Abstract
IgE contributes to disease exacerbations but not to baseline airway hyperresponsiveness (AHR) in human asthma. In rodent allergic airway disease (AAD), mast cell and IgE dependence for the induction of AHR has only been observed when mice are immunized with a relatively weak allergen without adjuvant. To evaluate the role of IgE in murine AAD that is induced by a potent allergen, we inoculated BALB/c and FVB/N background wild-type and IgE- or FcεRIα-deficient mice intratracheally with large or limiting doses of house dust mite extract (HDM) and evaluated AHR, pulmonary eosinophilia, goblet cell metaplasia, serum IgE, and lung mastocytosis. We found that neither IgE nor FcεRIα contributed to AAD, even in mice inoculated with the lowest dose of HDM, which readily induced detectable disease, but did not increase serum IgE or pulmonary mast cell levels. In contrast, high doses of HDM strikingly increased serum IgE and pulmonary mast cells, although both AHR and airway mast cell degranulation were equally elevated in wild-type and IgE-deficient mice. Surprisingly, allergen challenge of mice with severe AAD and pulmonary mastocytosis failed to acutely increase airway resistance, lung Newtonian resistance, or hysteresis. Overall, this study shows that, although mice may not reliably model acute asthma exacerbations, mechanisms that are IgE and FcεRIα independent are responsible for AHR and airway inflammation when low doses of a potent allergen are inhaled repetitively.
Keywords: asthma, IgE, house dust mite, airway hyperresponsiveness, mast cell
Clinical Relevance
An implication of this work is that those infants born to mothers receiving omalizumab would not be expected to enjoy a decreased risk of allergic airway disease, as defined by airway hyperresponsiveness, even if they themselves were to continue this therapy.
Mast cells are potent members of the innate immune system that produce numerous mediators involved in the manifestation or induction of allergic disease. Stimulation may result in immediate and/or delayed responses depending on the immunological context and the type of the stimulus. Mast cells can acquire the additional capacity for antigen-specific responses by binding Ig, particularly IgE, which increases their sensitivity to stimuli and augments production of mediators.
Although many acute asthma exacerbations are readily attributable to mast cell activation (with and without atopy), the role of IgE and mast cells in the induction of underlying asthma is unknown. Several animal models address this question experimentally in mice. Although they vary substantially in approach, these studies generally have reported that IgE and/or mast cells promote the pathogenesis of disease when: (1) mice are immunized with an antigen that has relatively weak allergenicity, typically ovalbumin (OVA); (2) relatively mild lung disease is induced; (3) allergic sensitization is first achieved passively by injection of monoclonal IgE; or (4) allergic sensitization is first achieved actively through a site of inoculation other than the airway, such as skin or peritoneum (1–18). In addition, some of these studies were performed in mouse strains that are relatively resistant to the development of a Th2 response and airway hyperresponsiveness (AHR).
To maximize the clinical relevance of murine allergic airway disease (AAD), we induced this disorder in mice that are genetically prone to the development of allergy and AHR by inoculating them repetitively with a small dose of a clinically relevant, potent allergen, house dust mite extract (HDM). We found that this induces a mild to moderate AAD, characterized by AHR, pulmonary eosinophilia, and goblet cell metaplasia that is independent of IgE and FcεRIα. These findings were consistent between the BALB/c and FVB/N congenic backgrounds. In addition, we found no evidence for increased IgE production, pulmonary mastocytosis, or pulmonary mast cell degranulation when mice were inoculated with the lowest doses of HDM capable of inducing convincing AHR and airway inflammation.
When mice were inoculated with high doses of HDM or OVA to induce intense disease that was characterized by pulmonary mastocytosis and increased IgE levels, we again found that AAD was induced equally in wild-type (WT) and IgE-deficient mice. In addition, we observed striking pulmonary mast cell activation that, surprisingly, was IgE independent. These observations are consistent with the failure of treatment with an anti-IgE monoclonal antibody, such as omalizumab, to improve the baseline pulmonary function in human asthma (19). Some of the results of these studies have been reported in the form of an abstract at a meeting of the American Academy of Immunology (20).
Materials and Methods
Mice
Animals 7–8 weeks old were used. IgE deficiency was available first on the FVB/N background and then the BALB/c background. Mice deficient in FcεRIα were BALB/c, as were mice that were doubly deficient in complement component 3 and the FcR common γ chain. Animals were bred and used in a specific pathogen-free environment using barrier isolation with protocols approved by the Cincinnati Children’s Hospital and Medical Center’s Institutional Animal Care and Use Committee (Cincinnati, OH).
Reagents
Lyophilized HDM was from Greer (Lenoir, NC), resuspended in sterile saline, stored at −80°C. Grade V OVA and methacholine were from Sigma-Aldrich (St. Louis, MO). Alum was prepared as described previously (21).
Measurement of Proteins
Total IgE, murine mast cell protease 1 (MMCP1), and HDM-specific IgG1 were quantitated by ELISA, as detailed in the first section of the online supplemental information labeled E1 in the supplemental Materials and Methods in the online supplement.
Active Sensitization and Induction of AAD
Figure legends contain the most pertinent information (see Figure E2 for details).
Passive Sensitization and Strong Mast Cell Stimulation
Anti-trinitrophenyl (TNP) IgE (IGELb4; 10 μg) was injected intravenously 1 day before mice were killed. For in vivo assessments, mice received 100 μg of TNP-BSA intraperitoneally to provoke anaphylaxis. For ex vivo assessments, sections of lung were exposed to TNP-BSA for 90 minutes at 1 μg/ml 3 days after sensitization.
Measurement of Pulmonary Function by Unrestrained Barometric Plethysmography Immediately after Antigen Administration
Mice were anesthetized with isoflurane, inoculated intratracheally with antigen and placed into a plethysmographic chamber. After anesthesia waned over a few seconds, continuous monitoring was begun—calculating the average enhanced pause (Penh) every 60 seconds.
Determination of Airway Responsiveness to β-Methacholine by Barometric Plethysmography
Determination of airway responsiveness to β-methacholine by barometric plethysmography was as described previously (22), except that an Aeroneb Lab Nebulizer (Aerogen, Galway, Ireland) generated aerosol particles of 2.5–4.0 μm in diameter.
Invasive Measurement of Pulmonary Function after Exposure to Allergen
The flexiVent system (Scireq, Montreal, PQ, Canada) was used to quantitate airway resistance and pulmonary hysteresis. The time periods assessed ranged from 1 to 60 minutes after exposure. Please see the first section of the online supplemental information labeled E1 for details of these protocols.
Determination of Airway Responsiveness to β-Methacholine by Forced Oscillation
Determination of airway responsiveness to β-methacholine by forced oscillation was performed as described previously (23), with the exception of nebulizing each methacholine dose for 15 seconds.
Ex Vivo Determination of Airway Responsiveness by Precisely Cut Lung Slices
Ex vivo determination of airway responsiveness by precisely cut lung slices was performed as described previously (24).
Bronchoalveolar Lavage and Quantitation of Bronchoalveolar Lavage Cells
Bronchoalveolar lavage (BAL) and quantitation of BAL cells were performed as described previously (25).
Goblet Cell Enumeration
Histologic slides were prepared as previously described (25). The epithelial cells of all airways (sized 80–160 cells), in one lung section per mouse, were counted and percent goblet cells calculated.
Method of Flow Cytometry
Lung cells were isolated per the first section of the online supplemental information labeled E1. 106 live cells were stained in 100 μl of staining buffer/reaction. The first section of the online supplemental information, E1, lists the reagents used. Four staining reactions per mouse were pooled before analyzing on an LSRFortessa cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ). Florescence-minus-one determinations were prepared for cells stained for FcεRIα, IgE, cKit, and CD45. Peritoneal lavage fluid provided positive control for mast cells. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Methacholine-Induced Saliva Production
A gauze pad (previously weighed in an Eppendorf tube [Denville Scientific, Holliston, MA]) was placed orally before flexiVent (Scireq) interrogation, then reweighed in the same Eppendorf tube.
Statistical Analysis
Figures show means (±SEM). Significance testing was performed by Student’s t test for simple groups and by ANOVA for methacholine and carbachol challenges.
Results
Neither IgE nor FcεRIα Contribute to AAD in Mice Inoculated with Low Doses of HDM
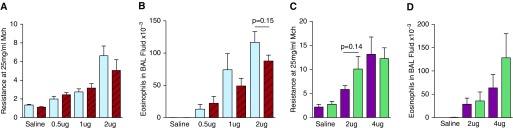
To determine the importance of IgE and FcεRIα in the development of AAD in response to limiting doses of HDM, WT, and IgE-deficient FVB/N mice, and WT and FcεRIα-deficient BALB/c mice were inoculated intratracheally every other day, seven times, with saline or 0.5, 1, 2, or 4 μg of HDM. Increases in eosinophilia and AHR to methacholine were observed with HDM doses as low as 0.5 μg and increased in a dose-dependent manner. No significant differences were found consistently between WT and IgE-deficient FVB/N or between WT and FcεRIα-deficient BALB/c mice with regard to AHR, as measured by unrestrained plethysmography or by forced oscillation, BALF eosinophilia, or goblet cell metaplasia (Figure 1, Figure E3). Additional experiments were performed to evaluate whether increases in IgG1 might provide for a compensatory mechanism in the generation of disease (Figure E4). After inoculating BALB/c mice with 4 μg HDM every other day, seven times, we found that IgE deficiency substantially inhibited production of HDM-specific IgG1, whereas FcεRIα deficiency did not, compared with WT mice. Importantly, AHR was not different between these two deficient groups.
Figure 1.
Intratracheal inoculation with limiting doses of house dust mite extract (HDM) induces IgE- and FcεRIα-independent allergic airway disease (AAD). Wild-type (WT) and IgE-deficient FVB/N mice (A and B) and WT and FcεRIα-deficient BALB/c mice (C and D) were inoculated intratracheally with saline or HDM every other day for 14 days. Amounts of HDM shown denote protein delivered per inoculation. Resistance units are cm H2O × s/ml. (A and B) n = 5–15/group; three experiments pooled. Mice per each experimental group from left to right: 15, 14, 6, 5, 13, 13, 11, and 11. (C and D) n = 11–20/group; four experiments pooled. Mice per each experimental group from left to right: 20, 17, 15, 11, 12, and 13. FVB/N WT, blue bars; FVB/N IgE-KO, red bars; BALB/c WT, purple bars; BALB/c FcεRIα-KO, green bars. Data presented are means (±SEM). BAL, bronchoalveolar lavage; KO, knockout; Mch, methacholine.
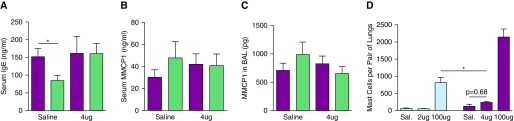
To determine whether IgE and FcεRIα influenced mast cell infiltration into the lungs and airway mast cell responses, we evaluated pulmonary mast cell numbers, serum IgE levels, and MMCP1 levels in FVB/N WT, BALB/c WT, and BALB/c FcεRIα-deficient mice inoculated with saline or 2, 4, or 100 μg of HDM. Only the highest dose of HDM induced an appreciable increase in lung mast cell number in either FVB/N or BALB/c mice (Figure 2). Consistent with this, 4-μg doses of HDM failed to significantly increase serum or BAL fluid (BALF) MMCP1 levels or serum IgE level (Figure 2). Taken together with the data in Figure 1, these observations demonstrate that airway inoculation with limiting doses of HDM induces AHR, airway eosinophilia, and airway epithelial cell metaplasia in the absence of evidence of airway mast cell hyperplasia or activation.
Figure 2.
Intratracheal inoculation with limiting doses of HDM fails to increase serum IgE or murine mast cell protease 1 (MMCP1) levels or pulmonary mast cells in BALB/c mice, whereas high doses of HDM induce pulmonary mastocytosis in BALB/c and FVB/N mice. WT and FcεRIα-deficient BALB/c mice were inoculated intratracheally as in Figure 1 and killed 4 hours after the seventh inoculation (A–C); n = 12–17/group 2 experiments pooled. Mice per each experimental group from left to right: 8, 7, 10, and 8. (D) WT mice were were inoculated intratracheally as in Figure 1 and killed 1 day after the seventh inoculation; lungs were digested and the cells obtained were assessed by flow cytometry; n = 4/group in two separate experiments. Sal., saline; BALB/c WT, purple bars; BALB/c FcεRIα-KO, green bars; FVB/N WT, blue bars. *P < 0.05. Data presented are means (±SEM).
IgE Does Not Contribute to AAD in Mice Inoculated with a High Dose of HDM
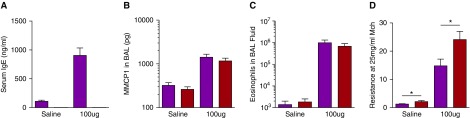
To determine whether mast cells and IgE play more of a role in the induction of AAD in mice inoculated with a high dose of a potent allergen, BALB/c WT and IgE-deficient mice were inoculated intratracheally (every other day, seven times) with 100 μg of HDM per dose. BAL eosinophils and MMCP1 levels, as well as responsiveness to methacholine, increased strikingly in response to this dose, along with serum IgE levels (in WT mice). No difference was observed, however, in MMCP1 and eosinophil levels between the WT and IgE-deficient mice (Figure 3). The increase in airway resistance in response to methacholine was higher in IgE-deficient mice inoculated with HDM than in WT mice inoculated with HDM (Figure 3); however, the fold increase in airway resistance of HDM-treated mice over the corresponding saline-treated group after methacholine challenge was equivalent (Figure E5). We investigated the mechanism for the observed substantial mast cell degranulation in the absence of IgE by comparing WT BALB/c mice to mice that lacked both complement component 3 and the γ chain that is shared by all activating Fc receptors, including FcεRIα. Figure E6 shows that the MMCP1 content of BALF, AHR, HDM-specific IgG1 production, and mast cell numbers were the same between WT mice and those mice deficient in both complement and activating Fc receptors.
Figure 3.
Intratracheal inoculation with high doses of HDM induces IgE-independent AAD. WT and IgE-deficient BALB/c mice were inoculated intratracheally with 100 μg of HDM as in Figure 1 and assessed 1 day after the seventh inoculation. (A–D) Serum IgE level, MMCP1 content of BAL fluid, pulmonary eosinophilia, and airway hyperresponsiveness (AHR), respectively; n = 11–13/group pooled from two experiments. Mice per each experimental group from left to right: 11, 11, 12, and 13. BALB/c WT, purple bars; BALB/c IgE-knockout, red bars. *P < 0.05. Data presented are means (±SEM).
IgE Is Not Required for AAD in a Mast Cell–Dependent Model of AAD
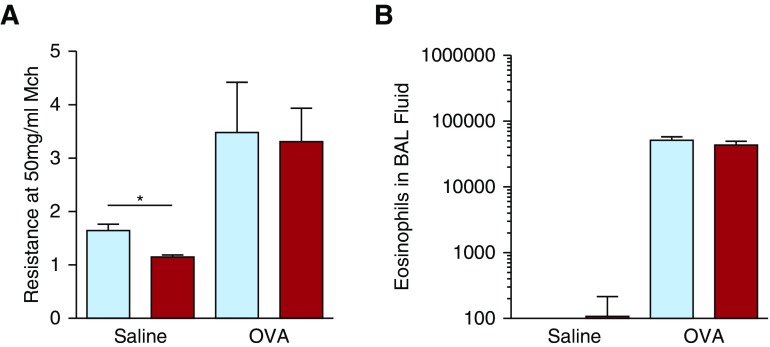
Because mast cell degranulation occurred independently of IgE in mice inoculated with HDM, we evaluated the IgE dependence of a model of AAD that has been shown to consistently depend on mast cells. AAD is induced in this model by inoculating mice intraperitoneally with OVA without adjuvant, followed by intranasal inoculation with OVA (Model B in Figure E2). AHR and pulmonary eosinophilia developed independently of IgE in these FVB/N mice (Figure 4 and Figure E7).
Figure 4.
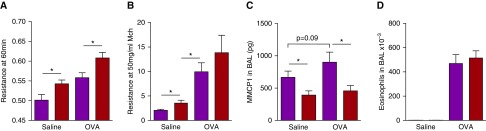
Induction of AAD by ovalbumin (OVA) inoculation is IgE-independent. WT and IgE-deficient FVB/N mice were injected three times intraperitoneally, once every 3 days, with 50 μg of OVA, then inoculated intranasally weekly with 20 μg of OVA for 9 weeks. (A and B) AHR and pulmonary eosinophilia, respectively; n = 11–12/group pooled from two experiments. Mice per each experimental group from left to right: 12, 12, 11, and 12. FVB/N WT, blue bars; FVB/N IgE-knockout, red bars. *P < 0.05. Data presented are means (±SEM).
Inoculation with OVA Fails to Induce an Acute IgE-Dependent Increase in Airway Resistance in OVA-Immune Mice
To evaluate whether allergen inhalation induces IgE-dependent changes in pulmonary function in mice with AAD, BALB/c WT and IgE-deficient mice were first injected intraperitoneally with saline or with 50 μg of OVA in the presence of alum, followed by three intratracheal inoculations with saline or with 500 μg of OVA (model C in Figure E2). This induced severe disease in the group treated with OVA. At 3 days after the third airway treatment, mice were anesthetized with isoflurane and treated a fourth time intratracheally with either saline or 500 μg of OVA and immediately placed into a plethysmographic chamber for monitoring. Mice with AAD rapidly differentiated from saline-treated mice over 3 hours of monitoring, but IgE deficiency was associated with higher, rather than lower, Penh values (Figure E8A). Mice received another three intratracheal treatments the following week to maintain disease. At 3 days after their seventh airway treatment, all mice were exposed to aerosolized OVA, then mechanically ventilated after a 60-minute delay and assessed for airway resistance, airway responsiveness to methacholine, hysteresis, pulmonary eosinophilia, MMCP1 content of BALF, serum IgE level, and pulmonary mastocytosis. Figure 5 shows that baseline resistance was higher for OVA- than for saline-treated WT and IgE-deficient mice, and was higher in the IgE-deficient mice regardless of treatment—consistent with the data in Figure 3. However, if resistance data are represented as fold increase over the corresponding saline-treated group, WT and IgE-deficient groups are again equivalent, both with initial resistance measurements at 60 minutes and after methacholine challenge (Figures E8B and E8C). Pulmonary eosinophilia, goblet cell metaplasia, and hysteresis were not affected by IgE deficiency (Figures 5 and Figures E8F and E9). However, despite good induction of IgE synthesis and pulmonary mast cells in this model (Figures E8D and E8E), the MMCP1 content of BALF was not increased at this point in disease development in either WT or IgE-deficient mice, although IgE-deficient mice had lower levels than WT mice (Figure 5C).
Figure 5.
High doses of OVA induce disease in BALB/c mice that is not accentuated by IgE, and lung function is not affected 60 minutes after exposure to antigen. OVA (50 μg) with 2 mg alum was injected intraperitoneally into WT and IgE-deficient mice on Days 0, 14, and 28, which were then inoculated intratracheally with 500 μg of OVA on Days 35, 36, 37, 40, 42, 43, and 44. Control mice were injected and inoculated with saline. On Day 47, all mice were exposed to a 1% OVA aerosol for 20 minutes, then mechanically ventilated to measure airway resistance by forced oscillation 60 minutes after initiation of OVA aerosol (A). Methacholine challenge was then performed (B). Pulmonary eosinophilia and MMCP1 in BAL fluid were measured (C and D); n = 11–13/group pooled over six experiments. Mice per each experimental group from left to right: 11, 12, 13, and 13. BALB/c WT, purple bars; BALB/c IgE-knockout, red bars. *P < 0.05. Data presented are means (±SEM).
Anaphylaxis Increases the Response to Methacholine in Saline-Treated, but Not HDM-Inoculated Mice
Active immunization can have multiple stimulatory and inhibitory effects on airway responses. Because of this, we wanted to use an approach that circumvents peripheral immunological tolerance. We induced severe AAD in BALB/c WT mice by administering 100 μg of HDM intratracheally every other day, seven times, to induce pulmonary mastocytosis. At 1 day after the seventh dose, on Day 13, we assessed mice for AHR by unrestrained barometric plethysmography, which allowed saline-treated and HDM-treated mice to be divided into subgroups with equivalent AHR (Figure E10A). On Day 15, those mice that would undergo anaphylaxis were passively sensitized to TNP by injection with monoclonal anti-TNP IgE antibody, whereas those mice that would not undergo anaphylaxis were injected with saline.
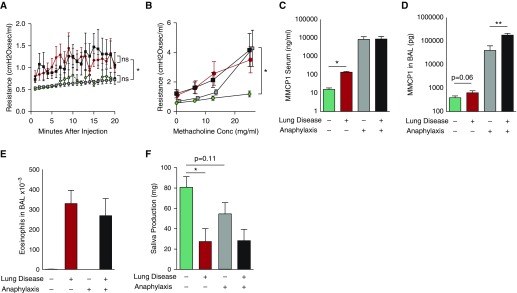
On Day 16, we then evaluated the immediate effects of systemic TNP-BSA injection on airway resistance, airway Newtonian resistance, airway responsiveness, hysteresis, and serum and BALF MMCP1 levels. Pulmonary eosinophilia, serum IgE levels, and saliva produced in response to methacholine challenge were additionally measured. Passive sensitization and TNP challenge did not affect airway resistance or Newtonian resistance (Figure 6A; Figures E10B and E10C). Such sensitization and TNP challenge did significantly increase levels of MMCP1 in the serum of saline-treated mice and in the BALF of HDM-treated mice (Figures 6C and 6D). Equivalent AHR was seen in mice treated with HDM, regardless of induction of anaphylaxis (Figure 6B). Thus, strong IgE-mediated mast cell degranulation does not immediately increase airway resistance or AHR in mice that have severe AAD when methacholine is administered by aerosol.
Figure 6.
IgE-mediated anaphylaxis does not cause acute changes in lung function in mice with AAD. BALB/c WT mice were inoculated intratracheally every other day for a total of seven doses with 100 μg of HDM or saline. On Day 15, mice were injected intravenously with saline or anti-trinitrophenyl (TNP) IgE monoclonal antibody. On Day 16, all mice were mechanically ventilated, injected intraperitoneally with TNP-BSA, and then monitored for 20 minutes while pulmonary function was measured (A). Mice were then challenged with increasing concentrations (Conc) of inhaled methacholine (B). The MMCP1 content of serum and BAL fluid was measured (C and D). Pulmonary eosinophilia (E) and saliva produced by methacholine challenge (F) were measured. Four mice in each saline-treated group; six mice in each HDM-treated group. Data pooled from two experiments. No lung disease + no shock, green circles and bars; no lung disease + shock, gray squares and bars; +lung disease + no shock, red circles and bars; +lung disease + shock, black squares and bars. *P < 0.05, **P < 0.01. Data presented are means (±SEM). ns, not significant.
In mice treated with saline, AHR was seen in those undergoing shock only at the highest dose of methacholine. We anticipated unexpected results upon methacholine challenge in this experiment, due to the altered cardiovascular status in anaphylactic mice. As a way to help explain our findings, the effects of methacholine on saliva production were measured. In Figure 6F, mice with severe AAD produced less than half the saliva produced by mice without AAD, regardless of whether anaphylaxis was induced. In saline-treated mice, anaphylaxis was associated with a trend toward decreased saliva production (P = 0.11). In addition, acute IgE-mediated mast cell activation had no effect on hysteresis or pulmonary eosinophilia (Figure E10D; Figure 6E).
IgE Cross-Linking Does Not Promote Airway Smooth Muscle Contraction in HDM-Immune Mice
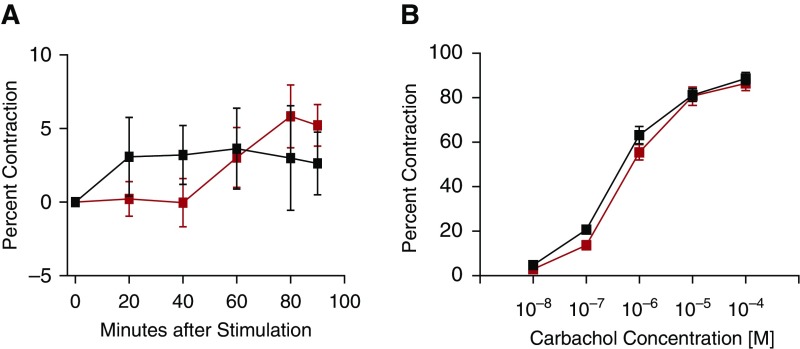
To isolate the effects of IgE-mediated mast cell activation on airway smooth muscle contractility, and increase the sensitivity of our detection of contraction, we pursued ex vivo airway contraction assays that use microscopy to measure airway size. Because these studies were performed in collaboration with a laboratory in another city, lungs from mice were inflated with agarose and shipped to our collaborators overnight on ice. We first verified that this approach provided good tissue viability (Figure E11). All experimental mice were treated with 100 μg of HDM intratracheally every other day for a total of seven doses to induce severe AAD and pulmonary mastocytosis. On Day 13, all mice were injected with IgE anti-TNP monoclonal antibody. On the following day, the mice were anesthetized and their lungs were inflated with agarose and shipped immediately on ice. Precision-cut lung slices were prepared and incubated in medium that contained either TNP-BSA or BSA. Slices were monitored for contraction for 90 minutes and then immediately subjected to carbachol challenge (Figures 7A and 7B; Figure E12). Results show that airway contraction was not observed in those slices exposed to TNP until carbachol challenge was administered. Immediate antecedent exposure to TNP-BSA did not increase airway contractility to carbachol as compared with exposure to BSA alone.
Figure 7.
IgE cross-linking does not provoke airway contraction or increase smooth muscle contractility ex vivo. BALB/c WT mice were inoculated intratracheally every other day for a total of seven doses with 100 μg of HDM. On Day 13, all mice were injected with anti-TNP IgE; in the late evening of Day 14, their lungs were inflated with agarose and shipped directly overnight on ice to our collaborating laboratory that expertly performs this assay. On Day 15, slices of the left lung were made and incubated in medium. On Day 16, these slices were exposed to medium that contained TNP-BSA or BSA, monitored for contraction for 90 minutes (A), then immediately subjected to carbachol challenge (B). A total of 11 mice in the BSA group, 12 mice in the TNP-BSA group, pooled from 4 experiments. BSA incubation, red squares; TNP-BSA incubation, black squares. Data presented are means (±SEM).
Discussion
Allergic asthma is a serious medical problem that has garnered substantial attention from the scientific community based upon its prevalence, complexity, and lack of a cure. Early investigations primarily used OVA and rodents to model this disease, and often assessed the significance of components causing immediate hypersensitivity reactions. Because asthma is traditionally characterized by episodic bronchospasm with normal lung function between episodes, it was logical to assume that induction of underlying disease was dependent on repeated episodes of IgE-mediated mast cell stimulation that classically define the allergic status of patients. However, asthma is more broadly defined by an increased sensitivity of the airway to contract upon stimulation that may be conferred by immune cells, structural lung cells, and/or by allergens directly. Ultimately, cytokines have been shown to underpin this process, and can be produced through a variety of mechanisms and cell types.
Although studies of severe AAD have shown directly or indirectly that IgE does not substantially promote AAD in mice (26, 27), AHR in the mouse can be made to depend on IgE when IgE is administered passively before its cognate antigen is administered to the animal’s airway. Expression of CD23 (the low-affinity IgE receptor) by nonhematopoietic cells has recently been shown to promote induction of murine AAD when active sensitization to OVA is achieved by peritoneal injection with alum and when small amounts of antigen are delivered to the airway. Administering CD23-blocking Ig inhibits AAD that is induced in this way, but also inhibits AAD that is induced by repetitive administration of OVA in small amounts to the airway without antecedent systemic sensitization (1, 12). Similarly, FcεRIα expression on mast cells was shown to be important in the induction of AAD when OVA was repetitively administered to the airway in small amounts without antecedent systemic sensitization (8). Mast cells themselves have been shown to be important in the induction of AAD when mice are sensitized to OVA by peritoneal injection without adjuvant, and then AAD is induced by inhaling doses of antigen administered intranasally (10).
Although AAD dependence on IgE and/or mast cells has been most often associated with mild disease, weak allergens, and induction of airway disease that follows prior sensitization at a distant site, much less is known about the roles of IgE when AAD is induced by inhalation of a potent, clinically relevant antigen, such as HDM (3). To evaluate this, we used a mouse AAD model in which disease is induced by repeated intratracheal inoculation with very small doses of HDM without adjuvant.
We find that IgE and FcεRIα neither promote nor inhibit AAD when mice that are genetically prone to the development of AHR are immunized in this way. Surprisingly, such disease induction does not increase systemic IgE level, pulmonary mast cells, or evidence of mast cell activation. In addition, surprisingly, we find that the IgG1 response to HDM given at low dose depends on IgE and possibly FcεRIα, the former accentuating such a response and the latter possibly inhibiting such a response. However, because there was no difference in AHR between these two groups, which demonstrated such an obvious difference in IgG1 levels, we also conclude that IgG1 is unlikely to substantially promote or inhibit the onset of AAD caused by low doses of HDM. This highlights the potency of other cell types that appear to provide for AAD in the absence of measurable immediate hypersensitivity reactions. This is consistent with the finding that omalizumab does not decrease the baseline AHR to methacholine in adults with asthma (19).
A potential limitation of these experiments that used low-dose HDM is that we did not administer high doses of methacholine for invasive measurement of AHR. However, our conclusions about these experiments are very likely correct, because: (1) we use mouse strains that demonstrate AHR robustly when AAD is present; (2) mice consistently produce abundant saliva upon nebulization after the 25 mg/ml methacholine aerosol; and (3) IgE is not noticeably produced with low doses of HDM.
Because there may be value in measuring AHR by more than one method, we performed methacholine challenge by plethysmography in unrestrained, unanesthetized mice before they were killed. Although this technique has been criticized (28), is prone to error despite very careful calibration, is confounded by behavior of the mouse while in the chamber, and potentially confounded by nasal physiology, we have found it consistently diagnostic in severe AAD. We were pleased to find that this technique could be used to readily identify AHR in mice treated with small doses of HDM (Figure E3). A very smooth dose–response curve between HDM and AHR was seen in FVB/N WT mice, BALB/c WT mice, and BALB/c FcεRIα-deficient mice. FVB/N IgE-deficient mice demonstrated an erratic dose–response curve by unrestrained plethysmography that was not, however, recapitulated by the more accurate, sensitive, and reliable invasive measurements of airway resistance performed the following day.
Similarly, when pulmonary mastocytosis, increased serum IgE levels, and intense allergic disease are induced by high doses of HDM or OVA, IgE does not increase mast cell degranulation. Other factors that have a good chance of providing such stimulation to mast cells include IgG, complement, and cytokines. We investigated the first two of these factors simultaneously by comparing WT mice to mice that lack both complement and all activating conventional receptors for Ig. Finding equivalent disease parameters, including the MMCP1 content of BALF, we suspect that the local cytokine milieu or direct contact with T cells may be largely responsible for this degranulation of mast cells. Even when we used a mast cell–dependent model of allergic sensitization, AAD was induced independently of IgE. These findings highlight both the innate nature of mast cells and their capacity to contribute to AAD.
Given that IgE did not contribute to the induction of AAD in our hands, we wanted to see if we could model the acute changes in human lung function that follow allergen exposure. The literature on this topic is diverse, includes studies of rats, mice, guinea pigs, and sheep, and reports changes in pulmonary function that occur within minutes or hours of allergen exposure (29–31). Mice, however, may be less able to develop increased airway resistance or AHR through this mechanism, because their mast cells release more 5-hydroxytryptamine (5-HT) than histamine, their airway smooth muscle is resistant to the effects of histamine, and 5-HT is thought to induce airway contraction indirectly (32–34).
Although airway contraction is the cause of asthma exacerbations in humans and increased airway resistance in mice, other changes in pulmonary function might also occur after exposure to allergen. Notably, in biologic drug trials, humans with asthma can demonstrate a substantial improvement in their baseline obstruction by spirometry, and yet have unchanged symptoms scores (35). Conversely, such drugs can also improve symptom scores, while not improving baseline obstruction (19). In addition, because immediate hypersensitivity reactions cause extravasation of fluid, such fluid may alter pulmonary mechanics beyond the airway (36). Thus, we thought it worthwhile to also measure the hysteresis of mice undergoing acute allergen challenge using a traditional pressure–volume loop delivered by modern, highly sensitive equipment. To our knowledge, this has not been reported in the literature.
To induce substantial numbers of intrapulmonary mast cells, we first used a high-dose OVA protocol to look at early and late time points (30). We find that delivering a large intratracheal dose of OVA does not immediately change the breathing pattern of sensitized, unrestrained, conscious WT mice more than that of IgE-deficient mice. When delivering a small dose of OVA by aerosol to the same mice, increased airway resistance or hysteresis is not provoked 1 hour after exposure even when using very sensitive methods of detection. However, despite strong mastocytosis, we did not see obvious signs of mast cell activation in this model, as disclosed by MMCP1 content of BALF.
Because we were unsure as to how well pulmonary cells were acutely stimulated by OVA in the prior experiment, we sought to maximally stimulate them by inducing IgE-mediated systemic anaphylaxis by passive means that circumvent mechanisms of peripheral tolerance. Using high doses of HDM to induce severe disease with pulmonary mastocytosis and using TNP-BSA for provocation of mice sensitized with IgE anti-TNP monoclonal antibody, we find no change in the hysteresis, airway resistance, or Newtonian resistance of lung tissue, even when pulmonary mast cells are intensely degranulated by antigen-induced IgE cross-linking. Measurement of AHR in this experiment provided both expected and unexpected results. Although anaphylaxis did not change AHR in mice treated with HDM, anaphylaxis did strikingly increase AHR in mice treated with saline. One possible explanation of this result is suggested by changes in the amount of saliva produced in response to methacholine.
Both groups treated with HDM show significant decrement in the amount of saliva produced during methacholine challenge. Because methacholine and other cholinergic agents stimulate saliva secretion, this observation suggests that the amount of methacholine reaching systemic circulation is reduced in mice that have HDM-induced AAD. A possible explanation for this is that the substantial mucus burden of these mice decreases the amount of aerosolized methacholine that reaches the alveoli for interstitial and systemic absorption. This proposed difference in methacholine absorption between saline-treated and HDM-treated mice might explain how IgE/mast cell–mediated shock increased responsiveness to methacholine in saline-treated mice, but not in HDM-treated mice.
The striking increase in airway resistance upon exposure to methacholine of saline-treated mice undergoing IgE-mediated shock may also have its roots in the effective dose of methacholine delivered. Mice in shock may have decreased capacity to clear methacholine from their lung tissue, leading to a higher accumulation of methacholine after successive doses are administered. This view is supported by a somewhat lower saliva production in saline-treated mice undergoing shock compared with those not undergoing shock. An alternate explanation would be that mast cell–derived factors, such as 5-HT, were already providing a small degree of stimulation that was then accentuated by methacholine—pushing beyond a “tipping point.”
Wanting to remove the confounding factors of airway mucus and cardiac status from our experiments, and wanting to increase the sensitivity of detecting airway smooth muscle contraction, we turned to ex vivo assays in which the airways are imaged by microscopy. This approach isolates the component of airway narrowing that is attributable to smooth muscle and allows calculation of airway contractility. We find that ex vivo stimulation of pulmonary mast cells does not induce airway contraction or increase airway contractility to carbachol, which is consistent with our in vivo studies.
Although mice may not reliably model the acute changes in lung function that characterize IgE-dependent exacerbations of human asthma, our study provides new and important information. From a methodological standpoint, our data suggest that the cardiac status and the burden of airway mucus may confound the measurement of AHR in laboratory animals. From a biological standpoint, we show that inhalation of small and large quantities of a potent, clinically relevant allergen induces AAD that is IgE independent, even though inoculation with a large allergen dose induces substantial IgE production and pulmonary mast cell numbers. In addition, we demonstrate substantial pulmonary mast cell activation in the absence of IgE in the setting of severe AAD induced by either potent allergen or innocuous antigen. This underscores the innate nature of mast cells. As for implications for human disease, these experiments inform us that the earliest manifestation of allergic asthma likely depends exclusively on a cytokine response, rather than an antibody response, when potent allergen is inhaled repetitively at low doses into the lungs. This may have relevance for the prevention of human allergic asthma.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases grant T32 AI060515 and University of Cincinnati’s Fellow to Faculty Award; all flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by grants NIH AR-47363, NIH DK78392, and NIH DK90971.
Author Contributions: Study conception and design—C.G.M. and F.D.F.; acquisition, analysis, and interpretation of data—C.G.M., J.A.J., Z.Z., R.A.P., and F.D.F.; drafting of the manuscript—C.G.M., R.A.P., and F.D.F.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0356OC on July 12, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Palaniyandi S, Liu X, Periasamy S, Ma A, Tang J, Jenkins M, Tuo W, Song W, Keegan AD, Conrad DH, et al. Inhibition of CD23-mediated IgE transcytosis suppresses the initiation and development of allergic airway inflammation. Mucosal Immunol. 2015;8:1262–1274. doi: 10.1038/mi.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizutani N, Nabe T, Yoshino S. IgE/antigen-mediated enhancement of IgE production is a mechanism underlying the exacerbation of airway inflammation and remodelling in mice. Immunology. 2015;144:107–115. doi: 10.1111/imm.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boer JD, Yang J, van den Boogaard FE, Hoogendijk AJ, de Beer R, van der Zee JS, Roelofs JJ, van ’t Veer C, de Vos AF, van der Poll T. Mast cell–deficient kit mice develop house dust mite–induced lung inflammation despite impaired eosinophil recruitment. J Innate Immun. 2014;6:219–226. doi: 10.1159/000354984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JY, Kim JW, Kim JS, Kim SJ, Lee SH, Kwon SS, Kim YK, Moon HS, Song JS, Park SH, et al. Inhibitory effects of anti–immunoglobulin E antibodies on airway remodeling in a murine model of chronic asthma. J Asthma. 2010;47:374–380. doi: 10.3109/02770901003801972. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs B, Sjöberg L, Möller Westerberg C, Ekoff M, Swedin L, Dahlén SE, Adner M, Nilsson GP. Mast cell engraftment of the peripheral lung enhances airway hyperresponsiveness in a mouse asthma model. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1027–L1036. doi: 10.1152/ajplung.00227.2012. [DOI] [PubMed] [Google Scholar]
- 6.Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, Buhl R, Taube C. Mast cell–derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 7.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell–derived TNF contributes to airway hyperreactivity, inflammation, and Th2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, Takeda K, Loader J, Miyahara N, Kodama T, et al. Mast cells, Fc epsilon RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172:6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 9.Mayr SI, Zuberi RI, Zhang M, de Sousa-Hitzler J, Ngo K, Kuwabara Y, Yu L, Fung-Leung WP, Liu FT. IgE-dependent mast cell activation potentiates airway responses in murine asthma models. J Immunol. 2002;169:2061–2068. doi: 10.4049/jimmunol.169.4.2061. [DOI] [PubMed] [Google Scholar]
- 10.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, Ishizaka K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 12.Haczku A, Takeda K, Hamelmann E, Loader J, Joetham A, Redai I, Irvin CG, Lee JJ, Kikutani H, Conrad D, et al. CD23 exhibits negative regulatory effects on allergic sensitization and airway hyperresponsiveness. Am J Respir Crit Care Med. 2000;161:952–960. doi: 10.1164/ajrccm.161.3.9905046. [DOI] [PubMed] [Google Scholar]
- 13.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999;21:480–489. doi: 10.1165/ajrcmb.21.4.3659. [DOI] [PubMed] [Google Scholar]
- 14.Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness—a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell–deficient mice. Proc Natl Acad Sci USA. 1997;94:1350–1355. doi: 10.1073/pnas.94.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamelmann E, Oshiba A, Schwarze J, Bradley K, Loader J, Larsen GL, Gelfand EW. Allergen-specific IgE and IL-5 are essential for the development of airway hyperresponsiveness. Am J Respir Cell Mol Biol. 1997;16:674–682. doi: 10.1165/ajrcmb.16.6.9191469. [DOI] [PubMed] [Google Scholar]
- 17.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;97:1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non-anaphylactogenic anti-IgE antibody. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, Bao W, Fowler-Taylor A, Matthews J, Busse WW, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–593. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 20.McKnight CG, Perkins C, Finkelman FD. IgE and FceRIα do not promote allergic airway disease in mice inoculated intratracheally with house dust mite extract. Presented at the 98th American Academy of Immunology Annual Meeting. May 2014, Pittsburgh, PA. Abstract HYP7P.316. J Immunol. 2014;192(1 Suppl):119.31. [Google Scholar]
- 21.Tsitoura DC, Blumenthal RL, Berry G, Dekruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 22.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 23.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med. 2011;208:853–867. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper PR, Mesaros AC, Zhang J, Christmas P, Stark CM, Douaidy K, Mittelman MA, Soberman RJ, Blair IA, Panettieri RA. 20-HETE mediates ozone-induced, neutrophil-independent airway hyper-responsiveness in mice. PLoS One. 2010;5:e10235. doi: 10.1371/journal.pone.0010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13–independent allergic airway inflammation. J Allergy Clin Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adler A, Cieslewicz G, Irvin CG. Unrestrained plethysmography is an unreliable measure of airway responsiveness in BALB/c and C57BL/6 mice. J Appl Physiol (1985) 2004;97:286–292. doi: 10.1152/japplphysiol.00821.2003. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JE, Peng R, Harris P, Burns L, Renteria L, Lundblad LK, Fine JS, Bauer CM, Stevenson CS. House dust mite models: will they translate clinically as a superior model of asthma? J Allergy Clin Immunol. 2013;132:242–244. doi: 10.1016/j.jaci.2012.12.1571. [DOI] [PubMed] [Google Scholar]
- 30.Nabe T, Matsuya K, Akamizu K, Fujita M, Nakagawa T, Shioe M, Kida H, Takiguchi A, Wakamori H, Fujii M, et al. Roles of basophils and mast cells infiltrating the lung by multiple antigen challenges in asthmatic responses of mice. Br J Pharmacol. 2013;169:462–476. doi: 10.1111/bph.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haile S, Lefort J, Eum SY, Dumarey C, Huerre M, Heusser C, Vargaftig BB. Suppression of immediate and late responses to antigen by a non-anaphylactogenic anti-IgE antibody in a murine model of asthma. Eur Respir J. 1999;13:961–969. doi: 10.1034/j.1399-3003.1999.13e06.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 33.Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell–cholinergic nerve interaction in mouse airways. J Physiol. 2009;587:3355–3362. doi: 10.1113/jphysiol.2009.173054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eum SY, Norel X, Lefort J, Labat C, Vargaftig BB, Brink C. Anaphylactic bronchoconstriction in BP2 mice: interactions between serotonin and acetylcholine. Br J Pharmacol. 1999;126:312–316. doi: 10.1038/sj.bjp.0702304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 36.Lowe K, Alvarez DF, King JA, Stevens T. Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit Care Med. 2010;38:1458–1466. doi: 10.1097/CCM.0b013e3181de18f0. [DOI] [PMC free article] [PubMed] [Google Scholar]