Abstract
Our previous results showed that oligodendrocyte development is regulated by both nociceptin and its G-protein coupled receptor, the nociceptin/orphanin FQ receptor (NOR). The present in vitro and in vivo findings show that nociceptin plays a crucial conserved role regulating the levels of the glutamate/aspartate transporter GLAST/EAAT1 in both human and rodent brain astrocytes. This nociceptin-mediated response takes place during a critical developmental window that coincides with the early stages of astrocyte maturation. GLAST/EAAT1 upregulation by nociceptin is mediated by NOR and the downstream participation of a complex signaling cascade that involves the interaction of several kinase systems, including PI-3K/AKT, mTOR and JAK. Because GLAST is the main glutamate transporter during brain maturation, these novel findings suggest that nociceptin plays a crucial role in regulating the function of early astrocytes and their capacity to support glutamate homeostasis in the developing brain.
INTRODUCTION
Nociceptin/orphanin FQ (nociceptin) is an endogenous heptadecapeptide generated by cleavage of its precursor protein pre-pronociceptin (Meunier et al., 1995). Our previous findings suggested that nociceptin regulates oligodendrocyte development and may prevent precocious brain myelination (Eschenroeder et al., 2012). The present study provides evidence indicating that nociceptin also plays an important role in maturing rodent and human astrocytes, stimulating the expression of the glutamate transporter GLAST/EAAT1. Nociceptin binds to a G-protein coupled receptor known as the nociceptin receptor (NOR) or opioid receptor like-1 (ORL1), the most recently discovered member of the opioid receptor family. Interestingly, although nociceptin shows structural similarity to dynorphin A (Lapalu et al., 1997), it does not bind to the classical μ-, δ- or κ- opioid receptors. Likewise, NOR shows no binding affinity for the classical endogenous opioids, including dynorphin A, β-endorphin, and enkephalin (Meunier et al., 1995; Reinscheid et al., 1995). A number of roles for nociceptin and NOR in the central nervous system (CNS) have been investigated, the most well studied being pain modulation. The differing effects between supraspinal and spinal administration of nociceptin, hyperalgesia and analgesia, respectively, make the nociceptin system a unique target among the opioid receptor family (Heinricher et al., 1997; Pan et al., 2000; Rizzi et al., 2006). NOR activation has also been shown exert anxiolytic effects in different models of stress (Köster et al., 1999; Jenck et al., 2000) and NOR knockout mice exhibit increased learning ability, memory, and hippocampal long term potentiation (Mamiya et al., 1998; Yu and Xie, 1998; Noda et al., 2000). Furthermore, NOR-deficient mice also develop altered responses to morphine (Ueda et al., 1997, 2000; Rizzi et al., 2000; Mamiya et al., 2001; Chung et al., 2006), nicotine (Sakoori and Murphy, 2009), heroin (Kallupi et al., 2017), ethanol (Kallupi et al., 2013), and cocaine (Marquez et al., 2008, 2013; Kallupi et al., 2017), indicating a possible role of the nociceptin system in drug addiction.
Interestingly, early studies showed that the levels of pre-pronociceptin mRNA expression are significantly elevated during embryonic and early postnatal life (Ikeda et al., 1998). However, little is known about the role of nociceptin in brain development. The present studies establish a role for this molecule in developing astrocyte s. Once considered as simple support cells, research in the recent decades demonstrated that astrocytes are instead a complex cell population that plays a number of crucial roles; including among others being responsible for guiding neuronal migration (Rakic, 1995; Komuro and Rakic, 1998), control of synapse number and maintenance (Ullian et al., 2001), regulation of neuronal activity (Halassa and Haydon, 2010), brain glycogen storage and energy production (Pellerin and Magistretti, 1994; Brown and Ransom, 2007), water distribution (Nielsen et al., 1997), antioxidant metabolism (Aschner, 2000), ion buffering (Bevan et al., 1985; Bordey and Sontheimer, 2000), and induction and maintenance of the blood brain barrier (Janzer and Raff, 1987; Hawkins and Davis, 2005). However, one of the most critical roles played by astrocytes, both in the developing and the adult brain, is the regulation of neurotransmitter concentrations. Due to its excitotoxic effects at high concentrations, excess glutamate is rapidly removed after synaptic transmission by a set of transport proteins expressed in astrocytes. The glutamate-aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1), respectively named EAAT1 and EAAT2 in humans, belong to the family of excitatory amino acid transporters (EAATs). Importantly, GLAST/EAAT1 is the main transporter during brain maturation and several lines of evidence indicate that GLAST/EAAT1 function during early brain development is fundamental beyond protection from glutamate-induced cytotoxicity and regulation of glutamatergic activity. This is because glutamate levels are known to regulate a variety of crucial developmental events that include among others, the proliferation and survival of neural progenitors (Zafra et al., 1991; LoTurco et al., 1995; Luk et al., 2003; Luk and Sadikot, 2004; Brazel et al., 2005; Mattson, 2008; Jansson and Akerman, 2014), neuronal migration (Komuro and Rakic, 1993, 1998; Behar et al., 1999), and dendritic outgrowth (Mattson et al., 1988; Voss et al., 2007; Whitney et al., 2008; Jansson et al., 2013). The critical developmental role of glutamate transporters is further evidenced by the observation that GLT-1/GLAST double knockout mice exhibit gross malformation of the brain due to incorrect migration and maturation of neurons as well as differentiation of astrocytes (Matsugami et al., 2006).
The present in vitro and in vivo studies show that GLAST/EAAT1 expression in developing rodent and human astrocytes is stimulated by nociceptin. These novel findings support the idea that the nociceptin system plays a crucial role in regulating glutamate homeostasis in the maturing brain.
MATERIALS AND METHODS
Materials
Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN) and Charles River (Wilmington, MA) Laboratories. LY294002 (2-Morpholin-4-yl-8-phenylchromen-4-one), rabbit anti-pAKT (Cat# 4060), rabbit anti-EAAT1/GLAST (Cat# 5684 and 5685) and anti-EAAT2/GLT-1 (Cat# 3838) antibodies, were all obtained from Cell Signaling Technology (Danvers, MA). Nociceptin and BAN-ORL 24 ((2R)-1-(phenylmethyl)-N-[3-(spiro[isobenzofuran-1(3H),4′-piperdin]-1-yl)propyl-2-pyrrolidinecarboxamide), were purchased from Tocris Bioscience (Ellisville, MO). Rapamycin was a generous gift from the lab of Dr. Sarah Spiegel. JAK inhibitor I (2-(1,1-Dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one) was purchased from EMD Millipore (Temecula, CA). Chicken anti-GFAP (Cat# PA1-10004), rabbit anti-nociceptin (Cat# PA3-204), TFB-TBOA ((3S)-3-[[3-[[4-(Trifluoromethyl)benzoyl]amino]phenyl]methoxy]-L-aspartic acid), and Super Signal West Dura chemiluminescence reagent were obtained from ThermoFisher Scientific (Rockford, IL). Guinea pig anti-nociceptin (Cat# GP10107) and rabbit anti-NOR (Cat# RA14140) antibodies were from Neuromics, Inc. (Edina, MN). Rabbit anti-aldehyde dehydrogenase 1, family member L1 (ALDH1L1) antibody (Cat# NBP2-25143) was from Novus Biologicals (Littleton, CO). Rabbit anti-GFAP (Cat# G9269) and mouse anti-β actin (Cat# A5316) antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Dulbecco’s Modified Eagle Medium/Ham’s F12 (DMEM-F12), Dulbecco’s Modified Eagle Medium (DMEM), N2 supplement, anti-rabbit AlexaFluor®488 (Cat# A11008), anti-chicken AlexaFluor® 594 (Cat# A11042) and the Tyramide Signaling Kit (Cat# T20950) were purchased from Life Technologies (Frederick, MD). Digoxigenin-labeled probes for in situ hybridization were obtained from Integrated DNA Technologies (Skokie, IL). All electrophoresis supplies were from Bio-Rad laboratories (Hercules, CA). D-[3,4-3H]-aspartic acid (12.9 Ci/mmol) was obtained from Perkin Elmer (Waltham, MA). Except for the horseradish peroxidase (HRP)-conjugated anti-guinea pig antibody (ThermoFisher, Cat# A18769), all HRP-labeled secondary antibodies and the anti-digoxigenin antibody (Cat# 200-002-156) were purchased from Jackson ImmunoResearch (West Grove, PA).
Human and Rat Astrocyte Cultures
Human cortical astrocytes prepared from fetal brain tissue provided by Advanced Bioscience Resources (Rockville, MD) were cultured as previously described (Kordula et al., 1998). Fetal brain tissue was from de-identified subjects and subjected to number 4 IRB-exempt protocol. All animal use and isolation of astrocytes were conducted in accordance with the National Institutes of Health (NIH) recommendations and approved by the Virginia Commonwealth University Animal Care and Use Committee (IACUC). Astrocyte cultures were prepared from postnatal day 3 (PD3) Sprague Dawley rat pups according to a modification of the method of McCarthy and de Vellis (McCarthy and de Vellis, 1980) as follows. After decapitation, the cerebral hemispheres were rapidly dissected out, and the meninges removed. The tissue was then finely minced (~2mm pieces) and forced through two consecutive nylon meshes of 118μm and 75μm, respectively. The filtrate was collected and centrifuged at 1000xg for 5 min. The pellet obtained from 1/2 brain was resuspended in DMEM-F12 containing 10% fetal bovine serum (FBS), penicillin (100IU/mL), and streptomycin (100μg/mL) and placed in a 75cm2 culture flask maintained for 5 days at 37°C under 5% CO2, with medium replacement after three days. On the 6th day, the flasks were shaken at 300 rpm for 2 hrs to remove non-astroglial cells, followed by passage of the attached astrocytes into 75cm2 flasks. After reaching 80% confluence, astrocytes were harvested and plated in 8-well glass chamber slides or 24-well plates and the medium changed every 2 days until use. Neuronal, microglial and oligodendroglia contamination of these cultures, as assessed by immunocytochemistry, was less than 5%.
Cell culture treatments with nociceptin, and NOR and kinase inhibitors
All cell culture treatments were carried out in FBS-free chemically defined medium (CDM) [DMEM-F12 supplemented with 50μg/mL holo-transferrin, 5μg/mL insulin, 20nM progesterone, 100μM putrescine, 30nM sodium selenite]. For studies of nociceptin effects, astrocytes were incubated for 24 hrs in CDM alone (controls), or CDM supplemented with 1μM nociceptin, 100nM NOR inhibitor BAN-ORL24 (NOR-I) or a combination of the two. For kinase inhibitor experiments, cells were treated with 1μM nociceptin, and one of the following kinase inhibitors: 30μM LY294002 [Phosphatidylinositol-3-Kinase (PI-3K) inhibitor], 1μM JAK Inhibitor I [Janus Kinase (JAK) inhibitor], 25nM rapamycin [mammalian target of rapamycin (mTOR) inhibitor] 50μM PD98059 [extracellular-signal related kinase (ERK)1/2 inhibitor], 10μM SB202190 [p38 mitogen activated protein kinase (MAPK) inhibitor], 30μM KN93 [Ca2+/calmodulin-dependent protein kinase II (CamKII) inhibitor], 10μM KT5720 [protein kinase A (PKA) inhibitor], 10μM SQ22536 (adenylyl cyclase inhibitor), 100nM Go6983 [protein kinase C (PKC) inhibitor] or a combination of nociceptin and the kinase inhibitor. For the short-term signaling experiments, cells were preincubated for 15 min in the presence or absence of the appropriate kinase inhibitors.
In vivo administration of NOR inhibitor
Sprague-Dawley rats were injected intraperitoneally with BAN-ORL24 in phosphate-buffered saline (PBS) at a dose of 1mg/kg/day on 2 different timelines as indicated in Fig. 9. Pups were administered the NOR inhibitor from PD3 to PD9 (Group 1) or PD9 to PD14 (Group 2). All controls were similarly injected with vehicle alone. At the end of the respective time periods, the rats were anesthetized with isofluorane and sacrificed. The brains were harvested, flash frozen on dry ice and kept at −80°C until use.
Figure 9. In vivo treatment with an NOR inhibitor or genetic ablation of NOR affect GLAST brain expression.
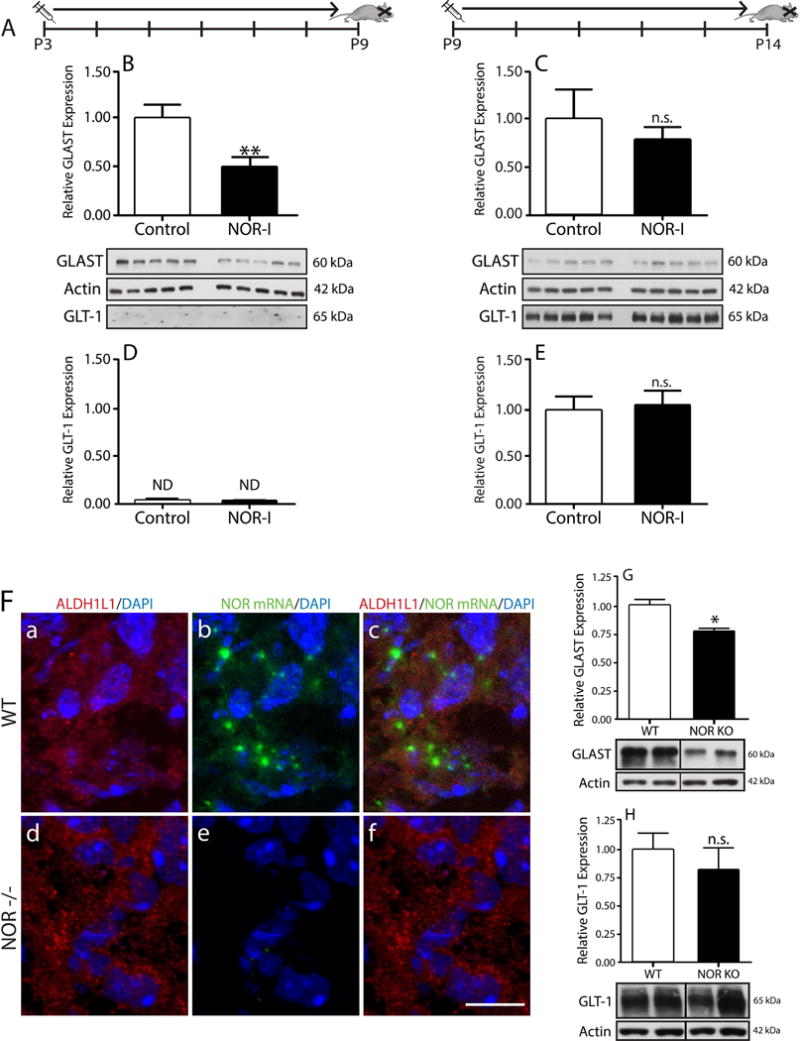
(A) Rat pups were given daily IP injections of BAN-ORL24 (NOR-I) on two different timelines: from postnatal day 3 to 9, or postnatal day 9 to 14. Total homogenates prepared from brains collected at the end of each timeline were subjected to western blot analysis for GLAST and GLT-1. (B and C) GLAST expression in total brain homogenates of animals injected from (B) postnatal day 3 to 9 and (C) postnatal day 9 to 14. (D and E) GLT-1 levels in brain homogenates from pups injected from (D) postnatal day 3 to 9 and (E) postnatal day 9 to 14. The results are expressed as change relative to control values and represent the mean ± SEM from at least 5 animals. **p<0.01; n.s., not significant; ND, not detected. (F) 5-day-old WT and NOR KO mouse cortical brain tissue slices were subjected to immunohistochemical staining with (a and d) anti-ALDH1L1 antibody and in situ hybridization using (b and e) a digoxigenin-labeled probe for NOR mRNA, as indicated under “Methods”. Notice the presence of NOR mRNA in ALDH1L1-labeled astrocytes in WT mice (a-c) and lack of NOR mRNA in the NOR KO animals (d-f). Scale bar: 10μm. (G and H) Total brain homogenates from 14-day-old WT and NOR knockout mice were subjected to western blot analysis to evaluate expression of (G) GLAST and (H) GLT-1, using β-actin as loading control. Results are represented as change relative to WT and are the mean ± SEM from 4 animals, * p<0.01.
Immunoblotting
Western blot analyses were done as described previously (Sanchez et al., 2008; Eschenroeder et al., 2012). Protein concentration in the samples was determined by Bradford assay (Bio-Rad). After solubilization in the appropriate volume of Laemmli buffer (60 mM Tris–HCl buffer, pH 6.8, containing 10% glycerol, 2% sodium dodecyl sulfate (SDS), and 5% 2-mercaptoethanol), proteins from the cell cultures (6 μg/lane) or tissue homogenates (5 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 12% acrylamide and then transferred to nitrocellulose for 1 hr at 100V. Non-specific binding was blocked with 3% nonfat dry milk and 0.05% Tween-20 in PBS (blocking solution) for 1 hr at room temperature. The membranes were then incubated overnight at 4°C with the appropriate primary antibodies in blocking solution at the following dilutions: anti-GFAP (1:1,000), anti-EAAT1/GLAST (1:1,000), anti-EAAT2/GLT-1 (1:1,000), anti-actin (1:1,000), anti-pAKT (1:2,000). After rinsing, the membranes were re-blocked for 30 min and incubated for 2 hrs in blocking solution with the appropriate HRP-conjugated secondary antibody. Extensive rinsing was followed by detection with Super Signal West Dura Reagent. The relative expression levels of the immunoreactive bands were determined by scanning analysis of the X-ray films using the N.I.H. Image J program, and the relative density values divided by β-actin levels to correct for sample loading differences.
Determination of nociceptin concentrations by dot blot analysis
Nociceptin concentrations during postnatal brain development as well as nociceptin secretion by cultured cells were both determined by dot blot analysis. For this, different aliquots of total brain homogenates or astrocyte-conditioned culture media were pipetted onto a nitrocellulose membrane using a 96-well Schliecher and Schuell Minifold vacuum dot blot apparatus. A parallel set of nociceptin standards (25-200 femtomoles) was included in the same membrane for quantification of the samples. The membrane was dried for 2 hrs under vacuum before being rehydrated in PBS and subsequently incubated for 1 hr in blocking solution (3% nonfat dry milk and 0.05% Tween-20 in PBS). After overnight incubation with anti-nociceptin antibody (1:2,000) in blocking solution, the membrane was extensively washed, re-blocked, and subjected to incubation with HRP-conjugated secondary antibody and detection by chemiluminescence. Following scanning of the X-ray films, relative values were obtained by using the NIH ImageJ program and the concentration of nociceptin in different sample dilutions was determined by extrapolation to the standard curve. Dilution values falling within the linear range of the standard curve were used for quantitation. Results were expressed as femtomoles/μg of protein for total brain homogenates or nM for secreted nociceptin concentrations in the culture media. Protein concentrations were determined using the Bradford assay (Bio-Rad).
Immunocytochemical analysis
For in vitro studies, cultured cells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature. For immunohistochemistry, rats were anesthetized using a 2.5% solution of Avertin and then transcardially perfused with PBS followed by 4% PFA. Brains were removed and cryopreserved overnight in 30% sucrose before flash freezing in OCT and cut into 30μm-thick sections. Fetal brain tissue samples from de-identified subjects, subjected to number 4 IRB-exempt protocol, were obtained from the Department of Pathology at Virginia Commonwealth University Medical Center. Non-specific binding was blocked by incubation in PBS containing 5% normal goat serum and 0.3% Triton® X-100 (blocking buffer) for 1 hr. Samples were incubated overnight at 4°C with the appropriate primary antibody in blocking buffer at the following dilutions: anti-GFAP (1:160), anti-EAAT1/GLAST (1:100), anti-NOR (1:150), anti-nociceptin (1:250). Samples were then rinsed with PBS, re-blocked for 30 min, and incubated for 2 hrs at room temperature in the presence of the appropriate secondary antibody. NOR was detected using a tyramide signal amplification system (Life Technologies) according to the manufacturer’s instructions, using the rabbit anti-NOR antibody at a sub-threshold concentration that allowed for co-labeling with rabbit anti-ALDH1L1 by traditional immunofluorescence. Cell cultures and tissue slides were mounted with DAPI-containing Vectashield and visualized and imaged using Eppendorf, Zeiss Confocal LSM710 or Zeiss AxioImager A1 microscopes. Lack of bleeding in samples stained with both fluorophores was confirmed by the fact that no staining of the sample was observed in the red channel in the absence of excitation of red fluorophore, indicating that the green fluorophore emission was not bleeding into the red channel. Additionally, single AlexaFluor 488 staining shows no bleed through into the red channel and single AlexaFluor 594 labeling shows no signal when the sample is observed in the green channel.
Fluorescent in situ hybridization for NOR mRNA
In situ hybridization was performed by modification of a previously published protocol (Wang et al., 2000). Cell cultures and tissue sections were prepared as indicated above for immunohistochemistry. After quenching of endogenous peroxidase using 3% H2O2 in PBS for 20 min, samples were rinsed and acetylated (1.25% triethanolamine, 0.25% acetic anhydride in ddH2O) for 20 min at RT. Following extensive rinsing in PBS, samples were permeabilized with 0.1% Triton X-100 in PBS for 20 min. Tissues sections were re-fixed in 4% PFA for 20 min and rinsed 3 times in PBS before proceeding with prehybridization. Samples were incubated in hybridization buffer [50% Formamide, 5xSSC (1X SCC; 150 mM NaCl, 15 mM Na citrate), 10% dextran sulfate, 500ug/mL salmon sperm DNA, 1% Triton X-100] for 1 hr at 37°C. A 50-mer deoxyoligonucleotide probe for rat NOR mRNA with the sequence 5′-CAG GGA TCT CCA CCA GGC ACT CGA TCT CTT CAT CTT CCA CTT GTG CTG AA-3′ or a 49-mer mouse/human NOR mRNA with the sequence 5′-GAT CTC CAC CAG GCA CTC GAT CTC TTC ATC CTC CAC TTG TGC TGA GCC C-3′, both with a 3′end digoxigenin tag, were synthesized and purified by IDT. These sequences correspond to the rat and mouse/human NOR extracellular region located between transmembrane domains 4 and 5. Hybridization was done using 1ng/uL of digoxigenin labeled-oligonucleotide probe in hybridization buffer at 37°C for 18 hrs. After hybridization, samples were washed twice with 50% formamide/5xSSC at 37°C for 10 min, then once with 2xSSC at 37° for 10 min, once with 2xSSC at RT for 10 min, twice in 0.2xSSC at RT for 10 min, and finally rinsed three times in PBS at RT for 5 min.
The samples then underwent immunohistochemical detection of ALDH1L1 and digoxigenin, as previously described with minor modifications. Samples were then consecutively incubated for 1 hr at RT with blocking buffer (5% NGS and 0.1% Triton X-100 in PBS), and for 2 hrs with a mixture of mouse anti-digoxigenin (5 μg/ml) and anti-ALDH1L1 (1:1000) antibodies in blocking buffer. After rinsing in PBS, samples were re-blocked for 20 min before the appropriate fluorescently conjugated secondary antibodies were added for 2hr at RT, then extensively rinsed with PBS and finally mounted with DAPI-containing Vectashield.
Aspartate uptake assay
Aspartate uptake assay was carried out using a modification of previously published protocols (Matos et al., 2008; Escartin et al., 2011). Cells were pre-incubated for 24 hrs in CDM alone, CDM with 1μM nociceptin, CDM with 100nM BAN-ORL24, or CDM with 100nM BAN-ORL24 and 1μM nociceptin. The cells were then pretreated for 10 min before the start of the uptake assay with CDM alone, CDM with 300μM DHK or CDM with 1μM TFB-TBOA. The different groups of cells were then incubated in BSS-P (140mM NaCl, 5mM KCl, 1.2mM CaCl2, 1.2mM MgSO4, 0.5mM KH2PO4, 5mM glucose, 5mM PIPES, pH 7.2) containing 0.5μCi of 3H-D-aspartic acid and 100μM non-radioactive D-aspartic acid for different times at 37°C under 5% CO2. At the end of the incubation, the culture plates were placed on ice and carefully rinsed three times in ice-cold BSS-P. Cells were then lysed with 200μl 0.5N NaOH and aliquots used for measurement of radioactivity by liquid scintillation counting and protein determination by the Bradford assay (Bio-Rad).
NOR knockout mice
The NOR knockout (KO) mice were raised in the laboratory of Dr. Kabirullah Lutfy at Western University of Health Sciences (Pomona, CA). Mice lacking NOR (Nishi et al., 1997) and their wild-type controls were originally obtained from Dr. Hiroshi Takeshima (Department of Biochemistry, Tohoku Graduate School of Medicine, Sendai, Miyagi 980-8575, Japan). Male and female heterozygous mice were generated from mating of KO and wild-type mice and fully backcrossed on a C57BL/6J mouse strain for 12 generations. Heterozygous breeding pairs were then mated to generate mice lacking NOR and their wild-type controls. Mice were provided ad libitum access to water and food in a 12hr light/dark cycle. Pups were genotyped employing a standard polymerase chain reaction (PCR) protocol, using samples obtained from ear snips. All the experimental procedures were conducted according to the NIH guidance and were approved by the IACUC at Western University of Health Sciences.
Statistical analysis
Statistical analysis was performed by the nonparametric tests Mann-Whitney (for two group comparisons) or one-way analysis of variance on ranks (Kruskal-Wallis test) when comparing more than two experimental groups. All analyses were carried out using the GraphPad Prism program (La Jolla, CA). Differences were considered statistically significant when P values were <0.05.
RESULTS
Postnatal rat brain maturation is accompanied by a progressive decrease in nociceptin expression
Our previous findings suggested a role for nociceptin in oligodendrocyte maturation and early myelination (Eschenroeder et al., 2012). Further support for the involvement of nociceptin in brain development is the observation that mRNA levels encoding pre-pronociceptin, the precursor of nociceptin, are higher in the immature than in the adult rodent brain (Ikeda et al., 1998; Neal et al., 2001). Moreover, our analysis of nociceptin protein levels in cerebral hemispheres from rat pups, revealed the highest expression between postnatal days 2 and 5, with peak values of 797.2±34.3 and 936.0±113.5 fmol/μg of protein, respectively (Fig. 1). However, the expression of this molecule gradually decreases, reaching values at 21 days after birth that are about 6-fold lower than those observed in the 5-day-old pups. Interestingly, the highest levels of postnatal nociceptin expression overlap with the peak of astrocyte development that is observed in rodents between 3 and 5 days of age (Mission et al., 1991), raising the possibility that the nociceptin system may play a role in astrocyte maturation.
Figure 1. Postnatal brain maturation is accompanied by a progressive decrease in nociceptin expression.
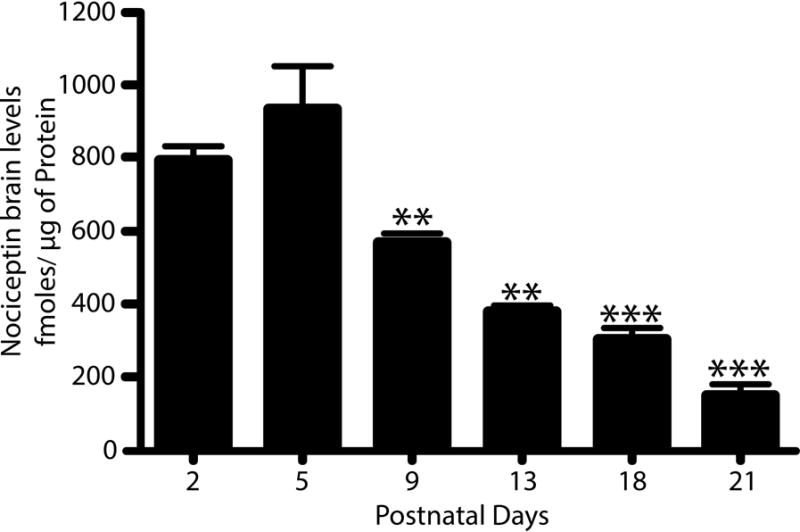
Nociceptin levels were measured by dot blot analysis of total homogenates prepared from cerebral hemispheres of rat pups at 2-, 5-, 9-, 13-, 18-, and 21- postnatal days. Purified nociceptin was used to generate a standard curve as indicated under “Methods”. The results, expressed as fmoles of nociceptin/μg of protein, are the average ± SEM from at least 3 animals per age, PD5 vs. PD2, not significant (n.s.); PD5 vs. PD9 and PD13, **p<0.01; PD5 vs. PD18 and PD21, ***p<0.001.
Nociceptin and NOR expression in developing rodent and human astrocytes
Previous findings indicated the presence of both NOR protein and mRNA in cultured astrocytes from rat spinal cord (Fu et al., 2007). Support for a role of nociceptin in developing brain astrocytes, was next provided by immunocytochemical staining of rat brains at postnatal day 5 (PD5), a timing that corresponds to the third trimester of CNS development in utero for humans (Fig. 2). In these studies cortical astrocytes were identified by their labeling with cytoplasmic ALDH1L1 (Fig. 2). Co-staining with ALDH1L1 and anti-nociceptin (Fig. 2, A-C) or anti-NOR (Fig. 2, D-F) antibodies clearly indicated the presence of nociceptin and NOR in developing rat astrocytes. To further substantiate the presence of the nociceptin system in astrocytes, we carried out in situ hybridization studies which demonstrated the expression of NOR mRNA in these cells (Fig. 2, G-I and Fig. S2). Importantly, these findings are not limited to rodents as localization of both components of the nociceptin system is also observed in astrocytes of the human developing brain. Analysis of fetal brains at gestational week 23, also showed the presence of both nociceptin and NOR in human cortical astrocytes identified by their labeling with ALDH1L1 (Fig. 3, A-F). Moreover, as in the rat brain, in situ hybridization studies clearly indicated NOR mRNA expression in astrocytes of the developing human brain (Fig. 3, G-I and Fig. S2).
Figure 2. In vivo expression of nociceptin (Noci) and nociceptin receptor (NOR) in developing rat astrocytes.
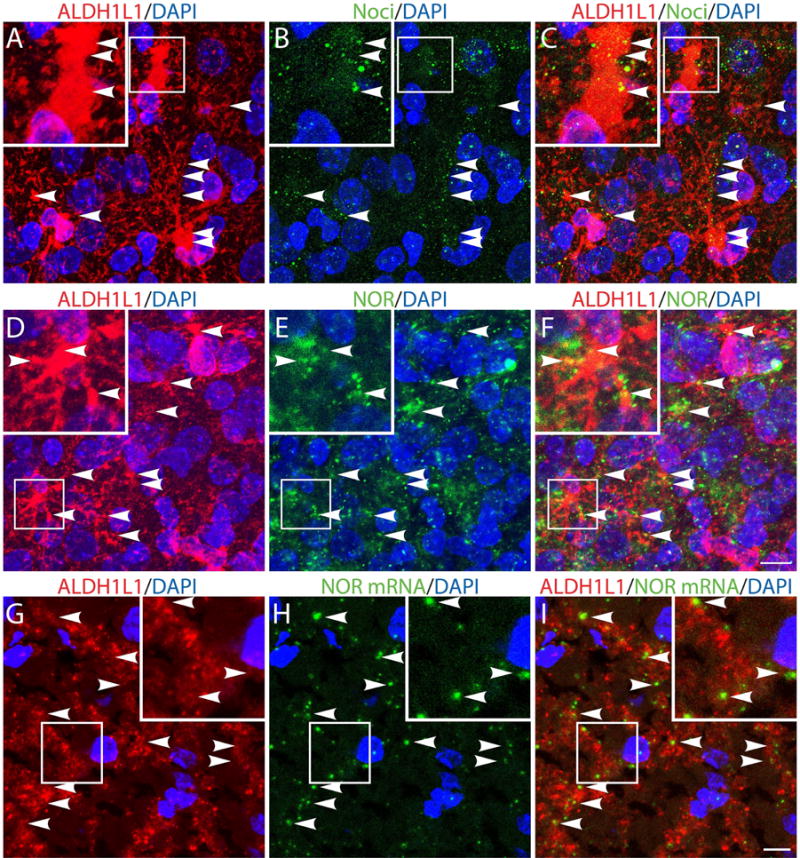
(A-F) Cortical brain tissue slices from 5-day-old rats were subjected to immunohistochemistry using (A and D) anti-ALDH1L1 together with (B) anti-Noci or (E) anti-NOR antibodies. (G-I) Cortical brain slices were subjected to immunohistochemical staining with (G) anti-ALDH1L1 antibody and to in situ hybridization using (H) a digoxigenin-labeled probe for NOR mRNA, as indicated under “Methods”. Notice the presence of nociceptin (C), NOR protein (F) and NOR mRNA in the ALDH1L1-labeled astrocytes (I). Nuclei were counterstained with DAPI. Scale bar: 10μm. Controls for immunocytochemistry using the appropriate normal sera as well as in situ hybridization controls using nonsense deoxyoligonucleotide probes are shown in Supplemental Figures 1 and 2, respectively.
Figure 3. In vivo expression of nociceptin (Noci) and nociceptin receptor (NOR) in developing human astrocytes.
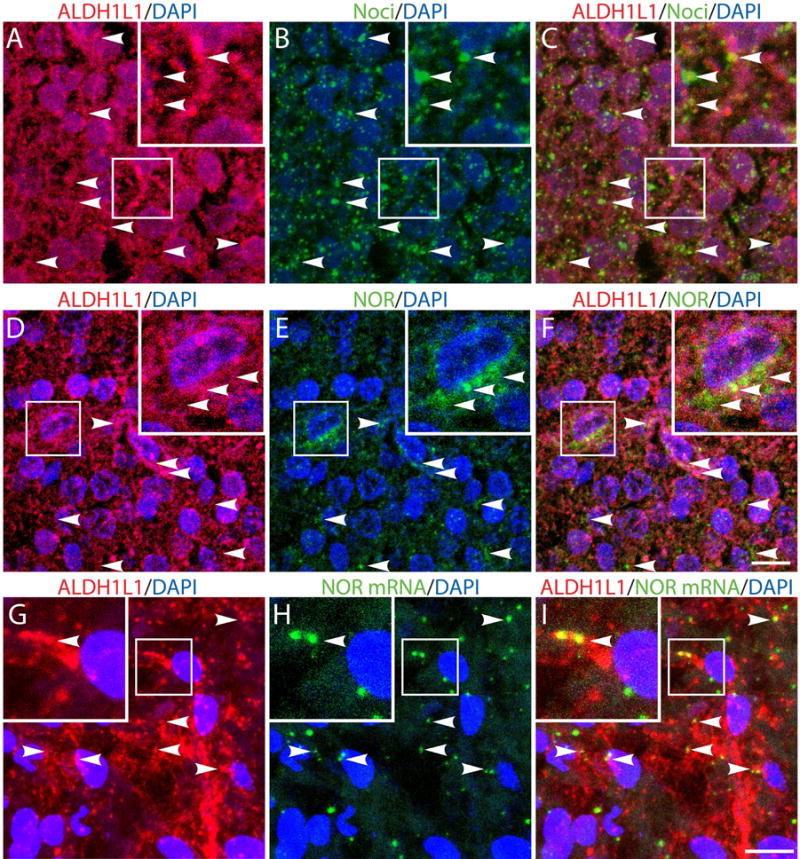
(A-F) Gestational week 23, fetal cortical brain tissue slices were subjected to immunohistochemistry using (A and D) anti-ALDH1L1 together with (B) anti-Noci or (E) anti-NOR antibodies. (G-I) Cortical brain slices were subjected to immunohistochemical staining with (G) anti-ALDH1L1 antibody and to in situ hybridization using (H) a digoxigenin-labeled probe for NOR mRNA, as indicated under “Methods”. Notice the presence of nociceptin (C), NOR protein (F) and NOR mRNA in the ALDH1L1-labeled astrocytes (I). Scale bar: 10μm. Controls for immunocytochemistry using the appropriate normal sera as well as in situ hybridization controls using nonsense deoxyoligonucleotide probes are shown in Supplemental Figures 1 and 2, respectively.
Interestingly, the expression of astrocytic NOR appears to be developmentally regulated. As shown in Figure 4, analysis of rat brain at different postnatal ages (PD2-PD21) demonstrated the highest levels of astrocytic NOR in the 2- and 5-day-old animals. However, NOR expression in astrocytes subsequently decreases with brain maturation, following a pattern that is similar to that of nociceptin brain levels (Fig. 1).
Figure 4. Astrocytic NOR expression during postnatal rat brain development.
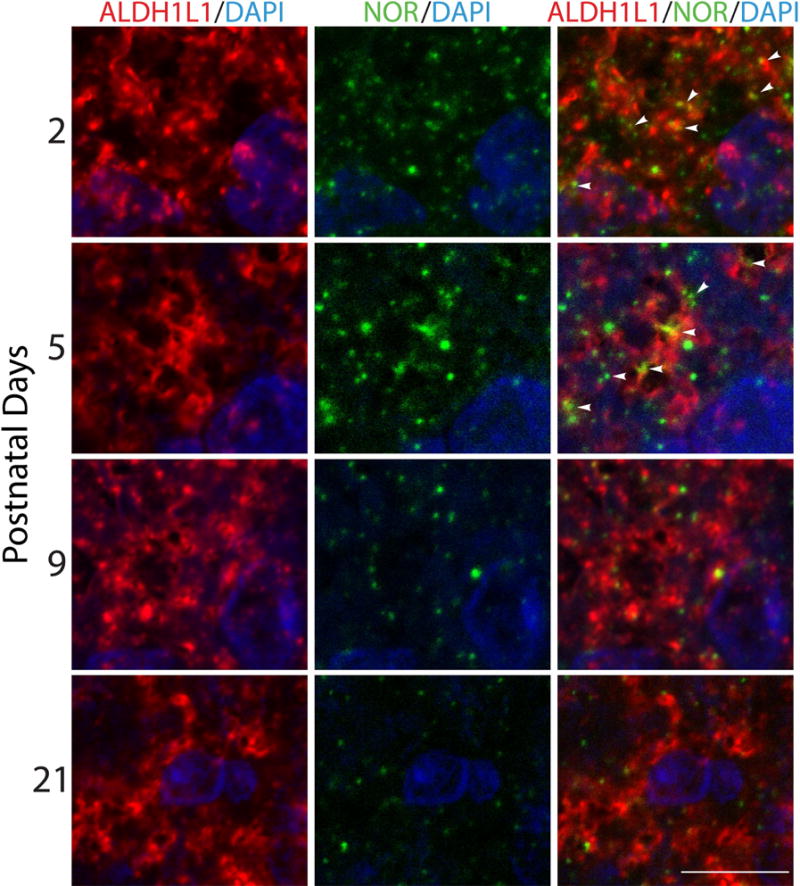
Cortical tissue slices from 2-, 5-, 9-, and 21-postnatal day rat pups were subjected to immunohistochemistry with anti-ALDH1L1 (red) and anti-NOR (green) antibodies to assess astrocytic NOR localization throughout postnatal development. Notice that NOR expression in astrocytes is developmentally regulated and decreases with brain maturation. Scale bar: 10μm.
The nociceptin system regulates GLAST expression in rat and human developing astrocytes
The findings described above pointed to a potential role of nociceptin in developing astrocytes. Thus, we next decided to investigate this possibility by directly testing the potential effects of this peptide in cultured cells. We first examined if the nociceptin system was also expressed when primary rat and human astrocytes were isolated and cultured in chemically defined medium (CDM). For this, rat (Fig. 5, A-F) and human (Fig. 5, G-L) cultured cells were subjected to immunocytochemical analysis with anti-ALDH1L1 (A, D, G, and J) and antibodies for nociceptin (B and H) or NOR (E and K). The results indicated that similar to the above in vivo observations, nociceptin and NOR are indeed present in both rat and human cultured astrocytes.
Figure 5. Nociceptin and NOR are present in cultured primary human and rat brain astrocytes.
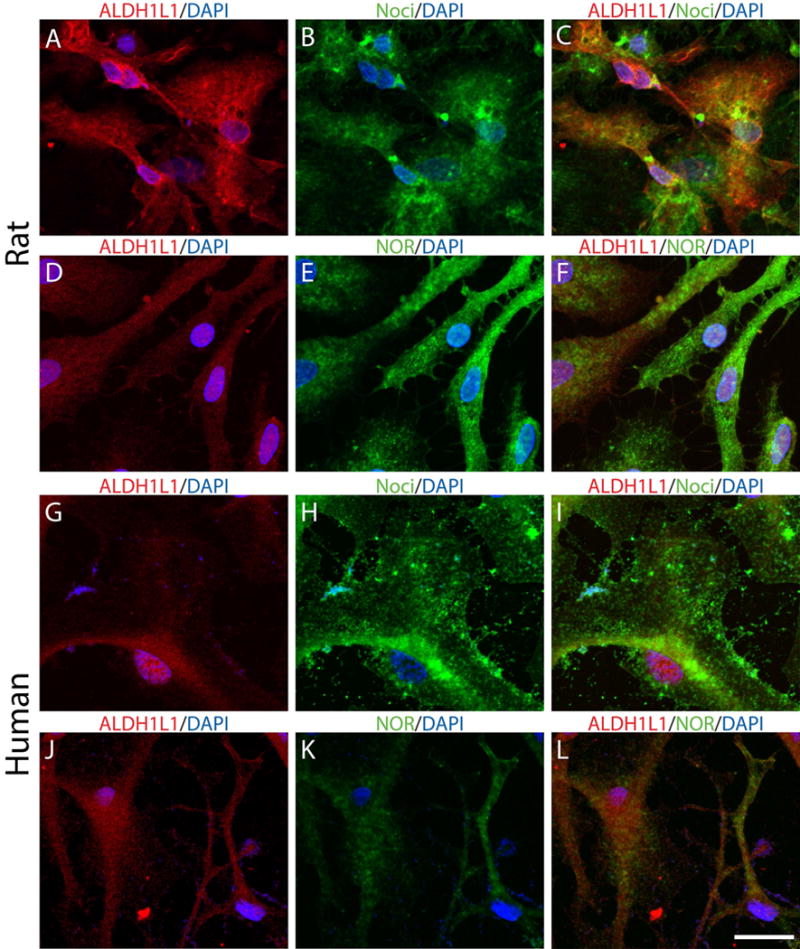
(A-F) Primary rat astrocytes were cultured in chemically defined medium (CDM). Immunocytochemistry was used to analyze the expression of (A and D) ALDH1L1 together with (B) nociceptin or (E) NOR. (G-L) Primary human astrocytes were also cultured in CDM alone and similarly stained for (G and J) ALDH1L1 and (H) nociceptin or (K) NOR expression. Scale Bar: 50μm.
As depicted in Figure 6, western blot analysis showed that incubation of the rat astrocytes in the presence of increasing concentrations of nociceptin did not affect expression of the cytoskeletal astroglial marker GFAP (Fig. 6A), but unexpectedly resulted in a dose-dependent increase in the levels of the glutamate transporter GLAST (Fig. 6B). Important to note is that these cultures exhibit a high molecular weight form of GLAST that has been previously shown (Schlag et al., 1998; Ye and Sontheimer, 2002) to be characteristic of immature astrocytes. Others have demonstrated that the “classical” low molecular weight GLAST is on the other hand the predominant form that is expressed when the maturation of astrocytes is induced by treatment with dibutyryl cAMP (Schlag et al., 1998; Duan et al., 1999; Susarla et al., 2004; Filosa et al., 2009; Carbone et al., 2012). Importantly, an interesting developmental expression pattern of these GLAST bands is observed in vivo. As shown in Figure 6C, western blot of total brain homogenates reveals that the higher molecular weight form of GLAST predominates before PD9, an age at which the high and low molecular weight bands appear to be present in equivalent amounts. However, after this time, the low molecular weight band, believed to be the monomeric form of GLAST, is more prominent.
Figure 6. Treatment of astrocytes with nociceptin results in a dose-dependent increase in GLAST expression.
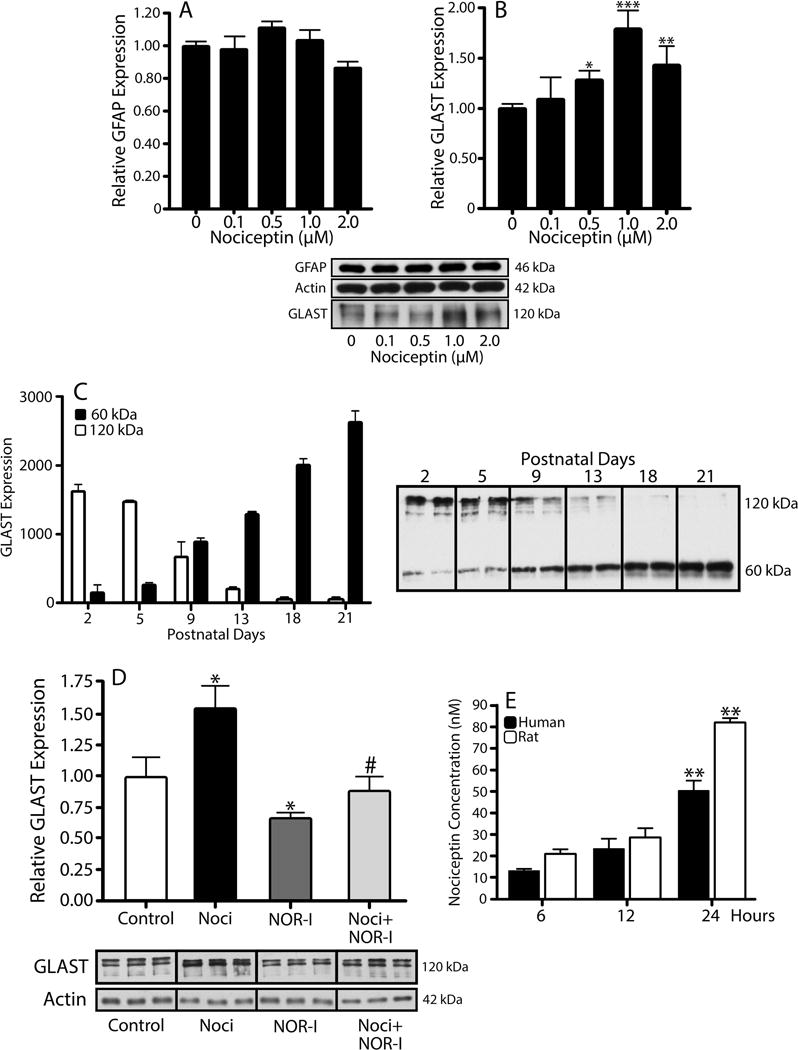
Astrocytes isolated from 3-day-old rat brains were treated for 24 hrs with increasing concentrations of nociceptin. (A) GFAP and (B) GLAST expression were evaluated by western blot analysis, using β-actin as loading control. Results are expressed as change relative to control values and represent the mean ± SEM, n=3, *p<0.05, **p<0.01, ***p<0.001. (C) GLAST levels in total brain homogenates from 2-, 5-, 9-, 13-, 18-, and 21-day-old rats were determined using western blot. Each gel lane was loaded with 5μg of protein and levels for the 60 kDa (black bars) and 120 kDa (open bars) molecular forms of GLAST correspond to the mean ± SEM from 3 animals per age. (D) Western blot analysis was used to determine relative GLAST levels after a 24 hr incubation in CDM alone, 1μM nociceptin (Noci), 100nM BAN-ORL24 (NOR-I), or 1μM Noci + 100nM NOR-I. The results are expressed as change relative to control values and represent the mean ± SEM from at least 3 experiments; control vs. Noci and control vs. NOR-I, *p<0.05; Noci vs. Noci + NOR-I, #p<0.03. (E) Secretion of endogenous nociceptin was assessed by dot blot analysis of medium collected from cultured rat (open bars) and human (black bars) astrocytes after 6, 12, and 24 hrs in CDM alone. The results are the mean ± SEM from 6 different cultures. Rat, 6 hrs and 12 hrs vs. 24 hrs, **p<0.01. Human, 6 hrs vs. 24 hrs, **p<0.01.
To further substantiate the specificity of nociceptin action, the cells were then cultured in CDM alone, CDM with 1μM nociceptin, CDM with 100nM NOR inhibitor BAN-ORL24 (NOR-I), or CDM supplemented with a combination of both nociceptin and NOR-I (Fig. 6D). As described above, a 24-hr incubation with nociceptin resulted in a significant elevation in GLAST expression. However, this effect was abrogated upon co-incubation with NOR-I, indicating that activation of NOR is a necessary step in the nociceptin-dependent upregulation of GLAST. Interestingly, Figure 6D also shows that NOR-I treatment itself causes a small but statistically significant decrease in GLAST levels. Since no effects on cell viability were detected, this could be attributed to an inhibitory effect on the action of endogenously produced nociceptin. This possibility is supported by the observation that as already indicated above (Figs. 2, 3, and 5), both rat and human in vivo and cultured astrocytes express nociceptin. Furthermore, as depicted in Figure 6E, analysis of medium collected from both rat and human cells grown in CDM alone, indicate that astrocytes indeed secrete nociceptin. The results indicated that the concentration of endogenous nociceptin released by both rat and human astrocytes ranges from 12 to 20nM at 6 hrs to a maximal value of 50 to 80nM after 24 hrs in culture (Fig 6E). This information combined with the reported EC50 value for NOR of 90nM in neurons (Connor et al., 1996), suggests 10% saturation at 6 hours and 50% saturation of NOR under basal culture conditions after 24 hours. Thus, while the cells indeed produce a significant amount of nociceptin, the addition of exogenous peptide is still able to further stimulate the expression of GLAST.
The stimulatory effect of nociceptin is also clearly observed when rat astrocytes are analyzed by immunocytochemistry (Fig. 7, A-L) and the results expressed as percentage of GFAP(+) cells that are also GLAST positive under the different culture conditions (Fig. 7M). About 42% of GFAP(+) cells in the nociceptin-treated cultures (Fig. 7, D-F) expressed GLAST in comparison to only 18% for the control astrocytes in CDM alone (Fig. 7, A-C) and approximately 9-5% for those co-incubated with NOR-I (Fig. 7, G-I) or a combination of nociceptin and NOR-I (Fig. 7, J-L). The small but statistically significant decrease observed upon treatment with NOR-I alone may reflect, as indicated above, the effect of endogenously produced nociceptin.
Figure 7. GLAST/EAAT1 expression increases after nociceptin treatment in both rat and human developing astrocytes.
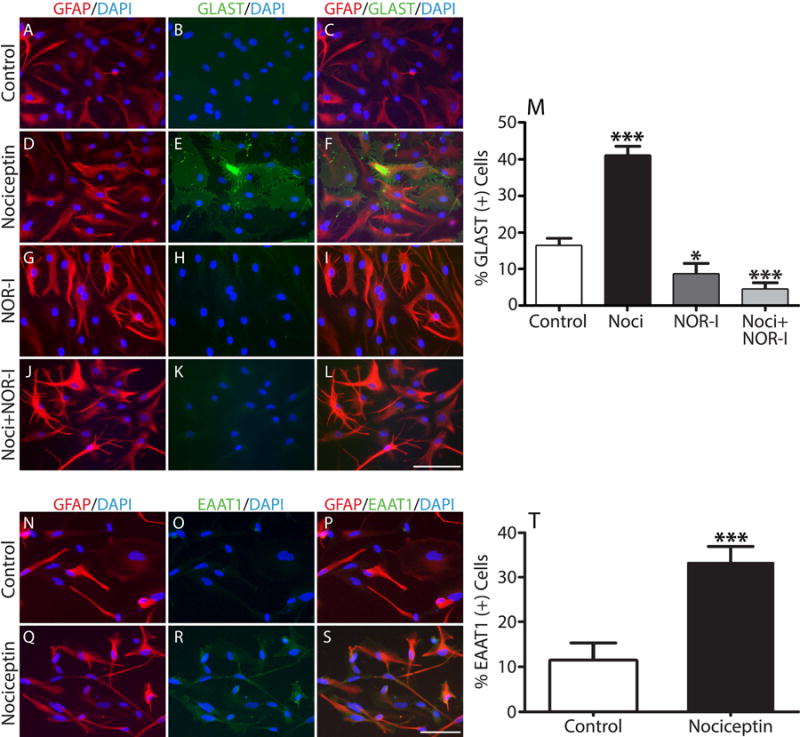
(A-L) Immunocytochemistry with anti-GFAP (red) and anti-GLAST/EAAT1 (green) antibodies was used to evaluate GLAST expression in cultures treated for 24 hrs with (A-C) CDM alone (controls), (D-F) 1μM nociceptin, (G-I) 100nM NOR-I or (J-L) 100nM NOR-I + 1μM nociceptin. Scale Bar: 100μm. (M) Determination of GLAST(+) cells as % of GFAP(+) cells/field. Results are the mean ± SEM from 4 independent experiments in which ten fields/well, 3 wells/condition, containing approximately 100 cells per field were analyzed. Control vs. Noci, *** p<0.001; control vs. NOR-I, * p<0.05; Noci vs. Noci + NOR-I, *** p<0.001. (N-T) Immunocytochemistry of developing human fetal astrocytes with anti-GFAP (red) and anti-GLAST/EAAT1 (green) antibodies. Cells were incubated for 24 hrs in (N-P) CDM alone or (Q-S) CDM supplemented with 1μM nociceptin. Scale Bar: 100μm. (T) Determination of EAAT1(+) cells as % of GFAP(+) cells/field. Results are the mean ± SEM from 3 independent experiments in which ten fields/well, 3 wells/condition, containing approximately 100 cells per field were analyzed, control vs. nociceptin, *** p<0.001.
Furthermore, the stimulatory effect of nociceptin on GLAST expression is not restricted to the rat cells, since a similar result was also observed in studies using cultured fetal human astrocytes. As shown in Figure 7, N-T, a significantly greater percentage of the human cells also express GLAST upon treatment of the cultures with nociceptin (~37%) (Fig. 7, Q-S) when compared to those that were incubated under basal control conditions in CDM alone (~12%) (Fig. 7, N-P). Together with the previous observations in the rat cells, these findings point to a conserved function of nociceptin in regulating GLAST expression in developing astrocytes.
The nociceptin system regulates GLAST activity
Importantly, the elevation of GLAST protein levels induced by nociceptin is indeed accompanied by an increase in transporter activity. As depicted in Figure 8A, cells exhibited increased aspartate uptake when transporter activity was assayed after a 24 hour pre-treatment with nociceptin. This uptake was not affected by treatment with the GLT-1 selective inhibitor DHK, but was in contrast reduced to background levels by the GLT-1/GLAST inhibitor TFB-TBOA (Fig. 8B). These observations indicate that nociceptin-induced increase in aspartate transport is indeed mediated by GLAST.
Figure 8. 3H-Aspartate uptake increases in primary rat astrocytes treated with nociceptin.
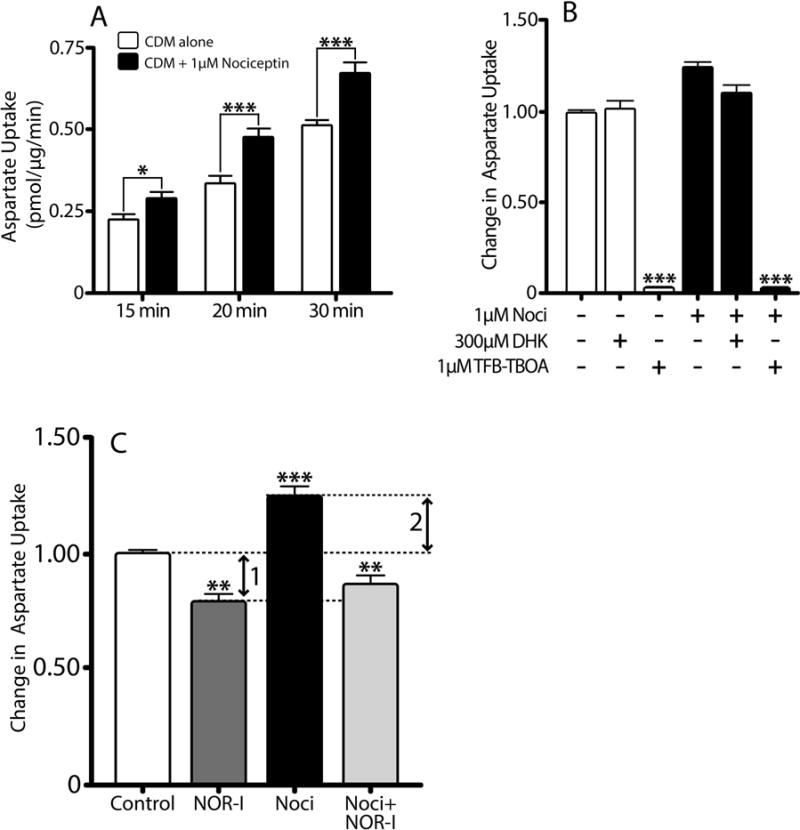
(A) Aspartate uptake in primary rat astrocytes after 24 hr pre-incubation in CDM alone (controls, open bars) or CDM with 1μM nociceptin (black bars) was determined using a 3H-D-aspartate uptake assay, as described under Methods. Results expressed as pmol/μg protein/min and are the mean ± SEM from at least 10 replicates/condition, *p<0.05, ***p<0.001. (B) Aspartate uptake was assessed in cell cultures after 24 hours pre-treatment in CDM alone (white bars) or CDM supplemented with 1μM nociceptin (black bars). Prior to the assay, parallel controls and nociceptin-treated cultures were in addition pre-incubated for 10 min with either 300μM DHK or 1μM TFB-TBOA. Results are expressed as change in uptake over 30 min and represent the mean ± SEM from at least six replicates/condition, control vs control with TFB-TBOA and nociceptin vs. nociceptin with TFB-TBOA, ***p<0.0001. (C) Aspartate uptake was assessed in primary rat cultures treated for 24 hrs with CDM alone (control), or CDM supplemented with either 100nM NOR-I, 1μM nociceptin, or 1μM nociceptin with 100nM NOR-I. Results are expressed as change in uptake over 30 min and represent the mean ± SEM from at least six replicates/condition. Control vs. NOR-I, **p<0.01; control vs. nociceptin, ***p<0.001; nociceptin vs. nociceptin with NOR-I, ***p<0.001. Arrow 1: aspartate uptake due to endogenously produced nociceptin. Arrow 2: aspartate uptake due to exogenously added nociceptin.
Furthermore, the increase in transporter activity observed after a 24-hr pre-treatment with nociceptin is not seen if the cells are pre-incubated with a combination of nociceptin and NOR-I. Important to note is that as indicated previously (Fig. 6E), the cultured cells produce and secrete nociceptin. This raises the question of whether the aspartate uptake in the control cells, pre-incubated in CDM alone, reflects a contribution of endogenous nociceptin to GLAST expression. As shown in Figure 8C, support for this possibility, stems from the observation that pre-treatment of control cells with NOR-I alone results in a small but statistically significant decrease in aspartate uptake (arrow 1), which parallels the previously described decrease in GLAST protein levels (Fig. 6D) as well as the decrease in GLAST(+) cells (Fig. 7 M) after treatment of cultured astrocytes with NOR-I. Therefore, it is possible to hypothesize that the aspartate uptake measured after cells are pre-treated for 24 hours with nociceptin reflects the increase in GLAST expression due to both exogenously added (arrow 2) as well as endogenously produced nociceptin (arrow 1). Altogether, the results presented up to this point indicated that nociceptin-dependent increases in GLAST levels are accompanied by elevated transporter activity and these effects may be part of an autocrine loop that contributes to glutamate homeostasis in the developing brain.
In vivo regulation of GLAST expression by the nociceptin system
The results described above raised the question of whether nociceptin effects on GLAST expression are also observed in vivo. To investigate this problem, we focused on the first two weeks of life which not only show the highest levels of postnatal nociceptin expression, as already indicated in Figure 1, but also represent a major time period of astrocyte proliferation and differentiation in rodents (Mission et al., 1991; Rice and Barone, 2000). To evaluate the role of nociceptin in vivo, rats pups were administered the blood brain barrier permeable NOR-I, BAN-ORL24 (1mg/kg/day), using two separate schedules (Fig. 9A). In the first group, pups received daily injections from PD3 to PD9, a time period that includes the peak of both astrocyte development and postnatal nociceptin expression. A second group of animals was administered the NOR-I from PD9 to PD14, a window that does not include the peak of astroglial development but encompasses other events such as the start of myelination. Comparison with vehicle-injected control pups, indicated that GLAST expression was significantly decreased in the brains of animals treated with NOR-I from PD3 to PD9 (Fig. 9B). However, no significant differences between controls and NOR-I-treated rats were detected when the inhibitor was administered between PD9 to PD14 (Fig. 9C). Altogether, these results point to a role of the nociceptin system that is restricted to the first 9-10 days of postnatal rat brain development. In contrast, no effects of NOR-I were detected when the same animals were examined for the brain expression of GLT-1 (Fig. 9, D and E). Notice that only background levels of GLT-1 are observed at PD9 (Fig. 9D) as the expression of this glutamate transporter is known to begin after the first postnatal week (Ullensvang et al., 1997; Schreiner et al., 2014).
To further substantiate these observations we determined GLAST levels in wild-type (WT) and NOR KO mice. Analogous to the rat and human cells, mouse astrocytes also express NOR mRNA (Fig. 9F, a-c), which is clearly absent in the NOR KO animals (Fig. 9F, d-f). In these animals, a role of the nociceptin system in controlling GLAST expression is further supported by the observation that NOR KO mice exhibit decreased GLAST brain levels (Fig. 9G). Furthermore, similar to the observations on GLT-1 expression in the NOR-I injected pups (Figs. 9, D and E), the unaffected GLT-1 levels in NOR KO animals (Fig. 9H), supports the notion that nociceptin effects are restricted to GLAST expression. There is a possibility that changes in GLAST expression in these experiments may be due to unpredicted effects of nociceptin on astrocyte proliferation. However, cultured astrocytes treated with either nociceptin or NOR-I did not display differences in Ki-67 levels, a marker of proliferation (data not shown). Altogether, these results further substantiate the notion that nociceptin signaling plays a crucial role in controlling GLAST expression in developing astrocytes.
The stimulation of GLAST expression by nociceptin involves a PI-3K/AKT, mTOR and JAK- mediated pathway
We next thought to investigate the molecular mechanisms that regulate GLAST expression downstream of NOR activation. Testing of a number of specific enzyme inhibitors (data not shown) determined that neither ERK, PKA, PKC, p38MAPK, CamKII, nor adenylate cyclase play any significant role in the nociceptin-dependent up-regulation of GLAST. However, significant results were obtained when investigating the role of a PI-3K/AKT-dependent pathway. In these studies, cells were pre-incubated for 2 hrs in DMEM-F12 medium alone to downregulate potential previous signaling induced by endogenously produced nociceptin. As shown in Figure 10A, incubation in CDM alone elicits Ser473 AKT phosphorylation, an action most likely resulting from the presence of insulin which is known to stimulate AKT activation in the majority of cell types. However, only treatment with nociceptin-containing CDM resulted in sustained AKT phosphorylation during the entire 60-min incubation. This nociceptin-dependent stimulation of AKT phosphorylation is abolished by co-incubation of the cells with LY294002, an inhibitor of PI-3K, the kinase that phosphorylates AKT. Furthermore, the role of a PI-3K/AKT pathway downstream of NOR activation is substantiated by the finding that PI-3K inhibition indeed blocks the capacity of nociceptin to increase GLAST expression, as shown by both immunocytochemical staining (Fig. 10B) and western blot analysis (Fig. 10C) of the cells. In addition, as shown in Figure 11, the stimulatory effect of nociceptin is also blocked by rapamycin, an inhibitor of the mTOR kinase complex that is a key regulator frequently downstream of PI-3K/AKT activation. Treatment with rapamycin abolishes the capacity of nociceptin to increase both the percentage of astrocytes expressing GLAST (Fig. 11A) as well as the elevation of GLAST levels detected by western blotting (Fig. 11B). Furthermore, the stimulatory effect of nociceptin on GLAST expression was also blocked by the Janus kinase (JAK) inhibitor I (Figs. 11, A and B). Moreover, this inhibitor also blocks the sustained nociceptin-dependent stimulation of AKT phosphorylation observed at 60 min (Fig. 11C), suggesting that JAK acts upstream of PI-3K. This is particularly interesting as a JAK/STAT pathway was implicated in regulating GLAST expression in a model of perinatal hypoxia (Raymond et al., 2011). Furthermore, JAK/STAT signaling is believed to control the differentiation of nestin(+) pluripotent cells into GFAP(+) astrocytes (Sriram et al., 2004; Gautron et al., 2006). While the precise interaction of JAK and PI-3K/AKT downstream of NOR activation remains to be investigated, altogether these observations show that nociceptin regulates GLAST expression in developing astrocytes by a complex mechanism that involves PI-3K/AKT, mTOR and JAK activation (Fig. 12).
Figure 10. Involvement of PI-3K/AKT in the nociceptin-dependent upregulation of GLAST expression.
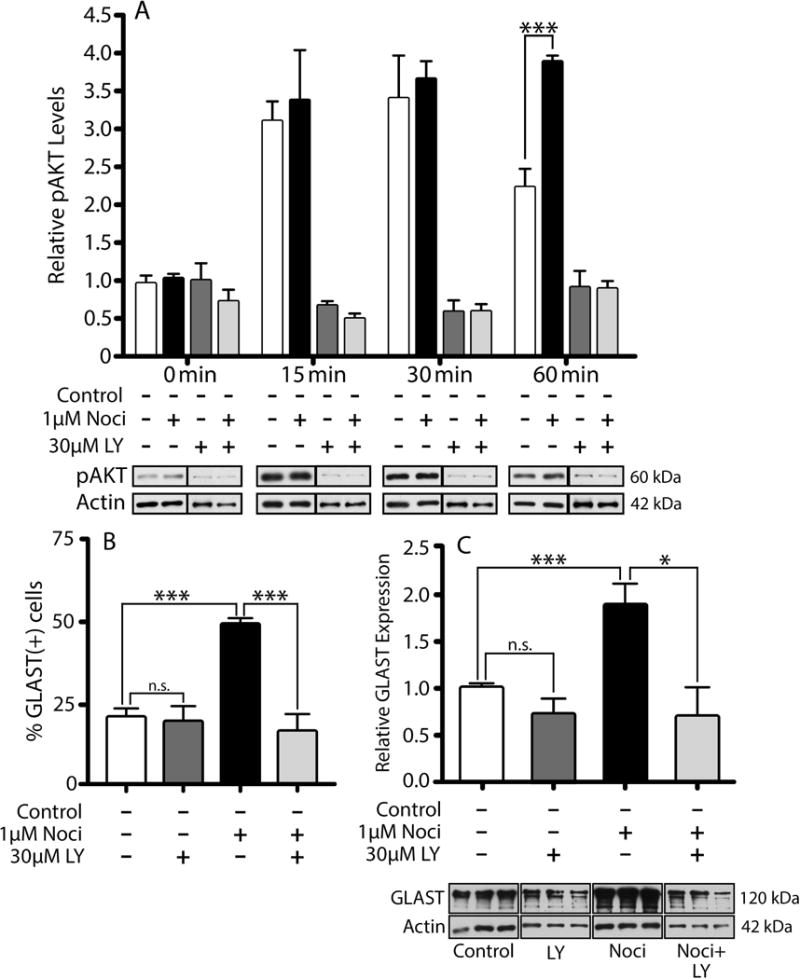
(A) Astrocyte cultures were first pre-incubated for 2 hrs in DMEM-F12 alone; and then for increasing times (0-60 min) in CDM alone (white bars), CDM with 1μM nociceptin (Noci), CDM with 30μM LY294002 (LY) (PI-3K inhibitor) or CDM with 1μM Noci and 30μM LY. Cell lysates were then subjected to western blot analysis with anti-phosphorylated AKT (pAKT) antibody. The results are the mean ± SEM from 3 different cultures; control vs. Noci at 60 min, ***p<0.001. (B) Immunocytochemistry was used to evaluate GLAST expression in rat astrocytes after a 24 hr treatment with CDM alone, or CDM supplemented with one of the following: 1μM Noci, 30μM LY, or 1μM Noci + 30μM LY. The bar graph shows the number of GLAST positive cells as a % of the GFAP(+) cells/field under each condition. Results are the mean ± SEM from twelve fields/well, 3 wells/condition. Control vs. LY, n.s.; control vs. Noci,***p<0.001; Noci vs. Noci + LY, ***p<0.001. (C) Astrocytes treated for 24 hrs as in (B), were subjected to western blot analysis for GLAST. GLAST levels in the bar graph are expressed as change relative to control values. The results are the mean ± SEM, n=3, Control vs. LY, n.s.; control vs. Noci,***p<0.001; Noci vs. Noci + LY, ***p<0.05.
Figure 11. The nociceptin dependent upregulation of GLAST is also mediated by mTOR and JAK signaling complexes.
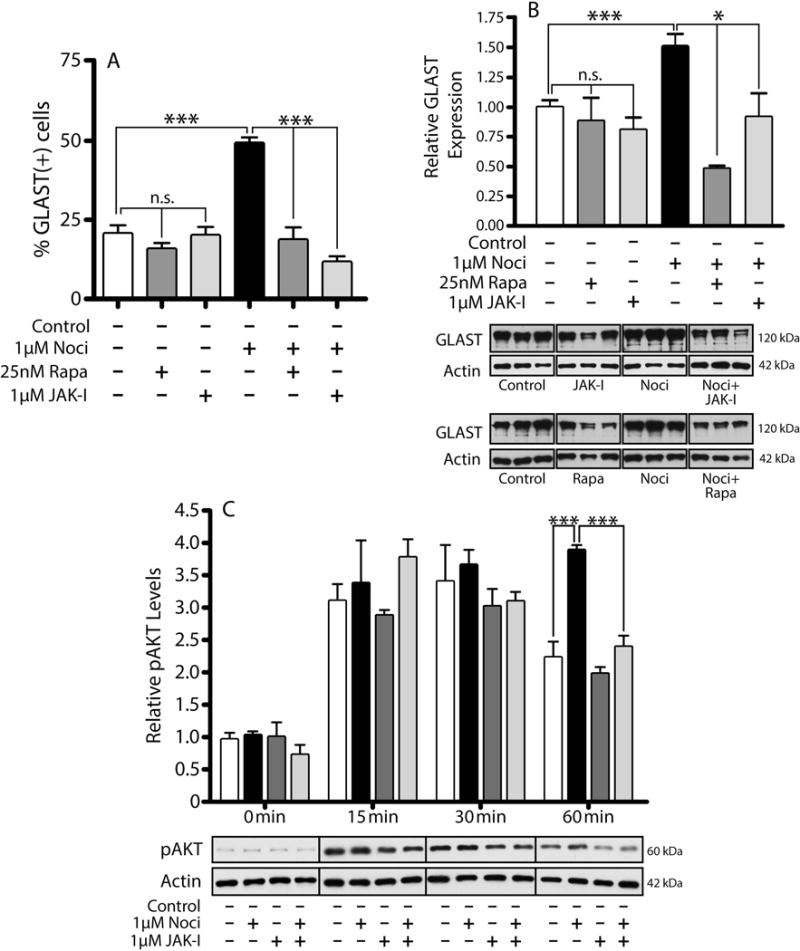
(A) Immunocytochemistry was used to evaluate GLAST expression in rat astrocytes after a 24 hr incubation in CDM alone, or CDM supplemented with one of the following: 25nM Rapamycin (Rapa), 1μM JAK Inhibitor I (JAK-I), 1μM Noci, 1μM Noci + 25nM Rapa, or 1μM Noci + 1μM JAK-I. The bar graph shows the number of GLAST positive cells as a % of the GFAP(+) cells/field under each condition. Results are the mean ± SEM from twelve fields/well, 3 wells/condition. Control vs. Rapa and JAK-I, n.s.; control vs. Noci ***p<0.001; Noci vs. Noci + Rapa and Noci + JAK-I, ***p<0.001. (B) Astrocytes treated for 24 hrs in the conditions listed above, were subjected to western blot analysis for GLAST. GLAST levels in the bar graph are expressed as change relative to control values. The results are the mean ± SEM, n=3. Control vs. Rapa and JAK-I, n.s.; control vs. Noci ***p<0.001; Noci vs. Noci + Rapa and Noci + JAK-I, *p<0.05. (C) Astrocyte cultures were first pre-incubated for 2 hrs in DMEM-F12 alone; and then for increasing times (0-60 min) in CDM alone, CDM + 1μM Noci, CDM + 1μM JAK-I, or CDM + 1μM Noci + 1μM JAK-I. Cell lysates were then subjected to western blot analysis for pAKT. The results are expressed as change relative to control and are the mean ± SEM from 3 different cultures; control vs. Noci at 60 min, ***p<0.001; controls vs. JAK-I, n.s.; Noci vs. Noci + JAK-I, ***p<0.001.
Figure 12. Proposed signaling pathway of nociceptin-mediated GLAST expression.
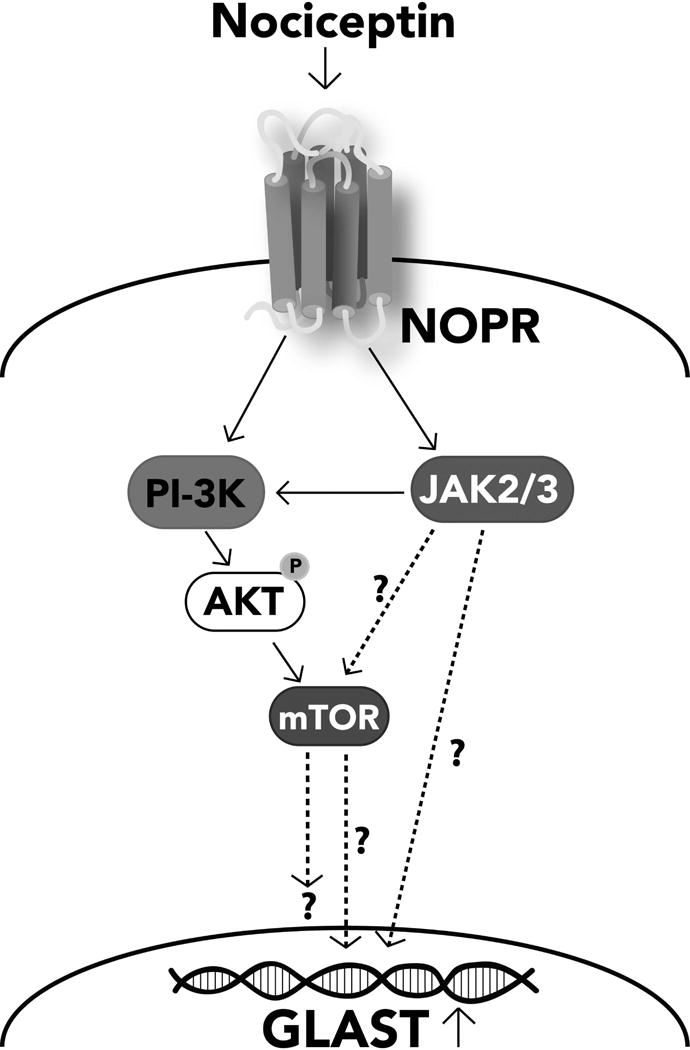
The effects of different inhibitors (Figs. 10 and 11) indicate that nociceptin effects on GLAST expression are mediated by a signaling cascade that involves the interaction of PI-3K/AKT, mTOR and JAK. Solid lines represent experimentally supported interactions. Dashed arrows represent possible interactions that require further investigation.
DISCUSSION
The studies undertaken in this investigation uncovered a novel role for the nociceptin system during brain maturation. Our in vitro and in vivo findings showed that nociceptin plays a conserved role stimulating the expression of the glutamate transporter GLAST/EAAT1 in both rodent and human developing astrocytes. This regulatory effect is mediated by NOR and the downstream participation of a complex signaling cascade that involves the interaction of several kinase systems, including PI-3K/AKT, mTOR and JAK. The observation that the cells produce and secrete nociceptin suggests the existence of a nociceptin mediated autocrine loop crucial to the regulation GLAST expression and glutamate homeostasis in developing astrocytes. Previous studies (Saito et al., 1997; Zaveri et al., 2006) showed that nociceptin can influence neuronal differentiation and neurite outgrowth; and findings from our laboratory suggested a role of this peptide in oligodendrocyte development (Eschenroeder et al., 2012). The present results further implicate the nociceptin system as a critical regulatory player during CNS formation and brain maturation.
An important observation of these studies is the specificity of timing for these nociceptin effects. The in vivo findings indicate that, overlapping with the temporal elevation of nociceptin brain concentration and astroglial NOR expression, the capacity of nociceptin to increase GLAST levels in the rat brain is restricted to a developmental window that encompasses the first 9-10 days of life. This observation is particularly significant because this postnatal period in rodents, equivalent to the third trimester in humans (Workman et al., 2013), is known to be representative of a timing at which a major number of glutamatergic synapses is formed in the mammalian brain. Importantly, this crucial developmental event immediately follows the rapid generation of astrocytes (Miller and Gauthier, 2007) and occurs at a time at which astrocytic GLAST/EAAT1 is still the dominant transporter responsible for local control of glutamate levels (Danbolt, 2001). This early GLAST/EAAT1 function is fundamental beyond protection from glutamate-induced cytotoxicity and regulation of glutamatergic activity, as results from different laboratories have pointed to glutamate as a pivotal regulator of crucial aspects of brain maturation. Glutamate has been shown to control the proliferation and survival of neural progenitors (LoTurco et al., 1995; Brazel et al., 2005) and to stimulate the rate of neuronal migration (Komuro and Rakic, 1993, 1998; Behar et al., 1999). Activation of glutamate receptors by subtoxic levels can also result in opening of voltage-dependent Ca2+ channels, with subsequent suppression of growth cone function and dendritic outgrowth (Mattson et al., 1988); and both metabotropic and ionotropic glutamate receptors are thought to play an important role in the cell contact-mediated suppression of sprouting that occurs prior to the establishment of action potential activity (Miskevich et al., 2002). Direct activation of NMDA, AMPA and KA receptors by glutamate increases both branching and length of process outgrowth (Komuro and Rakic, 1993; Voss et al., 2007; Whitney et al., 2008; Jansson et al., 2013), and studies from Kwon and Sabatini (Kwon and Sabatini, 2011) showed that glutamate is also able to induce the de novo growth of functional spines in the developing cortex. In addition, glutamate signaling was shown to directly control transcription factor activation in developing oligodendrocytes (Pende et al., 1997; Sato-Bigbee et al., 1999). Importantly, these multiple glutamate effects are concentration-dependent, underscoring the role of nociceptin as a regulator of astrocytic GLAST/EAAT1 expression and the potential importance of this function in brain development. This raises the question of whether alterations in nociceptin system activity may disrupt glutamate-dependent steps during brain maturation, in particular those that could result in abnormal synapse plasticity and connectivity. In support of this possibility, studies have shown that NOR knockout animals exhibit a variety of abnormal responses; including those involved in spatial memory and learning (Noda et al., 2000), feeding behavior and neuronal excitability (Koizumi et al., 2009; Farhang et al., 2010) as well as responses to drug effects (Rizzi et al., 2000; Ueda et al., 2000; Mamiya et al., 2001; Sakoori and Murphy, 2004, 2009; Chung et al., 2006; Marquez et al., 2008, 2013, Kallupi et al., 2013, 2017). It remains to be determined whether such findings could be solely explained by direct effects of nociceptin on neuronal cells, or might also reflect among other possibilities, an underlying alteration in network connectivity resulting from early defects in glutamate homeostasis and brain development.
While the present observations indicate that the effects of nociceptin on astroglial GLAST/EAAT1 expression are restricted to a specific window of early brain development, different reports raise the question of whether nociceptin plays a similar role at a later time under pathological conditions. Such may be the case of epilepsy, a disease characterized by the presence of elevated levels of extracellular glutamate (Cavus et al., 2005). An intriguing outcome from studies using the kainic acid model of epilepsy, is the presence of abnormally elevated levels of nociceptin (Bregola et al., 1999, 2002b; Aparicio et al., 2004; Bayrakdar et al., 2013) followed in time by upregulation of GLAST expression (Nonaka et al., 1998). However, the possibility of a positive role of nociceptin in controlling GLAST levels in epilepsy is difficult to assess at this time as studies in animal models have indicated contradictory results pointing to both protective (Gutiérrez et al., 2001; Carmona-Aparicio et al., 2007; Bayrakdar et al., 2013) as well as permissive (Bregola et al., 2002a; b; Binaschi et al., 2003; Aparicio et al., 2004) roles of the nociceptin system in this disease. Nevertheless, one could argue that the increase in nociceptin levels in epileptic subjects could result in a compensatory rise in the level of GLAST to protect the brain from further excitotoxic effects of glutamate.
The present results also further emphasize the developmental differences between GLAST/EAAT1 and GLT-1/EAAT2. In agreement with the observations from other laboratories (Ullensvang et al., 1997; Schreiner et al., 2014), we were unable to detect in the current study GLT-1 expression in the younger group of pups (postnatal days 3 to 9). Interestingly, while GLT-1 is readily detectable in the brain of older animals, no significant differences in GLT-1 expression were observed at any age group between controls and NOR inhibitor-treated rats or between wild-type and NOR KO mice, indicating that, in contrast with GLAST, GLT-1 is not regulated by the nociceptin system. These findings add to results from others demonstrating that GLAST and GLT-1, which have almost equal affinities for glutamate and similar structures, are on the other hand differentially regulated and maintain unique expression patterns both during development and in the adult brain (Wadiche et al., 1995; Rothstein et al., 1996; Bar-Peled et al., 1997; Furuta et al., 1997; Ullensvang et al., 1997; Schlag et al., 1998; Gegelashvili et al., 2000; Perego et al., 2000; Danbolt, 2001; Mim et al., 2005; Schreiner et al., 2014).
Interestingly, nociceptin may also regulate GLAST levels in another glial cell type, GLAST is also expressed in oligodendrocytes and their precursors (Domercq and Matute, 1999; Pitt et al., 2003; DeSilva et al., 2009). The present findings in astrocytes, together with our previous results indicating the presence of NOR in developing oligodendrocytes (Eschenroeder et al., 2012), raise the possibility that nociceptin may also play a role regulating GLAST expression in these glial cells. Furthermore, there is evidence to suggest that GLAST activation plays an important role in oligodendrocyte maturation (Martinez-Lozada et al., 2014). Studies are in progress to investigate this possibility and the extent to which nociceptin-dependent regulation of oligodendroglial GLAST may play a role in controlling developmental myelination.
In summary, our earlier studies implicated NOR in the maturation of oligodendrocytes and the present results show that nociceptin can control the expression of GLAST in developing astrocytes. The presence of nociceptin and NOR, in maturing human and rat astrocytes makes the system an interesting target for future neurodevelopmental studies. The multiple roles of the nociceptin system in combination with the critical functions of astrocytic glutamate transport and the high levels of nociceptin expression observed early in development strongly support a role for nociceptin in brain maturation, a problem that warrants further investigation.
Supplementary Material
Cortical brain tissue slices from (A-C) 5-day-old rats and (G-I) gestational week 23, human were subjected to in situ hybridization using a nonsense deoxyoligonucleotide probe (instead of the NOR mRNA-antisense specific probe) to control for non-specific deoxyoligonucleotide binding. (D-F) Rat and (J-L) human tissue were incubated with NOR mRNA antisense probe followed by double immunohistochemistry using (D and J) normal rabbit (Rb) serum and (E and K) normal mouse (Ms) serum in place of anti-ALDH1L1 and anti-digoxigenin antibodies, respectively.
Figure S1. Immunohistochemical controls in rat and human brain slices. Cortical brain tissue slices from 5-day-old rats and gestational week 23, human were subjected to double immunohistochemistry using (A and G) normal rabbit (Rb) serum (instead of rabbit anti-ALDH1L1 antibody) and (B and H) normal Guinea pig (GP) serum (in place of GP anti-Noci antibody) to control for non-specific labeling in the studies of Noci localization in astrocytes (Figs. 2 and 3). Rat and human tissue slices were also subjected to double immunohistochemistry using (D and J) normal rabbit (Rb) serum (instead of rabbit anti-ALDH1L1 antibody) and (E and K) normal rabbit (Rb) serum (in place of Rb anti-NOR antibody) to control for non-specific labeling in the studies of NOR localization in astrocytes (Figs. 2 and 3).
Main Points.
Nociceptin increases expression of the glutamate transporter GLAST in human and rat astrocytes
This effect is restricted to a critical maturational window, suggesting a role of nociceptin in controlling glutamate homeostasis in the developing brain.
Acknowledgments
This work was supported by the National Multiple Sclerosis Society grant RG 1501-2891 and a sub-award (C S-B) from NIH CTSA grant UL1TR00058 from the Virginia Commonwealth University (VCU) Center for Clinical and Translational Research and NIH grant RO1AI093718 (TK). Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Grant P30 CA016059.
References
- Aparicio LC, Candeletti S, Binaschi A, Mazzuferi M, Mantovani S, Di Benedetto M, Landuzzi D, Lopetuso G, Romualdi P, Simonato M. Kainate seizures increase nociceptin/orphanin FQ release in the rat hippocampus and thalamus: A microdialysis study. J Neurochem. 2004;91:30–37. doi: 10.1111/j.1471-4159.2004.02633.x. [DOI] [PubMed] [Google Scholar]
- Aschner M. Neuron-astrocyte interactions: implications for cellular energetics and antioxidant levels. Neurotoxicology. 2000;21:1101–7. [PubMed] [Google Scholar]
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. J Neurochem. 1997;69:2571–80. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- Bayrakdar ET, Bojnik E, Armagan G, Kanit L, Benyhe S, Borsodi A, Yalcin A. Kainic acid-induced seizure activity alters the mRNA expression and G-protein activation of the opioid/nociceptin receptors in the rat brain cortex. Epilepsy Res. 2013;105:13–19. doi: 10.1016/j.eplepsyres.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu Q-YY, Colton CA, Barker JL. Glutamate Acting at NMDA Receptors Stimulates Embryonic Cortical Neuronal Migration. J Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Chiu SY, Gray PT, Ritchie JM. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc London Ser B, Biol Sci. 1985;225:299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Binaschi A, Zucchini S, Bregola G, Rodi D, Mazzuferi M, Reinscheid RK, Simonato M. Delayed epileptogenesis in nociceptin/orphanin FQ-deficient mice. Neuroreport. 2003;14:825–827. doi: 10.1097/00001756-200305060-00009. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia. 2000;30:27–38. doi: 10.1002/(sici)1098-1136(200003)30:1<27::aid-glia4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Nuñez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131:55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Bregola G, Candeletti S, Romualdi P, Simonato M. Limbic seizures increase pronociceptin mRNA levels in the thalamic reticular nucleus. Neuroreport. 1999;10:541–6. doi: 10.1097/00001756-199902250-00018. [DOI] [PubMed] [Google Scholar]
- Bregola G, Zucchini S, Frigati L, Candeletti S, Romualdi P, Reinscheid R, Simonato M. Involvement of the neuropeptide orphanin FQ/nociceptin in kainate and kindling seizures and epileptogenesis. Epilepsia. 2002a;43:18–19. doi: 10.1046/j.1528-1157.43.s.5.43.x. [DOI] [PubMed] [Google Scholar]
- Bregola G, Zucchini S, Rodi D, Binaschi A, D’Addario C, Landuzzi D, Reinscheid R, Candeletti S, Romualdi P, Simonato M. Involvement of the neuropeptide nociceptin/orphanin FQ in kainate seizures. J Neurosci. 2002b;22:10030–8. doi: 10.1523/JNEUROSCI.22-22-10030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–71. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M. Riluzole neuroprotection in a Parkinson’s disease model involves suppression of reactive astrocytosis but not GLT-1 regulation. BMC Neurosci. 2012;13:38. doi: 10.1186/1471-2202-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Aparicio L, Peña F, Borsodi A, Rocha L. Effects of nociceptin on the spread and seizure activity in the rat amygdala kindling model: Their correlations with 3H-leucyl-nociceptin binding. Epilepsy Res. 2007;77:75–84. doi: 10.1016/j.eplepsyres.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK. Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther. 2006;318:262–267. doi: 10.1124/jpet.106.103960. [DOI] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol. 1996;119:1614–8. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- DeSilva TM, Kabakov AY, Goldhoff PE, Volpe JJ, Rosenberg PA. Regulation of glutamate transport in developing rat oligodendrocytes. J Neurosci. 2009;29:7898–908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Mol Brain Res. 1999;67:296–302. doi: 10.1016/s0169-328x(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci. 1999;19:10193–10200. doi: 10.1523/JNEUROSCI.19-23-10193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Joon Won S, Malgorn C, Auregan G, Berman AE, Chen P-C, Deglon N, Johnson JA, Won Suh S, Swanson RA. Nuclear Factor Erythroid 2-Related Factor 2 Facilitates Neuronal Glutathione Synthesis by Upregulating Neuronal Excitatory Amino Acid Transporter 3 Expression. J Neurosci. 2011;31:7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of μ-opioid- and nociceptin/orphanin FQ receptors in cell development: Implications for drug addiction treatment during pregnancy. Glia. 2012;60:125–136. doi: 10.1002/glia.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59:190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa A, Paixão S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zhu ZH, Wang YQ, Wu GC. Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience. 2007;144:275–285. doi: 10.1016/j.neuroscience.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, De Smedt-Peyrusse V, Layé S. Characterization of STAT3-expressing cells in the postnatal rat brain. Brain Res. 2006;1098:26–32. doi: 10.1016/j.brainres.2006.04.115. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Leff P, Romo-Parra H, Acevedo R, Antón B. Orphanin-FQ/nociceptin inhibits kindling epileptogenesis and enhances hippocampal feed-forward inhibition. Neuroscience. 2001;105:325–333. doi: 10.1016/s0306-4522(01)00196-8. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–55. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The Blood-Brain Barrier / Neurovascular Unit in Health and Disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J Neurophysiol. 1997;78:3351–3358. doi: 10.1152/jn.1997.78.6.3351. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Watanabe M, Ichikawa T, Kobayashi T, Yano R, Kumanishi T. Distribution of prepro-nociceptin/orphanin FQ mRNA and its receptor mRNA in developing and adult mouse central nervous systems. J Comp Neurol. 1998;399:139–151. doi: 10.1002/(sici)1096-9861(19980914)399:1<139::aid-cne11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Jansson LC, Akerman KE. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J Neural Transm. 2014:819–836. doi: 10.1007/s00702-014-1174-6. [DOI] [PubMed] [Google Scholar]
- Jansson LC, Louhivuori L, Wigren H-K, Nordström T, Louhivuori V, Castrén ML, Åkerman KE. Effect of glutamate receptor antagonists on migrating neural progenitor cells. Eur J Neurosci. 2013;37:1369–82. doi: 10.1111/ejn.12152. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–7. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura aM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Scuppa G, de Guglielmo G, Calò G, Weiss F, Statnick MA, Rorick-Kehn LM, Ciccocioppo R. Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology. 2017;42:695–706. doi: 10.1038/npp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Nociceptin/Orphanin FQ Decreases Glutamate Transmission and Blocks Ethanol-Induced Effects in the Central Amygdala of Naive and Ethanol-Dependent Rats. Neuropsychopharmacology. 2013;39:1–12. doi: 10.1038/npp.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M, Cagniard B, Murphy NP. Endogenous nociceptin modulates diet preference independent of motivation and reward. Physiol Behav. 2009;97:1–13. doi: 10.1016/j.physbeh.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordula T, Rydel RE, Brigham EF, Horn F, Heinrich PC, Travis J. Oncostatin M and the interleukin-6 and soluble interleukin-6 receptor complex regulate alpha1-antichymotrypsin expression in human cortical astrocytes. J Biol Chem. 1998;273:4112–4118. doi: 10.1074/jbc.273.7.4112. [DOI] [PubMed] [Google Scholar]
- Köster A, Montkowski A, Schulz S, Stübe EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H-B, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–4. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalu S, Moisand C, Mazarguil H, Cambois G, Mollereau C, Meunier JC. Comparison of the structure-activity relationships of nociceptin and dynorphin A using chimeric peptides. FEBS Lett. 1997;417:333–6. doi: 10.1016/s0014-5793(97)01318-5. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJS, Davis MBE, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–50. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Sadikot AF. Glutamate and regulation of proliferation in the developing mammalian telencephalon. Dev Neurosci. 2004;26:218–228. doi: 10.1159/000082139. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T. Enhancement of spatial attention in nociceptin/orphanin FQ receptor-knockout mice. Brain Res. 1998;783:236–40. doi: 10.1016/s0006-8993(97)01406-6. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Noda Y, Ren X, Nagai T, Takeshima H, Ukai M, Nabeshima T. Morphine tolerance and dependence in the nociceptin receptor knockout mice. J Neural Transm. 2001;108:1349–1361. doi: 10.1007/s007020100012. [DOI] [PubMed] [Google Scholar]
- Marquez P, Hamid A, Lutfy K. The role of NOP receptors in psychomotor stimulation and locomotor sensitization induced by cocaine and amphetamine in mice. Eur J Pharmacol. 2013;707:41–45. doi: 10.1016/j.ejphar.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Waggener CT, Kim K, Zou S, Knapp PE, Hayashi Y, Ortega A, Fuss B. Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIβ’s actin-binding/-stabilizing domain. Glia. 2014;62:1543–58. doi: 10.1002/glia.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: Involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T, Tanaka K. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci U S A. 2006;103:12161–12166. doi: 10.1073/pnas.0509144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing Is Everything: Making Neurons versus Glia in the Developing Cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Mim C, Balani P, Rauen T, Grewer C. The glutamate transporter subtypes EAAT4 and EAATs 1-3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J Gen Physiol. 2005;126:571–89. doi: 10.1085/jgp.200509365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskevich F, Lu W, Lin S-Y, Constantine-Paton M. Interaction between metabotropic and NMDA subtypes of glutamate receptors in sprout suppression at young synapses. J Neurosci. 2002;22:226–238. doi: 10.1523/JNEUROSCI.22-01-00226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mission JP, Takahashi T, Caviness VS. Ontogeny of radial and other astroglial cells in murine cerebral cortex. Glia. 1991;4:138–48. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- Neal CR, Akil H, Watson SJ. Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat. 2001;22:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Mamiya T, Manabe T, Nishi M, Takeshima H, Nabeshima T. Role of nociceptin systems in learning and memory. Peptides. 2000;21:1063–1069. doi: 10.1016/s0196-9781(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Nonaka M, Kohmura E, Yamashita T, Shimada S, Tanaka K, Yoshimine T, Tohyama M, Hayakawa T. Increased transcription of glutamate-aspartate transporter (GLAST/GluT-1) mRNA following kainic acid-induced limbic seizure. Mol Brain Res. 1998;55:54–60. doi: 10.1016/s0169-328x(97)00361-6. [DOI] [PubMed] [Google Scholar]
- Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron. 2000;26:515–522. doi: 10.1016/s0896-6273(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, Pietrini G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J Neurochem. 2000;75:1076–1084. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci U S A. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Li P, Mangin J-M, Huntsman M, Gallo V. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–71. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Sci (New York, NY) 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Bigoni R, Marzola G, Guerrini R, Salvadori S, Regoli D, Calo G. The nociceptin/orphanin FQ receptor antagonist, [Nphe1]NC(1-13)NH2, potentiates morphine analgesia. Neuroreport. 2000;11:2369–2372. doi: 10.1097/00001756-200008030-00007. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, Zeilhofer HU, Regoli D, Caló G. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: Pharmacological and genetic evidences. Pain. 2006;124:100–108. doi: 10.1016/j.pain.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Saito Y, Maruyama K, Saido TC, Kawashima S. Overexpression of a neuropeptide nociceptin/orphanin FQ precursor gene, N23K/N27K, induces neurite outgrowth in mouse NS20Y cells. J Neurosci Res. 1997;48:397–406. doi: 10.1002/(sici)1097-4547(19970601)48:5<397::aid-jnr2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56:896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: Perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Bigbee C, Pal S, Chu AK. Different neuroligands and signal transduction pathways stimulate CREB phosphorylation at specific developmental stages along oligodendrocyte differentiation. J Neurochem. 1999;72:139–147. doi: 10.1046/j.1471-4159.1999.0720139.x. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schreiner AE, Durry S, Aida T, Stock MC, Rüther U, Tanaka K, Rose CR, Kafitz KW. Laminar and subcellular heterogeneity of GLAST and GLT-1 immunoreactivity in the developing postnatal mouse hippocampus. J Comp Neurol. 2014;522:204–224. doi: 10.1002/cne.23450. [DOI] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: Key signaling pathway for ast. J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Susarla BTS, Seal RP, Zelenaia O, Watson DJ, Wolfe JH, Amara SG, Robinson MB. Differential regulation of GLAST immunoreactivity and activity by protein kinase C: Evidence for modification of amino and carboxyl termini. J Neurochem. 2004;91:1151–1163. doi: 10.1111/j.1471-4159.2004.02791.x. [DOI] [PubMed] [Google Scholar]
- Ueda H, Inoue M, Takeshima H, Iwasawa Y. Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci. 2000;20:7640–7. doi: 10.1523/JNEUROSCI.20-20-07640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H. Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett. 1997;237:136–138. doi: 10.1016/s0304-3940(97)00832-x. [DOI] [PubMed] [Google Scholar]
- Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–61. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Voss OP, Milne S, Sharkey J, O’Neill MJ, McCulloch J. Molecular mechanisms of neurite growth with AMPA receptor potentiation. Neuropharmacology. 2007;52:590–597. doi: 10.1016/j.neuropharm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Kinetics of a human glutamate transporter. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang J, Zhu C, Cao X, Wu G. Evidence of the synthesis of opioid receptor like 1 receptor in nociceptinergic neurons in rat brain suggests the existence of autoreceptor: a confocal double staining study. Neurosci Lett. 2000;290:193–6. doi: 10.1016/s0304-3940(00)01356-2. [DOI] [PubMed] [Google Scholar]
- Whitney NP, Peng H, Erdmann NB, Tian C, Monaghan DT, Zheng JC. Calcium-permeable AMPA receptors containing Q/R-unedited GluR2 direct human neural progenitor cell differentiation to neurons. FASEB J. 2008;22:2888–900. doi: 10.1096/fj.07-104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–83. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-C, Sontheimer H. Modulation of glial glutamate transport through cell interactions with the extracellular matrix. Int J Dev Neurosci. 2002;20:209–217. doi: 10.1016/s0736-5748(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Yu TP, Xie CW. Orphanin FQ/nociceptin inhibits synaptic transmission and long-term potentiation in rat dentate gyrus through postsynaptic mechanisms. J Neurophysiol. 1998;80:1277–1284. doi: 10.1152/jn.1998.80.3.1277. [DOI] [PubMed] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT, Waleh N, Toll L. Regulation of the prepronociceptin gene and its effect on neuronal differentiation. Gene. 2006;384:27–36. doi: 10.1016/j.gene.2006.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cortical brain tissue slices from (A-C) 5-day-old rats and (G-I) gestational week 23, human were subjected to in situ hybridization using a nonsense deoxyoligonucleotide probe (instead of the NOR mRNA-antisense specific probe) to control for non-specific deoxyoligonucleotide binding. (D-F) Rat and (J-L) human tissue were incubated with NOR mRNA antisense probe followed by double immunohistochemistry using (D and J) normal rabbit (Rb) serum and (E and K) normal mouse (Ms) serum in place of anti-ALDH1L1 and anti-digoxigenin antibodies, respectively.
Figure S1. Immunohistochemical controls in rat and human brain slices. Cortical brain tissue slices from 5-day-old rats and gestational week 23, human were subjected to double immunohistochemistry using (A and G) normal rabbit (Rb) serum (instead of rabbit anti-ALDH1L1 antibody) and (B and H) normal Guinea pig (GP) serum (in place of GP anti-Noci antibody) to control for non-specific labeling in the studies of Noci localization in astrocytes (Figs. 2 and 3). Rat and human tissue slices were also subjected to double immunohistochemistry using (D and J) normal rabbit (Rb) serum (instead of rabbit anti-ALDH1L1 antibody) and (E and K) normal rabbit (Rb) serum (in place of Rb anti-NOR antibody) to control for non-specific labeling in the studies of NOR localization in astrocytes (Figs. 2 and 3).


