Summary
T helper type 2 (Th2) cells, type 2 innate lymphoid cells (ILC2s) and eosinophil progenitors have previously been described to produce interleukin‐5 (IL‐5) in the airways upon allergen provocation or by direct administration of IL‐33. Eosinophilic airway inflammation is known to be associated with IL‐5‐dependent eosinophil development in the bone marrow, however, the source of IL‐5 remains unclear. T helper cells, ILC2s and CD34+ progenitors have been proposed to be involved in this process, therefore, we investigated whether these cells are taking part in eosinophilopoiesis by producing IL‐5 locally in the bone marrow in IL‐33‐driven inflammation. Airway exposure with IL‐33 led to eosinophil infiltration in airways and elevated eotaxin‐2/CCL24. Importantly, IL‐5 production as well as expression of the IL‐33 receptor increased in ILC2s in the bone marrow under this treatment. A small but significant induction of IL‐5 was also found in CD34+ progenitors but not in T helper cells. Similar results were obtained by in vitro stimulation with IL‐33 where ILC2s rapidly produced large amounts of IL‐5, which coincided with the induction of eosinophil hematopoiesis. IL‐33‐mediated eosinophil production was indeed dependent on IL‐5 as both airway and bone marrow eosinophils decreased in mice treated with anti‐IL‐5 in combination with IL‐33. Interestingly, the responsiveness of ILC2s to IL‐33 as well as IL‐33‐induced eotaxin‐2/CCL24 were independent of the levels of IL‐5. In summary, we demonstrate for the first time that IL‐33 acts directly on bone marrow ILC2s, making them an early source of IL‐5 and part of a process that is central in IL‐33‐driven eosinophilia.
Keywords: bone marrow, eosinophilia, IL‐33, IL‐5, ILC2
Abbreviations
- BAL
bronchoalveolar lavage
- CCR3
CC‐chemokine receptor 3
- ILC
innate lymphoid cell
- IL
interleukin
- rm
recombinant murine
- Th
T helper
- WT
wild‐type
Introduction
Eosinophils are granulocytes that are involved in host defence against parasites and play a central role in the pathogenesis of allergic diseases.1, 2 They develop in the bone marrow, from CD34+ hematopoietic progenitor cells, in a process tightly regulated by interleukin‐3 (IL‐3), granulocyte–macrophage colony‐stimulating factor and IL‐5.3 Although many factors contribute to the development of eosinophils, IL‐5 is essential for maturation, survival, proliferation and priming for migration to target tissues.2, 4 Similarly, eotaxin‐1/CCL11 and eotaxin‐2/CCL24, ligands for the cell surface CC‐chemokine receptor 3 (CCR3), regulate eosinophil homing to tissues both in cooperation with IL‐5 and through IL‐5‐independent pathways.4, 5, 6 IL‐5 is typically considered a product of type 2 polarized T helper (Th) cells, however, eosinophils and their progenitor cells also produce a wide range of cytokines that influence hematopoietic differentiation, including IL‐5.7, 8, 9, 10, 11 IL‐5 is also produced by other innate immune cells, and during the last decade, type 2 innate lymphoid cells (ILC2s) have emerged as a new important source of type 2 cytokines in response to IL‐33.12, 13, 14, 15 A positive relationship between IL‐33 and eosinophilia has been demonstrated in several studies, including reports of lower baseline levels of eosinophils in peripheral blood in gene knockout mice that lack IL‐33 or the IL‐33 receptor ST2.16 Moreover, studies of ST2‐deficient mice in allergic inflammatory settings have revealed that disruption of IL‐33 signalling pathways results in impaired eosinophilic airway inflammation and reduced levels of type 2 cytokines upon allergen challenge.17 Interestingly, it was recently shown that IL‐33 not only supports IL‐5 production but also promotes eosinophil development by enhancing IL‐5 receptor (IL5Rα) expression in early ST2‐expressing precursor cells in the bone marrow.16 However, several aspects of IL‐5‐driven eosinophilopoiesis still remain unresolved, including the cellular source of IL‐5 in the bone marrow. It has proved challenging to dissect the exact pathways by which cellular subtypes regulate specific hematopoietic processes and several different cell types have been suggested to be involved in the IL‐5 production in the bone marrow.7, 10, 18 Interestingly, one study that found increased IL‐5 in bone marrow after allergen challenge demonstrated that CD3+ T cells were the major IL‐5‐producing cell type in sensitized mice, whereas CD34+ cells were responsible for the IL‐5‐production in the bone marrow at baseline in non‐sensitized mice.10 Other studies have also linked T cells to bone marrow IL‐5 where increased numbers of IL‐5+ CD3+ cells were detected in bone marrow aspirates from individuals with allergic asthma after airway allergen challenge,19 and adoptive transfer of IL‐5 transgenic CD3+ lymphocytes resulted in increased bone marrow eosinophilia in allergen sensitized and challenged mice.20 In addition to IL‐5, T cells in the bone marrow are also important mediators of progenitor differentiation by secretion of IL‐3 and granulocyte–macrophage colony‐stimulating factor.21 Indeed, T‐cell‐deficient mice have been reported to accumulate myeloid progenitors in the bone marrow, which was reversed by reconstitution of the CD4+ Th cell pool.22 However, a role of Th cells in the bone marrow in eosinophilic inflammation that is not driven by an adaptive allergic response has not yet been reported. Furthermore, although there is evidence for a contribution of ILC2s in eosinophilic airway inflammation, their role in the bone marrow remains to be determined. In this study, we compared the ability of Th cells, CD34+ progenitors and ILC2s to produce IL‐5 in vivo in the bone marrow using a model of IL‐33‐driven eosinophilia. We demonstrate that IL‐33‐induced eosinophilic airway inflammation is accompanied by increased eosinophils in the bone marrow, where ST2 expression was identified on early CD34+ progenitors, a subset of Th cells and on all ILC2s in the bone marrow. Th cells and ILC2s, but not CD34+ progenitors, up‐regulated ST2 expression upon IL‐33, however, IL‐5 production only increased in CD34+ progenitors and ILC2s in response to IL‐33 in vivo and in vitro. Importantly, ILC2s responded more rapidly and strongly towards IL‐33 than the CD34+ progenitors did, and culture supernatants of IL‐33 stimulated ILC2s increased CD34+ IL5Rα + eosinophil progenitors in naive bone marrow cultures. Hence, we propose that IL‐33 and ILC2s, in addition to IL‐5, are important regulators of eosinophil development in the bone marrow.
Materials and methods
Mice
Wild‐type (WT) C57BL/6J mice were purchased from Charles River (Sulzfeld, Germany) and housed in pathogen‐free conditions where they were given food and water ad libitum. Male mice between 8 and 11 weeks of age were included in the study. All procedures were approved by the Animal Ethics Committee at the University of Gothenburg, Gothenburg, Sweden (permit number 126/14).
IL‐33‐induced airway inflammation
IL‐33‐driven inflammation was induced as previously described.14, 15 Briefly, naive mice were given 1 μg recombinant murine IL‐33 (rmIL‐33; PeproTech, Rocky Hill, NJ) in PBS on days 1, 3 and 5 by intranasal administration. Control mice were given PBS. Samples were collected on day 6, 24 hr after the final challenge. In some experiments, mice were given 50 μg anti‐mouse IL‐5 antibodies or isotype control (BD Biosciences, San Jose, CA) by intraperitoneal injection 1 hr before the first intranasal challenge, and in some experiments, mice were given 50 μg anti‐mouse eotaxin‐2/CCL24 antibodies or isotype control (R&D Systems, Minneapolis, MN) by intranasal administration 1 hr before all three intranasal challenges with IL‐33.
Collection of blood, bronchoalveolar lavage and bone marrow
Sample collection was performed as previously described in detail in Johansson et al.15 Blood was collected in tubes containing 2 mm EDTA (Sigma‐Aldrich, St Louis, MO) in PBS for differential cell count or in empty tubes for serum processing. Bronchoalveolar lavage (BAL) was collected from tracheotomized mice by instillation of sterile PBS followed by gentle aspiration. Mediators were analysed in cell‐free BAL, and BAL cells were processed for differential cell count. Bone marrow cells from left and right femurs were flushed out with 10 ml wash buffer (2% fetal calf serum in PBS) and further processed for flow cytometric analysis, in vitro stimulation or differential cell count.
Differential cell count
Bone marrow cells and whole blood were lysed with red blood cell lysis solution consisting of 0·1 mm EDTA (Sigma‐Aldrich) in distilled water supplemented with 0·8% NH4Cl (Merck Chemicals, Darmstadt, Germany) and incubated on ice for 10 min. Eosinophils were identified by histological examination of cytospin preparations (as previously described in ref. 15) from bone marrow, blood and BAL that were stained with Hemacolor Rapid stain (Merck Chemicals) according to the manufacturer's protocol.
Cytokine and chemokine analysis
Mediator levels in cell‐free BAL (eotaxin‐1/CCL11, eotaxin‐2/CCL24 and TARC/CCL17), serum (eotaxin‐1/CCL11, eotaxin‐2/CCL24 and IL‐5) and ILC2 culture supernatants (IL‐5) were analysed using DuoSet® ELISA kits (R&D Systems) according to the manufacturer's protocol. Absorbance was measured on a Varioskan™ LUX multimode microplate reader (ThermoFisher Scientific, Vantaa, Finland).
Flow cytometric analysis of bone marrow cells
Following red blood cell lysis, bone marrow cells were incubated 15 min on ice with 2% mouse serum (Dako, Glostrup, Denmark). Antibodies for surface antigens were incubated for 30 min at 4° in the dark, followed by fixation with 4% paraformaldehyde 15 min at room temperature (dark). Cells were washed in wash buffer and immediately analysed on a BD FACSVerse™ Flow Cytometer running BD facsuite™ software (BD Biosciences). For analysis of intracellular IL‐5, all steps in the staining procedure were performed with solutions supplemented with monensin (4 μl/6 ml), a protein transport inhibitor (BD GolgiStop™; BD Biosciences) until fixation of cells. After fixation, cells were washed with wash buffer and permeabilized with 0·1% saponin (Sigma‐Aldrich) in Hanks' balanced salt solution (HyClone™; GE Healthcare Life Sciences, South Logan, UT). Antibodies for intracellular antigens were incubated for 40 min at room temperature (dark) followed by washing and flow cytometric analysis. Data were analysed with flowjo Software (Tree Star Inc, Ashland, OR). Gating strategies and antibodies for flow cytometry are shown in the Supplementary material (Fig. S1 and Table S1, respectively).
In vitro stimulation of bone marrow cells
Bone marrow cells from naive mice were subjected to red blood cell lysis, counted and seeded at a concentration of 1 × 106/ml in complete cell culture medium (RPMI‐1640 medium, 10% fetal calf serum, 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 55 μm 2‐mercap toethanol). Then, 100 ng/ml rmIL‐33 (PeproTech) was added to stimulated wells. Cells were stimulated for 3, 6, 12 and 24 hr before harvest for intracellular FACS analysis of IL‐5. Monensin (BD GolgiStop™) was added (4 μl/6 ml) 3 hr before harvest to all wells.
ILC2 sorting and stimulation
Following red blood cell lysis, bone marrow cells were seeded in tissue culture flasks for 4 hr in complete cell culture medium for removal of adherent cells. Lineage‐negative cells were enriched from suspension cells by magnetic depletion according to the manufacturer's protocol (Lineage Cell Depletion Kit, MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). The lineage negative fraction was seeded overnight at a concentration of 1 × 106 cells/ml in complete cell culture medium supplemented with rmIL‐2 and rmIL‐7 (PeproTech; 50 ng/ml of each). Cells were harvested the following day and antibodies for surface antigens were incubated for 30 min followed by one wash in PBS supplemented with 1% fetal calf serum, and immediately subjected to FACS sorting on a BD FACS Aria Flow Cytometer running bd facs diva version 6.0 Software. Sorted ILC2s (Lin− CD45+ ICOS+ CD25+ ST2+) were seeded (3500–4800/well) in 200 μl complete cell culture medium supplemented with rmIL‐2, rmIL‐7, rmIL‐25 (R&D Systems), rmIL‐33 (50 ng/ml of each) and recombinant murine thymic stromal lymphopoietin (R&D Systems; 20 ng/ml). Cells were allowed to expand over 14 days while fresh culture medium was added every other day. The expanded ILC2s were rested in complete cell culture medium supplemented with rmIL‐2 and rmIL‐7 (50 ng/ml of each) for 2 days before stimulation with rmIL‐33 (100 ng/ml) for 20 hr and cell‐free supernatants were stored for IL‐5 measurements. In some experiments, cell‐free culture supernatants from IL‐33‐stimulated ILC2s were added at a concentration of 5% (corresponding to a final concentration of approximately 25 ng/ml IL‐5) to naive bone marrow cells. Unstimulated controls were given plain culture media, the bone marrow cells were cultured for 9 hr followed by FACS analysis of eosinophil progenitors.
Statistical analysis
Statistical testing by non‐parametric Mann–Whitney U‐test or paired t‐test was performed using graphpad prism 6 software (GraphPad Software Inc, La Jolla, CA). Statistical significance was defined as *P < 0·05, **P < 0·01 and ***P < 0·001.
Results
IL‐33 elicits airway, blood and bone marrow eosinophilia in vivo
We and others have previously shown that exogenous IL‐33 administration by intraperitoneal injection or by intranasal route (Fig. 1a) gives rise to elevated eosinophils in circulating blood and in peripheral sites such as the lung and BAL.14, 15, 16, 23, 24 Consistent with these reports, mice that were given intranasal IL‐33 demonstrated significantly increased eosinophil numbers in the bone marrow (Fig. 1b,c), blood (Fig. 1d) and BAL (Fig. 1e) as determined by differential cell count (representative contrast stained cells in Fig. 1b). Analysis of eosinophil‐attracting chemokines in serum (Fig. 1f,g) and BAL (Fig. 1h,i) revealed that IL‐33‐challenged mice had elevated levels of eotaxin‐2/CCL24, but not eotaxin‐1/CCL11, in the circulation and locally in the airways, indicating that bone marrow eosinophils are recruited to the airways in response to IL‐33. Indeed, pre‐treatment with anti‐eotaxin‐2/CCL24 antibodies 1 hr before IL‐33 challenges decreased BAL eosinophils (Fig. 1j).
Figure 1.
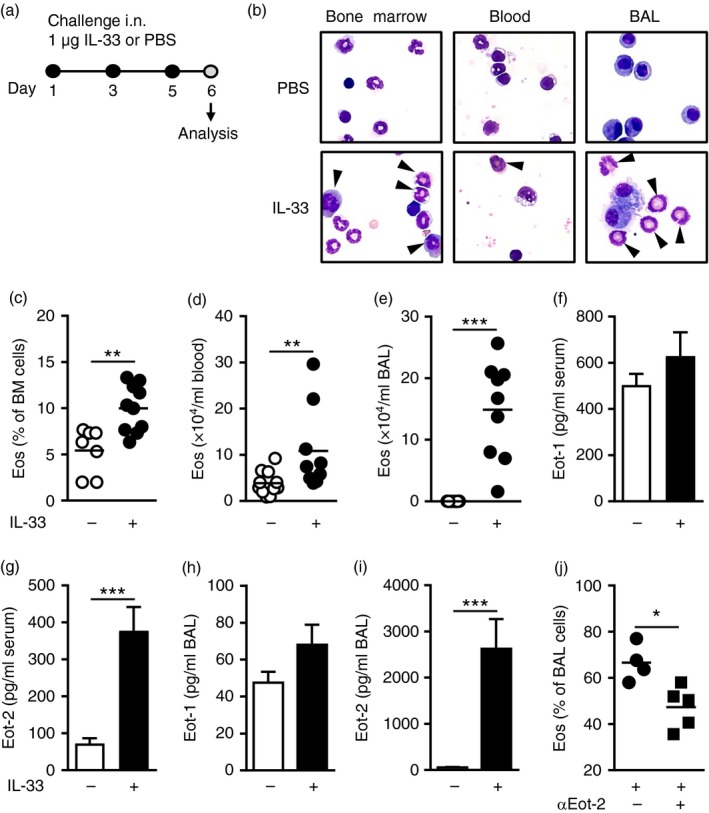
IL‐33 elicits airway, blood and bone marrow eosinophilia. (a) Experimental model of IL‐33‐induced inflammation. (b) Contrast‐stained cells; arrows indicate eosinophils. (c) Percentage eosinophils in bone marrow, and total eosinophils in blood (d) and bronchoalveolar lavage (BAL) (e) from IL‐33‐ or PBS‐treated mice. Eotaxin‐1/CCL11 (f) and eotaxin‐2/CCL24 (g) protein levels in serum, and eotaxin‐1/CCL11 (h) and eotaxin‐2/CCL24 (i) protein levels in BAL from IL‐33‐ or PBS‐treated mice. (j) Percentage eosinophils in BAL from mice treated with IL‐33 in combination with anti‐eotaxin‐2/CCL24 (αEot‐2) or isotype control. Data are shown as means (±SEM in f–i) (n = 4 to n = 14 per group). *P < 0·05, **P < 0·01, and ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
IL‐33 promotes eosinophil maturation in the bone marrow
To investigate eosinophil hematopoiesis locally in the bone marrow under IL‐33 treatment, bone marrow cells from IL‐33‐challenged mice or PBS‐treated controls were isolated and analysed by flow cytometry (Fig. 2a; gating strategies of bone marrow populations are outlined in the Supplementary material, Fig. S1a–c). Flow cytometric analysis of mature eosinophils, defined as CD45+ SSChi CD34− IL5Rα lo CCR3hi cells, confirmed a marked increase in IL‐33‐challenged mice (Fig. 2b). These cells expressed high levels of the eotaxin receptor, CCR3, allowing the mature eosinophils to migrate to the airways in response to increased eotaxin expression. In addition, the mature eosinophils expressed high levels of Siglec‐F, a well‐characterized marker of murine eosinophils, demonstrating that the phenotype is consistent with other studies of eosinophils in mice (see Supplementary material, Fig. S1a). CD34+ progenitor cells (CD45+ SSClo CD34+) were also found at significantly increased numbers in the bone marrow of IL‐33‐challenged mice (Fig. 2c), however, no differences were found in the levels of immature eosinophils, defined as CD45+ SSCint CD34− IL5Rα hi CCR3neg/lo cells, at this time point (Fig. 2b).
Figure 2.
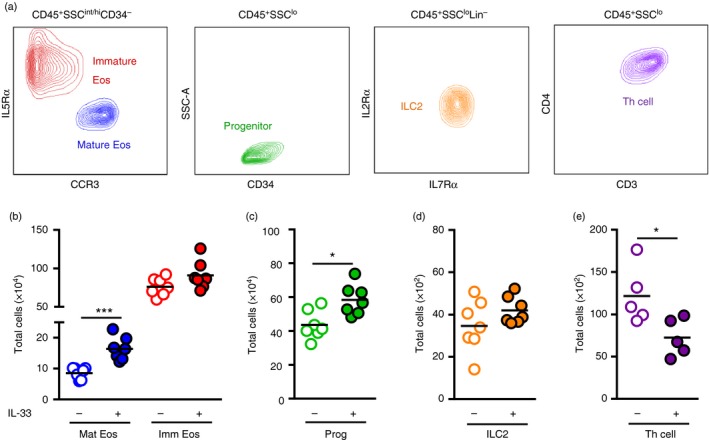
IL‐33 promotes eosinophil maturation in bone marrow and alters T helper (Th) cells but not type 2 innate lymphoid cells (ILC2s). (a) Mature eosinophils (Mat Eos; CD45+ SSChi CD34− IL5Rα lo CCR3hi), immature eosinophils (Imm Eos; CD45+ SSCint CD34− IL5Rα hi CCR3neg/lo), progenitors (Prog; CD45+ SSClo CD34+), ILC2s (CD45+ SSClo Lin− CD19− CD11c− NK1.1− FcԑRI− IL2Rα + IL7Rα +) and T helper cells (Th cell; CD45+ SSClo CD3+ CD4+) in bone marrow identified by flow cytometry. Total number of Mat Eos and Imm Eos (b), Prog (c), ILC2s (d), and Th cells (e) in the bone marrow of IL‐33‐treated or PBS‐treated mice. Data are shown as means (n = 5 to n = 7 per group). *P < 0·05, and ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
IL‐33 alters the number of Th cells but not ILC2s in the bone marrow
Further examination of non‐eosinophilic bone marrow populations demonstrated that IL‐33 administration had no effect on the proportion of ILC2s (CD45+ SSClo Lin− IL7Rα + IL2Rα +) in the bone marrow (Fig. 2d). However, Th cells (CD45+ SSClo CD3+ CD4+) decreased upon IL‐33 challenge (Fig. 2e), which opens the possibility that IL‐33 might be involved in T‐cell trafficking. Interestingly, expression of the T‐cell activation marker, IL‐2Rα/CD25, increased on bone marrow Th cells in IL‐33‐challenged mice compared with PBS‐treated controls (see Supplementary material, Fig. S2a–c). However, although the percentage of CD25‐expressing cells increased, the total number of activated CD25+ Th cells decreased in the bone marrow upon IL‐33 challenge (see Supplementary material, Fig. S2d), suggesting that Th cells leave the bone marrow upon activation. At the same time, elevated levels of the T‐cell chemoattractant, TARC/CCL17, were found in the airways of IL‐33‐challenged mice (see Supplementary material, Fig. S2e), which further supports the possibility that IL‐33 induces T‐cell migration.
Mature eosinophils, ILC2s and Th cells in bone marrow respond to IL‐33 through IL33R/ST2
To examine whether the investigated bone marrow populations have the ability to respond directly to IL‐33 signalling, we analysed expression of the IL‐33 receptor (ST2). ST2 expression was found on a majority of mature eosinophils (Fig. 3a,b), a population that expanded upon IL‐33 challenge. In sharp contrast, no ST2‐positive cells were found among immature eosinophils (Fig. 3b). However, a subpopulation of CD34+ progenitors displayed positive ST2 expression (Fig. 3b), although the expression remained unchanged in response to IL‐33 challenge. Interestingly, Johnston et al.16 recently described how ST2 expression on bone marrow precursors precedes IL5Rα expression. Consistent with this, expression analysis of the progenitor marker, CD34, in combination with IL5Rα, demonstrated a distinct ST2‐expressing subpopulation of early progenitors that have not committed to the eosinophil lineage (CD34+ IL5Rα −) (see Supplementary material, Fig. S3a–d). As these cells commit, seen by decreasing CD34 and increasing IL5Rα expression, ST2 expression drops drastically (see Supplementary material, Fig. S3d), and among cells that have lost CD34 expression, but demonstrate high IL5Rα, ST2 expression was completely absent (see Supplementary material, Fig. S3d). As a further indicator of eosinophil differentiation, expression of the eotaxin receptor CCR3 was highest in the presumed late CD34− IL5Rα + cell population compared with CD34+ cells (see Supplementary material, Fig. S3e). Whereas a slight increase of CD34+ IL5Rα − cells was found under IL‐33 treatment, the number of CD34+ IL5Rα + and CD34− IL5Rα + cells remained the same in IL‐33‐challenged mice and PBS‐treated control mice (see Supplementary material, Fig. S3b).
Figure 3.
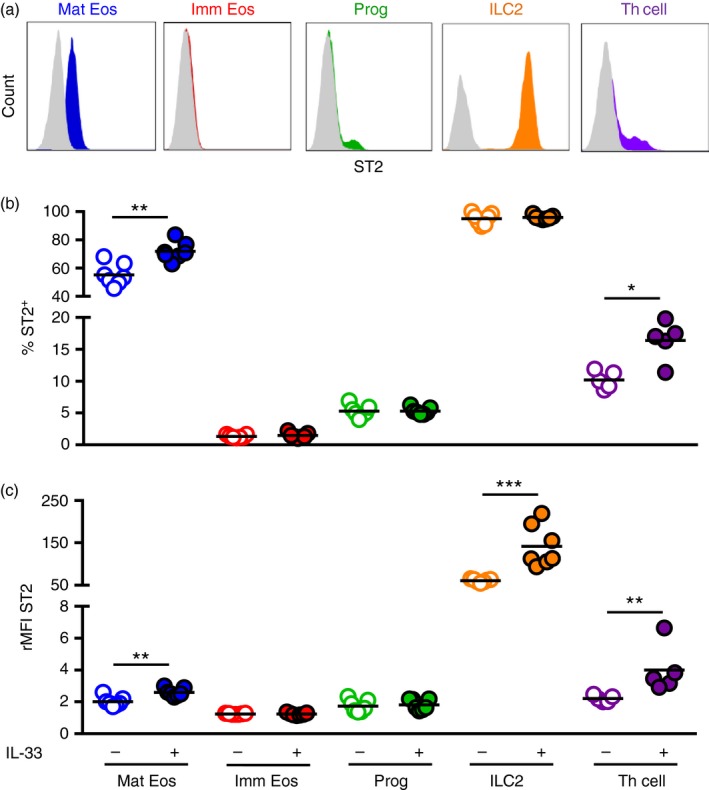
Mature eosinophils (Mat Eos), type 2 innate lymphoid cells (ILC2s) and T helper (Th) cells in bone marrow respond to IL‐33 through IL33R/ST2. (a) Representative IL‐33 receptor (ST2) expression on investigated bone marrow populations. Percentage ST2+ cells (b) and ST2 receptor density (rMFI) (c) within indicated populations in bone marrow from IL‐33‐treated or PBS‐treated mice. Data are shown as means (n = 5 to n = 7 per group). *P < 0·05, **P < 0·01, and ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
Among the cell populations examined, ILC2s showed the highest ST2 expression (Fig. 3a). Indeed, all lineage‐negative cells expressing the IL‐2 and IL‐7 receptors in the bone marrow were ST2+ cells (Fig. 3b), and the density of the receptor on the cell surface increased three‐fold in response IL‐33 administration (Fig. 3c). A subpopulation of Th cells also expressed ST2 (Fig. 3b,c), which increased in IL‐33‐challenged mice compared with PBS‐treated mice, suggesting that IL‐33 might act directly on Th cells to induce chemotaxis to peripheral sites of inflammation.
IL‐33‐induced eosinophil production, but not recruitment, is dependent on IL‐5
As a result of airway administration of IL‐33, significantly elevated levels of IL‐5 were found systemically in serum (Fig. 4a). Importantly, by pre‐treating mice with anti‐IL‐5 antibodies 1 hr before the first intranasal IL‐33 challenge (Fig. 4b), a significant decrease of eosinophils was found in the bone marrow (Fig. 4c) and BAL (Fig. 4e). A wider spread of eosinophils was recorded in the peripheral blood of IL‐33‐challenged mice at this time point (Fig. 4d), however, in PBS‐treated control mice, representing homeostatic conditions, anti‐IL‐5 treatment led to a significant decrease of circulating eosinophils, as previously shown.16 Indeed, consistent with our results, two recent studies reported elevated IL‐5 in the serum of IL‐33‐treated mice and where IL‐33‐induced eosinophilia was impaired upon neutralization of IL‐5.8, 16 However, interestingly, we also found that the expression of eotaxin‐2/CCL24 in serum (Fig. 4f) and BAL (Fig. 4g) was independent of the levels of IL‐5 because no significant difference was found in IL‐33‐challenged mice pre‐treated with anti‐IL‐5 or isotype control. This suggests that IL‐33‐induced eosinophil production, but not recruitment, is dependent on IL‐5. Indeed, lower numbers of mature bone marrow eosinophils in anti‐IL‐5‐treated mice were confirmed by flow cytometric analysis (see Supplementary material, Fig. S4a). Furthermore, no differences were found in the numbers of CD34+ progenitors or ILC2s in IL‐33‐challenged mice under anti‐IL‐5 treatment (see Supplementary material, Fig. S4b,c). However, Th cells accumulated in the bone marrow in lack of IL‐5 (see Supplementary material, Fig. S4d), which indicates an involvement of IL‐5 in T‐cell trafficking. Indeed, the elevated levels of the T‐cell‐recruiting chemokine TARC/CCL17 in the airways of IL‐33‐challenged mice were unaffected by anti‐IL‐5 treatment (see Supplementary material, Fig. S2f), suggesting that IL‐5 locally in the bone marrow, might be involved in migration of bone marrow T cells. ST2 expression was also found to be affected by the presence of IL‐5 in the bone marrow; ST2 expression decreased significantly on mature eosinophils in anti‐IL‐5/IL‐33‐treated mice (see Supplementary material, Fig. S4e). Interestingly, the expression of ST2 was found to increase in CD34+ progenitors in lack of IL‐5 (see Supplementary material, Fig. S4f), possibly highlighting the tightly regulated relationship between IL‐33 and IL‐5 signalling in bone marrow progenitors. In bone marrow ILC2s, ST2 expression (shown as mean fluorescence index) increased upon IL‐33 and this up‐regulation was independent of the levels of IL‐5 because no significant difference was found in ST2 receptor expression in IL‐33‐challenged mice that received anti‐IL‐5 or isotype control (see Supplementary material, Fig. S4g). This was also the case for Th cells where no change in ST2 expression was found in anti‐IL‐5‐treated mice (see Supplementary material, Fig. S4h).
Figure 4.
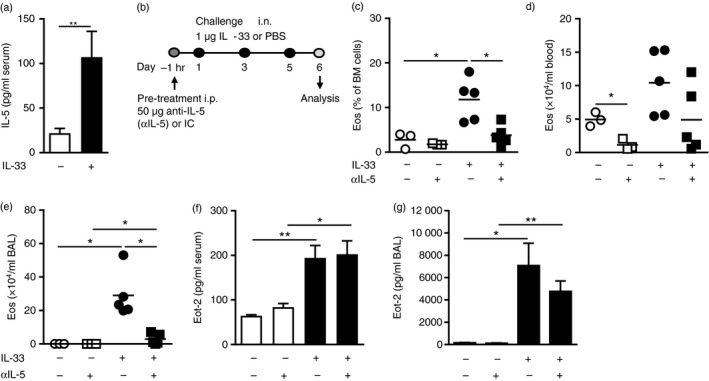
IL‐33‐mediated eosinophil hematopoiesis, but not recruitment, is driven by IL‐5. (a) IL‐5 protein in serum from IL‐33‐treated or PBS‐treated mice. (b) Model of IL‐33‐induced inflammation including anti‐IL‐5 (αIL‐5) antibody pre‐treatment. (c) Percentage eosinophils in bone marrow, and total eosinophils in blood (d) and bronchoalveolar lavage (BAL) (e) and, eotaxin‐2/CCL24 protein in serum (f) and BAL (g) from αIL‐5‐ or isotype‐pre‐treated mice challenged with IL‐33 or PBS. Data are shown as means (± SEM in a, f–g) (n = 3 to n = 8 per group). *P < 0·05, and **P < 0·01.
ILC2s and CD34+ progenitors, but not Th cells, produce IL‐5 in the bone marrow in vivo
By having identified that different populations in the bone marrow express ST2, which was altered upon IL‐33 challenge, we investigated whether these cell populations were able to produce IL‐5 in IL‐33‐induced inflammation in vivo. Interestingly, not previously shown, examination by intracellular flow cytometry revealed a dramatic increase of IL‐5+ ILC2s in the bone marrow of IL‐33‐challenged mice (Fig. 5a). Furthermore, CD34+ progenitor cells were also found to increase their IL‐5 production in response to IL‐33 compared with PBS‐treated controls (Fig. 5a), however, no induction of IL‐5 was found in Th cells (Fig. 5a).
Figure 5.
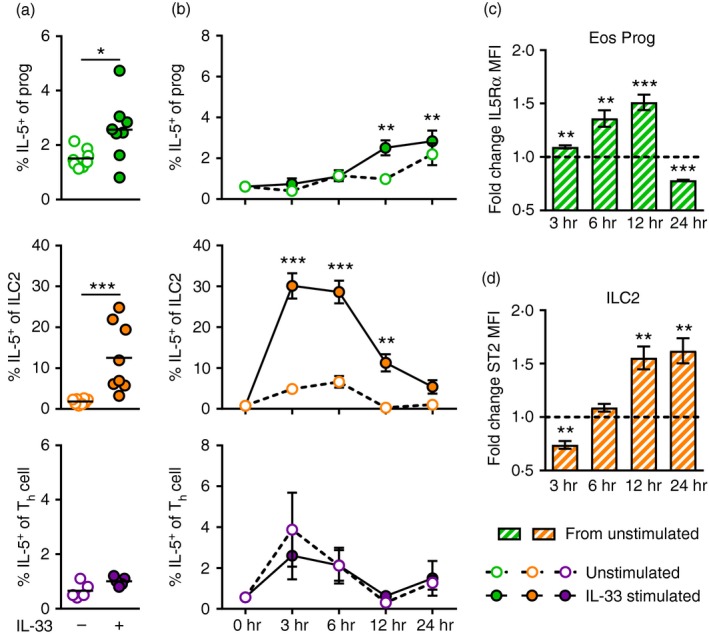
Bone marrow type 2 innate lymphoid cells (ILC2s) and CD34+ progenitors, but not T helper (Th) cells, produce interleukin‐5 (IL‐5) in response to IL‐33 in vivo and in vitro. (a) Percentage IL‐5+ progenitors, ILC2s and Th cells in vivo in bone marrow of IL‐33‐ or PBS‐treated mice. (b) Percentage IL‐5+ progenitors, ILC2s and Th cells of in vitro stimulated bone marrow cells from naive mice. Cells were stimulated with IL‐33 for 3, 6, 12 or 24 hr or left unstimulated as medium control. Fold change of IL5Rα expression (MFI) on eosinophil progenitors (CD34+ IL5Rα +) (c) and ST2 expression (MFI) on ILC2s (d) in IL‐33 stimulated wells compared with unstimulated wells. Data are shown as means (± SEM in b–d) (n = 3 to n = 7 per group). *P < 0·05, **P < 0·01, and ***P < 0·001. [Colour figure can be viewed at wileyonlinelibrary.com]
Bone marrow ILC2s rapidly produce IL‐5 in response to IL‐33 stimulation in vitro
To examine IL‐5 production in these populations further, we set up an in vitro system where naive bone marrow cells were stimulated with IL‐33 for 3, 6, 12 or 24 hr and analysed by intracellular flow cytometry to monitor IL‐5 production over time. These experiments revealed that the CD34+ progenitors induced a small but significant increase of IL‐5 after 12 hr of IL‐33 stimulation, which was maintained at 24 hr of stimulation (Fig. 5b). Similar to the results from the in vivo investigation, no significant induction of IL‐5 was found in Th cells in response to IL‐33 (Fig. 5b). In contrast, an early induction of IL‐5 was found in ILC2s, which demonstrated a dramatic increase of intracellular IL‐5 already at 3 hr of IL‐33 stimulation (Fig. 5b). The high levels of IL‐5+ ILC2s were maintained at 6 hr and decreased first at 12 hr of stimulation. Interestingly, at the 12 hr time point, ST2 expression increased by 50% in the ILC2 population, possibly reflecting a compensatory upregulation of the receptor in response to decreasing IL‐33 levels in the cultures (Fig. 5d). Importantly, the functional relevance of IL‐33‐induced IL‐5 production was demonstrated by increasing IL5Rα expression in eosinophil progenitors (CD34+ IL5Rα +) (Fig. 5c). Indeed, the observed elevation of IL5Rα receptor intensity coincided with increasing IL‐5+ ILC2s, and at 24 hr of stimulation both ILC2‐derived IL‐5 and IL5Rα expression on eosinophil progenitors had decreased. Further analysis of bone marrow‐derived ILC2s was enabled by FACS sorting followed by in vitro expansion, where ILC2s were found to secrete large amounts of IL‐5 in response to IL‐33 (Fig. 6a,b). Importantly, by adding supernatants from stimulated ILC2 cultures to naive bone marrow, eosinophil differentiation was induced as seen by increased numbers of eosinophil progenitors (Fig. 6c) and upregulation of IL5Rα expression (Fig. 6d). Together, these findings demonstrate that IL‐5‐producing ILC2s support IL‐33‐driven eosinophil hematopoiesis in the bone marrow, which adds a previously unrecognized role of ILC2s in eosinophilic inflammation.
Figure 6.
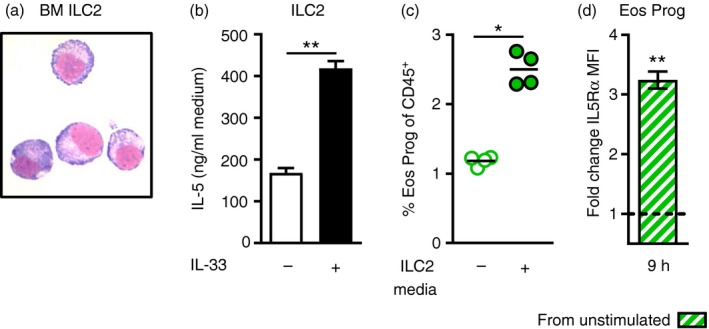
Culture supernatants from bone marrow type 2 innate lymphoid cells (ILC2s) stimulate eosinophil differentiation. (a) FACS‐sorted bone marrow ILC2s (Lin− CD45+ ICOS+ CD25+ ST2+) stained with Hemacolor rapid stain. (b) IL‐5 protein in cell‐free culture supernatants from in vitro expanded ILC2s stimulated with IL‐33 for 20 hr or left unstimulated as medium control. (c) Percentage eosinophil progenitors (CD34+ IL5Rα +) in naive bone marrow cultures supplemented with 5% culture supernatant from stimulated ILC2s or plain medium as control. (d) Fold change of IL5Rα expression (MFI) in stimulated wells (5% ILC2 media) compared with control wells. Data are shown as means (± SEM in b and d) (n = 4 or n = 5 per group). *P < 0·05, and **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In recent years, IL‐33 has emerged as a central player in allergic immune responses where we and others have demonstrated that IL‐33 increases in the airways in mice upon allergen challenge, which activates inflammatory cells to release mediators that in turn contribute to the development of tissue eosinophilia.15, 25, 26 Importantly, IL‐33 has also been demonstrated to take part in the immunopathogenesis of human allergy and asthma where several studies have identified IL33 and IL1RL1 (ST2 gene) to be associated with these diseases.27, 28, 29, 30, 31 Indeed, elevated IL‐33 levels have been found in airway samples from individuals with allergic asthma compared with healthy controls, where increased IL‐33 expression was correlated with asthma severity.32, 33, 34
Although several studies have reported that intranasal IL‐33 delivery in mice results in elevated eosinophil numbers in the airways and the circulation, in the current study we show for the first time a functional effect in the bone marrow compartment under IL‐33 stimulation. We analysed bone marrow cells by flow cytometry, which enabled identification of eosinophils of different maturity as well as IL‐5‐producing cells. Signalling via IL5Rα and CCR3 is crucial for eosinophil development and trafficking, therefore commonly used markers for detection of human and mouse eosinophils.35, 36, 37 Analysis of these receptors proved to be a successful strategy and we were able to demonstrate that IL‐33 administration induced eosinophil differentiation in the bone marrow. Interestingly, ST2 expression on bone marrow eosinophils was clearly related to cell maturation as a majority of mature eosinophils were ST2+, however, no ST2 expression was found on immature eosinophils. In addition, although a subpopulation of CD34+ progenitors expressed ST2, we found a sudden decrease in receptor expression as soon as the cells were committed to the eosinophil lineage (i.e. acquired IL5Rα expression), suggesting a fine‐tuned interplay between IL‐33 and IL‐5 in hematopoietic differentiation, which has been described in further detail by others.16, 24, 38 In a recent study, ST2 expression in mature granulocytes including eosinophils, basophils and mast cells, was compared with the expression of the ST2 receptor in precursor cells.8 Eosinophil progenitors, basophil progenitors and mast cell progenitors were all found to express ST2 at higher levels than the mature cells. The functional relevance of this observation was documented by increased IL‐33‐induced cytokine production in vitro (IL‐1β, IL‐6, IL‐9 and IL‐13) by the progenitors compared with the mature granulocytes.8 Importantly, functional implications of ST2 expression on CD34+ progenitors have previously been reported in humans where CD34+ ST2+ cells in peripheral blood were found to produce IL‐5 in response to IL‐33 stimulation.9 This demonstrates that in addition to being precursors of myeloid cells with inflammatory roles, IL‐33‐responsive CD34+ cells may themselves act as cytokine‐producing effector cells that contribute to eosinophil development. Indeed, increased IL‐5‐producing CD34+ IL5Rα + cells were found in sputum from individuals with severe asthma39 and the number of CD34+ ST2+ cells, along with mature eosinophils and CD34+ IL5Rα + progenitors, increased in the sputum of subjects with allergic asthma after inhaled allergen challenge.9 Of interest for the current study, bone marrow aspirates from individuals with allergic asthma have shown that even though no difference was found in CD34+ cell numbers after airway allergen challenge, IL‐5‐responsive CD34+ IL5Ra+ progenitors increased significantly in the bone marrow.36, 40, 41 In our experimental model of IL‐33‐driven eosinophilia we found no significant differences in CD34+ IL5Ra+ cells in the bone marrow upon IL‐33 challenge, however, the number of CD34+ cells and progenitor‐derived downstream differentiation products such as eosinophils were detected at significantly increased levels in IL‐33‐challenged mice compared with PBS‐treated controls, which suggests that the production of hematopoietic stem cells in the bone marrow is a highly dynamic yet tightly regulated process.
In addition to myeloid cells, in our analysis of ST2 expression in bone marrow lymphocytes we found indisputably high expression levels in ILC2s and a more modest ST2+ subpopulation of Th cells. Interestingly, an ST2‐expressing population of lineage− Sca1+ c‐Kit− CD25+ cells has previously been described within the mouse bone marrow that, consistent with our findings, was reported to produce IL‐5 when cultured in vitro in the presence of IL‐33 and IL‐7.42 In our study, in addition to being ST2+ cells, the ILC2s expressed Sca‐1 and ICOS but mostly negative for c‐Kit and KLRG1 (see Supplementary material, Fig. S1b). Furthermore, the number of ILC2s remained unchanged in the bone marrow of IL‐33‐challenged mice compared with PBS‐treated control mice, whereas the Th cells were found at significantly lower numbers after IL‐33 challenge. This finding is in contrast to the role that was previously suggested for T lymphocytes in the context of allergen‐driven eosinophilia, where allergen challenge is thought to induce trafficking of IL‐5‐producing T cells to the bone marrow.10, 19, 43 However, it is likely that different pathways are activated upon innate cytokine signalling compared with adaptive allergen stimulation.
In our model of IL‐33‐driven eosinophilia, eosinophils were recruited from the bone marrow to the airways upon IL‐33 challenge. Indeed, we found that IL‐33 challenge alone proved to be sufficient to induce eotaxin‐2/CCL24 expression in the airways of naive mice at comparable levels reported in models of allergen‐driven eosinophilic inflammation,44 and blocking of eotaxin‐2/CCL24 by neutralizing antibodies decreased airway eosinophils in IL‐33‐challenged mice. Interleukin‐33‐induced eotaxin‐2/CCL24 production has previously been investigated in mice, where IL‐33 was reported to polarize alveolar macrophages toward an alternatively activated phenotype that were able to produce high levels of eotaxin‐2/CCL24 (and TARC/CCL17) in vitro in response to IL‐33 stimulation together with IL‐13.45 Eotaxin‐2/CCL24 expression has been described to be dependent on IL‐13,46, 47 and we and others have previously shown that IL‐13 is produced by airway ILC2s in response to IL‐33 stimulation in vivo.15, 48 It is possible that airway ILC2s are contributing to the expression of eotaxin‐2/CCL24 by producing IL‐13 in our model of IL‐33‐induced eosinophilia. Interestingly, the expression of IL‐33‐induced eotaxin‐2/CCL24 was unaffected by neutralizing IL‐5 antibodies and no significant changes in the upregulation of ST2 on bone marrow ILC2s under anti‐IL‐5 treatment were found. One might speculate that IL‐33‐responsiveness of airway ILC2s, similar to ILC2s in the bone marrow, is independent of IL‐5. This would explain the high levels of eotaxin‐2/CCL24 that were maintained under anti‐IL‐5 treatment.
A recent study claimed that allergen inhalation in a mouse model caused the release of IL‐33 in the airways, which stimulated production of IL‐5 by lung ILC2s; the IL‐5 reached systemically high levels that stimulated eosinophil development in the bone marrow.49 We also found elevated IL‐5 systemically in serum from mice that were treated with IL‐33 compared with PBS‐treated mice. Although we cannot rule out that various tissues such as the lung might contribute to elevated IL‐5 in the circulation, we do believe that there is a local source of IL‐5 in the bone marrow that is driving eosinophil development. To address this hypothesis, we examined intracellular IL‐5 production in vivo and can for the first time demonstrate that CD34+ progenitors and ILC2s, but not Th cells, induce IL‐5 locally in the bone marrow in IL‐33‐challenged mice. In the in vitro setting, we were able to monitor IL‐5 production over time in these populations as well as at an earlier time point that might reveal which cells are involved in the initiation of eosinophil hematopoiesis. Indeed, bone marrow ILC2s were the predominant source of IL‐5, which coincided with the expansion of IL‐5‐responsive CD34+ eosinophil progenitors.
In conclusion, we conducted a comparative investigation of three cellular candidates in the bone marrow, known to produce IL‐5 in peripheral blood and airways in the context of eosinophilic inflammation, to determine whether these cells are supporting eosinophil development locally in the bone marrow. By examining IL‐33‐responsiveness and intracellular IL‐5 production we show that although ST2 receptor expression was found in all three cell types, ILC2s stood out by rapidly producing large amounts of IL‐5 upon IL‐33 stimulation, which induced expansion of CD34+ IL5Rα + eosinophil progenitors in vitro. This novel finding identifies IL‐5‐producing bone marrow ILC2s as a new important mediator of IL‐33‐driven eosinophil development, which might have implications in antigen‐independent induction of eosinophilic asthma.
Disclosures
This study was funded by Herman Krefting Foundation for Asthma and Allergy research, the Swedish Heart and Lung Foundation and Vårdalstiftelsen. The authors declare that they have no conflicts of interest.
Author contributions
KJ and MR conceived the study and designed experiments. KJ, CM and PR performed the experiments. KJ and MR wrote the paper. All authors read and approved the manuscript before submission.
Supporting information
Figure S1. Gating strategy of bone marrow populations by flow cytometry.
Figure S2. T helper cell activation in bone marrow.
Figure S3. ST2 expression precedes interleukin‐5 receptor Rα expression in bone marrow progenitors.
Figure S4. Lack of interleukin‐5 (IL‐5) prevents eosinophil maturation and T helper cell migration, but does not affect IL‐33‐responsiveness of bone marrow type 2 innate lymphoid cells.
Table S1. Antibodies used for flow cytometric analysis..
Acknowledgements
We thank Frida Dahlberg for experimental assistance.
References
- 1. Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull 2000; 56:985–1003. [DOI] [PubMed] [Google Scholar]
- 2. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006; 24:147–74. [DOI] [PubMed] [Google Scholar]
- 3. Ema H, Suda T, Nagayoshi K, Miura Y, Civin CI, Nakauchi H. Target cells for granulocyte colony‐stimulating factor, interleukin‐3, and interleukin‐5 in differentiation pathways of neutrophils and eosinophils. Blood 1990; 76:1956–61. [PubMed] [Google Scholar]
- 4. Humbles AA, Conroy DM, Marleau S, Rankin SM, Palframan RT, Proudfoot AE et al Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo . J Exp Med 1997; 186:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang M, Hogan SP, Mahalingam S, Pope SM, Zimmermann N, Fulkerson P et al Eotaxin‐2 and IL‐5 cooperate in the lung to regulate IL‐13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol 2003; 112:935–43. [DOI] [PubMed] [Google Scholar]
- 6. Radinger M, Johansson AK, Sitkauskiene B, Sjostrand M, Lotvall J. Eotaxin‐2 regulates newly produced and CD34 airway eosinophils after allergen exposure. J Allergy Clin Immunol 2004; 113:1109–16. [DOI] [PubMed] [Google Scholar]
- 7. Johansson AK, Sjostrand M, Tomaki M, Samulesson AM, Lotvall J. Allergen stimulates bone marrow CD34+ cells to release IL‐5 in vitro; a mechanism involved in eosinophilic inflammation? Allergy 2004; 59:1080–6. [DOI] [PubMed] [Google Scholar]
- 8. Tsuzuki H, Arinobu Y, Miyawaki K, Takaki A, Ota SI, Ota Y et al Functional interleukin‐33 receptors are expressed in early progenitor stages of allergy‐related granulocytes. Immunology 2017; 150:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M et al CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol 2009; 123:472–8. [DOI] [PubMed] [Google Scholar]
- 10. Minshall EM, Schleimer R, Cameron L, Minnicozzi M, Egan RW, Gutierrez‐Ramos JC et al Interleukin‐5 expression in the bone marrow of sensitized Balb/c mice after allergen challenge. Am J Respir Crit Care Med 1998; 158:951–7. [DOI] [PubMed] [Google Scholar]
- 11. Willebrand R, Voehringer D. IL‐33‐induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM‐CSF. PLoS One 2016; 11:e0163751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol 2011; 12:21–7. [DOI] [PubMed] [Google Scholar]
- 13. Barlow JL, McKenzie AN. Type‐2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol 2014; 14:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y et al Pulmonary innate lymphoid cells are major producers of IL‐5 and IL‐13 in murine models of allergic asthma. Eur J Immunol 2012; 42:1106–16. [DOI] [PubMed] [Google Scholar]
- 15. Johansson K, Malmhall C, Ramos‐Ramirez P, Radinger M. MicroRNA‐155 is a critical regulator of type 2 innate lymphoid cells and IL‐33 signaling in experimental models of allergic airway inflammation. J Allergy Clin Immunol 2017; 139(3):1007–1016.e9. [DOI] [PubMed] [Google Scholar]
- 16. Johnston LK, Hsu CL, Krier‐Burris RA, Chhiba KD, Chien KB, McKenzie A et al IL‐33 precedes IL‐5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol 2016; 197:3445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu DK, Llop‐Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE et al IL‐33, but not thymic stromal lymphopoietin or IL‐25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013; 131:187–200 e1‐8. [DOI] [PubMed] [Google Scholar]
- 18. Tomaki M, Zhao LL, Lundahl J, Sjostrand M, Jordana M, Linden A et al Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL‐5 and IL‐5 receptor α . J Immunol 2000; 165:4040–50. [DOI] [PubMed] [Google Scholar]
- 19. Wood LJ, Sehmi R, Dorman S, Hamid Q, Tulic MK, Watson RM et al Allergen‐induced increases in bone marrow T lymphocytes and interleukin‐5 expression in subjects with asthma. Am J Respir Crit Care Med 2002; 166:883–9. [DOI] [PubMed] [Google Scholar]
- 20. Johansson AK, Sergejeva S, Sjostrand M, Lee JJ, Lotvall J. Allergen‐induced traffic of bone marrow eosinophils, neutrophils and lymphocytes to airways. Eur J Immunol 2004; 34:3135–45. [DOI] [PubMed] [Google Scholar]
- 21. Dent AL, Kaplan MH. T cell regulation of hematopoiesis. Front Biosci 2008; 13:6229–36. [DOI] [PubMed] [Google Scholar]
- 22. Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood 2005; 105:1484–91. [DOI] [PubMed] [Google Scholar]
- 23. Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M et al IL‐33‐mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen‐induced allergic airway inflammation. J Immunol 2013; 190:4489–99. [DOI] [PubMed] [Google Scholar]
- 24. Dyer KD, Percopo CM, Rosenberg HF. IL‐33 promotes eosinophilia in vivo and antagonizes IL‐5‐dependent eosinophil hematopoiesis ex vivo . Immunol Lett 2013; 150:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hardman CS, Panova V, McKenzie AN. IL‐33 citrine reporter mice reveal the temporal and spatial expression of IL‐33 during allergic lung inflammation. Eur J Immunol 2013; 43:488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louten J, Rankin AL, Li Y, Murphy EE, Beaumont M, Moon C et al Endogenous IL‐33 enhances Th2 cytokine production and T‐cell responses during allergic airway inflammation. Int Immunol 2011; 23:307–15. [DOI] [PubMed] [Google Scholar]
- 27. Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM et al Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 2009; 41:342–7. [DOI] [PubMed] [Google Scholar]
- 28. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S et al A large‐scale, consortium‐based genomewide association study of asthma. N Engl J Med 2010; 363:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE et al Meta‐analysis of genome‐wide association studies of asthma in ethnically diverse North American populations. Nat Genet 2011; 43:887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonnelykke K, Sleiman P, Nielsen K, Kreiner‐Moller E, Mercader JM, Belgrave D et al A genome‐wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 2014; 46:51–5. [DOI] [PubMed] [Google Scholar]
- 31. Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, Lyon HN et al Genome‐wide association studies of asthma in population‐based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One 2012; 7:e44008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prefontaine D, Lajoie‐Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ et al Increased expression of IL‐33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009; 183:5094–103. [DOI] [PubMed] [Google Scholar]
- 33. Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J et al Increased IL‐33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol 2010; 125:752–4. [DOI] [PubMed] [Google Scholar]
- 34. Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL‐33 and soluble ST2 in young asthmatic children. J Asthma 2013; 50:803–9. [DOI] [PubMed] [Google Scholar]
- 35. Sehmi R, Dorman S, Baatjes A, Watson R, Foley R, Ying S et al Allergen‐induced fluctuation in CC chemokine receptor 3 expression on bone marrow CD34+ cells from asthmatic subjects: significance for mobilization of haemopoietic progenitor cells in allergic inflammation. Immunology 2003; 109:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sehmi R, Wood LJ, Watson R, Foley R, Hamid Q, O'Byrne PM et al Allergen‐induced increases in IL‐5 receptor α‐subunit expression on bone marrow‐derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J Clin Invest 1997; 100:2466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME. IL‐5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol 2014; 193:4043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stolarski B, Kurowska‐Stolarska M, Kewin P, Xu D, Liew FY. IL‐33 exacerbates eosinophil‐mediated airway inflammation. J Immunol 2010; 185:3472–80. [DOI] [PubMed] [Google Scholar]
- 39. Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM et al Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol 2016; 137:75–86 e8. [DOI] [PubMed] [Google Scholar]
- 40. Wood LJ, Inman MD, Watson RM, Foley R, Denburg JA, O'Byrne PM. Changes in bone marrow inflammatory cell progenitors after inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med 1998; 157:99–105. [DOI] [PubMed] [Google Scholar]
- 41. Dorman SC, Sehmi R, Gauvreau GM, Watson RM, Foley R, Jones GL et al Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med 2004; 169:565–72. [DOI] [PubMed] [Google Scholar]
- 42. Brickshawana A, Shapiro VS, Kita H, Pease LR. Lineage– Sca1+ c‐Kit– CD25+ cells are IL‐33‐responsive type 2 innate cells in the mouse bone marrow. J Immunol 2011; 187:5795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gauvreau GM, Ellis AK, Denburg JA. Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin Exp Allergy 2009; 39:1297–306. [DOI] [PubMed] [Google Scholar]
- 44. Malmhall C, Alawieh S, Lu Y, Sjostrand M, Bossios A, Eldh M et al MicroRNA‐155 is essential for TH2‐mediated allergen‐induced eosinophilic inflammation in the lung. J Allergy Clin Immunol 2014; 133:1429–38, 38 e1‐7. [DOI] [PubMed] [Google Scholar]
- 45. Kurowska‐Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S et al IL‐33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009; 183:6469–77. [DOI] [PubMed] [Google Scholar]
- 46. Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI et al IL‐13 induces eosinophil recruitment into the lung by an IL‐5‐ and eotaxin‐dependent mechanism. J Allergy Clin Immunol 2001; 108:594–601. [DOI] [PubMed] [Google Scholar]
- 47. Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N et al Identification of a cooperative mechanism involving interleukin‐13 and eotaxin‐2 in experimental allergic lung inflammation. J Biol Chem 2005; 280:13952–61. [DOI] [PubMed] [Google Scholar]
- 48. Piehler D, Eschke M, Schulze B, Protschka M, Muller U, Grahnert A et al The IL‐33 receptor (ST2) regulates early IL‐13 production in fungus‐induced allergic airway inflammation. Mucosal Immunol 2016; 9:937–49. [DOI] [PubMed] [Google Scholar]
- 49. Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, Kita H. IL‐33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy 2016; 71:977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy of bone marrow populations by flow cytometry.
Figure S2. T helper cell activation in bone marrow.
Figure S3. ST2 expression precedes interleukin‐5 receptor Rα expression in bone marrow progenitors.
Figure S4. Lack of interleukin‐5 (IL‐5) prevents eosinophil maturation and T helper cell migration, but does not affect IL‐33‐responsiveness of bone marrow type 2 innate lymphoid cells.
Table S1. Antibodies used for flow cytometric analysis..


