Abstract
A high circulating concentration of the non proteinogenic amino acid homocysteine has been implicated as a risk factor for Alzheimer’s Disease and its prodromal stage, mild cognitive impairement. Furthermore, hyperhomocysteinaemia has been directly attributed to a deficiency in vitamins B12, folate, and B6. Several studies have demonstrated decrease in progression of mild cognitive impairement to Alzheimer’s Disease, and some have even shown an improvement in cognition after vitamin supplements with B12 and folate. Plausible mechanisms linking hyperhomocysteinaemia to Alzheimer’s and cognitive impairement have been hypothesized and demonstrated in hyperhomocysteinemic mice models. However, some studies have not elucidated any benefit of vitamin supplements in subjects with cognitive impairment. Hence, multicentric clinical studies need to be conducted to substantiate the mechanisms of neuronal degeneration due to hyperhomocysteinaemia and to demonstrate the beneficial effect of folate, B6 and B12 supplements on cognition.
Keywords: Homocysteine, B12, Folate, Mild cognitive impairment, Alzheimer’s disease
Introduction
With the current increase in geriatric population due to increased longevity, cognitive decline is becoming more prevalent and there is a need to investigate the possible causes, especially modifiable ones, so as to be able to institute preventive measures.
Cognitive functions can be defined as cerebral activities that lead to knowledge. They are a group of varied brain functions that are attributable to different areas of the brain and include memory, learning, spatial orientation, reasoning, judgement and evaluation. The areas of the brain involved in these functions include frontal and temporal lobes, basal ganglia, limbic system [thalamus, hypothalamus, hippocampus, cingulate gyrus, amygdala]. A decline of these functions has been recognised as a progressive condition which starts as mild cognitive impairment (MCI) and progresses to Alzheimer’s Disease (AD).
Several promoting factors of cognitive decline have been identified. These can be non-modifiable and modifiable. The non-modifiable include factors like age and genetic predisposition. For example, it is generally known that individuals carrying the ε4 allele of apolipoprotein E (ApoE) gene are at greater risk of developing AD than those carrying the ε3 allele, whereas the ε2 allele decreases risk [1]. However, the use of ApoE genotyping in the diagnosis of MCI that evolves into AD is limited due to its low sensitivity and specificity. Moreover, diagnosing AD or MCI is just the tip of the iceberg; preventing further cognitive decline or, better still, improving cognition is the current therapeutic target which represents the major volume of this iceberg. Hence, it is important to identify modifiable factors and ascertain the means to modify them. Amongst the modifiable factors is circulating homocysteine (hcy) concentration.
Homocysteine is a homologue of cysteine, and is biosynthesized from methionine by removal of a terminal methyl group. It can be recylced into methionine or converted into cysteine with the aid of B-vitamins, which act as essential co-factors for the enzymes methionine synthase and cystathionine synthase. It follows, therefore that deficiency of these vitamins [B12, folate (B9) and pyridoxine (B6)] causes hyperhomocysteinemia (Hhcy) [2]. Hence, they also are implicated in cognitive decline.
Homocysteine, a non proteinogenic amino acid, has also been implicated as a risk factor for several types of vascular disease including cerebrovascular disease [3]. But the presence of cerebrovascular disease on its own is generally not associated with major cognitive impairment [4]. Likewise, Alzheimer’s pathology has been shown to be present in a significant proportion of non-demented elderly [5]. Hcy has been implicated in mild cognitive impairment [MCI] as well as its end-stage condition, Alzheimer’s disease [AD] by several scientists. It may, therefore, be surmised that hcy promotes cognitive decline by more than one mechanism, i.e. not by its vascular pathology alone.
This review attempts to assess the current status of the role of hcy, folate and B12 in the causation and amelioration of cognitive decline.
Homocysteine, MCI and AD
One of the early reports was by McCaddon et al. in 1998, who established the positive causality between high serum hcy levels and early onset of AD. They enrolled 60 subjects aged 65 years or over in their study, of which 30 were controls and 30 had clinically diagnosed AD as per DSM-III criteria [DSM-III is the Diagnostic and Statistical Manual of Mental Disorders]. It was shown that patients with diagnosed AD had Hcy levels significantly higher than that of the control group [mean Hcy of patients = 21.9 µmol/L; mean Hcy in controls 12.2 µmol/L; p < 0.0001] [6].
Wang et al. [7] randomly selected 370 individuals aged 75 years and above and not on vitamin B12 and folate supplements, measured their serum vitamin B12 and folate levels and followed them up for 3 years to detect incidence of AD as per DSM-III-R criteria. They observed that subjects who had lower B12 [<150 pmol/L]/lower folate levels [<10 nmol/L] had a twice as high relative risk of developing AD [RR = 2.1; 95% CI] than those with higher levels of these vitamins. This association was even stronger in subjects with a good baseline cognition [RR = 3.1; 95% CI]. The pattern remained similar when the cut-off was changed to 250 pmol/L for B12 and 12 nmol/L for folate [7].
Williams et al. [8] demonstrated that hippocampal width which is known to decrease with age also relates to plasma homocysteine in patients of AD so that there is a relation between the atrophy of the medial temporal lobe and Hhcy.
At the same time, Kruman et al. elucidated that folate deficiency and hyperhomocysteinemia impaired DNA repair in hippocampal neurons, sensitizing them to amyloid toxicity, one of the well-known features of AD. They cultured hippocampal neuron tissue in a medium deficient in folic acid and methionine, and hence high in hcy; this lead to the death of hippocampal neurons. The neurons incubated in a control medium had less DNA damage than the ones incubated in hcy. Further analyses showed that amyloid beta (Aß) protein alone caused reversible oxidative damage involving an increase in 8-oxyguanine, but that cells incubated in a folate or methionine deficient medium could not repair DNA after exposure to Aß. This lead to the inference that Hcy interfered with DNA repair. To further elucidate this, purines and thymidines were added, resulting in decreased cell death.
Another effect of folate/methionine deficient medium was increased misincorporation of Uracil. So if Hcy affects DNA repair by affecting availability of purines and thymidines, then DNA damage is promoted. When this DNA damage cannot be repaired, the cell switches to apoptosis. This hypothesis that Aß deposits along with folate deficiency and Hhcy cause neuronal death was confirmed by tests on transgenic mutant mice by the same researchers [9].
In the same year, Mallroy et al. [10] found significantly higher serum homocysteine levels (as compared to healthy controls) in three categories of patients—stroke, vascular dementia and AD. This was after allowance for confounding factors [age, sex, smoking, hypertension, cholesterol, creatinine and nutritional measures].
In 2004, Qadri et al. [11] demonstrated that subjects in the lowest tertile of folate had a high odds ratio for MCI [3.1] and dementia [3.8]. They also showed that HHcy conferred an odds ratio of 4.3 for development of dementia.
Linear regression models given by Schaffer et al. [12] revealed that homocysteine was consistently and strongly associated with worse neurobehavioral test performance and that subjects in the highest quartile of homocysteine levels were more than two times as likely to be in the lowest quartile of neurobehavioral test scores than those in the lowest quartile.
Thus, it was established that there is a direct association linking high serum homocysteine levels, low folate and low vitamin B12 levels with MCI and AD. In studies conducted in our laboratory on the relationship of vitamin supplements with homocysteine, we demonstrated that homocysteine was lowered by nutritional modification with folate alone (5 mg/day) or a combination of folate (1.5 mg/day) and B12 (0.5 mg/day) [13].
But does supplementation with these vitamins and consequent lowering of homocysteine actually change the progression of cognitive decline?
In 2006, McCaddon presented a case series in which a baseline homocysteine and cognitive assessment was followed by vitamin supplements and then homocysteine estimation and cognitive assessment were repeated. The results, which are summarized in the Table 1, indicate a role of vitamin supplements to not only prevent further decline of cognition but also to improve cognitive status [14].
Table 1.
Effect of vitamin supplements on homocysteine and cognitive scores
| Case no. | Homocysteine in µmol/L | MMSE or alternate score | Vitamin supplement with duration | Repeat MMSE (or alt score) |
Repeat homocysteine in µmol/L |
|---|---|---|---|---|---|
| 1 | 20.1 | 12/28 | B12 & folate 1 month |
28/30 | 7.5 |
| 2 | 27.5 | Moderate to severe dementia | B12 & folate 3 months |
Mild confusion | 6.6 |
| 3 | 15.6 | 12/28 [6CIT] | B12 & folate 3 months |
28/30 | 9.6 |
| 4 | 14.6 | 8/28 [6CIT] 16/39 [TICs-m] |
B12
6 months |
21/39 | 8.3 |
6CIT Six item cognitive impairment test, TICs-m Telephonic interview for cognitive status modified
Aisen et al., in 2008, conducted an interesting case control study in where they enrolled patients of mild to moderate AD (MMSE = 14–26) and with normal levels of homocysteine, vitamin B12 and folate. These 409 subjects were randomly assigned to two groups—one receiving high dose of vitamins (5 mg folate, 25 mg B6 and 1 mg B12) and the other receiving a placebo for 24 months. 168 subjects successfully completed the study. The authors then measured homocysteine and the cognitive subscale ADAS-cog (Alzheimer’s Disease cognitive scale). They observed that though these vitamin supplements significantly reduced both homocysteine levels and the rate of brain atrophy, it did not significantly affect the cognitive scales. They concluded that vitamin supplements do not slow cognitive decline in mild to moderate AD, which was at variance with the findings of most other studies as mentioned above. One could also conclude, from their findings, that in the absence of hyperhomocysteinemia, vitamin B supplements do not have a role in slowing cognitive decline [15].
From amongst the trials in the Cochrane Dementia and Cognitive Improvement Specialized Register Group, Malouf and Grimley conducted a meta-analysis of all double-blind, placebo-controlled randomized trials in healthy elderly subjects (with some form of cognitive impairment) on folate supplementation with or without B12. They found that folate (750 mcg/day) with or without B12 was effective in reducing homocysteine but did not have any beneficial effects on measures of cognition or mood. In a randomized controlled trial in healthy elderly subjects with Hhcy, they observed that a daily supplement of 800 mcg of folate over 3 years resulted in better global functioning (p = 0.033), better memory storage (p = 0.006) and better information processing speed (p = 0.016). In another trial on subjects with cognitive impairment and Hhcy, and who were given cholinesterase inhibitors as well as 1 mg of folate daily, had a better overall response (p = 0.02) than those on cholinesterase inhibitors alone [16].
In 2010, Smith et al., conducted a randomized, double-blind, controlled trial of the effects of high dose vitamin B supplement (folate 0.8 mg/day, B12 0.5 mg/day and B6 20 mg/day) on the rate of brain atrophy as measured by MRI volumetric scan in subjects over 70 years old. They demonstrated that the rate of atrophy was significantly lower (p = 0.001) in the group receiving active treatment as compared to those on placebo. They also elucidated that this atrophy was related to the baseline homocysteine levels so that those in the placebo group with a baseline hcy > 13 µmol/L had a 53% higher rate of atrophy than those in the active treatment group with similar homocysteine levels. Moreover, a greater rate of atrophy was associated with a lower final cognitive test score [17].
One of the mainstays of AD is the deposition of amyloid plaques and the presence of tau proteins and amyloid proteins, specifically β amyloids, in the brain. So Smach et al. measured plasma hcy along with CSF levels of hcy, folate, Aβ 1–42 and tau proteins in 133 subjects, of which 70 had AD, 33 had non AD dementia and 30 were age-matched controls. They elucidated that CSF concentrations of folate, Aβ 1–42 and tau proteins was significantly different between the groups with CSF folate being lower in AD patients than in controls (p < 0.02). They also demonstrated that there was no correlation between Aβ 1–42 or tau and folate or homocysteine irrespective of the groups. In addition, they found an inverse correlation between hcy and folate in the CSF (p < 0.0005). They, therefore, concluded that folate had a role in worsening of AD [18].
To further demonstrate the role of hcy in progression from MCI to AD, Zheng et al. studied the circulating levels of hcy and brain derived neurotrophic factor (BDNF), in patients with Apo ε4 allele. Plasma hcy was found to increase and serum BDNF to decrease in those cases of MCI that converted to AD. As compared to Apo ε4 allele, hcy as well as BDNF had a better sensitivity, specificity and positive predictive value for progression to AD [19].
Recently, Lee et al. elucidated that there were significant effects of multivitamin supplements on cognitive function (p = 0.021), hcy (p = 0.001) and depression (p = 0.001) in older patients of MCI. Hence multivitamin supplements could be used not only to improve cognitive function, but also to reduce depression in MCI [20].
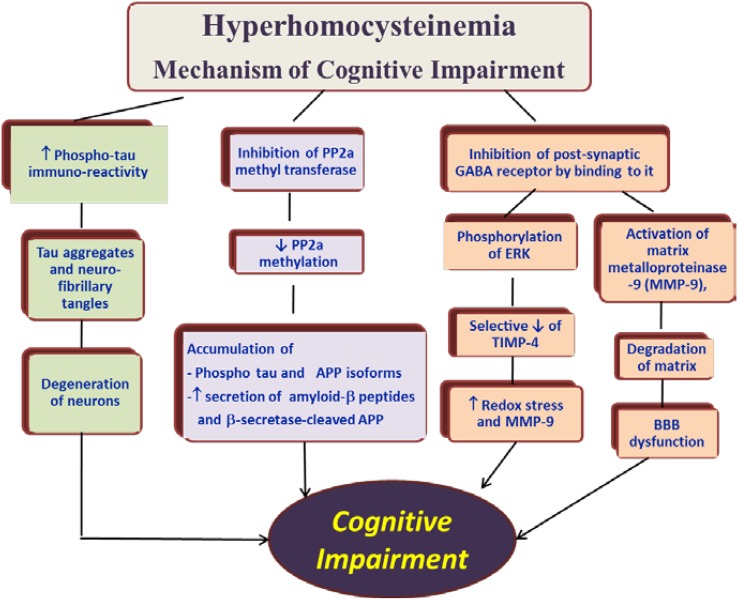
Mechanism of Cognitive Pathology Due to Hhcy
That remodelling of tissues due to Hhcy is mediated through matrix metalloproteinases (MMPs) has been amply demonstrated in several studies. Different MMPs are activated in different tissues. In the brain, Lominadze et al. demonstrated that Hhcy causes disruption of the blood brain barrier and microvascular leak in brain of mice through the action of MMP 9 [21].
Another experiment by Kumar et al. elucidated that the expression of MMPs 2 and 9, and that of tissue inhibitors of metalloproteinases (TIMPs) 3 and 4 was enhanced in hyperhomocysteinemic mice, probably through its inhibition of γ amino butyric acid (GABA) [22].
In a study we conducted on mice heterozygous for cystathionine-β-synthetase gene (CBS+/−) and these CBS+/− mice in whom the gene for MMP 9 has been ablated (CBS+/−/MMP9−/−), we demonstrated that ablating MMP 9 gene ameliorates the cognitive decline observed in the hyperhomocysteinemic CBS+/− mice, indicating that hcy causes cognitive decline through the action of MMP 9 [23].
Yet another study demonstrated that folate deficiency caused hcy-dependent inhibition of methylation of phosphatases and resultant inhibition of phosphatase activity. This in turn lead to increase in phosphor-tau immunoreactivity [24].
On basis of the available literature on the subject, we can schematically summarize the mechanisms by which Hhcy causes neuronal damage as shown in Fig. 1.
Fig. 1.
Nonvascular mechanisms of neurotoxicity due to hyperhomocysteinemia. PP2a, protein phosphatase 2a, APP amyloid precursor protein
Conclusion
Over the past several decades, scientists have demonstrated that Hhcy is a risk factor for cognitive decline, and that decreasing homocysteine by supplements of the B vitamins results in slowing of the progress of cognitive decline as well as improvement of cognition per se. At the same time, a few studies have not observed any benefit of these vitamins in amelioration of cognitive impairment. Hence, due to these unequivocal reports, current therapeutic regimen for MCI or AD do not routinely include supplements of the B vitamins. Moreover, the mechanism of action of Hhcy in causation of cognitive decline has not been amply demonstrated in humans, though there are several studies in mice models of Hhcy.
Hence, there is a need for more multicentric clinical studies demonstrating the mechanism of Hhcy and deficiency of vitamins B6, B9 and B12 in causing cognitive decline. This would encourage clinicians to include vitamins in the management of cognitive impairment and lead to decrease in morbidity in the geriatric population which is a growing section of every population.
Compliance with Ethical Standards
Conflicts of Interest
None.
Human Participants/Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Since this is a review, there is no patients’ informed consent involved.
Contributor Information
Seema Bhargava, Email: bhargavaseema6@gmail.com.
Annsh Bhandari, Email: annshbhandari@gmail.com.
Sangeeta Choudhury, Email: dr.sangeeta.sgrh@gmail.com.
References
- 1.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava S, Srivastava LM. Homocysteine—Marker of the millenium—a review of its evolution and clinical implication. J Med Sci. 2001;4(2):104–116. [Google Scholar]
- 3.Bhargava S, Parakh R, Manocha A, Kankra M, Aggarwal CS, Menon G, et al. High prevalence of hyperhomocysteinemia among Indian patients of vascular disease. Clin Chim Acta. 2006;374(1–2):160–162. doi: 10.1016/j.cca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer Disease—The Nun Study. JAMA. 1997;277(10):813–817. doi: 10.1001/jama.1997.03540340047031. [DOI] [PubMed] [Google Scholar]
- 5.Neuropathology Group Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357(9251):169–175. doi: 10.1016/S0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 6.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(SICI)1099-1166(199804)13:4<235::AID-GPS761>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B12 and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56:1188–1194. doi: 10.1212/WNL.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 8.Williams JH, Pereira EA, Budge MM, Bradley KM. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing. 2002;31:440–444. doi: 10.1093/ageing/31.6.440. [DOI] [PubMed] [Google Scholar]
- 9.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33:2351–2356. doi: 10.1161/01.STR.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- 11.Qadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, et al. Homocysteine, folate and vitamin B-12 in mild cognitive impairment, Alzheimer’s Disease and vascular dementia. Am J Clin Nutr. 2004;80:114–122. doi: 10.1093/ajcn/80.1.114. [DOI] [PubMed] [Google Scholar]
- 12.Schafer JH, Glass TA, Bolla KI, Mintz M, Jedlicka AE, Shwartz BS. Homocysteine and cognitive function in a population-based study of older adults. J Am Geriatr Soc. 2005;53:381–388. doi: 10.1111/j.1532-5415.2005.53153.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhargava S, Ali A, Bhargava EK, Manocha A, Kankra M, Das S, et al. Lowering homocysteine and modifying nutritional status with folic acid and vitamin B12 in Indian patients of vascular disease. J Clin Biochem Nutr. 2012;50(3):222–226. doi: 10.3164/jcbn.11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCaddon A. Homocysteine and cognitive impairment—a case series in a general practice setting. Nutr J. 2006;5:6. doi: 10.1186/1475-2891-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malouf M, Grimley EJ, Areosa SA. Folic acid with or without B12 for cognition and dementia. Cochrane Database Syst Rev 2003; (4):CD004514. [DOI] [PubMed]
- 17.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE. 2010;5(9):e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smach MA, Jacob N, Golmard JL, Charfeddine B, Lammouchi T, Ben Othman L, et al. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer’s disease or dementia: a case control study. Eur Neurol. 2011;65(5):270–278. doi: 10.1159/000326301. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, Kong X, Cui Y, Wei Y, Zhang J, Wei W. Conversion from MCI to AD in patients with the APOE ε4 genotype: prediction by plasma HCY and serum BDNF. Neurosci Lett. 2016;626:19–24. doi: 10.1016/j.neulet.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Lee HK, Kim SY, Sok SR. Effects of Multivitamin Supplements on Cognitive Function, Serum Homocysteine Level, and Depression of Korean Older Adults With Mild Cognitive Impairment in Care Facilities. J Nurs Scholarsh. 2016;48(3):223–231. doi: 10.1111/jnu.12201. [DOI] [PubMed] [Google Scholar]
- 21.Lomnadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290(3):H1206. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, et al. Homocysteine reduces blood flow to brain due to vascular resistance in carotid artery. Neurochem Int. 2008;53(6–8):214–219. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhargava S, Pushpakumar S, Metreveli N, Givvimani S, Tyagi SC. MMP-9 gene ablation mitigates hyperhomocysteinemia-induced cognition and hearing dysfunction. Mol Biol Rep. 2014;41(8):4889–4898. doi: 10.1007/s11033-014-3425-x. [DOI] [PubMed] [Google Scholar]
- 24.Chan AY, Alsaraby A, Shea TB. Folate deprivation increases tau phosphorylation by homocysteine-induced calcium influx and by inhibition of phosphatase activity: alleviation by S-adenosyl methionine. Brain Res. 2008;1199:133–137. doi: 10.1016/j.brainres.2008.01.008. [DOI] [PubMed] [Google Scholar]