Abstract
The rapid increase in bacterial resistance to antibiotics is a global healthcare crisis. Non-antibiotic pharmaceuticals that have attained approval by the United States Food and Drug Administration have the potential to be repurposed as bacterial resistance-modifying agents and therefore could become valuable resources in our battle against antibiotic-resistant microbes. Amoxapine is a tetracyclic antidepressant used in the treatment of major depressive disorder. Here we demonstrate the ability of amoxapine to resensitize methicillin-resistant Staphylococcus aureus strain ATCC 43300 to oxacillin in both agar diffusion and broth microdilution assays. Amoxapine also reduced the bacterial cleavage of nitrocefin in a dose-dependent manner, suggesting that it may exert its adjuvant effects through reduction of beta-lactamase activity.
Keyword: Microbiology
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is responsible for an increasingly large number of infection-related illnesses and deaths each year. In 2015, MRSA was responsible for over 11,000 deaths in the United States alone [1]. The development of antibiotic resistance by bacteria is an inevitable consequence of antibiotic use and the process of natural selection, however the spread of resistance is likely accelerated by the frequent overuse and misuse of medically valuable antibiotic compounds [2, 3]. While the prevalence of antibiotic resistance in S. aureus and other pathogenic bacteria is growing, efforts to discover and develop novel antibiotic compounds remains a lengthy, laborious, and costly endeavor [4]. This combination of scientific, economic, and regulatory challenges has resulted in a dramatic reduction in the number of antibiotic compounds approved by the United States Food and Drug Administration (FDA) over the last 3 decades, causing the World Health Organization and others to warn of an impending post-antibiotic era [5, 6, 7].
Rather than developing novel antibiotic compounds that will inevitably be rendered ineffective by the rise of bacterial resistance, some researchers and pharmaceutical companies have explored alternative strategies to combat antibiotic-resistant microbes. One strategy is to develop formulations that include adjuvant compounds, which increase the efficacy of existing antibiotics [8, 9, 10, 11]. Each of the thousands of FDA-approved pharmaceutical compounds has the potential to act as a resistance-modifying agent, and yet most remain underutilized resources for adjuvant molecule exploration. The existing compounds of the FDA-approved pharmacopeia also have well-established toxicity profiles and are relatively inexpensive compared to de novo drug development. The exploration of existing drugs for repurposing as antibiotic adjuvants has the potential to provide an additional line of defense in the antibiotic resistance crisis and ultimately could save countless lives [12, 13].
Amoxapine (Fig. 1A) is a tricyclic antidepressant of the dibenzoxazepine class that was developed in the early 1970s and marketed under the brand names of Asendin, Asendis, Defanyl, and Demolox [14]. It continues to be prescribed to treat the symptoms of depression. Here we demonstrate the potential for amoxapine to act as a resistance-modifying agent for MRSA strain ATCC 43300. At sublethal concentrations, amoxapine potentiates the activity of oxacillin against S. aureus in both agar diffusion and broth dilution assays. We also demonstrate that amoxapine reduces the cleavage of nitrocefin, a chromogenic beta-lactam compound, by MRSA strain ATCC 43300.
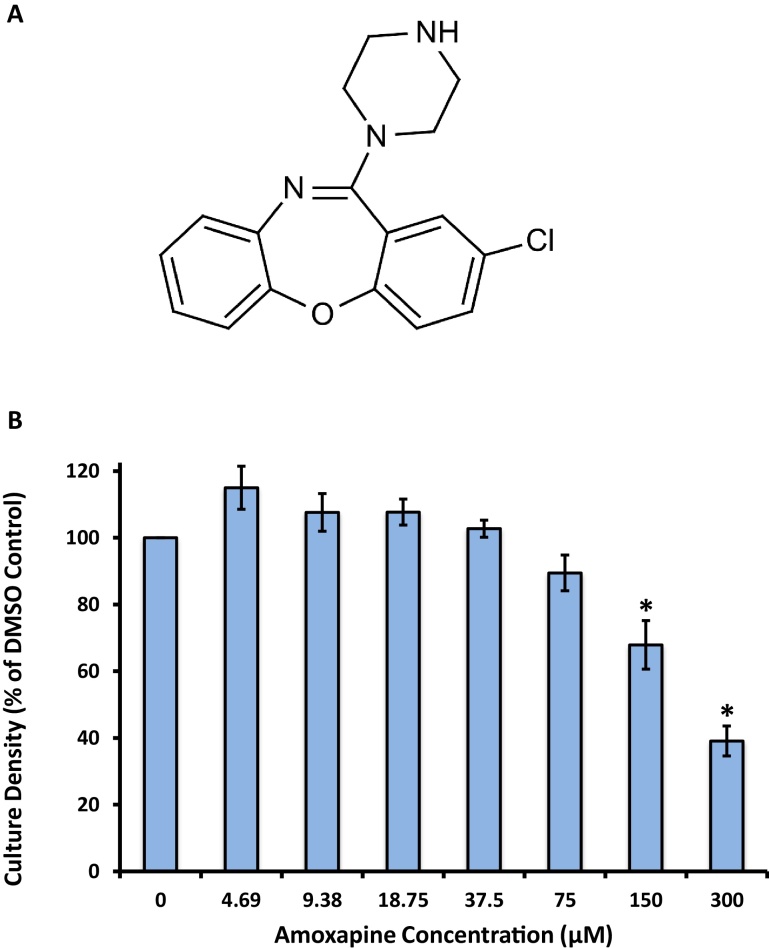
Fig. 1.
Growth of S. aureus is not completely inhibited by amoxapine. (A) Chemical structure of amoxapine, CAS 14028-44-5. (B) Optical densities (OD600) of 3 mL S. aureus cultures after 16–18 h of incubation at 37 °C with shaking at 180 rpm in the presence of amoxapine. Culture densities are expressed as a percentage of the DMSO control. The mean of 4 technical replicates collected from 2 independent experiments is shown. Error bars represent SEM. * indicates p < 0.05 vs DMSO control.
2. Materials and methods
2.1. Bacterial strains and media
Bacterial strains used in the study were methicillin-sensitive S. aureus (MSSA) strain ATCC 29213 and MRSA strain ATCC 43300 (ATCC, Manassas, VA, USA). Bacteria were cultured on Muller Hinton II broth (MHB) or agar (MHA) at 37 °C (Becton Dickson and Company, Sparks, MD, USA).
2.2. The effect of amoxapine on the growth of S. aureus
Amoxapine, CAS number 14028-44-5 (TCI, Tokyo, Japan) stocks were prepared at 30 mM in DMSO. In an effort to limit the effect of DMSO on bacterial growth, the final solvent concentration was maintained at 1% (v/v) for all experimental and control cultures. An overnight culture of MSSA was diluted to 2 × 106 CFU/mL in 3 mL cultures of MHB containing 1:2 serially diluted concentrations of amoxapine ranging from 300 μM to 0.59 μM or 1% DMSO without amoxapine. Each experimental condition was prepared in duplicate. Following 16–18 h of incubation at 37 °C with continuous shaking at 180 RPM, the turbidity of each culture was assessed by measuring OD600 and expressed as a percentage of the solvent control.
2.3. Agar diffusion assay with amoxapine-infused plates
Following autoclave sterilization, flasks of MHA were allowed to cool to approximately 60 °C. Either DMSO or amoxapine was aseptically added to the liquid, mixed on a magnetic stir plate to ensure even distribution, and poured into 100 mm plastic petri dishes. Control plates contained 1% (v/v) DMSO and amoxapine-infused plates contained 300 μM amoxapine and 1% (v/v) DMSO. Overnight cultures of MSSA and MRSA were diluted to approximately 5 × 108 CFU/mL in fresh MHB and plated as a continuous lawn using sterile polyester-tipped applicators. Following inoculation, oxacillin-impregnated (1 μg, OX 1) Sensi-Disc (Becton Dickson and Company, Franklin Lakes, NJ, USA) were applied to the agar surface with gentle pressure. Following 16–18 h of incubation at 37 °C, the diameter of the zones of inhibited bacterial growth were measured using digital calipers.
2.4. Broth microdilution assay
Oxacillin (Acros Organics, Thermo Fisher Scientific, Waltham, MA, USA) minimum inhibitory concentrations (MIC) were determined in the presence and absence of amoxapine using the broth microdilution assay described by the Clinical and Laboratory Standards Institute [15]. Briefly, a 1:2 dilution series of oxacillin was prepared in 4 concentrations of amoxapine. Final concentrations of amoxapine were 0 μM, 75 μM, 150 μM, and 300 μM in 1% DMSO (v/v). Lastly, overnight cultures of MSSA and MRSA were diluted in MHB and inoculated to a final concentration of 5 × 104 CFU/well. The total volume in each microtiter well was 100 μL. MICs were determined by visual inspection following 16–18 h of static incubation at 37 °C. Using unaided eye observation, we determined the MIC to be the lowest concentration of oxacillin that completely inhibited bacterial growth [15]. Each experimental condition was prepared in duplicate.
2.5. Nitrocefin cleavage assay
In order to increase the bacterial production of beta-lactamase prior to our assay, a culture of MRSA, freshly diluted to 2 × 108 CFU/mL, was incubated for 30 minutes at 37 °C with shaking at 180 RPM in the presence of 10 μg/mL oxacillin. The cells were washed 3 times by low speed centrifugation and resuspension in 0.1 M potassium phosphate buffer, pH 7.0 to remove the oxacillin. The concentration of washed cell suspensions was adjusted to provide 1 × 107 CFU/well. The final reaction volume of 100 μL contained 1 × 107 CFU/well MRSA, 50 μg/mL nitrocefin (Oxoid, Thermo Fisher Scientific, Waltham, MA, USA), 0 μM, 75 μM, 150 μM, or 300 μM amoxapine, and 1% DMSO (v/v). Five technical replications of each experimental condition were included on each microtiter plate. Cleavage of nitrocefin was monitored in a Synergy H1 microplate reader (BioTek Instruments, Winooski, VT, USA) by measuring OD486 for 2 h at 37 °C with 5 s of shaking prior to each absorbance reading. Starting absorbance values were subtracted from the 2 h absorbance values to derive ΔOD486.
2.6. Statistics
Two-tailed Student’s t-test analyses between control and treatments were performed using Microsoft Excel. We judged statistical significance to be p < 0.05. Error bars represent standard error of the mean.
3. Results
3.1. Amoxapine treatment does not completely inhibit the growth of S. aureus
In order to determine the effect of amoxapine treatment on the growth of MSSA in broth culture, we conducted a series of 1:2 serial dilutions of amoxapine in a final concentration of 1% DMSO. Following 16–18 h of growth, both the 150 μM and 300 μM conditions exhibited statistically significant reductions in final culture density (OD600) compared to the DMSO control (Fig. 1B). Importantly, while 300 μM amoxapine did cause a 60% reduction in the growth of S. aureus in broth culture, it did not completely inhibit bacterial growth, and thus, we did not determine the minimum inhibitory concentration for amoxapine in this study.
3.2. Amoxapine increases the sensitivity of S. aureus to oxacillin in an agar diffusion assay
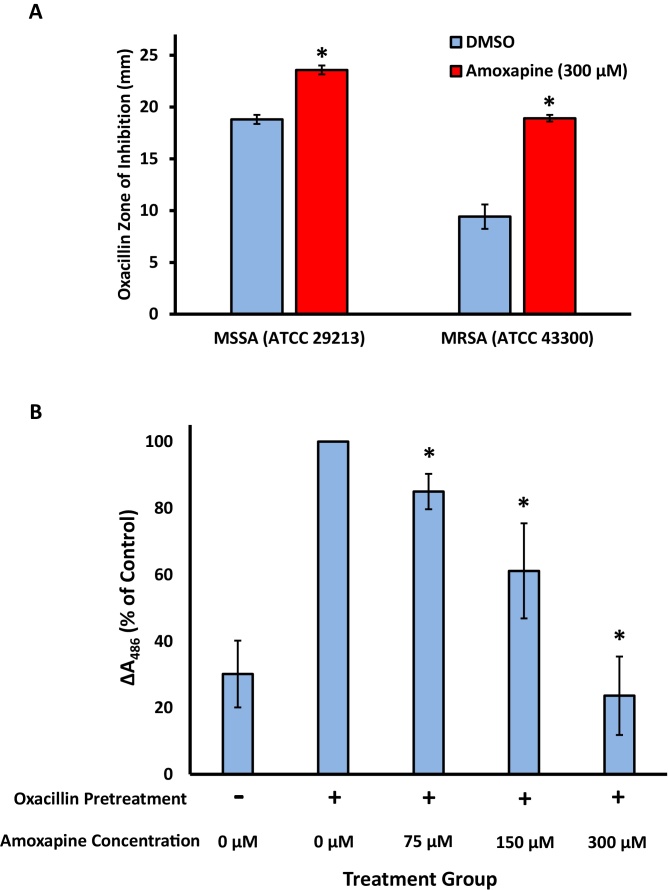
We used a modified disc-diffusion assay to explore the potential of amoxapine to sensitize S. aureus to the beta-lactam antibiotic oxacillin. As our amoxapine titration experiment failed to establish a concentration that completely inhibited bacterial growth, we incorporated amoxapine into MHA plates at a final concentration of 300 μM, the highest concentration tested in our study. For both MSSA and MRSA, the oxacillin zone of inhibition was significantly larger on the amoxapine-containing plates compared to the DMSO-containing control plates (Fig. 2A). Relative to MSSA, the effect of amoxapine was more robust in MRSA, more than doubling the average zone of inhibition from 9.4 mm to 18.9 mm.
Fig. 2.
Effects of amoxapine on oxacillin disc-diffusion sensitivity and nitrocefin cleavage. (A) Zones of inhibited methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) growth by oxacillin. MHII agar plates contained either 1% DMSO (blue bars) or 300 μM amoxapine (red bars). For MSSA, the mean of 3 technical replicates collected from a single experiments is shown. For MRSA, the mean of 5 technical replicates collected from 2 independent experiments is shown. (B) Following a pretreatment with oxacillin to increase β-lactamase production, washed cultures of MRSA were incubated at 37 °C in the presence of amoxapine and nitrocefin, a chromogenic β-lactamase substrate in a 96-well microtiter plate. Absorbance of the cultures at 486 nm was monitored for a period of 2 h. The change in absorbance at 486 nm is expressed as a percentage of the oxacillin-induced DMSO control. The mean of 4 independent biological replicates, each performed with 5 technical replicates, is shown. Error bars represent SEM. * indicates p < 0.05 vs DMSO control.
3.3. Amoxapine increases the sensitivity of MRSA to oxacillin in a microdilution assay
To better quantify the impact of amoxapine on the sensitivity of MSSA and MRSA to oxacillin, we conducted static microdilution assays in 96-well microtiter plates. We determined the MIC of oxacillin in 3 concentrations of amoxapine (Table 1). In the absence of amoxapine, MRSA is highly resistant to oxacillin, with an MIC of 16 μg/mL. Increasing amounts of amoxapine resulted in a dose-dependent resensitization of MRSA to oxacillin. The presence of 150 μM amoxapine reduced the oxacillin MIC 8–16 fold from the DMSO control to 1–2 μg/mL, and 300 μM amoxapine reduced the oxacillin MIC 128 fold to 0.125 μg/mL. In contrast to the MRSA, treatment of MSSA did not result in a robust reduction of oxacillin MIC values (Table 1). The presence of 300 μM amoxapine reduced the oxacillin MIC of MSSA 2 fold from the DMSO control, but the 150 μM and 75 μM amoxapine treatments had no effect on oxacillin MIC.
Table 1.
Minimum inhibitory concentrations of oxacillin against methicillin-sensitive and methicillin-resistant S. aureus in the presence of amoxapine. Data represent the results of 3 independent experiments performed in duplicate. An MIC range is presented for conditions in which results varied.
| Amoxapine Concentration | MSSA (ATCC 29213) | MRSA (ATCC 43300) |
|---|---|---|
| Oxacillin MIC (μg/ml) | Oxacillin MIC (μg/ml) | |
| 0 μМ | 0.25 | 16 |
| 75 μМ | 0.25 | 4–8 |
| 150 μМ | 0.25 | 1–2 |
| 300 μМ | 0.125 | 0.125 |
Data represent the results of 3 independent experiments performed in duplicate. An MIC range is presented for conditions in which results varied.
3.4. Amoxapine reduces the cleavage of nitrocefin by MRSA
One common mechanism of microbial resistance to beta-lactam antibiotics is mediated through bacterial synthesis and secretion of beta-lactamase enzymes into the extracellular environment. Thus, we sought to investigate the potential contribution of beta-lactamase inhibition to our observed amoxapine-mediated increase in MRSA oxacillin sensitivity. We measured extracellular beta-lactamase activity by monitoring the cleavage of nitrocefin, a chromogenic cephalosporin that serves as an enzymatic substrate. Nitrocefin contains a beta-lactam ring that is susceptible to hydrolysis by beta-lactamase enzymes. This cleavage causes a shift in the light absorbance properties of nitrocefin, allowing the detection of beta-lactamase activity by monitoring the change in absorbance at 486 nm.
Prior to amoxapine exposure, we induced elevated expression of beta-lactamase enzymes by treating MRSA cultures with oxacillin for 30 min. Pretreatment with oxacillin approximately tripled the amount of extracellular beta-lactamase activity (Fig. 2B, column 1 and 2). Following the removal of oxacillin by 3 rounds of centrifugation and resuspension in phosphate buffer, amoxapine and nitrocefin were added to the cultures simultaneously. The presence of amoxapine caused a statistically significant, dose-dependent reduction in the cleavage of nitrocefin compared to the DMSO control over 2 h of incubation (Fig. 2B).
4. Discussion
Amoxapine causes a reduction in the growth of S. aureus at concentrations greater than or equal to 150 μM (Fig. 1B), however it does not completely inhibit growth at 300 μM, the highest concentration tested our studies (Fig. 1, Fig. 2 and Table 1). While amoxapine did not completely inhibit bacterial growth, the antidepressant compound did exhibit the ability to suppress oxacillin resistance in both agar diffusion and broth microdilution assays (Table 1, Fig. 2A). The ability of 300 μM amoxapine to reduce MRSA sensitivity to oxacillin by 128 fold to 0.125 μg/mL is particularly noteworthy, as the amoxapine-sensitized MIC falls well below the 2 μg/mL oxacillin MIC breakpoint for MSSA [16].
The mid-micromolar concentrations of amoxapine (75–300 μM) required to confer adjuvant activity in our studies are much higher than the peak serum/plasma concentrations in human subjects, which are in the nanomolar range [17, 18, 19]. Additionally, these concentrations of amoxapine are elevated compared to the effective concentrations of previously described compounds that increase the efficacy of β-lactam antibiotics in vitro; approximately 10–20 μM of clavulanic acid, sulbactam, or tazobactam is sufficient to potentiate the action of amoxicillin against S. aureus [20].
Our use of mid-micromolar concentrations of amoxapine increase the possibility of non-specific bacterial sensitization. However, two lines of evidence suggest that amoxapine’s resensitization of MRSA functions through a resistance-related mechanism. Firstly, the adjuvant effect of amoxapine on MRSA was far more robust than its effects on MSSA in both the agar diffusion and microdilution assays (Table 1, Fig. 2A). Secondly, the nitrocefin cleavage assay demonstrates that amoxapine is exerting resistance modification activity, in part, by interfering with some aspect of the bacterial beta-lactamase system. Amoxapine clearly reduced the cleavage of nitrocefin, a cephalosporin compound containing a beta-lactam ring, in a dose-dependent manner (Fig. 2B) and thus, may be acting as a direct inhibitor of beta-lactamase, as is the case with previously described adjuvant compounds: clavulanic acid, sulbactam, and tazobactam [8]. However, due to our measurement of nitrocefin cleavage by intact MRSA rather than cell lysate or purified beta-lactamase enzyme, we did not determine the specific mechanism by which amoxapine inhibits nitrocefin cleavage. Therefore, we cannot rule out inhibition of beta-lactamase synthesis or export as potential amoxapine modes of action.
Finally, this study included a single strain of MRSA (ATCC 43300) and therefore may not be applicable to all MRSA strains. The future exploration of additional MRSA strains with diverse mechanisms of resistance would provide valuable insights into the mechanism of amoxapine-mediated resensitization.
5. Conclusion
Despite an undefined mode of action, amoxapine exhibits promise as a resistance-modifying agent for MRSA and warrants further exploration in other strains of antibiotic-resistant bacteria. Further exploration of tetracyclic antidepressants could also yield exciting molecules with adjuvant activity.
Declarations
Author contribution statement
Tyler J. Wilson: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Meghan S. Blackledge: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Patrick A. Vigueira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by High Point University funds.
Additional information
No additional information is available for this paper.
Acknowledgments
We thank Jordan Smith for his willingness to share his wisdom.
References
- 1.Gross M. Antibiotics in crisis. Curr. Biol. 2013;23:R1063–R1065. doi: 10.1016/j.cub.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 2.Costelloe C., Metcalfe C., Lovering A., Mant D., Hay A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. Br. Med. J. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 3.Clatworthy A.E., Pierson E., Hung D.T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 4.Quadri F., Mazer-amirshahi M., Fox E.R., Hawley K.L., Pines J.M., Zocchi M.S., May L. Antibacterial drug shortages from 2001 to 2013: implications for clinical practice. Clin. Infect. Dis. 2015;60:1737–1742. doi: 10.1093/cid/civ201. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B. The future of antibiotics. Crit. Care. 2014;18 doi: 10.1186/cc13948. 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes P. The global challenge of new classes of antibacterial agents: an industry perspective. Curr. Opin. Pharmacol. 2015;24:7–11. doi: 10.1016/j.coph.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Luepke K.H., Suda K.J., Boucher H., Russo R.L., Bonney M.W., Hunt T.D., Mohr J.F. Past, present, and future of antibacterial economics: increasing bacterial resistance. Limited antibiotic pipeline, and societal implications. Pharmacotherapy. 2017;37:71–84. doi: 10.1002/phar.1868. [DOI] [PubMed] [Google Scholar]
- 8.Drawz S.M., Bonomo R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Cleaver L., Wang W., Podoll J.D., Walls S., Jolly A., Wang X. Tetracyclic indolines as a novel class of β-lactam-selective resistance-modifying agent for MRSA. Eur. J. Med. Chem. 2017;125:130–142. doi: 10.1016/j.ejmech.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worthington R.J., Melander C. Overcoming resistance to β-lactam antibiotics. J. Org. Chem. 2014;78:4207–4213. doi: 10.1021/jo400236f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris T.L., Worthington R.J., Melander C. Potent small-molecule suppression of oxacillin resistance in methicillin-resistant Staphylococcus aureus. Angew. Chem. Int. Ed. 2012;51:11254–11257. doi: 10.1002/anie.201206911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 13.Chong C.R., Sullivan D.J., Jr. New uses for old drugs. Nature. 2007;448:645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry I.B., Husain N., Khan S., Badshah S., Deakin B., Kapur S. Amoxapine as an antipsychotic: comparative study versus haloperidol. J. Clin. Psychopharmacol. 2007;27:575–581. doi: 10.1097/jcp.0b013e31815a4424. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . 9th edn. Wayne; Pennsylvania: 2012. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, CLSI document M07-A9. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute . Wayne; Pennsylvania: 2014. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement, CLSI document M100-S24. [Google Scholar]
- 17.Calvo B., Garcia M.J., Pedraz J.L., Marino E.L., Dominguez-Gil A. Pharmacokinetics of amoxapine and its active metabolites. Int. J. Clin. Pharmacol. Ther. Toxicol. 1985;23(4):180–185. [PubMed] [Google Scholar]
- 18.Kobayashi A., Sugita S., Nakazawa K. Determination of amoxapine and its metabolites in human serum by high-performance liquid chromatography. Neuropharmacology. 1985;24(12):1253–1256. doi: 10.1016/0028-3908(85)90162-5. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi H., Yokota S., Shimada S., Ohtani Y., Miura S., Kubo H. Pharmacokinetics of amoxapine and its active metabolites in healthy subjects. Curr. Ther. Res. 1993;53(4):427–434. [Google Scholar]
- 20.Paukner S., Hesse L., Preželj A., Šolmajer T., Urleb U. In vitro activity of LK-157, a novel tricyclic carbapenem as broad-spectrum β-lactamase inhibitor. Antimicrob. Agents Chemother. 2009;53:505–511. doi: 10.1128/AAC.00085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]