Abstract
Background
Children exposed to alcohol prenatally may suffer from behavioral and cognitive alterations that adversely affect their quality of life. Animal studies have shown that perinatal supplementation with the nutrient choline can attenuate ethanol’s adverse effects on development; however, it is not clear how late in development choline can be administered and still effectively reduce the consequences of prenatal alcohol exposure. Using a rodent model, this study examined whether choline supplementation is effective in mitigating alcohol’s teratogenic effects when administered during adolescence/young adulthood.
Methods
Sprague–Dawley rats were exposed to alcohol (5.25 g/kg/d) during the third trimester equivalent brain growth spurt, which occurs from postnatal day (PD) 4 to 9, via oral intubation. Sham-intubated and nontreated controls were included. Subjects were treated with 100 mg/kg/d choline chloride or vehicle from PD 40 to 60, a period equivalent to young adulthood in the rat. After the choline treatment had ceased, subjects were tested on a series of behavioral tasks: open field activity (PD 61 to 64), Morris water maze spatial learning (PD 65 to 73), and spatial working memory (PD 87 to 91).
Results
Ethanol-exposed subjects were overactive in the activity chambers and impaired on both the spatial and the working memory versions of the Morris water maze. Choline treatment failed to attenuate alcohol-related overactivity in the open field and deficits in Morris water maze performance. In contrast, choline supplementation significantly mitigated alcohol-related deficits in working memory, which may suggest that choline administration at this later developmental time affects functioning of the prefrontal cortex.
Conclusions
The results indicate that adolescent choline supplementation can attenuate some, but not all, of the behavioral deficits associated with early developmental alcohol exposure. The results of this study indicate that dietary intervention may reduce some fetal alcohol effects, even when administered later in life, findings with important implications for adolescents and young adults with fetal alcohol spectrum disorders.
Keywords: Fetal Alcohol, Fetal Alcohol Spectrum Disorders, Treatment, Nutrition
The Adverse effects of prenatal alcohol exposure can be modified by numerous factors, including genetics, developmental timing and pattern of alcohol exposure, postnatal environment, and nutrition (Abel and Hannigan, 1995; Gemma et al., 2007; May et al., 2013). Both clinical and preclinical studies indicate that nutritional deficiencies may exacerbate ethanol’s teratogenic effects, whereas nutritional supplements may reduce fetal risk to alcohol’s damaging consequences (Guerrini et al., 2007; Keen et al., 2010; Rufer et al., 2012; Summers et al., 2008; Wang et al., 2009). Although it is not surprising that prenatal nutrition can alter alcohol’s effects on the developing fetus, less is known of how the postnatal nutritional intake of an individual with fetal alcohol spectrum disorders (FASD) influences postnatal brain and behavioral development.
Choline is an essential nutrient known to play an important role in brain development (Food and Nutrition Board 1998; Jiang et al., 2014; Zeisel, 2006). Animal studies illustrate that perinatal choline supplementation leads to long-lasting changes in brain and consequent behavior, including structural, neurochemical, and electrophysiological changes that are expressed as enhancements in cognitive functioning (McCann et al., 2006; Meck and Williams, 2003). Moreover, perinatal choline supplementation can protect against several other types of central nervous system (CNS) insults, even when the insults occur later in life (Guo-Ross et al., 2002; Wong-Goodrich et al., 2008, 2011; Yang et al., 2000).
Several animal studies have demonstrated that postnatal choline supplementation can reduce the severity of prenatal alcohol effects. Although choline supplementation during prenatal alcohol exposure attenuates a range of adverse outcomes, including brain weight reductions, motor impairments, and cognitive alterations (Thomas et al., 2009, 2010), when administered postnatally and after alcohol exposure has ceased, choline still improves cognitive outcome. For example, choline supplementation prior to postnatal day (PD) 30 can reduce the severity of alcohol-related overactivity in the open field (Ryan et al., 2008; Thomas et al., 2004), as well as deficits in spatial learning (Thomas et al., 2007), reversal learning (Thomas et al., 2004), trace classical conditioning (Thomas and Tran, 2012; Wagner and Hunt, 2006), and working memory (Thomas et al., 2000). These findings have led to clinical trials to investigate whether these preclinical data translate to preschool-aged clinical populations with FASD (Wozniak et al., 2013).
However, many individuals are not identified with FASD until they enter school (Streissguth et al., 2004). Previously, we have demonstrated that choline can reduce the severity of spatial learning deficits in rodents when administered from either PD 11 to 20 or PD 21 to 30 (Ryan et al., 2008). However, as many individuals with FASD are currently adolescents or young adults, one question is whether the developmental window for choline to be effective closes or whether choline can still exert some beneficial effects when administered after PD 30, during a period of development equivalent to adolescence. Indeed, recent data indicate that choline supplementation initiated just prior to the adolescent period (beginning on PD 26 or even later) in otherwise typically developing rats can influence neural plasticity and behavior (Corriveau and Glenn, 2012; Glenn et al., 2012; Holmes et al., 2002; McCall et al., 2015; Teather and Wurtman, 2005). Choline administration can also reduce the severity of insults induced later in life. For example, Holmes and colleagues (2002) reported that choline can reduce the severity of seizure-induced hippocampal damage, even when administered after PD 35.
The present experiment examined whether choline supplementation during adolescence/early adulthood (PD 40 to 60) will be effective in attenuating visuospatial learning deficits, as measured with the Morris water maze, and overactivity, as measured in the open field activity chamber, in subjects exposed to alcohol during the third trimester brain growth spurt.
MATERIALS AND METHODS
This study incorporated a 3 (EtOH, Sham, and Nonintubated Control) × 2 (Choline, Vehicle) × 2 (Male and Female) design. All procedures included in this study were approved by the San Diego State University Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Subjects were Sprague–Dawley rats, offspring from the breeding colony at San Diego State University Animal Care Facilities. Gestational day (GD) 0 was determined by the presence of a seminal plug the morning after housing opposite sex pairs overnight. Animals were housed in standard shoebox cages with microisolator tops (26.7 cm × 48.3 cm × 20.3 cm), with Alpha-dri sani chip bedding (Shepherd Specialty Papers, Milford, NJ). Pregnant females were then singly housed in the nursery which was controlled for temperature (21°C), humidity (40 to 60%), and light (06:00-to 18:00-hour light–dark schedule) with ad lib access to food (LabDiet 5001 [Richmond, IN] containing 2.25 g choline chloride/kg diet) and water. The cages were monitored for the presence of pups beginning on GD 20. After birth, litters were pseudorandomly culled to 8 pups, maintaining 4 males and 4 females when possible. To control for possible litter effects, no more than 1 pup per sex per litter was assigned to a particular treatment group. A total of 12 to 13 subjects per sex and group were generated.
Subjects were exposed to alcohol during the neonatal brain growth spurt (PD 4 to 9), a period of brain development equivalent to the human third trimester (Dobbing and Sands, 1979). On PD 4, pups were randomly assigned to 1 of 3 treatment groups: EtOH-exposed, sham intubation (Sham), and nonintubated control (Cont). The EtOH group was exposed to 5.25 g/kg/d alcohol via intubation from PD 4 to 9. For the EtOH group, subjects were gavaged twice a day, 2 hours apart, with a milk formula containing 11.9% v/v EtOH for a total daily dose of 5.25 g/kg. In addition, EtOH subjects received an additional 2 intubations of milk formula alone, each 2 hours apart, after the initial EtOH intubations, to minimize growth differences between the EtOH and control groups. The sham group received 4 daily intubations, 2 hours apart, with no liquid infused, since intubating with milk formula causes them to grow at a faster rate than nontreated controls. The nonintubated controls were minimally handled and had no intubations.
Peak blood alcohol level was determined on PD 6, 1.5 hours after the last alcohol feed. Twenty milliliters of blood was collected from a tail clip from all subjects and analyzed using the Analox Alcohol Analyzer (Model AM1; Analox Instruments, Lunenburg, MA). In addition, on PD 6, all pups were given paw tattoos for identification, with predetermined codes that allowed investigators to remain blind to treatment condition. On PD 21, all subjects were weaned; on PD 27, all subjects were housed separated by sex; and on PD 35, subjects were double housed. From PD 40 to 60, subjects received subcutaneous injections of choline chloride (100 mg/kg/d) or saline vehicle. The alcohol and choline doses were based on previous results, where we and others have reported that choline can mitigate EtOH’s effects (Thomas and Tran, 2012; Thomas et al., 2007).
All behavioral testing was initiated after the choline treatment had ceased, beginning on PD 61. Activity levels were measured via an automated open field (Hamilton-Kinder; Kinder Scientific Company, LLC, Poway, CA) containing a grid of infrared beams that tracked the subjects’ movement. The plexiglass open field (45 cm [w] × 45 cm [l] × 30.5 cm [h]) was contained within a sound-resistant chamber with a fan. White noise was introduced into the testing rooms to provide further masking of sounds. All data were collected during dark cycle, beginning at 18:00 hours.
Beginning on PD 61, subjects were placed in the activity room 30 minutes prior to testing to allow subjects to acclimate to the testing environment. The activity chambers were cleaned prior to testing each of the subjects to eliminate any olfactory cues. Activity was recorded for 1 h/d for 4 consecutive days. Data included total number of photo beam breaks, total distance traveled, number of rearings, and time spent in the center of the open field.
On PD 67 to 75, spatial learning was tested in subjects with the Morris water maze. The Morris maze apparatus consisted of a large circular tank (178-cm diameter, 61-cm height) painted white and filled with water (26°C). Powdered milk was added to the tank so the water was opaque. During testing, the environment in the room, such as posters on the walls, shelving, and a sink, provided salient landmarks for the rats. The experimenter stood behind a white curtain, to eliminate any variation in the visual cues. A plexiglass escape platform was placed 3 to 4 cm under the surface of the water, hidden by the opacity of the water. A tracking video camera was placed above the tank which directly recorded the data into the Menu2020 tracking program (HVS Image Ltd., Buckingham, UK). All testing occurred within the subjects’ light cycle (06:00 to 18:00).
During the spatial learning version of the Morris maze, the escape platform was placed in 1 of the 4 quadrants; the location was counterbalanced across treatment groups. The location of the escape platform remained constant for each subject throughout the testing period. At the start of each trial, the subject was placed in the water facing the wall of the tank. The start position was varied pseudorandomly to control for learning of motor strategies. Once the subject was placed in the tank, the experimenter moved behind a curtain so that they did not serve as a cue. If the subject failed to find the hidden platform within 60 seconds, the experimenter guided the subject to the platform. Subjects were tested for 6 consecutive days, 4 trials/d with an intertrial interval (ITI) of 4 minutes. One day following acquisition (PD 73), subjects were tested on a probe trial. During the probe trial, the hidden platform was removed and the subjects were allowed to swim for 60 seconds.
Subjects were also tested on a cued version of the Morris maze, during which all subjects were tested for their ability to swim to a platform clearly visible above the surface of the water, while distal visual cues were occluded with white curtains. This test was to ensure that the animals were able to visually navigate their environment and to determine whether any performance measures, such as sensory, motor, or motivational factors, might influence group differences. Each subject was tested for 4 trials a day for 2 consecutive days (PD 74 to 75). The position of the visible platform was pseudorandomly changed so that each subject was tested in all 4 different platform locations each day.
Subjects were tested on the working memory version of the Morris water maze on PD 87 to 91. For this task, each session consisted of 2 trials, an acquisition and a test trial. Subjects were tested for 2 sessions each day, 1 in the morning (09:00) and 1 in the afternoon (15:00). The location of the hidden platform was changed during each session. During the acquisition trial, the subject located the hidden platform by trial and error, closely followed by the test trial. On testing days 1 to 3, subjects were tested with a 0-second ITI. After spending 20 seconds on the escape platform following the acquisition trial, the subject was removed from the platform and placed immediately back into the Morris water maze for the test trial at a predetermined start position. On testing days 4 to 6, subjects were tested with a 60-second ITI following the acquisition trial. Once the subject found the escape platform during the acquisition trial, they spent 20 seconds on the escape platform, but were then removed and placed in a drying cage for the 60-second ITI and then replaced in the tank for the test trial at a predetermined start position.
Data were analyzed with analyses of variance with group (EtOH, Sham, and Control), treatment (Choline and Vehicle), and sex (Male and Female) as between-subject variables. For body weight, day served as a within-subject repeated measure. For activity data, day and 5-minute bin served as within-subject repeated measures. For Morris maze testing, day and trial served as within-subject repeated measures. All data were analyzed with SPSS software (IBM, Armonk, NY) with Newman–Keuls (p < 0.05) follow-ups for post hoc analyses.
RESULTS
Body Growth
On PD 4, there were no significant differences in body weight among the groups. However, beginning on PD 5, the EtOH-exposed group showed a significant lag in growth, producing a main effect of group, F(2, 135) = 24.0, p < 0.001, and a day by group interaction, F(16, 1,080) = 22.7, p < 0.001. Post hoc analyses confirmed that the EtOH-exposed groups weighed significantly less than control groups on all days from PD 5 through PD 12. All groups gained weight over days, producing a significant main effect of day, F(8, 1,080) = 5,625, p < 0.001, and male rats grew at a faster rate than the female rats, producing a main effect of sex, F(1, 135) = 11.2, p < 0.001, and a day by sex interaction, F(8, 1,080) = 2.7, p < 0.05 (Fig. 1A). The lag in growth due to EtOH exposure was evident in both sexes, but slightly more pronounced among females, producing a significant group by day by sex interaction, F(16, 1,080) = 2.5, p < 0.01 (data not shown). From PD 15 to 30, the body weights of the EtOH-exposed groups caught up with that of controls, so that by PD 30, there were no significant effects of group.
Fig. 1.

Mean (±SEM) body weight during ethanol (EtOH) exposure (PD 4 to 12; A) and choline (Chol) treatment (PD 40 to 60; B). EtOH-exposed subjects began to lag in growth as compared to both the control groups, beginning on PD 5 and continuing through PD 12. EtOH-exposed subjects caught up in body weight by PD 30.
On PD 40 to 60, during the period of choline supplementation, there was a significant main effect of day, F(20, 2,700) = 6,665, p < 0.001, as all groups gained weight over days. Male rats grew faster than the female rats, producing a significant main effect of sex, F(1, 135) = 883.3, p < 0.001, and a day by sex interaction, F(20, 2,700) = 971.4, p < 0.001. There was a significant main effect of choline treatment, F(1, 135) = 3.9, p < 0.05, on body weight, as choline-treated subjects weighed less than vehicle-treated groups, but this effect was evident on PD 40, before treatment was initiated, suggesting that this was a spurious effect and not directly related to choline treatment.
Open Field Activity
Ethanol exposure during the third trimester equivalent increased locomotor activity and choline supplementation did not significantly attenuate this effect. As seen in Fig. 2, subjects exposed to EtOH traveled a greater distance in the open field compared to controls, producing a main effect of group, F(2, 135) = 10.6, p < 0.001. EtOH-exposed subjects also exhibited less habituation within session compared to the controls, producing a bin by group interaction, F(22, 1,485) = 3.9, p < 0.001. All subjects habituated both within and between sessions producing main effects of day, F(3, 405) = 137.3, p < 0.001, bin, F(11, 1,485) = 1,382.5, p < 0.001, and a day by bin interaction, F(33, 4,455) = 10.5, p < 0.001. Males were also less active compared to females, indicated by a main effect of sex, F(1, 135) = 8.4, p < 0.005. In addition, females consistently exhibited more locomotor activity and less habituation compared to males, resulting in a significant bin by sex interaction, F(11, 1,485) = 4.1, p < 0.001, and a day by sex interaction, F(3, 348) = 3.0, p < 0.05 (data not shown). Similar effects were seen for distance traveled in the center and periphery of the open field chamber, as well as rearing.
Fig. 2.

Mean (±SEM) distance traveled in the open field activity chamber during each testing day. Ethanol-exposed subjects exhibited significantly more locomotor activity compared to both control groups.
Similar to locomotor activity, EtOH exposure during the third trimester equivalent increased the time spent in the center of the chamber (Fig. 3) and choline did not attenuate this effect. EtOH-exposed subjects spent significantly more time in the center than either of the controls, confirmed by a main effect of group, F(2, 135) = 7.9, p < 0.001, and post hoc analyses. This reduction in thigmotaxis may indicate alterations in emotionality and/or may be reflective of overall increased activity and exploration. EtOH-exposed groups habituated more slowly than controls across bins, producing a bin by group interaction, F(22, 1,485) = 2.1, p < 0.005. Subjects habituated both within and between session, producing a main effect of day, F(3, 405) = 17.9, p < 0.001, and bin, F(11, 1,485) = 250.6, p < 0.001, and a day by bin interaction, F(33, 4,455) = 3.8, p < 0.001. Finally, overall, males spent more time in the center of the chamber at the beginning of training sessions, but female subjects habituated less across bins, producing a bin by sex interaction, F(11, 1,485) = 8.8, p < 0.001 (data not shown). However, there were no interactions of sex with group or treatment factors.
Fig. 3.

Mean (+SEM) daily time spent in the center of the open field activity chamber. The ethanol-exposed subjects spent significantly more time in the center compared to either of the control groups. Choline supplementation did not modify this effect. **Significantly different from both control groups.
Morris Water Maze Spatial Memory
Alcohol exposure during the third trimester equivalent caused modest, but significant impairments in Morris water maze performance and choline did not improve the outcome. EtOH-exposed subjects exhibited longer path lengths to find the platform, producing a main effect of group, F(2, 135) = 4.0, p < 0.05 (Fig. 4B). Post hoc analyses of this effect confirmed that the EtOH-exposed groups had significantly longer path lengths compared to both control groups. As the subjects learned the task, they exhibited shorter path lengths both within and between sessions, producing main effects of trial, F(3, 405) = 63.9, p < 0.001, day, F(5, 675) = 134.5, p < 0.001 (Fig. 4A), and a day by trial interaction, F(15, 2,025) = 2.7, p < 0.001. Although there was no significant interaction of day by group, there were no significant effects of EtOH on day 1 of testing (mean path lengths for groups varied from 11.5 to 12.2 m with SEMs ranging from 0.5 to 0.7), but by days 5 and 6, EtOH-exposed subjects took significantly longer path lengths compared to both control groups (Fig. 4A). Similar results were observed with time to find the platform (data not shown).
Fig. 4.

Mean (±SEM) distance traveled to find the hidden platform for each day (A) and collapsed across days (B) during Morris water maze testing. Ethanol (EtOH) exposure produced modest, but significant, impairments in spatial learning performance. Choline (Chol) did not affect Morris water maze learning. **Significantly different from both control groups.
During the probe trial, the hidden platform was removed from the Morris water maze tank and the subject was allowed to swim freely for 60 seconds to provide a measure of spatial memory. There were no significant group or treatment effects on percentage of time spent in the target quadrant (Fig. 5); similarly, there were no effects of group or treatment in passes through the platform area (data not shown). Finally, during visual platform testing, the extra-maze cues are occluded and the platform was raised above the water and clearly marked. This task was used to determine whether any potential group or treatment differences were due to sensory, motor, or motivational factors. All subjects exhibited shorter path length across days, F(1, 135) = 67.7, p < 0.001, but there were no effects of either group or treatment on this variable.
Fig. 5.
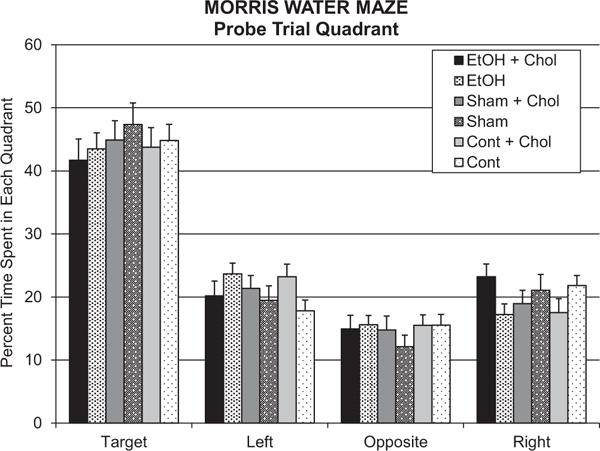
Mean (+SEM) percent time spent in each quadrant of the Morris water maze during the probe trial. All groups spent significantly more time in the target quadrant, an effect that was not altered by either ethanol or choline treatment.
Morris Water Maze Spatial Working Memory
In contrast to open field activity and reference spatial learning, choline was effective in attenuating the severity of alcohol-related deficits in spatial working memory. On testing days 1 to 3, subjects were tested with a 0-second ITI, and on testing days 4 to 6, subjects were tested with a 60-second ITI. During the 0-second ITI sessions, there were no significant effects of group or treatment on the training trials. However, early developmental alcohol exposure led to longer path length during the test trials, an effect mitigated with choline supplementation, as indicated by a group by treatment interaction, F(2, 83) = 3.4, p < 0.05 (Fig. 6A). The EtOH-exposed group not treated with choline took significantly longer path lengths to find the platform compared to all other groups, including the EtOH-exposed group treated with choline. In fact, performance of the EtOH-exposed group treated with choline was not significantly different from that of controls. Similar effects were observed for latency to find the platform. There were no main or interactive effects of sex during the 0-second ITI sessions.
Fig. 6.

Mean (+SEM) path length to find the hidden platform in the 0-second intertrial interval (ITI) working memory version (A) and the 60-second ITI working memory version (B) of Morris water maze. Performance was significantly impaired among subjects exposed to ethanol and not treated with choline. During the 60-second ITI, this pattern was significant among females (C), but failed to reach significance among the males (D). Ethanol-exposed subjects treated with choline performed at levels that did not differ significantly from that of controls. ***Significantly different from all other groups, **significantly different from all groups except Sham + Choline.
A similar pattern was seen with the 60-second ITI trials (Fig. 6B). Follow-up analyses of a group by treatment interaction, F(2, 83) = 5.1, p < 0.01, indicated that the EtOH group that did not receive choline had longer path lengths during the test trial compared to the EtOH-exposed group treated with choline, as well as the control groups. This effect was similar in both sexes, but the EtOH effect was more robust in females, producing a significant interaction of sex by group by treatment, F(2, 83) = 3.9, p < 0.05, as well as a main effect of sex, F(1, 83) = 4.4, p < 0.05. Follow-up analyses within each sex illustrated that EtOH significantly impaired performance among females and that choline mitigated this effect, producing a significant effect of group, F(2, 41) = 3.3, p < 0.05, and a group by treatment interaction, F(2, 41) = 6.9, p < 0.01 (Fig. 6C). EtOH-exposed females were significantly impaired compared to all other groups, except the Sham + Choline group. The pattern was similar among males, but did not reach statistical significance (Fig. 6D).
DISCUSSION
The results of this study indicate that alcohol exposure during the third trimester equivalent led to significant behavioral alterations, including overactivity and impaired spatial and working memory. Postnatal choline supplementation during adolescence/young adulthood reduced some, but not all, of the alcohol-related behavioral alterations. This study is the first to demonstrate that choline supplementation beginning as late as PD 40 and continuing through PD 60 can reduce the severity of some adverse outcomes that comprise fetal alcohol spectrum disorders.
Alcohol exposure during the brain growth spurt increased activity levels in the open field activity chambers. Hyperactivity is a commonly described symptom of prenatal alcohol exposure (Mattson and Riley, 1998), although the prominence depends on the method of evaluation (Glass et al., 2014). Animal models of FASD have more consistently reported overactivity in many different tasks (Schneider et al., 2011). We found that early developmental alcohol exposure increased locomotion, rearings, and time spent in the center of the chambers, indicating that early developmental alcohol exposure may change overall activity level, exploration, and the emotionality of subjects. Previously, we reported that choline supplementation from PD 4 to 30 attenuated open field overactivity associated with early developmental alcohol exposure (Monk et al., 2012; Thomas et al., 2004); however, choline from PD 40 to 60, in the present study, failed to mitigate EtOH-related changes in activity level. The results of this study indicate that choline supplementation may have began too late in development to reduce the severity of alcohol-induced increases in activity.
Similar effects were seen with spatial learning performance on the Morris water maze. First, alcohol-related deficits in Morris water maze acquisition in the present study were somewhat modest and there were no significant effects of alcohol on spatial memory measured during the probe trial. Although spatial learning deficits are consistently reported following early developmental alcohol exposure, in both clinical and animal studies, variability in outcome can depend on age of testing, gender, and alcohol dose and timing of administration (for review, see Berman and Hannigan, 2000). We previously found more robust deficits in Morris water maze acquisition and spatial memory during the probe trial following third trimester equivalent alcohol when subjects were tested 3 weeks earlier, on PD 45 (Ryan et al., 2008). When tested on the Morris water maze at PD 145, EtOH-related deficits were only evident in females (Thomas et al., 2007). Thus, the severity and sex dependency of EtOH effects likely depends on the age of testing. Importantly, in all of our previous studies which utilized choline supplementation prior to PD 30, choline mitigated EtOH’s effects on learning tasks (Thomas and Tran, 2012; Thomas et al., 2000, 2004, 2010; Wagner and Hunt, 2006) and we have not seen sex effects of choline administration on any of our behavioral measures examined to date. For example, choline supplementation attenuates spatial learning deficits, when choline supplementation occurs from PD 10 to 30 (the same dose and duration of choline supplementation used in the present study, but earlier in development; Thomas et al., 2007). Interestingly, Ryan and colleagues (2008) demonstrated that choline supplementation can effectively attenuate alcohol-related spatial memory deficits even when administered from PD 11 to 20 or from PD 21 to 30. These results indicated that choline supplementation may be effective in attenuating behavioral alterations associated with early developmental alcohol exposure at an older age than initially suspected (after PD 20). However, the current study suggests that choline supplementation from PD 40 to 60 is too late to mitigate the detrimental effects of alcohol on simple spatial learning.
In contrast, early developmental alcohol exposure produced deficits in the spatial working memory version of the Morris water maze, and choline did attenuate these deficits. Alcohol-related deficits were statistically significant in both sexes with the 0-second ITI, but were only significant among the females with the 60-second ITI. It is interesting that choline was effective in attenuating EtOH’s adverse effects on spatial working memory, but not reference spatial learning. Alcohol-related deficits in spatial working memory may be caused by dysfunction of the hippocampus and/or the prefrontal cortex (Arnsten et al., 2010; Funahashi, 2006). Given that choline did not improve spatial learning, which depends on the functional integrity of the hippocampus, it may be that choline affects only the prefrontal cortex during this later period of development. Prenatal alcohol exposure has been shown to disrupt development of the prefrontal cortex (for review, see Norman et al., 2009) and given the late development of this CNS region, it is reasonable to suggest that choline may more selectively target the prefrontal cortex when administered during the adolescence/young adulthood. This is also consistent with the role of the prefrontal cortex in working memory, behavioral flexibility, and the learning of a rule, all of which could contribute to the performance on the spatial working memory task (Kesner and Churchwell, 2011). Together, the data would suggest that when administered postnatally, but prior to PD 30, choline may target the hippocampus and possibly the prefrontal cortex, but when administered after PD 30, choline may be acting in a more regionally selective manner within the CNS.
However, it is also possible that differences in choline’s effects were related to the difficulty of the tasks. It is notable that the alcohol-related spatial learning deficits were rather modest in severity, and there was no indication of spatial memory deficits during the probe trial. The greater sensitivity of the working memory version of the Morris water maze, a more demanding task, as compared to the reference spatial memory Morris maze may indicate that the choline effects only became evident upon the greater demand of, or the behavioral flexibility required, in the working memory task. It is also possible that choline could have effects if administered at different doses. The present study utilized 100 mg/kg/d choline chloride, as we have found that dose to be effective when administered earlier, but it may be that the dose–response effect of choline is altered when administered later in development. Finally, it should be noted that the working memory task was the last to be tested. Although timing between choline supplementation and behavioral testing could influence choline’s effects, we have not found this to be the case with any of our other studies and have found beneficial effects of choline shortly after choline supplementation as well as months later, when choline is administered earlier in development.
In the current study, choline supplementation from PD 40 to 60 was effective in attenuating alcohol-related deficits. These data suggest that the critical period for choline supplementation to reduce alcohol-related alterations in working memory extends beyond PD 30. Previous studies with typically developing rats suggested that the critical periods for choline supplementation to improve cognitive functioning ended by PD 30 (Meck et al., 2008), and consistent with this finding, we did not find beneficial effects of choline supplementation among control subjects. However, beneficial effects of postnatal choline supplementation beyond PD 30 have been documented within a damaged nervous system. For example, Holmes and colleagues (2002) found that choline supplementation reduces the severity of hippocampal damage induced by seizures when administered after PD 35. In addition, Guseva and colleagues (2008) found that dietary choline supplementation beginning on PD 28 and continuing through PD 42, prior to a traumatic brain insult, could mitigate spatial memory deficits associated with the insult. These studies indicate that the critical effective period for choline supplementation may be much larger, when administered in subjects with brain dysfunction.
Although the exact mechanism by which choline reduces alcohol-related behavioral problems is still unknown, it is known that choline affects development of the hippocampus and prefrontal cortex in typically developing rodents (for reviews, see Jiang et al., 2014; McCann et al., 2006). Prenatal choline supplementation in the rat has been shown to induce morphological, neurochemical, and electrophysiological changes in the hippocampus and cortex (McCann et al., 2006). For example, choline is a precursor to the neurotransmitter acetylcholine, and prenatal choline supplementation leads to long-lasting changes in cholinergic neurotransmission in typically developing rats (Cermak et al., 1998). Our laboratory has found that perinatal choline supplementation from PD 4 to 30 alters cholinergic receptors in the hippocampus of subjects exposed to alcohol during development (Monk et al., 2012). Acetylcholine is essential for proper development of both neuronal and non neuronal cells, and is important for continued neuronal plasticity as well as functional recovery from injury (Conner et al., 2005). Given the behavioral pattern observed and the role of cholinergic influence on the prefrontal cortex in spatial working memory (Croxson et al., 2011; Deiana et al., 2011), it is possible that adolescent choline supplementation influences cholinergic functioning in the prefrontal cortex. However, choline also is a precursor to major constituents of cell membranes, phosphatidylcholine and sphingomyelin, and can influence the signaling and structural integrity of the cells (Craciunescu et al., 2003; Zeisel, 2011). Finally, choline also serves as a methyl donor, influencing DNA methylation and subsequent gene expression (Blusztajn and Mellott, 2012; Jiang et al., 2014). Early developmental alcohol alters global DNA methylation in the hippocampus and prefrontal cortex, an effect that is modified with choline supplementation from PD 2 to 20 (Otero et al., 2012). Choline supplementation from GD 7 to 21 has also been shown to modify alcohol’s epigenetic effects (Bekdash et al., 2014). Most of the studies examining the effects of choline on brain development have focused on prenatal and early choline supplementation; thus, it is not clear which mechanisms are being affected by choline supplementation during PD 40 to 60, but it may be a combination of mechanisms.
This study has important clinical implications. Choline supplementation can be effective in reducing some, but not all, deficits associated with early alcohol exposure, even when treatment began at PD 40 and continued through PD 60. Given that FASD is not typically diagnosed at birth and that the damage may not become evident until later in life, this study illustrates that a dietary intervention starting later in life still may be effective in attenuating some of the deficits induced by early developmental alcohol exposure. In particular, choline supplementation during later development may improve performance on tasks that depend on the functional integrity of the prefrontal cortex.
Acknowledgments
This study was funded by AA012446.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
References
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:445–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: a new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash R, Zhang C, Sarkar D. Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress. Alcohol Clin Exp Res. 2014;38:2323–2330. doi: 10.1111/acer.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Mellott TJ. Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem. 2012;12:82–94. doi: 10.2174/187152412800792706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak JM, Holler T, Jackson DA, Blusztajn JK. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998;12:349–357. doi: 10.1096/fasebj.12.3.349. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Corriveau JA, Glenn MJ. Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2012;103:60–68. doi: 10.1016/j.pbb.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Kyriazis DA, Baxter MG. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat Neurosci. 2011;14:1510–1512. doi: 10.1038/nn.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behav Brain Res. 2011;221:389–411. doi: 10.1016/j.bbr.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Gemma S, Vichi S, Testai E. Metabolic and genetic factors contributing to alcohol induced effects and fetal alcohol syndrome. Neurosci Biobehav Rev. 2007;31:221–229. doi: 10.1016/j.neubiorev.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Glass L, Graham DM, Deweese BN, Jones KL, Riley EP, Mattson SN. Correspondence of parent report and laboratory measures of inattention and hyperactivity in children with heavy prenatal alcohol exposure. Neurotoxicol Teratol. 2014;42:43–50. doi: 10.1016/j.ntt.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Adams RS, McClurg L. Supplemental dietary choline during development exerts antidepressant-like effects in adult female rats. Brain Res. 2012;1443:52–63. doi: 10.1016/j.brainres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini I, Thomson AD, Gurling HD. The importance of alcohol misuse, malnutrition and genetic susceptibility on brain growth and plasticity. Neurosci Biobehav Rev. 2007;31:212–220. doi: 10.1016/j.neubiorev.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Guo-Ross SX, Clark S, Montoya DA, Jones KH, Obernier J, Shetty AK, White AM, Blusztajn JK, Wilson WA, Swartzwelder HS. Prenatal choline supplementation protects against postnatal neurotoxicity. J Neurosci. 2002;22:RC195. doi: 10.1523/JNEUROSCI.22-01-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva MV, Hopkins DM, Scheff SW, Pauly JR. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25:975–983. doi: 10.1089/neu.2008.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Yang Y, Liu Z, Cermak JM, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK. Seizure-induced memory impairment is reduced by choline supplementation before or after status epilepticus. Epilepsy Res. 2002;48:3–13. doi: 10.1016/s0920-1211(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, West AA, Caudill MA. Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol Metab. 2014;25:263–273. doi: 10.1016/j.tem.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Keen CL, Uriu-Adams JY, Skalny A, Grabeklis A, Grabeklis S, Green K, Yevtushok L, Wertelecki WW, Chambers CD. The plausibility of maternal nutritional status being a contributing factor to the risk for fetal alcohol spectrum disorders: the potential influence of zinc status as an example. BioFactors. 2010;36:125–135. doi: 10.1002/biof.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning MA, Blankenship J, Buckley D, Hoyme HE, Adnams CM. Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr. 2013;34:314–325. doi: 10.1097/DBP.0b013e3182905587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall N, Mahadevia D, Corriveau JA, Glenn MJ. Adult emotionality and neural plasticity as a function of adolescent nutrient supplementation in male rats. Pharmacol Biochem Behav. 2015;132:125–135. doi: 10.1016/j.pbb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2008;1:1–11. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22:1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero NK, Thomas JD, Saski CA, Xia X, Kelly SJ. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin Exp Res. 2012;36:1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer ES, Tran TD, Attridge MM, Andrzejewski ME, Flentke GR, Smith SM. Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders. PLoS One. 2012;7:e47499. doi: 10.1371/journal.pone.0047499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Summers BL, Henry CM, Rofe AM, Coyle P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behav Brain Res. 2008;186:230–238. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Teather LA, Wurtman RJ. Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats. Learn Mem. 2005;12:39–43. doi: 10.1101/lm.83905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol. 2009;31:303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121:120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran T. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, Wang J, Zhou SF, Li Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Liu YB, Blusztajn JK, Williams CL. Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood. Hippocampus. 2011;21:584–608. doi: 10.1002/hipo.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Mellott TJ, Glenn MJ, Blusztajn JK, Williams CL. Prenatal choline supplementation attenuates neuropathological response to status epilepticus in the adult rat hippocampus. Neurobiol Dis. 2008;30:255–269. doi: 10.1016/j.nbd.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, Georgieff MK. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res. 2013;33:897–904. doi: 10.1016/j.nutres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermak JM, Tandon P, Sarkisian MR, Stafstrom CE, Neill JC, Blusztajn JK, Holmes GL. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. J Neurosci. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol. 2011;22:624–628. doi: 10.1016/j.semcdb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


