognosis. Clin Nephrol. 1977;8:459–471. [PubMed] [Google Scholar]
- 5.van der Peet J., Arisz L., Brentjens J.R., Marrink J., Hoedemaeker P.J. The clinical course of IgA nephropathy in adults. Clin Nephrol. 1977;8:335–340. [PubMed] [Google Scholar]
- 6.Joshua H., Sharon Z., Gutglas E., Rosenfeld J., Ben-Bassat M. IgA-IgG nephropathy: a clinicopathologic entity with slow evolution and favorable prognosis. Am J Clin Pathol. 1977;67:289–295. doi: 10.1093/ajcp/67.3.289. [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli C., Traversi L., Feliciani A., Cesana B.M., Banfi G., Tarantino A. Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int. 2001;60:1948–1954. doi: 10.1046/j.1523-1755.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 8.Berger J. Recurrence of IgA nephropathy in renal allografts. Am J Kidney Dis. 1988;12:371–372. doi: 10.1016/s0272-6386(88)80027-1. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt R.J., Julian B.A., Bhathena D.B. IgA nephropathy: presentation, clinical course, and prognosis in children and adults. Am J Kidney Dis. 1984;4:192–200. doi: 10.1016/s0272-6386(84)80071-2. [DOI] [PubMed] [Google Scholar]
- 10.Wyatt R.J., Julian B.A., Baehler R.W. Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. J Am Soc Nephrol. 1998;9:853–858. doi: 10.1681/ASN.V95853. [DOI] [PubMed] [Google Scholar]
- 11.Moldoveanu Z., Wyatt R.J., Lee J.Y. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 12.Lee H., Kim D.K., Oh K.H. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS One. 2012;7:e51225. doi: 10.1371/journal.pone.0051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knoop T., Vikse B.E., Svarstad E., Leh S. Mortality in patients with IgA nephropathy. Am J Kidney Dis. 2013;62:883–890. doi: 10.1053/j.ajkd.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radford M.G., Donadio J.V., Bergstralh E.J., Grande J.P. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 16.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842. doi: 10.1016/s0272-6386(97)90456-x. [DOI] [PubMed] [Google Scholar]
- 17.Geddes C.C., Rauta V., Gronhagen-Riska C. A tricontinental view of IgA nephropathy. Nephrol Dial Transplant. 2003;18:1541–1548. doi: 10.1093/ndt/gfg207. [DOI] [PubMed] [Google Scholar]
- 18.Arroyo A.H., Bomback A.S., Butler B. Predictors of outcome for severe IgA nephropathy in a multi-ethnic U.S. cohort. Clin Nephrol. 2015;84:145–155. doi: 10.5414/CN108556. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt R.J. Are we ready for targeted therapy for IgA nephropathy. Lancet. 2017;389:2083–2084. doi: 10.1016/S0140-6736(17)30820-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment for patients after diagnostic biopsy.
Associations between treatment after diagnosis and time to death using univariate Cox proportional hazards models.
Association between clinical variables at diagnostic biopsy and treatments after diagnosis and time from biopsy to death using multivariable Cox proportional hazards models.
Kaplan-Meier curves for CKD stage at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.
Kaplan-Meier curves for the 24-hour urinary protein excretion group at the time of diagnosis and time from biopsy to death for southeastern U.S. patients with IgA nephropathy.