Abstract
It is well known that dietary intakes play a pivotal role in pathogenesis of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH); however, the role of each component of diet has not yet been elucidated. Our objective was to evaluate the effects of onion consumption on prevention of NAFLD/NASH development. Sprague–Dawley rats were fed either high-fat, high sugar diet (model group), or high-fat, high sugar diet plus 7% onion powder (model + onion), or chow diet ad libitum for 7 weeks. Serum levels of fasting glucose, triglyceride, cholesterol, liver enzymes, insulin, and hepatic tumor necrosis factor-alpha (TNF-α) gene expression were determined. Hepatic histology was examined by H&E stain. Model + onion group had significantly lower hepatic steatosis, ballooning, lobular inflammation, and portal inflammation (p < 0.001), lower hepatic TNF-α gene expression (p < 0.001), lower plasma levels of ALT (p = 0.026), AST (p = 0.041), insulin (p < 0.001), TG (p = 0.041), and glucose (p = 0.009) compared with model group; however, weight gain, food intake, plasma total cholesterol and LDL levels were not significantly different between these two groups. Our data indicate that regular consumption of onion can prevent NAFLD even in the presence of the other risk factors such as obesity, hypercholesterolemia, and high energy, fat, and sugar intakes.
Keywords: Onion, NAFLD, Fatty liver, Prevention, Experimental model
Introduction
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of hepatic disorders ranging from simple triglyceride (TG) accumulation in hepatocytes through hepatic steatosis with inflammation, which is called as nonalcoholic steatohepatitis (NASH), to cirrhosis [1]. Although the exact pathophysiology of NAFLD has not yet elucidated, there is a consensus on the “multiple hit” hypothesis [2]. According to this hypothesis, multiple factors interact for the development of NAFLD. The first hit is the accumulation of TG in hepatocytes mostly due to insulin resistance (IR) [3]. The subsequent hits include a combination of oxidative stress, lipid peroxidation, and release of inflammatory mediators [4]. Although some dietary interventions have shown promising effects in the treatment of NAFLD [5–9], there are scarce studies evaluating the effects of dietary intakes in prevention of the disease.
Given the high content of phytochemicals, such as flavonoids, fructo-oligosaccharides (FOS), thiosulphinates and other sulphur compounds [10], Onion is an excellent source of antioxidants, immunomodulators, antimicrobs, and prebiotics [11]. Thus, we hypothesized that its regular consumption can inhibit the pathogenesis of NAFLD. To examine this hypothesis, we designed an experimental study evaluating the effects of regular consumption of onion on the development of a high fat, high sugar NAFLD/NASH model.
Materials and Methods
Animals and Diets
Eighteen male Sprague–Dawley rats (weighted 120–150 g), which were purchased from Pasteur Institute (Karaj, Iran), were housed individually in wire bar-floor cages. Body weights (BW) in grams were recorded on arrival and every 2 week thereafter. Food intake was also monitored twice a week. The animals were allowed 1 week of acclimatization in a standard environment at 22 °C, 50% humidity and 12-h light/dark cycles with free access to food and water. During the first week, all animals were fed a standard laboratory chow diet (Pasture Institute, Iran) and afterwards, they were randomly assigned to three groups: first group fed a standard chow diet (control group) with 10% of energy derived from fat, 30% from protein, and 60% from carbohydrates, second group fed a high-fat, high sugar diet (model group) [12] with 59% of energy derived from fat, 30% from carbohydrates, and 11% from protein and finally the third group fed high-fat, high sugar diet with 7% (w/w) onion powder (model + onion group) including 59% of energy derived from fat, 31% from carbohydrates, and 10% from protein. Onion powder was prepared according to the methods used by Hamauzu et al. [13]. All animals were fed ad libitum. The diets were prepared weekly and stored as vacuum packed (500 g) at −20 °C. Packs taken for use were thawed in the refrigerator at 4 °C. The food was offered daily, and the remains were weighed and removed after 48 h.
After 7-week feeding period, animals were killed by exsanguination (under light pentobarbital anesthesia). All animal procedures were carried out in accordance with the National Nutrition and Food Technology Research Institute (NNFTRI). The study protocol was approved at NNFTRI ethics committee with ethics code of NNFTRI 1393-568.
Tissue and Blood Preparation
Animals were sacrificed by exsanguination of blood from their heart under chloroform anesthesia. Five milliliters blood were collected in heparinized tubes; then, centrifuged (3500 rpm, 15 min, at 6 °C) to obtain the plasma. Fasted plasma glucose was measured immediately, and the remaining samples were kept at −80 °C before biochemical analysis.
After blood sampling, the livers were excised, washed with cold physiologic saline (0.9%), and dried. One part of each hepatic lobe was preserved in 10% buffered-formalin solution for histopathologic examination. Other liver samples were placed in liquid nitrogen tank, and then kept at −80 °C for gene expression evaluation.
RNA Extraction and Quantitative RT-PCR
Total RNA was purified using RNeasy Plus Mini Kits (Qiagen) according to the manufacturer’s instructions and cDNA synthesized with Superscript II reverse transcriptase (Invitrogen). Quantitative real-time PCR was performed using the Bio-Rad Laboratories MJ mini Opticon real-time PCR system, using IQ SYBR Green Supermix (Bio-Rad).
The PCR mix contained 2 µl cDNA, 1 µl of the appropriate forward and reverse primers, and 2 µl SYBR Green PCR Master mix in a total volume of 25 ml. PCR consisted of 50 cycles of denaturation at 94 °C for 30 s, annealing at melting temperature (Tm) for 30 s, and extension at 72 °C for 60 s. Primer sequences for each target gene, their source as well as their optimal PCR annealing temperatures are as follows: GAPDH forward primer 5′-GTGCTGAGTATGTCGTGGAGTCTA-3′ and reverse 5′-TCTCGTGGTTCACACCCATCAC-3′ (Tm 60 °C), and TNF-α forward primer 5′-ACT GAA CTT CGG GGT GAT TG-3′ and reverse 5′-GCT TGG TGG TTT GCT ACG AC-3′ (Tm 60 °C). Primer specificity was confirmed from the product size by agarose gel electrophoresis and the specificity of the PCR products checked by melt curve analysis.
Biochemical Assessments
Plasma concentrations of alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured using optimized UV at 340 nm, and insulin concentrations were measured using a rat insulin radioimmunoassay kit at 4 °C (Linco Research Inc, St Charles, MO). Plasma Glucose and TG were measured calorimetrically, Gamma glutamyl transferase (GGT), and Alkaline phosphatase (ALP) assessed photometrically, total Cholestrol, HDL, and LDL cholesterol were examined enzymatically all by using a commercial kit (Parsazmoon, Tehran, Iran).
Histopathology
Five sections from different lobes of each liver were submitted and processed through ethyl alcohol and xylene series, and embedded in paraffine blocks. Slides were stained with Hematoxyline Eosin and Masson’s Trichome and viewed under light microscopy by Nikon E 200. The grading was defined as follow: for hepatic steatosis: grade 0, no fat; grade 1, steatosis occupying less than 33% of the hepatic parenchyma; grade 2, 34–66% of the hepatic parenchyma; grade 3, more than 66% of the hepatic parenchyma; for inflammatory cell infiltration: grade 0: none; grade 1, 1–2 foci/field; grade 2, 3–4 foci/field; grade 3, more than 4 foci/field [14]; for ballooning: minimal, mild, and marked [15].
Statistical Analysis
Results are expressed as median (interquartile range) and using nonparametric tests included Mann–Whitney, Kruskal–Wallis and Chi square tests. p < 0.05 was considered for significance level. All statistical analyses were performed with the use of either GraphPad Prism Software Version 5.00 (GraphPad Software, SanDiego, CA), or SPSS 20.0 software (Chicago, IL, USA).
Results
Body Weight, Food Consumption
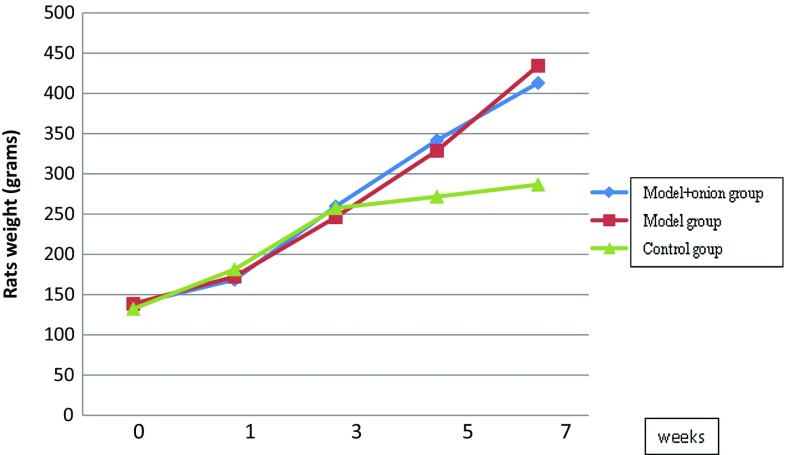
Total animals weight were measured and compared between three groups at week one, three, five and seven. Animal weights in different groups during the study are shown in Fig. 1. Weight gain differences among the groups were significantly different after week 3 (p = 0.003 for week 5, p = 0.002 for week 7 and p = 0.003 for total weight gain). Weight gain was not significantly different between model group and model + onion group; however, the weight gain was significantly lower in control group in comparison to the other two groups (p = 0.002).
Fig. 1.
Mean weight of animals in nonalcoholic fatty liver disease model group, control group, and model +7% onion group (n = 6 in each group) at weeks 1, 3, 5, and 7; from 3rd week, the mean weight of control group was significantly less than two other groups (p = 0.002)
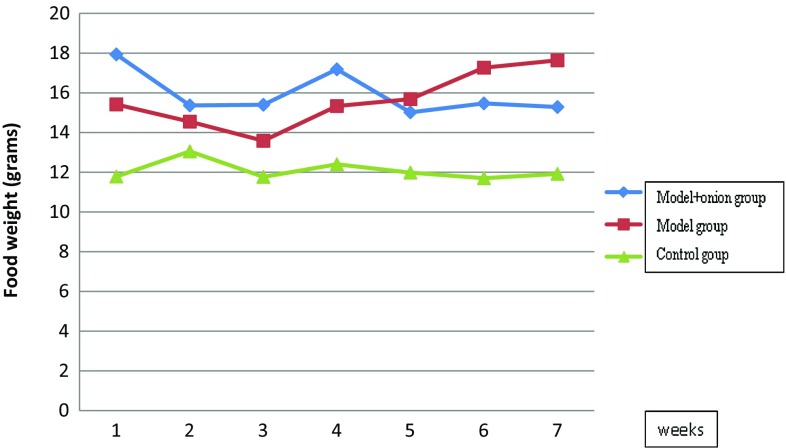
Animals’ food intake during the study is shown in Fig. 2. Kruskal–Wallis test showed that food intake was different among three groups in every week (p = 0.05); however, this near significant difference was related only to the significant lower food intake in control group (p < 0.05), and was not present between the model and model + onion groups.
Fig. 2.
Mean food intake in animals in nonalcoholic fatty liver disease model group, control group, and model +7% onion group (n = 6 in each group) at the end of each week. At weeks one to seven food intake of control group was significantly less than two other groups (p < 0.05)
Biochemical Assessments
Model + onion group had significantly lower plasma levels of ALT (p = 0.026), AST (p = 0.041), Insulin (p < 0.001), TG (p = 0.041), and glucose (p = 0.009) compared with model group; however, total cholesterol and LDL levels were not significantly different between these two groups (Table 1).
Table 1.
Plasma levels of liver enzymes, glycemic indices, and lipid profiles in study groups
| Model group [median (IQR)] | Model + onion group [median (IQR)] | Control group [median (IQR)] | p value* | |
|---|---|---|---|---|
| ALT (IU/l) | 36 (28.2–65.7)b | 26 (20.4–29.2)a | 20.5 (15.5–23.7)a | 0.01 |
| AST (IU/l) | 45 (42.2–52)b | 20 (14.5–30.5)a | 21.5 (18.5–24.5)a | 0.015 |
| GGT (IU/l) | 12.3 (5.3–18)b | 5.7 (4.1–6.9)a,b | 4 (2.7–7.5)a | 0.05 |
| ALP | 895 (498.5–1074)b | 980 (827–1255)b | 430 (342.5–630)a | 0.01 |
| Glucose (mg/dl) | 179.3 (168–191)b | 147 (136.2–155.7)a | 152.5 (137.7–159.7)a | 0.01 |
| Insulin (pmol/l) | 442 (391–505)b | 201 (182–229)a | 199 (176–231)a | 0.01 |
| TG (mg/dl) | 159.5 (143.7–168.7)b | 135.5 (106.2–156.5)a | 108 (97.7–118.7)a | 0.004 |
| Cholesterol (mg/dl) | 145 (120–171.5)b | 135.5 (112.5–155.7)a,b | 104 (96.5–118)a | 0.05 |
| HDL-c (mg/dl) | 27.5 (22.2–35.2)a | 22 (18.2–35.7)a | 30 (26.5–37)a | 0.36 |
| LDL-c (mg/dl) | 87.4 (62.2–109)b | 80.9 (58.3–101.4)b | 39 (31–56.2)a | 0.02 |
* Kruskal–Wallis test
a,bIn every row different scripts show significant difference
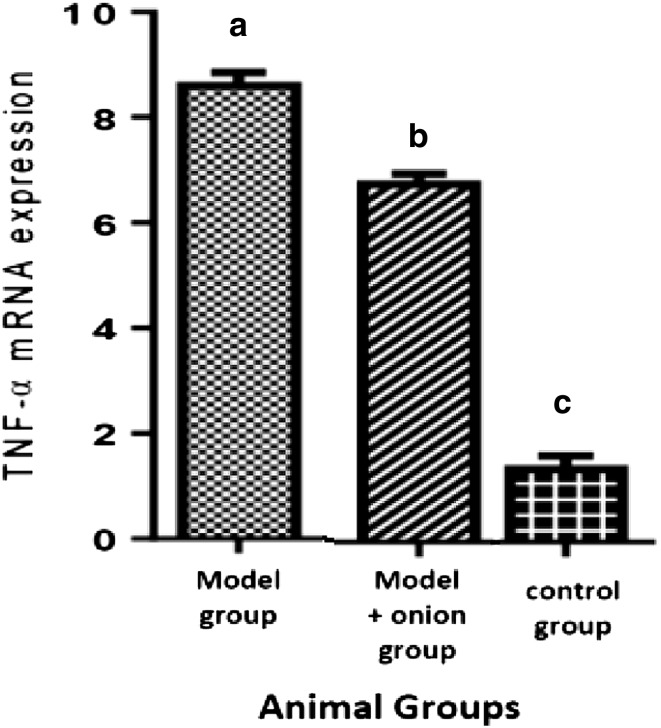
Hepatic TNF-α Gene Expression
Hepatic TNF-α gene expression was significantly decreased in model + onion group in comparison to model group (Fig. 3).
Fig. 3.
TNF-α mRNA expression comparison between three groups differences among three groups were statistically significant (p < 0.05)
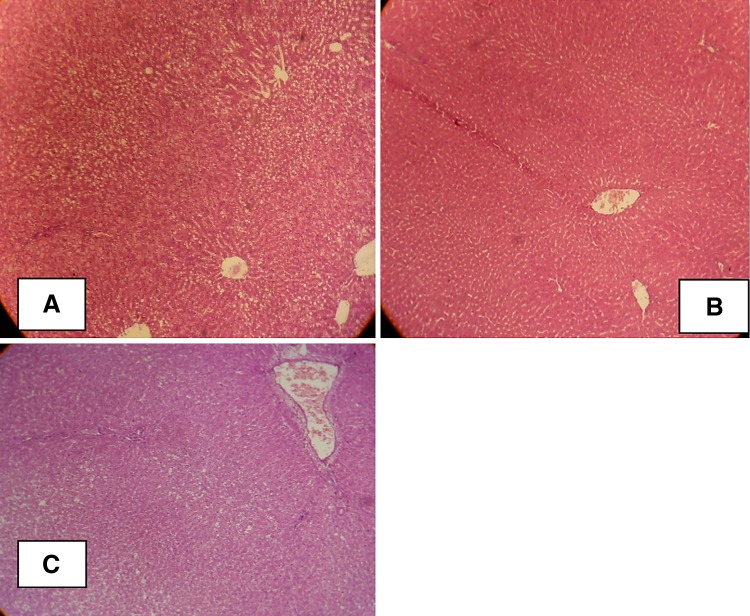
Histological Analysis
Hepatic histopathological features of the three groups are shown in Table 2, and Fig. 4. Model + onion group had significantly lower hepatic steatosis, ballooning, lobular inflammation, and portal inflammation in comparison to the model group.
Table 2.
Histopathological characteristics of model, control and model + onion groups (n = 6 in each group)
| Group | Stage | p value | ||
|---|---|---|---|---|
| Low | High | |||
| Steatosis n (%) | Model | 1 (16.7) | 5 (83.3) | 0.01 |
| Model + onion | 4 (66.7) | 2 (33.3) | ||
| Control | 6 (100) | 0 | ||
| Ballooning n (%) | Model | 1 (16.7) | 5 (83.3) | 0.004 |
| Model + onion | 2 (33.3) | 4 (66.7) | ||
| Control | 6 (100) | 0 | ||
| Lobular inflammation n (%) | Model | 2 (33.3) | 4 (66.7) | 0.05 |
| Model + onion | 4 (66.7) | 2 (33.3) | ||
| Control | 6 (100) | 0 | ||
| Portal inflammation n (%) | Model | 2 (33.3) | 4 (66.7) | 0.05 |
| Model + onion | 4 (66.7) | 2 (33.3) | ||
| Control | 6 (100) | 0 | ||
Low = 0 or 1 stage
High = 2 or 3 stage
Fig. 4.
Hepatic pathology in rats from control group ×100 (a), model group ×100 (b), and model + onion group ×100 (c). The liver samples were stained with haematoxylin and eosin
Discussion
To our knowledge, this is the first study that has evaluated the effects of onion powder consumption on the prevention of NAFLD. Since it is not convenient to evaluate the effects of dietary intakes in prevention of NAFLD in human, we designed this study in the experimental model of the disease. Our results have shown that regular consumption of onion powder can prevent development of NAFLD even at the presence of other risk factors such as obesity and high energy, fat, and sugar intakes.
The beneficial effects of onion consumption on the NAFLD risk factors such as hyperlipidemia and oxidative stress [16, 17], hyperglycemia [18, 19], and inflammation [20] have been shown previously. These effects are attributed to the high content of flavonoids, and prebiotics in the onion. Two flavonoid subgroups are found in onion, the anthocyanins, and flavanols such as quercetin and its derivatives [11, 21].
A recent study has shown that oral administration of quercetin to hyperlipidemia rats is highly effective in decreasing the levels of serum lipids, hepatic enzymes, steatosis, and inflammation through regulating the expressions of Sirt1, NF-kB p65 and iNOS [22]. Another recent study has reported that quercetin significantly decrease hepatic damage enzymes, lipoperoxidation, DNA damage and macrovesicular steatosis, ballooning and inflammatory process in an experimental model of NASH [23]. Furthermore, Marcolin et al. [24] indicated that oral administration of quercetin attenuates steatohepatitis in an experimental model of NASH via down regulation of profibrotic and proinflammatory gene pathways.
Moreover, the beneficial effects of prebiotics in modulation of gut microbiota, and attenuation of NAFLD have been shown recently [7, 25]. Prebiotics can increase the number of prebiotics in the gut lumen, which results in reduction in inflammation through reduction in pathogenic bacteria, enhancement of intestinal permeability, and immune regulation [7].
This study has several advantages; we used a diet induced model of NAFLD, which is similar to the disease development in the human. We evaluated the effects of onion consumption as a food, which is available in every season with a cheap price, and can be used in every population at risk of NAFLD development. Finally, we have shown these effects in the prevention of disease, and the results have shown that the effects will remain even in the presence of other risk factors for NAFLD development.
In conclusion, our results indicate that regular consumption of onion can prevent from development of NAFLD at least partially through improvement in serum glycemic indices, and TG, and down regulation of hepatic TNF-α gene expression, which plays a pivotal role in both inflammation and insulin resistance. These beneficial effects were observed even in the presence of main risk factors of NAFLD development such as obesity, hypercholesterolemia, and high energy, fat, and sugar intakes.
Acknowledgements
This study was supported by a Grant from the National Nutrition and Food Technology Research Institute of the Shahid Beheshti University.
Funding
This study was funded by National Nutrition and Food Technology Research Institute (Grant Number 568).
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 3.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 4.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaemi A, Taleban FA, Hekmatdoost A, Rafiei A, Hosseini V, Amiri Z, et al. How much weight loss is effective on nonalcoholic fatty liver disease? Hepat Mon. 2013;13:e15227. doi: 10.5812/hepatmon.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res (New York, NY) 2014;34:143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. 2014;99:535–542. doi: 10.3945/ajcn.113.068890. [DOI] [PubMed] [Google Scholar]
- 8.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res (New York, NY) 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;114:796–803. doi: 10.1017/S0007114515002433. [DOI] [PubMed] [Google Scholar]
- 10.Slimestad R, Fossen T, Vagen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths G, Trueman L, Crowther T, Thomas B, Smith B. Onions—a global benefit to health. Phytother Res PTR. 2002;16:603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- 12.Emamat H, Nouri M, Foroughi F, Rismanchi M, Hekmatdoost A. An accessible and pragmatic experimental model of nonalcoholic fatty liver disease. Middle East J Dig Dis. 2016;8:1–7. doi: 10.15171/mejdd.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamauzu Y, Nosaka T, Ito F, Suzuki T, Torisu S, Hashida M, et al. Physicochemical characteristics of rapidly dried onion powder and its anti-atherogenic effect on rats fed high-fat diet. Food Chem. 2011;129:810–815. doi: 10.1016/j.foodchem.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Vidyavati HG, Manjunatha H, Hemavathy J, Srinivasan K. Hypolipidemic and antioxidant efficacy of dehydrated onion in experimental rats. J Food Sci Technol. 2010;47:55–60. doi: 10.1007/s13197-010-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajitha G, Augusti K, Jose R. Prophylactic effects of garlic oil and onion oil fractions as compared to vitamin E on rats orally fed with lead acetate solution. Ind J Clin Biochem. 2016;31:260–269. doi: 10.1007/s12291-015-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal alpha-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int J Mol Sci. 2011;12:3757–3769. doi: 10.3390/ijms12063757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bang MA, Kim HA, Cho YJ. Alterations in the blood glucose, serum lipids and renal oxidative stress in diabetic rats by supplementation of onion (Allium cepa. Linn) Nutr Res Pract. 2009;3:242–246. doi: 10.4162/nrp.2009.3.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez-Prieto MA, Rodriguez Lanzi C, Lembo C, Galmarini CR, Miatello RM. Garlic and onion attenuates vascular inflammation and oxidative stress in fructose-fed rats. J Nutr Metab. 2011;2011:475216. doi: 10.1155/2011/475216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stajner D, Milic N, Canadanovic-Brunet J, Kapor A, Stajner M, Popovic BM. Exploring Allium species as a source of potential medicinal agents. Phytother Res PTR. 2006;20:581–584. doi: 10.1002/ptr.1917. [DOI] [PubMed] [Google Scholar]
- 22.Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem Toxicol. 2013;52:53–60. doi: 10.1016/j.fct.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Marcolin E, Forgiarini LF, Rodrigues G, Tieppo J, Borghetti GS, Bassani VL, et al. Quercetin decreases liver damage in mice with non-alcoholic steatohepatitis. Basic Clin Pharmacol Toxicol. 2013;112:385–391. doi: 10.1111/bcpt.12049. [DOI] [PubMed] [Google Scholar]
- 24.Marcolin E, San-Miguel B, Vallejo D, Tieppo J, Marroni N, Gonzalez-Gallego J, et al. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J Nutr. 2012;142:1821–1828. doi: 10.3945/jn.112.165274. [DOI] [PubMed] [Google Scholar]
- 25.Eslamparast T, Eghtesad S, Poustchi H, Hekmatdoost A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J Hepatol. 2015;7:204–212. doi: 10.4254/wjh.v7.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]