Abstract
Background
Rising prevalence of childhood obesity and type 2 diabetes mellitus (T2DM) is an emerging public health issue.
Objectives
To investigate the association of maternal hyperglycemia exposure during pregnancy with obesity and abnormal glucose tolerance in offspring, and the age at occurrence.
Methods
We searched MEDLINE and EMBASE for observational studies on obesity and diabetes in offspring of diabetic mothers (gestational diabetes mellitus (GDM), type 1 diabetes mellitus (T1DM) and T2DM), and those on non-diabetic mothers. We performed fixed effect meta-analysis for all studies except when heterogeneity was detected. The quality of studies was evaluated using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS)
Results
Twenty observational studies were included involving a total of 26,509 children. Offspring of GDM mother had higher BMI z-score in childhood (pooled MD: 0.14, 95%CI: 0.04–0.24, seven studies, 21,691children, low quality of evidence). Offspring of T1DM mothers had higher BMI z-score from prepubertal to adolescent (pooled MD: 0.35, 95% CI: 0.13–0.58, three studies, 844 children, low quality of evidence) compared with control. After adjustment for maternal pre-pregnancy BMI, this association remained in offspring of T1DM, but disappeared in those of GDM mothers. Offspring of GDM mother had higher 2-hour plasma glucose from prepubertal to early adulthood (pooled MD: 0.43 mmol/L, 95% CI: 0.18–0.69, five studies, 890 children), while those of T1DM mothers had higher rate of T2DM in 2–5 years old to early adulthood (pooled odds ratio [OR], 6.10: 95% CI: 1.23–30.37, two studies, 448 children, very low quality of evidence) compared with control. As there was only one study with offspring of T2DM mothers, evidence is sparse.
Limitations
Only observational studies were included, with a few adequately adjusted for covariables.
Conclusions
Exposure to maternal hyperglycemia was associated with offspring obesity and abnormal glucose tolerance especially in offspring of T1DM mothers, but the evidence relies on observational studies with low quality of evidence only.
Introduction
Prevalence of type 2 diabetes mellitus (T2DM) has increased globally, impacting on health and economies worldwide [1]. In particular, the increased prevalence of T2DM in children, adolescents and young adults [2], combined with increased childhood obesity, is a serious public health concern [1]. In 2014, 42 million children (6.1%) under 5 years old were overweight or obese worldwide, up from 31 million (4.8%) in 1990 [3]. It was predicted that if current trends continue, the number of children under 5 years old who are overweight or obese would rise to 70 million by 2025 [3]. Notably, overweight and obese children are likely to be obese as adults, and will have non-communicable diseases (NCDs) such as T2DM at a younger age [4].
Fuel-mediated teratogenesis is a well-known hypothesis regarding intrauterine exposure to maternal diabetes [5]. Exposure to hyperglycemia in utero is believed to be associated with offspring obesity and impaired glucose tolerance [5], which is one of the most important viewpoints in preventing sharp rise in obesity and type 2 diabetes in the near future.
To date, two systematic reviews have shown the association between maternal diabetes and childhood obesity [6, 7]. In both reviews, maternal hyperglycemia was shown to be a risk factor for obesity or overweight in offspring, but the association was attenuated or no longer apparent after adjusting for covariates, especially maternal BMI [6, 7]. To clarify this association, it is necessary to consider the differences in the timing and degree of hyperglycemic intrauterine exposure, the difference in the genetic predisposition of obesity and T2DM, and the difference in the evaluation age of offspring. At present, there is no systematic review on the association between maternal hyperglycemia and childhood abnormal glucose tolerance.
Therefore, we aimed to systematically review current findings on children with obesity and glucose metabolism born to diabetic mothers to clarify whether intrauterine hyperglycemia exposure increases the risk of obesity and abnormal glucose tolerance of offspring according to the type of maternal diabetes. We also sought to clarify at which age the impact of intrauterine maternal diabetes on child obesity or abnormal glucose tolerance emerged.
Methods
Data sources and searches
Reporting procedures for this systematic review were consistent with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines [8]. An information specialist conducted a comprehensive literature search of EMBASE and MEDLINE for reports on obesity and diabetes among offspring born to diabetic mothers published between January 1946 and December 2016. Then, we conducted hand searching and checked the references lists of the retrieved articles. We also searched all abstracts published by the American Diabetes Association and the European Association for the Study of Diabetes for their annual meetings in the last five years for inclusion. No language restrictions were applied. The detailed search strategy is shown in S1 Table.
Study selection
We included studies that evaluated the association between intrauterine exposure to maternal hyperglycemia and offspring obesity and diabetes.
The inclusion criteria are as follows:
Exposures to maternal diabetes, including GDM, pre-pregnancy type 1 diabetes mellitus (T1DM) and T2DM. Studies with unclear diabetes status (e.g., only ‘pre-existing diabetes’) and with no information on whether the mothers had diabetes before or after their pregnancies were excluded;
Non-diabetic control group;
Offspring were from a singleton pregnancy;
Offspring were over 2 years old;
One of the following outcomes was described:
Primary outcomes: the prevalence of obesity or overweight, BMI z-score, the prevalence of DM, secondary outcomes: fasting plasma glucose and 2-hour plasma glucose, and the prevalence of abnormal glucose tolerance (DM, impaired fasting glucose (IFG), impaired glucose tolerance (IGT)).
The definition of obesity or overweight is a BMI of >85th or >95th percentile for age and sex. The 2-hour plasma glucose (2hPG) and glucose tolerance were determined by a 2-hour oral glucose tolerance test.
Study designs such as randomized controlled trials (RCTs) and prospective or retrospective cohort studies were considered for inclusion. If offspring outcomes were described at multiple ages, we used findings for the longest duration of follow-up. The Pima Indian cohort was excluded as they have a genetically high incidence of obesity and T2DM. Reports were limited to human studies without any language restriction.
Data extraction and quality assessment
Eligible titles and abstracts were screened independently by two researchers (MK and CM). After screening, full-text articles of relevant studies were obtained. Any disagreements were resolved through discussion or consulting a third reviewer (EO).
Data were independently extracted by two researchers (MK and CM) from included reports using a data extraction form. Extracted data included the following: first author’s name, year of publication, study design, study duration, number of participants, maternal characteristics (pre-pregnancy BMI), exposure (maternal diabetes status and evidence of diagnosis), offspring characteristics (age, sex and ethnicity), outcome and covariates. We contacted authors directly for additional data relating to missing information. We divided age of offspring into four categories: 2 to 6 years old, 7 to 9 years old, 10 to 15 years old, 16 to 19 years, and over 20 years old.
The quality of studies was evaluated using the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) [9]. The RoBANS tool was developed for assessing the risk of bias of non-randomized studies, and comprised six domains: selection of participants, confounding variables, measurement of exposure, blinding of outcomes, incomplete outcome data and selective outcome reporting. The risk of bias for each domain was classified as low risk, high risk and unclear risk.
Data synthesis and analysis
Data from included studies were pooled and meta-analysis was performed. For dichotomous data, odds ratios (ORs) were calculated using a fixed-effects model. When heterogeneity was detected, a random-effects model was used. For continuous data, mean differences (MDs) with 95% confidence interval (CI) were used. Heterogeneity between studies was evaluated using the I2 statistics. We regarded heterogeneity as substantial if I2 was greater than 75%. All statistical analyses used 95% CI and a p-value with a cut-off point of 0.05. All statistical analyses were performed using Review Manager version 5 software (RevMan 5.3; The Cochrane Collaboration, Oxford, UK).
If the domain of participant selection was determined by RoBANS to be high risk, we conducted sensitivity analysis and excluded the research from the meta-analysis [10]. We extracted data by age of offspring. If there was no significant subgroup difference, we synthesized the data. If there was a significant subgroup difference, we interpreted the data according to age.
Evidence grading
GRADE
We evaluated the quality of evidence with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach using GRADEpro GDT available at http://guidelinedevelopment.org.
Quality ratings were made for the BMI z-scores, as well as the ORs for obesity or overweight, DM and the abnormal glucose tolerance of mothers with GDM and controls, and mothers with T1DM.
Results
Literature search
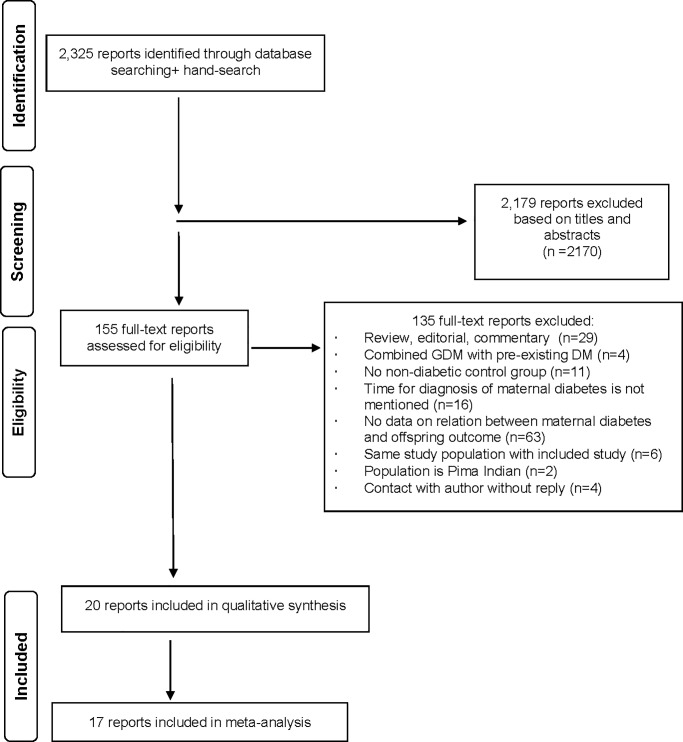
We identified 2,325 reports through our database searches (Fig 1). Of these, 2,170 were not relevant and were excluded based on the title and abstract, leaving 155 articles for full-text evaluation. Of the 155 studies, 132 were excluded due to outcomes of interest not reported, control group not included and timing of maternal diagnosis not reported. We translated one study and contacted the authors of six studies.
Fig 1. PRISMA flow diagram of search and study selection.
Finally, we selected 20 studies to be included in the current systematic review. Of these, 14 studies included offspring of mothers with GDM, eight studies focused on offspring of mothers with T1DM and one study included offspring of mothers with T2DM.
Study characteristics
The characteristics of the 20 included studies [11–30] are summarized in Table 1.
Table 1. Characteristics of included studies.
| Offspring·of·mothers·with·gestational·diabetes·mellitus¤ | |||||||||
| Autdor, year¤ |
GDM·criteria, Treatment (Tx)¤ |
Offspring·characteristics¤ | Outcome¤ | GDM·group¤ | Control·group¤ | P·value¤ | Adjustment¤ | ||
| Prospective·study¤ | |||||||||
| Boerschmann, 2010¤ | OGTT* two·of·three:·fasting≧90mg/dl,1h·180mg/dl, 2h≧155mg/dl Tx:NA¤ |
Age = 11·years Germany Race/ethnicity: NA Sex: NA¤ |
BMI>90th percentile¤ | N = 77¤ | n = 24¤ | N = 148¤ | n = 23¤ | NA** | Not·done¤ |
|
Catalano, 2009¤ |
National·Diabetes·Group·criteria Tx:diet(+insulin)¤ |
Age = 6.1–11.9 years USA Race/ethnicity:White,African American,Hispanic Sex: NA¤ |
BMI·z-score | N = 37 | 0.9±1.4 | N = 52 | 0.31±1.16 | 0.03 | Not·done |
| FPG**** ¤ | N = 23¤ | 4.9±0.3¤ | N = 26¤ | 4.8±0.2¤ | 0.33¤ | Not·done¤ | |||
|
Davis, 2013¤ |
Self-report Tx:NA¤ |
Age = 8–13·years USA Race/ethnicity: Hispanic Sex: female,GDM: 49%, control:42%¤ |
BMI·z-score | N = 47¤ | 2.2±0.4 | N = 163¤ | 2.1±0.4 | NS | Not·done |
| FPG**** | 4.99±0.38 | 4.94±0.34 | NS | Not·done | |||||
| 2hPG***** ¤ | 7.19±0.96¤ | 7.03±1.02¤ | NS¤ | Not·done¤ | |||||
|
Holder, 2014¤ |
Responded·to a·validated questionnaire Tx:NA¤ |
Age = 4–20·years (Mean age = GDM:15, Control:15) USA Race/ethnicity: NA Sex: NA¤ |
BMI·z-score | N = 45¤ | 2.37±0.54 | N = 219 | 2.26±0.58 | 0.27 | Not·done |
| FPG**** | 5.27±0.5 | N = 210¤ | 5.11±0.5 | 0.01 | Not·done | ||||
| 2hPG*****¤ | 7±1.44¤ | 6.33±1.11¤ | 0.005¤ | Not·done¤ | |||||
| Pirkola, 2010¤ | National·guidelines·in·Finland Tx:Diet,SMBG,(+insulin)¤ |
Age = 16·years Finland Race/ethnicity: NA Sex: NA¤ |
BMI>85th·percentile¤ | N = 84¤ | n = 18¤ | N = 661¤ | n = 113¤ | NA¤ | Not·done¤ |
|
Tam, 2010¤ |
WHO·criteria·1999 Tx:NA¤ |
Age = 15·years Hong Kong Race/ethnicity:Chinese Sex: NA¤ |
BMI≧85th· percentile | N = 63 | N = 63 | N = 101 | n = 26 | 0.51 | Not·done |
| DM | N = 42¤ | n = 1 | N = 87¤ | n = 0 | 0.77 | Not·done | |||
| FPG**** | 4.6±0.3 | 4.7±0.3 | 0.51 | Not·done¶ | |||||
| 2hPG*****¤ | 6±1.5¤ | 5.6±1.4¤ | 0.16¤ | Not·done¤ | |||||
| Whitaker, 1998¤ | Carpenter·and·Cousta·criteria Tx:diet¤ |
Age = 8–10·years USA Race/ethnicity: NA Sex: NA¤ |
BMI·z-score | N = 58¤ | 0.39±0.94 | N = 257¤ | 0.45±0.93 | 0.4 | Not·done¶ |
| BMI>85th·percentile¤ | n = 11¤ | n = 62¤ | 0.4¤ | Not·done¤ | |||||
|
Wright, 2009¤ |
Carpenter·and Cousta·criteria Tx:diet,·exercise,(+insulin)¤ |
Age = 3·years USA Race/ethnicity:White,Black, Hispanic,Other Sex: female,GDM:45%, control:49%¤ |
BMI·z-score |
N = 51¤ | 0.47±1.2 | N = 1053¤ | 0.44±1.02 | 0.68 |
-0.08±0.15(p = 0.61) |
| BMI>85th·percentile¤ | n = 9¤ | n = 169¤ | 0.52¤ | Not·done¤ | |||||
| Retrospective·study¤ | |||||||||
|
Buzinaro,2008¤ |
medical·record·and·questionnaire Tx:diet(+insulin)¤ |
Age = <17·years (Mean age = GDM:15,control:12) Brazil Race/ethnicity: NA Sex: NA¤ |
BMI>85th·percentile | N = 23¤ | n = 12 | N = 27¤ | n = 4 | NA** | Not·done¤ |
| FPG****¤ | 5.17±0.34¤ | 5±0.39¤ | NA** | Not·done¤ | |||||
|
Clausen,2008¤ |
OGTT*, OGTT·at ·east·two·of·seven·glucose·values ·exceeded·the·mean·3SD·values·for·a·reference·group·of·normal-weight·non-pregnant·women· without·a·family·history·of·diabetes Tx:NA¤ |
Age = 18–27·years Denmark Race/ethnicity: NA Sex: female, GDM: 46%, control:51%¤ |
DM | N = 168¤ | n = 7 | N = 128¤ | n = 1 | 0.77 | Not·done |
| IGT | n = 19 | n = 3 | |||||||
| IFG | n = 10 | n = 1 | |||||||
| FPG**** | 5.5±0.9 | 5.1±0.4 | <0.001 | Not·done | |||||
| 2hPG*****¤ | 5.9±2.1¤ | 5.3±1.3¤ | 0.005¤ | Not·done¤ | |||||
|
Gillman,2003¤ |
Self-report Tx:NA¤ |
Age = 9–14·years USA Race/ethnicity: NA Sex: female, GDM: 50%, control:54%¤ |
BMI·z-score | N = 463¤ | 0.33±1.01 | N = 14416¤ | 0.15±0.12 | <0.001 | Not·done |
| BMI>95th·percentile¤ | n = 45¤ | n = 958¤ | NA**¤ | OR·1.2(0.8–1.7) ¤ | |||||
|
Page,2014¤ |
NA¤ | Age = 5–16·years USA Race/ethnicity:Mexican·American Sex: female, GDM: 52%,control:27%¤ |
BMI·z-score¤ |
N = 25¤ | 0.95±0.2¤ | N = 37¤ | 0.25±0.2¤ | 0.02¤ | Not·done¤ |
|
Patel, 2012¤ |
By questionnaire Tx:NA¤ |
Age = 15.5·years UK Race/ethnicity: NA Sex: NA¤ |
BMI z-score |
N = 27¤ | 0.37±1.11 | N = 4834¤ | -0.22±0.97 | NA**¤ | -0.15±0.19¤ |
| FPG****¤ | 5.4±0.47¤ | 5.21±0.38¤ | NA**¤ | Not·done¤ | |||||
|
Pham,2013¤ |
National·Diabetes·Group·criteria,·switched·to·the·Carpenter·and·Coustan·criteria, Tx:participated·in·an·educational· class·focusing·on·diet·modification,postprandial·exercise,blood ·glucose·monitoring,appropriate ·weight·gain. |
Age = 2–4·years USA Race/ethnicity: South·Asian, Asian, Black, Latina, White, Other Sex: NA¤ |
BMI>85th·percentile¤ |
N = 255¤ | n = 61¤ | N = 1838¤ | n = 432¤ | NA**¤ | Not·done¤ |
| Offspring·of·mothers·with·type 1·diabetes·mellitus¤ | |||||||||
| Author, year¤ |
T1DM·criteria, Treatment¤ |
Offspring·characteristics¤ | Outcome¤ | T1DM·group¤ |
Control·group¤ |
P·value¤ | Adjustment¤¤ | ||
| Prospective·study¤ | |||||||||
|
Boerschmann, 2010¤ |
WHO·criteria. insulin¤ |
Age = 11·years Germany Race/ethnicity: NA Sex: NA¤ |
BMI>90th·percentile | N = 284¤ | n = 45¤ | N = 148¤ | n = 23¤ | NA**¤ | Not·done¤ |
|
Buinauskiene, 2004¤ |
WHO·criteria.¤ | Age = 2–5·years Lithuania Race/ethnicity: NA Sex: female T1DM 45%, control:45%¤ |
DM¤ | N = 51¤ | n = 1¤ | N = 109¤ | n = 1¤ | NS***¤ | Not·done¤ |
| Lindsay, 2010¤ | medical·records, insulin¤ |
Age = 7.4·years UK,Scotland Race/ethnicity: NA Sex: NA¤ |
BMI·z-score | N = 100 | 0.69±1.2 | N = 45 | 0.28±0.7 | 0.22 |
TIDM:0.67±0.11 Control:0.33±0.16 (p = 0.08) |
| BMI>90th·percentile | n = 22 | n = 0 | 0.001 | Not·done | |||||
| FPG**** | N = 53 | 4.5±0.3 | N = 19 | 4.5±0.4 | NA** | Not·done | |||
| 2hPG*****¤ | N = 34¤ | 5.1±1.3¤ | N = 12¤ | 5.7±0.8¤ | NA**¤ | Not·done¤ | |||
| Rodrigues, 1998¤ | NS¤ | Age = 18–27·years Denmark Race/ethnicity: NA Sex: NA¤ |
BMI>95th·percentile¤ | N = 17¤ | n = 7¤ | N = 18¤ | n = 0¤ | NA**¤ | Not·done¤ |
| Vlachov, 2015¤ | medical·records¤ | Age = 13–19.8·years Denmark Race/ethnicity: NA Sex: female, T1DM: 59%, control: 60%¤ |
BMI·z-score | N = 278¤ | 0.69±1.27 | N = 303¤ | 0.24±1.14 | <0.001 | TIDM·0.44·higher ·than·control(p<0.001) |
| FPG***** | 5.4±0.4 | 5.3±0.4 | 0.021 | TIDM·0.1·higher ·than·control(p = 0.008) | |||||
| 2hPG****¤ | 6.4±1.3¤ | 6.1±1.2¤ | 0.009¤ | TIDM· 0.2 ·higher ·than·control(p = 0.136)¤ | |||||
| Retrospective·study¤ | |||||||||
| Clausen, 2008¤ | fulfilled·three·criteria: onset·of ·diabetes·at·age≦40·years, classical·history, and·insulin·treatment·starting≦6·months ·after· diagnosis¤ | Age = 5.9–9·years USA Race/ethnicity: NA Sex: female, T1DM 46%,control:51%¤ |
IGT | N = 160¤ | n = 8 | N = 128¤ | n = 3 | NS*** | Not·done |
| IFG | n = 6 | n = 1 | NS*** | Not·done | |||||
| DM | n = 10 (T1·7,T2·3) |
n = 1 | NS*** | Not·done | |||||
| FPG**** | 5.2±0.5 | 5.1±0.4 | NS*** | Not·done | |||||
| 2hPG*****¤ | 5.8±1.6 | 5.3±1.3 | NS*** | Not·done¤ | |||||
| Hunter, 2004¤ | Onset·before·30·years·and·one·and·more·of·the·following: autoantibody·positive (GAD, insulin-associated·protein·2,or ·islet cells), ketoacidosis·at presentation,·normal ·BMI·at·diagnosis·no·first-degree ·relative·with· type 2· diabetes, and ·commencement· of· insulin ·therapy· at· diagnosis, insulin¤ |
Age = 5–10·years New Zealand Race/ethnicity: NA Sex: female, T1DM: 24%, control: 47%¤ |
BMI·z-score | N = 17¤ | 0.7±0.6 | N = 15¤ | -0.2±0.6 | 0.22 | Not·done |
| FPG****¤ | 4.9±0.1¤ | 4.9±0.1¤ | NS***¤ | Not·done¤ | |||||
| Manderson, 2002¤ | medical record database and standard questionnaire, insulin¤ |
Age = 5–11·years UK Race/ethnicity: NA Sex: female T1DM: 57%, control: 41%¤ |
BMI·z-score | N = 61¤ | 0.59±1.35 | N = 57¤ | 0.6±1.21 | 0.96 | Not done |
| FPG****¤ | 4.35±0.32¤ | 4.44±0.28¤ | 0.16¤ | Not done¤ | |||||
| Offspring·of·mothers·with·type 2·diabetes·mellitus¤ | |||||||||
| Author, year |
T2DM criteria, Treatment¤ |
Offspring·characteristics¤ | Outcome¤ | T2DM·group¤ |
Control·group¤ |
P·value¤ | Adjustment¤ | ||
| Retrospective·study¤ | |||||||||
| Hunter, 2004¤ | BMI>30kg/m2·at·diagnosis·had ·one· or· more ·of ·the· following: no·insulin· therapy requirement·non-ketoacidosis ·prone, and· the· presence· of ·acanthosis· nigricans.¤ |
Age = 5–10·years New Zealand Race/ethnicity: NA Sex: female, T2DM: 20%, control: 47%¤ |
BMI·z-score | N = 17¤ | 3.2±0.7 | N = 15¤ | -0.2±0.6 | <0.001 | Not·done¤ |
| FPG****¤ | 5±0.1¤ | 4.9±0.1¤ | NS¤ | Not·done¤ | |||||
*OGTT, oral glucose tolerance test;
**NA, not applicable;
***NS,not significant,
****FPG, fasting plasma glucose;
***** 2hPG, two hours plasma glucose
Offspring of mothers with gestational diabetes mellitus
Fourteen studies of offspring of GDM mothers and controls were included in this review, involving a total of 25,336 children [11–17, 20–22, 24, 26, 27, 29]. Eight studies were prospective cohort [11, 13, 15–17, 20, 26, 29] and six were retrospective cohort [12, 14, 21, 22, 24, 27]. Eight studies were conducted in the USA [11, 13–17, 24, 27], one each in Germany [26], Finland [29], Hong Kong [20], Brazil [21], Denmark [22] and the UK [12]. Ethnicities of offspring were White [11, 13, 24], Hispanic [11, 13, 16], African American [13], Mexican American [14], Black [11, 24], South Asian [24] and Chinese [20]. The age of offspring ranged from 3 to 27 years old. The sex of offspring was available in five studies [11, 14, 16, 22, 27] and the proportion of boys and girls was about the same. The GDM diagnostic criteria were available in all studies, except for one [14].Two studies [11, 15] used Carpenter-Coustan criteria, two studies [13, 24] used National Diabetes Data Group criteria, one study [20] used WHO criteria, three studies [22, 26, 29] used other criteria and five studies [12, 16, 17, 21, 27] were based on self-report. GDM treatments were included in five studies [11, 13, 15, 21, 29].
Offspring of mothers with type 1 diabetes mellitus
Eight studies of offspring of T1DM mothers and controls were included in this review, involving a total of 4,957 children [18, 19, 22, 23, 25, 26, 28, 30]. Five studies were prospective cohort [23, 25, 26, 28, 30], and three were retrospective cohort [18, 19, 22]. Two studies were conducted in the UK [19, 28] [19, 28]and Denmark[23, 30], one each in Lithuania [25], Germany [26], the USA [22] and New Zealand [18]. The age of offspring ranged from 3 to 27 years old.
Offspring of mothers with type 2 diabetes mellitus
Only one study of offspring of T2DM mothers and controls was included in this review [18]. The study, involving 32 children, was a retrospective cohort study conducted in New Zealand and the age of offspring ranged from 5 to 10 years old.
Quality evaluation
Risk of bias
The results of risk of bias assessment using RoBANs are summarized in S2 Fig. As for selection of participants, 55% studies were a low risk of bias, 15% at high risk and the risk was unclear for 30%. For incomplete outcome data, 30% studies were a low risk of bias, 30% at high risk and the risk was unclear for 40%. For selective outcome reporting, 5% studies were a low risk of bias, 25% at high risk and the risk was unclear risk for 75%.
Association
Offspring of mothers with gestational diabetes mellitus
Obesity and overweight
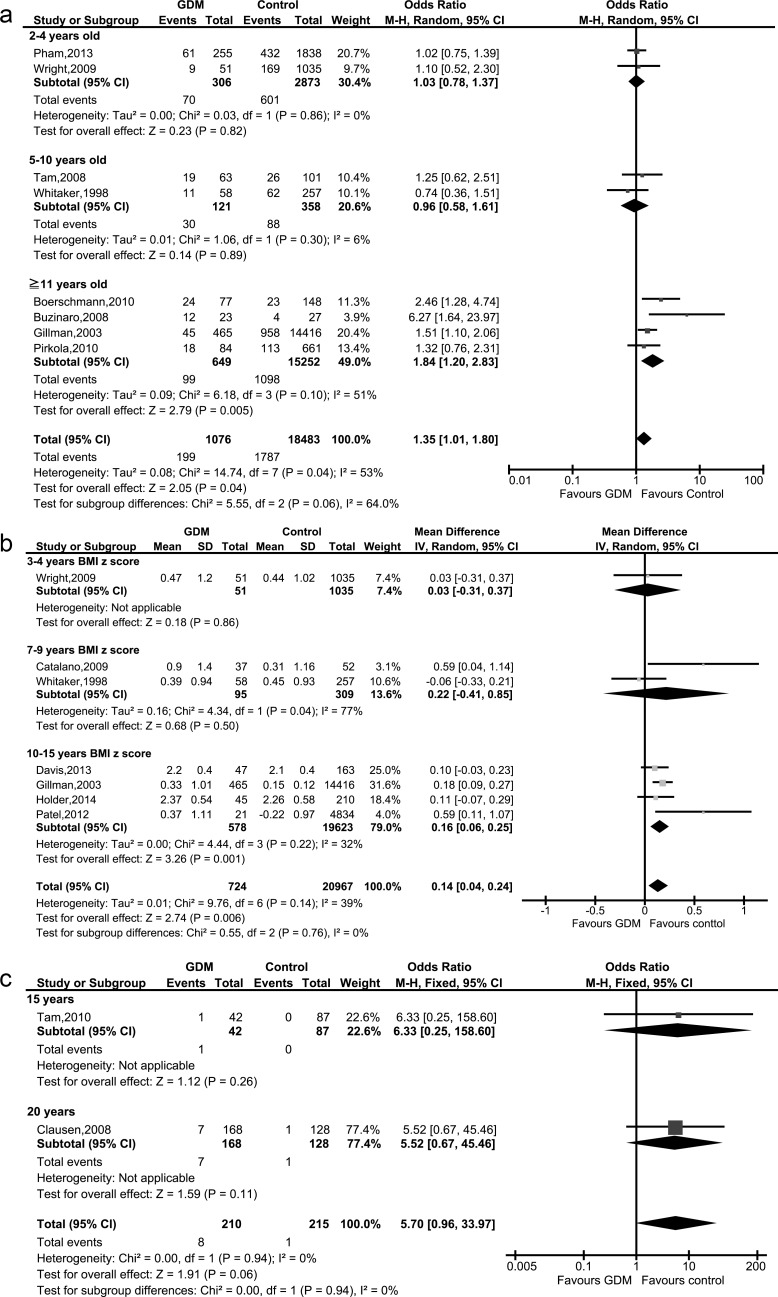
Eight studies of GDM mothers’ offspring and controls were included in this review, involving a total of 19,559 children [11, 15, 20, 21, 24, 26, 27, 29]. A forest plot of the crude odds ratios of obesity or overweight of mothers with GDM and controls is shown in Fig 2A.
Fig 2.
Forest plots of metabolic parameters in offspring of mothers with gestational diabetes mellitus and controls: (a), obesity or overweight, (b) BMI z-scores, (c) diabetes.
The rate of childhood obesity or overweight was statistically significantly higher in the offspring of GDM mothers (p value = 0.04, pooled OR: 1.35; 95% CI: 1.01–1.80; I2 = 53%; eight studies, 19,559 children). Quality of evidence was judged as ‘low’ (S2 Table).
Only one study [27] was adjusted for covariates including maternal pre-pregnancy BMI. Therefore, we could not conduct an adjusted OR. Unadjusted OR for overweight in adolescent was 1.4 (95% CI: 1.0–1.9) and adjusted OR was 1.2 (95% CI: 0.8–1.7).
BMI z-scores
Eight studies of GDM mothers’ offspring and controls were included in this review, involving a total of 21,753 children [11–17, 27]. A forest plot of BMI z-scores in the offspring of GDM mothers and controls is shown in Fig 2B.
Unadjusted BMI z-scores in the offspring of GDM mothers was significantly (p value = 0.006) higher than in the controls (pooled MD: 0.14; 95%CI: 0.04–0.24; I2 = 39%; seven studies, 21,691 children) (Fig 2B). Quality of evidence was judged as low’ (S2 Table). In a sensitivity analysis, overall unadjusted BMI z-scores of the offspring of GDM mothers including Page et al. was significantly (p value = 0.03) higher than the controls (pooled MD: 0.26; 95%CI: 0.03–0.49; I2 = 92%; eight studies, 21,753 children) (S3A Fig), we excluded Page et al. [14] because the ‘selection of participants’ domain was high risk [31] and reported as main results. Heterogeneity decreased from 92% to 39% but significance did not change after sensitivity analysis (S3A Fig). Only two studies [11, 12] were adjusted for covariates including maternal pre-pregnancy BMI. Using the adjusted data, the association was no longer significant (pooled MD: -0.11; 95% CI: -0.33–0.12; two studies, 5,941 children).
Diabetes
Two studies of GDM mothers’ offspring and controls were included in this review, involving a total of 425 children [20, 22]. There was no significant difference in the rate of childhood diabetes between GDM and controls (pooled OR: 5.70; 95% CI: 0.96–33.97; I2 = 0%; two studies, 425 children) (Fig 2C)
From the viewpoint of abnormal glucose tolerance (total T2DM, IGT and IFG), no meta-analysis was conducted because of subgroup differences were detected (p value<0.05). There was no significant difference in children aged 15 years old (OR: 1.17; 95% CI: 0.37–3.74; 129 children). However, a significantly higher rate of abnormal glucose tolerance (p = 0.0001) was observed in offspring with GDM aged 20 years old (OR: 6.71; 95% CI: 2.55–17.65; 296 children, one study) (Table 2, S1A Fig). Quality of the evidence was judged as ‘very low’ and was downgraded due to the serious extent of loss to follow-up (S2 Table 2).
Table 2. Abnormal glucose tolerance and plasma glucose in offspring of mothers with gestational diabetes mellitus, type1 diabetes and controls.
| Offspring·of·mothers·with·gestational·diabetes·mellitus¤ | |||||
| Outcome·or·subgroup·title¤ | No.·of ·studies¤ | No.·of·children¤ | No.·of· control·children¤ | Statistical·methods¤ | Effect·size¤ |
| Abnormal·glucose·tolerance | |||||
| 15· years | 1 | 42 | 87 | Odds·ratio (M-H,Fixed, 95%CI) | 1.17 [0.37, 3.74] |
| 20· years¤ | 1¤ | 168¤ | 128¤ | Odds·ratio (M-H,Fixed, 95%CI)¤ | 6.71 [2.55, 17.65]¤ |
| Fasting·plasma·glucose | |||||
| 7–10·years | 2 | 70 | 189 | Std.·Mean·Difference (Ⅳ,Random,95%CI) | 0.07 [-0.02, 0.16] |
| 15·years | 4 | 137 | 5158 | Std.·Mean·Difference (Ⅳ,Random,95%CI) | 0.09 [-0.07, 0.26] |
| 20·years¤ | 1¤ | 168¤ | 128¤ | Std.·Mean·Difference (Ⅳ,Random,95%CI)¤ | 0.40 [0.25,0.55]¤ |
| 2h·plasma·glucose | |||||
| 7 ·to·20·years¤ | 4¤ | 302¤ | 588¤ | Std. Mean Difference (Ⅳ, Random, 95%CI)¤ | 0.43 [0.18, 0.69]¤ |
| Offspring of mothers with type 1 diabetes mellitus¤ | |||||
| Outcome·or·subgroup·title¤ | No.·of· studies¤ | No· of·children¤ | No.·of·control·children¤ | Statistical·methods¤ | Effect·size¤ |
| Abnormal·glucose·tolerance | |||||
| 2–5·years,20·years¤ | 2¤ | 211¤ | 237¤ | Odds·ratio (M-H, Fixed, 95%CI)¤ | 3.48 [1.87, 6.49]¤ |
| Fasting·plasma·glucose | |||||
| 7–10·years | 2 | 114 | 76 | Std.·Mean·Difference (Ⅳ,Random,95%CI) | -0.07 [-0.16, 0.03] |
| 15·years | 1 | 278 | 303 | Std.·Mean·Difference (Ⅳ,Random,95%CI) | 0.10 [0.03, 0.17] |
| 20·years¤ | 1¤ | 160¤ | 128¤ | Std· Mean·Difference (Ⅳ, Random,95%CI)¤ | 0.10 [-0.00,0.20]¤ |
| 2h·plasma·glucose | |||||
| 7–10·years | 1 | 34 | 12 | Std.·Mean·Difference (Ⅳ,Random,95%CI) | -0.60 [-1.23, 0.03] |
| 15·years | 1 | 278 | 303 | Std.·Mean·Difference (Ⅳ, Random,95%CI) | 0.30 [0.10, 0.50] |
| 20·years¤ | 1¤ | 160¤ | 128¤ | Std.·Mean·Difference (Ⅳ,Random,95%CI)¤ | 0.50 [0.17,0.83]¤ |
Plasma glucose
Seven studies of GDM mothers’ offspring and controls were included in this review, involving a total of 5850 children for fasting plasma glucose (FPG) and 890 children for 2hPG [12, 13, 16, 17, 20–22].
For FPG, no meta-analysis not conducted because of subgroup differences were detected. No significant difference was found between GDM and controls in 7- to 10-year-olds (pooled MD: 0.07 mmol/L; 95% CI: -0.02–0.16; I2 = 0%; two studies, 259 children) and in 15-year-olds (pooled MD: 0.09 mmol/L:, 95% CI: -0.07–0.26; I2 = 76%; four studies, 5,295 children). However, significantly higher FPG (p<0.00001) was found in the offspring of GDM mothers aged 20 years old (MD: 0.40 mmol/L; 95% CI: 0.25–0.55; 296 children) (Table 2, S1B Fig).
In the offspring of GDM mothers, 2hPG was significantly higher (p = 0.0009) than in the controls (pooled MD: 0.43 mmol/L; 95% CI: 0.18–0.69; I2 = 35%; four studies, 890 children) (Table 2, S1C Fig).
Offspring of mothers with type 1 diabetes mellitus
Obesity and overweight
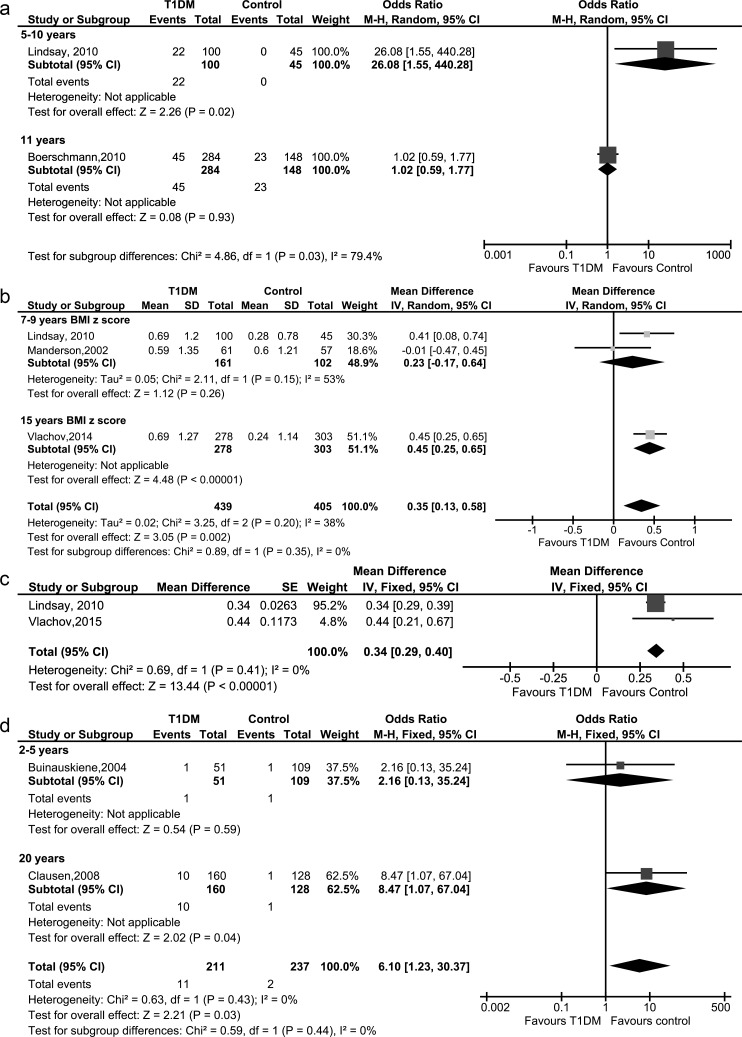
Two studies of offspring of T1DM mothers and controls were included in this review, involving a total of 577 children [26, 28].
No meta-analysis was conducted because of subgroup differences were detected. The rate of childhood obesity or overweight was significantly higher (p = 0.02) in the offspring of T1DM mothers among offspring aged 5 to 10 years old (OR: 26.08; 95% CI: 1.55–440.28; one study, 145 children), but there was no significant difference in 11 years old (OR: 1.02; 0.59–1.77; one study, 432 children) (Fig 3A). Quality of evidence was judged as ‘low’ for 5- to 10-year-olds and ‘very low’ among 11-year-olds (S2 Table).
Fig 3.
Forest plots of metabolic parameters in offspring of mothers with type 1 diabetes mellitus and controls: (a) obesity or overweight, (b) unadjusted BMI z-scores, (c) adjusted BMI z-scores, (d) diabetes.
BMI z-scores
Four studies of offspring of T1DM mothers and controls were included in this review, involving a total of 876 children [18, 19, 28, 30].
Unadjusted BMI z-scores of the offspring of T1DM mothers was significantly higher (p value = 0.002) than the controls (pooled MD: 0.35, 95% CI: 0.13–0.58, I2 = 38%, three studies, 844 children)(Fig 3B). Quality of the evidence was judged as ‘low’ (S2 Table). In a sensitivity analysis, overall unadjusted BMI z-scores of the offspring of T1DM mothers including Hunter et al. was significantly higher (p value = 0.002) than the controls (pooled MD: 0.45, 95% CI: 0.17–0.73, I2 = 64%, four studies, 876 children) (S3B Fig), we excluded Hunter et al. [18] because the domain of participant selection had high risk of bias [31] and reported as main results. Heterogeneity decreased from 64% to 38% but significance did not change after sensitivity analysis (S3B Fig).
Two studies [28, 30] were adjusted by maternal pre-pregnancy BMI. Adjusted BMI z-scores of the offspring of T1DM mothers were also significantly higher than those of the controls (pooled MD: 0.34; 95% CI: 0.29–0.40; two studies, 726 children) (Fig 3C).
Diabetes
Two studies of offspring of T1DM mothers and controls were included in this review, involving a total of 448 children [22, 25]. The rate of DM was significantly higher (p = 0.03) in the offspring of T1DM mothers. (pooled OR: 6.10; 95% CI: 1.23–30.37; I2 = 20%; two studies, 448 children) (Fig 3D). Quality of evidence was judged as ‘very low’ and was downgraded due to selective-outcome reporting (S2 Table).
From the viewpoint of abnormal glucose tolerance (total T2DM, IGT and IFG), a significantly (p<0.0001) higher rate of abnormal glucose tolerance was reported in offspring with T1DM (pooled OR: 3.48; 95% CI: 1.87–6.49; I2 = 0%; two studies, 448 children) (Table 2, S1D Fig). Quality of evidence was judged as ‘low’ (S2 Table).
Plasma glucose
Five studies of offspring of T1DM mothers and controls were included in this review, involving a total of 1091 children for FPG and 915 children for 2hPG[18, 19, 22, 28, 30]. We excluded Hunter et al. [18]from the meta-analysis because the domain of participant selection was high risk.
For FPG, no meta-analysis was conducted because of subgroup differences were detected. No significant difference was found between T1DM and controls in 7- to 10-year-olds (pooled MD: -0.07 mmol/L; 95% CI:-0.16–0.03; I2 = 0%; two studies, 190 children), and in 20-year-olds (MD: 0.10 mmol/L; 95% CI:-0.00–0.20; 288 children). Significantly higher FPG was found in the offspring of T1DM mothers aged 15-year-olds (p value = 0.003, MD: 0.10 mmol/L; 95% CI: 0.03–0.17; 581 children) (Table 2, S1E Fig).
For 2hPG, no meta-analysis was conducted because of subgroup differences were detected. No significant difference was observed between the T1DM group and controls in 7- to 10-year-olds (MD: -0.60 mmol/L; 95% CI: -1.23–0.03; 46 children). However, significantly higher 2hPG was found in the offspring of T1DM mothers aged 15-year-olds (p value = 0.004, MD: 0.30 mmol/L; 95% CI: 0.10–0.50; 581 children) and 20-year-olds (MD: 0.50 mmol/L; 95% CI: 0.17–0.83; 288 children) (Table 2, S1F Fig).
Offspring of mothers with type 2 diabetes mellitus
Offspring BMI z-scores, obesity and overweight
We found only one study with BMI z-scores including the offspring of T2DM mothers [18]. Unadjusted BMI z-scores of the offspring of T2DM mothers aged 5–10 years old was significantly higher than the controls (MD: 3.40; 95% CI: 2.87–3.93; 25 children).
We found no studies for the rate of childhood obesity or overweight including the offspring of T2DM mothers.
Diabetes, plasma glucose
We found no studies with rates of childhood T2DM, including the offspring of T2DM mothers. Only one study was included for offspring FPG of mothers with T2DM and controls. FPG was significantly higher than in the controls (p value = 0.005, MD: 0.10 mmol/L; 95% CI: 0.03–0.17, 32 children)[18].
Discussion
In this meta-analysis, we have shown that offspring of GDM mothers had a significantly higher risk of obesity or overweight in childhood than offspring of non-diabetic mothers. In addition, we found that offspring of GDM mothers had a higher 2hPG after glucose load from prepubertal to early adulthood compared with offspring of non-diabetic mothers. The offspring of T1DM mothers had significantly higher BMI z-scores from prepubertal to adolescent than offspring of non-diabetic mothers independently of maternal obesity. Also, the risk of diabetes or AGT was higher in those of T1DM mothers than in controls after the data of 2–5 year olds and 20 year olds were synthesized, while T1DM mothers generally do not have strong genetic background of T2DM. These findings suggested that childhood overweight/obesity or diabetes in offspring of T1DM mothers is likely due to the influence of intrauterine exposure to hyperglycemia.
In the studies on offspring of GDM mothers and controls (S2 Table), the evidence was judged to be of low quality (obesity and overweight, BMI z-score) and very low quality (DM, AGT), while in those on offspring of T1DM mothers and controls (S2 Table), the evidence was judged to be of low quality (BMI z-score, AGT) and very low quality (obesity and overweight, DM). These outcomes were downgraded due to observational studies bias limitation and wide 95%CI with small sample size.
Philipps et al. reported in their meta-analysis that unadjusted BMI z-scores of the offspring of GDM mothers were significantly higher than those of controls (pooled MD: 0.28; 95% CI: 0.05–0.51; six studies)[7], although it was no longer significant after adjustment for maternal pre-pregnancy BMI using three studies (two on GDM). Kim et al. reported that eight of 12 studies had significantly high crude ORs of childhood obesity (>95th percentile) or overweight (>85th percentile) for offspring of GDM mothers[6]. Kim et al. also mentioned only one study which was adjusted for covariates including maternal pre-pregnancy BMI and showed the association was no longer significant after adjustment [6, 27]. Since these two systematic reviews [6, 7] were published, 10 other studies have been reported, of which six [12, 14, 16, 17, 24, 30] were included in this review. We found that GDM mothers had a significantly higher BMI z-scores in childhood than offspring of non-diabetic mothers although the association was no longer significant after adjustment for maternal pre-pregnancy BMI, which supported previous reviews [6, 7]. On the other hand, a recent study showed that offspring of GDM mothers were significantly more likely to be overweight at an early age than those born to non-diabetic mothers [32]. Excessive weight gain during pregnancy is also related to large for gestational age at birth and childhood obesity [33]. Since there are many potential confounding factors for child overweight/obesity including maternal pre-pregnancy BMI, weight gain during pregnancy, duration or intensity of breastfeeding etc., further studies that adjust for those confounding factors are needed to clarify the association between intrauterine exposure to hyperglycemia and overweight/obesity in offspring of GDM mothers.
To detect mild glucose tolerance in the offspring of GDM mothers earlier, such as in school ages, it may be necessary to examine blood tests after glucose load. Regarding AGT among the offspring of GDM mothers, the influence of intrauterine exposure of hyperglycemia was suggested, although genetic factors of T2DM could not be distinguished because of high genetic predisposition to T2DM related to GDM.
We need to take into account the level of plasma glucose and the effects of therapeutic interventions on hyperglycemia of diabetic mothers, although in this systematic review information on maternal treatment during pregnancy was insufficient. Landon et al. showed that there was no apparent reduction in the rates of obesity or fasting glucose in treated offspring compared with control group at age 5 to 10 years, although the study showed only treated female children had lower fasting glucose [34]. We have shown that the offspring of T1DM mothers had significantly higher BMI z-scores from prepubertal to adolescent period compared with offspring of non-diabetic mothers.
We also showed that BMI z-scores of the offspring of T1DM mothers were significantly higher than those of controls after adjustment for maternal pre-pregnancy BMI. This means the intrauterine exposure of hyperglycemia would directly affect offspring higher BMI z-scores besides hereditary determinants. Hummel et al. reported that independent risk factor for childhood overweight in offspring of T1DM mothers was short breast-feeding duration and high birth weight, while maternal type 1 diabetes was not an independent predictor [35], which showed that intrauterine hyperglycemia might affect childhood overweight via birth size.
We have shown that the offspring of T1DM mothers had a higher risk of diabetes after the data of 2- to 5-year-olds and 20-year-olds were synthesized. Compared with the offspring of GDM mothers, the offspring of T1DM mothers seemed to be affected by metabolic effect from early age, regardless of low genetic predisposition to obesity or T2DM. One likely explanation is that offspring of women with T1DM are generally considered to be exposed to intrauterine hyperglycemia during the whole pregnancy period, while offspring of women with GDM are exposed to hyperglycemia only during the second half of the pregnancy. Early pregnancy period is thought to be a sensitive period in terms of metabolic effect on the offspring [36]. Another explanation is that offspring of T1DM mothers were exposed to a relatively high plasma glucose level compared with those of mothers with GDM. The possibility that the etiology of DM/ATG might be due to diminution of beta cells caused by pre-onset of T1 DM could not be denied. Sobngwi et al. found that offspring of mothers with T1DM had a higher occurrence of IGT compared with those of fathers with T1DM when they were around 20 years old, [37] suggesting that an exposure to hyperglycemia in utero is associated independent of the effect of genetic factors for T1DM. There were no reports about the effect of maternal diabetic control on offspring’s metabolic state after growth. The effect of the intervention into maternal blood glucose on offspring’s metabolic state should be assessed in the future.
Limitations
This review has several limitations. First, the evidence of this review relies only on observational studies. Therefore, we could not confirm the causal relationship between intrauterine exposure to maternal hyperglycemia and offspring obesity or impaired glucose tolerance.
Second, most of the data included in this meta-analysis were crude data with no adjustment, because only a few studies in this review adequately adjusted for covariables [11, 12, 27, 28, 30]. Genetic, demographic, socioeconomic and lifestyle factors including postnatal growth and breastfeeding can influence obesity and diabetes, and should be adjusted for [38, 39].
Conclusions
Exposure to maternal hyperglycemia during pregnancy might be associated with offspring obesity and abnormal glucose tolerance, although the association depends on the duration and intensity of intrauterine exposure to hyperglycemia, and the evidence relies only on observational studies with low quality of evidence. As for offspring of mothers with T2DM, we have to assess the actual status. To explore a causal relationship, well-designed prospective trials that consider the genetic background, the timing and strength of intrauterine exposure to hyperglycemia, other related factors and long-term results are warranted.
Supporting information
Forest plots of metabolic parameters in offspring of mothers with gestational diabetes mellitus, type 1diabetes mellitus and controls: (a) abnormal glucose tolerance (GDM), (b) fasting plasma glucose (GDM), (c) 2h plasma glucose (GDM), (d) abnormal glucose tolerance (T1DM), (e) fasting plasma glucose (T1DM), (f) 2h plasma glucose (T1DM).
(TIF)
(TIF)
Forest plots of sensitivity analysis: (a) BMI z-score (GDM), (b) BMI z-score (T1DM).
(TIF)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
The authors thank Ms. Miwako Segawa and Mr Toshiyuki Swa from the Department of Health Policy, National Center for Child Health and Development, for conducting the electronic search. The authors thank Ms. Chiemi Kataoka and Ms. Yuko Serizawa from the Department of Health Policy, National Center for Child Health and Development, for helping prepare the full texts needed for conducting the systematic review. The authors thank Dr Julian Tang for proofreading the manuscript and editing it for language. The authors thank Dr Yuji Hiramatsu for research leader for Research Promotion and Practical Use for Women’s Health.
Abbreviations
- MD
mean difference
- OR
odds ratio
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- GDM
gestational diabetes mellitus
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- AGT
abnormal glucose tolerance
- FPG
fasting plasma glucose
- 2hPG
two-hour plasma glucose
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from Research Promotion and Practical Use for Women’s Health, Japan Agency for Medical Research and Development (AMED) (grant number 15gk0210010h0101/ 16gk02100012h0001) and a grant from the National Center for Child Health and Development (grant number 26A-5), Japan.
References
- 1.Federation. ID. IDF Diabetes Atlas– 7th Edition. http://www.diabetesatlas.org/ (accessed May 15, 2016).
- 2.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. The lancet Diabetes & endocrinology. 2014;2(1):56–64. Epub 2014/03/14. doi: 10.1016/s2213-8587(13)70112-8 . [DOI] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. Epub 2014/06/02. doi: 10.1016/S0140-6736(14)60460-8 ; PubMed Central PMCID: PMCPmc4624264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatib O. Noncommunicable diseases: risk factors and regional strategies for prevention and care. East Mediterr Health J. 2004;10(6):778–88. Epub 2005/12/13. . [PubMed] [Google Scholar]
- 5.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29(12):1023–35. Epub 1980/12/01. . [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, England JL, Sharma JA, Njoroge T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res. 2011;2011:541308 Epub 2011/10/01. doi: 10.1155/2011/541308 ; PubMed Central PMCID: PMCPmc3179897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011;54(8):1957–66. Epub 2011/06/01. doi: 10.1007/s00125-011-2180-y . [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA: the journal of the American Medical Association. 2000;283(15):2008–12. Epub 2000/05/02. . [DOI] [PubMed] [Google Scholar]
- 9.Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Journal of clinical epidemiology. 2013;66(4):408–14. Epub 2013/01/23. doi: 10.1016/j.jclinepi.2012.09.016 . [DOI] [PubMed] [Google Scholar]
- 10.James M. Robins AR, Scharfstein Daniel O. Statistical Models in Epidemiology, the Environment, and Clinical Trials. 2000:pp 1–94. [Google Scholar]
- 11.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. American journal of hypertension. 2009;22(2):215–20. doi: 10.1038/ajh.2008.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S, Fraser A, Davey Smith G, Lindsay RS, Sattar N, Nelson SM, et al. Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes care. 2012;35(1):63–71. doi: 10.2337/dc11-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–13. Epub 2009/09/18. doi: 10.3945/ajcn.2008.27416 ; PubMed Central PMCID: PMCPmc2762159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page KA, Romero A, Buchanan TA, Xiang AH. Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. The Journal of pediatrics. 2014;164(4):807–10. doi: 10.1016/j.jpeds.2013.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998;101(2):E9 [DOI] [PubMed] [Google Scholar]
- 16.Davis JN, Gunderson EP, Gyllenhammer LE, Goran MI. Impact of gestational diabetes mellitus on pubertal changes in adiposity and metabolic profiles in Latino offspring. The Journal of pediatrics. 2013;162(4):741–5. doi: 10.1016/j.jpeds.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holder T, Giannini C, Santoro N, Pierpont B, Shaw M, Duran E, et al. A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia. 2014. [DOI] [PubMed] [Google Scholar]
- 18.Hunter WA, Cundy T, Rabone D, Hofman PL, Harris M, Regan F, et al. Insulin sensitivity in the offspring of women with type 1 and type 2 diabetes. Diabetes care. 2004;27(5):1148–52. [DOI] [PubMed] [Google Scholar]
- 19.Manderson JG, Mullan B, Patterson CC, Hadden DR, Traub AI, McCance DR. Cardiovascular and metabolic abnormalities in the offspring of diabetic pregnancy. Diabetologia. 2002;45(7):991–6. doi: 10.1007/s00125-002-0865-y [DOI] [PubMed] [Google Scholar]
- 20.Tam WH, Ma RCW, Yang X, Li AM, Ko GTC, Kong APS, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes care. 2010;33(6):1382–4. doi: 10.2337/dc09-2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buzinaro EF, Berchieri CB, Haddad ALM, Padovani CR, Pimenta WdP. [Overweight in adolescent offspring of women with hyperglycemia during pregnancy]. Sobrepeso na adolescencia de filhos de maes que tiveram disturbios glicemicos na gestacao. 2008;52(1):85–92. [DOI] [PubMed] [Google Scholar]
- 22.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes care. 2008;31(2):340–6. Epub 2007/11/15. doi: 10.2337/dc07-1596 . [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues S, Ferris AM, Perez-Escamilla R, Backstrand JR. Obesity among offspring of women with type 1 diabetes. Clinical and investigative medicine Medecine clinique et experimentale. 1998;21(6):258–66. [PubMed] [Google Scholar]
- 24.Pham MT, Brubaker K, Pruett K, Caughey AB. Risk of childhood obesity in the toddler offspring of mothers with gestational diabetes. Obstet Gynecol. 2013;121(5):976–82. Epub 2013/05/03. doi: 10.1097/AOG.0b013e31828bf70d . [DOI] [PubMed] [Google Scholar]
- 25.Buinauskiene J, Baliutaviciene D, Zalinkevicius R. Glucose tolerance of 2- to 5-yr-old offspring of diabetic mothers. Pediatric diabetes. 2004;5:143–6. doi: 10.1111/j.1399-543X.2004.00054.x [DOI] [PubMed] [Google Scholar]
- 26.Boerschmann H, Pfluger M, Henneberger L, Ziegler A-G, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes care. 2010;33:1845–9. doi: 10.2337/dc10-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221–6. Epub 2003/03/04. . [DOI] [PubMed] [Google Scholar]
- 28.Lindsay RS, Nelson SM, Walker JD, Greene SA, Milne G, Sattar N, et al. Programming of adiposity in offspring of mothers with type 1 diabetes at age 7 years. Diabetes care. 2010;33:1080–5. doi: 10.2337/dc09-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pirkola J, Pouta A, Bloigu A, Hartikainen A-L, Laitinen J, Jarvelin M-R, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes care. 2010;33:1115–21. doi: 10.2337/dc09-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlachova Z, Bytoft B, Knorr S, Clausen TD, Jensen RB, Mathiesen ER, et al. Increased metabolic risk in adolescent offspring of mothers with type 1 diabetes: the EPICOM study. Diabetologia. 2015;58(7):1454–63. Epub 2015/05/01. doi: 10.1007/s00125-015-3589-5 . [DOI] [PubMed] [Google Scholar]
- 31.Scharfstein JMRRO. Sensitivity Analysis for Selection bias and unmeasured Confounding in missing Data and Causal inference models. Statistical Models in Epidemiology, the Environment, and Clinical Trials 2000:pp 1–94. [Google Scholar]
- 32.Hakanen T, Saha MT, Salo MK, Nummi T, Harjunmaa U, Lipiainen L, et al. Mothers with gestational diabetes are more likely to give birth to children who experience early weight problems. Acta Paediatr. 2016;105(10):1166–72. Epub 2016/05/14. doi: 10.1111/apa.13468 . [DOI] [PubMed] [Google Scholar]
- 33.Leng J, Li W, Zhang S, Liu H, Wang L, Liu G, et al. GDM Women's Pre-Pregnancy Overweight/Obesity and Gestational Weight Gain on Offspring Overweight Status. PloS one. 2015;10(6):e0129536 Epub 2015/06/23. doi: 10.1371/journal.pone.0129536 ; PubMed Central PMCID: PMCPMC4476720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes care. 2015;38(3):445–52. Epub 2014/11/22. doi: 10.2337/dc14-2159 ; PubMed Central PMCID: PMCPmc4338507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes care. 2009;32(5):921–5. Epub 2009/02/21. doi: 10.2337/dc08-1943 ; PubMed Central PMCID: PMCPMC2671121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annual review of physiology. 2012;74:107–30. Epub 2011/09/14. doi: 10.1146/annurev-physiol-020911-153245 ; PubMed Central PMCID: PMCPMC4132057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobngwi E, Boudou P, Mauvais-Jarvis F, Leblanc H, Velho G, Vexiau P, et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361(9372):1861–5. Epub 2003/06/06. doi: 10.1016/S0140-6736(03)13505-2 . [DOI] [PubMed] [Google Scholar]
- 38.Gungor NK. Overweight and obesity in children and adolescents. Journal of clinical research in pediatric endocrinology. 2014;6(3):129–43. Epub 2014/09/23. doi: 10.4274/Jcrpe.1471 ; PubMed Central PMCID: PMCPmc4293641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos MB, Welsh J. Childhood obesity: update on predisposing factors and prevention strategies. Current gastroenterology reports. 2010;12(4):280–7. Epub 2010/06/22. doi: 10.1007/s11894-010-0116-1 ; PubMed Central PMCID: PMCPmc3056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plots of metabolic parameters in offspring of mothers with gestational diabetes mellitus, type 1diabetes mellitus and controls: (a) abnormal glucose tolerance (GDM), (b) fasting plasma glucose (GDM), (c) 2h plasma glucose (GDM), (d) abnormal glucose tolerance (T1DM), (e) fasting plasma glucose (T1DM), (f) 2h plasma glucose (T1DM).
(TIF)
(TIF)
Forest plots of sensitivity analysis: (a) BMI z-score (GDM), (b) BMI z-score (T1DM).
(TIF)
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.