Abstract
Cold shock triggers an immediate rise in the cytosolic free calcium concentration ([Ca2+]cyt) in Arabidopsis thaliana and this cold-induced elevation of [Ca2+]cyt is inhibited by lanthanum or EGTA. It is suggested that intracellular calcium mainly contributes to the cold-induced [Ca2+]cyt response by entering into the cytosol. Two calcium-permeable mechanosensitive channels, MCA1 and MCA2 (mid1-complementing activity), have been identified in Arabidopsis. Here, we demonstrate that MCA1 and MCA2 are involved in a cold-induced increase in [Ca2+]cyt. The cold-induced [Ca2+]cyt increase in mca1 and mca2 mutants was markedly lower than that in wild types. The mca1 mca2 double mutant exhibited chilling and freezing sensitivity, compared to wild-type plants. Expression of At5g61820, At3g51660, and At4g15490, which are not regulated by the CBF/DREB1s transcription factor, was down-regulated in mca1 mca2. These results suggest that MCA1 and MCA2 are involved in the cold-induced elevation of [Ca2+]cyt, cold tolerance, and CBF/DREB1-independent cold signaling.
Introduction
Calcium ions are used as secondary messengers in eukaryotic cells. The cytosolic Ca2+ concentration, [Ca2+]cyt, fluctuates in response to a variety of stimuli, including mechanical stimulation, hormones, pathogens, light, and abiotic stresses such as low temperature1–3. The stimulus-specific spatiotemporal patterning of [Ca2+]cyt dynamics is called the Ca2+ signature4, and to create these signatures, Ca2+ influx channels and Ca2+ efflux transporters that permit transient increases in [Ca2+]cyt are required5.
How plant cells generate stimulus-specific Ca2+ signals remains unknown. To identify the spatiotemporal patterning of [Ca2+]cyt dynamics, recombinant aequorin has been introduced as a reporter of [Ca2+]cyt changes in plant systems6. In Arabidopsis plants expressing aequorin in the cytoplasm, low temperature triggers an immediate and transient rise in [Ca2+]cyt6–8. The final temperature and cooling rate are important for sensing low temperature in Arabidopsis9. In mammals, many TRP (transient receptor potential) channels, which are a specific class of ion channels, function as intracellular Ca2+ release channels10. Some of these channels also function as thermosensors10, and TRPA1 seems to act as a sensor for cold11–13. Although no proteins with high similarity to TRP channels have been identified in land-plant genomes, the genes for Cr-TRP proteins are encoded in the genomic sequence of the alga Chlamydomonas reinhardtii and show functional properties that are similar to those of mammalian TRP channels14.
Two Ca2+-permeable mechanosensitive channels, named MCA1 and MCA2 (mid1-complementing activity 1 and 2), have been identified in Arabidopsis15–19. Both MCA1 and MCA2 complement deficiency of Ca2+ uptake in yeast cells lacking a Ca2+ channel composed of the Mid1 and Cch1 subunits15,16. It should be noted that this complementation activity is detected under conditions that allow the Mid1/Cch1 channel to function as the sole Ca2+ influx system in yeast cells, suggesting that MCA1 and MCA2 can directly mediate Ca2+ influx in the cells lacking both Mid1 and Cch1. Electrophysiological studies have shown that both MCA1 and MCA2 produce stretch-activated currents when expressed in Xenopus laevis oocytes17. These results with yeast cells and Xenopus oocytes suggest that MCA1 and MCA2 mediate Ca2+ influx as mechanosensitive channels, and are not accessory factors that facilitate Ca2+ influx. Overexpression of MCA1 enhances an increase in [Ca2+]cyt upon hypoosmotic shock15. The mca2 mutant exhibits a defect in Ca2+ uptake from the roots16. Structurally, MCA1 and MCA2 have 74% identity and 89% similarity in amino acid sequences15. Both have a single transmembrane segment and an EF-hand-like motif and coiled-coil motif in the N-terminal region, as well as a plac8 motif in the C-terminal region15,18. MCA1-GFP and MCA2-GFP are localized to the plasma membrane15. MCA1 and MCA2 form a homotetramer19,20. Topological analysis has indicated that the EF-hand-like motif, the coiled-coil motif, and the plac8 motif are present in the cytoplasm18, suggesting that both channels recognize intracellular Ca2+. The MCA genes are conserved in the plant kingdom21, and an increase in [Ca2+]cyt as a result of hypo-osmotic shock is mediated by MCA proteins in rice and tobacco22,23.
Application of the patch-clamp technique has demonstrated that Ca2+-permeable channels are transiently activated by cold shock in Arabidopsis mesophyll cells7. In plants, extracellular freezing causes dehydration and mechanical stresses on the plasma membrane, and cold-acclimated plant plasma membranes become resistant to mechanical stress24. Expression of CBF2 is induced not only by cold, but also by mechanical stress25. Therefore, it is assumed that mechanical stress may be one of the factors involved in cold acclimation.
Three CBF/DREB1 (C-repeat binding factor/DRE binding factor 1) transcription factors have been extensively studied. They belong to the AP2/ERF (Apetala/ethylene-responsive factor) superfamily and are important factors for cold acclimation in plants26. CBF/DREB1 genes are rapidly and transiently induced after cold treatment27, and overexpression of CBF/DREB1 constitutively enhances freezing tolerance28,29. Under cold stress, CBF/DREB1 proteins bind to CRT/DRE cis-elements in the promoter of cold-regulated (COR) genes and induce transcription28. However, gene expression analyses reveals that only 6.5% of the total COR genes are regulated by CBF/DREB130. In addition to CBF/DREB1 genes, 27 transcription factors that were up-regulated at an early stage after cold treatment were considered as first-wave transcription factors30. Use of the cbf1/2/3 triple mutant showed that six first-wave transcription factors are partially regulated by CBF/DREB1, whereas the transcription factors HSFC1, ZAT12, and CZF1, which regulate cold-regulated genes30,31, are not regulated by CBF/DREB132. As acclimated cbf1/2/3 triple mutants are more tolerant of freezing stress than non-acclimated ones33, and the expression of a large number of cold-regulated genes is not affected by the cbf1/2/3 triple mutation32, a CBF/DREB1-independent pathway may control cold tolerance. Overexpression of HSFC1 enhances cold tolerance without an increase in expression of CBF1, CBF2, or CBF330, suggesting that HSFC1 is one of the important transcription factors controlling non-CBF/DREB1 regulons and cold tolerance.
Here, we demonstrate that MCA1 and MCA2 are involved in a transient rise in [Ca2+]cyt upon cold shock. The cold-induced increase in [Ca2+]cyt was smaller in the mca1 and mca2 mutants than in the Col-0 wild type. The mca1 mca2 double mutant exhibited increased sensitivity to chilling and freezing stresses. These results suggest that MCA1 and MCA2 are involved in cold-induced Ca2+ influx and that the reduced [Ca2+]cyt increase caused by the mca1 and mca2 mutations affects cold acclimation. As the CBF/DREB1 genes and their regulon genes were not down-regulated in the mca1 mca2 mutant, MCA may not be involved in the regulation of CBF/DREB1-dependent cold signaling.
Results
MCA1 and MCA2 are involved in a cold-induced [Ca2+]cyt increase
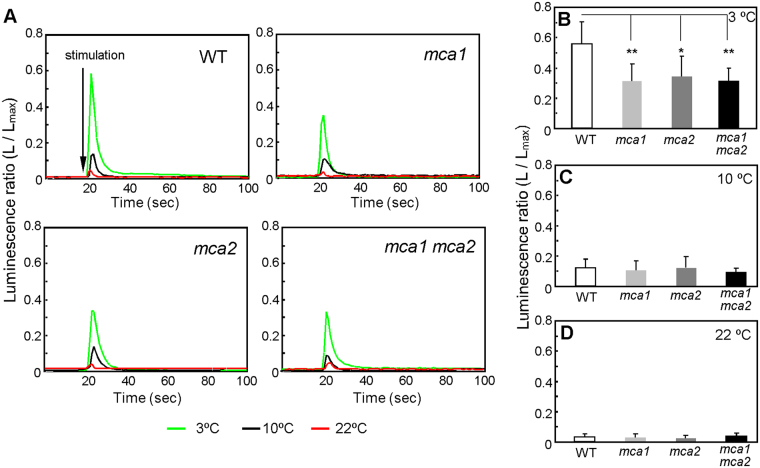
To monitor changes in [Ca2+]cyt (the cytosolic concentration of Ca2+), Arabidopsis seedlings expressing aequorin, a Ca2+ indicator15, that had been immersed in MS medium (400 µl) at 22 °C were exposed to low temperatures by the addition of MS medium (500 µl) kept at 3, 10, or 22 °C. [Ca2+]cyt in the wild type was significantly increased by a 3 °C shock (Fig. 1A green line and B), moderately by a 10 °C shock (Fig. 1A black line and C), and just a little by a 22 °C shock (Fig. 1A red line and D). On the other hand, the magnitude of the cold-induced [Ca2+]cyt increase was markedly lower in the mca1, mca2, and mca1 mca2 mutants (Fig. 1). Small increases observed in response to the 22 °C shock in both the wild type and the mutants could be a consequence of mechanical stress rather than cold stress, because MS medium (a fluid) was added to induce the response. These results suggest that MCA1 and MCA2 contribute to a [Ca2+]cyt increase upon cold shock.
Figure 1.
Transient cold-induced increase in cytosolic Ca2+ is lower as a result of the mca mutation. (A) Relative luminescence of plants harboring aequorin was measured before and after the addition (indicated by the vertical arrow) of precooled solution (3 °C or 10 °C) or room temperature solution (22 °C). The figures are of representative data. The peak luminescence after the addition of solution at 3 °C (B, n ≥ 10), 10 °C (C, n ≥ 9), and 22 °C (D, n ≥ 17) is shown. Data represent the means ± SD. n indicates the number of seedlings. *p < 0.05; **p < 0.005 versus the wild type. Significance was determined using unpaired Student’s t tests.
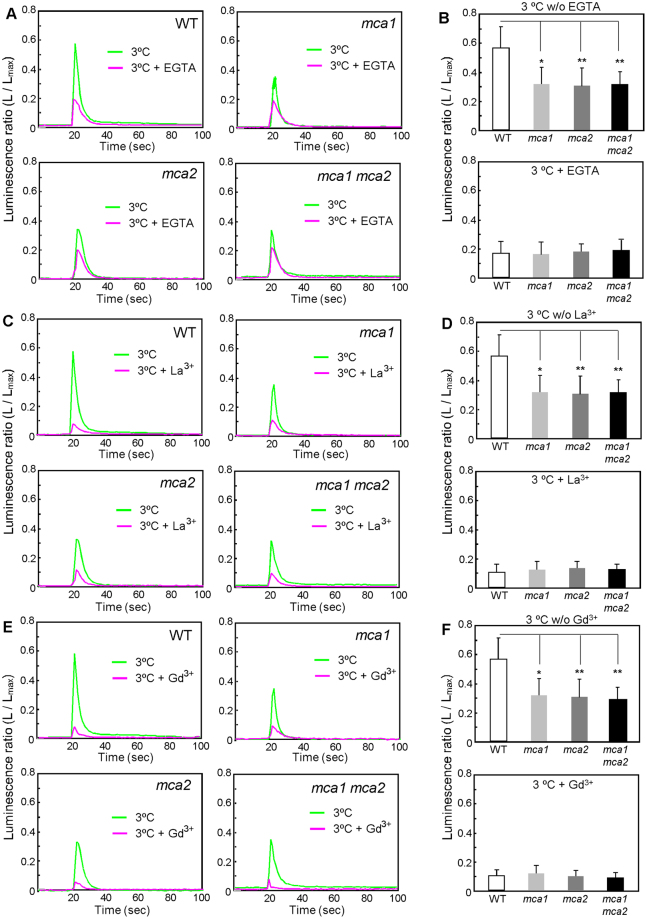
Since MCA1 and MCA2 are present in the plasma membrane15,16, the cold-induced [Ca2+]cyt increase could be brought about by Ca2+ influx. To examine this possibility, we preincubated seedlings for 30 min in MS medium including either a Ca2+ chelator, EGTA, or a plasma membrane ion channel blocker, La3+ or Gd3+, and then monitored changes in [Ca2+]cyt upon cold shock. As expected, the cold-induced [Ca2+]cyt increase was inhibited by EGTA (Fig. 2A,B), La3+ (Fig. 2C,D), and Gd3+ (Fig. 2E,F) in the wild type and in all the mca mutants, although the inhibition rates of the wild type were greater than those of the mca mutants. It should also be noted that significant [Ca2+]cyt increases remained in all the mca mutants, as well as in the wild type, suggesting that there is another cold-induced Ca2+ transport system(s) that is insensitive to the blockers we used in the plasma membrane, or that is in the intracellular compartment.
Figure 2.
Effect of channel blockers and a Ca2+ chelator on the cold-shock-induced [Ca2+]cyt increase. Thirty minutes before cold shock, 5 mM EGTA (A), 1 mM La3+ (C), or 1 mM Gd3+ (D) was added to the medium. Then, the relative luminescence of a plant harboring aequorin was measured, as in Fig. 1, before and after the application of precooled solution (3 °C). The peak luminescence after the addition of the solution at 3 °C with or without 5 mM EGTA (B, n ≥ 17), 1 mM La3+ (D, n ≥ 9), or 1 mM Gd3+ (F, n ≥ 9) is shown. Data represent the means ± SD. *p < 0.05; **p < 0.005 versus the wild type in each treatment. Significance was determined using unpaired Student’s t tests.
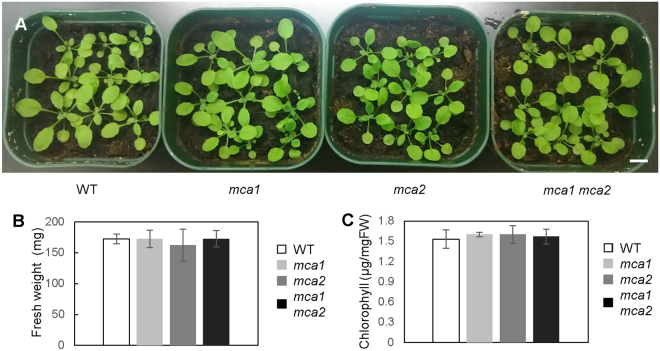
Even though the mca mutants exhibited a reduced cold-induced [Ca2+]cyt increase, the mutants looked healthy when they grew under normal conditions (Fig. 3A). To examine whether the mca mutation affects plant growth under normal conditions, fresh weight and chlorophyll contents were measured (Fig. 3B,C). The mca1, mca2, and mca1 mca2 plants had similar values, as did the wild type, suggesting that plant development in the aerial part is unaffected by MCA1 and MCA2.
Figure 3.
The growth of the mca1 mca2 mutant is normal at room temperature. (A) Representative 3-week old plants of wild type, mca1, mca2, and mca1 mca2 mutants (nine plants in each pot) are displayed. The bar indicates 1-cm length. Fresh weight (B) and chlorophyll content (C) of each plant were measured. Values represent the means ± SD (n = 10). No significant difference was observed with an unpaired Student’s t-test.
Mutations in MCA1 and MCA2 result in cold sensitivity
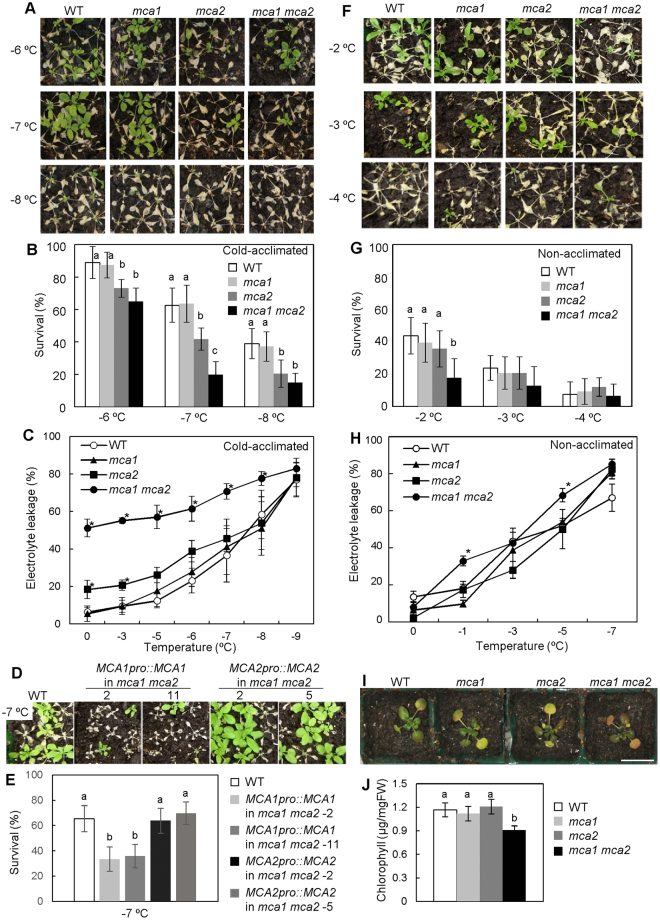
Since the mca mutants exhibited a reduced cold-induced [Ca2+]cyt increase, we investigated their cold sensitivity. Three-week-old plants were incubated at 4 °C for 1 week to acclimate to cold stress. These plants were then exposed to freezing temperatures (Fig. 4A,B). Before this exposure, mca1, mca2, mca1 mca2 and the wild-type plants looked healthy (Fig. 3A). After this exposure, the survival of the mca1 mutant was similar to that of the wild type. On the other hand, the mca2 mutant exhibited a freezing-sensitive phenotype (Fig. 4A,B). Furthermore, the mca1 mca2 double mutant was more sensitive to freezing stresses than the mca2 mutant (Fig. 4A,B). Electrolyte leakage from the mca1 mca2 double mutant was much higher (approximately 50%) than that of the mca single mutants and the wild type, even before it was subjected to freezing temperatures, and it increased as the freezing temperature was lowered (Fig. 4C). At every freezing temperature we employed (−3 to −9 °C), the leakage was greatest in the double mutant. To confirm whether the freezing sensitivity of the mca1 mca2 mutant was caused by the mutation in MCA1 or MCA2, complement lines were produced. MCA1pro::MCA1 or MCA2pro::MCA2 was expressed in the mca1 mca2 mutant (Fig. 4D,E). Because the own promoter was used for expression of MCA1 or MCA2, the expression level of MCA1 in the complement lines, MCA1pro::MCA1 in mca1 mca2, was similar to that of wild type and the mca2 mutant (Figure S1). The expression level of MCA2 in the complement lines, MCA2pro::MCA2 in mca1 mca2, was slightly higher than that of WT and mca1 (Figure S1). The sensitivity of MCA2pro::MCA2-expressing mca1 mca2 mutant was recovered (Fig. 4E). On the other hand, the survival ratio of MCA1pro::MCA1-expressing mca1 mca2 was similar to that of mca2 (Fig. 4E). The wild-type, mca1, mca2, and mca1 mca2 plants without cold acclimation were also treated with a freezing temperature for 1 h (Fig. 4F). Before acclimation, the cold sensitivity of the mca1 mca2 plants was slightly greater than that of wild-type, mca1 and mca2 plants (Fig. 4G). Furthermore, electrolyte leakage of the mca1 mca2 mutant was a little higher than that of the wild type (Fig. 4H). These results suggest that MCA mainly functions in the regulation of cold tolerance during cold acclimation.
Figure 4.
The mca1 mca2 double mutant exhibited sensitivity to cold stress. (A) Freezing sensitivity of the mca1 mca2 double mutant after cold acclimation. Three-week-old plants were incubated at 4 °C for 1 week, then plants were used for freezing treatment. Photographs are representative plants 7 days after 4-h exposure to the indicated temperature. (B) Survival rates were determined for 9 plants after freezing treatment at the indicated temperature. The survival ratio was calculated from 9 plants per pots. Data represent the means ± SD calculated from the data of 9 independent experiments. Differences between the values of each treatment were evaluated by one-way ANOVA followed by the Tukey-Kramer test. Difference of alphabet letters at each temperature indicates statistically significant difference (p < 0.05). (C) Electrolyte leakage from cold-acclimated wild-type, mca1, mca2, and mca1 mca2 plants after exposure to the indicated temperature (programmed to cool at a rate of 2 °C h−1). Data represent the means ± SD (n = 4 leaves, each from a different plant). *p < 0.05 compared with the value of wild type at each temperature (one-way ANOVA followed by the Tukey-Kramer test). Data were representative experiments from 3 biological independent experiments. (D) Freezing sensitivity of the mca1 mca2 double mutant harboring MCA1pro::MCA1 (lines #2 and #11) or MCA2pro::MCA2 (lines #2 and #5). Photographs are representative plants 7 days after 4-h exposure to −7 °C. (E) The survival ratio at −7 °C was calculated from 9 plants per pots. Data represent the means ± SD calculated from the data of 9 independent experiments. Difference between values of each treatment were evaluated by one-way ANOVA followed by the Tukey-Kramer test. Difference of alphabet letters at each temperature indicates statistically significant difference (p < 0.05). (F) Freezing sensitivity of the mca1 mca2 double mutant without cold acclimation. Three-week-old plants were treated with a freezing temperature. Photographs are representative plants after freezing treatment. Photographs are representative plants 7 days after 1-h exposure to the indicated temperature. (G) The survival ratio was calculated from 9 plants per pots. Difference between values of each treatment were evaluated by one-way ANOVA followed by the Tukey-Kramer test. Difference of alphabet letters at each temperature indicates statistically significant difference (p < 0.05). (H) Electrolyte leakage from non-acclimated wild-type, mca1, mca2, and mca1 mca2 plants after exposure to the indicated temperature. Data represent the means ± SD (n = 3 leaves, each from a different plant). *p < 0.05 compared with the value of wild types at each temperature (ANOVA followed by the Tukey-Kramer test). (I) Photographs are of representative wild type, mca1, mca2, and mca1 mca2 mutants after incubation at 4 °C for 1 month. Five-day-old plants were incubated at 4 °C for 1 month. (J) The chlorophyll content of the plants was determined. Values represent the means ± SD (n = 7 plants per each genotype). Difference of alphabet letters indicates a significant difference (p < 0.05) as determined by one-way ANOVA followed by the Tukey-Kramer test.
To examine whether the mutant exhibits chilling sensitivity, wild-type, mca1, mca2, and mca1 mca2 plants were incubated at 4 °C for 1 month under continuous light conditions. The leaves of the mca1 mca2 double mutant looked unhealthy (Fig. 4I). Thus, to quantify chilling sensitivity, chlorophyll content was measured. The chlorophyll content in the mca1 mca2 double mutant was approximately three-fourths that of the wild type (Fig. 4J). No detectable difference was observed between the wild type and the mca single mutants. These results suggest that the double mutation in MCA1 and MCA2 results in hypersensitivity to cold stress in Arabidopsis plants.
Down-regulation of cold-inducible genes is governed by a non-CBF/DREB1 regulon
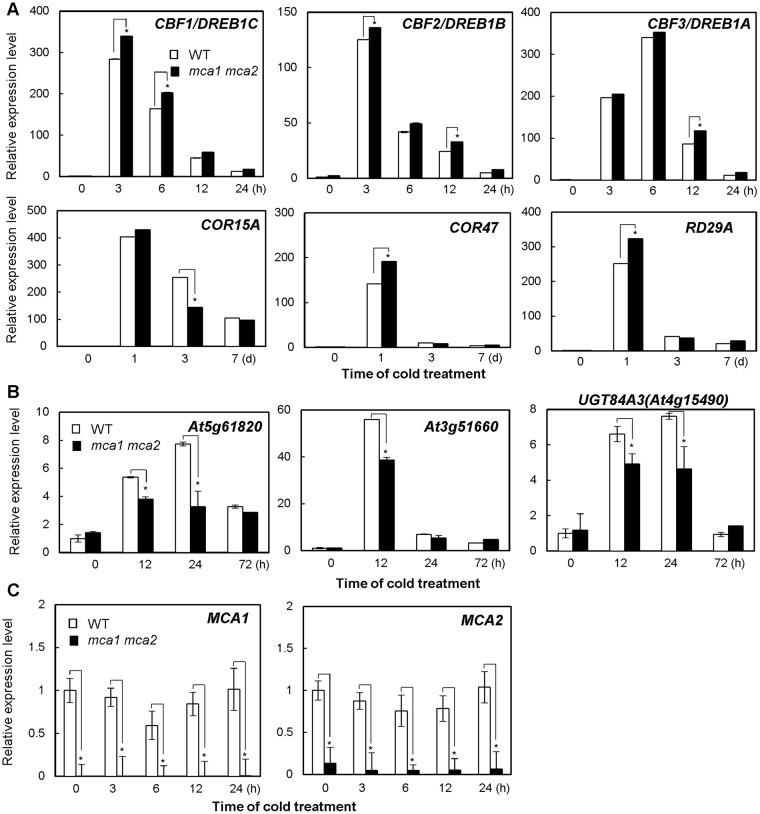
The expression of the CBF/DREB1 genes and their regulon genes, COR15 A, COR47, and RD29A, was investigated. To perform this, three-week-old wild-type, mca1, mca2, and mca1 mca2 plants were exposed to cold at 4 °C for appropriate periods, and RNA prepared from the plants was subjected to a quantitative RT-PCR analysis. Interestingly, the expression of these genes was slightly up-regulated in the mca1 mca2 double mutant, especially soon after the start of the cold treatment (Fig. 5A). This increase could be the consequence of a compensatory response caused by a lack of the function of MCA1 and MCA2 and suggests that both proteins may control another cold signaling pathway. CBF/DREB1 and its regulatory genes are only partly responsible for the acquisition of tolerance to freezing stress for cold acclimation30. Therefore, we examined the expression of cold-inducible genes that are governed by HSFC1 but not by CBF230, such as At5g61820, At3g51660, and At4g15490, which encode an unknown protein, a tautomerase/MIF superfamily protein, and a UDP-glycosyltransferase superfamily protein, respectively. Figure 5B shows that the expression of the three genes was significantly down-regulated in the mca1 mca2 double mutant. Expression of MCA1 and MCA2 themselves was unchanged upon cold shock (Fig. 5C). According to these results, it is plausible that MCA1 and MCA2 mediate cold tolerance by participating in a pathway other than the CBF/DREB1 pathway.
Figure 5.
The expression levels of several cold-regulated genes, which are not regulated by CBF/DREB1, were reduced in the mca1 mca2 double mutant. (A) Relative mRNA transcript levels of CBF/DREB1 and its regulon genes, COR15A, COR47, and RD29A, in wild-type and mca1 mca2 plants were determined by quantitative RT-PCR analyses. Three-week-old plants grown at 24 °C were incubated at 4 °C for the indicated time. Values represent the means ± SD (n = 3) from representative experiments from 3 biologically independent experiments. (B) Relative expression levels of cold-regulated genes that are not CBF/DREB1-regulon genes30 were determined by quantitative RT-PCR analyses. Values represent the means ± SD (n = 3) from representative experiments from 3 biologically independent experiments. (C) The expression levels of MCA1 and MCA2 in wild types and mca1 mca2 double mutants were investigated by quantitative RT-PCR analyses. An asterisk indicates a significant difference from wild-type plants at each point (p < 0.05) as determined by unpaired Student’s t-tests.
Discussion
In the present study, we have demonstrated that MCA1 and MCA2 play a role in the transient rise in [Ca2+]cyt upon cold shock and are also involved in chilling and freezing tolerance. The mca1 mca2 double mutant exhibited a lower cold-induced increase in [Ca2+]cyt than the wild type (Fig. 1), as well as an increased sensitivity to cold stress (Fig. 4). Although the mca1 and mca2 single mutants exhibited a lower cold-induced increase in [Ca2+]cyt like the double mutant, the mca1 single mutant did not show a cold sensitive phenotype similar to that of the mca2 single and mca1 mca2 double mutants. We speculate that a reason for this discrepancy may be a difference in the spatial expression patterns of MCA1 and MCA2 in Arabidopsis plants16, as explained in more detail in a later paragraph. As for the regulation of gene expression, CBF/DREB1 genes and their regulon genes were not down-regulated in the mca1 mca2 double mutant (Fig. 5), suggesting that MCA may not regulate CBF/DREB1-dependent cold signaling.
Different stimuli produce different patterns of Ca2+ elevation and oscillations with different frequencies, and these are called Ca2+ signatures. As shown in Fig. 1, low-temperature stress stimulates a transient increase in [Ca2+]cyt34. MCA1 and MCA2 have been identified as plasma membrane proteins involved in Ca2+ influx in response to mechanical stimuli, such as touch, gravity, flexure, and turgor15,16. In the mca1 or mca2 mutant, the magnitude of the cold-induced [Ca2+]cyt increase was lower, by approximately 40%, than that in the wild type (Fig. 1). These results suggest that MCA1 and MCA2 are partially involved in Ca2+ influx in response to cold shock. Application of a mechanosensitive Ca2+ channel blocker, Gd3+, prevents the induction of cold-regulated genes35. As two Ca2+ channel inhibitors, La3+ and Gd3+, still reduced the cold-induced [Ca2+]cyt increase in the mca mutants, and the mca mutations were unable to block [Ca2+]cyt increases completely (Fig. 2), other cold-activatable Ca2+ transport system(s) must exist in the plasma membrane and/or organellar membranes. Indeed, it is reported that the vacuole, the major intracellular Ca2+ store, is involved in a cold-induced Ca2+ release8. In the present study, we did not calibrate the bioluminescent intensity of aequorin for [Ca2+]cyt because of difficulties in the precise calibration, although we noted a report describing a successful calibration specific for the isoform of aequorin and temperature that the authors used8. It should be mentioned that although the present study has clearly suggested the involvement of MCA1 and MCA2 in cold-induced [Ca2+]cyt increases, it remains to be examined whether complementation lines of mca1, mca2 and mca1 mca2 mutants expressing aequorin show a wild-type level of cold-induced [Ca2+]cyt increases.
Plants employ several kinds of mechanisms to control Ca2+-regulated gene expression36. However, it is still unclear how cold-induced [Ca2+]cyt increases are recognized. One possible mechanism involves calmodulin-binding transcription factors (CAMTAs). CAMTAs possess calmodulin (CaM)-binding domains37 and CAMTAs play a role in the regulation of gene expression in response to Ca2+ signals38. CAMTA3 is a positive regulator of CBF2/DREB1C expression and binds to the consensus sequence of a CGCG core motif, a cis-element for CAMTAs, in the promoter of CBF2/DREB1C39. The camta2 camta3 double mutant is sensitive to freezing temperatures39. Microarray analyses demonstrated that the expression level of HSFC1 (At3g24520) in the camta1/2/3 mutant is lower than that in the wild type40. CAMTA is one of possibilities how increased [Ca2+]cyt is recognized.
As shown in Fig. 1, the mca1 mca2 double mutation reduced about 40% of the [Ca2+]cyt increase. The transient increase in [Ca2+]cyt may be conducted by other Ca2+-permeable channels that are responsible for 60% of the transient increase. One such channel could be AtGLR3.4, a member of the Arabidopsis homologs of ionotropic glutamate receptors, whose expression is up-regulated under cold stress41. Another such channel could be the cyclic nucleotide-gated ion channel (CNGC) family. In rice, the expressions of 10 out of 16 CNGC genes are induced under cold stress42.
The mca1 mca2 double mutant exhibited increased sensitivity to chilling and freezing stresses, even though the single mutants did not exhibit a severe phenotype (Fig. 4). Both MCA1 and MCA2 complement a Ca2+ uptake deficiency of yeast cells lacking a Ca2+ channel composed of Mid1 and Cch115,16, and generate stretch-activated currents in Xenopus oocytes17. Even though MCA1 and MCA2 have similar functions as Ca2+-permeable mechanosensitive channels, their spatial expression patterns are not necessarily the same in whole plants16. MCA1p::GUS and MCA2p::GUS are expressed in vascular tissues of cotyledons, leaves and primary roots in common. On the other hand, MCA1p::GUS is expressed in the promeristem and adjacent elongation zone of the primary root, while MCA2p::GUS is not. MCA2p::GUS is expressed in mesophyll cells of cotyledons and leaves, but MCA1p::GUS is not. In addition, MCA2p::GUS is expressed more than MCA1p::GUS at the center of rosettes in a region corresponding to the shoot apical meristem. To survive freezing stress, the shoot apical meristem should be protected to recover plant growth43. Based on the observation of differences in the spatial expression patterns of MCA1p::GUS and MCA2p::GUS in whole plants, it is possible to speculate that the differences may allocate MCA1 and MCA2 a role in the acquisition of tolerance to cold stress. This allocation could explain why only the double mutant becomes hypersensitive to chilling and freezing stresses.
According to microarray analyses, several genes were up-regulated in HSFC1-overexpressed plants, but not in CBF2-overexpressed plants30. Some HSFC1-dependent and CBF2-independent cold-regulated genes, such as At5g61820, At3g51660, and At4g15490, encoding an unknown protein, a tautomerase/MIF superfamily protein, and the UDP-glycosyltransferase superfamily protein UGT84A3, respectively, were down-regulated in the mca1 mca2 double mutant (Fig. 5). It is possible that the MCA1/2-regulated Ca2+ signal is transduced to a HSFC1-dependent pathway to enhance cold tolerance. This possibility warrants further study.
In conclusion, two mechanosensitive Ca2+ channels, MCA1 and MCA2, are involved in a cold-induced transient [Ca2+]cyt increase in Arabidopsis, and in the regulation of cold tolerance through a pathway other than the CBF/DREB1-dependent pathway.
Methods
Plant materials
The Columbia-0 (Col-0) of Arabidopsis and its isogenic, transgenic lines mca1-null, mca2-null, and mca1-null mca2-null were previously described15,16. The complementation lines MCA1pro::MCA1 in mca1-null mca2-null and MCA2pro::MCA2 in mca1-null mca2-null were also previously described15,16.
Monitoring of [Ca2+]cyt changes following cold shock treatment
Apoaequorin-expressing seedlings grown at 22 °C on MS medium supplemented with 0.8% agar and 1% sucrose under 16-h light conditions at 40–60 µM m−2 s−1 light intensity were used to monitor [Ca2+]cyt changes upon cold shock. A seedling was harvested 14 days after sowing and incubated overnight at 22 °C in 2 ml of MS medium containing 2.5 µM coelenterazine in the dark to reconstitute aequorin. The seedling was transferred to fresh MS medium (400 µl) kept at 22 °C in a tube (Microtech-Nition, #NU-063, Funabashi, Japan) and received an additional 500 µl of the same medium kept at 3, 10, or 22 °C. Luminescence (L) from aequorin in the whole seedlings was measured using a luminometer (Microtech-Nition, Model NU-2500). At the end of each monitoring, 1 ml of 20% ethanol/2 M CaCl2 solution was added to the medium (0.9 ml total) to measure the maximum luminescence (Lmax). The luminescence ratios (L/Lmax) are presented in Figs 1 and 2.
Plant freezing and chilling assay
Wild-type (ecotype Col-0), mca1, mca2, and mca1 mca2 plants were grown at 24 °C for 3 weeks in soil with fluorescent lighting (16 h/8 h light/dark photoperiod). These plants were then incubated at 4 °C for 1 week for acclimation to low temperatures. For non-acclimation, 3-week-old plants were treated with the freezing temperature without incubation at 4 °C. Whole-plant freezing assays were performed as previously described44. Briefly, plants were incubated at 0 °C for 1 h, and the temperature was lowered by 2 °C h−1 until it reached to the indicated temperature, and then held at the desired temperature for 1 h or 4 h for non-acclimated plants or cold-acclimated plants, respectively, in the incubator (IN602, Yamato Scientific Co., Ltd., Tokyo, Japan). After cold acclimation, the plants were incubated at 4 °C overnight and transferred to 24 °C. The survival ratio was determined 1 week after the freezing test.
For the chilling assay, 5-day-old plants were incubated at 4 °C. After incubation for 1 month under constant illumination, the chlorophyll content of the plants was determined. Eighty percent acetone was added to leaves ground with liquid nitrogen. The mixture was shaken at 4 °C. After centrifugation, the absorbances of the supernatant at 663 nm and 646 nm were measured. Total chlorophyll content was calculated as 17.3 A646 + 7.18 A66345.
Electrolyte leakage from fully developed rosettes of leaves of three-week-old plants was measured as previously described46,47. The sample was incubated in a refrigerated circular bath (TRL-11P, Thomas Kagaku Kikai, Co., Ltd., Japan). The conductivity was measured with a conductivity meter (CD-4302, Lutron Electronic Enterprise Co., Ltd., Taipei, Taiwan).
RNA preparation and quantitative RT-PCR
Three-week-old wild-type (ecotype Col-0), mca1, mca2, and mca1 mca2 plants were subjected to cold treatment at 4 °C for the indicated time. Isolation of total RNA, cDNA synthesis, and quantitative RT-PCR were performed as previously described47. The primers used to detect CBF/DREB1 and its regulon genes were also previously described47. Other genes were detected with gene-specific primers for At5g61820 (5′-GAGGCACCTGCGAGAAGCTTGAG-3′ and 5′ GTAACCATCTTCCCGTTTCTGTC-3′), At3g51660 (5′-GACCTCAAAACTTAGTGATGGTG-3′ and 5′-TTAACTTGTTTGGTGATGCCTCC-3′), At4g15490 (5′-CCTCCCATGGAAGGGACATTTGTAGA-3′ and 5′-ACAAGCAATCGCAGGATGAGCCA-3′), MCA1 (5′-AAGATTGCCACTGCAGCATCC-3′ and 5′-ACGCCATTAGCTCATTACATGCTTC-3′), and MCA2 (5′-AAGATCATTGCAACACCGTGGA-3′ and 5′-GTGTCTTCAAGCAAAGACAAGGTTC-3′).
Electronic supplementary material
Acknowledgements
We thank Ms. Rieko Nozawa and Ms. Yuri Nemoto of the University of Tsukuba, Dr. Masataka Nakano of Tokyo Gakugei University for technical support, and Ms. Yumiko Higashi of Tokyo Gakugei University for secretarial help. This research was supported by Grants-in-Aid for Scientific Research on Innovative Areas (23120509 and 25120708 to HI; JP16H01458 to KM) and KAKENHI (JP16K07390 to KM) from the Ministry of Education, Culture, Sports, Science & Technology of Japan, and a Cooperative Research Grant from the Plant Transgenic Design Initiative, Gene Research Center, University of Tsukuba (to HI).
Author Contributions
Hidetoshi Iida and Kenji Miura contributed to designing the experiments. Kendo Mori, Na Renhu, Maho Naito, Aki Nakamura, and Hayato Shiba performed the experiments, and collected and analyzed the data. Tsuyoshi Yamamoto, Takuya Suzaki, Hidetoshi Iida, and Kenji Miura contributed to data interpretation and preparation of the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Kendo Mori and Na Renhu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17483-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hidetoshi Iida, Email: iida@u-gakugei.ac.jp.
Kenji Miura, Email: miura.kenji.ga@u.tsukuba.ac.jp.
References
- 1.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:s401–417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 3.White PJ, Broadley MR. Calcium in plants. Ann. Bot. 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monshausen GB. Visualizing Ca2+ signatures in plants. Curr. Opin. Plant Biol. 2012;15:677–682. doi: 10.1016/j.pbi.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 5.McAinsh MR, Pittman JK. Shaping the calcium signature. New Phytol. 2009;181:275–294. doi: 10.1111/j.1469-8137.2008.02682.x. [DOI] [PubMed] [Google Scholar]
- 6.Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 7.Carpaneto A, et al. Cold transiently activates calcium-permeable channels in Arabidopsis mesophyll cells. Plant Physiol. 2007;143:487–494. doi: 10.1104/pp.106.090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight MR. Signal transduction leading to low-temperature tolerance in Arabidopsis thaliana. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:871–875. doi: 10.1098/rstb.2002.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karashima Y, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aubdool AA, et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014;5:5732. doi: 10.1038/ncomms6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Camino D, et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias-Darraz L, et al. A transient receptor potential ion channel in Chlamydomonas shares key features with sensory transduction-associated TRP channels in mammals. Plant Cell. 2015;27:177–188. doi: 10.1105/tpc.114.131862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa Y, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA. 2007;104:3639–3644. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka T, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–1296. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuichi T, Iida H, Sokabe M, Tatsumi H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal. Behav. 2012;7:1022–1026. doi: 10.4161/psb.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamano S, et al. Transmembrane topologies of Ca2+-permeable mechanosensitive channels MCA1 and MCA2 in Arabidopsis thaliana. J. Biol. Chem. 2015;290:30901–30909. doi: 10.1074/jbc.M115.692574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano M, Iida K, Nyunoya H, Iida H. Determination of structural regions important for Ca2+ uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast. Plant Cell Physiol. 2011;52:1915–1930. doi: 10.1093/pcp/pcr131. [DOI] [PubMed] [Google Scholar]
- 20.Shigematsu H, et al. Structural characterization of the mechanosensitive channel candidate MCA2 from Arabidopsis thaliana. PLoS ONE. 2014;9:e87724. doi: 10.1371/journal.pone.0087724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H. Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 2013;18:227–233. doi: 10.1016/j.tplants.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Kurusu T, et al. Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol. 2012;12:11. doi: 10.1186/1471-2229-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurusu T, et al. Involvement of the putative Ca2+-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca2+ uptake, Ca2+-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J. Plant Res. 2012;125:555–568. doi: 10.1007/s10265-011-0462-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki T, Kawamura Y, Uemura M. Cryobehavior of the plasma membrane in protoplasts isolated from cold-acclimated Arabidopsis leaves is related to surface area regulation. Plant Cell Physiol. 2008;49:944–957. doi: 10.1093/pcp/pcn068. [DOI] [PubMed] [Google Scholar]
- 25.Zarka DG, Vogel JT, Cook D, Thomashow MF. Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol. 2003;133:910–918. doi: 10.1104/pp.103.027169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miura K, Furumoto T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013;14:5312–5337. doi: 10.3390/ijms14035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmour SJ, et al. Low temperature regulation of the Arabisopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, et al. Regulation of the Arabidopsis CBF regulon by a complex low-temperature regulatory network. Plant J. 2015;82:193–207. doi: 10.1111/tpj.12796. [DOI] [PubMed] [Google Scholar]
- 31.Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao C, et al. Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol. 2016;171:2744–2759. doi: 10.1104/pp.16.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia Y, et al. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in. Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- 34.Knight MR, Knight H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012;195:737–751. doi: 10.1111/j.1469-8137.2012.04239.x. [DOI] [PubMed] [Google Scholar]
- 35.Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 2002;31:629–638. doi: 10.1046/j.1365-313X.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 36.Galon Y, Finkler A, Fromm H. Calcium-regulated transcription in plants. Mol. Plant. 2010;3:653–669. doi: 10.1093/mp/ssq019. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Whalley HJ, Knight MR. Combining modelling and experimental approaches to explain how calcium signatures are decoded by calmodulin-binding transcription activators (CAMTAs) to produce specific gene expression responses. New Phytol. 2015;208:174–187. doi: 10.1111/nph.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whalley HJ, Knight MR. Calcium signatures are decoded by plants to give specific gene responses. New Phytol. 2013;197:690–693. doi: 10.1111/nph.12087. [DOI] [PubMed] [Google Scholar]
- 39.Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Park S, Gilmour SJ, Thomashow MF. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013;75:364–376. doi: 10.1111/tpj.12205. [DOI] [PubMed] [Google Scholar]
- 41.Meyerhoff O, et al. AtGLR3.4, a glutamate recepteer-like gene is sensitive to touch and cold. Planta. 2005;222:418–427. doi: 10.1007/s00425-005-1551-3. [DOI] [PubMed] [Google Scholar]
- 42.Nawaz Z, Kakar KU, Saand MA, Shu QY. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics. 2014;15:853. doi: 10.1186/1471-2164-15-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearcec RS, McDonald I. Ultrastructural damage due to freezing followed by thawing in shoot meristem and leaf mesophyll cells of tall fescue (Festuca arundinacea Schreb.) Planta. 1977;134:159–168. doi: 10.1007/BF00384966. [DOI] [PubMed] [Google Scholar]
- 44.Miura K, Ohta M, Nakazawa M, Ono M, Hasegawa PM. ICE1 Ser403 is necessary for protein stabilization and regulation of cold signaling and tolerance. Plant J. 2011;67:269–279. doi: 10.1111/j.1365-313X.2011.04589.x. [DOI] [PubMed] [Google Scholar]
- 45.Zakhleniuk OV, Raines CA, Lloyd J. C. pho3: a phosphorus-deficient mutant of Arabidopsis thaliana (L.) Heynh. Planta. 2001;212:529–534. doi: 10.1007/s004250000450. [DOI] [PubMed] [Google Scholar]
- 46.Miura K, Ohta M. SIZ1, a small ubiquitin-related modifier ligase, controls cold signaling through regulation of salicylic acid accumulation. J. Plant Physiol. 2010;167:555–560. doi: 10.1016/j.jplph.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Miura K, et al. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell. 2007;19:1403–1414. doi: 10.1105/tpc.106.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.