Abstract
The aim of this study was to formulate a biodegradable implant capable of imparting local anti-tumor activity through the sustained release of the chemotherapeutic agent, 5-fluorouracil (5-FU). Thus injectable pellets (< 1.2 mm diameter) made from poly(lactide co-glycolide (PLGA) and loaded with 5-FU at varying drug:polymer ratios were fabricated using hot-melt extrusion and tested for their ability to provide sustained release of 5-FU in in vitro and in vivo settings. In addition, these formulations were compared against soluble 5-FU for their antitumor activity in vivo as well as for their toxicity. It was demonstrated that the release rate of 5-FU from PLGA pellets was directly related to the percentage of 5-FU in the pellets. PLGA pellets loaded with 50% w/w 5-FU exhibited comparable, and significantly enhanced, antitumor activity (as measured by tumor volumes and survival) in vivo in a thymoma and colon cancer model, respectively, when compared to an equivalent bolus dose (120 mg/kg) of soluble 5-FU. We concluded that 5-FU-loaded PLGA pellets were more effective and specifically less erythrotoxic than 5-FU bolus injections and therefore may prove to be of benefit as an intraoperative adjunct therapy for patients with cancers that are sensitive to 5-FU and who are undergoing tumor resection.
Keywords: 5-FU, 5-fluorouracil, Poly(lactic/glycolic) acid (PLGA, PLA), controlled/sustained release/delivery, thymoma cancer, colon cancer
Introduction
5-FU is a pyrimidine analog that has been used for the treatment of various types of cancer, including, most notably, colorectal cancer. Due to its rapid elimination and therefore short biological half-life, 5-FU is usually administered as multiple bolus injections or continuous infusion, the latter of which has been shown to be preferable in clinical settings.[1, 2] One explanation for the observed enhanced therapeutic effects of continuous infusions is that a sustained presence of 5-FU is required to provide optimal tumor cytotoxicity. An in vitro study using colon adenocarcinoma cells demonstrated that the LD50 for 5-FU delivered continuously was approximately 100-fold lower compared to when the drug was delivered in a pulsatile manner.[3] In addition, patients receiving continuous infusions of 5-FU experienced less hematological toxicity than patients receiving multiple bolus doses.[4] It has been known for over two decades that recurrence of gastrointestinal cancers often occurs at the site of resection.[5] Therefore, developing formulations that release 5-FU in a sustained fashion may provide more efficient and safer alternatives to current 5-FU delivery methods, especially in the local treatment of cancers at sites of tumor resection.[6]
Poly(lactide co-glycolide) (PLGA) is a polymer often used as a platform for sustained drug delivery due to its safety profile, biocompatibility and biodegradability.[7] Although PLGA microparticles have been a common means of drug delivery in preclinical studies, such a system is not appropriate in the context of 5-FU. This is due to the low loading capacity and rapid release kinetics of 5-FU due to its small molecular weight and overall hydrophilic properties.[8–10] In order to avoid the rapid loss of 5-FU during preparation and to minimize the burst release upon implantation, water-free fabrication methods of larger sized drug delivery platforms are desirable. The thermostable nature of 5-FU makes it a candidate drug for loading into PLGA pellets using hot-melt extrusion. Thus, hot-melt extrusion[11] was used to create 5-FU-loaded PLGA pellets with different percentage loadings (30, 40 and 50% 5-FU w/w). PLGA pellet formulations (diameters − 1.2 mm) were generated by combining appropriate ratios of PLGA polymer and 5-FU powder in the presence of a constant percentage (10% w/w) of the plasticizer, polyethylene glycol (PEG) and hot-melt extruding at 85˚C. Each formulation was tested for both in vitro and in vivo 5-FU release rates and displayed increasing rates of release with increasing percentage loading of 5-FU, and possessing release kinetics potentially suitable for imparting in vivo antitumor activity. It was also shown that prepared 5-FU-loaded PLGA pellets could provide sustained 5-FU release in vitro and in vivo and that the rates of release were faster in vivo, suggesting that mechanical forces and/or the biological milieu accelerated the degradation of the pellets. PLGA pellets loaded with 50% w/w 5-FU were capable of imparting significant antitumor activity against two independent tumor types (thymoma (EL4) and colon carcinoma (CT26)) in vivo when subcutaneously injected proximal to palpable tumors. The degree of tumor regression was comparable to, or greater than, that caused by the equivalent dose of soluble 5-FU delivered systemically (120 mg/kg). In addition, the 5-FU-loaded pellets were significantly less erythrotoxic than an equivalent dose of soluble 5-FU.
Materials and methods
Reagents
PLGA (lactic acid: glycolic acid) 50:50 with the 1-dodecanol end, was purchased from Durect (Birmingham, AL). Polyethylene glycol (PEG) 400 and 5-fluorouracil (5-FU) were purchased from Sigma-Aldrich (St. Louis, MO). PEG was used as a plasticizer to reduce the glass transition state of PLGA. A red food colorant, NO. 7700 FD&C Red #40, was purchased from Warner Jenkinson (St. Louis, MO). The food colorant was used to facilitate in observing mixture homogeneity and in the approximation of the dilution factor in in vitro release experiments.
Fabrication of 5-FU-loaded PLGA pellets
The fabrication of 5-FU-loaded PLGA 50:50 pellets was performed by mixing 5-FU with PLGA, PEG (10% w/w) and 10 mg of FD&C dye. The percentages by weight of 5-FU in the pellets were 30%, 40% and 50%. The mixture was fabricated into pellets by hot-melt extrusion (HAAKE MiniCTW, Thermo Fisher Scientific, Waltham, MA) at the temperature of 85°C. During extrusion, the diameter of the extrudate was controlled to be less than 1.2 mm. The extrudate was cut into 10 mg pieces. Unless otherwise stated PLGA pellets loaded with 5-FU were stored in polypropylene tubes with desiccant at − 20°C until ready for use (< 2 months).
Characterization of 5-FU loaded PLGA pellets
Quantitative analysis of 5-FU and differential scanning calorimetry (DSC) on 5-FU-loaded pellets
The concentration of 5-FU in pellet samples was assessed using high-performance liquid chromatography (HPLC), Water 2690 Separations Module, (Waters, Milford, MA) with an ultraviolet (UV) detector (Water 2487 Dual λ Absorbance Detector, Waters). 5-FU was detected at the wavelength of 260 nm. The stationary phase was a Symmetry Shield™ RP 18, 5 μm HPLC column (Waters) with a diameter x length of 4.6 x 150 mm. The mobile phase was 10% methanol in water. The flow rate of the mobile phase used for analysis was 1 ml/min which had the retention time of 5-FU at 3 minutes.[12]
DSC was performed on 5-FU powder and PLGA pellets loaded with 50% w/w 5-FU was performed using a Q20 differential scanning calorimeter (TA Instruments, New Castle, Delaware).
Loading and in vitro release study for 5-FU loaded pellets
Loading of, and release from, 5-FU-loaded pellets were quantitatively determined using HPLC. To determine loading, 5-FU-loaded pellets were submerged in 1 ml of dichloromethane (Sigma) for 5 minutes until pellets were completely dissolved and 5 ml of water was added to the dichloromethane to extract 5-FU. The mixture was vortexed for 1 minute and incubated at room temperature overnight for complete extraction. The mixture was then centrifuged at 5,000 x g for 20 minutes to remove dichloromethane droplets from the water phase. The aqueous phase containing 5-FU was then separated, diluted 100-fold and quantitatively analyzed for 5-FU using HPLC.
Loading efficiency was calculated by the following formulation:
For in vitro release studies, 5-FU pellets (10 mg) were submerged in 1 ml 0.1 mM phosphate buffer saline (PBS) (pH 7.4) and incubated at 37°C and 300 RPM. Samples were collected at indicated time points and fresh PBS was added after each sample collection. Each quantification of 5-FU concentration was added to the previous and presented as cumulative release.
Effect of percent 5-FU loading and storage conditions on 5-FU release kinetics from pellets
The effect of percent w/w 5-FU in PLGA 50:50 pellets, storage temperature (room temperature or −20°C), storage duration (0, 6 and 8 weeks after fabrication) and desiccation (presence versus absence) on the release rates of 5-FU from pellets was investigated. PLGA pellets loaded with 5-FU at 30%, 40% and 50% w/w were stored at the indicated temperatures, durations and with or without desiccation. Surface areas of all pellets in this study were recorded and no significant differences were observed from batch to batch, with a range recorded of 34.12 – 36.28 mm2.
In vivo studies
Cell line and cell culture
EL4, a murine thymoma cell line, was selected as an initial tumor model to test the efficacy of 5-FU loaded PLGA pellets due to the sensitivity of these types of cells to 5-FU.[13] EL4 cells were obtained from the American Type Culture Collection (ATCC: Manassas, VA) and maintained in RPMI-1640 medium (Gibco, Life technologies, Grand Island, NY). The media was supplemented with 10% v/v fetal calf serum (Atlanta Biologicals, Lawrenceville, GA), 10 mM HEPES (Gibco), 50 μg/ml gentamycin sulfate (Cellgro, Manassas, VA), 1 mM sodium pyruvate (Gibco), 1 mM Glutamax™ (Gibco) and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich). CT26, a murine colon cancer cell line, was purchased from ATCC. CT26 cells were maintained in RPMI-1640 medium supplemented as described above except that 2-mercaptoethanol was not added. Cells were incubated at 37°C and 5% CO2 in a humidified incubator (New Brunswick™ Galaxy® 170S, Eppendorf, Humburg, Germany).
Animals
6–8-week old female C57BL/6J and BALB/CJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were allowed to acclimatize for one week prior to performing experiments. A 12-hour light-dark cycle was maintained in the housing room. Mice were given ad libitum access to food and water. Animal husbandry and veterinary care were provided by the Office of Animal Resources at the University of Iowa. All animal protocols used in these studies were in agreement with and approved by Institutional Animal Care and Use Committee (IACUC).
In vivo release study
PLGA pellets loaded with 50% w/w 5-FU were implanted subcutaneously (SC) into the right flank of C57BL/6J mice using 16 gauge needles. Mice were injected while under anesthesia (87.5 mg/kg ketamine; 2.5 mg/kg xylazine (Office of Animal Resources, University of Iowa, Iowa City, IA) delivered intraperitoneally (IP)). Subsequent to implantation, mice were randomly assigned into treatment groups, each group representing a predetermined time point of the in vivo release study. A group of 5 mice was euthanized at each time point (6 hours, 1, 2, 5, 7, 14, 21 and 28 days after implantation). Pellets were then extracted from mice and 5-FU was extracted as described above. The amount of 5-FU released was calculated by subtracting 5-FU left in pellets from average loading of 5-FU in pellets. In addition, an in vitro release study was performed in parallel using incubation settings described above (see Loading and release study).
In vivo toxicity study
In vivo toxicity of 5-FU-loaded (50% w/w) PLGA pellets was assessed by monitoring blood cell counts (red blood cells (RBCs), lymphocytes and granulocytes), body weights and general signs of distress (hunching, lethargy and ruffled fur). Twenty C57BL/6J mice were randomized into 4 groups (5 mice per group). One group remained untreated while each of the other three groups were treated with a dose of 120 mg/kg 5-FU in one of the following three forms: 5-FU-loaded PLGA pellets (injected SC); soluble form injected IP; soluble form injected SC. The results obtained were compared to the untreated control mice. Blood samples were collected (via submandibular bleeds) at one week before administration of 5-FU, and then one week, three weeks and five weeks after administration. Data obtained prior to 5-FU treatment and from untreated mice were used as control baselines. RBCs were counted using an automated cell counter, Countess II Fl (Thermo Fisher Scientific). WBC were counted using FACScan flow cytometry (Becton-Dickinson, Franklin Lakes, NJ) using 123count eBeads (eBioscience, Affymetrix inc., San Diego, CA: used as per manufacturer’s instructions) at a known concentration as an internal reference and analyzed using FlowJo software (TreeStar, Inc., Ashland, OR). Mouse weights and general health status were monitored at least 3 times per week. Mice exhibiting signs of distress (see above) were sacrificed and their deaths were recorded.
In vivo antitumor efficacy study
The antitumor efficacy of 5-FU-loaded PLGA pellets was assessed in C57BL/6J mice challenged with EL4 murine thymoma cells and BALB/CJ mice challenged with CT26 murine colon cancer cells. The optimal formulation was determined in the EL4 model and subsequently applied to the CT26 model. Mice were challenged SC with either 2 x 106 EL4 cells/mouse or 1 x 106 CT26 cells/mouse, on the shaved rear right flank. Once a palpable tumor was detectable (Day 5) then treatments were commenced. Prior to treatments the mice were placed into groups so that the mean tumor volumes of each group were similar. Mice were monitored for tumor volume, weight and survival for 60 days after the treatment. Tumor volume was calculated by the equation for determining the volume of an ellipsoid: [(Diameter 1 x Diameter 2 x Height) x (π/6)].
Statistical analysis
In vitro release profiles were analyzed by a linear mixed-effect model. Covariates (percent by weight (% w/w) of 5-FU in pellets, storage duration (week), storage temperature (°C) and the presence of desiccant) were applied to this model as well as a random intercept for the sampling time point to account for correlation from repeated measurements. Correlation of in vitro and in vivo release was analyzed by the Pearson correlation. The percent cumulative release of 5-FU served as an outcome variable. Cell counts and weight data were also analyzed using a linear mixed-effect model. This model was applied with an initial outcome parameter, the type of 5-FU treatment and the interaction between time and treatment type (time*treatment) as covariates and a random slope and intercept for sampling time points. Survival data of mice were analyzed using Kaplan-Meier estimation. Multiple comparisons of survival data were performed using log-rank test with Tukey adjustment. P-values were 2-sided and considered as statistically significant when they were less than 0.05. Graphs were plotted using GraphPad Prism 7 (GraphPad Software, Inc.). Statistical analysis was performed using Statistical Analysis Software (SAS Institute, Inc.).
Results and discussion
Fabrication and characterization of 5-FU-loaded PLGA pellets
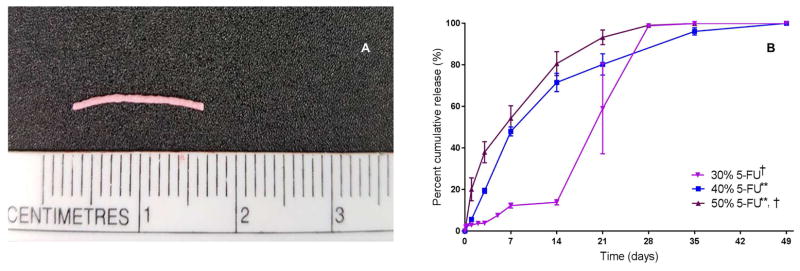
PLGA pellets loaded with varying amounts of 5-FU (30%, 40% or 50% w/w) were fabricated using hot melt extrusion as described in the methods section (example shown in Figure 1A). All 5-FU-loaded PLGA pellets exhibited approximately 100% loading efficiency (Error! Reference source not found.). DSC revealed that 5-FU (in the 5-FU-loaded PLGA pellets) exhibited an endothermic peak at 280˚C versus 285˚C (which is the melting point for 5-FU) for the pure powder form of 5-FU, suggesting that 5-FU was dispersed in the pellets as solid crystals. The 5-FU release kinetics of pellets made from PLGA 50:50 (containing 10% PEG as a plasticizer) and loaded with varying amounts of 5-FU were compared in vitro (Figure 1B). It was discovered that the rate of release correlated to the percent loaded 5-FU with the release rate increasing significantly with increased loading (p < 0.05). With 5-FU being a mildly hydrophilic molecule (approximately 12 mg/ml in water at pH 7 [14]), an increase in percent 5-FU in the formulation therefore likely increased the hydrophilicity of the formulation and accordingly enhanced the rate of water absorption, polymer hydrolysis and erosion, and consequently, the 5-FU release rate.[15] PLGA pellets loaded with 30% 5-FU (w/w) showed a tri-phasic release pattern with a small initial burst release within one day followed by a diffusion-predominate release until day 14. The literature indicates that the release of 5-FU from PLGA devices can be bi-phasic[16, 17] or tri-phasic[16]. A possible explanation for the burst release witnessed between days 14 and 28 for the 30% w/w 5-FU loaded pellets is that later stage acid catalyzed degradation of these PLGA pellets occurred due to a build-up of acidic oligomers. This acid build-up was possibly a result of the 30% w/w 5-FU loaded pellets being less porous (due to lower loading of 5-FU) than the 40% and 50% w/w 5-FU loaded pellets. For the pellets loaded with 40% and 50% w/w 5-FU, the temporal distinction between the diffusion-predominant and polymer degradation-predominant phases is likely to be less clear and as a result the release profiles manifest as biphasic.
Figure 1. Characterization of 5-FU-loaded PLGA pellets.
(A) Photo of 5-FU-loaded PLGA pellet; (B) Cumulative 5-FU release profiles of 5-FU-loaded pellets (n = 5) containing different percentages (30% (3 mg), 40% (4 mg) and 50% (5 mg) w/w) of 5-FU. The average percent cumulative release from pellets loaded with 40% 5-FU and 50% 5-FU was increasingly and significantly higher than from pellets loaded with 30% 5-FU; p < 0.05 as determined using a linear mixed-effect model. ** indicates differences from 30% 5-FU-loaded pellets were statistically significant. † indicates differences from 40% 5-FU-loaded pellets were statistically significant.
In vitro versus in vivo release of 5-FU-loaded PLGA pellets
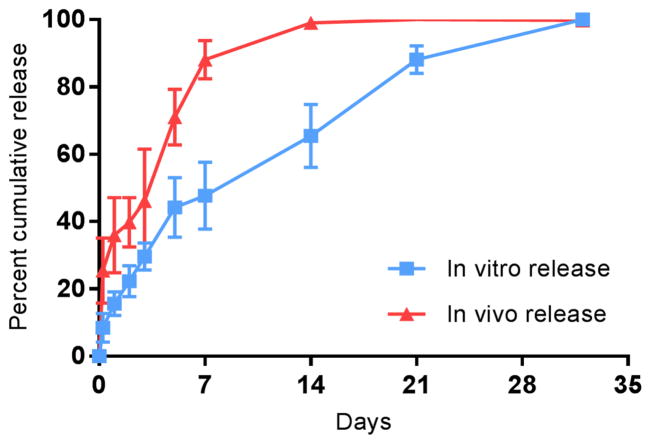
To investigate the differences between in vitro and in vivo release rates of 5-FU from PLGA pellets, an in vivo release study was conducted in C57BL/6J mice in parallel with an in vitro release study, comparing PLGA pellets loaded with 50% 5-FU. The release data shown in Figure 2 demonstrate that the pellets provided faster release of 5-FU in vivo than pellets under in vitro conditions, where approximately 80% of the 5-FU was released in vivo within the first 7 days versus approximately 50% being released in vitro over the same period. It was demonstrated that although in vivo and in vitro release rates were different, they were highly correlated (rs = 0.98, p < 0.01) and that future in vitro release data obtained with PLGA pellets loaded with 50% 5-FU could subsequently be used as a predictor for in vivo release using the following equation: Percent cumulative release (In vitro) = −0.01X2 +2.22X + 1.21 where X is percent cumulative release (in vivo). The finding that in vivo release was faster than in vitro release was entirely expected and has been demonstrated in previously published work involving independent formulations.[18, 19] Enzymes[20, 21], biological surfactants[22] and mechanical forces[23] are likely responsible for enhancing 5-FU release rate in vivo. Lysozymes[20], esterases, and lipases[21] have shown the ability to catalyze the hydrolysis of PLGA in vitro. Trypsin[24] and other serum proteins[22] such as albumin, lysozymes, ribonucleases and β-lactoglobulin act as surface-active agents that help accelerate the degradation of PLGA from pellets.
Figure 2. In vitro versus in vivo release studies.
In vitro versus in vivo percent cumulative release of 5-FU from 5-FU-loaded PLGA pellets (50% w/w). The in vivo release study was performed twice with the use of 5 pellets per time point. In this experiment, in vivo release was faster than in vitro release. Complete release of 5-FU from pellets occurred within two weeks of the pellets being implanted in mice. A strong correlation existed between the in vitro and in vivo release (Pearson correlation coefficient = 0.98, p < 0.01)
In vivo antitumor efficacy of 5-FU-loaded PLGA pellets in a 5-FU sensitive thymoma mouse tumor model
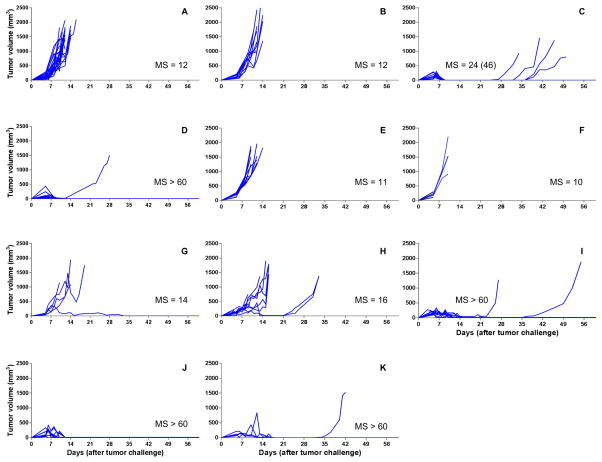
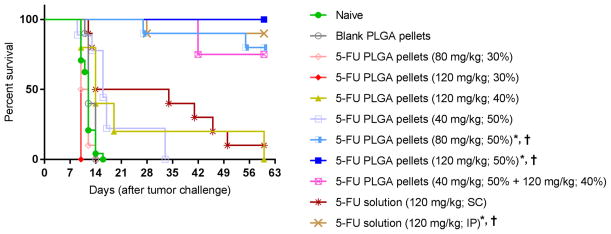
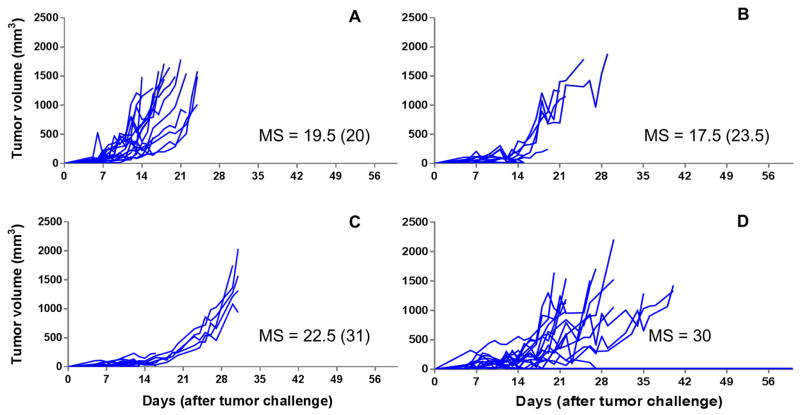
We next investigated whether the in vivo release rate observed with 5-FU-loaded PLGA pellets was sufficient to affect local tumor killing. To determine this, the therapeutic effect of 5-FU-loaded pellets on mice challenged with EL4, a 5-FU sensitive thymoma cell line, was tested.[13] Mice, possessing palpable tumors, were treated with different doses of 5-FU (40 mg/kg, 80 mg/kg and 120 mg/kg) using pellets containing varying w/w percentages of 5-FU/PLGA, and their effects on tumor development were compared to untreated mice and mice treated with either empty pellets or soluble 5-FU (delivered by IP or SC injection) (Figure 3). The results demonstrated that 100% of mice treated with 120 mg/kg 5-FU delivered by 5-FU-loaded PLGA pellets (50% w/w) experienced complete remission of EL4 tumors without recurrence (Figure 3J), while 90% of mice treated with 120 mg/kg soluble 5-FU IP also experienced complete remission without recurrence (Figure 3D). Dose dependency was demonstrated with 50% w/w 5-FU-loaded PLGA pellets since 120 mg/kg, 80 mg/kg and 40 mg/kg, displayed decreasing tumoricidal activity, respectively (Figure 3H, 3I and 3J). Mice treated with the 50% w/w 5-FU-loaded PLGA pellets at doses of 80 mg/kg and 120 mg/kg had significantly extended survivals over untreated mice and mice treated with blank PLGA pellets (Figure 4, p < 0.05). All other pellet based treatments had no significant effect on tumor regression or survival indicating that antitumor efficacy is not only dosedependent but also dependent on the kinetics of 5-FU release. To explain the latter, mice treated with the same dose (120 mg/kg) of 5-FU had reduced antitumor activity when pellets with lower loading percentages (40% and 30%) of 5-FU were used (Figure 3F and 3G). Based on the results obtained with C57BL/6J mice, we selected 120 mg/kg 5-FU-loaded PLGA pellets (50% w/w) for further studies in CT26 challenged BALB/CJ mice (see below) as well as for cytotoxicity studies in C57BL/6J mice.
Figure 3. Spider plots for tumor volumes of EL4 challenged C57BL/6J mice receiving different 5-FU formulations.
Mice received the following treatments (on Day 5 post tumor challenge, when palpable tumor was present): A: naive mice without treatment (n = 24); B: mice treated with blank PLGA pellets (n = 10); C: mice treated with 5-FU solution by SC injection (120 mg/kg) (n = 10); D: mice treated with 5-FU solution by IP injection (120 mg/kg) (n = 10); E: mice treated with 5-FU-loaded pellets (30% w/w of 5-FU, 80 mg/kg) (n = 10); F: mice treated with 5-FU-loaded pellets (30% w/w 5-FU, 120 mg/kg) (n = 3); G: mice treated with 5-FU-loaded pellets (40% w/w 5-FU, 120 mg/kg) (n = 5); H: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 40 mg/kg) (n = 9); I: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 80 mg/kg) (n = 10); J: mice treated with 5-FU-loaded pellets (50% w/w 5-FU, 120 mg/kg) (n = 10); K: mice treated with 5-FU-loaded pellets (40% w/w 5-FU, 120 mg/kg) combined with 5-FU-loaded pellets (50% w/w 5-FU, 40 mg/kg) (n = 4). Median survival times (MS, days) using all-cause mortality are shown besides the spider plots of each group. The median survival times when events were death from no efficacy are equal to those when all-cause mortality was used, unless otherwise indicated in parentheses.
Figure 4. Survival curve of EL4-challenged mice treated with 5-FU-loaded pellets.
C57BL/6J mice were challenged with EL4 cells and then once palpable tumor was detectable (Day 5) the mice were treated with the indicated formulation. Kaplan-Meier estimator was used to estimate the survival function of treated mice. Events were all-cause mortality. Multiple comparisons were performed using the log-rank test with Tukey adjustment. * and † designate groups whose survival was statistically different from survival of tumor-challenged untreated mice (naive) and mice treated with blank PLGA pellets, respectively.
In vivo toxicity of 5-FU-loaded PLGA pellets
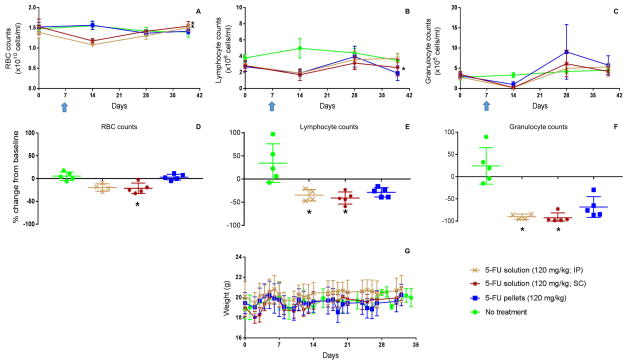
The above findings demonstrated that 5-FU-loaded PLGA pellets delivered locally could provide efficient antitumor activity comparable to that of systemically delivered 5-FU. We next, using C57BL/6J mice, assessed the in vivo cytotoxicity of soluble 5-FU (120 mg/kg) delivered either by IP (systemic) or SC injection versus 5-FU delivered in PLGA pellets (50% w/w) as defined by body weight and red and white blood cell counts (Figure 5). Using a linear mixed effect model, it was shown that the 5-FU-loaded PLGA pellets had no significant impact on RBC count and hence were not erythrocytotoxic, while soluble 5-FU delivered by IP or SC injection significantly reduced the RBC count (p < 0.01) (Figure 5A). With respect to the effects of soluble 5-FU, this study agreed with separate reports using BALB/C mice demonstrating that the nadir of blood counts occurred a week after a single dose of 120 mg/kg IP 5-FU.[25–27] When delivered in solution form, 5-FU significantly reduced lymphocyte counts (Figure 5E) and granulocyte counts (Figure 5F) within the first week of treatment compared to untreated mice (p < 0.05) while there were no significant differences in lymphocyte nor granulocyte counts between mice treated with 5-FU-loaded PLGA pellets and mice receiving no treatment (Figures 5E & 5F). However, there was an observed trend toward the 5-FU-loaded pellets being cytotoxic with respect to lymphocytes and granulocytes. Such a trend was not observed for RBCs. Thus, 5-FU-loaded PLGA pellets had an advantage over soluble 5-FU (IP or SC) in that they were not erythrocytotoxic and were less leukocytotoxic.
Figure 5. Comparative in vivo toxicities of 5-FU in soluble (IP and SC) versus pellet form.
C57BL/6J mice were treated with indicated treatments on day 7 (indicated with arrow) of study. (A) RBC counts over time and (D) statistical analysis of differences in RBC counts on day 14 (7 days post treatment); (B) lymphocyte counts over time and (E) statistical analysis of differences in lymphocyte counts on day 14 (7 days post treatment); (C) granulocyte counts over time and (F) statistical analysis of differences in granulocyte counts on day 14 (7 days post treatment); and (G) mouse body weights were measured as described in methods section. * designates groups whose counts were statistically different from counts of untreated mice (p < 0.05).
With respect to body weights, no significant impact of any treatment was observed (Figure 5G). Although an independent study performed by another group showed the negative impact of 5-FU on mouse weight, the impact was only marginal and thus the results were largely in agreement with the study presented here. Their study involved BALB/CJ mice and showed that when treated with a single dose of 100 mg/kg of 5-FU there was a marginal reduction in mouse weight (< 5% of total body weight) on days 1, 3 and 4 post-treatment (p < 0.05) with mouse weight recovering to the normal weight range within five days.[28] Figure 5G also shows weight loss, albeit not significant, after a few days of the treatment and the rapid recovery of mice weight within a week.
In vivo antitumor efficacy of 5-FU loaded PLGA pellets in a mouse colon cancer model
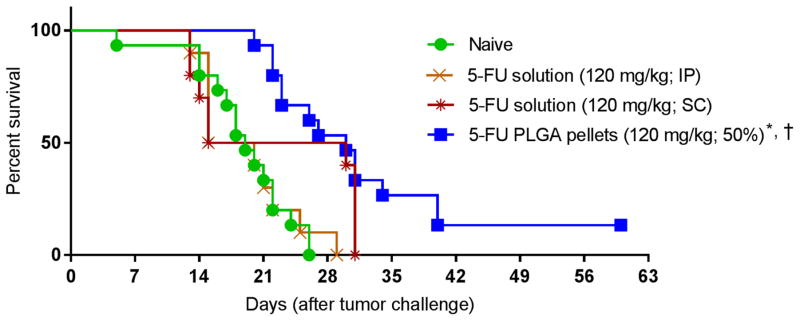
Since 5-FU is often used in the treatment of patients with colon cancer we investigated the antitumor efficacy of the optimal 5-FU-loaded pellet formulation (determined above) in a mouse model for colon cancer. Mice were challenged with the syngeneic colon cancer cell line, CT26, and upon the appearance of palpable tumors these mice were treated with soluble 5-FU (delivered by IP or SC (peritumoral) injection) or 5-FU-loaded PLGA pellets (50% w/w) at a dose of 120 mg/kg per mouse, delivered peritumorally. Tumor volume data (Figure 6) revealed that 5-FU-loaded pellets could extend the median survival time significantly when compared to untreated mice or mice treated with soluble 5-FU delivered by IP injection. Although mice treated with soluble 5-FU delivered SC had significantly extended median survival when death from primary causes (tumor volume) was used for analysis, this was not so when analysis was performed using death from all causes since 20% of these mice died prematurely (presumably due to the toxicity of the treatment, however, no cause was officially determined). Thus, unlike 5-FU-loaded PLGA pellets which were both efficient and effective, soluble 5-FU lacked significant effectiveness when delivered SC compared to untreated mice. When survival curves were analyzed, it was found that mice treated with 5-FU-loaded pellets had significantly extended survival over untreated mice (p < 0.01) as well as mice treated with soluble 5-FU delivered IP at the same dose (p < 0.05) (Figure 7).
Figure 6. Spider plots for tumor volumes of CT-26-challenged BALB/CJ mice receiving different 5-FU formulations.
Mice received indicated treatments (on Day 5 post tumor challenge, when palpable tumor was present). A: naive mice without treatment (n = 16); B: 5-FU solution IP (120 mg/kg) (n = 10); C: 5-FU solution SC (120 mg/kg) (n = 10); D: 5-FU-loaded PLGA pellets (50% w/w 5-FU, 120 mg/kg) (n = 10). Median survival times (MS, days) using all-cause mortality are shown besides the spider plots of each group. The median survival times when events were death from no efficacy are equal to those when all-cause mortality was used, unless otherwise indicated in parentheses.
Figure 7. Survival curve of CT26-challenged mice treated with 5-FU-loaded pellets.
BALB/CJ mice were challenged with CT26 cells and then once palpable tumor was detectable (Day 5) the mice were treated with the indicated formulation. Kaplan-Meier estimator was used to analyze survival of mice. The log-rank test with Tukey adjustment was used for multiple comparison. Events were death from all causes. * and † designate group was statistically different from the untreated group and mice treated with soluble 5-FU (IP), respectively (p < 0.05).
Factors affecting in vitro release of 5-FU loaded PLGA pellets
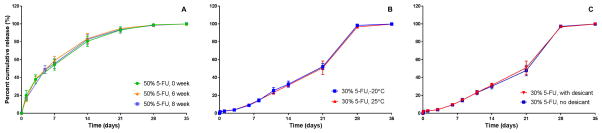
Should 5-FU-loaded pellet formulations similar to those described here be a potential mode of treatment of local tumors in cancer patients, it would be of importance that these pellets maintain their release kinetics profiles under various storage conditions for extended time periods. Thus, the in vitro release profiles of the 5-FU-loaded PLGA pellets were assessed after experiencing various storage conditions (see Figure 8). Storage of pellets for up to 8 weeks at −20˚C or 25˚C had no effect on 5-FU release rates. Nor did storing the pellets with or without desiccant for 8 weeks. These results suggest that 5-FU-loaded PLGA pellets are robust in terms of maintaining their release profiles for at least 2 months of diverse storage conditions.
Figure 8. Effect of storage conditions on release profiles of 5-FU-loaded PLGA pellets.
Release profiles of 5-FU from: (A) PLGA pellets loaded with 5-FU (50% w/w) stored at −20˚C with desiccant for indicated periods. (B) PLGA pellets loaded with 5-FU (30% w/w) stored at indicated temperature for 8 weeks. (C) PLGA 50:50 pellets loaded with 5-FU (30% w/w) stored at −20˚C with or without desiccant as indicated for 8 weeks. (Error bars represent SD).
Conclusion
In order to effectuate a local delivery platform that provides sustained release of 5-FU capable of affecting antitumor efficacy with minimal off-target cytotoxicity, 5-FU-loaded PLGA pellets were developed. Hot-melt extrusion was successfully used to fabricate 5-FU-loaded PLGA pellets with a 100% loading efficiency. This study showed that the percentage of 5-FU in the formulation dictated the release rate of 5-FU from the pellets with percentage loading being directly proportional to 5-FU release rate. It was found that 5-FU-loaded PLGA pellets containing 50% 5-FU w/w at the dose of 120 mg/kg was both efficacious and effective against both thymoma and colon cancer challenged mice. Importantly, these pellets were significantly more therapeutic than IP-delivered 5-FU in colon cancer challenged mice. In addition, 5-FU loaded PLGA pellets had an advantage over either SC or IP administration of soluble 5-FU in terms of their lower erythrocytotoxicity. Therefore, we conclude that 5-FU-loaded PLGA pellets are a promising delivery system capable of enhancing the antitumor efficacy and lowering the toxicity of 5-FU when compared to 5-FU delivered in soluble form. Such pellet-based formulations may prove beneficial as effective modes of tumor therapy for patients undergoing resection or who have inoperable tumors, particularly for those patients with cancers known to be sensitive to 5-FU treatment such as colon cancer.
Table 1.
Loading of 5-FU in PLGA pellets. All formulations contained 10% w/w of PEG 400.
| Percent 5-FU in PLGA formulation | Amount of 5-FU per pellet (mg/pellet) | Loading efficiency (% ± SD), n = 5 |
|---|---|---|
| 30% | 3 | 101.77 ± 3.96 |
| 40% | 4 | 102.51 ± 6.11 |
| 50% | 5 | 113.08 ± 7.82 |
Acknowledgments
We acknowledge support from the National Cancer Institute at the National Institutes of Health (5P30CA086862) and the Lyle and Sharon Bighley Chair of Pharmaceutical Sciences. Nattawut Leelakanok acknowledges support from The Royal Thai Government Scholarship. We would like to thank Kareem Ebeid for his assistance in generating the differential scanning calorimetry data and Behnoush Khorsand for her guidance in operating the hot-melt extruder.
Footnotes
Conflicts of interest
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piedbois P, Rougier P, Buyse M, et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301–8. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 2.Fang L, Xin W, Ding H, et al. Pharmacokinetically guided algorithm of 5-fluorouracil dosing, a reliable strategy of precision chemotherapy for solid tumors: a meta-analysis. Sci Rep. 2016:625913. doi: 10.1038/srep25913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters GJ, Lankelma J, Kok RM, et al. Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol. 1993;31(4):269–76. doi: 10.1007/BF00685670. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS. Toxicity of 5-fluorouracil. Oncology (Williston Park) 1999;13(7 Suppl 3):33–4. [PubMed] [Google Scholar]
- 5.Jacquet P, Stuart OA, Dalton R, et al. Effect of intraperitoneal chemotherapy and fibrinolytic therapy on tumor implantation in wound sites. J Surg Oncol. 1996;62(2):128–34. doi: 10.1002/(SICI)1096-9098(199606)62:2<128::AID-JSO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Yuan H, Zheng Ba, Tu S. Clinical research of intraperitoneal implantation of sustained-release 5-fluorouracil in advanced colorectal cancer. World J Surg Oncol. 2015:13320. doi: 10.1186/s12957-015-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3(3):1377–97. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Pu Y, Wang S, et al. Pharmacokinetic study and effectiveness evaluation of slow-release PLGA-5-fluorouracil microsphere. Cancer Chemother Pharmacol. 2013;71(2):351–9. doi: 10.1007/s00280-012-2016-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupte A, Ciftci K. Formulation and characterization of Paclitaxel, 5-FU and Paclitaxel + 5-FU microspheres. Int J Pharm. 2004;276(1–2):93–106. doi: 10.1016/j.ijpharm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Bae JW, Go DH, Park KD, et al. Thermosensitive chitosan as an injectable carrier for local drug delivery. Macromolecular Research. 2006;14(4):461–65. [Google Scholar]
- 11.Follonier N, Doelker E, Cole ET. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained release capsules containing high loadings of freely soluble drugs. Drug Dev Ind Pharm. 1994;20(8):1323–39. [Google Scholar]
- 12.Alsarra IA, Alarifi MN. Validated liquid chromatographic determination of 5-fluorouracil in human plasma. J Chromatogr B. 2004;804(2):435–39. doi: 10.1016/j.jchromb.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 13.Geary SM, Lemke CD, Lubaroff DM, et al. The combination of a low-dose chemotherapeutic agent, 5-fluorouracil, and an adenoviral tumor vaccine has a synergistic benefit on survival in a tumor model system. PLoS One. 2013;8(6):e67904. doi: 10.1371/journal.pone.0067904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanco MD, Guerrero S, Benito M, et al. In Vitro and In Vivo Evaluation of a Folate-Targeted Copolymeric Submicrohydrogel Based on N-Isopropylacrylamide as 5-Fluorouracil Delivery System. Polymers. 2011;3(3):1107. [Google Scholar]
- 15.Fredenberg S, Wahlgren M, Reslow M, et al. The mechanisms of drug release in poly(lactic-coglycolic acid)-based drug delivery systems—A review. Int J Pharm. 2011;415(1–2):34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 16.Shakeri-Zadeh A, Shiran MB, Khoee S, et al. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29(4):548–56. doi: 10.1177/0885328214536940. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Li Y, Ooi CP. 5-Fluorouracil encapsulated HA/PLGA composite microspheres for cancer therapy. J Mater Sci Mater Med. 2012;23(10):2453–60. doi: 10.1007/s10856-012-4723-2. [DOI] [PubMed] [Google Scholar]
- 18.Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. J Control Release. 2008;127(2):137–45. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza S, Faraj JA, Giovagnoli S, et al. In vitro–in vivo correlation from lactide-co-glycolide polymeric dosage forms. Progress in Biomaterials. 2014;3(2):131–42. doi: 10.1007/s40204-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Cai Q, Yan N, et al. In vitro hydrolytic and enzymatic degradation of nestlike-patterned electrospun poly(D,L-lactide-co-glycolide) scaffolds. J Biomed Mater Res A. 2010;95(3):755–65. doi: 10.1002/jbm.a.32896. [DOI] [PubMed] [Google Scholar]
- 21.Kemme M, Prokesch I, Heinzel-Wieland R. Comparative study on the enzymatic degradation of poly(lactic-co-glycolic acid) by hydrolytic enzymes based on the colorimetric quantification of glycolic acid. Polym Test. 2011;30(7):743–48. [Google Scholar]
- 22.Kazakov VN, Sinyachenko OV, Trukhin DV, et al. Dynamic interfacial tensiometry of biologic liquids — does it have an impact on medicine. Colloids Surf Physicochem Eng Aspects. 1998;143(2–3):441–59. [Google Scholar]
- 23.Xiong Y, Yu Z, Lang Y, et al. In vitro stress effect on degradation and drug release behaviors of basic fibroblast growth factor--poly(lactic-co-glycolic-acid) microsphere. Drug Des Devel Ther. 2016:10431–40. doi: 10.2147/DDDT.S93554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Q, Shi G, Bei J, et al. Enzymatic degradation behavior and mechanism of Poly(lactide-coglycolide) foams by trypsin. Biomaterials. 2003;24(4):629–38. doi: 10.1016/s0142-9612(02)00377-0. [DOI] [PubMed] [Google Scholar]
- 25.Anderson NM, Berberovic Z, Berndl E, et al. Cytopenia induction by 5-fluorouracil identifies thrombopoietic mutants in sensitized ENU mutagenesis screens. Exp Hematol. 2012;40(1):48–60. doi: 10.1016/j.exphem.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadal JC, van Groeningen CJ, Pinedo HM, et al. Schedule-dependency of in vivo modulation of 5-fluorouracil by leucovorin and uridine in murine colon carcinoma. Invest New Drugs. 1989;7(2–3):163–72. doi: 10.1007/BF00170853. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney SE, Davis JM, Murphy EA, et al. Effects of 5-fluorouracil chemotherapy on fatigue: Role of MCP-1. Brain Behav Immun. 2013;27(1):155–61. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song MK, Park MY, Sung MK. 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J Cancer Prev. 2013;18(4):322–9. doi: 10.15430/JCP.2013.18.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]