Abstract
In order to highlight the potential of photodynamic antimicrobial chemotherapy in case of infections by antibiotic resistant-strains, a new antimicrobial peptide conjugate has been synthesized, consisting of a derivative of polymyxin B and a cationic porphyrin covalently attached together to a spacer. A polymyxin-derived moiety was subjected to a primary structural modification in the replacement of four diaminobutyrate residues with lysine ones. This modification was done in order to strongly reduce bactericidal activity, with the aim to eliminate the potential rise of polymyxin-resistant strains. Despite this modification, this new conjugate displayed a strong photobactericidal activity against Gram-positive as well as Gram-negative bacteria. It was further shown that this conjugate was able to strongly stick to the cell walls of either kind of strain, thus helping to inactivate bacteria through the production of reactive oxygen species under light irradiation.
Keywords: PACT, cationic porphyrin, polymyxin B, targeting, lysine, antimicrobial peptide
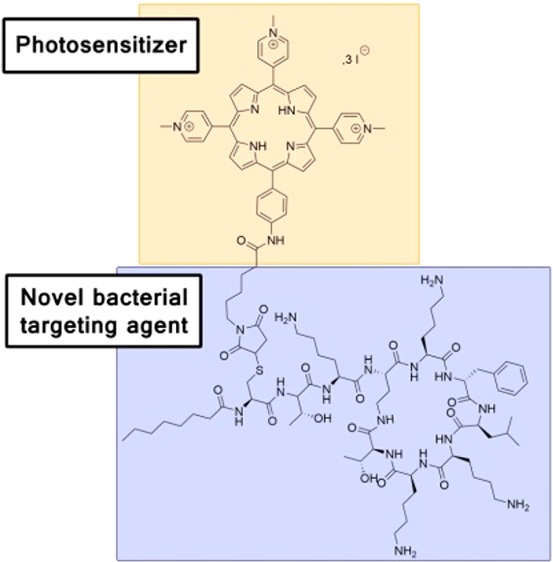
Since the introduction of penicillin in the 1940s, the era of conventional antimicrobial drugs (commonly known as antibiotics) was considered as miraculous, given that these treatments had significantly decreased the death rates from bacterial infections.1 Despite the well-known rise of resistant bacteria, these drugs have been wrongly used all around the world, due in particular to faulty communications and common beliefs.2 In addition to bacterial resistance, leading to the emergence of superbugs or multidrug resistant (MDR) strains, the pace of new antibiotic discoveries has gradually slowed down in recent years.3,4 The issues due to microbial resistance are currently recognized worldwide by health organizations.5 Consequently, alternative strategies to reduce the impact of microbial infections have been investigated. Among them, photodynamic antimicrobial chemotherapy (PACT) appeared as a promising alternative since, contrary to antibiotherapy, it did not induce bacterial resistance.6 This technique relies on the light activation of photosensitizers (PS), which, in the presence of dioxygen (3O2) and under appropriate irradiation, leads to a local production of cytotoxic reactive oxygen species (ROS) like singlet oxygen (1O2), hydrogen peroxide, or oxygen-centered radicals. However, besides its advantages, the major drawback of PACT lies in its lack of specificity since photoinduced ROS may be deleterious to bacteria and infected tissues as well.7 Thus, in order to take advantage of the promising potential of PACT, many researchers have successively improved this alternative to antibiotics by using chemistry8−10 or delivery methods.11,12 In a previous work, a conjugate consisting of a cationic porphyrin (efficient PACT-tested PS13) covalently bound to a polymyxin derivative (antimicrobial peptide,141, Figure 1A) has been developed (5, Figure 1B).15 In addition to a selective interaction with bacteria, this conjugate showed a better photobactericidal activity than the tetra(N-methylpyridyl)porphyrin alone (TMPyP, Figure 1-C). The polymyxin derivative, on which this conjugate was based, retained most of the antibiotic activity of genuine polymyxin, ensured by binding to the lipid A of the Gram-negative bacteria cell wall.16,17 Such an active conjugate might ease a transition to PACT through a light-enhanced antibacterial activity. Despite the promising results obtained with this conjugate, we investigated an approach aimed at decreasing the antibacterial activity of the peptidic moiety while preserving its target specificity. The rationale behind this modification was to reduce the potential rise of microbial resistance against polymyxin B. Even if a peptidic modification would raise some uncertainties about targeting, such an efficient photosensitizer devoid of resistance-inducing properties would provide a considerable opportunity for PACT. To reach this objective, a new polymyxin derivative was designed by switching all the remaining dab residues with lysine (expect residue #4, the dab residue involved in the cycle formation) (1Lys, Figure 1D). Such a compound may offer a good interaction but a reduced biological activity.18 Based on a strategy described in a previous communication,15 we report here the synthesis, characterization, and photobactericidal properties of a conjugate made of a cationic porphyrin covalently bound to a lysine analogue of polymyxin B (5Lys) (Figure 1-E).
Figure 1.
Structures of (A) initial polymyxin derivative (1); (B) the previous cationic porphyrin–polymyxin B conjugate (5); (C) tetrakis(N-methylpyridyl)porphyrin (TMPyP); (D) Lysine analogue of polymyxin (1Lys); and (E) studied conjugate based on the lysine analogue (5Lys).
The impact of lysine residue in polymyxin has been only studied with nonapeptides,19 whereas 5Lys is more related to the initial structure of polymyxins. Nevertheless, Liao and co-workers have shown that the structural fold of the lysine analogue shares similarities with original PMB.20 However, all these studies have already underscored the sufficient interaction between Gram-negative bacteria and lysine-inspired peptides.21 The synthesis of this conjugate was simply achieved by following a protocol already described in a previous communication (Figure S1).15 The amino acid switching did not bring any additional difficulty and the final compound was clearly characterized by HRMS and NMR (Figures S2–S6). As expected, this conjugate 5Lys displayed photophysical properties similar to previously synthesized compound 5 (Figures S7–S8, Table S1), indicating that any potential difference between their biological activities must be associated with the peptidic modification.
Bactericidal properties of compounds 1Lys and 5Lys have been tested against three bacterial strains (E. coli, P. aeruginosa, and S. aureus) in the dark and in the presence of light, and compared with the properties of the parent compounds 1 and 5 (Table 1).15 Compound 1Lys, in which four dab residues of compound 1 were switched for lysine, displayed a strongly reduced activity against Gram-negative bacteria. A MBC of 50 μM was recorded with compound 1Lys against E. coli, vs 5 μM with compound 1; the same shift was observed with compound 5Lys in comparison with compound 5, with MBC equal to 25 and 1.2–5 μM, respectively, against the same strain in the dark. Compounds 1Lys and 5Lys were completely ineffective in the dark against P. aeruginosa (even at 100 μM), while the parent compounds 1 and 5 showed MBC of 10 μM in the same conditions. We found that, in the presence of light, the bactericidal activity of compound 5Lys was restored to the level found with compound 5, with MBC of 0.8 vs 0.5 μM, respectively, against E. coli. A similar effect was recorded against P. aeruginosa, although with a higher value of MBC, 8 vs 2.5 μM. We showed in the previous article that compound 5 was endowed with photobactericidal properties against S. aureus.15 The present results show that the Dab/Lys switches had little influence on MBC, 1.2 vs 0.8 μM for compounds 5Lys and 5, respectively, against this Gram-positive strain. Since the enhanced activity of compound 5 against this strain was based on its strong positive charge, this result seems to be consistent as 5Lys has an equal charge but longer side chains. Table 1 also shows that compound 5Lys was more active than the cationic porphyrin TMPyP alone against the three bacteria studied, which suggests a synergic effect between the two moieties despite the weak activity of the Lys analogue of polymyxin.
Table 1. MBCs (μM) against S. aureus, P. aeruginosa, and E. coli under Two Different Conditions at 37 °C; 20 h of White Light Irradiation (4.83 mW/cm2) and in the Darka.
| MBC
(μM) |
||||||
|---|---|---|---|---|---|---|
|
S. aureus |
P.
aeruginosa |
E. coli |
||||
| compd | light | dark | light | dark | light | dark |
| 1(15) | >50 | >50 | 10.0 | 10.0 | 5.0 | 5.0 |
| 5(15) | 0.8 | 2.5 | 10.0 | 0.5 | 1.2–5.0 | |
| 1Lys | >100 | >100 | 50.0 | 50.0 | ||
| TMPyP | 5.0 | 20.0 | 18.0 | |||
| 5Lys | 1.2 | 8.0 | >100 | 0.8 | 25.0 | |
These assays were performed in tryptic soy culture medium, with bacteria in their log phase. The minimal bactericidal concentration corresponds to the concentration of the active compound for which 99.99% of the bacteria have been killed (i.e., 4 log reduction compared to the untreated control). Four replicates were performed for each condition.
These results were confirmed through an additional set of experiments (Figure 2). For these assays, a large amount of bacteria were harvested and directly put in contact with the compounds in PBS. After 30 min of incubation in the different solutions, bacteria were washed twice with PBS, suspended in the same solution, and then irradiated under the same light conditions as before. The bacterial densities and the survival rate were monitored as a function of irradiation time. Whereas compound 5Lys seemed to be slightly less effective than 5 against S. aureus (Figure 2A), the results against Gram-negative bacteria were interesting. Indeed, 5Lys showed an efficiency similar to that of conjugate 5 against E. coli (Figure 2C), but a better efficacy against P. aeruginosa (Figure 2B). Despite these slight discrepancies, 5Lys offers a wide-spectrum of bactericidal effect starting from the first income of light. Based on the results from both experiments, 5 and 5Lys seem to have a close photobactericidal capacity, whereas the activity of 5Lys is significantly weaker than 5 in the dark, in accordance with the main purpose of this study.
Figure 2.
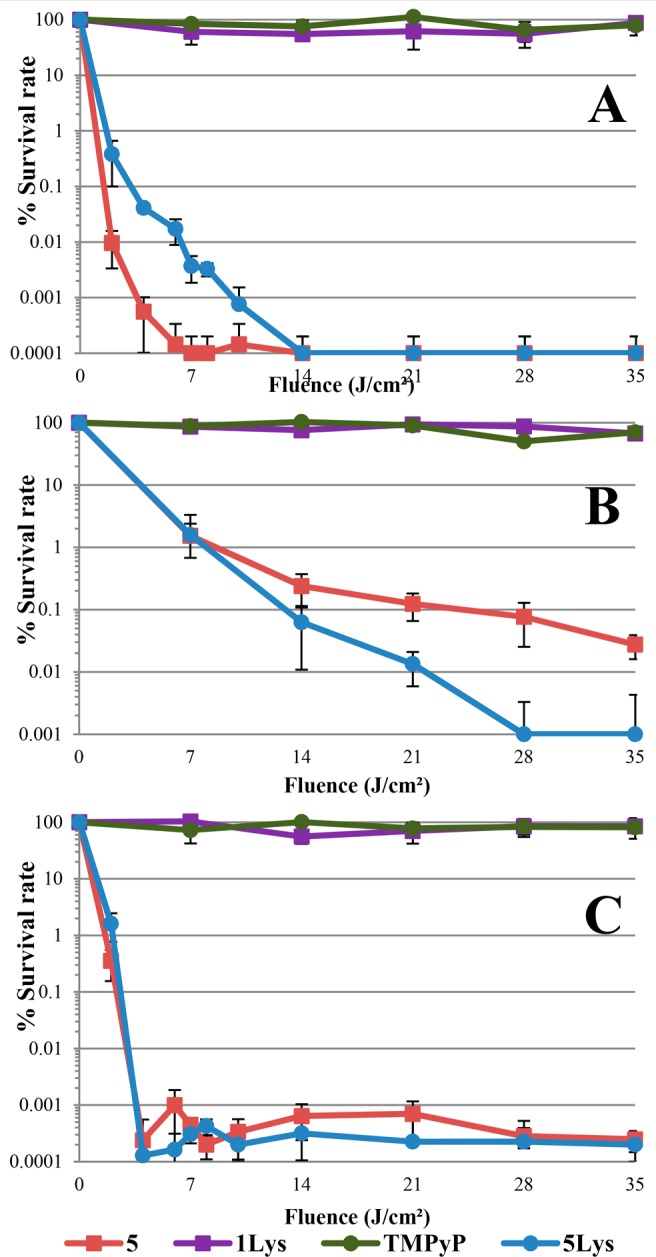
Photodynamic inactivation of S. aureus (A), P. aeruginosa (B), and E. coli (C) in their stationary phase. After 30 min in the different solutions of 5Lys (10 μM for S. aureus and E. coli, 1 μM for P. aeruginosa in PBS 1×), bacteria were washed three times with PBS. Then, they were irradiated by white LED (4.83 mW/cm2, 7 J/cm2 corresponds to 24 min) at 37 °C. Bacterial survival rates (% of initial densities) were plotted against cumulative light fluence at different irradiation times.
Because of the peptidic modification, supplementary biological assays were necessary. Even if the biological assays have proven a synergistic effect of the two different moieties of compound 5Lys, an uncertainty remains about the potential interaction of this compound with bacteria. Then, flow cytometry experiments were tried out in the same experimental condition as before (Figure 3).15 Propidium iodide (PI) was added as a marker of membrane integrity. The analysis with S. aureus has shown an unexpected result. In spite of the good amount of labeled bacteria (Q2 + Q4 = 23.8%), which was already observed with compound 5, the repeated washings did not reduce the percentage of the labeled population. Thus, this result may indicate a strong attachment of this lysine analogue to this strain. Among the numerous studies focused on the structure–activity relationship of polymyxin, some of them have already highlighted an increase of activity against Gram-positive strains after a peptidic modification.22−24 Most of these studies assigned this new efficiency to a decrease of the cationic charge or an increase of the lipophilic trend, which is not the case here. Anyhow, compound 5Lys seems to bind to the Gram-positive bacterium without inducing any bactericidal activity.
Figure 3.

Flow cytometry analyses of bacterial strains after contact with 5Lys. The bacteria were incubated in a solution of 5Lys (1 μM) for 30 min at 37 °C, then washed with saline. PI was added (0.01 mg/mL) in order to appreciate the bacterial viability. Q1 refers to bacteria with permeable membrane (PI positive). Q2 refers to double positive bacteria (PI positive and PS positive). Q3 and Q4 refers to live bacteria (PI negative) of which Q4 are labeled with PS. Based on three independent experiments, the average population repartition is specified for each condition.
The cytogrammes obtained from the experiments with P. aeruginosa show a less well-defined distribution of the populations. Anyway, more than 30% of the bacteria seem to be strongly labeled by 5Lys. Even if it did not show a bactericidal effect against this strain, the peptidic moiety of this compound still has a significant affinity for the bacterial outer membrane. Moreover, this membrane seems to be weakened as a consequent amount of bacteria is PI positive (Q2 + Q4 = 64.5%). This effect is less pronounced than with previous conjugate 5 (80%).15 Despite its lack of bactericidal activity, the lysine-based compound 5Lys showed a capacity to weaken the outer membrane of P. aeruginosa. Thus, this retained ability offers an opportunity for ROS to inactivate the bacteria, in accordance with low MBCs after light irradiation. Similar behaviors were already described for few polymyxin derivatives (such as PMBN19), and they are generally assigned to a lack of fatty acid chain, which leads to a weaker uptake in the outer membrane (OM). Thus, lysine residues may hinder the inclusion of the hydrophobic part of 5Lys into the OM. Anyway, this result was also confirmed by experiments against E. coli, where at least 30% of bacteria were labeled by 5Lys. Thus, in spite of less efficient interaction than with previously synthesized compound 5, conjugate 5Lys still has a good affinity for bacteria and has the ability to weaken the outer membrane, leading to an enhanced photobactericidal activity.
Confocal microscopy analyses were performed to visualize interactions between bacteria and 5Lys (Figure 4). The pictures with bacteria alone in contact with 5Lys revealed a significant fluorescence, which is very similar to that obtained with the previous conjugate 5. As the peptidic modification could lead to a new and unexpected interaction with mammal cells, the selectivity of the new conjugate 5Lys was also investigated using normal dermal human fibroblasts (NDHF) incubated together with bacterial strains. As the cytotoxicity of 5Lys on NDHF was similar to 5 (Table S2), the lysine analogue of polymyxin 5Lys did not seem to have any affinity for NDHF cells since confocal imaging showed no additional fluorescence emission from these cells. Moreover, in compliance with cytometry flow experiments, both bacterial strains incubated with NHDF cells have kept a noticeable fluorescence. Thus, despite a slightly weaker bacterial interaction, the ability of this new conjugate to selectively target bacteria has been retained.
Figure 4.
Confocal laser scanning microscopy imaging of S. aureus (left) and P. aeruginosa (right) after contact with 5Lys (100×). NHDF cells and bacteria were mixed together before treatment with 5Lys (40×). Inocula were suspended into solution of 1 μM for 30 min in 37 °C, then washed three times with PBS.
This additional investigation of a new targeting agent for PACT has highlighted promising interest for the lysine analogue of polymyxin. Indeed, peptide 1Lys and the peptidic moiety of conjugate 5Lys have shown a significant loss of activity in the dark. Nevertheless, affinity of 5Lys for bacteria and its ability to weaken the bacterial membrane has been mostly preserved. Unfortunately, the substantial peptidic modification prevents us from finding a clear interpretation as lipid A targeting is not ensured anymore. Anyhow, by using this peptide through a conjugate with a cationic porphyrin, ROS production led to very low MBCs. An incertitude remains about the establishment of the resistance from the bacteria that are in contact with such compound, as the bacterial inactivation seems to be induced by PACT only. Such conjugate should resolve this question in further studies and should challenge the limits of resistance to PACT.
Experimental Procedures
Based on our previous work, identical materials (including devices, bacterial strains, and mammalian cell lines) and methods have been used in order to provide homogeneous experimental conditions.15
Chemical Synthesis
Synthesis of 5-(4-(Maleimidohexanoamidophenyl)-10,15,20-tri(4-N-methylpyridinium)porphyrin Triiodide (4) and Synthesis of PMB-Lysine Derivative (1Lys)
The used protocols have already been described in the previous communication. From the peptidic preparation, the Fmoc-Dab(Boc)-OH has been switched with Fmoc-Lys(Boc)-OH during the sequential peptide formation. Likewise, Fmoc-Lys-O-allyl was synthesized using similar conditions as those used for the synthesis of Fmoc-Dab-O-allyl. HRMS (ESI+) [C62H109N15O13S]: [M + 2H]2+ calcd 652.9103, found 652.9088.
Synthesis of the Final Conjugate Porphyrin-PMB (5Lys)
Compound 4 (41.6 mg, 33.2 μmol, 1 equiv) and 1Lys (43.3 mg, 33.2 μmol, 1 equiv) were dissolved in a phosphate buffer solution (0.5 M) at pH 6.5. The mixture was gently stirred overnight at room temperature. The crude was directly purified by reverse phase chromatography. The freeze-dried final product 5Lys was obtained as a brown powder with 69% yield (58.5 mg, 22.9 μmol). UV–visible (MeOH), λmax (nm) (ε log L·mol–1·cm–1): 427 (5.31), 519 (4.14), 556 (3.86), 594 (3.73), 650 (3.36). HRMS (ESI+) [C116H157I3N24O16S]: [M]3+ calcd 724.7305, found 724.7334.
Acknowledgments
The authors thank the Conseil Régional du Limousin for financial support (FLG) and are indebted to Dr. Michel Guilloton for help in manuscript editing and Claire Carrion (BISCEm – University of Limoges) and Dr. Cyril Colas (ICOA-University of Orleans), respectively, for confocal microscopy imaging and ESI and HRMS analyses.
Glossary
ABBREVIATIONS
- MDR
multidrug resistant
- MBC
minimal bactericidal concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NHDF
normal human dermal fibroblast
- ROS
reactive oxygen species
- PACT
photodynamic antimicrobial chemotherapy
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PMB
polymyxin B
- PS
photosensitizer
- TPP
meso-tetrakis(phenyl)porphyrin
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00360.
Synthetic route, MS and NMR analyses, photophysical properties, confocal laser scanning microscopy imaging of NHDF (alone or with bacteria), and cytotoxicity of 5Lys on NHDF (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Prescott L. M.; Willey J.; Sherwood L.; Woolverton C. J.. Microbiologie, 4th ed.; De Boeck, 2013. [Google Scholar]
- Cabral C.; Ingram J.; Lucas P. J.; Redmond N. M.; Kai J.; Hay A. D.; Horwood J. Influence of Clinical Communication on Parents’ Antibiotic Expectations for Children with Respiratory Tract Infections. Ann. Fam. Med. 2016, 14 (2), 141–147. 10.1370/afm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C. L. The Antibiotic Resistance Crisis. Pharm. Ther. 2015, 40 (4), 277–283. [PMC free article] [PubMed] [Google Scholar]
- Wainwright M.; Maisch T.; Nonell S.; Plaetzer K.; Almeida A.; Tegos G. P.; Hamblin M. R. Photoantimicrobials—are We Afraid of the Light?. Lancet Infect. Dis. 2017, 17 (2), e49–e55. 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- Tavares A.; Carvalho C. M. B.; Faustino M. A.; Neves M. G. P. M. S.; Tomé J. P. C.; Tomé A. C.; Cavaleiro J. A. S.; Cunha Â.; Gomes N. C. M.; Alves E.; Almeida A. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8 (1), 91–105. 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J.; Gomer C. J.; Henderson B. W.; Jori G.; Kessel D.; Korbelik M.; Moan J.; Peng Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90 (12), 889–905. 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosselli R.; Ruiz-González R.; Moret F.; Agnolon V.; Compagnin C.; Mognato M.; Sella V.; Agut M.; Nonell S.; Gobbo M.; Reddi E. Synthesis, Spectroscopic, and Photophysical Characterization and Photosensitizing Activity toward Prokaryotic and Eukaryotic Cells of Porphyrin-Magainin and -Buforin Conjugates. J. Med. Chem. 2014, 57 (4), 1403–1415. 10.1021/jm401653r. [DOI] [PubMed] [Google Scholar]
- Liu F.; Soh Yan Ni A.; Lim Y.; Mohanram H.; Bhattacharjya S.; Xing B. Lipopolysaccharide Neutralizing Peptide–Porphyrin Conjugates for Effective Photoinactivation and Intracellular Imaging of Gram-Negative Bacteria Strains. Bioconjugate Chem. 2012, 23 (8), 1639–1647. 10.1021/bc300203d. [DOI] [PubMed] [Google Scholar]
- Johnson G. A.; Ellis E. A.; Kim H.; Muthukrishnan N.; Snavely T.; Pellois J.-P. Photoinduced Membrane Damage of E. Coli and S. Aureus by the Photosensitizer-Antimicrobial Peptide Conjugate Eosin-(KLAKLAK) 2. PLoS One 2014, 9 (3), e91220. 10.1371/journal.pone.0091220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B. L.; Feese E.; Sadeghifar H.; Argyropoulos D. S.; Ghiladi R. A. orphyrin-Cellulose Nanocrystals: A Photobactericidal Material That Exhibits Broad Spectrum Antimicrobial Activity. Photochem. Photobiol. 2012, 88 (3), 527–536. 10.1111/j.1751-1097.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- Anaya-Plaza E.; van de Winckel E.; Mikkilä J.; Malho J.-M.; Ikkala O.; Gulías O.; Bresolí-Obach R.; Agut M.; Nonell S.; Torres T.; Kostiainen M. A.; de la Escosura A. Photoantimicrobial Biohybrids by Supramolecular Immobilization of Cationic Phthalocyanines onto Cellulose Nanocrystals. Chem. - Eur. J. 2017, 23, 4320–4326. 10.1002/chem.201605285. [DOI] [PubMed] [Google Scholar]
- Yin R.; Hamblin M. R. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22 (18), 2159–2185. 10.2174/0929867322666150319120134. [DOI] [PubMed] [Google Scholar]
- Velkov T.; Roberts K. D.; Thompson P. E.; Li J. Polymyxins: A New Hope in Combating Gram-Negative Superbugs?. Future Med. Chem. 2016, 8 (10), 1017–1025. 10.4155/fmc-2016-0091. [DOI] [PubMed] [Google Scholar]
- Le Guern F.; Sol V.; Ouk C.; Arnoux P.; Frochot C.; Ouk T.-S. Enhanced Photobactericidal and Targeting Properties of a Cationic Porphyrin Following Attachment of Polymyxin B. Bioconjugate Chem. 2017, 28, 2493. 10.1021/acs.bioconjchem.7b00516. [DOI] [PubMed] [Google Scholar]
- Zavascki A. P.; Goldani L. Z.; Li J.; Nation R. L. Polymyxin B for the Treatment of Multidrug-Resistant Pathogens: A Critical Review. J. Antimicrob. Chemother. 2007, 60 (6), 1206–1215. 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- Caruso E.; Banfi S.; Barbieri P.; Leva B.; Orlandi V. T. Synthesis and Antibacterial Activity of Novel Cationic BODIPY Photosensitizers. J. Photochem. Photobiol., B 2012, 114, 44–51. 10.1016/j.jphotobiol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Velkov T.; Thompson P. E.; Nation R. L.; Li J. Structure–Activity Relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53 (5), 1898–1916. 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubery H.; Ofek I.; Cohen S.; Fridkin M. Structure-Function Studies of Polymyxin B Nonapeptide: Implications to Sensitization of Gram-Negative Bacteria. J. Med. Chem. 2000, 43 (16), 3085–3092. 10.1021/jm0000057. [DOI] [PubMed] [Google Scholar]
- Liao S.-Y.; Ong G.-T.; Wang K.-T.; Wu S.-H. Conformation of Polymyxin B Analogs in DMSO from NMR Spectra and Molecular Modeling. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1995, 1252 (2), 312–320. 10.1016/0167-4838(95)00127-G. [DOI] [PubMed] [Google Scholar]
- Thomas C. J.; Surolia A. Kinetics of the Interaction of Endotoxin with Polymyxin B and Its Analogs: A Surface Plasmon Resonance Analysis. FEBS Lett. 1999, 445 (2–3), 420–424. 10.1016/S0014-5793(99)00150-7. [DOI] [PubMed] [Google Scholar]
- Chihara S.; Ito A.; Yahata M.; Tobita T.; Koyama Y. Chemical Synthesis, Isolation and Characterization of α-N-Fattyacyl Colistin Nonapeptide with Special Reference to the Correlation between Antimicrobial Activity and Carbon Number of Fattyacyl Moiety. Agric. Biol. Chem. 1974, 38 (3), 521–529. 10.1080/00021369.1974.10861184. [DOI] [Google Scholar]
- Witzke N. M.; Heding H. Broad-Spectrum Derivatives of Polymyxin B and Colistin. J. Antibiot. 1976, 29 (12), 1349–1350. 10.7164/antibiotics.29.1349. [DOI] [PubMed] [Google Scholar]
- Weinstein J.; Afonso A.; Moss E.; Miller G. H. Selective Chemical Modifications of Polymyxin B. Bioorg. Med. Chem. Lett. 1998, 8 (23), 3391–3396. 10.1016/S0960-894X(98)00612-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




