Abstract
5-fluorouracil (5-FU) is a chemotherapeutic agent that has been used for the treatment of a variety of malignancies since its initial introduction to the clinic in 1957.1 Due to its short biological half-life, multiple dosings are generally required to maintain effective 5-FU plasma concentrations throughout the therapeutic period. Clinical studies have shown that continuous 5-FU administration is generally superior to bolus injection as exhibited by lower toxicities and increased therapeutic efficacy.2,3 Optimal therapeutic efficacy, however, is often compromised by the limiting therapeutic index. Whilst oral formulations are also used, these suffer from the drawbacks of variable bioavailability and first pass metabolism. As a result, sustained release formulations of 5-FU have been investigated in an effort to mimic the kinetics of continuous infusion particularly for situations where local delivery is considered appropriate. The biocompatible, biodegradable and highly tunable synthetic polymer, poly(D,L-lactide-co-glycolide) (PLGA), is widely used as a vector for sustained drug delivery, however, issues such as insufficient loading and inappropriate burst release kinetics have dogged progress into the clinic for small hydrophilic drugs such as 5-FU. This review provides introductory information about the mechanism of action, pharmacokinetic and physicochemical properties, and clinical use of 5-FU that have contributed to the development of PLGA-based 5-FU release platforms. In addition, this review provides information on fabrication methods used for a range of 5-FU-loaded PLGA formulations and discusses factors affecting the release kinetics of 5-FU as well as the in vitro and in vivo antitumor or antiproliferative efficacy of these platforms.
Keywords: 5-FU; 5-fluorouracil; Poly(lactic/glycolic) acid (PLGA, PLA); controlled/sustained release/delivery; microparticles; nanoparticles
Introduction
5-FU is a rationally designed fluoropyrimidine antimetabolite that has been used for a number of clinical applications including cancer therapy and antiproliferative treatment. Although used to treat a wide range of cancers off-label, such as head and neck, cervical and esophageal cancers, 5-FU is most commonly used to treat gastrointestinal cancers such as colorectal cancer, gastric adenocarcinoma and pancreatic cancer. A meta-analysis of patients with resectable gastric cancer, where 5-FU has shown great promise, revealed that 5-FU could increase the 5 year survival rate from 49.6% (surgery alone) to 55.3% when used as an adjuvant therapy.4 In the case of advanced colorectal cancers, 5-FU delivered either intravenously (IV) (often in association with leucovorin) or orally in a prodrug form, capecitabine, can significantly increase survival, albeit marginally (by 2–3 months), when used in combination with other chemotherapeutic agents, such as irinotecan.5,6 These modest increases in survival indicate the need for further improvements. US and European guidelines recommended adding monoclonal antibody therapies (targeting either vascular endothelial growth factor or epidermal growth factor receptors) to the regimen of 5-FU (+ leucovorin) plus oxaliplatin or irinotecan to improve the treatment efficacy of metastatic colon cancer.5 Meanwhile, pharmacokinetically guided dose adjustments have been tested clinically and proven to be a reliable method to improve the efficacy and reduce the toxicity of 5-FU treatment.7,8 The common toxic side-effects of 5-FU include myelosuppression, hair-loss, nausea and diarrhea whilst, more rarely (1–18% of patients), cardiotoxicity may occur.9 Due to the short plasma half-life and the rapid elimination of 5-FU, alternative modes of delivery are sought particularly for situations involving localized therapy subsequent to resection.10 It has long been recognized that the recurrence of gastrointestinal cancers often occurs at the surgical site, emphasizing a need for intraoperative adjuvant therapy at the site of resection.11 Formulating delivery systems that ensure prolonged exposure of tumor cells to 5-FU at doses above the minimum effective concentration has shown improved tumor cell killing over equivalent doses of 5-FU delivered intraperitoneally (i.e. systemic delivery) in mice and may be a promising way forward in the clinic.12 Hence, this review focuses on modes of improving the antitumor and antiproliferative efficacies of 5-FU through the development of PLGA-based delivery systems providing sustained release kinetics.
Physicochemical properties of 5-FU
5-FU, or 5-fluoro-1H–pyrimidine-2,4-dione, is a small molecule (MW: 130.08) and occurs as a white odorless crystalline powder.13 It is a uracil analog possessing a fluorine atom instead of a hydrogen atom at the C-5 position.14 It is usually synthesized as the base form but it is also available as TRIS and sodium salts.15,16 Also, 5-FU is a weak acid (pKa of 7.93)17 and possesses low solubility in water (12 –13 mg/ml at pH = 7).18 Its solubility can be enhanced to 50 mg/ml in aqueous solutions at pH ~ 9, however, although 5-FU is not sensitive to hydrolysis under neutral conditions, the stability of 5-FU decreases drastically in basic and highly acidic aqueous solutions.19 The solubility of 5-FU in methanol is 3.79 mg/ml at 25°C whilst in ethanol at 25°C its solubility is very poor (0.35 mg/ml).20 The n-Octanol/Water Partition Coefficient of 5-FU is 0.1123 (logP = −0.9496) at 32°C (pH 7.4) and is therefore considered to be hydrophilic despite it having low solubility in water.21 In terms of stability, 5-FU in a solid form is heat-stable and exhibits one-stage decomposition at 200°C.22 This allows 5-FU to be formulated into polymeric platforms by techniques involving heating, such as spray drying23 and compression-heat molding24, without significant detriment to its biological activity.
Mechanisms of action of 5-FU
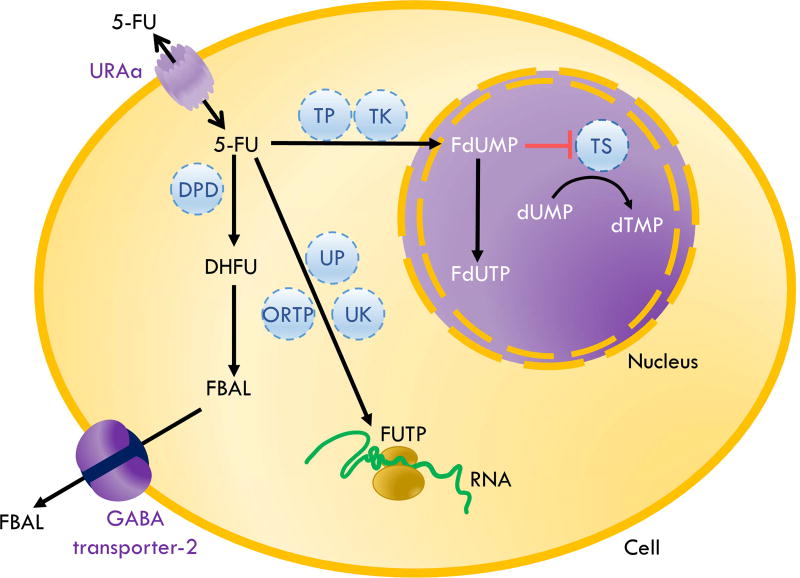
In order to affect cell viability, 5-FU, a type 1A prodrug, requires intracellular conversion at the site of action by enzymes, such as orotate phosphoribosyltransferase and thymidine phosphorylase, to active metabolites, fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP).14 FdUMP inhibits the activity of thymidylate synthase by binding to the nucleotide-binding site, irreversibly preventing the binding of the normal substrate, deoxyuridine monophosphate, leading to a depletion in deoxythymidine triphosphate which consequently results in cell death due to impaired DNA synthesis and repair (Figure 1).14 FdUTP and FUTP can be misincorporated into DNA and RNA, respectively, also leading to cell death.14 Leucovorin (folinic acid) is a drug that enhances the inhibition of thymidylate synthase by FdUMP by stabilizing a trimeric complex of FdUMP, thymidylate synthase and leucovorin and is usually used in combination with 5-FU in cancer treatment.14 As an antimetabolite, 5-FU exerts its effects during S-phase of dividing cells.25
Figure 1. Schematic of metabolic fate and mechanism of action of 5-FU.
The uracil transporter, URAa, facilitates 5-FU entry into cells. After entering the cells, the majority of 5-FU is catabolized by dihydropyrimidine dehydrogenase (DPD) into an inactive metabolite, dihydrofluorouracil (DHFU) which is further metabolized to fluoro-beta-alanine (FBAL). FBAL is transported out of cells by the GABA transporter-2 and then eliminated by urinary excretion. Approximately 10% of 5-FU taken up by cells is metabolized into active forms, namely, fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP). FUTP, a metabolite from the metabolism by multiple enzymes including orotate phosphoribosyltransferase (ORTP) or uridine phosphorylase (UP) and uridine kinase (UK), incorporates into RNA and causes RNA damage. FdUMP, a metabolite from the metabolism by thymidine phosphorylase (TP) and thymidine kinase (TK), forms a stable complex with thymidylate synthase (TS), an enzyme normally responsible for the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). The inhibition of TS by 5-FU leads to deoxynucleotide pool imbalances which in turn results in DNA damage and apoptosis.
In addition to the direct cytotoxic effects of 5-FU mentioned above, it has been reported that 5-FU can also impart antitumor activity indirectly by bolstering antitumor immune responses. To explain, 5-FU has demonstrated in vivo toxicity against myeloid-derived suppressor cells (MDSCs) in tumor challenged mice.26 MDSCs are immunosuppressive cells that accumulate in the spleen, blood, lymph nodes, bone marrow and at tumor sites of tumor bearing individuals and, importantly, suppress the activation of CD8+ T cells (or cytotoxic T cells).27 MDSCs are known to express relatively low levels of thymidylate synthase rendering them more susceptible to the cytotoxic effects of 5-FU.28
Pharmacokinetics and pharmacodynamics of 5-FU
5-FU is generally administered IV to patients in order to avoid the diminishing effects of first pass metabolism associated with enteral administration.29 Tissue distribution of 5-FU depends on route of administration, the presence of tumor and dose of 5-FU.30 The apparent volume of distribution (Vd) of 5-FU in humans is 0.266 L/kg 31 implicating that, subsequent to administration, 5-FU is distributed in aqueous extracellular fluid throughout the body. Murine studies have shown 5-FU to be distributed to the cerebrospinal fluid 13 and other tissues such as bone marrow, brain, intestine, kidney, liver, lung, lymph, and muscle.30,32 It has been reported that 5-FU enters cells by facilitated diffusion using a uracil transporter (UraA).33 Once inside the cell, 5-FU molecules follow an anabolic (15–20%) or catabolic fate (80–85%), the former generating products that mimic uridine triphosphate (UTP) or deoxyuridine monophosphate (dUMP) that are capable of incorporating into RNA or inhibiting thymidylate synthase, respectively, leading to cell death, whilst the latter results in the inactivation of 5-FU via the cytosolic enzyme dihydropyrimidine dehydrogenase (DPD) (Figure 1). The ubiquitous expression of DPD throughout the body results in significant extrahepatic clearance of 5-FU.34 Another characteristic of 5-FU is its nonlinear clearance kinetics which means an increased dose of 5-FU does not result in a proportional increase in plasma concentrations.35 The rapid catabolism of 5-FU by the ubiquitous DPD results in a short elimination half-life of approximately 10–20 minutes with approximately 90% of 5-FU following a metabolic fate.36 A large proportion (60–90%) of the administered dose of 5-FU is excreted in urine as alpha-fluoro-beta-alanine (post-metabolism) which is transported out of cells via GABA transporter-237, whilst approximately 10% and 2–3% is excreted in an unchanged form in urine and bile, respectively.38,39
The levels of 5-FU in plasma have a strong correlation with its biological effects.7 Plasma concentrations of 5-FU can fluctuate over a 24-hour period due to circadian variations in DPD activity.40,41 These fluctuations exist even when the administration rate of 5-FU is controlled as in continuous IV infusion.42 Although administration of 5-FU at a constant rate cannot reduce interpatient variability, it is still preferred to IV bolus injection because multiple bolus IV injections cause higher fluctuations in plasma concentrations of 5-FU than IV infusion. An in vitro study showed that the LD50 of 5-FU for a range of human epithelial cancer cell lines including 2 colon adenocarcinomas was 30–120 µg/ml when cells received pulsed exposures compared to 0.5–1 µg/ml with constant exposure.43 In addition, a meta-analysis of 6 randomized trials involving > 1200 patients with advanced colon cancer revealed higher tumor response rates and greater survival rates in patients receiving continuous infusion of 5-FU compared to those receiving multiple 5-FU intravenous bolus doses.2,44 This can be explained by the fact that the concentration of 5-FU, when delivered by continuous infusion, remains higher than the minimum effective concentration for longer durations.45,46 Thus, IV infusion, which causes less dose fluctuations compared to multiple IV bolus injections, ensures that tumor cells are exposed to 5-FU at effective concentrations for longer periods, resulting in superior antitumor efficacy. Furthermore, patients receiving IV bolus administration of 5-FU were more likely to experience severe hematologic toxicity compared to patients receiving continuous IV infusion.2 Thus, in terms of developing an implantable drug delivery device, one that can release 5-FU with controlled, or at least sustained, release kinetics and is biodegradable and biocompatible would be of great potential benefit to patients undergoing tumor resection.
Clinical use and dosing of 5-FU
5-FU has been used clinically since 195744 and is approved by the United States Food and Drug Administration (USFDA) as an intravenous drug for the treatment of basal cell carcinoma, breast cancer, colorectal cancer, gastric adenocarcinoma, pancreatic cancer and squamous cell carcinoma of the head and neck.47 It is also delivered as a topical agent for the treatment of actinic keratosis48 and corneal squamous cell carcinoma.49 In addition, 5-FU can be used intraoperatively or postoperatively as an adjunct to glaucoma surgery to prevent conjunctiva scarring at the operation site.50 Other ophthalmic pathologies, such as proliferative vitreoretinopathy, can also be treated using 5-FU.51 When used for the treatment of cancer, 5-FU treatment alone has demonstrated low response rates. Bolus and continuous infusions of patients with advanced colon cancer have independently yielded 14% and 23% tumor response rates, respectively.2 In a separate clinical study, involving both elderly and young patients with metastatic colorectal cancer, marginal, albeit significant, enhancement in overall and progression-free survival in both elderly and young patients was observed when continuous infusion of 5-FU was compared to bolus delivery.52 Regardless of whether bolus or continuous delivery is applied, it has long been recognized that 5-FU treatment alone is inadequate even when delivered in combination with leucovorin/folinic acid which is known to stabilize the FdUMP binding to thymidylate synthase.53 Therefore, 5-FU is mostly used in combination with other anticancer regimens ± leucovorin, such as leucovorin and oxaliplatin (FOLFOX regimen)54, leucovorin and irinotecan (FOLFIRI regimen)55 or docetaxel and oxaliplatin (or cisplatin) (DP(O)F regimen).56 These combinations improve response rates to 50–60% and increase median overall survival times compared to 5-FU alone by an extra 6–12 months.54–56 More recently, the combination of 5-FU, leucovorin, oxaliplatin (mFOLFOX6) plus bevacizumab (anti-vascular endothelial growth factor-A) was shown to provide an overall median survival of 34.1 months in patients with metastatic colorectal cancer in a phase IIb trial.57 Further improvements are still nevertheless required to improve survival outcome as well as decrease the negative side effects associated with chemotherapy. Implementing novel 5-FU delivery systems that supply prolonged exposure of 5-FU directly to tumor cells may have advantages for patients at sites of resection, where a concentrated local delivery that does not generate high plasma levels may provide strong localized antitumor efficacy against residual disease, and minimal systemic cytotoxicity. Such a treatment could be of significant benefit to patients undergoing resection of ostensibly curable (through surgery alone) early stage colon tumors since it is recognized that approximately 11% of these patients experience recurrence at the site of resection.58
Currently, 5-FU can be dosed by three major independent approaches. First, the dose can be adjusted based on patient weight. The initial dose recommended by the manufacturer is 12 mg/kg daily for 4 consecutive days with a daily maximum dose of 800 mg.13 Second, 5-FU can be adjusted based on body surface area (mg/m2). Although dose adjustment according to either weight or body surface area is uncomplicated, it is associated with significant interpatient variability with respect to resultant 5-FU plasma levels.7 This variability is primarily a result of variations from patient to patient in DPD activity possibly due to genetic polymorphisms.59,60 Hence the development of the third approach, pharmacokinetically guided (PKG) dose adjustment, which involves administering doses such that the concentration of 5-FU in the plasma is within the therapeutic range.61 This dosing approach was assessed in a phase III clinical trial where metastatic colon cancer patients were given either constant doses of 5-FU (infusion at 1,500 mg/m2 (plus 200 mg/m2 leucovorin infusion)) based on body surface area for 8 hours per week or, alternatively, the patients were given the first dose based on body surface area and then subsequent doses were adjusted to achieve targeted 5-FU exposure (area under the concentration-time curve: AUC) at 20–25 mg.h/L.62 These adjustments were based on weekly measurements of 5-FU in the plasma and using a 5-FU dose-adjustment algorithm. More recently, a meta-analysis was performed on head and neck or colon cancer patients comparing 5-FU treatments based on a body surface area algorithm versus a PKG dose algorithm.8 The patients treated with 5-FU doses based on the PKG dose algorithm experienced significantly increased response rates and reduced toxicities. Thus, it would appear that for colon cancer patients, PKG dose adjustment would be a preferable treatment option, however, it is as yet not widely used in clinical practice possibly due to a lack of consistent results with a wide range of cancer types and a paucity of high quality evidence.8 Therefore, there is an impetus to investigate the development of 5-FU delivery systems that are compatible with the PKG dose adjustment approach. This will be challenging since most of the 5-FU sustained release delivery systems that are being developed depend highly on dosing based on actual weight with non-adjustable release rates while the PKG dose adjustment approach requires adjustable dosing.
5-FU-loaded PLGA formulations
Aside from being an adjunct therapy following tumor resection, delivery systems involving modified drug release also offer advantages for patients with unresectable tumors.63 For instance, the localized release of 5-FU in a sustained fashion at the site of an unresectable tumor, such as a brain tumor, provides the opportunity for the eradication of the tumor, potentially preventing invasion and metastasis as well as diminishing the side effects associated with systemic drug delivery.64–66 Prolonged, and even controlled, release of 5-FU can be achieved by incorporating 5-FU into tunable polymer matrices, resulting in formulations that can release 5-FU for sustained durations.
Many polymer types have been investigated in the quest to develop novel formulations mediating sustained 5-FU release with improved antitumor efficacy.67 Of these polymers, poly(D,L-lactide-co-glycolide) (PLGA) possesses many desirable properties that make it an attractive candidate for use in such systems. PLGA is approved by the USFDA as well as the European Medicines Agency for clinical use.68 It is biodegradable and biocompatible, since it spontaneously hydrolyzes into two monomers, lactic acid and glycolic acid, which are endogenous substances that can be utilized by Krebs cycle.68 In addition, PLGA does not stimulate inflammatory responses per se.69 PLGA is also a candidate polymer for gastroretentive oral formulations because of its bioadhesive properties that allows binding to gastrointestinal mucosa.70 This property extends the residence time of the encapsulated drug in the gastrointestinal tract thereby extending the absorption period. However, oral delivery suffers the drawback of drug loss through first pass metabolism which consequently introduces variability in terms of bioavailability.71–73 Another advantage of using PLGA polymers is that they can be processed into a range of shapes and sizes and are compatible with a wide variety of organic solvents.74 PLGA-based platforms such as microparticles can be readily co-loaded with distinct biomolecules75 or drugs76 and have been shown to be capable of differential release kinetics of drugs from the same particle.76 Finally, PLGA is highly tunable with respect to molecular weight, functionalized end groups and lactide:glycolide ratios.74 With these properties, PLGA has become a popular polymer for use in drug delivery systems and has been used to deliver molecules with diverse physicochemical properties including small molecules, macromolecules, as well as hydrophilic and hydrophobic molecules.68 More of a challenge to overcome is the low loading and high burst release often associated with certain PLGA formulations, such as microparticles, when small hydrophilic molecules such as 5-FU are used.77–81 This review focuses on the implementation of PLGA-based platforms to deliver 5-FU in a sustained fashion and what efforts have been made to improve formulation parameters.
The release of 5-FU from PLGA formulations can be bi-phasic or tri-phasic. For example, several studies have reported bi-phasic release of 5-FU from PLGA particles82–85 and PLGA chamber microdevices10 while others have reported tri-phasic release from PLGA particles63 and PLGA magnetic carrier systems.86,87 Tri-phasic release involves an initial burst release, which is diffusion dominated, followed by a diffusion mediated release and finally a polymer erosion mediated release. The initial burst release of 5-FU from PLGA nanoparticles and microparticles involves the relatively fast release of a large quantity of the drug (typically 15 – 30%) upon immersion into aqueous surroundings.70,88 This is due to drug particles or crystals deposited at, or near, the surface of PLGA particles dissolving and diffusing into the surrounding fluid.70,89 Although a high burst release may have advantages for treatments requiring a loading dose, it can however deplete the drug pool within the PLGA particles too rapidly, resulting in truncated durations of drug release which is clearly undesirable for most antitumor applications. The magnitude of the burst release can be decreased by increasing drug-polymer interaction which will increase the amount of drug molecules that are dissolved or encapsulated in the polymer matrix. Simultaneously, polymer-solvent and drug-solvent interactions must be decreased to reduce the escape of drugs from the polymer matrix.83 The second phase of the release involves an approximately constant release rate of drug molecules. During this second phase, the drug molecules deeper within the PLGA formulation start to diffuse out. As the diffusion path length increases with time, the release of drug molecules should be slower. However, the decrease in drug release is compensated by an increase in diffusion rate because drug molecules become more mobile due to increasing polymer degradation.70 The final rapid drug release phase is the result of erosion and disintegration of the construct/particles, leading to complete drug release.70 In bi-phasic release, the effect of diffusion and polymer degradation are inseparable so the release has no clear cut boundary between the second and the third phase.74
1. PLGA microparticles loaded with 5-FU
Microparticles are defined by the International Union of Applied Physical Chemistry as particles with dimensions of 0.1 – 100 µm in diameter.90 However, it is important to note that many examples exist of published research where particles in the range of 0.1 µm to < 1 µm are defined as nanoparticles or sub-micron sized particles and therefore microparticles are defined in those publications as particles with the dimensions of 1 – 100 µm in diameter. Many articles also define particles as microparticles even when their size falls in the range of 100 – 1000 µm. For the purposes of this review we are defining microparticles as 0.1 – 1000 µm in diameter and will specifically stipulate the size where appropriate. In pharmaceutical fields, microparticles are generally formulated by microencapsulation techniques and can be categorized into two major classes, microcapsules and microspheres.91 Microcapsules are microparticles that contain an internal reservoir of entrapped substances, surrounded by a solid shell.90,91 Microspheres are monolithic-type microparticles that contain no distinct outer layer and drug molecules are embedded, dissolved, or bonded throughout the polymeric matrix.90,91 Many researchers, however, have used the term microparticles, microcapsules and microspheres interchangably.92 In this review we will attempt to delineate between microspheres and microcapsules, the latter of which have been far less studied.
In terms of in vivo sustained delivery of drug-loaded microparticles an optimal size range is considered to be 10 – 200 µm in diameter. The rationale for this size range is that particles < 10 µm in diameter are likely to be vulnerable to removal by phagocytosis by immature dendritic cells or tissue macrophages. Another drawback is that, in the context of achieving sustained delivery lasting at least 4 – 5 days, these smaller microparticles tend to release the majority of their payload too quickly. The upper limit of 200 µm is possibly a little nebulous and could expand out to larger diameters depending on other factors such as route of administration and the specifics of the desired release kinetics. Due to the damage that microparticles can cause to muscle, intramuscular delivery of particles in the size range of 10 – 200 µm can generally be ruled out and therefore subcutaneous delivery would be considered more appropriate.93 It has been reported that PLGA microspheres larger than 300 µm have unpredictable release rates due to heterogeneous degradation rates of the core and the surface of the particles.94 Microspheres < 300 µm generally experience homogeneous degradation by hydrolysis. Ultimately, the optimal size will be determined by end-user preferences such as the desired release rate and the desired duration of release. Factors determining release rate will include the MW of PLGA, molar ratio of lactic acid to glycolic acid, end-group functionalization, and the degree of loading of the drug.81 In the case of 5-FU, being small and hydrophilic, the greater the loading the faster the rate of release.
PLGA microparticles can be fabricated by several methods including emulsion-solvent evaporation/extraction, phase separation (coacervation), electrospraying, supercritical fluid technology, and spray drying. More detailed explanations of these various techniques along with their advantages and disadvantages have been recently reviewed elsewhere.74,81,95–97 PLGA microparticles loaded with 5-FU are often formulated using emulsion solvent evaporation/extraction despite the many associated drawbacks such as poor loading and the use of toxic organic solvents. For this method, single emulsions or double emulsions are created by dispersing drugs (in the case of single emulsions) or aqueous drug solutions (in the case of double emulsions) in a PLGA organic solvent solution (oil phase). The oil phase is then dispersed into a water phase containing surfactant. After dispersion, emulsions are evaporated or extracted with water or quenching media to remove the solvent. Oil-in-water and water-in-oil-in-water emulsions are the most common types of emulsion used for formulating 5-FU-loaded microparticles. The free base form of 5-FU is considered preferable to the salt form as this increases hydrophobicity of the drug and therefore efficiency of loading. However, due to the hydrophilic nature of 5-FU combined with its small size the loading efficiency as well as loading are often very low. As an alternative, in order to improve loading efficiency, solid-in-oil-in-water emulsions have been used.98
Spray drying is another method commonly used to fabricate 5-FU-loaded PLGA microparticles which generally yields higher loading than the emulsion solvent evaporation method since it does not rely on dispersing 5-FU solutions in a water phase and therefore circumvents escape of 5-FU to the water phase. Instead, spray drying is a one step continuous production process that results in the collection of dry particles after spraying the atomized liquid form into a hot inert gas or hot air. Table 1 summarizes the formulation methods used by researchers to fabricate 5-FU-loaded PLGA microparticles as well as their characteristics. It can be seen that many of these microparticles were of sizes possibly too small (< 10 µm) for sustained delivery purposes. Nevertheless for both completeness and potential interest we have included these formulations in the Table.
Table 1.
Characteristics of 5-FU-loaded PLGA microspheres from previous studies
| Fabrication method (particle type) |
PLA:PGA ratio |
Organic phase |
Surfactant | Mean diameter (µm, ±SD) |
Loading efficiency (%) |
Loading (mg/mg) |
% release (time) | Route of administration |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Spray dry (microsphere) | 50:50, | DCM | - | 1.1 ± 0.5 | 52 | 0.047 | 100 (24 h) | - | 23 |
|
| |||||||||
| Spray dry (microsphere) | 75:25 | DCM | - | 1.7 ± 0.9 | 74 | 0.067 | 100 (90 h) | - | 23 |
| 85 (24 h) | |||||||||
|
| |||||||||
| Spray dry (microsphere) | 100:0 | DCM | - | 1.5 ± 0.8 | 56 | 0.05 | 100 (130 h) | 23 | |
| 70 (24 h) | |||||||||
|
| |||||||||
| Emulsion-solvent extraction (S/O/W) | 50:50 | DCM | PVA | 47 | - | 0.26 | 100 (21 d) | - | 63 |
| Burst approx. 25–30 (1 d) | |||||||||
|
| |||||||||
| Spray dry | 50:50 | DCM, chloroform | - | 1.4±0.9 | - | 0.081 | - | lung | 100 |
|
| |||||||||
| Spray dry | 65:35 | DCM, chloroform | - | 1.3±0.7 | - | 0.078 | 90 (24 h) | lung | 100 |
|
| |||||||||
| Spray dry | 75:25 | DCM, chloroform | - | 1.5±0.4 | - | 0.076 | 80 (24 h) | lung | 100 |
|
| |||||||||
| Spray dry | 85:15 | DCM, chloroform | - | 1.4±0.6 | - | 0.075 | - | lung | 100 |
|
| |||||||||
| Double emulsion-solvent evaporation W/O/W | - | DCM | PVA | 10–20 | 70 | 0.0039 | 75–80 (35 d) | - | 82 |
| 15–20 (24 h) | |||||||||
|
| |||||||||
| Spray dry | 50:50 | DCM, chloroform | - | 2.01 | 99.99 | 0.0799 | 70 (24 h) | - | 83 |
|
| |||||||||
| Spray dry | 65:35 | DCM, chloroform | - | 2.23 | 97.42 | 0.0779 | 70 (24 h) | - | 83 |
|
| |||||||||
| Spray dry | 85:15 | DCM, chloroform | - | 2.06 | 91.51 | 0.0732 | 40 (24 h) | - | 83 |
|
| |||||||||
| Emulsion-solvent evaporation | 75:25 | DMSO, DCM | PVA | 55±5 | 48.4 | - | 50–85 (35 d) | - | 84* |
| 10–20 (24 h) | |||||||||
|
| |||||||||
| Emulsion-solvent evaporation | 50:50 | Acetone.Liquid paraffin | lecithin | 4.4±0.6 | - | 0.10 | 85–90 (21 d) | Ocular implant | 89 |
| 70 (24 h) | |||||||||
|
| |||||||||
| Emulsion-solvent extraction | 50:50 | DCM, acetone | PVA | 25 | - | 0.398 | 168 hours | - | 98 |
|
| |||||||||
| Emulsion-solvent evaporation | 50:50 | DCM | PVA | 2.5 | 19 | 0.015 | 3 weeks | - | 104 |
|
| |||||||||
| Multiple emulsion-solvent evaporation | 80:20 and 75:25 (1:1) | DCM | PVA | 515.3 ±129.1 | 82.3 | 0.040 | 60 days | - | 184 |
|
| |||||||||
| Multiple emulsion-solvent evaporation | 80:20 and 75:25 (2:1) | DCM | PVA | 775.0 ±156.6 | 86.5 | 0.041 | 60 days | - | 184 |
|
| |||||||||
| Emulsion-solvent evaporation | 50:50 | DCM | Ethylene glycol, glycerol, PVA | 100–150 | 89.85 | - | 30 days | SC injection | 88 |
|
| |||||||||
| Emulsion-solvent extraction | 50:50 | DCM, acetone | PVA | 48±20 | - | 0.23 | 20 days | Surgical implant | 107† |
|
| |||||||||
| Spray dry | 75:25 | DCM | - | Less than 100 | 20 | - | 30 days | IT injection | 109 |
|
| |||||||||
| Emulsion-solvent evaporation | 50:50 | DCM, ethyl-acetate | - | - | 95.5 | - | 25 days | SC implant | 110* |
|
| |||||||||
| Emulsion-solvent extraction | - | DCM | PVA | 20–30 | 22.6 | - | 10 days | - | 111* |
Abbreviations: DCM – dichloromethane; PVA – polyvinyl alcohol; SC – subcutaneous; IT – intratumoral; W/O/W – water-oil-water; S/O/W – solid-oil-water; PLA – polylactic acid; PGA – polyglycolic acid
human study
mixed materials
Studies with monolithic microparticles/microspheres
The in vitro release kinetics of 5-FU from PLGA microparticles can depend on multiple factors such as the size of the microspheres, drug loading parameters (e.g. solid versus soluble; amount of drug loaded), polymer degradation rates, drug diffusion, hydrophobicity and molecular weight of the PLGA polymers and experimental conditions.63 The effect of the size of PLGA microspheres on rate of release of 5-FU has been studied in vitro using microspheres prepared using a solid-in-oil-in-water solvent extraction method.99 This study found that 5-FU release rates positively correlated with the size of microparticles, where the size range involved mean diameters of 29 to 78 µm. This positive correlation is not in agreement with Fick’s laws of diffusion since 5-FU release is primarily diffusion-dependent and therefore the release rate should decrease with an increasing diffusion path length (i.e. increasing particle size). A potential reason for this apparent anomaly is that larger particles had a greater accumulation of acids resulting from PLGA degradation, however, this was shown not to be the case through differential scanning calorimetry. Instead it was found that drug loading increased with increasing particle size and, because the 5-FU was loaded as crystals it meant that larger particles became more porous during release thereby contributing to higher rates of release of 5-FU. A mathematical model was devised that took into account 5-FU diffusion and PLGA degradation and was capable of predicting 5-FU release rates from microspheres of varying sizes. The findings from this article are promising in terms of potential clinical translation of 5-FU-loaded PLGA microparticles in that they provide evidence of sustained (21 days) drug release at a predictable rate and also that intraparticle acidity often associated with monolithic PLGA microparticles was likely abrogated by the presence of 5-FU crystals (as explained above).
The solubility of 5-FU is very important because its hydrophilicity strongly influences both loading efficiency into, and release rates from, PLGA particles.98 It has been demonstrated that 5-FU solubility in dispersed solvents affects its physical state (e.g. fully dissolved, partially dissolved or deposited as crystals) within the PLGA matrix of microspheres.91 As alluded to above99, these differences in physical states affect release kinetics since crystallized 5-FU requires dissolution of the crystal before 5-FU can diffuse out of the particles while completely dissolved 5-FU can begin diffusing out of particles immediately.91 Refining the size of the raw 5-FU powder, which on average contains crystals of approximately 275 µm in diameter, by pulverizing them to sizes < 20 µm prior to encapsulation ensures sufficient distribution of 5-FU within the PLGA matrix of microparticles of sizes ranging from 20 – 100 µm.98 These researchers also found that manipulating the manufacturing parameters such as organic:water phase ratios significantly impacted the release profiles of the resultant microparticles with particles being loaded with 5-FU crystals using an organic:water phase ratio of 1:33 having a longer release duration (18 days) than particles made using an organic:water phase ratio of 1:61 (48 hours). Drug diffusion and PLGA degradation can be influenced by the lactide:glycolide ratio. Lactide monomers are more hydrophobic than glycolide monomers and therefore a higher lactide:glycolide ratio results in a slower rate of water penetration into particles, reducing both the 5-FU diffusion rate and the PLGA hydrolysis rate.100 A study showed that the in vitro release of 5-FU from submicron sized particles formulated from PLGA 50:50 was faster than from particles made from PLGA 90:10.101
External conditions that govern 5-FU release from microparticles include osmolarity, pH and temperature of the release media. It has been reported that increasing the osmolarity of the release media decreases the water uptake rate by the particles, consequently decreasing the release rate of 5-FU.63 The pH of the release media has been shown to have a positive correlation with the release rate and this effect can be mainly explained by the change in the glass transition state at different pH conditions.63 In acidic conditions (pH 1.3–4.5), PLGA has a glass transition temperature at 40°C, whilst in basic conditions (pH 7.4–10.8) the glass transition temperature is 30°C. Therefore, PLGA at 37°C, in acidic conditions, generally appears in a glass form where there is limited mobility of PLGA side chains, thus impeding the movement of 5-FU. This explains the finding that 5-FU release was very rapid in basic conditions but was slower in acidic conditions.63 High temperatures (e.g. ≥ 60°C) can also affect the movement of PLGA side chains. PLGA side chains can be highly mobile at high temperatures allowing 5-FU to diffuse out of particles at a greater rate when compared to cooler temperatures.98 These factors emphasize the importance of using settings that mimic physiological conditions when performing in vitro release studies, otherwise the results will be less translationally relevant. Radiation is another external condition that can affect the release of 5-FU from PLGA particles. Studies have shown that ultrasound increased protein release from PLGA porous disks102 while gamma-irradiation103 accelerated the release of 5-FU from PLGA particles. Both studies speculated that these forms of radiation mechanically degrade PLGA which causes an increase in the release of 5-FU.
The duration of release of 5-FU also depends on the amount of 5-FU that can be loaded into particles. The double emulsion solvent evaporation method, which can be used to prepare 5-FU-loaded particles70, yields low 5-FU loading because the drug can rapidly diffuse across the phase boundary into the external water phase during the solvent evaporation step.98 In order to overcome this problem, a common approach is to decrease the solubility of 5-FU in a dispersed phase to prevent the diffusion of 5-FU across phases.98 This can be achieved by co-loading 5-FU with hydrophobic drugs e.g. paclitaxel.104 Solvents used for formulation can also affect the loading. If the solvent evaporates too slowly then PLGA precipitation is also slow and cannot efficiently trap 5-FU within the particles.70 In addition, for double emulsions, the difference in osmotic pressure between the inner and outer water phases can lead to the rupture of primary emulsions that contain 5-FU. This pressure can be reduced by optimizing the PLGA:5-FU ratio to yield particles with surfaces that can tolerate the pressure difference.70 Increasing the volume of the external water phase leads to an increase in particle size and encapsulation efficiency.70
The levels of 5-FU found in the body over time subsequent to in vivo administration of 5-FU-loaded microparticles depends on the release kinetics of 5-FU from the microparticles, the pharmacokinetics of 5-FU as well as the route of administration of the microparticles. In vitro release kinetics of drugs (including 5-FU) from PLGA microparticles are usually an underestimate of the in vivo release kinetics for a number of reasons. One reason is that the rate of hydrolysis is faster in vivo due to the presence of hydrolytic enzymes.105,106 Another reason can be the inability of the in vitro system to adequately represent mechanical loads. Most in vitro studies underestimate or fail to take into account the mechanical compression that occurs inside the body. This can result in a discrepancy in the duration of in vitro and in vivo release.
Preclinical and clinical studies have demonstrated that 5-FU-loaded PLGA microspheres possess antitumor properties. A phase 1 clinical study demonstrated that implantation of 5-FU-loaded PLGA microspheres into the walls of the surgical bed after surgical resection of tumor in combination with external beam radiotherapy for the treatment of glioblastoma was safe.107 However, a randomized, multicenter phase 2 study conducted later by the same group of investigators did not find significant clinical benefits of 5-FU-loaded PLGA microspheres with radiotherapy over the radiotherapy alone.66 A trend was observed, nevertheless, in favor of the 5-FU-loaded microspheres with respect to enhanced median overall survival (15.2 months vs 13.5 months) and currently preclinical studies are in progress in an attempt to improve the formulation.108 In a separate preclinical study in nude mice it was found that 5-FU-loaded PLGA microspheres fabricated by spray drying had a stronger antitumor effect against HCT-116 human colorectal cancer than 5-FU solution when injected intratumorally at equivalent doses.109 The higher efficacy was likely to have been due to the 5-FU-loaded PLGA microspheres allowing increased duration of exposure of the tumor cells to 5-FU. This group measured plasma concentration of 5-FU and the non-compartmental pharmacokinetic analysis showed that encapsulation of 5-FU into PLGA microspheres increased the half-life of 5-FU to 195 hours meaning that tumor cells were exposed to 5-FU for a longer period of time. Vd increased from 33.14 L to 2,055 L and AUC decreased from 724 mg/L to 148 mg/L when soluble 5-FU and 5-FU-loaded microspheres were respectively compared. This indicates that deep tissue infiltration of 5-FU from microspheres increased and that systemic exposure to 5-FU decreased.109
5-FU-loaded PLGA particles can be loaded into other formulations in order to further fine tune the release kinetics of 5-FU. For example, it has been reported that 5-FU-loaded PLGA particles can be successfully incorporated into commercial hemostatic gelatin sponges.110 These particles were absorbed into gelatin sponges before solvent evaporation to ensure homogeneous particle distribution. The loading capacity of 5-FU when 5-FU-loaded PLGA particles were absorbed into gelatin sponges was higher than that obtained for soluble 5-FU loaded into sponges. Encapsulation of 5-FU into PLGA particles also prolonged the release of 5-FU from the sponges from 2 hours in 5-FU-loaded sponges to 25 days in 5-FU-loaded PLGA particle-absorbed sponges. An in vivo study using CT26 colon cancer challenged mice demonstrated that 5-FU-loaded PLGA particles absorbed into sponges, and delivered subcutaneously, had a longer 5-FU elimination half-life and resulted in greater survival than 5-FU-loaded sponges. Another study used a chitosan hydrogel, instead of a gelatin sponge, as an injectable carrier for 5-FU-loaded PLGA particles.111 The 5-FU-loaded chitosan gel exhibited a 12 hour release profile while 5-FU-loaded PLGA particles incorporated into chitosan gel provided a 10 day 5-FU release profile.
2. PLGA nanoparticles loaded with 5-FU
According to the European commission, the definition of nanomaterial is “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range of 1–100 nm”.112 Nanomaterials possess properties that they do not exhibit in bulk form, e.g., improved strength, greater flexibility, increased surface energy and surface reactivity, enhanced thermal conductivity and greater electric conductivity.113 Nanoparticles are, by definition, nanomaterials and are particles of any shape with measurements of 1 – 100 nm in at least 2 dimensions, thus tubes and fibers can also be considered nanoparticles.90 Round-shaped nanoparticles can be categorized into two types, nanospheres and nanocapsules. Nanospheres are spherical nanoparticles that have no outer layer or external membrane. Nanocapsules are nanoparticles that have a cavity for entrapping molecules inside the polymeric shell.90 PLGA nanoparticles can be fabricated by methods used to fabricate PLGA microparticles (see above). Additional methods include nanoprecipitation and salting out.74 For nanoprecipitation, a drug and polymer in an organic solvent is gradually dropped into an aqueous solution containing surfactant (e.g. Pluronic F68). The organic solvent evaporates under reduced pressure, resulting in drug-loaded nanoparticles. For the salting out method, water/oil emulsions containing polymer, solvents, salts (e.g. magnesium acetate tetrahydrate) and stabilizers are fabricated. Then water is added to diffuse the solvent out, resulting in nanoparticle formation.74 PLGA nanoparticles have proven capable of providing sustained release of molecules with diverse physicochemical properties such as docetaxel114, siRNA115 and rifampicin.116
2.1 PLGA nanospheres loaded with 5-FU
5-FU-loaded nanospheres are usually formulated by an emulsion-solvent evaporation method (table 2) which, in general, yields low drug loading. The loading efficiency of 5-FU into nanoparticles versus microparticles is lower because of the higher surface area to volume ratio of the former which contributes to higher amounts of 5-FU lost during formulation.70 As is often the case with microparticles the burst release of 5-FU from nanoparticles can be very large, often greater than 60%.117 Nevertheless the advantage associated with PLGA nanoparticles, as with nanoparticles in general, is that they can be administered intravenously so as to target tumors via the enhanced permeation and retention (EPR) effect and have the potential to be surface decorated with tumor targeting ligands.118 However, very few in vitro studies have reported on 5-FU-loaded nanoparticles designed for the purposes of tumor targeting through the EPR effect117 and no in vivo studies have been documented to date.
Table 2.
Characteristics of 5-FU-loaded PLGA nanospheres from previous studies
| Fabrication method |
PLA:PGA ratio |
Organic phase |
Surfactant | Diameter (nm±SD) |
Encapsulation efficiency (%) |
Loaded drug amount (mg/mg) |
Release duration (hours) |
Route of administration |
Ref |
|---|---|---|---|---|---|---|---|---|---|
| Double emulsion-solvent evaporation* | 75:25 | Acetic ether | Poloxamer 188 | 81.4–87.7 | 64 | 0.13 | 288 | Oral | 70 |
| Emulsion-solvent evaporation* | 50:50 | Acetone | Lutrol F68 | 117.8 ± 15.6 | 59.1 | 0.0439 | 120 | - | 85 |
| Double emulsion-solvent evaporation* | 50:50 | DCM | Pluronic F-68 | 150 | 66.6 | - | 168 | - | 101 |
| Double emulsion-solvent evaporation* | 90:10 | DCM | Pluronic F-68 | 190 | 65.5 | - | 168 | - | 101 |
| Double emulsion-solvent evaporation | 50:50 | DCM | PVA | 185–300 | 17.13–38.37 | 0.024–0.068 | 120 | - | 185 |
| Double emulsion-solvent evaporation | 85:15 | DCM | PVA | 126.9 ±64.3 | 8 | - | 360 | IV | 119 |
| Double emulsion-solvent evaporation | 50:50 | DCM | Pluronic F-68 | 100–200 | 0.7313 | 0.2023 | 2.5 | - | 186 |
PdI are provided.
Abbreviations: IV - intravenous; DCM – dichloromethane; PVA - polyvinyl alcohol; PLA – polylactic acid; PGA – polyglycolic acid
Nanoparticles can provide sustained release of 5-FU over a long period of time (at least 5 days, see table 2). A pharmacokinetics study in Sprague-Dawley rats demonstrated that 5-FU could be released in a sustained fashion from nanoparticles. This study administered 5-FU nanoparticles by an intragastric route and found that the elimination half-life of 5-FU increased from 0.36 hours to 2.35 hours and the plasma concentration of 5-FU remained higher than 1 µg/ml for 24 hours.70
The release and efficacy of 5-FU from 5-FU-loaded PLGA nanoparticles has the potential to be improved by ultrasound.102,119 IV administration to glioma-challenged mice of 5-FU-loaded PLGA nanoparticles covalently bound to microbubbles resulted in enhanced localization of nanoparticles to ultrasound treated subcutaneous solid tumors compared to tumors not treated with ultrasound or compared to mice administered with noncovalently linked microbubbles and nanoparticles (plus ultrasound). In addition, enhanced survival of mice was observed compared to mice treated with soluble 5-FU. This study demonstrates how ultrasound can lead to enhanced effective delivery of 5-FU to tumors thereby enhancing antitumor efficacy whilst reducing systemic distribution of 5-FU to healthy tissues.
2.2 PLGA nanofibers loaded with 5-FU
Nanofibers are fibers that have diameters ≤ 100 nm.120 Because nanofibers have large specific surface areas and high porosities, they have a very large surface area-to-volume ratio.120 This explains why the release of drugs loaded into nanofibers depends solely on drug diffusion, as opposed to polymer degradation. Currently, nanofibers can be fabricated by three methods: electrospinning, self-assembly and phase-separation.121 The process of electrospinning is a simple procedure that can be used with various types of polymers and is the most preferred method for the fabrication of PLGA nanofibers. 122,123 Electrical potential is applied to the polymer solution, leading to the elongation of the polymer, yielding nanofibers of 50–1000 nm in length. Self-assembly is a method used to generate nanofibers from amphiphilic polypeptides that contain hydrophilic head groups and hydrophobic tail groups. The reduction of cysteine in the peptides to free the thiol groups followed by the reduction of pH to < 4 results in self-assembly of amphiphilic polypeptides into cylindrical micelles or nanofibers of micron length.121 Phase separation involves five steps which include polymer dissolution, phase separation and gelation, solvent extraction, freezing, and lyophilization. Nanofibers have been used in filtration, spill containment, decontamination, catalysis and are also useful scaffold materials in tissue engineering and wound dressing.120,122 In addition, PLGA nanofibers can be used as delivery platforms for anticancer drugs such as daunorubicin and, as will be discussed below, 5-FU.123
PLGA nanofibers co-loaded with 5-FU and oxaliplatin were implanted onto the tumor surface of colon cancer challenged mice and shown to enhance their survival compared to mice treated IV with free oxaliplatin and 5-FU.124 A separate study demonstrated the benefit of coating stents with 5-FU-loaded nanofibers to prevent recurrence of obstruction in gastrointestinal cancer. Gastrointestinal stents are minimally invasive options for acute decompression in gastrointestinal cancer with obstruction.125 However, the effect of uncoated stents is temporary since tumors continue to grow and obstruction recurs. It was shown that 5-FU-loaded polylactide nanofiber-coated polydioxanone colonic stents were able to reduce tumor volume in mice.126 In another study, 5-FU-loaded PLGA nanofibers and blank PLGA nanofibers were coated as alternate layers onto an esophageal stent.127 The stents coated with only 5-FU-loaded PLGA nanofibers demonstrated a relatively brief 6 day release period of 5-FU with total drug loading of 15.66 µg/cm3, while the stents coated with alternating layers of blank PLGA nanofibers and 5-FU-loaded PLGA nanofibers had a drug loading of 10.62 µg/cm3 and exhibited a 21 day release period. Although drug loading decreased as additional PLGA nanofiber layers were added, these blank PLGA nanofiber layers retarded the release of 5-FU from the formulation presumably by preventing the formation of pores in 5-FU-loaded PLGA nanofibers.127
3. PLGA magnetic carrier systems for 5-FU
Polymeric magnetic carrier systems are targeting drug delivery systems incorporated with magnetic materials such as iron oxides (Fe2O3 and Fe3O4), metal alloys (Fe, Co and Ni) or iron cobalt alloys.128 The application of an external magnetic field or magnetic implant is used to target magnetic carrier systems to, and retain them at, a desired location.129 Magnetic particles in the nanosize range are of particular interest because at dimensions < 20 nm they exhibit superparamagnetic behavior, which is the ability to become powerfully magnetized under an external magnetic field (e.g. during magnetic resonance imaging).128 Polymeric magnetic systems provide a number of advantages which include: 1) the system can be delivered and retained at the target site by applying a magnetic field. Magnetic particles that penetrate into interstitial spaces may remain at the target site even after the removal of the magnetic field.130 2) Polymeric magnetic carrier systems are considered biocompatible because iron can be eliminated by normal iron metabolic pathways. Also, an in vivo study using a mouse macrophage cell line demonstrated that the combination of magnetic materials and PLGA were not toxic.131 The main disadvantage with using magnetic carrier systems is that target sites are generally restricted to superficial tissues such as skin and joints. This is because the magnetic moments of the magnetic carrier systems are small and therefore not strong enough to resist the blood flow, thus being unable to be retained, at targeted deeper tissues.132 Another disadvantage of polymeric magnetic carrier systems is the possibility of side effects such as thrombosis at the site of catheterization or vascular obstruction. PLGA-based magnetic carrier systems have been developed to deliver 5-FU in a sustained fashion and are mostly fabricated as particles using a multiple-emulsion solvent evaporation method (Table 3).
Table 3.
Characteristics of 5-FU-loaded magnetic PLGA nanoparticles from previous studies
| Fabrication method |
PLA:PGA ratio |
Organic phase |
Surfactant | Mean diameter (nm) |
Encapsulation efficiency (%) |
Loaded drug amount (mg/mg) |
Release duration (days) |
Route of injection |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| Multiple emulsion-solvent evaporation | 50:50 | DCM | PVA | 90.7–316.6 | 83.4 | 0.043 | 25 | - | 86 |
| Multiple emulsion-solvent evaporation | 50:50 | DCM | Glycerin, span 60 | 67.2 | 63.8 | 0.073 | 35 | IV | 87 |
| Emulsion-solvent evaporation | - | Aceto-nitrile | Span 80 | 800 | - | 0.20 | - | PT | 187 |
| Nanoprecipitation | - | Liquid paraffin, aceto-nitrile | Span 80 | 200–1500 | - | - | - | - | 131 |
Abbreviations: SC – subcutaneous; IV – intravenous; DCM – dichloromethane; PLA – polylactic acid; PGA – polyglycolic acid; PT – peritumoral
In vitro release experiments demonstrated that 5-FU-loaded PLGA magnetic particles (90–320 nm diameter) provided sustained release of 5-FU (Table 3). The initial burst release was dependent on PLGA concentration, 5-FU amount and surfactant (PVA) concentration in the formulation.86 These factors also affected particle size, drug loading and encapsulation efficiency. Sustained release of 5-FU-loaded PLGA magnetic nanoparticles was also confirmed in vivo. A pharmacokinetic study in rabbits demonstrated that 5-FU-loaded magnetic PLGA nanoparticles administered IV could provide sustained release of 5-FU for at least 7 days and extend the elimination half-life of 5-FU to 22 minutes.87 This study also demonstrated that 5-FU-loaded magnetic nanocapsules administered at the dose of 3 mg/kg had significantly higher therapeutic activity in a murine CT26 colon cancer model than 5-FU in solution at the same dose. In addition, this study reported that the antitumor activity of 5-FU-loaded magnetic PLGA nanoparticles was potentiated by applying a magnetic field at the tumor site.87
3. PLGA implantable matrices loaded with 5-FU
3.1 PLGA films loaded with 5-FU
Polymeric film coating has been used to improve the appearance, stability and release profiles of solid dosage forms, especially tablets.133 Films are also used to coat medical devices to provide drug release or prevent infection.134 Polymeric films can be formulated by several methods.135,136 PLGA films loaded with 5-FU have been successfully fabricated by the solvent casting method137 and spray technique69 using PLGA 50:50. For the solvent casting method, solutions of polymer and other ingredients are mixed, dried under vacuum, and the resulting film is cast onto a desired surface. The spray technique involves a solution of drug, polymer and excipients that are sprayed onto a suitable material (e.g. glass, polyethylene film or Teflon sheets). Often 5-FU-loaded PLGA film formulations are layered to form discs and these are discussed further in section 3.2.
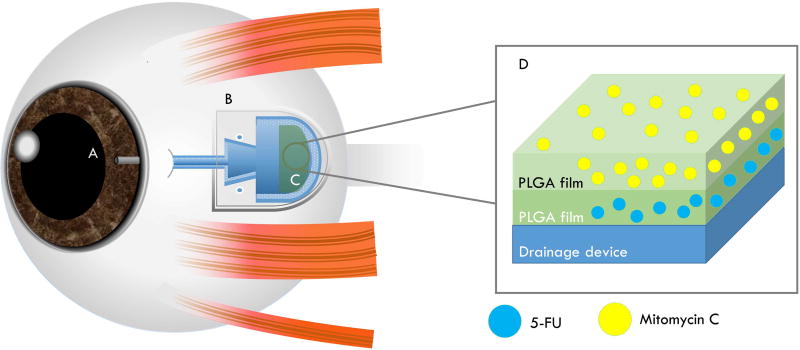
Both 5-FU and mitomycin C can mitigate side effects of glaucoma drainage devices and it has therefore been a goal to combine delivery of these drugs with the drainage device (Figure 3). One group used a modified method of spin coating to create double layered “breath figure” (or honey-comb-like) PLGA films capable of co-delivering mitomycin C (top layer) and 5-FU (bottom layer) with effective cytotoxicity towards fibroblasts for 28 days in vitro.69 The same group then demonstrated the benefits of coating the Ahmed drainage device with a similar formulation in vivo (rabbits).138 It was proposed that such films could be used in the future to coat glaucoma drainage devices used for glaucoma patients so as to reduce both inflammation and fibrosis.69
Figure 3. Schematic of a glaucoma drainage device coated with a 5-FU-loaded PLGA film.
The glaucoma drainage device is implanted to control the intraocular pressure in glaucoma treatment. Drugs such as mitomycin C and 5-FU have been used post-operatively to reduce the fibrosis. One type of draining device, the Ahmed glaucoma valve (pictured), has been tested preclinically in rabbits in combination with a PLGA coating designed to gradually release mitomycin C and 5-FU and shown to be effective.138 (A) An opening/tube of the device that drains intraocular fluid at the anterior chamber of the eye. (B) The end-plate of the drainage device attached to sclera. This part collects intraocular fluid which is reabsorbed back to the eye by passive diffusion. (C) PLGA film coating containing 5-FU and mitomycin C. (D) Cross-sectional view of the PLGA films loaded with mitomycin C and 5-FU. Thus, 5-FU-loaded PLGA film coating the device can help in preventing scarring and fibrosis.183
3.2 PLGA wafers and discs loaded with 5-FU
Wafers are thin slices of material sometimes referred to as thin films.139 Discs are drug delivery systems in a disc-shape that may have convex or concave bases.140 The semantic and practical differences between wafers and discs seems unclear and although they may be similar, we have delineated between the two in deference to the respective authors and to avoid any potential ambiguity. In the pharmaceutical field, wafers are extensively used for buccal drug delivery135,141,142, wound dressing143,144 and cancer treatment. The first USFDA-approved wafer for cancer treatment was the Gliadel® wafer which is a carmustine (bis-chloroethylnitrosourea or BCNU) loaded anticancer implant fabricated from poly[bis(p-carboxyphenoxy)propane with sebacic acid145 for the treatment of malignant glioma after resection.146,147 Although the role of this wafer for clinical use is controversial, a meta-analysis showed that carmustine-loaded wafers provided a sustained release of carmustine and improved survival in patients newly diagnosed with glioblastoma (Hazard ratio = 0.63 [95% confidence interval = 0.49–0.81]).148 There have been attempts to improve the efficacy of the wafer by loading carmustine into other types of wafer, such as PLGA149,150, so that the release profile of the drug can be modified, or by replacing carmustine with other anticancer agents, such as 5-FU. The rationale for replacing carmustine is that it drains out of brain tissue rapidly, reducing exposure time to cancer.151 Also the carmustine wafer can increase intracranial pressure and cause cerebrospinal fluid leakage.152 Thus alternative drug delivery formulations are being investigated.153,151
5-FU is one alternative to carmustine due to its size and hydrophilicity which promotes 5-FU accumulation in brain tissue.151 In one study, 5-FU-loaded PLGA wafers were fabricated from 5-FU loaded PLGA 50:50 microparticles using a compression molding method at room temperature.154 The microparticles were formulated by either mechanically mixing 5-FU with PLGA or by an emulsion solvent evaporation method using acetone as a solvent. The PLGA wafers fabricated from both types of 5-FU-loaded microparticles provided sustained release of 5-FU in vitro for at least 10 days. The release rate and initial burst release of 5-FU increased with increased 5-FU loading. The burst release resulted from the disintegration of 5-FU-loaded PLGA microparticles at the surface of the wafers. Microparticles fabricated by the emulsion solvent evaporation technique had a lower burst release than wafers incorporated with microparticles fabricated by the mechanical mixing method. Subsequent release was mainly due to disintegration of the microparticles deeper within the wafer as confirmed by SEM microphotographs which showed water channels within the wafer.154 Another study confirmed the sustained release of 5-FU from PLGA wafers fabricated from the physical mixing of 5-FU and PLGA by compression molding.155 This in vitro study showed initial fast degradation of PLGA after 6 hours followed by gradual degradation. SEM imaging showed pores at the surface and penetrating inside the PLGA wafers. This indicated that degradation of the PLGA wafer occurred throughout the wafer, not only at the surface.155 This result was in contrast to the findings for wafers formulated from poly[bis(p-carboxyphenoxy)propane with sebacic acid where degradation occurred only at the surface.156
Drug-loaded discs are generally prepared by compression140,157–159 and have been used as gastric floating systems140 and local implantable systems157 that provide sustained release.140 In the case of 5-FU, they can be prepared by compression or prepared from 5-FU-loaded films. In one study, 5-FU-loaded PLGA discs were formulated with PLGA 50:50 using a compression molding method, resulting in discs with 3 mm radii.158 The discs released approximately 70% of loaded 5-FU within the first 3 hours and were able to provide sustained release of 5-FU for 5 days. Factors determining the release rate of 5-FU from the formulation included the loaded amount of 5-FU and the glycolide:lactide ratio in the PLGA polymer. Because glycolide monomers are more hydrophilic than lactide monomers, a higher glycolide:lactide ratio resulted in a faster rate of PLGA hydrolysis. In addition, because 5-FU is a hydrophilic molecule, increasing the amount of 5-FU resulted in higher burst releases from those formulations.
There have been several studies where 5-FU-loaded PLGA discs have been fabricated from 5-FU-loaded PLGA films. One study fabricated 5-FU-loaded discs by compressing multilayers of 5-FU-loaded PLGA films together.137 The films were formulated by solvent casting using PLGA 63:37 and dimethyl chloride (DCM), then cut into discs and subsequently studied for release. The single layered discs released approximately 65% of loaded 5-FU during the first 2 hours as an initial burst release. The release continued for 5 days. To reduce the burst release, multilayered 5-FU discs were fabricated by compressing empty PLGA film and 5-FU-loaded PLGA film layers. An in vitro release study demonstrated that the burst release during the first 2 hours was reduced and the release duration of multilayered discs lasted 17 days. The study also demonstrated that the size of discs in terms of diameter was not related to their release kinetics.
3.4 PLGA millirods loaded with 5-FU
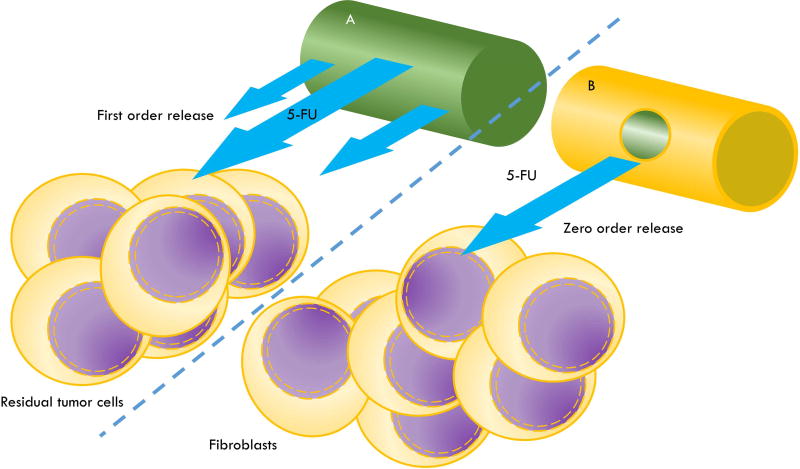
Millirods (minirods or minipellets) are drug delivery systems with a cylindrical shape, a diameter of approximately 1 mm or less and a length ranging from 1–10 mm. Millirods can be prepared by extrusion160–163, direct compression164 or a compression-heat molding process.24,51,165 Many studies have reported on the sustained release profiles of a variety of substances, including DNA166, proteins (e.g. recombinant human bone morphogenetic protein-2162, diphtheria and tetanus toxoids163) and vaccine antigens (e.g. Clostridium antigen161). One advantage of millirods is that they can be implanted using needles (e.g. gauge 14 tissue biopsy needles24), therefore avoiding the need for surgery. PLGA millirods have been fabricated and investigated mainly as peritumorally implanted delivery systems for anticancer agents such as doxorubicin164, paclitaxel167 and β-lapachone.165 In addition, millirods can be designed to drastically alter the release rates of the loaded drug as has been demonstrated with 5-FU-loaded PLGA millirods such that zero order24,168 and first order release kinetics could be achieved168 (Figure 2).
Figure 2. Schematic of 5-FU-loaded PLGA millirods with distinct release kinetics for the purpose of local antiproliferative effects.
Sustained local delivery of 5-FU from PLGA millirods (1 – 1.5 mm diameter), administered intraoperatively, can be designed to have antiproliferative potential for such purposes as the eradication residual tumor cells at the site of tumor resection or, alternatively, for preventing fibrosis during glaucoma treatment. (A) A 5-FU-loaded PLGA millirod (a homogenous mixture) that would generally be expected to release 5-FU with first order kinetics and is more likely to be suitable in situations where minimal time to reaching therapeutic concentrations is desired. (B) A 5-FU-loaded PLGA millirod (green) coated with drug-impermeable PLGA (without 5-FU) (yellow) and having a small hole (~0.7 mm diameter) drilled through the coating from where the 5-FU can diffuse out with zero order kinetics.168 Situations where zero order release kinetics are particularly desired are where it is vital that off-target effects are avoided such as in ocular therapies. This figure is not drawn to scale.
One study formulated 5-FU-loaded PLGA millirods from PLGA 50:50 by a compression-heat molding process.24 Physical mixtures of PLGA and 5-FU at various ratios were melted and compressed to yield 5-FU-loaded PLGA monolithic millirods. Then PLGA membranes containing NaCl particles were formulated by the solvent casting method, resulting in NaCl impregnated PLGA films. The films were wrapped around the 5-FU-loaded PLGA monolithic millirods using forceps. An in vitro release study showed that the amount of 5-FU loaded into the monolithic millirods directly affected the release pattern of 5-FU from the millirods. Millirods with 30% w/w 5-FU exhibited a high initial release and released 90% of loaded 5-FU within 40 hours while the millirods with 10% w/w 5-FU displayed low initial release and released only 30% of loaded 5-FU after 160 hours.24 This was explained by the fact that 5-FU in millirods dissolves and leaves interconnected holes in the PLGA matrix. Therefore, water channels inside the rods were created faster in the formulations with higher percentages of 5-FU. It was further discovered that the release from millirods was influenced by the concentration of NaCl impregnated into the PLGA film coat. Whilst the PLGA film alone (without NaCl) retarded release of 5-FU from the monolithic millirods, increasing amounts of NaCl in the film correlated with faster release of 5-FU. This is due to the hydrophilicity of NaCl causing water channels within the film as the salt dissolves. By varying the NaCl concentration, a sustained release pattern ranging from 5–30 days could be achieved.24
Aside from their potential as locally administered antitumor devices, another application for 5-FU-loaded PLGA millirods is as subconjunctival implants. Specifically, 5-FU has been shown to increase the success of glaucoma filtering surgery for patients designated to have a high risk of failure. However, because of the short half-life of 5-FU, treatment involves daily or weekly injections of the drug for two to five weeks which not only places a burden on the patient but also runs the risk of ocular injuries. Thus, safe delivery devices are required to reduce patient discomfort as well as provide effective doses of 5-FU over sustained periods. One study reported on the fabrication and characterization of 5-FU-loaded PLGA ocular implants made using a direct compression method.168 In vivo (rabbit), it was found that these 5-FU-loaded millirods (2.5 mm in length and 1.5 mm in thickness) released the drug too quickly (almost 100% release in 24 hours) unless they were also coated with PLGA and, additionally, had a central hole (0.7 mm) drilled through both coating and millirods. The coated millirods containing a central hole were found to release 5-FU with first order kinetics over a period of 200 hours, providing steady state concentrations of 5-FU in the conjunctiva within 24 hours that were in the therapeutic range without being cytotoxic. The reason for a lack of translation into the clinic of such a formulation has not been documented but may stem from a reluctance of clinicians to risk damaging a patient’s sight through unforeseen outcomes, such as an unexpectedly excessive release rate of 5-FU, which could result in permanent retinal damage. In short, the risks may be deemed to outweigh the benefits. Another possibility is that the in vivo release duration is still insufficient since it is desired that the release of 5-FU last for up to one month. More recently, promising in vivo (rabbit) results have been obtained using mitomycin c and 5-FU co-loaded PLGA films (see section 3.1), however, as yet there has been no translation into the clinic.138
3.5 PLGA chamber microdevices loaded with 5-FU
Advances in lithographic technology have enabled the fabrication of PLGA microdevices.10,169,170 In one study a micromold was fabricated by casting polydimethylsiloxane (PDMS) onto a lithographically patterned SU-8 mold. The PDMS mold was used to fabricate 5-FU-loaded PLGA microdevices with honeycomb structures and 5-FU was injected as a fine powder into the chamber. Then, the top of the device was sealed with a PLGA 50:50 membrane. The device dimensions were 10 × 10 × 1 mm and contained up to 10.7 mg of 5-FU/device. An in vitro experiment showed that 5-FU-loaded PLGA microdevices released approximately 86% of loaded 5-FU within the first 10 days.169 This study also demonstrated that peritumoral implantation of 5-FU-loaded PLGA microdevices improved the efficacy of the 5-FU treatment as demonstrated in mice challenged with murine sarcoma S180 cells. Tumor cell mass in mice implanted with the device containing 7.2 mg of 5-FU was significantly smaller than mice receiving 7.2 mg 5-FU/mouse as an IP injection.169
Another study by this group investigated the effect of the molecular weight and monomer composition of PLGA on 5-FU release.10 The study showed that devices fabricated from PLGA 50:50 provided faster release than devices made from PLGA 75:25 with the same molecular weight. Also, PLGA with lower molecular weight provided faster release than PLGA with higher molecular weight. The release durations from the devices fabricated from PLGA 50:50 (27 KDa), PLGA 50:50 (40 KDa) and 75:25 (27 KDa) were 15, 20 and 25 days, respectively. An antitumor efficacy study in mice challenged with murine sarcoma S180 cells showed that the three types of device provided the same antitumor efficacy within the first 15 days; however, tumor relapse occurred in the PLGA 50:50 (27 KDa) group.10 This group of researchers also conducted another study to observe the pharmacokinetics of 5-FU released from this device.170 Mice administered with 5-FU via IP injection (7.2 mg/mice) had a peak plasma concentration (Cmax) of 0.83 µg/ml and time to peak plasma concentration (Tmax) at 0.5 hours. The plasma concentration was lower than the limit of detection (50 µg/ml) within 2 hours. In contrast, 5-FU-loaded PLGA microdevices provided sustained release of 5-FU in vivo as seen by the pharmacokinetic parameters (Cmax: 0.16 µg/ml; Tmax: 6 days). The 5-FU-loaded PLGA microdevices provided sustained release of 5-FU in vivo for up to 12 days. Importantly, the pharmacokinetic study also showed that 5-FU-loaded PLGA microdevices provided higher 5-FU concentrations in the tumor and lower concentrations in the plasma than groups receiving 5-FU intraperitoneally.
4. PLGA hydroxyapatite formulations loaded with 5-FU
Hydroxyapatite (Ca10(PO4)6(OH)2) is an inorganic material found in bone and teeth and has been used as a material for bone regeneration.171 Hydroxyapatite is biocompatible and has a high affinity for chemicals and biomolecules. Therefore, it has been used as a localized drug delivery system, especially to bones.172,173 It also has activity against several tumor cell lines including gastric cancer174, hepatic cancer175,176 and colon cancer.177 Therefore, hydroxyapatite has been increasingly studied for its potential as an anticancer delivery system. Several anticancer agents (e.g. cisplatin178, doxorubicin179, paclitaxel180 and 5-FU171,181) have been successfully formulated with hydroxyapatite. However, some of the formulations, especially the 5-FU-loaded hydroxyapatite formulations failed to provide prolonged release with the release kinetics being immediate, resulting in complete release of 5-FU within 5 minutes.181
PLGA has been used to modify the release of 5-FU from hydroxyapatite formulations. One study used PLGA 85:15 as a matrix for 5-FU and hydroxyapatite.182 All three components were milled at high temperature to form a three-phase composite material which was then fabricated into cylindrical scaffolds by a nanocomposite deposition system. The study showed that PLGA helped retard the release of 5-FU from 5-FU-loaded PLGA hydroxyapatite scaffolds. Another study formulated 5-FU-loaded PLGA hydroxyapatite microparticles using an emulsion-solvent evaporation method84 (Table 1). The release kinetics of 5-FU depended on the amount of hydroxyapatite in the formulations. Hydroxyapatite reduced the initial release and also increased the release rate of 5-FU. This was because hydroxyapatite and 5-FU competitively diffused to the surface of particles. The particles with more hydroxyapatite, therefore, had less 5-FU at the surface. Also, hydroxyapatite caused pores in the particles, increasing the release rate of 5-FU.84
Conclusions and future directions
The chemotherapeutic agent, 5-FU, has a short elimination half-life in the body and therefore requires either multiple dosings or continuous infusions when used as a therapy for a range of cancers or proliferative disorders. These forms of delivery, usually administered systemically, are hampered by having narrow therapeutic windows such that doses that provide optimal antiproliferative efficacy are usually too toxic to be considered effective or safe. There are circumstances where local sustained delivery of 5-FU would be preferable to systemic dosings, such as: 1) intraoperative administration of a delivery device during tumor resection to enhance local residual tumor elimination, 2) the treatment of inoperable tumors, and 3) the treatment of other proliferative ailments such as fibrosis. Incorporation of 5-FU into PLGA matrices has proven to be an effective way to provide sustained release of 5-FU in vivo and has demonstrated promise as a local delivery-based therapy in preclinical studies and in clinical trials. Procedures such as intraoperative delivery of 5-FU-loaded PLGA-based devices at the time, and location, of tumor resection have the potential to significantly reduce the percent tumor recurrence at the site of operation in 5-FU sensitive cancers such as colon cancer. In essence, local sustained delivery of 5-FU from PLGA-based, or PLGA-coated, devices aim at maximizing the desired antiproliferative effect whilst minimizing unwanted side effects. PLGA allows the flexibility of fabricating multiple types of delivery systems including microparticles, nanoparticles and implantable matrices such as wafers. PLGA is USFDA-approved and can be tuned to release drugs at predetermined rates of zero or first order kinetics. It can be used to coat medical devices or be combined or functionalized with other agents, thereby making it a versatile and safe polymer capable of providing effective delivery of 5-FU to a range of patients with proliferative disorders. It is amenable to being part of hybrid systems involving magnetic nanoparticles or microbubbles thus increasing the delivery device’s tumor targeting and controllable release properties. The future outlook for 5-FU-loaded PLGA based delivery systems is quite possibly one involving adjunctive therapy where sustained and controlled release of 5-FU from strategically designed biocompatible PLGA devices provides significant therapeutic benefits by acting in synergy with other treatments against proliferative disorders such as cancer.
Table 4.
Characteristics of 5-FU loaded PLGA disc from previous studies
| Fabrication method | PLA:PLG ratio |
Organic phase |
Diameter (mm) |
Thickness (nm) |
Loaded drug amount (mg/mg) |
Release duration (days) |
Reference |
|---|---|---|---|---|---|---|---|
| Film coating | 50:50 | DCM | 8 | - | - | 28 | 69 |
| Film compression | 63:37 | DCM | 3 and 5 | 0.15 | 0.10 | 17 | 137 |
| Compression molding | 50:50 | - | 3 | - | 0.15–0.30 | 5 | 158 |
Abbreviations: DCM- dichloromethane; PLA – polylactic acid; PGA – polyglycolic acid
Acknowledgments
Nattawut Leelakanok would like to acknowledge the support from The Royal Thai Government Scholarship. The authors would also like to acknowledge Grerk Suthamtewakul, MD, and Rasheid Smith for assisting with the art illustrations in figure 1–3.
Abbreviations
- 5-FU
5-fluorouracil
- dUMP
Deoxyuridine monophosphate
- DCM
Dichloromethane
- EPR
Enhanced permeation and retention
- FdUMP
Fluorodeoxyuridine monophosphate
- FdUTP
Fluorodeoxyuridine triphosphate
- FUTP
Fluorouridine triphosphate
- GABA
Gamma aminobutyric acid
- IV
Intravenous
- MDSCs
Myeloid-derived suppressor cells
- PLGA
Poly(D,L-lactide-co-glycolide)
- PGA
Polyglycolic acid
- PLA
Polylactic acid
- PVA
Polyvinyl alcohol
- S/O/W
Solid-oil-water
- USFDA
United States Food and Drug Administration
- UraA
Uracil transporter
- UTP
Uridine triphosphate
- W/O/W
Water-oil-water
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Authors declare no conflicts of interest.
References
- 1.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179(4561):663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 2.Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, Hansen R, Zee B, Weinerman B, Pater J, Leichman C, Macdonald J, Benedetti J, Lokich J, Fryer J, Brufman G, Isacson R, Laplanche A, Levy E. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 3.Thrall BSMM, Wood P, King V, Rivera W, Hrushesky W. Investigation of the comparative toxicity of 5-FU bolus versus 5-FU continuous infusion circadian chemotherapy with concurrent radiation therapy in locally advanced rectal cancer. International Journal of Radiation Oncology*Biology*Physics. 2000;46(4):873–881. doi: 10.1016/s0360-3016(99)00456-3. [DOI] [PubMed] [Google Scholar]
- 4.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E MB. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA. 2010;303(17):1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 5.Edwards MS, Chadda SD, Zhao Z, Barber BL, Sykes DP. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012;14(2):e31–47. doi: 10.1111/j.1463-1318.2011.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saltz LB, Douillard JY, Pirotta N, Alakl M, Gruia G, Awad L, Elfring GL, Locker PK, Miller LL. Irinotecan plus fluorouracil/leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist. 2001;6(1):81–91. doi: 10.1634/theoncologist.6-1-81. [DOI] [PubMed] [Google Scholar]
- 7.Saif MW, Choma A, Salamone SJ, Chu E. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101(22):1543–1552. doi: 10.1093/jnci/djp328. [DOI] [PubMed] [Google Scholar]
- 8.Fang L, Xin W, Ding H, Zhang Y, Zhong L, Luo H, Li J, Yang Y, Huang P. Pharmacokinetically guided algorithm of 5-fluorouracil dosing, a reliable strategy of precision chemotherapy for solid tumors: a meta-analysis. Sci Rep. 2016;6:25913. doi: 10.1038/srep25913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorrentino MF, Kim J, Foderaro AE, AG T. 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J. 2012;19(5):453–458. doi: 10.5603/cj.2012.0084. [DOI] [PubMed] [Google Scholar]
- 10.Zheng NZ M, Du C, Wang S, Du C, Lu W. 5-Fluorouracil delivery from a novel three-dimensional micro-device: In vitro and in vivo evaluation. Arch Pharmacal Res. 2013;36(12):1487–1493. doi: 10.1007/s12272-013-0168-5. [DOI] [PubMed] [Google Scholar]
- 11.Jacquet P, Stuart OA, Dalton R, Chang D, Sugarbaker PH. Effect of intraperitoneal chemotherapy and fibrinolytic therapy on tumor implantation in wound sites. J Surg Oncol. 1996;62(2):128–134. doi: 10.1002/(SICI)1096-9098(199606)62:2<128::AID-JSO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Ren X, Zheng N, Gao Y, Chen T, Lu W. Biodegradable three-dimension micro-device delivering 5-fluorouracil in tumor bearing mice. Drug Deliv. 2012;19(1):36–44. doi: 10.3109/10717544.2011.635720. [DOI] [PubMed] [Google Scholar]
- 13.AHFS drug information essentials. Bethesda, MD: American Society of Health-System Pharmacists; 2008. [Google Scholar]
- 14.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 15.Plumb JA, Gardner MLG. Differential Toxicity and Pharmacokinetics of Sodium and Tris Salts of 5-fluorouracil in the Rat. ed. 1981:707–710. doi: 10.1042/cs0600707. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire L, Maletmartino M, Martino R, Deforni M, Lasserre B. The tris formulation of Fluorouracil is more cardiotoxic than the sodium-salt formulations. Oncol Rep. 1994;1(1):173–174. doi: 10.3892/or.1.1.173. [DOI] [PubMed] [Google Scholar]
- 17.Jang YH, Sowers LC, Çağin T, Goddard WA. First Principles Calculation of pKa Values for 5-Substituted Uracils. J Phys Chem A. 2001;105(1):274–280. [Google Scholar]
- 18.Blanco MD, Guerrero S, Benito M, Fern¨¢ndez A, Teij¨®n Cs, Olmo R, Katime I, Teij¨®n JM. In Vitro and In Vivo Evaluation of a Folate-Targeted Copolymeric Submicrohydrogel Based on N-Isopropylacrylamide as 5-Fluorouracil Delivery System. Polymers 2011 [Google Scholar]
- 19.Legay R, Massou S, Azéma J, Martino R, Malet-Martino M. Hydrolytic pathway of 5-fluorouracil in aqueous solutions for clinical use. J Pharm Biomed Anal. 2014;98:446–462. doi: 10.1016/j.jpba.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Windheuser JJ, Sutter JL, Auen E. 5-Fluorouracil and derivatives in cancer chemotherapy: Determination of 5-fluorouracil in blood. J Pharm Sci. 1972;61(2):301–303. doi: 10.1002/jps.2600610243. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Hirotani Y, Ogura N, Sasaki E, Kitagawa S. Enhancing effect of N-dodecyl-2-pyrrolidone on the percutaneous absorption of 5-fluorouracil derivatives. Chem Pharm Bull (Tokyo) 1998;46(5):831–836. doi: 10.1248/cpb.46.831. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Bei Y, Qi G, Ding Y. Thermal decomposition kinetics of 5-fluorouracil from thermogravimetric analysis. Korean J Chem Eng. 2008;25(5):980–981. [Google Scholar]
- 23.Blanco MDS RL, Teijón C, Olmo R, Teijón JM. 5-Fluorouracil-loaded microspheres prepared by spray-drying poly(D,L-lactide) and poly(lactide-co-glycolide) polymers: Characterization and drug release. J Microencaps. 2005;22(6):671–682. doi: 10.1080/02652040500161990. [DOI] [PubMed] [Google Scholar]
- 24.Qian F, Nasongkla N, Gao J. Membrane-encased polymer millirods for sustained release of 5-fluorouracil. J Biomed Mater Res. 2002;61(2):203–211. doi: 10.1002/jbm.10156. [DOI] [PubMed] [Google Scholar]
- 25.Jodrell DI, Stewart M, Aird R, Knowles G, Bowman A, Wall L, McLean C. 5-fluorouracil steady state pharmacokinetics and outcome in patients receiving protracted venous infusion for advanced colorectal cancer. Br J Cancer. 2001;84(5):600–603. doi: 10.1054/bjoc.2000.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 27.Schouppe E, Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Modulation of CD8(+) T-cell activation events by monocytic and granulocytic myeloid-derived suppressor cells. Immunobiology. 2013;218(11):1385–1391. doi: 10.1016/j.imbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Ghiringhelli F, Apetoh L. Enhancing the anticancer effects of 5-fluorouracil: current challenges and future perspectives. Biomed J. 2015;38(2):111–116. doi: 10.4103/2319-4170.130923. [DOI] [PubMed] [Google Scholar]
- 29.Lamont EB, Schilsky RL. The oral fluoropyrimidines in cancer chemotherapy. Clin Cancer Res. 1999;5(9):2289–2296. [PubMed] [Google Scholar]
- 30.Visser GW, Gorree GC, Peters GJ, Herscheid JD. Tissue distribution of [18F]-5-fluorouracil in mice: effects of route of administration, strain, tumour and dose. Cancer Chemother Pharmacol. 1990;26(3):205–209. doi: 10.1007/BF02897200. [DOI] [PubMed] [Google Scholar]
- 31.Climente-Marti M, Merino-Sanjuan M, Almenar-Cubells D, Jimenez-Torres NV. A Bayesian method for predicting 5-fluorouracil pharmacokinetic parameters following short-term infusion in patients with colorectal cancer. J Pharm Sci. 2003;92(6):1155–1165. doi: 10.1002/jps.10374. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y, Li J, Rong LF, Lu XW, Huang Y, Xu SY. Pharmacokinetics and tissue distribution of 5-fluorouracil encapsulated by galactosylceramide liposomes in mice. Acta Pharmacol Sin. 2005;26(2):250–256. doi: 10.1111/j.1745-7254.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Lu F, Li S, Jiang Y, Jiang J, Fan H, Lu G, Deng D, Dang S, Zhang X, Wang J, Yan N. Structure and mechanism of the uracil transporter UraA. Nature. 2011;472(7342):243–246. doi: 10.1038/nature09885. [DOI] [PubMed] [Google Scholar]
- 34.Warren BS, LaCreta FP, Kornhauser DM, Williams WM. Dose and flow dependence of 5-fluorouracil elimination by the isolated perfused rat liver. Cancer Res. 1987;47(20):5261–5265. [PubMed] [Google Scholar]
- 35.Schaaf LJ, Dobbs BR, Edwards IR, Perrier DG. Nonlinear pharmacokinetic characteristics of 5-fluorouracil (5-FU) in colorectal cancer patients. Eur J Clin Pharmacol. 1987;32(4):411–418. doi: 10.1007/BF00543978. [DOI] [PubMed] [Google Scholar]
- 36.Peters GJ, Lankelma J, Kok RM, Noordhuis P, van Groeningen CJ, van der Wilt CL, Meyer S, Pinedo HM. Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol. 1993;31(4):269–276. doi: 10.1007/BF00685670. [DOI] [PubMed] [Google Scholar]
- 37.Liu M, Russell RL, Beigelman L, Handschumacher RE, Pizzorno G. beta-alanine and alpha-fluoro-beta-alanine concentrative transport in rat hepatocytes is mediated by GABA transporter GAT-2. Am J Physiol. 1999;276(1 Pt 1):G206–210. doi: 10.1152/ajpgi.1999.276.1.G206. [DOI] [PubMed] [Google Scholar]
- 38.Bernadou J, Armand JP, Lopez A, Malet-Martino MC, Martino R. Complete urinary excretion profile of 5-fluorouracil during a six-day chemotherapeutic schedule, as resolved by 19F nuclear magnetic resonance. Clin Chem. 1985;31(6):846–848. [PubMed] [Google Scholar]
- 39.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47(8):2203–2206. [PubMed] [Google Scholar]
- 40.Milano G, Chamorey AL. Clinical pharmacokinetics of 5-fluorouracil with consideration of chronopharmacokinetics. Chronobiol Int. 2002;19(1):177–189. doi: 10.1081/cbi-120002597. [DOI] [PubMed] [Google Scholar]
- 41.Fleming G, Schumm P, Friberg G, Ratain M, Njiaju U, Schilsky R. Circadian variation in plasma 5-fluorouracil concentrations during a 24 hour constant-rate infusion. BMC Cancer. 2015;15(1):1–8. doi: 10.1186/s12885-015-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto H, Okumura H, Murakami H, Kubota H, Higashida M, Tsuruta A, Tohyama K, Hirai T. Fluctuation in Plasma 5-Fluorouracil Concentration During Continuous 5-Fluorouracil Infusion for Colorectal Cancer. Anticancer Res. 2015;35(11):6193–6199. [PubMed] [Google Scholar]
- 43.Calabro-Jones PM, Byfield JE, Ward JF, Sharp TR. Time-dose relationships for 5-fluorouracil cytotoxicity against human epithelial cancer cells in vitro. Cancer Res. 1982;42(11):4413–4420. [PubMed] [Google Scholar]
- 44.Lokich J. Infusional 5-FU: historical evolution, rationale, and clinical experience. Oncology (Williston Park) 1998;12(10 Suppl 7):19–22. [PubMed] [Google Scholar]
- 45.Link KH, Aigner KR, Peschau K, Warthona M, Schwemmle K, Danenberg PV. Concentration and time dependence of the toxicity of fluorinated pyrimidines to HT 29 colorectal carcinoma cells. Cancer Chemother Pharmacol. 1988;22(1):58–62. doi: 10.1007/BF00254182. [DOI] [PubMed] [Google Scholar]
- 46.Midena E, Lazzarini D, Catania AG, Moretto E, Fregona I, Parrozzani R. Cytostatic and cytotoxic effects of 5-fluorouracil on human corneal epithelial cells and keratocytes. Cornea. 2013;32(3):338–344. doi: 10.1097/ICO.0b013e31825d56c1. [DOI] [PubMed] [Google Scholar]
- 47.Fluorouracil. ed.: National Institue of Cancer. 2015. [Google Scholar]
- 48.Pomerantz H, Hogan D, Eilers D, Swetter SM, Chen SC, Jacob SE, Warshaw EM, Stricklin G, Dellavalle RP, Sidhu-Malik N, Konnikov N, Werth VP, Keri J, Lew R, Weinstock MA. Long-term Efficacy of Topical Fluorouracil Cream, 5%, for Treating Actinic Keratosis: A Randomized Clinical Trial. JAMA Dermatol. 2015 doi: 10.1001/jamadermatol.2015.0502. [DOI] [PubMed] [Google Scholar]
- 49.Dorbandt DM, Driskell EA, Hamor RE. Treatment of corneal squamous cell carcinoma using topical 1% 5-fluorouracil as monotherapy. Vet Ophthalmol. 2015 doi: 10.1111/vop.12290. [DOI] [PubMed] [Google Scholar]
- 50.Wormald R, Wilkins MR, Bunce C. Post-operative 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst Rev. 2001;(3):CD001132. doi: 10.1002/14651858.CD001132. [DOI] [PubMed] [Google Scholar]
- 51.Milne PJ, Gautier S, Parel JMA, Jallet V. Proceedings of SPIE - The International Society for Optical Engineering. 1997:137–146. [Google Scholar]
- 52.Folprecht G, Cunningham D, Ross P, Glimelius B, Di Costanzo F, Wils J, Scheithauer W, Rougier P, Aranda E, Hecker H, Kohne CH. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004;15(9):1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 53.Rustum YM. Biochemical rationale for the 5-fluorouracil leucovorin combination and update of clinical experience. J Chemother 2 Suppl. 1990;1:5–11. doi: 10.1080/1120009x.1990.11738998. [DOI] [PubMed] [Google Scholar]
- 54.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 55.Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 56.Wu F, Zhang HG, Ran FW, Zhang XR, Shi YK. [FOLFOX4 regimen versus DP(O)F regimen for advanced gastric cancer] Ai Zheng. 2008;27(4):413–417. [PubMed] [Google Scholar]
- 57.Hecht JR, Mitchell EP, Yoshino T, Welslau M, Lin X, Chow Maneval E, Paolini J, Lechuga MJ, Kretzschmar A. 5-Fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus sunitinib or bevacizumab as first-line treatment for metastatic colorectal cancer: a randomized Phase IIb study. Cancer Manag Res. 2015;7:165–173. doi: 10.2147/CMAR.S61408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sjövall A, Granath F, Cedermark B, Glimelius B, Holm T. Loco-regional Recurrence from Colon Cancer: A Population-based Study. Ann Surg Oncol. 2007;14(2):432–440. doi: 10.1245/s10434-006-9243-1. [DOI] [PubMed] [Google Scholar]
- 59.Diasio RB, Johnson MR. Dihydropyrimidine dehydrogenase: its role in 5-fluorouracil clinical toxicity and tumor resistance. Clin Cancer Res. 1999;5(10):2672–2673. [PubMed] [Google Scholar]
- 60.van Kuilenburg AB, De Abreu RA, van Gennip AH. Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem. 2003;40(Pt 1):41–45. doi: 10.1258/000456303321016150. [DOI] [PubMed] [Google Scholar]
- 61.Kline CL, Schiccitano A, Zhu J, Beachler C, Sheikh H, Harvey HA, Mackley HB, McKenna K, Staveley-O’Carroll K, Poritz L, Messaris E, Stewart D, Sivik J, El-Deiry WS. Personalized dosing via pharmacokinetic monitoring of 5-fluorouracil might reduce toxicity in early- or late-stage colorectal cancer patients treated with infusional 5-fluorouracil-based chemotherapy regimens. Clin Colorectal Cancer. 2014;13(2):119–126. doi: 10.1016/j.clcc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Gamelin E, Delva R, Jacob J, Merrouche Y, Raoul JL, Pezet D, Dorval E, Piot G, Morel A, Boisdron-Celle M. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(13):2099–2105. doi: 10.1200/JCO.2007.13.3934. [DOI] [PubMed] [Google Scholar]
- 63.Faisant NA J, Siepmann F, Benoit JP, Siepmann J. Effects of the type of release medium on drug release from PLGA-based microparticles: Experiment and theory. Int J Pharm. 2006;314(2):189–197. doi: 10.1016/j.ijpharm.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 64.Menei P, Venier MC, Gamelin E, Saint-Andre JP, Hayek G, Jadaud E, Fournier D, Mercier P, Guy G, Benoit JP. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of glioblastoma: a pilot study. Cancer. 1999;86(2):325–330. [PubMed] [Google Scholar]
- 65.Menei P, Jadaud E, Faisant N, Boisdron-Celle M, Michalak S, Fournier D, Delhaye M, Benoit J-P. Stereotaxic implantation of 5-fluorouracil-releasing microspheres in malignant glioma. Cancer. 2004;100(2):405–410. doi: 10.1002/cncr.11922. [DOI] [PubMed] [Google Scholar]
- 66.Menei P, Capelle L, Guyotat J, Fuentes S, Assaker R, Bataille B, Francois P, Dorwling-Carter D, Paquis P, Bauchet L, Parker F, Sabatier J, Faisant N, Benoit JP. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56(2):242–248. doi: 10.1227/01.neu.0000144982.82068.a2. discussion 242–248. [DOI] [PubMed] [Google Scholar]
- 67.Arias JL. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules. 2008;13(10):2340–2369. doi: 10.3390/molecules13102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 69.Ponnusamy TY H, John VT, Ayyala RS, Blake DA. A novel antiproliferative drug coating for glaucoma drainage devices. J Glaucoma. 2014;23(8):526–534. doi: 10.1097/IJG.0b013e318294869b. [DOI] [PubMed] [Google Scholar]
- 70.Li XX Y, Chen G, Wei P, Ping Q. PLGA nanoparticles for the oral delivery of 5-fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev Ind Pharm. 2008;34(1):107–115. doi: 10.1080/03639040701484593. [DOI] [PubMed] [Google Scholar]
- 71.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Yoshisue K, Kanie S, Nishimura T, Chikamoto J, Nagayama S. Effect of dimethylnitrosamine-induced liver dysfunction on the pharmacokinetics of 5-fluorouracil after administration of S-1, an antitumour drug, to rats. J Pharm Pharmacol. 2009;61(12):1643–1651. doi: 10.1211/jpp/61.12.0009. [DOI] [PubMed] [Google Scholar]
- 73.Nagata M, Hidaka Y, Hidaka M, Kawano Y, Iwakiri T, Okumura M, Arimori K. Effect of acute hepatic failure on the hepatic first-pass effect of 5-fluorouracil in rats. J Pharm Pharmacol. 2010;62(5):598–603. doi: 10.1211/jpp.62.05.0006. [DOI] [PubMed] [Google Scholar]
- 74.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed KK, Geary SM, Salem AK. Development and Evaluation of Biodegradable Particles Coloaded With Antigen and the Toll-Like Receptor Agonist, Pentaerythritol Lipid A, as a Cancer Vaccine. J Pharm Sci. 2016;105(3):1173–1179. doi: 10.1016/j.xphs.2015.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao Y, Wang B, Wang Y, Lou D. Dual drug release from core-shell nanoparticles with distinct release profiles. J Pharm Sci. 2014;103(10):3205–3216. doi: 10.1002/jps.24116. [DOI] [PubMed] [Google Scholar]
- 77.Song X, Zhao X, Zhou Y, Li S, Ma Q. Pharmacokinetics and disposition of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticles. Curr Drug Metab. 2010;11(10):859–869. doi: 10.2174/138920010794479682. [DOI] [PubMed] [Google Scholar]
- 78.Wieber A, Selzer T, Kreuter J. Characterisation and stability studies of a hydrophilic decapeptide in different adjuvant drug delivery systems: a comparative study of PLGA nanoparticles versus chitosan-dextran sulphate microparticles versus DOTAP-liposomes. Int J Pharm. 2011;421(1):151–159. doi: 10.1016/j.ijpharm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 79.Gaignaux A, Reeff J, Siepmann F, Siepmann J, De Vriese C, Goole J, Amighi K. Development and evaluation of sustained-release clonidine-loaded PLGA microparticles. Int J Pharm. 2012;437(1–2):20–28. doi: 10.1016/j.ijpharm.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Shin M, Kim HK, Lee H. Dopamine-loaded poly(D,L-lactic-co-glycolic acid) microspheres: new strategy for encapsulating small hydrophilic drugs with high efficiency. Biotechnol Prog. 2014;30(1):215–223. doi: 10.1002/btpr.1835. [DOI] [PubMed] [Google Scholar]
- 81.Han FY, Thurecht KJ, Whittaker AK, Smith MT. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front Pharmacol. 2016;7:185. doi: 10.3389/fphar.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hussain MB G, Hughes M, Akhtar S. Co-delivery of an antisense oligonucleotide and 5-fluorouracil using sustained release poly (lactide-co-glycolide) microsphere formulations for potential combination therapy in cancer. Int J Pharm. 2002;234(1–2):129–138. doi: 10.1016/s0378-5173(01)00950-4. [DOI] [PubMed] [Google Scholar]
- 83.Fu YJS SS, Su FH, Yu PC. Development of biodegradable co-poly(D,L-lactic/glycolic acid) microspheres for the controlled release of 5-FU by the spray drying method. Colloids Surf B Biointerfaces. 2002;25(4):269–279. [Google Scholar]
- 84.Lin YL Y, Ooi CP. 5-Fluorouracil encapsulated HA/PLGA composite microspheres for cancer therapy. J Mater Sci Mater Med. 2012;23(10):2453–2460. doi: 10.1007/s10856-012-4723-2. [DOI] [PubMed] [Google Scholar]
- 85.Laquintana VD N, Lopalco A, Lopedota A, Cutrignelli A, Lasorsa FM, Agostino G, Franco M. Translocator protein ligand-plga conjugated nanoparticles for 5-fluorouracil delivery to glioma cancer cells. Mol Pharm. 2014;11(3):859–871. doi: 10.1021/mp400536z. [DOI] [PubMed] [Google Scholar]
- 86.Ashjari MK S, Mahdavian AR. A multiple emulsion method for loading 5-fluorouracil into a magnetite-loaded nanocapsule: A physicochemical investigation. Polym Int. 2012;61(5):850–859. [Google Scholar]
- 87.Shakeri-Zadeh AS MB, Khoee S, Sharifi AM, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: In vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29(4):548–556. doi: 10.1177/0885328214536940. [DOI] [PubMed] [Google Scholar]
- 88.Lin QC Y, Yuan M, Ma L, Qiu M, Su J. Development of a 5-fluorouracil-loaded PLGA microsphere delivery system by a solid-in-oil-in-hydrophilic oil (S/O/hO) novel method for the treatment of tumors. Oncol Rep. 2014;32(6):2405–2410. doi: 10.3892/or.2014.3480. [DOI] [PubMed] [Google Scholar]
- 89.Chiang CHT SM, Lu DW, Yeh MK. In vitro and in vivo evaluation of an ocular delivery system of 5-fluorouracil microspheres. J Ocul Pharmacol Ther. 2001;17(6):545–553. doi: 10.1089/10807680152729239. [DOI] [PubMed] [Google Scholar]
- 90.Vert M, Doi Y, Hellwich K-H, Hess M, Hodge P, Kubisa P, Rinaudo M, Schue F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012) Pure Appl Chem. 2012;84(2):377–410. [Google Scholar]
- 91.Dash AK. Determination of the physical state of drug in microcapsule and microsphere formulations. J Microencapsul. 1997;14(1):101–112. doi: 10.3109/02652049709056471. [DOI] [PubMed] [Google Scholar]
- 92.Singh MN, Hemant KS, Ram M, Shivakumar HG. Microencapsulation: A promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65–77. [PMC free article] [PubMed] [Google Scholar]
- 93.Brazeau G, Sauberan SL, Gatlin L, Wisniecki P, Shah J. Effect of particle size of parenteral suspensions on in vitro muscle damage. Pharm Dev Technol. 2011;16(6):591–598. doi: 10.3109/10837450.2010.542161. [DOI] [PubMed] [Google Scholar]
- 94.Spenlehauer G, Vert M, Benoit JP, Boddaert A. In vitro and in vivo degradation of poly(D,L lactide/glycolide) type microspheres made by solvent evaporation method. Biomaterials. 1989;10(8):557–563. doi: 10.1016/0142-9612(89)90063-x. [DOI] [PubMed] [Google Scholar]
- 95.Wan F, Yang M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int J Pharm. 2016;498(1–2):82–95. doi: 10.1016/j.ijpharm.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 96.Nguyen DN, Clasen C, Van den Mooter G. Pharmaceutical Applications of Electrospraying. J Pharm Sci. 2016;105(9):2601–2620. doi: 10.1016/j.xphs.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 97.Meeus J, Lenaerts M, Scurr DJ, Amssoms K, Davies MC, Roberts CJ, Van Den Mooter G. The Influence of Spray-Drying Parameters on Phase Behavior, Drug Distribution, and In Vitro Release of Injectable Microspheres for Sustained Release. J Pharm Sci. 2015;104(4):1451–1460. doi: 10.1002/jps.24361. [DOI] [PubMed] [Google Scholar]
- 98.Boisdron-Celle MM, Ph;Benoit JP. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47(2):108–114. doi: 10.1111/j.2042-7158.1995.tb05760.x. [DOI] [PubMed] [Google Scholar]
- 99.Siepmann J, Faisant N, Akiki J, Richard J, Benoit JP. Effect of the size of biodegradable microparticles on drug release: experiment and theory. J Control Release. 2004;96(1):123–134. doi: 10.1016/j.jconrel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Hitzman CJE WF, Wattenberg LW, Wiedmann TS. Development of a respirable, sustained release microcarrier for 5-fluorouracil I: In vitro assessment of liposomes, microspheres, and lipid coated nanoparticles. J Pharm Sci. 2006;95(5):1114–1126. doi: 10.1002/jps.20591. [DOI] [PubMed] [Google Scholar]
- 101.Nair KLJ S, Nair SA, Kumar GS. Biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependence on the carrier, PLGA. International journal of nanomedicine. 2011;6:1685–1697. doi: 10.2147/IJN.S20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agrawal CM, Kennedy ME, Micallef DM. The effects of ultrasound irradiation on a biodegradable 50–50% copolymer of polylactic and polyglycolic acids. J Biomed Mater Res. 1994;28(8):851–859. doi: 10.1002/jbm.820280803. [DOI] [PubMed] [Google Scholar]
- 103.Faisant NS J, Oury P, Laffineur V, Bruna E, Haffner J, Benoit JP. The effect of gamma-irradiation on drug release from bioerodible microparticles: A quantitative treatment. Int J Pharm. 2002;242(1–2):281–284. doi: 10.1016/s0378-5173(02)00188-6. [DOI] [PubMed] [Google Scholar]
- 104.Gupte AC K. Formulation and characterization of Paclitaxel, 5-FU and Paclitaxel + 5-FU microspheres. Int J Pharm. 2004;276(1–2):93–106. doi: 10.1016/j.ijpharm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 105.Zhou X, Cai Q, Yan N, Deng X, Yang X. In vitro hydrolytic and enzymatic degradation of nestlike-patterned electrospun poly(D,L-lactide-co-glycolide) scaffolds. J Biomed Mater Res A. 2010;95(3):755–765. doi: 10.1002/jbm.a.32896. [DOI] [PubMed] [Google Scholar]
- 106.Kemme M, Prokesch I, Heinzel-Wieland R. Comparative study on the enzymatic degradation of poly(lactic-co-glycolic acid) by hydrolytic enzymes based on the colorimetric quantification of glycolic acid. Polym Test. 2011;30(7):743–748. [Google Scholar]
- 107.Menei PV MC, Gamelin E, Saint-Andŕe JP, Hayek G, Jadaud E, Fournier D, Mercier P, Gilles G, Benoit JP. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of glioblastoma: A pilot study. Cancer. 1999;86(2):325–330. [PubMed] [Google Scholar]
- 108.Wait SD, Prabhu RS, Burri SH, Atkins TG, Asher AL. Polymeric drug delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17(Suppl 2):i9–ii23. doi: 10.1093/neuonc/nou360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li JP Y, Wang S, Ding M, Chen D, Zhu M. Pharmacokinetic study and effectiveness evaluation of slow-release PLGA-5-fluorouracil microsphere. Cancer Chemother Pharmacol. 2013;71(2):351–359. doi: 10.1007/s00280-012-2016-6. [DOI] [PubMed] [Google Scholar]
- 110.Sun WC Y, Yuan W. Hemostatic absorbable gelatin sponge loaded with 5-fluorouracil for treatment of tumors. International Journal of Nanomedicine. 2013;8:1499–1506. doi: 10.2147/IJN.S41462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bae JWG DH, Park KD, Lee SJ. Thermosensitive chitosan as an injectable carrier for local drug delivery. Macromolecular Research. 2006;14(4):461–465. [Google Scholar]
- 112.Commission E. Commission recommendation of 18 October 2011 on the definition of nanomaterial. Off J Eur Union. 2011;L275:38–40. [Google Scholar]
- 113.He J-H, Wan Y-Q, Xu L. Nano-effects, quantum-like properties in electrospun nanofibers. Chaos, Solitons & Fractals. 2007;33(1):26–37. [Google Scholar]
- 114.Musumeci T, Ventura CA, Giannone I, Ruozi B, Montenegro L, Pignatello R, Puglisi G. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325(1–2):172–179. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 115.Pantazis P, Dimas K, Wyche JH, Anant S, Houchen CW, Panyam J, Ramanujam RP. Preparation of siRNA-encapsulated PLGA nanoparticles for sustained release of siRNA and evaluation of encapsulation efficiency. Methods Mol Biol. 2012;906:311–319. doi: 10.1007/978-1-61779-953-2_25. [DOI] [PubMed] [Google Scholar]
- 116.Terry AB, Salaam AD, Nyairo E, Thomas V, Dean DR. PLGA Nanoparticles for the Sustained Release of Rifampicin. ed 2014 [Google Scholar]
- 117.Guimaraes PP, Oliveira SR, de Castro Rodrigues G, Gontijo SM, Lula IS, Cortes ME, Denadai AM, Sinisterra RD. Development of sulfadiazine-decorated PLGA nanoparticles loaded with 5-fluorouracil and cell viability. Molecules. 2015;20(1):879–899. doi: 10.3390/molecules20010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Geary SM, Salem AK. Exploiting the tumor phenotype using biodegradable submicron carriers of chemotherapeutic drugs. Crit Rev Oncog. 2014;19(3–4):269–280. doi: 10.1615/critrevoncog.2014011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burke CWA E, Timbie K, Kilbanov AL, Price RJ. Ultrasound-activated agents comprised of 5FU-bearing nanoparticles bonded to microbubbles inhibit solid tumor growth and improve survival. Mol Ther. 2014;22(2):321–328. doi: 10.1038/mt.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.G B. Polymeric Nanofibers: Recent Technology Advancements Stimulating their Growth. J Textile Sci Eng. 2015;5(186) [Google Scholar]
- 121.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shin HJ, Lee CH, Cho IH, Kim YJ, Lee YJ, Kim IA, Park KD, Yui N, Shin JW. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17(1–2):103–119. doi: 10.1163/156856206774879126. [DOI] [PubMed] [Google Scholar]
- 123.Guimaraes PP, Oliveira MF, Gomes AD, Gontijo SM, Cortes ME, Campos PP, Viana CT, Andrade SP, Sinisterra RD. PLGA nanofibers improves the antitumoral effect of daunorubicin. Colloids Surf B Biointerfaces. 2015;136:248–255. doi: 10.1016/j.colsurfb.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J, Wang X, Liu T, Liu S, Jing X. Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. 2014:1–7. doi: 10.3109/10717544.2014.916768. [DOI] [PubMed] [Google Scholar]
- 125.Bonin EA, Baron TH. Update on the indications and use of colonic stents. Curr Gastroenterol Rep. 2010;12(5):374–382. doi: 10.1007/s11894-010-0136-x. [DOI] [PubMed] [Google Scholar]
- 126.Li G, Chen Y, Hu J, Wu X, Hu J, He X, Li J, Zhao Z, Chen Z, Li Y, Hu H, Li Y, Lan P. A 5-fluorouracil-loaded polydioxanone weft-knitted stent for the treatment of colorectal cancer. Biomaterials. 2013;34(37):9451–9461. doi: 10.1016/j.biomaterials.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 127.Park CGK MH, Park M, Lee JE, Lee SH, Park JH, Yoon KH, Bin Choy Y. Polymeric nanofiber coated esophageal stent for sustained delivery of an anticancer drug. Macromolecular Research. 2011;19(11):1210–1216. [Google Scholar]
- 128.Licciardi M, Scialabba C, Fiorica C, Cavallaro G, Cassata G, Giammona G. Polymeric Nanocarriers for Magnetic Targeted Drug Delivery: Preparation, Characterization, and in Vitro and in Vivo Evaluation. Mol Pharm. 2013;10(12):4397–4407. doi: 10.1021/mp300718b. [DOI] [PubMed] [Google Scholar]
- 129.Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, Pöttler M, Dürr S, Alexiou C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468(3):463–470. doi: 10.1016/j.bbrc.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 130.Kakar S, Batra D, Singh R, Nautiyal U. Magnetic microspheres as magical novel drug delivery system: A review. Journal of Acute Disease. 2013;2(1):1–12. [Google Scholar]
- 131.Wamocha HLM HE, Song Z, Chu HY, Chen YY, Asmatulu R, Yang SY, Ho JC. Cytotoxicity of release products from magnetic nanocomposites in targeted drug delivery. J Biomater Appl. 2013;27(6):661–667. doi: 10.1177/0885328211421989. [DOI] [PubMed] [Google Scholar]
- 132.Pondman KM, Bunt ND, Maijenburg AW, van Wezel RJA, Kishore U, Abelmann L, ten Elshof JE, ten Haken B. Magnetic drug delivery with FePd nanowires. J Magn Magn Mater. 2015;380:299–306. [Google Scholar]
- 133.Felton LA. Mechanisms of polymeric film formation. Int J Pharm. 2013;457(2):423–427. doi: 10.1016/j.ijpharm.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 134.Zilberman M, Malka A. Drug controlled release from structured bioresorbable films used in medical devices--a mathematical model. J Biomed Mater Res B Appl Biomater. 2009;89(1):155–164. doi: 10.1002/jbm.b.31200. [DOI] [PubMed] [Google Scholar]
- 135.Bala R, Pawar P, Khanna S, Arora S. Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig. 2013;3(2):67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Irfan M, Rabel S, Bukhtar Q, Qadir MI, Jabeen F, Khan A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharmaceutical Journal. doi: 10.1016/j.jsps.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dorta MJ, Oliva A, Munguia O, Llabres M, Farina JB. In-vitro release of fluoropyrimidines from PLGA film implants. J Pharm Pharmacol. 2002;54(6):757–763. doi: 10.1211/0022357021779096. [DOI] [PubMed] [Google Scholar]
- 138.Schoenberg ED, Blake DA, Swann FB, Parlin AW, Zurakowski D, Margo CE, Ponnusamy T, John VT, Ayyala RS. Effect of Two Novel Sustained-Release Drug Delivery Systems on Bleb Fibrosis: An In Vivo Glaucoma Drainage Device Study in a Rabbit Model. Translational Vision Science & Technology. 2015;4(3):4. doi: 10.1167/tvst.4.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganguly I, Abraham S, Bharath S, Madhavan V. Development of Fast Dissolving Sublingual Wafers of Promethazine Hydrochloride. Iranian Journal of Pharmaceutical Sciences. 2014;10(1):71–92. [Google Scholar]
- 140.Rossi A, Conti C, Colombo G, Castrati L, Scarpignato C, Barata P, Sandri G, Caramella C, Bettini R, Buttini F, Colombo P. Floating modular drug delivery systems with buoyancy independent of release mechanisms to sustain amoxicillin and clarithromycin intra-gastric concentrations. Drug Dev Ind Pharm. 2015:1–8. doi: 10.3109/03639045.2015.1054397. [DOI] [PubMed] [Google Scholar]
- 141.El-Mahrouk GM, El-Gazayerly ON, Aboelwafa AA, Taha MS. Chitosan lactate wafer as a platform for the buccal delivery of tizanidine HCl: in vitro and in vivo performance. Int J Pharm. 2014;467(1–2):100–112. doi: 10.1016/j.ijpharm.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 142.Shaikh RP, Pillay V, Choonara YE, Du Toit LC, Ndesendo VM, Kumar P, Khan RA. The application of a crosslinked pectin-based wafer matrix for gradual buccal drug delivery. J Biomed Mater Res B Appl Biomater. 2012;100(4):1029–1043. doi: 10.1002/jbm.b.32668. [DOI] [PubMed] [Google Scholar]
- 143.Matthews KH, Stevens HNE, Auffret AD, Humphrey MJ, Eccleston GM. Formulation, stability and thermal analysis of lyophilised wound healing wafers containing an insoluble MMP-3 inhibitor and a non-ionic surfactant. Int J Pharm. 2008;356(1–2):110–120. doi: 10.1016/j.ijpharm.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 144.Labovitiadi O, Lamb AJ, Matthews KH. In vitro efficacy of antimicrobial wafers against methicillin-resistant Staphylococcus aureus. Ther Deliv. 2012;3(4):443–455. doi: 10.4155/tde.12.27. [DOI] [PubMed] [Google Scholar]
- 145.Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Controlled Release. 2001;74(1–3):63–67. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- 146.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 147.Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr Oncol. 2007;14(5):189–194. doi: 10.3747/co.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xing WK, Shao C, Qi ZY, Yang C, Wang Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des Devel Ther. 2015;9:3341–3348. doi: 10.2147/DDDT.S85943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chae GS, Lee JS, Kim SH, Seo KS, Kim MS, Lee HB, Khang G. Enhancement of the stability of BCNU using self-emulsifying drug delivery systems (SEDDS) and in vitro antitumor activity of self-emulsified BCNU-loaded PLGA wafer. Int J Pharm. 2005;301(1–2):6–14. doi: 10.1016/j.ijpharm.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 150.Lee JS, An TK, Chae GS, Jeong JK, Cho SH, Lee HB, Khang G. Evaluation of in vitro and in vivo antitumor activity of BCNU-loaded PLGA wafer against 9L gliosarcoma. Eur J Pharm Biopharm. 2005;59(1):169–175. doi: 10.1016/j.ejpb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 151.Roullin V-G, Deverre J-R, Lemaire L, Hindré F, Venier-Julienne M-C, Vienet R, Benoit J-P. Anti-cancer drug diffusion within living rat brain tissue: an experimental study using [3H](6)-5-fluorouracil-loaded PLGA microspheres. Eur J Pharm Biopharm. 2002;53(3):293–299. doi: 10.1016/s0939-6411(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 152.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zembko I, Ahmed I, Farooq A, Dail J, Tawari P, Wang W, McConville C. Development of disulfiram-loaded poly(lactic-co-glycolic acid) wafers for the localised treatment of glioblastoma multiforme: a comparison of manufacturing techniques. J Pharm Sci. 2015;104(3):1076–1086. doi: 10.1002/jps.24304. [DOI] [PubMed] [Google Scholar]
- 154.Lee JSC GS, An TK, Khang G, Cho SH, Lee HB. Preparation of 5-fluorouracil-loaded poly(L-lactide-co-glycolide) wafer and evaluation of in vitro release behavior. Macromolecular Research. 2003;11(3):183–188. [Google Scholar]
- 155.Lee JS, Chae GS, Kim MS, Cho SH, Lee HB, Khang G. Degradation behaviour in vitro for poly(D,L-lactide-co-glycolide) as drug carrier. Biomed Mater Eng. 2004;14(2):185–192. [PubMed] [Google Scholar]
- 156.Gopferich A. Erosion of composite polymer matrices. Biomaterials. 1997;18(5):397–403. doi: 10.1016/s0142-9612(96)00151-2. [DOI] [PubMed] [Google Scholar]
- 157.Yoo JY, Kim JM, Khang G, Kim MS, Cho SH, Lee HB, Kim YS. Effect of lactide/glycolide monomers on release behaviors of gentamicin sulfate-loaded PLGA discs. Int J Pharm. 2004;276(1–2):1–9. doi: 10.1016/j.ijpharm.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 158.Kim JMS KS, Jeong YK, Lee HB, Kim YS, Khang G. Co-effect of aqueous solubility of drugs and glycolide monomer on in vitro release rates from poly(D,L-lactide-co-glycolide) discs and polymer degradation. J Biomater Sci Polym Ed. 2005;16(8):991–1007. doi: 10.1163/1568562054414676. [DOI] [PubMed] [Google Scholar]
- 159.Wang F, Lee T, Wang CH. PEG modulated release of etanidazole from implantable PLGA/PDLA discs. Biomaterials. 2002;23(17):3555–3566. doi: 10.1016/s0142-9612(02)00034-0. [DOI] [PubMed] [Google Scholar]
- 160.Metzmacher I, Radu F, Bause M, Knabner P, Friess W. A model describing the effect of enzymatic degradation on drug release from collagen minirods. Eur J Pharm Biopharm. 2007;67(2):349–360. doi: 10.1016/j.ejpb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 161.Lofthouse S, Nagahara S, Sedgmen B, Barcham G, Brandon M, Sano A. The application of biodegradable collagen minipellets as vaccine delivery vehicles in mice and sheep. Vaccine. 2001;19(30):4318–4327. doi: 10.1016/s0264-410x(01)00153-0. [DOI] [PubMed] [Google Scholar]
- 162.Maeda H, Sano A, Fujioka K. Controlled release of rhBMP-2 from collagen minipellet and the relationship between release profile and ectopic bone formation. Int J Pharm. 2004;275(1–2):109–122. doi: 10.1016/j.ijpharm.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 163.Higaki M, Azechi Y, Takase T, Igarashi R, Nagahara S, Sano A, Fujioka K, Nakagawa N, Aizawa C, Mizushima Y. Collagen minipellet as a controlled release delivery system for tetanus and diphtheria toxoid. Vaccine. 2001;19(23–24):3091–3096. doi: 10.1016/s0264-410x(01)00039-1. [DOI] [PubMed] [Google Scholar]
- 164.Weinberg BD, Ai H, Blanco E, Anderson JM, Gao J. Antitumor efficacy and local distribution of doxorubicin via intratumoral delivery from polymer millirods. J Biomed Mater Res A. 2007;81(1):161–170. doi: 10.1002/jbm.a.30914. [DOI] [PubMed] [Google Scholar]
- 165.Wang F, Blanco E, Ai Hua, Boothman DA, Gao J. Modulating lapachone release from polymer millirods through cyclodextrin complexation. J Pharm Sci. 95(10):2309–2319. doi: 10.1002/jps.20721. [DOI] [PubMed] [Google Scholar]
- 166.Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H, Nagai Y, Terada M. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet. Nat Med. 1999;5(6):707–710. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]
- 167.Danyuo Y E, Oberaifo O, Obayemi JD, Dozie-Nwachukwu SJ, Ani C, Odusanya OS, Zebaze Kana MG, Malatesta K, Soboyejo WO. Extended pulsated drug release from PLGA-based minirods. J Mater Sci Mater Med. 2017;28(4):61. doi: 10.1007/s10856-017-5866-y. [DOI] [PubMed] [Google Scholar]
- 168.Wang G, Tucker IG, Roberts MS, Hirst LW. In vitro and in vivo evaluation in rabbits of a controlled release 5-fluorouracil subconjunctival implant based on Poly(D,L-lactide-co-glycolide) Pharm Res. 1996;13(7):1059–1064. doi: 10.1023/a:1016062825360. [DOI] [PubMed] [Google Scholar]
- 169.Ren XZ N, Gao Y, Chen T, Lu W. Biodegradable three-dimension micro-device delivering 5-fluorouracil in tumor bearing mice. Drug Deliv. 2012;19(1):36–44. doi: 10.3109/10717544.2011.635720. [DOI] [PubMed] [Google Scholar]
- 170.Zheng N, Zhou M, Lu W. In vivo distribution of 5-Fluorouracil after peritumoral implantation using a biodegradable micro-device in tumor-bearing mice. Eur J Drug Metab Pharmacokinet. 2013;38(3):183–190. doi: 10.1007/s13318-012-0111-z. [DOI] [PubMed] [Google Scholar]
- 171.Yoon J, Kim D, Siregar A, Lee BY, Kwon K-Y, Byun J-H, Woo DK. 5-Fluorouracil-coated Hydroxyapatite Nanoparticles as Anticancer Drug Delivery Carriers. Bull Korean Chem Soc. 2015;36(2):445–446. [Google Scholar]
- 172.Andronescu E, Ficai A, Albu MG, Mitran V, Sonmez M, Ficai D, Ion R, Cimpean A. Collagen-hydroxyapatite/cisplatin drug delivery systems for locoregional treatment of bone cancer. Technol Cancer Res Treat. 2013;12(4):275–284. doi: 10.7785/tcrt.2012.500331. [DOI] [PubMed] [Google Scholar]
- 173.Quinlan E, Thompson EM, Matsiko A, O’Brien FJ, Lopez-Noriega A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J Control Release. 2015;207:112–119. doi: 10.1016/j.jconrel.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 174.Chen X, Deng C, Tang S, Zhang M. Mitochondria-dependent apoptosis induced by nanoscale hydroxyapatite in human gastric cancer SGC-7901 cells. Biol Pharm Bull. 2007;30(1):128–132. doi: 10.1248/bpb.30.128. [DOI] [PubMed] [Google Scholar]
- 175.Liu ZS, Tang SL, Ai ZL. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2003;9(9):1968–1971. doi: 10.3748/wjg.v9.i9.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu J, Liu ZS, Tang SL, He YM. Effect of hydroxyapatite nanoparticles on the growth and p53/c-Myc protein expression of implanted hepatic VX2 tumor in rabbits by intravenous injection. World J Gastroenterol. 2007;13(20):2798–2802. doi: 10.3748/wjg.v13.i20.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dey S, Das M, Balla VK. Effect of hydroxyapatite particle size, morphology and crystallinity on proliferation of colon cancer HCT116 cells. Mater Sci Eng C Mater Biol Appl. 2014;39:336–339. doi: 10.1016/j.msec.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 178.Uchida A, Shinto Y, Araki N, Ono K. Slow release of anticancer drugs from porous calcium hydroxyapatite ceramic. J Orthop Res. 1992;10(3):440–445. doi: 10.1002/jor.1100100317. [DOI] [PubMed] [Google Scholar]
- 179.Kunieda K, Seki T, Nakatani S, Wakabayashi M, Shiro T, Inoue K, Sougawa M, Kimura R, Harada K. Implantation treatment method of slow release anticancer doxorubicin containing hydroxyapatite (DOX-HAP) complex. A basic study of a new treatment for hepatic cancer. Br J Cancer. 1993;67(4):668–673. doi: 10.1038/bjc.1993.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Venkatasubbu GD, Ramasamy S, Reddy GP, Kumar J. In vitro and in vivo anticancer activity of surface modified paclitaxel attached hydroxyapatite and titanium dioxide nanoparticles. Biomed Microdevices. 2013;15(4):711–726. doi: 10.1007/s10544-013-9767-7. [DOI] [PubMed] [Google Scholar]
- 181.Scharnweber T, Santos C, Franke RP, Almeida MM, Costa ME. Influence of spray-dried hydroxyapatite-5-fluorouracil granules on cell lines derived from tissues of mesenchymal origin. Molecules. 2008;13(11):2729–2739. doi: 10.3390/molecules13112729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chu WSJ SY, Kim SG, Ha WS, Chi SC, Ahn SH. Fabrication of a biodegradable drug delivery system with controlled release made of PLGA/5-FU/hydroxyapatite. Rapid Prototyping Journal. 2008;14(5):293–299. [Google Scholar]
- 183.Hong C-H, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50(1):48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 184.Zheng W. A water-in-oil-in-oil-in-water (W/O/O/W) method for producing drug-releasing, double-walled microspheres. Int J Pharm. 2009;374(1–2):90–95. doi: 10.1016/j.ijpharm.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 185.Wang YL P, Peng Z, She FH, Kong LX. Microencapsulation of nanoparticles with enhanced drug loading for pH-sensitive oral drug delivery for the treatment of colon cancer. J Appl Polym Sci. 2013;129(2):714–720. [Google Scholar]
- 186.Seema D M. MMT-PLGA nanocomposites as an oral and controlled release carrier for 5 -Fluorouracil: A novel approach. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(2):332–341. [Google Scholar]
- 187.Misak HZ N, Song Z, Hwang S, Man KP, Asmatulu R, Yang SY. Skin cancer treatment by albumin/5-Fu loaded magnetic nanocomposite spheres in a mouse model. J Biotechnol. 2013;164(1):130–136. doi: 10.1016/j.jbiotec.2013.01.003. [DOI] [PubMed] [Google Scholar]