Abstract
Pregnant women and their unborn children are a population that is particularly vulnerable to bacterial infection. Physiological changes that occur during pregnancy affect the way women respond to such infections and the options that clinicians have for treatment. Antibiotics are still considered the best option for active infections and a suitable prophylaxis for prevention of potential infections, such as vaginal/rectal Streptococcus agalactiae colonization prior to birth. The effect of such antibiotic use on the developing fetus, however, is still largely unknown. Recent research has suggested that the fetal gut microbiota plays a critical role in fetal immunologic programming. Hence, even minor alterations in this microbiota may have potentially significant downstream effects. An ideal antibacterial therapeutic for administration during pregnancy would be one that is highly specific for its target, leaving the surrounding microbiota intact. This review first provides a basic overview of the challenges a clinician faces when administering therapeutics to a pregnant patient and then goes on to explore common bacterial infections in pregnancy, use of antibiotics for treatment/prevention of such infections and the consequences of such treatment for the mother and infant. With this background established, the review then explores the potential for use of bacteriophage (phage) therapy as an alternative to antibiotics during the antenatal period. Many previous reviews have highlighted the revitalization of and potential for phage therapy for treatment of a range of bacterial infections, particularly in the context of the increasing threat of widespread antibiotic resistance. However, information on the potential for the use of phage therapeutics in pregnancy is lacking. This review aims to provide a thorough overview of studies of this nature and discuss the feasibility of bacteriophage use during pregnancy to treat and/or prevent bacterial infections.
Keywords: bacteriophages, phage therapy, antenatal, perinatal, pregnancy, fetus, antibiotic prophylaxis, bacterial infections
Use of therapeutics in the antenatal period
Pregnancy induces a number of physiological changes in women, ranging from changes in energy metabolism (Herrera, 2000; Prentice and Goldberg, 2000) to maternal circulation (Hunter and Robson, 1992), and presents a number of unknown risks in relation to the pharmacokinetics of different therapeutics (Loebstein et al., 1997). Infection during pregnancy can have devastating outcomes for the mother and fetus and is a major cause of preterm birth (delivery at <37 weeks gestation) (Goldenberg et al., 2008). Minimizing harm to the developing fetus is of the utmost importance when treating maternal conditions during this period, however, the complex nature of pregnancy can make this a challenging task. Maternal treatment can often result in the fetus being unnecessarily treated and conversely, attempts to treat the fetus in utero by maternal administration of drugs can vary greatly depending on the placental transfer of the chosen compound (Pacifici, 2006). This is further confounded by the overall lack of knowledge of the interactions of various therapeutics during pregnancy, largely due to this population being excluded from most clinical trials of drug efficacy as a result of safety concerns (Ke et al., 2014).
A review by Anderson examined pharmacokinetic factors during pregnancy to assess the differences in drug absorption, metabolism, distribution and excretion (Anderson, 2006). The issue of drug transfer to the fetus in utero is of particular concern, given the poorly understood impacts that exposure may have during these early stages of development. The placenta plays a number of roles during pregnancy: not only does it act as a physical barrier, it also provides exchange of nutrients and oxygen and differentiates the maternal and fetal circulation. Drug transfer during pregnancy may occur across the placenta through passive diffusion or transporter systems (Syme et al., 2004). Improved knowledge of the mechanisms of such transfer and the extent of metabolism prior to contact with the fetus is important in understanding how drug administration during pregnancy may affect the neonate. With this in mind, one would think that drug safety for use in pregnancy would certainly be thoroughly investigated, however, a survey of the global clinical trials registries reveal that only 0.32% of active registered studies were pregnancy drug trials (Scaffidi et al., 2017). As the majority of drugs prescribed and used during pregnancy are off-label and often never formally tested for use in pregnancy (Ke et al., 2014), the exclusion of pregnant women from such trials is ethically questionable considering that the results may be of great relevance to this population; however, extensive consideration of the trial design is required for this population due to the greater inherent risks (Welch et al., 2015). In addition, as highlighted by Welch and colleagues, a depth of understanding must be attained to ensure women are able to make informed decisions about their own treatment and involvement in trials.
Antibiotics during pregnancy
Bacterial infections
The majority of antibiotic use during pregnancy is targeted at treating various urinary tract infections (UTIs), sexually transmitted infections (STIs) such as Treponema pallidum, Neisseria gonorrhoeae and Chlamydia trachomatis and the common food-borne pathogen Listeria monocytogenes (Ross, 1979; Brumfitt and Hamilton-Miller, 1981; Cram et al., 2002). Another pathogen associated with neonatal sepsis, Streptococcus agalactiae or Group B Streptococcus (GBS), is also targeted with antibiotics in an effort to prevent transmission to the neonate during birth (Verani et al., 2010). These pathogens are targeted in an effort to prevent poor obstetric outcomes, however, even narrow-spectrum antibiotics impact on a number of other bacterial species in addition to the target. Alternatively, broad-spectrum antibiotics are commonly used in the event of complications during pregnancy such as premature rupture of membranes (PROM) (Chapman, 1986). In utero infection is a major cause of preterm birth, with preterm births accounting for 11% of all live births (Blencowe et al., 2012), and is often associated with significant morbidity and mortality (Goldenberg et al., 2000). Major causes of infection-associated preterm birth include the Ureaplasma spp., for which treatment options are particularly limited due to their lack of a peptidoglycan layer rendering all β-lactam antibiotics ineffective (Viscardi, 2014). All of these bacterial infections and a number of others have resulted in widespread administration of antibiotics to pregnant women, the majority of which have limited data available on their relevant safety profiles and are broad-spectrum in their activity, affecting not only the target organisms, but also commensal bacteria.
Safety
With the above causes of infection and pharmacological considerations in mind, it has been reported that 80% of all prescriptions during pregnancy are antibiotics and approximately 20-25% of women will receive antibiotics during pregnancy (Heikkila, 1993; Santos et al., 2010; de Jonge et al., 2014; Bookstaver et al., 2015). A review of antibiotic use during pregnancy by Bookstaver et al. (2015) included an extensive list of antibiotics accompanied by their Food and Drug Administration (FDA) pregnancy category rating from A (no risk in pregnancy) to X (contraindicated in pregnancy). The list only contained categories B (generally safe to use) to D (avoid in pregnancy unless benefit outweighs risk). This further highlights the lack of knowledge we have on the effect of drugs, specifically antibiotics, which are widely used during pregnancy. In general β-lactams, macrolides, clindamycin and fosfomycin are considered safe during pregnancy, but additional studies are required for further assessment of other antibiotic classes that currently have minimal pregnancy data (Bookstaver et al., 2015).
In addition to selecting the appropriate antibiotic, the correct dose of antibiotics is essential to minimize toxicity and maximize efficacy. During pregnancy, pharmacokinetic properties of drugs can be expected to vary significantly in comparison with non-pregnant adults due to a number of physiological changes (Pariente et al., 2016). Further to this, the dose a fetus receives from maternal administration significantly differs depending on a number of factors, one being the placental barrier. Drugs must overcome the placental barrier to reach the fetus and have a therapeutic effect; the rate of this transplacental transfer is low for widely-used macrolide antibiotics (Philipson et al., 1973; Heikkinen et al., 2000; Ramsey et al., 2003). Heikkinen and others observed transfer of erythromycin, roxithromycin and azithromycin to be as low as 3, 4.3, and 2.6%, respectively, across a placental perfusion model (Heikkinen et al., 2000). Extensive studies by Kemp and colleagues have examined the in vivo effect of erythromycin, azithromycin and more recently, solithromycin, using a pregnant sheep model (Keelan et al., 2011; Kemp et al., 2014a,b). The clearance of Ureaplasma parvum intrauterine infection by IV administration was achieved by azithromycin and solithromycin (Miura et al., 2014). In contrast, neither intramuscular nor intra-amniotic administration of erythromycin was able to completely resolve U. parvum intra-amniotic infection (Kemp et al., 2014b). This highlights not only the importance of drug bioavailability but also efficacy against the pathogen.
Drug bioavailability to the fetus is important for treating intrauterine infections, while conversely, minimal transfer may be beneficial when treating maternal infection as the lack of transfer results in limited fetal exposure. Philipson and colleagues examined ampicillin (Philipson, 1977), cepharidine and cephazolin (Philipson et al., 1987): both studies concluded that higher doses were required during pregnancy to reach similar plasma levels as compared to after pregnancy. This raises questions regarding under and over dosing in this population; the former has the potential to impact on resistance in bacterial infections as sub-inhibitory doses can select for resistant isolates, and higher dosage raises issues of toxicity (Anderson, 2006; Pariente et al., 2016). Most antibiotics cross the placenta to some extent. β-lactams cross rapidly and equilibrate in maternal and cord plasma in what is termed “complete” transfer, while other antibiotics show “incomplete” transfer where concentrations are lower in the cord than maternal plasma (Pacifici, 2006).
Antibiotic resistance
Widespread antibiotic use is strongly associated with the development of multi-drug resistant (MDR) bacteria. The World Health Organisation (WHO) recently published a list of priority organisms that require urgent drug development to overcome resistance to current antibiotics (Willyard, 2017). Of the pathogens mentioned, N. gonorrhoeae is listed as “High Priority” for research and development of new antibiotics. While resistance is more prevalent in some bacterial species than others, continued widespread use coupled with a lack of new drug development is likely to continue to fuel the growth of MDR strains amongst numerous new species in the coming years (Norrby et al., 2005; Talbot et al., 2006; Ventola, 2015). In fact, some recent predictions suggest total human mortality attributed to MDR bacteria by 2050 could exceed that resulting from cancer (O'Neill, 2014).
Microbial dysbiosis
The majority of antibiotics have broad spectrum activity and affect not only the target bacteria but also commensal organisms. In addition to potentially promoting resistance amongst organisms other than the intended target/s, commensal disruption also results in a state of antibiotic-induced dysbiosis in which those bacteria unaffected by the antibiotic (potentially already resistant or protected within a microbial biofilm) populate the microbial community through lack of competition (Mendling, 2016). The resulting cascade of impacts possible is far reaching, from adverse effects on various maternal body site microbiomes, through to effects on the seeding of the neonatal microbiome in utero and soon after delivery (Mueller et al., 2015; Stinson et al., 2017). The latter in particular is of great significance when considering intrapartum antibiotic prophylaxis (IAP) which aims to delay antibiotic exposure until the point of delivery in an effort to reduce exposure and prevent neonatal infection through vertical transmission, particularly for GBS colonization (Schrag and Verani, 2013). This has successfully reduced incidence of GBS disease in neonates (Verani et al., 2010), however, a number of studies have now assessed the impact of this exposure on neonatal gut microbiomes.
Mazzola and colleagues noted the effect in IAP-exposed breastfed infants following analysis of infant stool samples, where 16S rDNA sequences associated with the Enterobacteriaceae were increased at seven days and remained in high abundance a month after delivery (Mazzola et al., 2016). IAP resulted in a reduction in Bifidobacterium spp. sequences in breastfed and mixed-fed infants at 7 days, but numbers returned to levels comparable with non-IAP exposed infants by 1 month of age. Similarly, another group examined the effect of IAP for GBS on the gut microbiota within the 1st month of life. This study selected microbial groups to examine including Lactobacillus spp., Bifidobacterium spp., and Bacteroides fragilis (Corvaglia et al., 2016). They observed a reduction in Bifidobacteria counts at 7 days of life in the IAP group compared to no IAP. However, breast milk-fed infants had increased Lactobacillus spp. counts regardless of IAP at both 7 and 30 days of life. Another study revealed differences in infant gut microbiota communities at 3 months of age following IAP exposure (Azad et al., 2016). Further disruption was evident in those who received IAP during cesarean section delivery, with taxonomic differences in microbial profiles persisting to 12 months in those cesarean section-delivered, formula-fed infants who received IAP. These studies highlight the potential effects of IAP on the neonatal gut microbiome and suggest that it can be modified and semi-restored through breastfeeding. However, the long-term impact of this initial microbial seeding displacement is not yet well understood (Azad et al., 2016; Corvaglia et al., 2016; Mazzola et al., 2016). Reviews on the significance of the fetal microbiome for infant health highlight the effect such prophylactic treatments have on the initial microbial diversity and the impact this may have on other health parameters (Dunlop et al., 2015; Mueller et al., 2015; Yang et al., 2016; Stinson et al., 2017). In light of this information, when considering IAP for a defined target, such as for the prevention of GBS vaginal/rectal transmission, the potential for use of targeted, non-antibiotic therapies warrants serious consideration.
Without a doubt, antibiotics represent a significant breakthrough in medical history. In an antenatal context they have prevented large amounts of maternal and neonatal morbidity, however, there is limited information available on their safety for use in pregnancy. The development of resistant bacteria, although not a major issue in pregnancy at present, is still a serious concern. Although substantial contention still exists (Perez-Munoz et al., 2017) surrounding recent evidence that the newborn likely develops its initial gut microbiota in utero and that this is seeded from the mother (Dunlop et al., 2015; Mueller et al., 2015; Stinson et al., 2017), regardless, it would certainly appear that targeted antimicrobial therapies, such as bacteriophages, would be of significant benefit during the antenatal period. This is particularly so in cases of prophylactic treatment, such as prevention of GBS infection. Broad-spectrum antibiotic use is certainly still warranted though when unknown potential pathogens are involved, in cases of PROM for instance (Seelbach-Goebel, 2013), but reserving usage to these emergency situations would likely be of significant benefit to both the mother and the developing fetus in terms of reducing microbial dysbiosis and potential associated adverse effects on fetal immune programming.
Bacteriophages
Bacteriophages are bacterial viruses also known as phages. They represent the most abundant organisms on Earth. They are so expansive in number that it has been postulated that there are likely a trillion phages for every single grain of sand on the planet (Keen, 2015). Unlike other viruses, bacteriophages are only able to infect bacteria and remain distinct from animal and plant viruses (Carlton, 1999). The co-discovery of these viruses was made in 1915 by Twort (1915) and independently 2 years later by d'Herelle who subsequently named them bacteriophages (D'Hérelle, 1917). Upon the realization that phages had the capability of killing bacteria, their use as a therapeutic was investigated almost immediately. In the century of research since their discovery, many breakthroughs, especially in molecular biology, have been made through the use of bacteriophages (Keen, 2015). Initial interest in these viruses was, however, overshadowed by the emergence of cheap and effective antibiotics which have since been the frontline in bacterial infection treatment. However, recent years have brought attention to the serious issue of emerging antibiotic resistance, which has revitalized the notion of using bacteriophages as therapeutic agents (Nobrega et al., 2015).
Mechanism of action
The overall mechanism of action that all phages undertake includes adsorption, injection of genetic material into host, replication, assembly and virion release. Given the breadth of phage taxa there are a number of variations to the following process, however, as an obligate pathogen the phage must find a host in which to replicate. The process of adsorption occurs in two steps, the first is the initial contact with the host surface through reversible electrostatic forces, allowing the phage to survey the host at closer proximity to locate specific receptors. In the case of tailed phages, capsid interaction with the host surface allows the tail components to search for and interact with specific receptors on the surface (Hu et al., 2013; Murata et al., 2017). If the specific host receptor is found, tailed phages will bind irreversibly to their target and complete the adsorption process. Alternatively, tailless and filamentous phages contain the necessary components for attachment at exposed surface sites and have evolved to use host-cell-encoded channels for genomic transfer (Peralta et al., 2013).
Once attached to the host, the genetic material of the phage, initially encased in the phage head or capsid, is injected into the host where it will then undergo replication. Larger phages, such as ϕKZ, contain genomes that encode their own necessary DNA replication requirements, while others may rely on the host (Ceyssens et al., 2014). The replication process involves synthesis of numerous copies of the genetic material, translation and the manufacture of phage components such as the head, tail and internal proteins. Once matured and assembled these virions are released from the host cell via internal lysis, after which the progeny may go on to infect other cells and continue the infection process, whilst the host is killed (Stent, 1963). Other methods of progeny release can also include budding and extrusion (Weinbauer, 2004).
Bacteriophage lifecycles include obligately lytic, lysogenic, pseudolysogenic and chronic infection (Clokie et al., 2011). Bacteriophages are often classified according to the best understood of these lifecycles as virulent (obligately lytic) or temperate (lysogenic lifestyle) (Dy et al., 2014). Recent review of phage terminology has highlighted terms that require classification, such as the intended use of “lytic and lysogenic” and these suggestions have been incorporated into this review (Hobbs and Abedon, 2016). Obligately lytic phages do not integrate with the host and only enter the lytic cycle. This cycle involves cessation of host component production and utilization of host products to replicate phage products, ultimately resulting in host cell death and release of assembled virions (Stent, 1963; Young, 2013; Roach and Donovan, 2015). This feature makes these phages the perfect subjects for bacteriophage therapy as the goal is to eradicate the target bacteria in a bactericidal fashion (Brussow, 2012). The lytic cycle acts to regulate bacterial populations and is beneficial in many ways, such as in the reduction of colonization by pathogens of mucosal surfaces. Barr et al. demonstrated that bacteriophage colonizing the mucosal surfaces of humans and animals limited bacterial populations and acted as an additional component of innate immunity (Barr et al., 2013). Their ubiquitous presence makes it difficult to believe they wouldn't play a significant role in our individual microbiomes and health. Indeed, Manrique and colleagues have demonstrated a shared “phageome” of humans and propose that it plays a major part in maintaining the structure and function of the gut microbiome, although the temperate phages discussed below are believed to be more abundant than obligately lytic phages in the human gut (Manrique et al., 2016).
Temperate phages, however, can undertake a lysogenic lifestyle which involves phage replication that does not directly result in virion production or release. These phages have the ability to integrate into the host genome or exist as a plasmid, enabling replication through host reproduction and resulting in bacterial daughter cells containing this phage genome (prophage). The temperate phage can remain as a prophage, coexisting in the lysogenized host which it leaves unharmed until conditions are unfavorable for growth, in which case the phage, if integrated, may then excise itself from the genome and enter a lytic cycle.
Both obligately lytic, and temperate phages may exhibit pseudolysogeny, a controversial topic but defined by some authors as a state of stalled development of a phage within its host, in which neither phage replication nor prophage formation occurs (Łoś and Wegrzyn, 2012). This state is triggered by conditions that cause sub-optimal growth of bacteria or starvation, allowing the co-existence of both the resulting inactive and unstable phage, and the starved host within low resource environments without mutual destruction (Brendan and Lenski, 1997). Pseudolysogeny is resolved with the change of nutrient conditions which enable growth, at which point the phage can either form a stable prophage or enter the lytic cycle. Chronic phage infection may also involve either temperate or non-temperate phages (Hobbs and Abedon, 2016), and refers to productive infection in which virions are released over time without host cell lysis. Filamentous phages have the ability to release progeny phage through extrusion via membrane transport complexes (Rakonjac et al., 2011).
Lysogeny makes temperate phages a potential public health risk as phage-encoded virulence genes or regulators can result in increased host pathogenesis as a result of phage expression. The process of phage conversion involves the expression of a different host phenotype as a result of temperate phage infection. Phage-encoded virulence factors are numerous, and thus temperate phage infection could result in increased pathogenicity of the lysogenized host bacteria (Clokie et al., 2011; Keen, 2015). Prime examples of phage-mediated virulence exist in Vibrio cholerae, in which the gene for production of the cholera toxin is contained on a phage (CTXϕ, an example of a chronic temperate phage) (Waldor and Mekalanos, 1996) and the same is evident in Escherichia coli with phage-encoded shiga-like toxin contributing to the symptoms of hemorrhagic colitis (O'Brien et al., 1984). As well as numerous toxin genes, temperate phages encode other traits that promote bacterial colonization, uptake and survival within a host cell (reviewed by Boyd) (Boyd, 2012).
Considering this brief overview of phage biology, we highlight that the obligately lytic phages currently provide the best option for therapeutic use due to their lethal effect on the bacterial host, as opposed to the co-existence with the host of temperate phages and the potential problem of lysogenic conversion.
Bacteriophage therapy
The lethal nature of obligately lytic bacteriophages is highly attractive for exploitation in treatment of bacterial infections. Certain characteristics of bacteriophages such as the specificity of their action, are a significant advantage over antibiotics when targeted therapy is applicable (Table 1). This specificity can improve treatment outcomes for patients in that only specific bacterial species within an individual host range are removed, leaving other beneficial microbes unaffected (Loc-Carrillo and Abedon, 2011).
Table 1.
Comparison of therapeutic characteristics of bacteriophages and antibiotics.
| Bacteriophages | Antibiotics |
|---|---|
| BACTERICIDAL AGENTS | |
|
|
| DOSAGE | |
|
|
| TOXICITY | |
|
|
| MICROBIOTA DISRUPTION | |
|
|
| RESISTANCE | |
|
|
| DISCOVERY | |
|
|
Information adapted from Loc-Carrillo and Abedon (2011).
Bacteriophages against antenatal pathogens
Urinary tract pathogens
Development of urinary tract infections (UTIs) can occur at any stage during pregnancy and often results in antibiotic intervention, which depending on the gestation, could have implications for fetal development (de Tejada, 2014). Numerous in vitro studies have outlined the level of activity that isolated bacteriophages have on specific pathogens and a number of cocktails have been developed to cover a broader host range and reduce risk of resistance forming (Lehman and Donlan, 2015; Galtier et al., 2016; Sybesma et al., 2016). Common causes of UTIs, E. coli and Klebsiella pneumoniae have been the target of a number of studies. Sybesma and others reported the activity of commercially available bacteriophage cocktails from the George Eliava Institute in Georgia against clinical E. coli (n = 41) and K. pneumoniae (n = 9) strains isolated from urine of patients with UTIs, where activity against all strains except one was observed (Sybesma et al., 2016). The ability to penetrate biofilms is of particular relevance when considering these as treatment options. One study found a significant reduction of uropathogenic E. coli biofilms within 2–12 h of phage administration in vitro (Chibeu et al., 2012). Additionally, a continuous-flow in vitro model using artificial urine was used to assess the prevention of biofilm formation by pre-treatment of urinary catheters with phages, which showed a significant reduction in common UTI species Pseudomonas aeruginosa and Proteus mirabilis (Lehman and Donlan, 2015).
The genitourinary tract is the major focus when considering applications for bacteriophage use antenatally, and in the context of UTI treatment during pregnancy the common antibiotic side-effect of vaginal dysbiosis/candidiasis can be prevented with such targeted therapies. In addition, it is well established that opportunistic pathogens residing in the vaginal microbiota during pregnancy can ascend through the cervix and establish florid infections of the fetal membranes (chorioamnionitis), amniotic cavity and ultimately the fetus (Goldenberg et al., 2000). The asymptomatic nature of such vaginal colonization can have devastating impacts on the immune-naïve fetus and neonate and in this context pathogen removal using specific bacteriophages could be implemented as a preventative measure.
Ureaplasma spp. are prime examples of such asymptomatic colonization's, with 40 to 80% of pregnant women colonized with these bacteria (Waites et al., 2005). Their role in preterm birth has been one of controversy, however, studies highlighted in the review by Capoccia et al. (2013) address the association between Ureaplasma spp. and adverse pregnancy outcomes. In addition, more recently, in a prospective cohort study of low-risk pregnant women, Payne and colleagues reported that it is U. parvum, not Ureaplasma urealyticum, that is of relevance to preterm birth and particularly U. parvum genotype SV6 (Payne et al., 2016). Intra-amniotic infection can lead to preterm birth and additional neonatal morbidity and mortality (van Waarde et al., 1997; Viscardi and Hasday, 2009; Kasper et al., 2011; Kallapur et al., 2013). Ureaplasma spp. are a potential target for bacteriophage therapy, however, their unique biological characteristics represent a challenge. They are unable to form a lawn or turbid culture due to their small cell size, making plaque assays and other standard phage techniques extremely difficult.
Galtier and colleagues examined phage therapy for UTIs in the form of uropathogenic E. coli eradication from the gut of mice using a cocktail of three virulent bacteriophages (Galtier et al., 2016). In this study they found that a single oral gavage dose of the bacteriophage cocktail was able to remove the target E. coli with less effect on microbial diversity than that of antibiotic administration. Another study found that a bacteriophage cocktail was effective against E. coli adhered to the urothelium, commonly the case in persistent UTIs (Sillankorva et al., 2011). While the assessment of bacteriophage activity in the context of pregnancy is lacking, we can appreciate the body of work regarding phage efficacy and selectivity in in vitro, animal and non-pregnant human models and move toward broadening the scope of this research.
P. aeruginosa represents another pathogen that causes UTIs from which isolation of MDR strains is becoming more frequent. Human examples of phage therapy often comprise case reports of persistent pathogens and instances of compassionate phage use. One such example includes a 67 year old woman administered a cocktail of six lytic phages in combination with antibiotic therapy to treat a UTI caused by P. aeruginosa. Antibiotic therapy alone had failed, however, combination of this with the phage cocktail resulted in eradication (Khawaldeh et al., 2011). Additionally, a clinical trial is currently underway to assess phage therapy in the context of UTI in transurethral resection of prostate patients (Leitner et al., 2017). While this trial is in men, the study design and context of UTIs make for a valuable addition to our current understanding of phage therapy in humans and will allow assessment of inter-patient variability which cannot be assessed from case studies. This represents significant progress toward gaining thorough and credible clinical trial data which will impact future phage therapeutics.
Group B streptococcus
Intrapartum antibiotic prophylaxis for S. agalactiae or Group B Streptococcus (GBS) vaginal/rectal colonization is standard practice in many countries in an attempt to reduce neonatal morbidity and mortality associated with GBS disease. These prophylactic strategies vary from risk-based to culture-based screening and result in identified women receiving intravenous (IV) antibiotics a minimum of 4 h prior to delivery and then every 4 h during labor in an effort to eradicate the bacteria (Verani et al., 2010). With 10 to 30% of pregnant women colonized with GBS, the potential for antibiotic exposure is widespread (Le Doare and Heath, 2013). This is a prime example of a single species target in which phage therapy could excel.
Exploitation of the key components of virulent phage, such as the lysins that result in bacterial cell lysis, have been pursued as therapeutic options (Gaca and Gilmore, 2016). Bacteriophage lysins are responsible for the final stage of the lytic cycle which involves disruption of the bacterial host envelope and release of phage progeny and cell contents, inevitably destroying the host (Grundling et al., 2001). Exogenously, the lysin enzyme alone is often sufficient to lyse bacteria as it has direct contact with the peptidoglycan layer (Loeffler et al., 2001). This property has been targeted by researchers in an attempt to uncover novel therapeutic strategies. Pritchard and colleagues examined the bifunctional peptidoglycan lysin of GBS bacteriophage B30 and found a synergistic killing activity between the lysozyme and endopeptidase functions (Pritchard et al., 2004). This study involved in vitro testing and characterization of the lysin molecules, which they aimed to use as treatment for vaginal GBS in an effort to prevent GBS disease transmission. The following year Cheng et al. examined GBS lysin activity in vivo using a mouse model (Cheng et al., 2005). It was found that GBS phage lysin, PlyGBS, efficiently killed all tested GBS serotypes in vitro, and in the mouse model significantly reduced bacterial colonization in both the vagina and oropharynx with a single dose. This study outlines promising results for use of bacteriophage lysins for the eradication of GBS in the vagina in particular. Bacteriophage-derived lysin reviews have highlighted the activity against Gram positive organisms (Fischetti, 2008; Fenton et al., 2010), however activity against Gram negative organisms has also been observed (Lood et al., 2015) and discussed (Yang et al., 2014; Trudil, 2015). These studies, however, rely on static compounds of the phage as opposed to the whole phage which has the potential to increase its concentration in vitro exponentially, therefore, these lysins are likely to face similar issues to antibiotics in that dose, host metabolism/excretion and potential resistance (although this is likely to be less of an issue for lysins) may limit therapeutic use.
Various in vitro studies have assessed whole bacteriophages which possess activity against GBS strains; as yet only temperate phages have been identified in this organism. Studies such as that by Domelier and others characterized the prophages present upon induction of clinical GBS strains and identified these as belonging to the family Siphoviridae (Domelier et al., 2009). Similarly, Bai and colleagues characterized a temperate phage, JX01, induced from a bovine mastitis isolate and reported similar taxonomic observations (Bai et al., 2013). This study went on to further assess characteristics of the phage and to report that an absorption of 90% of phage particles occurred after 2.5 min incubation, followed by a 30 min latent period prior to release of a burst size of 20 virions per infected cell. Another group examined the activity of induced and modified S. agalactiae phages in vitro and reported on their activity against pathogenic strains from the environment (Brnakova et al., 2005). They also went further to analyse horizontal transfer of genetic elements. To our knowledge no virulent GBS bacteriophages have been reported in the literature to date; these would represent the ideal candidates for phage therapy. Theoretically speaking, such phages should certainly exist.
Other antenatal and perinatal pathogens
Sepsis and meningitis resulting from bacterial infections are a significant burden in the neonatal population. A number of key pathogens have been targeted in phage therapy research and experimental trials of phage formulations tested. As is the case with the majority of phage studies, these have predominantly been in vitro or in vivo animal studies. Multi-drug resistant (MDR) K. pneumoniae is a major cause of neonatal disease and very difficult to treat. The action of a phage formulation active against MDR-K. pneumoniae within a septic mouse model has been described (Vinodkumar et al., 2005). These authors observed 100% rescue of the mice with immediate phage administration and 50% when treatment was delayed until mice were moribund. Considering therapeutic limitations faced by clinicians when treating MDR-infections, phage therapy could be a viable alternative. Similarly, a Polish study observed sterilization of cerebrospinal fluid of a MDR-K. pneumoniae infected child after oral phage administration (Stroj et al., 1999).
Regarding phage administration, Russian studies observed anti-phage antibody production in infants and children up to 15 years of age that was associated with time post-oral bacteriophage treatment (Pagava et al., 2011, 2012). Interestingly, patients less than 1 month of age did not produce antibodies until 30–60 days post-treatment and even then they were only detected in 20% of the participants. Children aged 1 month to 1 year of age showed antibody presence in 4–60% of cases 30–60 days post treatment and for children aged 1–15 the rate was 33.3–100%. These data suggest there may be an advantage to having naïve neonatal immune system when considering efficacy and immune clearance of phage preparations, however, a recent study by Roach and others observed conflicting results. Their findings suggest a synergy between the host immune system and phage lysis of target bacteria, termed “immunophage synergy.” This comes as a result of administering phage to treat P. aeruginosa infection in healthy compared to immunocompromised mice (Roach et al., 2017). In the antenatal and perinatal context the impact of limited immune defenses, such as those of a fetus or neonate, is a major consideration in terms of efficacy and further exploration into the specific immune components involved is warranted.
L. monocytogenes is a food-borne pathogen which causes sepsis, abortion and central nervous system dysfunction. While clinical cases are relatively rare, listeriosis is associated with high mortality, especially in immunocompromized patients such as pregnant women and neonates (Poulsen and Czuprynski, 2013). As a food-borne pathogen, prevention of infection at the production stage has seen bacteriophage approved for use as biocontrol agents by the Food and Drug Administration (FDA) (Lang, 2006). Klumpp and Loessner provide a thorough overview of Listeria spp. phages (Klumpp and Loessner, 2013) including the number of applications that have been achieved such as biocontrol on ready-to-eat foods (Guenther et al., 2009) and sprays for fresh fruit (Leverentz et al., 2004). Phage application as a preventative measure is currently more feasible than its use to treat human infection due to the intracellular nature of L. monocytogenes infection. Intracellular infection creates a barrier to phage therapy, however, studies are evaluating alternative phage access methods such as encapsulation in liposomes (Nieth et al., 2015) and avirulent co-infection models (Broxmeyer et al., 2002) in an attempt to overcome this problem. The issue of phage access during human infection with L. monocytogenes in particular, however, still remains a logistical challenge.
Route of administration and transfer
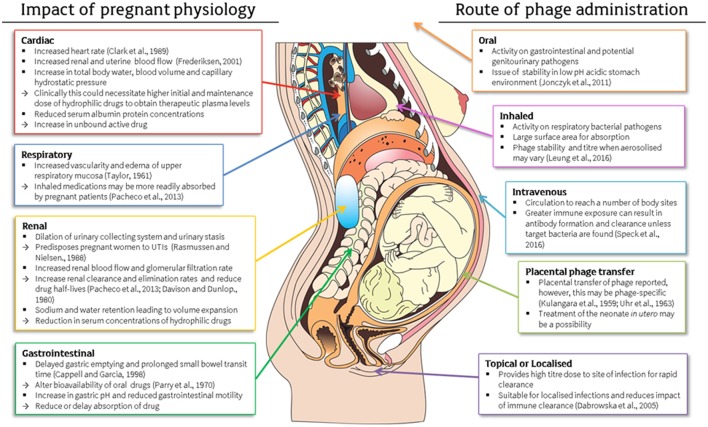
Availability of phages plays a key role in the efficacy of treatment. If just one phage can reach a bacterial cell of interest, then replication and release of exponential numbers of phage progeny will escalate the dose. Route of administration is dependent on the type and site of bacterial infection and this must be considered to ensure optimal results in terms of efficacy, distribution and clearance (Abedon, 2014). Once again, physiological differences during pregnancy can impact on therapeutic results through alterations in body systems (Costantine, 2014) including cardiac (Clark et al., 1989; Frederiksen, 2001; Pacheco et al., 2013), respiratory (Taylor, 1961; Pacheco et al., 2013), renal (Davison and Dunlop, 1980; Rasmussen and Nielsen, 1988; Pacheco et al., 2013) and gastrointestinal (Parry et al., 1970; Cappell and Garcia, 1998) systems for example (Figure 1).
Figure 1.
Physiological changes during pregnancy and their impact on drug pharmacokinetics and consideration of these factors in the application of antenatal phage therapy (Kulangara and Sellers, 1959; Taylor, 1961; Uhr et al., 1963; Parry et al., 1970; Davison and Dunlop, 1980; Rasmussen and Nielsen, 1988; Clark et al., 1989; Cappell and Garcia, 1998; Frederiksen, 2001; Dabrowska et al., 2005; Jonczyk et al., 2011; Pacheco et al., 2013; Costantine, 2014; Leung et al., 2016; Speck and Smithyman, 2016). This figure includes a licensed image obtained by the authors.
Intravenous phage administration
Systemic administration of phages by an IV route has the potential to treat multiple body sites, however a number of issues have been raised about the safety and efficacy of this application. A review by Speck and Smithyman highlighted and succinctly summarized these issues and previous successful IV phage applications (Speck and Smithyman, 2016). The main issues arising with IV use of phages can be summarized in the following categories; (i) clearance, (ii) adverse host response and (iii) host resistance; however, to some degree these are issues faced by all phage therapy routes.
The issue of clearance involves the rapid removal of phages from the bloodstream. The half-life of phages, however, has been described in the order of days as opposed to the hours observed for most antibiotics (Ochs et al., 1971). Further to this, Merril described selecting longer circulating phage variants for IV use (Merril et al., 1996). Equally, clearance is dependent on host bacteria presence, as the phage will persist only if they have access to their host bacteria to replicate in and subsequently lyse (Dubos et al., 1943).
Secondly, adverse host responses, such as shock, reported by early phage researchers (MacNeal, 1941) are likely due to the lack of purification of these initial preparations: current best practice involves extensive purification to ensure exclusion of bacterial components and endotoxins (Bonilla et al., 2016). The current state of bacteriophage preparation for IV use, however, has been refined significantly and has allowed successful IV administration and infection eradication as reported by numerous case studies in the US in the last year (Duplessis et al., 2017; Jennes et al., 2017; Schooley et al., 2017). Similarly, the effect of cell lysis and ensuing response to cell debris has been raised, however, this should be considered no different to the effects of bactericidal IV antibiotics which are regularly prescribed (Speck and Smithyman, 2016).
Lastly, the formation of phage-specific antibodies may act to clear the phage before they can exert an effect on the targeted pathogens. In response to this point we are reminded by the study of Matsuzaki et al. (2005) that phage kill extremely quickly and the time required for antibody formation may exceed what is needed to treat and resolve infection. Cases which involve prolonged treatment where antibodies are likely to form, however, may require administration of additional phages that do not have the same serological cross-reactivity (Abedon et al., 2011).
Considering the increased heart rate, uterine and renal blood flow observed in pregnancy the impact on IV phage administration may correlate with the findings observed for IV drug administration (Clark et al., 1989; Frederiksen, 2001). The increased cardiac output may require a greater dose to obtain initial plasma levels. Further to this, as phage are dose-independent in nature (replication upon bacterial interaction) the premise of adequate plasma levels is likely to be completely different to the way we view classical drugs. Attention should be focused on defining and refining such parameters in current phage studies of non-pregnant humans. Valuable information gained from each phage administration could be used to build individualized drug profiles for each phage which will likely influence future administration.
Local–topical, oral or inhaled phage administration
The thorough review by Dabrowska and others outlined numerous studies that have revealed the extent of bacteriophage penetration in vertebrates (Dabrowska et al., 2005). This article explored the ability of bacteriophages to enter the circulation by non-IV means (oral, intraperitoneal, intramuscular and topical) and disseminate into internal organs and the central nervous system. It was suggested that phage persistence and concentration in particular organs strongly depends on the presence or absence of susceptible bacteria.
The local delivery of bacteriophages is most appropriate where a site-specific infection or colonization is occurring (Ryan et al., 2011). In pregnancy, intravaginal application could be ideal for delivery of phages specific to genitourinary tract pathogens. Similarly in neonates, skin based administration may act to protect the infant's fragile skin and prevent colonization by pathogens. Such applications may come in the form of phage-impregnated gels, creams and pessaries, all of which have retained phage activity in such formulations as previously demonstrated (Brown et al., 2017).
Topical applications are ideal as a high titre of bacteriophage can be delivered directly to the source of infection with minimal host immune interference. Common examples of this topical approach include skin and sinus infections by organisms such as Staphylococcus aureus (Seth et al., 2013; Pincus et al., 2015; Drilling et al., 2017). In antenatal terms, however, the major sources of infection relate to genitourinary pathogens (Cunnington et al., 2013). As GBS is commonly isolated from both the vagina and rectum (Dillon et al., 1982) this represents a target site for topical applications. Intravaginal delivery of bacteriophage lysin was previously demonstrated by Cheng and colleagues in a mouse model (Cheng et al., 2005). Rectal phage delivery also has high potential for success with an early study reporting detection of phage in the blood as early as 10 min after administration (Sechter et al., 1989).
Respiratory pathogens can be targeted through inhalation of phage preparations and respiratory physiology during pregnancy is likely to enhance the absorption of inhaled drugs (Pacheco et al., 2013) and therefore potentially phages. The methods of production of powders and aerosolized phage, however, can result in titre reduction (Leung et al., 2016). Inhaled phage studies are summarized in a review by Bodier-Montagutelli et al. (2017). Respiratory phage delivery, however, is more relevant in perinatal and pediatric populations of developing nations where bacterial pneumonia accounts for 15% of deaths in children under 5 years (Liu et al., 2015). Respiratory delivery of phage and its potential for enhanced absorption could be beneficial during pregnancy as another route of administration and depending on the resulting access to the fetus, treat in utero infection.
Oral delivery has been successful for gastrointestinal tract and some systemic infections, however, the low pH of the stomach is a big issue for phage stability (Jonczyk et al., 2011). This problem may be overcome using microencapsulation techniques (Ma et al., 2008; Colom et al., 2015). Other suggestions have included the neutralization of stomach acid prior to oral delivery, with one study suggesting yogurt may have a synergistic effect in overcoming this unfavorable acidic environment (Miedzybrodzki et al., 2017). Similarly, proton-pump inhibitors such as rabeprazole have been used in Helicobacter pylori treatment and act to inhibit gastric acid production (Sharara, 2005). These approaches may be useful for oral phage administration during pregnancy. The physiological changes cause an increase in gastric pH which is a potential benefit, depending on the pH stability range of the phage, and reduced gastric emptying resulting in extended exposure to these pH conditions. If able to transit successfully through to the bowel, prolonged small intestine passage is likely. In light of new data suggesting transcytosis of phage across gut epithelial cells being possible in vitro, depending on the downstream consequences of this process which remain unknown, this could be beneficial (Nguyen et al., 2017). This also raises questions regarding the potential for transcytosis across the placenta.
Placental phage administration
When treating the mother and fetus, it is essential to have a thorough understanding of the placenta and the extent to which drug transfer occurs across this maternal/fetal barrier. Kulangara et al. (Kulangara and Sellers, 1959) examined the passage of coliphage and mycobacteriophages across the placental barrier of rats. Passage was achieved after injection into the uterine lumen as confirmed by presence of phage in the fetal fluids, however, maternal IV injection saw phage rarely reach the embryo. Conversely, transplacental transfer of bacteriophage was observed in a guinea pig model, where maternal IV bacteriophage administration resulted in the presence of bacteriophages in the fetal circulation (Uhr et al., 1963). Similarly, in a phage-display study an engineered T7 phage was injected into the tail vein of a pregnant rat and was subsequently recovered from fetal tissues 15 min after administration (Srivastava et al., 2004). The potential for virulent phage to target not only maternal infection, but also fetal, is extremely promising in the antenatal context. Mechanisms of this placental transfer of bacteriophage, however, are not well-understood at this stage and further studies are vital to progressing in utero treatment strategies.
Issues to consider
Successful therapies during pregnancy rely on a number of intercalating factors. First, is the aim of the therapy to eradicate maternal or fetal infection, or could both be feasible? Placental transfer, fetal immune response or lack thereof, maternal immune response, dose, route of administration and pharmacokinetics all play key roles in therapeutic considerations. Some of the greatest challenges in phage therapy have been linked to immune inactivation by antibodies. As mentioned throughout this review, these are all aspects that can be overcome by altering the way we approach phage therapy. Extensive characterization, purification and preparation of cocktails of phage to act specifically but also broadly amongst different strains of the same target of interest are likely to permit widespread killing of the entire species without the adverse reactions described in early literature. In addition, having a thorough understanding of the infection that is to be treated is also extremely important, as in the case of in utero infections, for example, if secondary pathogens are also present (but in much smaller numbers), then targeted removal of the primary pathogen may simply pave the way for a new infection to begin. Treatment protocols need to factor this scenario in and have suitable complementary interventions on hand, in this case most likely antibiotics. The issue of the potential for host inflammatory response due to release of bacterial cell components following cell lysis is obviously of concern, especially in an antenatal context due to the nature of parturition, however, antibiotic interventions can also result in similar scenarios. Further research into this is required, particularly the potential for phage preparations to be co-administered with anti-inflammatory agents such as cytokine-suppressive anti-inflammatory drugs (Ng et al., 2015).
Phage therapy has had a difficult time integrating with western medicine and its use during pregnancy is largely unexplored. This review highlights how little we currently know about the safety of the most common drugs used in pregnancy, antibiotics, and the potential benefits that phage research into antenatal and perinatal infection and colonization could have in the future. Although extensive phage research has been carried out, the majority of this research has not been in the western world. In a clinical context, many have questioned the credibility of past studies due to the lack of randomized double-blind clinical trials in general (Slopek et al., 1985, 1987). In addition, a number of studies reporting the use of phage therapy in pregnant women and pediatric contexts are inaccessible or only available in Russian (Samsygina and Boni, 1984; Terekhina et al., 2008; Pagava et al., 2011). To overcome these current perceptions of phage therapy a basic science approach needs to be taken initially, whereby candidate clinical bacteriophages are thoroughly characterized (molecularly and taxonomically) and purified, and potential bacterial resistance monitored at the molecular level in the target bacteria and by analysis of mutations occurring in the phages. Such an approach would likely result in the formulation of well-characterized, robust, efficacious phage cocktails suitable for use in randomized clinical trials. It is essential that standardized protocols be developed, not only for uniformity but so that accurate comparisons of data can be made. Furthermore, the refinement of clinical practice can be largely influenced by case studies, and despite patient heterogeneity, similarities in phage pharmacokinetics may be observed which are informative nonetheless and should not impede standard care.
The ethical implications of phage therapy thus far have not been greatly explored, as many instances of human phage therapy have been for compassionate use as a final option. The potential for phage therapy to be beneficial for treatment of pregnant women and neonates is great when we reflect on the pathogens afflicting this population, however, there will no doubt be a number of ethical considerations that need to be addressed which are specific to these patients. Optimal healthcare is a priority and this will ultimately come down to the decision of the patient, who needs to be fully informed of the risks vs. benefits.
Conclusion
The vulnerability of pregnant women and neonates as patients can make development and thorough testing of therapeutic agents difficult. This is evident in the little data available on antibiotic safety in pregnancy. Regardless of the ethical issues associated with this population, they remain at risk of infection and thus need access to safe, efficacious treatments. Antibiotics have numerous contraindications in pregnancy, have problems associated with resistance and new data suggests that antibiotic alteration of the vagina could influence the microbial seeding of the neonatal gut. Despite this, they still are certainly of use in pregnancy where an infection or potential infection of unknown etiology is in play, for example PROM. The potential for use of phage therapy is already fast accelerating with the emergence of antibiotic resistance, however, the targeted nature of this intervention would be especially beneficial in an antenatal and perinatal context where minimal disruption of the host microbiota is of importance. Through the numerous studies that have examined bacteriophages as therapeutic agents, we can see their vast and unique potential, especially in that their concentration and persistence is dependent on bacterial host prevalence and their site-specific localization appears to be somewhat independent of their route of administration. The potential for targeted treatment of infections in the mother and fetus is exciting and certainly warrants additional research into further development of antenatal in vitro and in vivo models of bacteriophage therapy to complement the many years of previous research in this field summarized within this review.
Author contributions
LF conceived the review topic and focus, drafted the manuscript and figures, and approved the final version to be published. Both BC and MP contributed to the structure and content, critically revised the drafted manuscript and approved the final version to be published.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
LF is supported by the Australian Government Research Training Program Scholarship and the Professor Gordon King Postgraduate Scholarship provided by WIRF. MP is supported by an NHMRC Project Grant [1077931].
References
- Abedon S. T. (2014). Phage therapy: eco-physiological pharmacology. Scientifica 2014:581639. 10.1155/2014/581639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedon S. T., Kuhl S. J., Blasdel B. G., Kutter E. M. (2011). Phage treatment of human infections. Bacteriophage 1, 66–85. 10.4161/bact.1.2.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. D. (2006). Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin. Drug Metab. Toxicol. 2, 947–960. 10.1517/17425255.2.6.947 [DOI] [PubMed] [Google Scholar]
- Azad M. B., Konya T., Persaud R. R., Guttman D. S., Chari R. S., Field C. J., et al. (2016). Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993. 10.1111/1471-0528.13601 [DOI] [PubMed] [Google Scholar]
- Bai Q., Zhang W., Yang Y., Tang F., Nguyen X., Liu G., et al. (2013). Characterization and genome sequencing of a novel bacteriophage infecting Streptococcus agalactiae with high similarity to a phage from Streptococcus pyogenes. Arch. Virol. 158, 1733–1741. 10.1007/s00705-013-1667-x [DOI] [PubMed] [Google Scholar]
- Barr J. J., Auro R., Furlan M., Whiteson K. L., Erb M. L., Pogliano J., et al. (2013). Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. U.S.A. 110, 10771–10776. 10.1073/pnas.1305923110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M. Z., Chou D., Moller A. B., Narwal R., et al. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- Bodier-Montagutelli E., Morello E., L'hostis G., Guillon A., Dalloneau E., Respaud R., et al. (2017). Inhaled phage therapy: a promising and challenging approach to treat bacterial respiratory infections. Expert Opin. Drug Deliv. 14, 959–972. 10.1080/17425247.2017.1252329 [DOI] [PubMed] [Google Scholar]
- Bonilla N., Rojas M. I., Netto Flores Cruz G., Hung S. H., Rohwer F., Barr J. J. (2016). Phage on tap-a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 4:e2261. 10.7717/peerj.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstaver P. B., Bland C. M., Griffin B., Stover K. R., Eiland L. S., Mclaughlin M. (2015). A review of antibiotic use in pregnancy. Pharmacotherapy 35, 1052–1062. 10.1002/phar.1649 [DOI] [PubMed] [Google Scholar]
- Boyd E. F. (2012). Bacteriophage-encoded bacterial virulence factors and phage–pathogenicity island interactions, in Advances in Virus Research, eds Łobocka M., Szybalski W. T. (Academic Press; ), 91–118. [DOI] [PubMed] [Google Scholar]
- Brendan J. M. B., Lenski R. E. (1997). Effect of resource enrichment on a chemostat community of bacteria and bacteriophage. Ecology 78, 2303–2315. 10.1890/0012-9658(1997)078[2303:EOREOA]2.0.CO;2 [DOI] [Google Scholar]
- Brnakova Z., Farkasovska J., Godany A. (2005). The use of bacteriophages in eliminating polyresistant strains of Staphylococcus aureus and Streptococcus agalactiae. Folia Microbiol. 50, 187–194. 10.1007/BF02931564 [DOI] [PubMed] [Google Scholar]
- Brown T. L., Thomas T., Odgers J., Petrovski S., Spark M. J., Tucci J. (2017). Bacteriophage formulated into a range of semisolid and solid dosage forms maintain lytic capacity against isolated cutaneous and opportunistic oral bacteria. J. Pharm. Pharmacol. 69, 244–253. 10.1111/jphp.12673 [DOI] [PubMed] [Google Scholar]
- Broxmeyer L., Sosnowska D., Miltner E., Chacon O., Wagner D., Mcgarvey J., et al. (2002). Killing of Mycobacterium avium and Mycobacterium tuberculosis by a mycobacteriophage delivered by a nonvirulent mycobacterium: a model for phage therapy of intracellular bacterial pathogens. J. Infect. Dis. 186, 1155–1160. 10.1086/343812 [DOI] [PubMed] [Google Scholar]
- Brumfitt W., Hamilton-Miller J. M. (1981). A new look at the aetiology of urinary infection. Infection 9, 214–216. 10.1007/BF01640717 [DOI] [PubMed] [Google Scholar]
- Brussow H. (2012). What is needed for phage therapy to become a reality in Western medicine? Virology 434, 138–142. 10.1016/j.virol.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Capoccia R., Greub G., Baud D. (2013). Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr. Opin. Infect. Dis. 26, 231–240. 10.1097/QCO.0b013e328360db58 [DOI] [PubMed] [Google Scholar]
- Cappell M. S., Garcia A. (1998). Gastric and duodenal ulcers during pregnancy. Gastroenterol. Clin. North Am. 27, 169–195. 10.1016/S0889-8553(05)70352-6 [DOI] [PubMed] [Google Scholar]
- Carlton R. M. (1999). Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. 47, 267–274. [PubMed] [Google Scholar]
- Ceyssens P. J., Minakhin L., Van Den Bossche A., Yakunina M., Klimuk E., Blasdel B., et al. (2014). Development of giant bacteriophage varphiKZ is independent of the host transcription apparatus. J. Virol. 88, 10501–10510. 10.1128/JVI.01347-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. T. (1986). Prescribing in pregnancy. Bacterial infections in pregnancy. Clin. Obstet. Gynaecol. 13, 397–416. [PubMed] [Google Scholar]
- Cheng Q., Nelson D., Zhu S., Fischetti V. A. (2005). Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49, 111–117. 10.1128/AAC.49.1.111-117.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibeu A., Lingohr E. J., Masson L., Manges A., Harel J., Ackermann H. W., et al. (2012). Bacteriophages with the ability to degrade uropathogenic Escherichia coli biofilms. Viruses 4, 471–487. 10.3390/v4040471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. L., Cotton D. B., Lee W., Bishop C., Hill T., Southwick J., et al. (1989). Central hemodynamic assessment of normal term pregnancy. Am. J. Obstet. Gynecol. 161, 1439–1442. 10.1016/0002-9378(89)90900-9 [DOI] [PubMed] [Google Scholar]
- Clokie M. R., Millard A. D., Letarov A. V., Heaphy S. (2011). Phages in nature. Bacteriophage 1, 31–45. 10.4161/bact.1.1.14942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom J., Cano-Sarabia M., Otero J., Cortes P., Maspoch D., Llagostera M. (2015). Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 81, 4841–4849. 10.1128/AEM.00812-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaglia L., Tonti G., Martini S., Aceti A., Mazzola G., Aloisio I., et al. (2016). Influence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J. Pediatr. Gastroenterol. Nutr. 62, 304–308. 10.1097/MPG.0000000000000928 [DOI] [PubMed] [Google Scholar]
- Costantine M. M. (2014). Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 5:65. 10.3389/fphar.2014.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram L. F., Zapata M. I., Toy E. C., Baker B., III. (2002). Genitourinary infections and their association with preterm labor. Am. Fam. Physician 65, 241–248. [PubMed] [Google Scholar]
- Cunnington M., Kortsalioudaki C., Heath P. (2013). Genitourinary pathogens and preterm birth. Curr. Opin. Infect. Dis. 26, 219–230. 10.1097/QCO.0b013e328360dc31 [DOI] [PubMed] [Google Scholar]
- Dabrowska K., Switala-Jelen K., Opolski A., Weber-Dabrowska B., Gorski A. (2005). Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 98, 7–13. 10.1111/j.1365-2672.2004.02422.x [DOI] [PubMed] [Google Scholar]
- Davison J. M., Dunlop W. (1980). Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 18, 152–161. 10.1038/ki.1980.124 [DOI] [PubMed] [Google Scholar]
- de Jonge L., Bos H. J., Van Langen I. M., De Jong-Van Den Berg L. T., Bakker M. K. (2014). Antibiotics prescribed before, during and after pregnancy in the Netherlands: a drug utilization study. Pharmacoepidemiol. Drug Saf. 23, 60–68. 10.1002/pds.3492 [DOI] [PubMed] [Google Scholar]
- de Tejada B. M. (2014). Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int. J. Environ. Res. Public Health 11, 7993–8009. 10.3390/ijerph110807993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hérelle F. (1917). Sur un microbe invisible antagoniste des bacillus dysentérique. Acad. Sci. Paris 165, 373–375. [Google Scholar]
- Dillon H. C., Jr., Gray E., Pass M. A., Gray B. M. (1982). Anorectal and vaginal carriage of group B streptococci during pregnancy. J. Infect. Dis. 145, 794–799. 10.1093/infdis/145.6.794 [DOI] [PubMed] [Google Scholar]
- Domelier A. S., Van Der Mee-Marquet N., Sizaret P. Y., Hery-Arnaud G., Lartigue M. F., Mereghetti L., et al. (2009). Molecular characterization and lytic activities of Streptococcus agalactiae bacteriophages and determination of lysogenic-strain features. J. Bacteriol. 191, 4776–4785. 10.1128/JB.00426-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drilling A. J., Ooi M. L., Miljkovic D., James C., Speck P., Vreugde S., et al. (2017). Long-term safety of topical bacteriophage application to the frontal sinus region. Front. Cell. Infect. Microbiol. 7:49. 10.3389/fcimb.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos R. J., Straus J. H., Pierce C. (1943). The multiplication of bacteriophage in vivo and its protective effect against an experimental infection with Shigella dysenteriae. J. Exp. Med. 78, 161–168. 10.1084/jem.78.3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop A. L., Mulle J. G., Ferranti E. P., Edwards S., Dunn A. B., Corwin E. J. (2015). Maternal microbiome and pregnancy outcomes that impact infant health: a review. Adv. Neonatal Care 15, 377–385. 10.1097/ANC.0000000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplessis C., Biswas B., Hanisch B., Perkins M., Henry M., Quinones J., et al. (2017). Refractory pseudomonas bacteremia in a 2-year-old sterilized by bacteriophage therapy. J. Pediatric. Infect. Dis. Soc. [Epub ahead of print]. 10.1093/jpids/pix056 [DOI] [PubMed] [Google Scholar]
- Dy R. L., Richter C., Salmond G. P., Fineran P. C. (2014). Remarkable mechanisms in microbes to resist phage infections. Annu. Rev. Virol. 1, 307–331. 10.1146/annurev-virology-031413-085500 [DOI] [PubMed] [Google Scholar]
- Fenton M., Ross P., Mcauliffe O., O'Mahony J., Coffey A. (2010). Recombinant bacteriophage lysins as antibacterials. Bioeng. Bugs 1, 9–16. 10.4161/bbug.1.1.9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. (2008). Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 11, 393–400. 10.1016/j.mib.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M. C. (2001). Physiologic changes in pregnancy and their effect on drug disposition. Semin. Perinatol. 25, 120–123. 10.1053/sper.2001.24565 [DOI] [PubMed] [Google Scholar]
- Gaca A. O., Gilmore M. S. (2016). A lysin to kill. eLife 5:e16111. 10.7554/eLife.16111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier M., De Sordi L., Maura D., Arachchi H., Volant S., Dillies M. A., et al. (2016). Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 18, 2237–2245. 10.1111/1462-2920.13284 [DOI] [PubMed] [Google Scholar]
- Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R. L., Hauth J. C., Andrews W. W. (2000). Intrauterine infection and preterm delivery. N. Engl. J. Med. 342, 1500–1507. 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- Grundling A., Manson M. D., Young R. (2001). Holins kill without warning. Proc. Natl. Acad. Sci. U.S.A. 98, 9348–9352. 10.1073/pnas.151247598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S., Huwyler D., Richard S., Loessner M. J. (2009). Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 75, 93–100. 10.1128/AEM.01711-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila A. M. (1993). Antibiotics in pregnancy–a prospective cohort study on the policy of antibiotic prescription. Ann. Med. 25, 467–471. 10.3109/07853899309147314 [DOI] [PubMed] [Google Scholar]
- Heikkinen T., Laine K., Neuvonen P. J., Ekblad U. (2000). The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG 107, 770–775. 10.1111/j.1471-0528.2000.tb13339.x [DOI] [PubMed] [Google Scholar]
- Herrera E. (2000). Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur. J. Clin. Nutr. 54(Suppl. 1), S47–S51. 10.1038/sj.ejcn.1600984 [DOI] [PubMed] [Google Scholar]
- Hobbs Z., Abedon S. T. (2016). Diversity of phage infection types and associated terminology: the problem with 'Lytic or lysogenic'. FEMS Microbiol. Lett. 363:fnw047. 10.1093/femsle/fnw047 [DOI] [PubMed] [Google Scholar]
- Hu B., Margolin W., Molineux I. J., Liu J. (2013). The bacteriophage T7 virion undergoes extensive structural remodeling during infection. Science 339, 576–579. 10.1126/science.1231887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S., Robson S. C. (1992). Adaptation of the maternal heart in pregnancy. Br. Heart J. 68, 540–543. 10.1136/hrt.68.12.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes S., Merabishvili M., Soentjens P., Pang K. W., Rose T., Keersebilck E., et al. (2017). Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—a case report. Critical Care 21, 129. 10.1186/s13054-017-1709-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonczyk E., Klak M., Miedzybrodzki R., Gorski A. (2011). The influence of external factors on bacteriophages–review. Folia Microbiol. 56, 191–200. 10.1007/s12223-011-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallapur S. G., Kramer B. W., Jobe A. H. (2013). Ureaplasma and BPD. Semin. Perinatol. 37, 94–101. 10.1053/j.semperi.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. C., Mechtler T. P., Bohm J., Petricevic L., Gleiss A., Spergser J., et al. (2011). In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J. Perinat. Med. 39, 331–336. 10.1515/jpm.2011.022 [DOI] [PubMed] [Google Scholar]
- Ke A. B., Rostami-Hodjegan A., Zhao P., Unadkat J. D. (2014). Pharmacometrics in pregnancy: an unmet need. Annu. Rev. Pharmacol. Toxicol. 54, 53–69. 10.1146/annurev-pharmtox-011613-140009 [DOI] [PubMed] [Google Scholar]
- Keelan J. A., Nitsos I., Saito M., Musk G. C., Kemp M. W., Timmins M., et al. (2011). Maternal-amniotic-fetal distribution of macrolide antibiotics following intravenous, intramuscular, and intraamniotic administration in late pregnant sheep. Am. J. Obstet. Gynecol. 204, 546.e510–e547. 10.1016/j.ajog.2011.02.035 [DOI] [PubMed] [Google Scholar]
- Keen E. C. (2015). A century of phage research: bacteriophages and the shaping of modern biology. Bioessays 37, 6–9. 10.1002/bies.201400152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. W., Miura Y., Payne M. S., Jobe A. H., Kallapur S. G., Saito M., et al. (2014a). Maternal intravenous administration of azithromycin results in significant fetal uptake in a sheep model of second trimester pregnancy. Antimicrob. Agents Chemother. 58, 6581–6591. 10.1128/AAC.03721-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. W., Miura Y., Payne M. S., Watts R., Megharaj S., Jobe A. H., et al. (2014b). Repeated maternal intramuscular or intraamniotic erythromycin incompletely resolves intrauterine Ureaplasma parvum infection in a sheep model of pregnancy. Am. J. Obstet. Gynecol. 207, 475.e1–475.e14. 10.1016/j.ajog.2014.02.025 [DOI] [PubMed] [Google Scholar]
- Khawaldeh A., Morales S., Dillon B., Alavidze Z., Ginn A. N., Thomas L., et al. (2011). Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J. Med. Microbiol. 60, 1697–1700. 10.1099/jmm.0.029744-0 [DOI] [PubMed] [Google Scholar]
- Klumpp J., Loessner M. J. (2013). Listeria phages: Genomes, evolution, and application. Bacteriophage 3:e26861. 10.4161/bact.26861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulangara A. C., Sellers M. I. (1959). Passage of bacteriophages from mother to foetus in the rat. Proc. Soc. Exp. Biol. Med. 101, 207–211. 10.3181/00379727-101-24885 [DOI] [PubMed] [Google Scholar]
- Lang L. H. (2006). FDA approves use of bacteriophages to be added to meat and poultry products. Gastroenterology 131, 1370. 10.1053/j.gastro.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Le Doare K., Heath P. T. (2013). An overview of global GBS epidemiology. Vaccine 31(Suppl. 4), D7–D12. 10.1016/j.vaccine.2013.01.009 [DOI] [PubMed] [Google Scholar]
- Lehman S. M., Donlan R. M. (2015). Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 59, 1127–1137. 10.1128/AAC.03786-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner L., Sybesma W., Chanishvili N., Goderdzishvili M., Chkhotua A., Ujmajuridze A., et al. (2017). Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 17, 90. 10.1186/s12894-017-0283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S. S., Parumasivam T., Gao F. G., Carrigy N. B., Vehring R., Finlay W. H., et al. (2016). Production of inhalation phage powders using spray freeze drying and spray drying techniques for treatment of respiratory infections. Pharm. Res. 33, 1486–1496. 10.1007/s11095-016-1892-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverentz B., Conway W. S., Janisiewicz W., Camp M. J. (2004). Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67, 1682–1686. 10.4315/0362-028X-67.8.1682 [DOI] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J. E., et al. (2015). Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385, 430–440. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- Łoś M., Wegrzyn G. (2012). Pseudolysogeny, in Advances in Virus Research, eds Łobocka M., Szybalski W. T. (Academic Press; ), 339–349. [DOI] [PubMed] [Google Scholar]
- Loc-Carrillo C., Abedon S. T. (2011). Pros and cons of phage therapy. Bacteriophage 1, 111–114. 10.4161/bact.1.2.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebstein R., Lalkin A., Koren G. (1997). Pharmacokinetic changes during pregnancy and their clinical relevance. Clin. Pharmacokinet. 33, 328–343. 10.2165/00003088-199733050-00002 [DOI] [PubMed] [Google Scholar]
- Loeffler J. M., Nelson D., Fischetti V. A. (2001). Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294, 2170–2172. 10.1126/science.1066869 [DOI] [PubMed] [Google Scholar]
- Lood R., Winer B. Y., Pelzek A. J., Diez-Martinez R., Thandar M., Euler C. W., et al. (2015). Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 59, 1983–1991. 10.1128/AAC.04641-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Pacan J. C., Wang Q., Xu Y., Huang X., Korenevsky A., et al. (2008). Microencapsulation of bacteriophage felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 74, 4799–4805. 10.1128/AEM.00246-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeal W. (1941). Specific therapeutic shock—the Hugh Young reaction. Arch. Surg. 43, 579–582. 10.1001/archsurg.1941.01210160040006 [DOI] [Google Scholar]
- Manrique P., Bolduc B., Walk S. T., Van Der Oost J., De Vos W. M., Young M. J. (2016). Healthy human gut phageome. Proc. Natl. Acad. Sci. U.S.A. 113, 10400–10405. 10.1073/pnas.1601060113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S., Rashel M., Uchiyama J., Sakurai S., Ujihara T., Kuroda M., et al. (2005). Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11, 211–219. 10.1007/s10156-005-0408-9 [DOI] [PubMed] [Google Scholar]
- Mazzola G., Murphy K., Ross R. P., Di Gioia D., Biavati B., Corvaglia L. T., et al. (2016). Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLoS ONE 11:e0157527. 10.1371/journal.pone.0157527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendling W. (2016). Vaginal microbiota. Adv. Exp. Med. Biol. 902, 83–93. 10.1007/978-3-319-31248-4_6 [DOI] [PubMed] [Google Scholar]
- Merril C. R., Biswas B., Carlton R., Jensen N. C., Creed G. J., Zullo S., et al. (1996). Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. U.S.A. 93, 3188–3192. 10.1073/pnas.93.8.3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedzybrodzki R., Klak M., Jonczyk-Matysiak E., Bubak B., Wojcik A., Kaszowska M., et al. (2017). Means to facilitate the overcoming of gastric juice barrier by a therapeutic Staphylococcal bacteriophage A5/80. Front. Microbiol. 8:467. 10.3389/fmicb.2017.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Payne M. S., Keelan J. A., Noe A., Carter S., Watts R., et al. (2014). Maternal intravenous treatment with either azithromycin or solithromycin clears Ureaplasma parvum from the amniotic fluid in an ovine model of intrauterine infection. Antimicrob. Agents Chemother. 58, 5413–5420. 10.1128/AAC.03187-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller N. T., Bakacs E., Combellick J., Grigoryan Z., Dominguez-Bello M. G. (2015). The infant microbiome development: mom matters. Trends Mol. Med. 21, 109–117. 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Zhang Q., Gerardo Galaz-Montoya J., Fu C., Coleman M. L., Osburne M. S., et al. (2017). Visualizing adsorption of cyanophage P-SSP7 onto marine Prochlorococcus. Sci. Rep. 7:44176. 10.1038/srep44176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P. Y., Ireland D. J., Keelan J. A. (2015). Drugs to block cytokine signaling for the prevention and treatment of inflammation-induced preterm birth. Front. Immunol. 6:166. 10.3389/fimmu.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen S., Baker K., Padman B. S., Patwa R., Dunstan R. A., Weston T. A., et al. (2017). Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 8:e01874-17. 10.1128/mBio.01874-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieth A., Verseux C., Barnert S., Suss R., Romer W. (2015). A first step toward liposome-mediated intracellular bacteriophage therapy. Expert Opin. Drug Deliv. 12, 1411–1424. 10.1517/17425247.2015.1043125 [DOI] [PubMed] [Google Scholar]
- Nobrega F. L., Costa A. R., Kluskens L. D., Azeredo J. (2015). Revisiting phage therapy: new applications for old resources. Trends Microbiol. 23, 185–191. 10.1016/j.tim.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Nord C. E., Finch R. (2005). Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect. Dis. 5, 115–119. 10.1016/S1473-3099(05)70086-4 [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Newland J. W., Miller S. F., Holmes R. K., Smith H. W., Formal S. B. (1984). Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226, 694–696. 10.1126/science.6387911 [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Davis S. D., Wedgwood R. J. (1971). Immunologic responses to bacteriophage phi-X 174 in immunodeficiency diseases. J. Clin. Invest. 50, 2559–2568. 10.1172/JCI106756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. (2014). Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 10.1371/journal.pmed.1002130 [DOI] [Google Scholar]
- Pacheco L. D., Costantine M. M., Hankins G. D. V. (2013). Physiologic changes during pregnancy. Clin. Pharmacol. Pregn. 5–16. 10.1016/B978-0-12-386007-1.00002-7 [DOI] [Google Scholar]
- Pacifici G. M. (2006). Placental transfer of antibiotics administered to the mother: a review. Int. J. Clin. Pharmacol. Ther. 44, 57–63. 10.5414/CPP44057 [DOI] [PubMed] [Google Scholar]
- Pagava K. I., Gachechiladze K. K., Korinteli I. A., Dzuliashvili M. G., Alavidze Z. I., Hoyle N., et al. (2011). [What happens when the child gets bacteriophage per os?]. Georgian Med. News 101–105. [PubMed] [Google Scholar]
- Pagava K. I., Metskhvarishvili G. D., Gachechiladze K. K., Korinteli I. A., Khoile N., Dzuliashvili M. G. (2012). [Some immunological changes in children with bacterial infections treated with bacteriophages]. Georgian Med. News 64–69. [PubMed] [Google Scholar]
- Pariente G., Leibson T., Carls A., Adams-Webber T., Ito S., Koren G. (2016). Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS Med. 13:e1002160. 10.1371/journal.pmed.1002160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry E., Shields R., Turnbull A. C. (1970). Transit time in the small intestine in pregnancy. J. Obstet. Gynaecol. Br. Commonw. 77, 900–901. 10.1111/j.1471-0528.1970.tb03423.x [DOI] [PubMed] [Google Scholar]
- Payne M. S., Ireland D. J., Watts R., Nathan E. A., Furfaro L. L., Kemp M. W., et al. (2016). Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth 16, 312. 10.1186/s12884-016-1110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta B., Gil-Carton D., Castano-Diez D., Bertin A., Boulogne C., Oksanen H. M., et al. (2013). Mechanism of membranous tunnelling nanotube formation in viral genome delivery. PLoS Biol. 11:e1001667. 10.1371/journal.pbio.1001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Munoz M. E., Arrieta M. C., Ramer-Tait A. E., Walter J. (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5, 48 10.1186/s40168-017-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson A. (1977). Pharmacokinetics of ampicillin during pregnancy. J. Infect. Dis. 136, 370–376. 10.1093/infdis/136.3.370 [DOI] [PubMed] [Google Scholar]
- Philipson A., Sabath L. D., Charles D. (1973). Transplacental passage of erythromycin and clindamycin. N. Engl. J. Med. 288, 1219–1221. 10.1056/NEJM197306072882307 [DOI] [PubMed] [Google Scholar]
- Philipson A., Stiernstedt G., Ehrnebo M. (1987). Comparison of the pharmacokinetics of cephradine and cefazolin in pregnant and non-pregnant women. Clin. Pharmacokinet. 12, 136–144. 10.2165/00003088-198712020-00004 [DOI] [PubMed] [Google Scholar]
- Pincus N. B., Reckhow J. D., Saleem D., Jammeh M. L., Datta S. K., Myles I. A. (2015). Strain specific phage treatment for Staphylococcus aureus infection is influenced by host immunity and site of infection. PLoS ONE 10:e0124280. 10.1371/journal.pone.0124280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K. P., Czuprynski C. J. (2013). Pathogenesis of listeriosis during pregnancy. Anim. Health Res. Rev. 14, 30–39. 10.1017/S1466252312000242 [DOI] [PubMed] [Google Scholar]
- Prentice A. M., Goldberg G. R. (2000). Energy adaptations in human pregnancy: limits and long-term consequences. Am/ J. Clin. Nutr. 71, 1226S-1232S. [DOI] [PubMed] [Google Scholar]
- Pritchard D. G., Dong S., Baker J. R., Engler J. A. (2004). The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 150, 2079–2087. 10.1099/mic.0.27063-0 [DOI] [PubMed] [Google Scholar]
- Rakonjac J., Bennett N. J., Spagnuolo J., Gagic D., Russel M. (2011). Filamentous bacteriophage: biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 13, 51–76. [PubMed] [Google Scholar]
- Ramsey P. S., Vaules M. B., Vasdev G. M., Andrews W. W., Ramin K. D. (2003). Maternal and transplacental pharmacokinetics of azithromycin. Am. J. Obstet. Gynecol. 188, 714–718. 10.1067/mob.2003.141 [DOI] [PubMed] [Google Scholar]
- Rasmussen P. E., Nielsen F. R. (1988). Hydronephrosis during pregnancy: a literature survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 27, 249–259. 10.1016/0028-2243(88)90130-X [DOI] [PubMed] [Google Scholar]
- Roach D. R., Donovan D. M. (2015). Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. 10.1080/21597081.2015.1062590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach D. R., Leung C. Y., Henry M., Morello E., Singh D., Di Santo J. P., et al. (2017). Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe 22, 38.e34–47.e34. 10.1016/j.chom.2017.06.018 [DOI] [PubMed] [Google Scholar]
- Ross J. M. (1979). Perinatal implications of the lower genital tract flora. Ciba Found. Symp. 69–83. [DOI] [PubMed] [Google Scholar]
- Ryan E. M., Gorman S. P., Donnelly R. F., Gilmore B. F. (2011). Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 63, 1253–1264. 10.1111/j.2042-7158.2011.01324.x [DOI] [PubMed] [Google Scholar]
- Samsygina G. A., Boni E. G. (1984). [Bacteriophages and phage therapy in pediatric practice]. Pediatriia 67–70. [PubMed] [Google Scholar]
- Santos F., Oraichi D., Berard A. (2010). Prevalence and predictors of anti-infective use during pregnancy. Pharmacoepidemiol. Drug Saf. 19, 418–427. 10.1002/pds.1915 [DOI] [PubMed] [Google Scholar]
- Scaffidi J., Mol B. W., Keelan J. A. (2017). The pregnant women as a drug orphan: a global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG 124, 132–140. 10.1111/1471-0528.14151 [DOI] [PubMed] [Google Scholar]
- Schooley R. T., Biswas B., Gill J. J., Hernandez-Morales A., Lancaster J., Lessor L., et al. (2017). Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 61:e00954-17. 10.1128/AAC.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag S. J., Verani J. R. (2013). Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 31(Suppl. 4), D20–D26. 10.1016/j.vaccine.2012.11.056 [DOI] [PubMed] [Google Scholar]
- Sechter I., Touitou E., Donbrow M. (1989). The influence of a non-ionic surfactant on rectal absorption of virus particles. Arch. Virol. 106, 141–143. 10.1007/BF01311045 [DOI] [PubMed] [Google Scholar]
- Seelbach-Goebel B. (2013). Antibiotic therapy for premature rupture of membranes and preterm labor and effect on fetal outcome. Geburtshilfe Frauenheilkd. 73, 1218–1227. 10.1055/s-0033-1360195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A. K., Geringer M. R., Nguyen K. T., Agnew S. P., Dumanian Z., Galiano R. D., et al. (2013). Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: a new approach to chronic wound care. Plast. Reconstr. Surg. 131, 225–234. 10.1097/PRS.0b013e31827e47cd [DOI] [PubMed] [Google Scholar]
- Sharara A. I. (2005). Rabeprazole: the role of proton pump inhibitors in Helicobacter pylori eradication. Expert Rev. Anti Infect. Ther. 3, 863–870. 10.1586/14787210.3.6.863 [DOI] [PubMed] [Google Scholar]
- Sillankorva S., Oliveira D., Moura A., Henriques M., Faustino A., Nicolau A., et al. (2011). Efficacy of a broad host range lytic bacteriophage against E. coli adhered to urothelium. Curr. Microbiol. 62, 1128–1132. 10.1007/s00284-010-9834-8 [DOI] [PubMed] [Google Scholar]
- Slopek S., Kucharewicz-Krukowska A., Weber-Dabrowska B., Dabrowski M. (1985). Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Arch. Immunol. Ther. Exp. 33, 241–259. [PubMed] [Google Scholar]
- Slopek S., Weber-Dabrowska B., Dabrowski M., Kucharewicz-Krukowska A. (1987). Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exp. 35, 569–583. [PubMed] [Google Scholar]
- Speck P., Smithyman A. (2016). Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 363:fnv242. 10.1093/femsle/fnv242 [DOI] [PubMed] [Google Scholar]
- Srivastava A. S., Chauhan D. P., Carrier E. (2004). In utero detection of T7 phage after systemic administration to pregnant mice. BioTechniques 37, 81–83. [DOI] [PubMed] [Google Scholar]
- Stent G. S. (1963). Molecular Biology of Bacterial Viruses. California: Freeman. [Google Scholar]
- Stinson L. F., Payne M. S., Keelan J. A. (2017). Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit. Rev. Microbiol. 43, 352–369. 10.1080/1040841X.2016.1211088 [DOI] [PubMed] [Google Scholar]
- Stroj L., Weber-Dabrowska B., Partyka K., Mulczyk M., Wojcik M. (1999). [Successful treatment with bacteriophage in purulent cerebrospinal meningitis in a newborn]. Neurol. Neurochir. Pol. 33, 693–698. [PubMed] [Google Scholar]
- Sybesma W., Zbinden R., Chanishvili N., Kutateladze M., Chkhotua A., Ujmajuridze A., et al. (2016). Bacteriophages as potential treatment for urinary tract infections. Front. Microbiol. 7:465. 10.3389/fmicb.2016.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme M. R., Paxton J. W., Keelan J. A. (2004). Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 43, 487–514. 10.2165/00003088-200443080-00001 [DOI] [PubMed] [Google Scholar]
- Talbot G. H., Bradley J., Edwards J. E., Jr., Gilbert D., Scheld M., Bartlett J. G. (2006). Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin. Infect. Dis. 42, 657–668. 10.1086/499819 [DOI] [PubMed] [Google Scholar]
- Taylor M. (1961). An experimental study of the influence of the endocrine system on the nasal respiratory mucosa. J. Laryngol. Otol. 75, 972–977. 10.1017/S0022215100058746 [DOI] [PubMed] [Google Scholar]
- Terekhina N. A., Padrul M. M., Makarova E. L. (2008). [Effect of bacteriophage on the serum level of iron and copper in pregnant women with pyelonephritis]. Klin. Lab. Diagn. 23–24, 33–24. [PubMed] [Google Scholar]
- Trudil D. (2015). Phage lytic enzymes: a history. Virol. Sin. 30, 26–32. 10.1007/s12250-014-3549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twort F. W. (1915). An investigation on the nature of ultra-microscopic viruses. Lancet 186, 1241–1243. 10.1016/S0140-6736(01)20383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Dancis J., Finkelstein M. S. (1963). Passage of bacteriophage phi X 174 across the placenta in guinea pigs. Proc. Soc. Exp. Biol. Med. 113, 391–394. 10.3181/00379727-113-28374 [DOI] [PubMed] [Google Scholar]
- van Waarde W. M., Brus F., Okken A., Kimpen J. L. (1997). Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur. Respir. J. 10, 886–890. [PubMed] [Google Scholar]
- Ventola C. L. (2015). The antibiotic resistance crisis: part 1: causes and threats. P T 40, 277–283. [PMC free article] [PubMed] [Google Scholar]
- Verani J. R., McGee L., Schrag S. J. (2010). Prevention of perinanatal group B streptococcal disease: revised guidelines from CDC, 2010. Recommend. Rep. 59, 1–32. [PubMed] [Google Scholar]
- Vinodkumar C. S., Neelagund Y. F., Kalsurmath S. (2005). Bacteriophage in the treatment of experimental septicemic mice from a clinical isolate of multidrug resistant Klebsiella pneumoniae. J. Commun. Dis. 37, 18–29. [PubMed] [Google Scholar]
- Viscardi R. M. (2014). Ureaplasma species: role in neonatal morbidities and outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 99, F87–F92. 10.1136/archdischild-2012-303351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscardi R. M., Hasday J. D. (2009). Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr. Res. 65, 84R−90R. 10.1203/PDR.0b013e31819dc2f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites K. B., Katz B., Schelonka R. L. (2005). Mycoplasmas and Ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18, 757–789. 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mekalanos J. J. (1996). Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272, 1910–1914. 10.1126/science.272.5270.1910 [DOI] [PubMed] [Google Scholar]
- Weinbauer M. G. (2004). Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28, 127–181. 10.1016/j.femsre.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Welch M. J., Lally R., Miller J. E., Pittman S., Brodsky L., Caplan A. L., et al. (2015). The ethics and regulatory landscape of including vulnerable populations in pragmatic clinical trials. Clin. Trials 12, 503–510. 10.1177/1740774515597701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willyard C. (2017). The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15. 10.1038/nature.2017.21550 [DOI] [PubMed] [Google Scholar]
- Yang H., Yu J., Wei H. (2014). Engineered bacteriophage lysins as novel anti-infectives. Front. Microbiol. 5:542. 10.3389/fmicb.2014.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I., Corwin E. J., Brennan P. A., Jordan S., Murphy J. R., Dunlop A. (2016). The infant microbiome: Implications for infant health and neurocognitive development. Nurs. Res. 65, 76–88. 10.1097/NNR.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. (2013). Phage lysis: do we have the hole story yet? Curr. Opin. Microbiol. 16, 790–797. 10.1016/j.mib.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]