Abstract
Background
Almost all men who present with advanced prostate cancer (CaP) and some men who fail therapy for clinically localized CaP are treated with androgen deprivation therapy (ADT). CaP cell lines are used to identify and characterize new agents for ADT or investigate mechanisms of ADT resistance. CaP cell lines are maintained in culture medium that contains fetal bovine serum, which contains testosterone (T). Androgen deprivation experiments are performed using media supplemented with androgen-free serum, such as charcoal stripped fetal bovine serum (CS-FBS). However, CS-FBS composition varies from batch to batch and variations may impact experimental reproducibility. Serum free media (SFM) may provide a better defined alternative to media supplemented with CS-FBS (CSM).
Methods
Cell growth of 6 human CaP cell lines was assessed using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Androgen levels were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Results
MTT assays showed 5 of 6 CaP cell lines grew after 6 days of culture in androgen- deprived SFM or CSM. LNCaP and VCaP growth was stimulated when cells were cultured in SFM or CSM supplemented with T. LNCaP, C4-2, LAPC-4 and VCaP cell growth was inhibited when cultured in SFM or CSM that with T and bicalutamide. LC-MS/MS data showed LAPC-4 cell produced similar DHT levels when cultured in T-supplemented SFM or CSM. Dutasteride impaired T to DHT metabolism in LAPC-4.
Conclusion
Media composition contributed to growth differences observed between CaP cells cultured in SFM or CSM. However, the differences in media composition did not impair CaP cell response to T-stimulated growth, bicalutamide growth inhibition, metabolism of T or dutasteride efficiency. SFM can be used as a better defined alternative to CSM for androgen deprivation experiments.
Keywords: Androgen deprivation, charcoal-stripped media, DHT, mass spectrometry, prostate cancer
Introduction
Prostate cancer (CaP) growth and progression rely on transactivation of the androgen receptor (AR) by the testicular androgens, testosterone (T) and dihydrotestosterone (DHT). Almost all men who present with advanced CaP and some men who fail therapy for clinically localized CaP are treated with androgen deprivation therapy (ADT). The goal of ADT is to suppress circulating androgen levels and deprive AR of T or DHT. However, ADT is not curative and CaP recurs as lethal castration-recurrent/resistant CaP (CRPC). One mechanism that contributes to resistance of CaP to ADT is intracrine metabolism, the conversion of weak adrenal androgens, dehydroepiandrosterone (DHEA) or androstenedione (ASD) to T or DHT [1,2]. Anti-androgens, such as enzalutamide, or anti-androgen metabolism drugs, such as the CYP17 inhibitor, abiraterone acetate extend patient survival by only several months [3,4]. Identification, development and characterization of new therapeutic agents to inhibit CaP growth or recurrence using various forms of ADT rely on using combinations of in vitro and in vivo models.
In vitro models include human CaP cell lines, such as the androgen-sensitive LAPC-4 [5] and LNCaP, and the androgen-independent LNCaP derivative, LNCaP-C4-2 (C4-2) [6]. Different cell lines require different culture conditions. However, one common component used for cell culture maintenance is fetal bovine serum (FBS). Culture media typically contain 10% FBS, which yields T concentrations 0.03 – 0.097 nM T [7], but serum composition varies from batch-to-batch [8]. One factor that contributes to FBS variation is that commercially available FBS compositions vary due to differences in genetic background and husbandry of cattle. Charcoal or charcoal and dextran are used to remove androgens and estrogens from FBS to produce charcoal-stripped FBS (CS-FBS), which is added commonly to growth media to produce androgen-free media. However, the stripping process removes molecules necessary for cell growth and metabolism. The stripping process is inconsistent and CS-FBS composition varies from batch-to-batch [9]. One alternative to using CS-FBS containing media for androgen deprivation is androgen-free defined media, such as serum-free media (SFM). The goal of these in vitro studies was to compare CaP cell growth and androgen metabolism in control media versus media altered to achieve androgen deprivation.
Materials and Methods
Cell culture and reagents
Human CaP lines, LAPC-4 [10], LNCaP-RPCI (LNCaP) [5,11], PC-3 (acquired from ATCC) and C4-2 [6,12] were cultured in RPMI 1640 (Corning, Corning, NY). VCaP (acquired from ATCC) was cultured in Dulbecco’s Modified Eagle Medium (DMEM, Corning). RPMI and DMEM were supplemented with 10% FBS (Corning) and 2 mM glutamine (Corning). CWR-R1 [13] was cultured in Richter’s Improved media (Corning) supplemented with 2% FBS, 10 mM nicotinamide, 20 ng/ml epidermal growth factor, 5 μg/ml Insulin/transferrin/selenium (ITS) and 5 μg/ml linoleic acid. Media that contained FBS were considered control media (CM). SFM (Serum Free Complete Media, Corning) contains bovine serum albumin (1g/L) and L-glutamine and other proprietary additives. Charcoal-stripped media (CSM) were identical to CM except that FBS was substituted with CS-FBS. CS-FBS was not purchased due to concerns for batch-to-batch variability. Instead, CS-FBS was generated in our laboratory from FBS that was incubated twice for 1 h with activated charcoal (Avantor, Center Valley, PA) and dextran (Amresco, Solon, OH) and sterilized using 0.2 μm filter (ThermoFisher Scientific, Waltham, MA). LC-MS/MS analysis confirmed CS-FBS androgen levels were below the limit of quantitation (BLQ). Cells were harvested using trypsin 0.05% (Corning). Dulbecco’s phosphate buffered saline (PBS; Corning) was used for all cell washes.
All cell lines were authenticated using genomic profiling in the Genomics Shared Resource. DNA profiles were acquired using 15 short tandem repeat (STR) loci and an amelogenin gender-specific marker. Test and control samples were amplified using the AmpFLSTR® Identifiler® Plus PCR Amplification Kit (Thermo Fisher Scientific, Waltham, MA) using the Verti 96-well Thermal Cycler (Applied Biosystems, Foster City, CA) in 9600 Emulation Mode (initial denature: 95°C 11 min, 28 cycles of denature: 94°C 20 sec and anneal/extend: 59°C 3 min, final extension: 60°C 10 min and hold: 12°C). PCR products were evaluated using the 3130xl Genetic Analyzer (Applied Biosystems) and analyzed using GeneMapper v4.0 (Applied Biosystems). Eight of the 15 STRs and amelogenin from the DNA profile for the cell lines were compared to the ATCC STR database (https://www.atcc.org/STR%20Database.aspx?slp=1) and the DSMZ combined Online STR Matching Analysis (http://www.dsmz.de/fp/cgi-bin/str.html). All matches > 80% were considered the same lineage.
Cell viability
A total of 1.0 × 104 LNCaP or LAPC-4 cells or 2.5 × 104 VCaP or 1.0 × 104 C4-2, CWR-R1 or PC-3 cells were plated per well in 24 well plates (Corning) and cultured in cell line-specific growth media for 24 h. All cell lines were cultured with CM, SFM or CSM alone or CM, SFM or CSM supplemented with 1 nM T (Steraloids, Newport, RI). Media were removed on day 0 (control) or 6 and 1 mg/ml 3-(4,5-dimethylthiazol-2-yl) methylthiazol-2-yl)-2,5-diphyltetrazolim bromide (MTT, Amresco) in fresh, respective media was added using 200 ul per well and incubated at 37°C for 4 h. After 4 h, equal volumes of 20% (w/v) SDS in double distilled H2O (Quality Biological, Gathersburg, MD) were added to wells and plates were incubated at 37°C overnight. Plates were read at 590 nm using a Tecan F200 Series (Tecan Group Ltd., Mannedorf, Switzerland) and Magellian 7.2 software (Tecan Group Ltd.). Data were expressed as percent growth. Percent was calculated by subtracting the mean Day 6 OD590 from the mean Day 0 OD590 and dividing by Day OD590 and multipling by 100. OD590 data were reported in Supplemental Figs. S1–5. Experiments were performed in triplicate.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
LAPC-4 cells were plated, 1 × 105 cells per well into 6-well plates for 6 day and 24 h experiments (2 6-well plates were combined to generate 1 sample). LAPC-4 cells used for 24 h studies were cultured in CM, SFM or CSM alone, or CM, SFM or CSM supplemented with 1 nM T. LAPC-4 cells used for 24 h experiments were cultured in CM, SFM or CSM alone or CM, SFM or CSM supplemented with 1 nM T or 1 μM dutasteride (SelleckChem, Houston, TX) separately or in combination. Media (total 24 mL from 2 6-well plates) were collected in 50 mL conical tubes (Corning) and cells were trypsinized and combined in 15 mL conical tubes (Corning). Cells were washed 3 times using PBS and centrifuged at 1200 RPM for 5 min. Cells were suspended after the last wash in 1 mL PBS and 50 μL of cell suspension were reserved to determine protein concentration using a BioRad Protein Determination Kit (BioRad Hercules, CA) and for western blot analysis. The remaining 950 μL of cell suspension were centrifuged at 1200 RPM for 5 min, PBS was removed, and cell pellets were stored at −80°C until analyzed for androgens.
Cell pellets and media were analyzed over 5 runs using a modification of a validated LC-MS/MS assay [14] for 5 androgens, which included T, DHT, DHEA, ASD and androsterone (AND). All androgens including the two internal standards, d3-T and d3-DHT were obtained from Steraloids. Dutasteride and dutasteride-13C6 were obtained from Toronto Research Chemicals (Toronto, ON, Canada). The modified assay used aqueous-based spiked calibration standards were used to document calibration ranges and confirm quality control (Supplemental Table S1). Androgen or dutasteride quality control samples were prepared in charcoal-stripped human postmenopausal female plasma (Bioreclamation, LLC, Westbury, NY). Calibration ranges, QC concentrations and overall performance of the assay were listed in Supplemental Table S1. Cell pellet samples were resuspended to total volume 1 mL in water and vortexed or sonicated to obtain a homogeneous suspension.
Sample extraction involved mixing 250 μL calibrator, quality control, plasma blank or study sample (cell pellet or media) with 750 μL HPLC-grade water,100 μL internal standard (IS) solution (75.0/225 pg/mL d3-T/d3-DHT and 3.0 ng/mL 13C6-dutasteride in 75% methanol) and 4.0 mL methyl-tert-butyl ether (MTBE, Omnisolve®, EMD Milipore, Billerica, MA) in 13 × 100 mm glass screw-top tubes. Androgens were analyzed in undiluted samples and dutasteride was analyzed twice, undiluted and diluted, to ensure concentrations were within range of each standard curve. Tubes were capped with teflon-lined caps, vortexed, rotated 15 min and centrifuged (Sorvall, model RT6000B, Thermo Scientific, Grand Island, NY) at 2,800 rpm and 4°C for 15–30 min to separate liquid phases. The aqueous phase was frozen in dry ice/acetone bath and the MTBE layer was poured into a clean glass 13 × 100 mm conical tube and evaporated at 37°C under nitrogen. The residue was reconstituted with 100 μL 60% methanol. Suspensions were centrifuged (Heraeus Multifuge X3R, Thermo Scientific) at 2,800 rpm at 4°C for 5 min to separate insoluble materials and approximately 13 μL of supernatant were injected for analysis. LC-MS/MS analyses of the extracted samples were performed using a Prominence UFLC System (Shimadzu Scientific Instruments, Kyoto, Japan), a QTRAP® 5500 mass spectrometer (AB Sciex, Framingham, MA), with an electrospray ionization source, and 2 10-port switching valves (Valco instruments Co. Inc., Houston, TX, model EPC10W).
Analytes were detected using multiple reaction monitoring (MRM) in positive ion mode controlled by AB SCIEX Analyst® software, version 1.6.2. Mass spectrometer conditions were ion spray voltage 5,250 volts, turbo gas temperature 700°C, gas 1 = 65, gas 2 = 60, curtain gas 20, collision-associated dissociation (CAD) gas medium and unit mass resolution for Q1 and Q3. Nitrogen was used for all gases and voltages for maximum parent/fragment ion pair intensities were optimized using direct infusion and flow injection analysis. Calibration curves were generated using analyte/IS area response ratios versus nominal concentrations (ng/mL) and weighted linear regressions with weighting factor 1/concentration2. The IS used for T, ASD and DHEA was d3-T and d3-DHT was used for DHT and AND. The IS used for dutasteride was 13C6-dutasteride. Back-calculated concentrations were generated using the formula x = (y − b)/m, where x is the back-calculated concentration, y is the analyte/IS peak area ratio, b is the y-intercept and m is the slope. Calibrator and quality control acceptance criteria required all acceptable concentrations to have accuracy deviations ≤15% from the nominal concentration, with relative standard deviations (% RSD) ≤15%, except at the lower limit of quantitation (LLOQ), which was allowed ≤20% deviation for both parameters. Values that were BLQ were treated as 0.0 ng/mL.
Analyte concentrations were represented as pmoles/mg protein. Cell lysates measured using LC-MS/MS (ng/mL) were multiplied by the total volume of lysate (1 mL), divided by 0.95 (to account for 50 μL removed for protein determination and western blot), normalized by the total protein of the cell pellet (mg), divided by the molecular weight of androgen (ng/nmole) and converted to pmoles. Media androgen concentrations (ng/mL) were multiplied by the total volume of media (24 mL), normalized against total protein of the cell culture (mg), divided by the molecular weight of the androgen (ng/nmole) and converted to pmoles. Cell pellet and media androgen concentrations were reported as combined concentrations in the Results section and separately in Supplemental Figs. S4 and S5. Experiments were performed in triplicate.
Western blotting
Western blots analyses were performed on all 3 biological replicates generated for LC-MS/MS analyses. Cells removed from −80°C storage were re-suspended in ubiquitin extraction lysis buffer (150 mM NaCl, 50 mM Tris-HCL, pH7.4, 5 mM EDTA, 1% NP40, 0.5% sodium deoxycholate [all reagents were purchased from Fisher, Pittsburgh, PA]) and 0.1% SDS (Quality Biological). Halt Protease Inhibitor Cocktail (Sigma, St. Louis, MO) was added to ubiquitin lysis buffer just before cells were lysed. Cells were freeze-thawed 3 times and centrifuged at 14,000 × g for 15 min. Supernatants were transferred to clean microfuge tubes. Protein was quantified using the Protein Determination Kit (BioRad) and analyzed in flat bottom 96 well plates using an EL800 University Microplate Reader (BioTek Instruments) and KC Junior software (Bio-Tek Instruments). SDS-polyacrylamide gel electrophoresis (PAGE) was performed using 4–15% Mini Trans-Blot cell (BioRad) and protein was transferred to Immuno-Blot PVDF Membrane for Protein Blot (BioRad) and blocked in 5% milk in Tris-buffered saline with Tween 20 (TBST, Amersham Bioscience, GE Healthcare Bio-Sciences, Pittsburgh, PA) for 30 min.
Membranes were incubated with AR targeted antibody (BD Pharmagen, N-terminal targeted AR antibody [BD Biosciences, San Jose, CA]) at 1:1000 overnight at room temperature. After incubation, blots were washed 3 times with TBST (Amersham Bioscience) for 10 min each. Washed blots were incubated with goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:1000 for 1 h at room temperature. Blots were washed with TBST 3 times 10 min each and protein expression was visualized using Pierce ECL Western Blotting Substrate (Life Technologies, Carlsbad, CA). Immunoblots were washed with TBST, blocked in 5% milk in TBST, re-probed for tubulin (1:1000 for 1 h at room temperature [Abcam, Cambridge, MA]) and incubated with goat anti-rabbit secondary antibody (1:1000 for 1 h at room temperature [Jackson ImmunoResearch Laboratories]). The western image was quantified using ImageStudio Lite (Li-Cor, Lincoln, NE) and data were expressed as relative AR/Tubulin expression.
Statistical Analysis
ASD, T, DHT and dutasteride levels in LAPC-4 cell pellets and media were modeled as a function of treatment (CM, SFM, CSM +/− 1 nM T, +/− 1 μM dutasteride, or +/− 1 nM T and 1 μM dutasteride) using a general linear model (GLM). Cell growth (MTT data) was modeled for each cell line as a function of treatment (CM, SFM, CSM +/− 1 nM T or +/− 10 μM bicalutamide (B), treatment duration (day 0 or day 6) and their interaction using a GLM. Mean differences of interest were evaluated using Holm-Bonferroni adjusted F-tests about the appropriate linear contrasts of model estimates. All model assumptions were verified graphically using quantile-quantile and residual plots, and transformations (i.e. Box-Cox) were applied as appropriate. All analyses were conducted using SAS v9.4 (Cary, NC) and nominal significance level 0.05. Statistical results were listed in Supplemental tables 2–6.
Results
CaP cell growth persisted when cultured in SFM or CSM media for 6 days
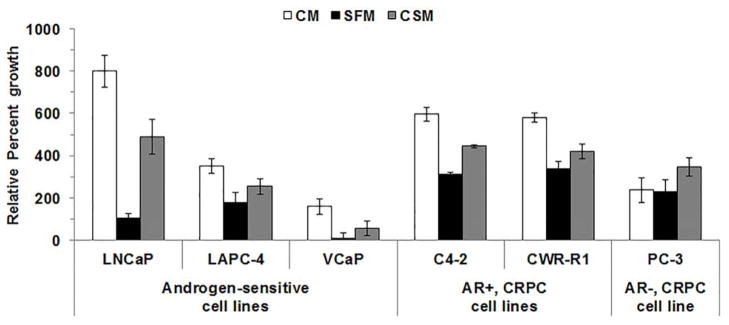
Six commonly used CaP cell lines were cultured in CM or were androgen deprived using SFM or CSM for 6 days and cell growth was measured. The 6 day time point was chosen because previous reports showed CaP cell growth slows or CaP cells undergo apoptosis 6 days after androgen deprivation [15,16]. All CaP cell lines grew (all p-values < 0.05 that compared Day 6 to Day) after 6 days of culture in CM, SFM or CSM (Fig. 1C; Supplemental Figs. S1A and S1B and Supplemental Table S2). All cell lines except, LAPC-4 cells cultured in CSM or PC-3 cells, grew slower in SFM or CSM compared to CM (all p-values < 0.05) Only LNCaP and VCaP cell growth was slowed during androgen deprivation using either SFM or CSM (all p-values < 0.05). However, LNCaP, VCaP and PC-3 cells grew slower when cultured for 6 days in SFM compared to CSM (all p-values < 0.05; Supplemental Table S2).
Figure 1. Androgen deprivation using SFM or CSM slowed LNCaP and VCaP cell growth.
CaP cells were cultured in CM, SFM or CSM. Cell growth was measured on days 0 and 6 using MT assay (OD590). Cell growth was expressed as percent growth. Data were presented as mean +/− SEM. P-values for statistical comparisons of among CaP cells cultured in CM, SFM or CSM were listed in Supplemental Table S2. P-values for statistical comparisons between Day 0 OD590 and Day 6 OD590 were listed in Supplemental Table S2.
T stimulated LNCaP and VCaP cell growth in SFM or CSM
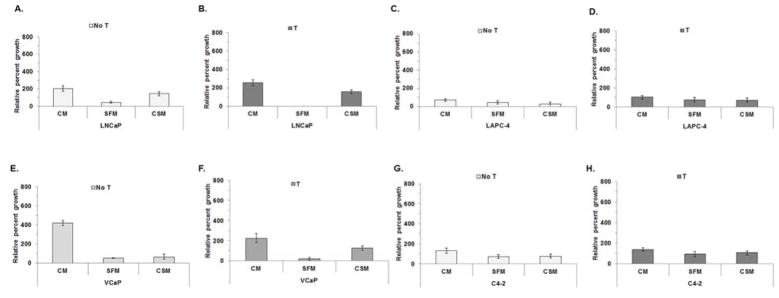
Since LNCaP and VCaP cell growth differed in CM, SFM or CSM, media were supplemented with T to determine if the differences were abrogated. PC-3 cells were not analyzed because PC-3 cells lack AR and do not respond to T. LNCaP growth was stimulated after treatment with SFM with T (all p-values < 0.05), but not CSM with T (Fig. 2A and B; Supplemental Fig. S2 A and B and Supplemental Table S3). LNCaP cells grew slower when cultured in SFM alone or SFM with T (all p-values <0.05) compared to LNCaP in CSM or CSM with T. VCaP cell growth was stimulated when cells were cultured in CM or SFM with T, but not in CSM with T, compared to VCaP cultured in CM or SFM without T (Fig. 2E and F; Supplemental Fig. S2 E, F and Supplemental table S3). T-stimulated growth differed among VCaP cells cultured in CM, SFM or CSM with T, which suggests media composition impacted VCaP growth. Growth differences were not observed among LAPC-4 or C4-2 cells cultured in CM, SFM or CSM alone or with T (Figs. 2C, D, G and H; Supplemental Figs. 2C, D, G and H and Supplemental Table S3).
Figure 2. SFM or CSM supplemented with T stimulated LNCaP and VCaP cell growth.
LNCaP (A and B), LAPC-4 (C and D), VCaP (E and F) or C4-2 (H and I) cells were cultured in CM, SFM or CSM alone or CM, SFM or CSM with 1 nM T. Cell growth was measured on days 0 and 6 using MTT assay (OD590). Data were presented as mean +/− SEM. P-values for statistical comparisons of cell growth between Day 0 and Day 6 were listed in Supplemental Table S3. Comparisons among CaP cells cultured in CM, SFM or CSM alone or CM with T, SFM with T, CSM with T were listed in Supplemental Table S3.
Bicalutamide treatment impaired T-stimulated CaP cell growth in SFM or CSM
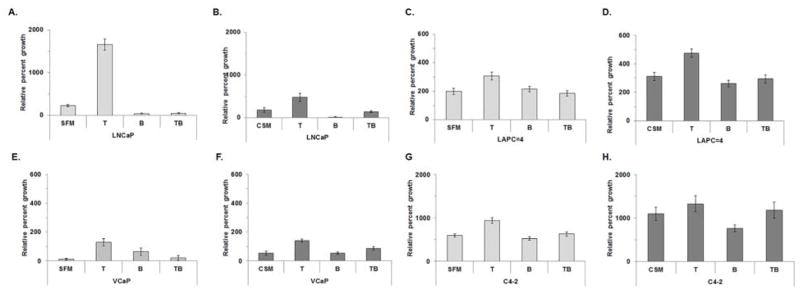
MTT experiments were performed using LNCaP, LAPC-4, VCaP and C4-2 cultured in SFM or CSM with T alone, bicalutamide alone or T and bicalutamide to confirm that cell growth observed after T treatment (Fig. 2) was due to T-stimulated AR activation. All CaP cells grew after 6 days (all p-values < 0.05; Supplemental Table S4), except for LNCaP cells cultured in SFM with bicalutimide or CSM with bicalutamide (Fig. 3A–H; Supplemental Fig. 3A–H and Supplemental Table S4). T treatment stimulated cell growth in LNCaP and VCaP in both SFM and CSM, LAPC-4 cell growth in CSM, and C4-2 cell growth in SFM. Bicalutamide treatment stunted LNCaP (Fig. 3A and B), LAPC-4 (Fig. 3 C and D; CSM with bicalutamide only), VCaP (Fig. 3 E and F) and C4-2 (Fig. 3 G and H) T-stimulated growth (all p-values < 0.05; Supplemental Table S4). T-stimulated LNCaP or LAPC-4 growth responses were different when LNCaP or LAPC-4 cells were cultured in SFM with T or CSM with T (p-values < 0.05). Bicalutamide treatment produced similar responses among LNCaP, LAPC-4 or VCaP treated with SFM with bicalutamide or CSM with bicalutamide, but not C4-2 cell cells treated with SFM with bicalutamide or CSM with bicalutamide (Fig. 3A–H; Supplemental Fig. 3A–H and Supplemental Table S4).
Figure 3. Bicalutamide treatment using SFM or CSM blunted T-stimulated growth in LNCaP, LAPC-4, VCaP or C4-2 cells.
LNCaP (A and B), LAPC-4 (C and D), VCaP (E and F) or C4-2 (H and I) cells were cultured in SFM or CSM alone or SFM or CSM with 1 nM T, SFM or CSM with 10 μM bicalutamide (B) or SFM or CSM with 1 nM T and 10 μM B. Cell growth was measured on days 0 and 6 using MTT assay read at OD590. Data were presented as mean +/− SEM. P-values for statistical comparisons of cell growth between Day 0 and Day 6 were listed in Supplemental Table S4. Cell growth comparisons among T and B and SFM vs. CSM treatments were listed in Supplemental Table S4.
Androgen levels were similar in LAPC-4 cells cultured for 6 days in SFM or CSM
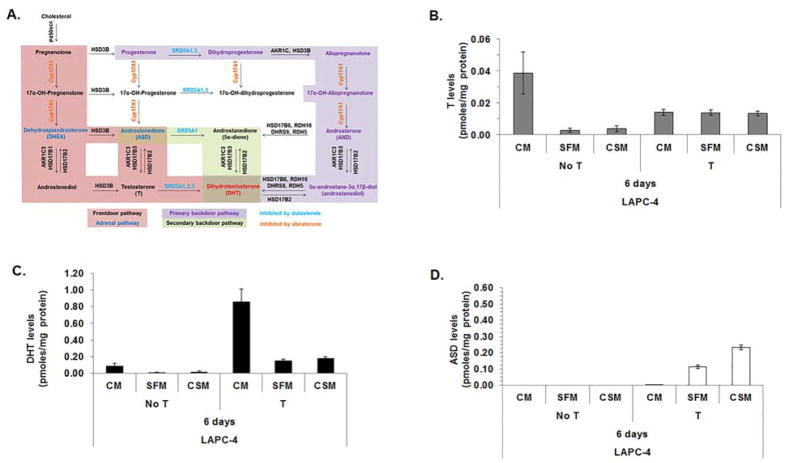
MTT assays revealed that CaP cells produced different growth responses when cultured in SFM or CSM. Therefore, LC-MS/MS was performed to determine if media composition impacted T metabolism. LAPC-4 cells were used because LAPC-4 cells express SRD5A enzymes that metabolize T to DHT (Fig. 4A, modified from [17]), unlike LNCaP or C4-2 cells, which cannot metabolize T to DHT [5]. LC-MS/MS revealed that LAPC-4 cells cultured for 6 days in CM alone produced higher levels of DHT (all p-values < 0.05; Supplemental Table S5) than LAPC-4 cells cultured for 6 days in SFM or CSM alone (Fig. 4B and C, and Supplemental Table S5). DHT levels were higher (all p-values < 0.05) in LAPC-4 cells cultured with CM with T compared to LAPC-4 cells cultured with SFM with T or CSM with T (Fig. 4C and Supplemental Table S5). LAPC-4 cells cultured in SFM alone or CSM alone produced similar T or DHT levels (all p-values > 0.05). LAPC-4 cells cultured for 6 days in SFM alone or CSM alone or SFM with T or CSM with T produced similar T or DHT levels (Fig. 4B and C and Supplemental Table S5), which suggested media composition did not affect LAPC-4 cell metabolism of T to DHT. The data suggested various nutrients or molecules present in CM and not in SFM or CSM facilitated metabolism of T to DHT.
Figure 4. Androgen levels were similar among LAPC-4 cells cultured 6 days in SFM or CSM.
Androgen metabolism pathways for DHT synthesis (A). LAPC-4 cells were analyzed using LC-MS/MS to measure T (B), DHT (C) and ASD (D) levels after LAPC-4 cells were cultured for 6 days in CM, SFM or CSM alone or cultured in CM, SFM or CSM with 1 nM T. Cell pellet and media androgen levels were combined. Cell pellet and media androgen levels were shown in Supplemental Fig. S1. Data were presented as mean pmoles/mg protein +/− SEM. P-values for statistical comparisons among LAPC-4 cells treated with or without T were listed in Supplemental Table S5.
ASD levels were measurable when LAPC-4 cells were cultured for 6 days with CM with T, SFM with T or CSM with T, but not when cells were cultured with CM, SFM or CSM alone (Fig. 4D and Supplemental Table S5). The data suggested LAPC-4 cells metabolized T to ASD when T or DHT levels were sufficient to activate AR.
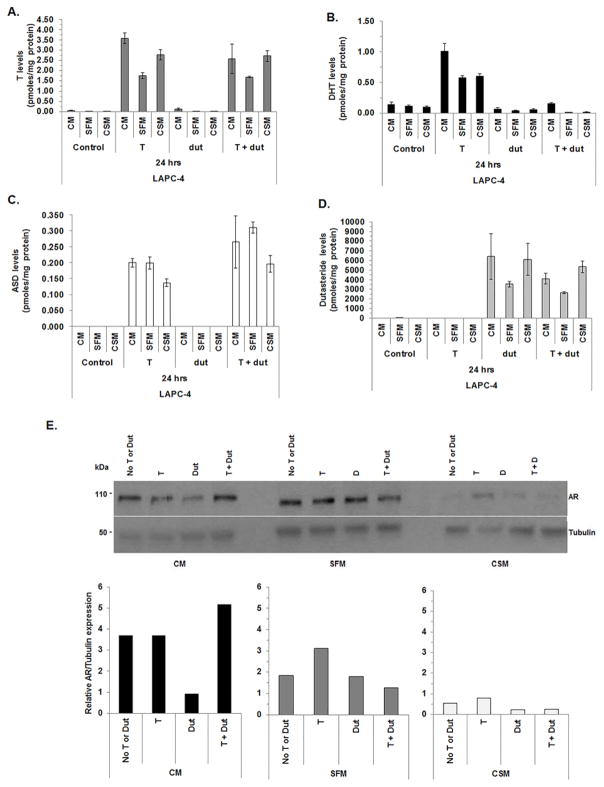
LAPC-4 cell T metabolism to DHT or dutasteride activity did not differ between LAPC-4 cells cultured with SFM or CSM media for 24 h
LC-MS/MS was used to assess acute changes in LAPC-4 cell androgen levels after LAPC-4 cells were cultured for 24 h in CM, SFM or CSM with T, dutasteride or T and dutasteride. Dutasteride, a dual 5α-reductase inhibitor, was used to determine if SRD5A enzymes contributed to intracellular DHT production. LAPC-4 cell T or DHT levels increased when CM, SFM or CSM were supplemented with T (Fig. 5A and B; note different Y-axis and Supplemental Table S6).These data were consistent with reports that show LAPC-4 cells 5α-reduce T to DHT [5]. DHT levels were lower (all p-values <0.05) when LAPC-4 cells were treated with CM, SFM or CSM supplemented with T and dutasteride (Fig. 5B and Supplemental Table S6) compared to LAPC-4 cells cultured with CM, SFM or CSM with T alone. These data suggested dutasteride inhibited SRD5A conversion of T to DHT. The data provide further evidence to show SRD5A isozymes were involved in T to DHT conversion. ASD levels were measurable when LAPC-4 cells were cultured with CM, SFM or CSM supplemented with T. LAPC-4 ASD levels were higher (all p-values < 0.05; Supplemental Table S6) after treatment with CM, SFM or CSM with T and dutasteride treatment compared to LAPC-4 cells cultured with CM, SFM or CSM supplemented with T (Fig. 5C and Supplemental Table S6). Dutasteride levels are similar among all LAPC-4 cells treated with dutasteride alone or T and dutasteride (Fig. 5D and Supplemental Table S6).
Figure 5. Metabolism of T to DHT was impaired in LAPC-4 cultured with dutasteride.
LAPC-4 cells were analyzed using LC-MS/MS to measure T (A), DHT (B). ASD (C) and dutasteride (dut; panel D) levels after LAPC-4 cells were cultured for 24 h in CM, SFM or CSM alone or cultured in CM, SFM or CSM with 1 nM T or CM, SFM or CSM with 1 μM Dut or CM, SFM or CSM with 1 nM T and 1 μM Dut. Cell pellet and media androgen levels were combined. AR protein expression was measured using western blot and quantitated using densitometry (E). Cell pellet and media androgen levels were shown in Supplemental Fig. S2. Data were presented as mean pmoles/mg protein +/− SEM. P-values for statistical comparisons among LAPC-4 cells treated with T, dut or T and dut combined were listed in Supplemental Table S6.
Quantitative western blot analysis revealed AR expression levels were lower in LAPC-4 cells treated with CM with dutasteride compared to CM alone or CM with T (Fig. 5E). AR expression levels appeared to recover when LAPC-4 cells were treated with T and dutasteride. AR expression levels were lower in LAPC-4 cells treated with CSM alone or CSM with T, dutasteride or T and dutasteride compared to SFM alone or SFM with T, dutasteride or T and dutasteride. However, AR expression levels appeared to increase when LAPC-4 cells were treated with SFM with T or CSM with T.
Discussion
The goal of the study was to determine if SFM could be used as an alternative to CSM to androgen deprive CaP cells. MTT assays revealed that media composition affected CaP cell growth, but did not impair CaP cell response to androgen deprivation, T treatment or bicatalumide activity. T metabolism to DHT was similar between LAPC-4 cells cultured in SFM or CSM and dutasteride activity was not affected by differences in SFM or CSM media composition.
CaP cell lines are used to study ADT resistance and androgen metabolism, and to characterize enzymatic pathways or properties because clinical specimens are difficult to acquire. Cell lines are readily available, easy to manipulate and easy to perform molecular or biochemical approaches to address various hypotheses. However, characterization and identification of the most suitable androgen-free tissue culture media is important for proper use of various CaP cell line models and interpretation of results. These studies showed that SFM, a defined androgen-free media with a consistent formula, can be used as an alternative to CSM to perform androgen deprivation experiments. SFM use avoids numerous potential confounders associated with CSM that are difficult to identify and control.
The LC-MS/MS method used to analyze LAPC-4 cell pellets and media measured 5 adrenal and testicular androgens, but did not include all potential metabolites and androgen-conjugates (glucuronides or sulfated metabolites). Therefore, DHT, T or ASD may have been metabolized further, which may account for some of the differences among treatment groups and 6 day or 24 h experiments. Dutasteride metabolism was not measured using this LC-MS/MS method; the metabolites generated from dutasteride metabolism may account for some of the observed changes in dutasteride levels.
The data showed LAPC-4 cells produced similar DHT levels after 6 days or 24 h of culture in SFM or CSM supplemented with T. However, overall androgen levels differed between short and long-term experiments, which suggested treatment duration may impact androgen metabolism. Androgen metabolism may reach steady state as CaP cells adjust to changes in media androgen levels, which suggests treatment time should be carefully considered for androgen deprivation or androgen metabolism experiments. The studies suggest CaP cells should be pre-cultured in media used for androgen deprivation experiments that contain T or DHT. CaP cell cultures propagated in SFM that contains androgen may provide a more reliable model because data generated using an adapted CaP cell line will not be influenced by acute changes that occur as CaP cells adapt to media composition changes.
Conclusions
SFM may be used as an alternative media to CSM for androgen deprivation or androgen metabolism studies. Variability in media composition could impact baseline CaP cell line growth and androgen metabolism, and response to androgen deprivation. Time points for androgen deprivation or androgen metabolism studies should be considered carefully because CaP cell lines adjust to androgen deprivation by 6 days in culture in androgen-deprived media.
Supplementary Material
Acknowledgments
This research was supported by P01-CA77739, Post-doctoral training Award W81XWH-15-1-0409, and, in part, by the NCI Cancer Center Support Grant to RPCI (P30-CA016056) for the Biostatistics and Bioinformatics, and Bioanalytics and Metabolomics and Pharmacokinetics Shared Resources.
Footnotes
This manuscript is an original unpublished work that was completed by all listed authors and authors have no conflicts of interest. Each author made substantive contributions toward the completion of these studies.
References
- 1.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Titus MA, Bai S, Kennerley BJ, Lih FB, Tomer KB, Wilson EM. Activation of the androgen receptor by intratumoral bioconversion of androstanediol to dihydrotestosterone in prostate cancer. Cancer Res. 2011;71(4):1486–1496. doi: 10.1158/0008-5472.CAN-10-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI. Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survivial (OS) in metastatic castration-resistant prostate cancer (mCRPC); Planned final analysis (FA) of Cou-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. J Clin Oncol. 2011:29. [Google Scholar]
- 4.Kantoff PW, Mohler JL. New developments in the management of prostate cancer. J Natl Compr Canc Netw. 2013;11(5 Suppl):653–657. doi: 10.6004/jnccn.2013.0194. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Godoy A, Azzouni F, Wilton JH, Ip C, Mohler JL. Prostate cancer cells differ in testosterone accumulation, dihydrotestosterone conversion, and androgen receptor signaling response to steroid 5alpha-reductase inhibitors. Prostate. 2013;73(13):1470–1482. doi: 10.1002/pros.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57(3):406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 7.Sedelaar JP, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69(16):1724–1729. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witzeneder K, Lindenmair A, Gabriel C, Holler K, Theiss D, Redl H, Hennerbichler S. Human-derived alternatives to fetal bovine serum in cell culture. Transfus Med Hemother. 2013;40(6):417–423. doi: 10.1159/000356236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, West C, Norton-Wenzel CS, Rej R, Davis FB, Davis PJ, Rej R. Effects of resin or charcoal treatment on fetal bovine serum and bovine calf serum. Endocr Res. 2009;34(4):101–108. doi: 10.3109/07435800903204082. [DOI] [PubMed] [Google Scholar]
- 10.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3(4):402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 11.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43(4):1809–1818. [PubMed] [Google Scholar]
- 12.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–2581. [PubMed] [Google Scholar]
- 13.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61(7):2892–2898. [PubMed] [Google Scholar]
- 14.Wilton JH, Titus MA, Efstathiou E, Fetterly GJ, Mohler JL. Androgenic biomarker profiling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. Prostate. 2014;74(7):722–731. doi: 10.1002/pros.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyprianou N, English HF, Isaacs JT. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990;50(12):3748–3753. [PubMed] [Google Scholar]
- 16.Godoy A, Montecinos VP, Gray DR, Sotomayor P, Yau JM, Vethanayagam RR, Singh S, Mohler JL, Smith GJ. Androgen deprivation induces rapid involution and recovery of human prostate vasculature. Am J Physiol Endocrinol Metab. 2011;300(2):E263–275. doi: 10.1152/ajpendo.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocking JJ, Fiandalo MV, Pop EA, Wilton JH, Azabdaftari G, Mohler JL. Characterization of Prostate Cancer in a Functional Eunuch. J Natl Compr Canc Netw. 2016;14(9):1054–1060. doi: 10.6004/jnccn.2016.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.