Abstract
Background
Gonadotropins induce follicular development that leads to ovulation and luteinization. In women, the level of gonadotropins, along with the expression of their receptors, changes dynamically throughout the menstrual cycle. This study aimed to clarify the mechanisms underlying these phenomena.
Methods
The literature was reviewed, including that published by the authors.
Main findings (Results)
Follicle‐stimulating hormone receptor expression in the granulosa cells was induced by androgens that were derived from growth differentiation factor‐9‐stimulated theca cells. In the theca cells, luteinizing hormone receptor (LHR) expression was noted from their appearance. In the granulosa cells, follicle‐stimulating hormone (FSH) stimulation was essential for LHR expression. However, FSH alone was not sufficient to respond to the luteinizing hormone (LH) surge for oocyte maturation, ovulation, and subsequent luteinization. To achieve these stages, various local factors that were derived from the granulosa and theca cells in response to FSH and LH stimulation had to work synergistically in an autocrine/paracrine manner to strongly induce LHR expression. Following the LH surge, the LHR expression decreased markedly; miRNAs were involved in this transient LHR downregulation. Following ovulation, LHR expression drastically increased again toward luteinization.
Conclusion
The expression of gonadotropin receptors is controlled by sophisticated and complicated systems; a breakdown of this system could lead to ovulation disorders.
Keywords: follicle‐stimulating hormone receptor, granulosa cells, luteinizing hormone receptor, receptor downregulation, theca cells
1. INTRODUCTION
Gonadotropin receptors consist of a follicle‐stimulating hormone receptor (FSHR) and a luteinizing hormone receptor (LHR), their expression of which is observed in numerous tissues.1, 2, 3, 4, 5 However, the main target of the ligands of these receptors is the gonads and these ligands and receptor systems play crucial roles in the reproductive function of both men and women. These functions include: steroid genesis in the testis and ovary; follicular development, ovulation, and luteinization in women; and spermatogenesis in men.6, 7 Physiologically, FSHR can bind solely to FSH, whereas LHR can bind to both luteinizing hormone (LH) and human chorionic gonadotropin (hCG). These receptors are members of the rhodopsin/β2 adrenergic receptor subfamily of seven G‐protein‐coupled transmembrane receptors. Thyroid‐stimulating hormone receptor also belongs to this subfamily. The characteristic structural features of the receptors that belong to this subfamily are seven transmembrane regions and a relatively large extracellular domain. The determination of the cDNA sequences of FSHR and LHR was achieved by Minegishi et al.8, 9
2. FOLLICLE‐STIMULATING HORMONE RECEPTOR EXPRESSION IN THE GRANULOSA CELLS
According to the result of a hormone‐binding experiment, FSHR expression was not observed in the primordial follicles. In the secondary follicle, along with the appearance of theca cells, FSHR was noted to exist on the surface of the granulosa cells.10 The FSHR expression increased as the follicle grew from the preantral to the antral stage and maintained a steady level in the follicle of a 3‐12 mm diameter. In the authors’ previous study, the mRNA expression of FSHR peaked in the mid‐follicular phase and its relative value was twice of that in the luteal phase.8
In a hypophysectomized rat that lacked FSH secretion, antral follicular formation was disturbed; however, FSHR expression was conserved. In FSHR knock‐out mice, antral follicular formation was inhibited.11 These findings suggest that FSH plays an essential role in antral follicular formation, but that FSHR expression is induced through a mechanism other than FSH stimulation.
2.1. Androgens
Several studies have suggested that androgen plays a major role in FSHR expression. In the study on a Rhesus monkey model, FSHR and androgen receptors were co‐expressed in the same follicles.12 Moreover, in the granulosa cells, the administration of testosterone to the Rhesus monkey induced FSHR mRNA expression and FSH‐stimulated expression of androgen receptors.13 These results indicate that, in the granulosa cells, FSHR is induced by androgen in a coordinated way. In a recent report on the primary culture of mouse granulosa cells, androgen induced FSHR expression at the protein level on the cell surface.14 Additionally, in a study on human ovarian granulosa cells, the expression of both FSHR and androgen receptors were the highest in the small antral follicle and gradually decreased as the follicle grew.15
2.2. Growth differentiation factor‐9
For androgen‐induced FSHR expression, the involvement of oocyte‐derived growth differentiation factor‐9 (GDF9) might be essential as it contributed to follicular growth from the preantral to the antral stage through the induction of androgen production in the theca cells.16 Additionally, in the study on an in vitro follicular culture system, when GDF9 in the oocyte was knocked down by the microinjection of the anti‐sense oligonucleotide of GDF9, FSHR expression in the granulosa cells was suppressed and apoptosis of the granulosa cells was induced. These effects of GDF9 knock‐down led to follicular growth arrest.17 These knock‐down effects of GDF9 were rescued by the addition of GDF9 itself or by the addition of an androgen. These results suggest that GDF9 is indispensable for FSHR expression in the granulosa cells and that this phenomenon is achieved via androgen signaling.
Collectively, during the growth from the preantral to the antral follicle, FSHR expression in the granulosa cells is, at least partly, controlled by oocyte‐derived GDF9 through androgen synthesis from the theca cells.
3. LUTEINIZING HORMONE RECEPTOR EXPRESSION IN THE GRANULOSA CELLS
With FSHR expression in the granulosa cells, the antral follicle could respond to FSH, and the follicular fluid was pooled inside the follicle at this stage. Follicular fluid contains various kinds of local factors that are secreted by the granulosa cells, theca cells, and oocyte. These local factors are involved in the maintenance of follicular growth. The most drastic change at this stage, in the granulosa cells, is LHR expression that is induced by FSH stimulation. Although LHR expression in the theca cells was already observed from its appearance at the secondary follicle,18, 19, 20, 21 LHR expression in the granulosa cells at this stage was equivocal before FSH stimulation.21 Once LHR expression was induced by FSH, multiple local factors coordinately enhanced its expression and encouraged follicular growth to prepare for the LH surge, followed by ovulation. For the progression of the follicles toward ovulation, the acquisition of sufficient numbers of LH receptors is the highest priority. Various local factors are involved at this stage. These are now discussed.
3.1. Activin
Activin is a growth factor that belongs to the TGF‐β superfamily and is expressed in the ovarian granulosa cells with concurrently expressed activin receptor. In rat granulosa cells, activin has been observed to strongly induce FSHR22, 23 and CYP19A124 expression. It also enhanced LHR expression that was induced by FSH.25 On the contrary, in the theca cells, activin markedly suppressed androgen production that was induced by insulin‐like growth factor (IGF)‐1 and LH.26 Moreover, with the growth of the follicle, activin production from the granulosa cells decreased. Simultaneously, the production of inhibin and follistatin (activin‐binding protein) from the granulosa cells increased.27 In the rat ovarian granulosa cell culture system, the addition of activin to the medium resulted in a drastic increase in FSHR expression. However, when the cells were stimulated with FSH, activin production rapidly decreased.28 Based on these findings, activin is considered to be secreted mainly from the granulosa cells before FSH installation and could be involved in the maintenance of the gonadotropin receptors. In a recent report involving human samples, the activin concentration in the follicular fluid was increased in follicles with a diameter that was >10 mm.29 However, FSHR expression decreased in the follicles at this stage. Therefore, the interaction between activin and FSHR expression is unclear after this stage.
3.2. Insulin‐like growth factor‐1
The IGF system is composed of a ligand, receptor, and binding protein and controls its bioactivity. Insulin also can bind to the IGF receptor and affect the system. In the ovary, IGF‐1 expression is identified exclusively within developing and mature follicles.30 The localization of IGF‐1 expression in the follicles corresponded to that of FSHR and aromatase.31 In human samples, IGF‐1 expression was observed only in the antral follicles with a 3‐5 mm diameter. On the contrary, its receptor could be found in most of the follicles from the primary to the preovulatory stage.18 In the rat granulosa cell culture system, the addition of IGF‐1 in the medium strongly enhanced LHR expression that was induced by FSH stimulation.32, 33, 34 In this phenomenon, IGF‐1 did not enhance the transcription of LHR mRNA, but elongated the half‐life of the mRNA. Similarly, FSHR expression was upregulated with the addition of IGF‐1.35 A double‐chamber co‐culture system with an artificial membrane was established that was seeded with theca cells on one side and granulosa cells on the other side. In this system, when the theca cells were stimulated with LH, LHR expression in the granulosa cells was increased. Similar LH stimulation also induced IGF‐1 expression in the theca cells. The former increase in LHR expression was diminished by the addition of IGF‐1 inhibitor. Based on these findings, LHR expression in the granulosa cells that are induced with FSH is enhanced by IGF‐1 that is derived from LH‐stimulated theca cells.
3.3. Estrogen
Estrogen is a steroid hormone and a final product of the hypothalamus–pituitary–ovary axis. Estrogen is produced and secreted by the granulosa cells as a result of FSH stimulation that activates CYP19A1 and converts androgen into estrogen. Estrogen receptors (ERs) are distributed throughout the body and one of the main target organs is the uterus. The granulosa cells also express ERs and estrogen contributes to follicular development in an autocrine/paracrine manner.36 In the ERβ‐knockout mice, the differentiation of the granulosa cells that were induced by FSH stimulation was abolished, aromatase activity was decreased, and LHR expression became insufficient. As a result, ovulation decreased and the expansion of the cumulus cells–oocyte complex (COC) became imperfect. These reactions were not evident in the ERα‐knockout mouse. Therefore, differentiation of the granulosa cells, according to follicular development, is thought to be controlled via the ERβ pathway.37 In the rat granulosa cell culture system, estrogen alone could not induce LHR and did not change FSHR expression, but it did enhance LHR expression that was induced by FSH. In this phenomenon, estrogen did not enhance the transcription of LHR mRNA, but stabilized LHR mRNA. Mevalonate kinase (Mvk) negatively correlated to this change. Co‐doped FSH and estrogen inhibited Mvk expression and its negative involvement with LHR. As a result, LHR expression was upregulated. This interaction of Mvk also was confirmed with the experiment on the overexpression of Mvk that diminished the positive estrogen effect.38 Mvk also could be involved in the transient downregulation of LHR after an LH surge.39
3.4. Interleukin‐6
Interleukin‐6 (IL‐6) is one of the cytokines that control humoral immunity. It was originally identified as a B‐cell differentiation factor.40 In previous reports, it was reported that the rat granulosa cells also can secrete IL‐641, 42 and that its secretion was increased by FSH stimulation.43 There were several reports on the effect of IL‐6 on follicular development. In one report, the addition of IL‐6 into the rat granulosa cell culture medium inhibited LHR expression.44, 45 In other reports, IL‐6 inhibited estradiol and progesterone production that were induced by FSH stimulation in the granulosa cells.46, 47 On the contrary, in another report, IL‐6 expression was increased during COC expansion and the addition of IL‐6 to the culture system accelerated the COC expansion.48 Based on these findings, the authors studied the effect of IL‐6 on LHR expression, as induced by FSH stimulation in the rat granulosa primary cell culture. In this primary culture, the addition of IL‐6 dramatically enhanced the LHR mRNA expression that was induced by FSH. Interleukin‐6 also enhanced the LHR expression that was induced by 8‐Br‐cAMP. Therefore, IL‐6 enhanced the signal downstream of cAMP. The LHR membrane protein that was induced by FSH also was highly enhanced by its association with IL‐6. The main signal transduction pathways of IL‐6 are mitogen‐activated protein kinase and Janus tyrosine kinase (JAK), signal transducer and activator of transcription (STAT) pathways. These enhancements were blocked by the JAK pathway inhibitor, but not by the ERK 1/2 inhibitor. Hence, this enhancement of IL‐6 might be mediated by the JAK/STAT pathway49 (Figure 1).
Figure 1.
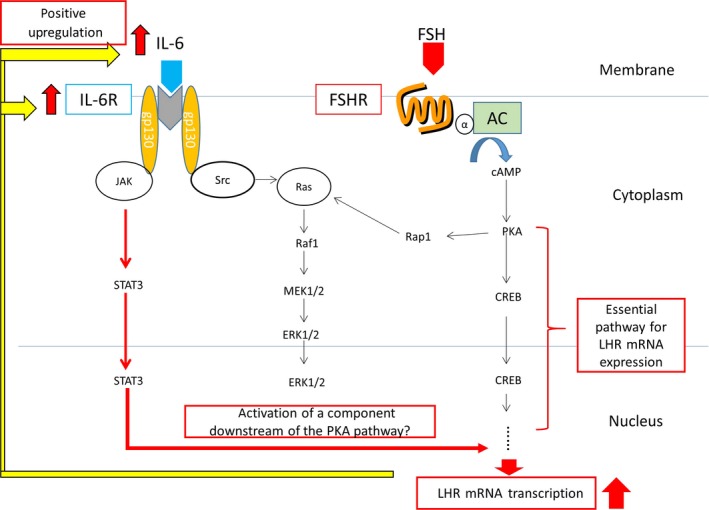
Schematic representation showing the enhancement of follicle‐stimulating hormone (FSH), induced luteinizing hormone receptor (LHR) expression that was elicited by interleukin (IL‐6). CREB, cAMP response element‐binding protein; ERK, extracellular‐signal‐regulated kinase; FSHR, follicle‐stimulating hormone receptor; JAK, Janus tyrosine kinase; PKA, protein kinase A; STAT, signal transducer and activator of transcription
4. TRANSIENT DOWNREGULATION OF LUTEINIZING HORMONE RECEPTOR AFTER A LUTEINIZING HORMONE SURGE
As mentioned above, during follicular development from the antral follicle, LHR expression was induced by FSH stimulation. This expression was enhanced further by various local factors that were derived from the granulosa and theca cells in a coordinated way. These changes are crucial for the preparation of a LH surge, which is absolutely necessary for oocyte maturation, ovulation, and transformation of the follicle and luteinization.
Until the LH surge, a constant increase in LHR expression continued. However, immediately after the LH surge, a sharp decrease in LHR mRNA expression in the preovulatory follicles was provoked.50, 51, 52 This downregulation was followed by LHR upregulation. The LHR expression peaked in the mid‐luteal phase. This transient downregulation of LHR that was induced by the LH surge could be reproduced in the cell culture system and this effect of LH could be mimicked by 8‐Br‐cAMP stimulation.53 In order to elucidate the precise mechanism underlying the downregulation of this receptor, the authors investigated the involvement of miRNAs in this phenomenon, which are small, non‐coding RNAs of ~22 nucleotides that bind to the 3′‐untranslated region of mRNAs. They are believed to negatively control post‐transcriptional gene expression. A miRNA microarray was performed for cyclopedic analysis of the miRNA expression profile of the rat ovaries during LHR downregulation. The result indicated that 23 candidate miRNAs that were highly expressed during this downregulation were recruited. Using a bioinformatics database for miRNAs and a clustering analysis, miR‐136‐3p was identified as a candidate for further investigation (Figure 2). In both the in vivo and in vitro studies, the miR‐136‐3p expression levels were increased 6 hour after hCG administration, concurrent with the downregulation of LHR mRNA expression. Moreover, the overexpression of miR‐136‐3p in the cultured granulosa cells provoked a significant downregulation of LHR mRNA, compared to that in the cells transfected with the negative control. In contrast, transfection with a miR‐136‐3p inhibitor increased the LHR mRNA levels. These data demonstrate that miR‐136‐3p participated in the downregulation of LHR mRNA by binding to LHR mRNA.54 For LHR downregulation, the involvement of another miRNA has been reported: the protein that invokes LHR downregulation was identified and is denominated as “LHR mRNA binding protein.” This protein has proven to be upstream of Mvk and the involvement of miR122 has been reported in the induction of Mvk.
Figure 2.
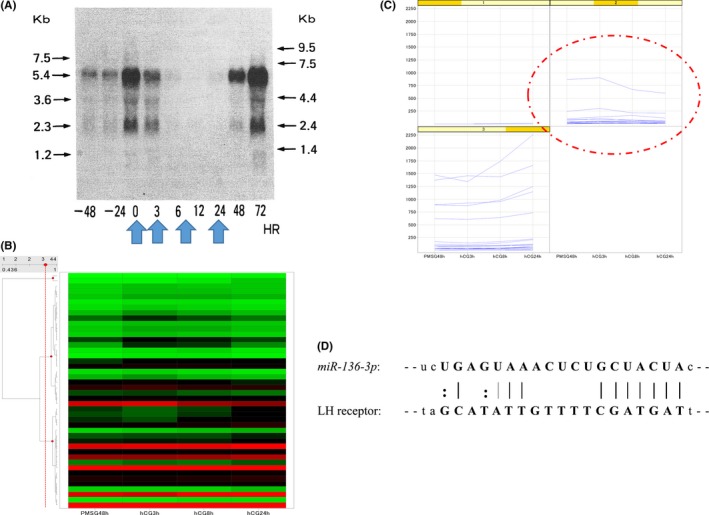
miRNA array analysis. (A) Northern blot analysis of rat luteinizing hormone receptor (LHR) in the ovarian samples after administration of pregnant mare serum gonadotropin (PMSG) and subsequent human chorionic gonadotropin (hCG) stimulation. The LHR mRNA expression was induced by PMSG stimulation that decreased after hCG addition. This downregulation of LHR was transient and recovered eventually. (B) Heat map with hierarchical clustering. Rat ovaries were removed at the indicated time points and a miRNA microarray analysis was performed. (C) Expression pattern of miRNA in each cluster. Twenty‐three candidate miRNAs that were highly expressed during downregulation were recruited. (D) Using a bioinformatics database for miRNAs and clustering analysis, miR‐136‐3p was identified as a candidate for further investigation. The arrangement of miR‐136‐3p and luteinizing hormone receptor (LHR) mRNA and the schematic representation of the predicted miR‐136‐3p binding site in the 3′‐untranslated region (UTR) of LHR mRNA are shown
5. LUTEINIZATION
As previously mentioned, following the transient downregulation, LHR expression increased again and peaked in the mid‐luteal phase.21, 55 The mechanism that underlies LHR upregulation at this stage is not fully understood. In a recent report on the human luteinized granulosa cells, the addition of RU486, a progesterone receptor antagonist, inhibited the increase in LHR expression.56 This result suggests that LHR expression in the luteal phase is, at least partly, controlled by progesterone that is secreted by the luteinized granulosa cells (Figure 3).
Figure 3.
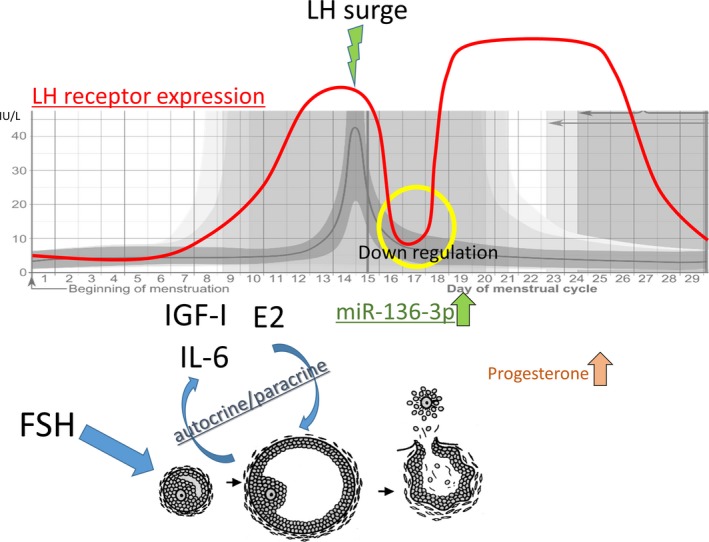
Schematic representation of the estimated luteinizing hormone (LH)/human chorionic gonadotropin receptor expression in the granulosa cells during the menstrual cycle. E2, estradiol; FSH, follicle‐stimulating hormone; IGF, insulin‐like growth factor; IL, interleukin
6. GONADOTROPIN RECEPTOR‐DEFICIENT MOUSE MODELS
6.1. Follicle‐stimulating hormone receptor knockout mouse
The FSHR model (FORKO) mouse was established by a group of researchers11 and has been characterized in detail.57 These mice demonstrated phenotypes that were similar to those that were recognized in the FSHβ knockout mice. The FORKO males had limited fecundity.58 Their testis was small in size and spermatogenesis was partially disturbed.59 On the contrary, the FORKO females were infertile. They had small ovaries and a thin uterus. In the ovaries, the primordial, primary, and secondary follicles could be recognized with the normal number of granulosa and theca cells. However, folliculogenesis was blocked before the antral follicular stage. The expression of LHR was not affected by the knockout of FSHR and ERs also existed.11 These results indicated that FSHR is essential for follicular development beyond the antral follicular stage.
6.2. Luteinizing hormone receptor knockout mouse
Two research groups have generated LHR knockout (LuRKO) mouse models independently.60, 61 Both the LuRKO males and females were infertile. In the LuRKO males, the testicular descent was blocked and the testis became diminished in size. In the testis, the number of Leydig cells was drastically decreased. The serum levels of gonadotropins were elevated and the testosterone level became lower. Spermatogenesis was blocked at round spermatid stage. The LuRKO females had small ovaries, a thin uterus, and their vaginal opening was delayed. In the ovary, antral follicular formation was recognized. However, the follicles at the preovulatory stage, as well as at the corpus luteum, were absent. Even by stimulation with a high dose of FSH, follicular development could not be recovered over the antral follicular stage. Therefore, the expression of LHR is indispensable for follicular maturation to ovulation.
7. TRANSCRIPTIONAL CONTROLS OF THE GONADOTROPIN RECEPTORS
7.1. Follicle‐stimulating hormone receptor knockout mouse
The promoter activity of FSHR has been reported to be varied among species of primates. However, the sequence homology of the 5′ flanking region of the receptor has been highly conserved through evolution.62 The information about the transcriptional control of FSHR and its expression and cause of organ specificity is still unknown. In the case of rats and humans, the 5′ flanking region of FSHR lacks the TATA box, CCAAT elements, and GC box motif. It also lacks the cAMP response element and the activating protein‐2 (AP‐2) binding site. The FSHR gene in rats, but not in humans, has an AP‐1 binding site. The human FSHR gene has incomplete half‐consensus estrogen receptor response element. One of the identified response elements is an E‐box consensus sequence (CACATG). Upstream stimulating factors 1 and 2 (USF1/2) were reported to bind to this E‐box sequence and to control FSHR transcription. The FSH stimulation caused a decrease in the USF1 expression, which led to a delay in the FSHR transcription.63
Activin and transforming growth factor‐β were reported to increase the transcription activity of the FSHR gene, but its mechanism is yet to be elucidated. For this activation, interaction with the E‐box is presumed.
7.2. Luteinizing hormone receptor
The promoter of the LHR gene contains binding sites of the specificity protein 1 (SP1), steroidogenic factor 1, and AP‐2. Among these binding sites, the SP1 binding site was reported to be essential for the activation of the LHR transcription that was induced by cAMP.64
One study reported that, for rat LHR expression, the 5′ flanking region from −171 to −137 bp is essential. It was identified that the early growth response gene‐1 (Egr‐1) was a transcription factor that binds to this promoter region and upregulates LHR expression. However, the expression of Egr‐1 was observed in the luteinized granulosa cells, but not in the preovulatory granulosa cells.65
Insulin‐like growth factor‐1, estradiol, and IL‐6 enhanced LHR expression that was induced by FSH stimulation. Among these, only IL‐6 could increase the promoter activity of LHR.49 The IGF‐1 and estradiol did not affect the promoter activity, but elongated the half‐life of the LHR mRNA.34, 38
In our previous report, TNF‐α diminished the LHR promoter activity and FSH‐induced LHR expression was decreased. In this phenomenon, nuclear factor‐κB p65 was translocated to the nucleus and interacted with the promoter region of LHR.66
8. CONCLUSION
Gonadotropins induce follicular development, leading to ovulation and luteinization. In women, the level of gonadotropins, along with the expression of their receptors, dynamically changes throughout the menstrual cycle. The FSHR expression is triggered by oocyte‐derived GDF9, which stimulates the theca cells to secrete androgens and which might play a key role in inducing FSHR in the granulosa cells. The level of FSHR expression peaks at the small antral follicular stage and decreases as follicular maturation proceeds. On the contrary, in the theca cells, LHR expression has been noted from its appearance. In the granulosa cells, FSH stimulation is essential for LHR expression. However, in the granulosa cells, FSH alone is not sufficient to respond to a LH surge for oocyte maturation, ovulation, and subsequent luteinization. In order to achieve these stages, various local factors, including IGF‐1, estrogen, and IL‐6, derived from the granulosa and theca cells in response to FSH and LH stimulation, have to work synergistically in an autocrine/paracrine manner to strongly induce LHR expression. Following a LH surge, LHR expression decreases markedly; miRNAs are involved in this transient downregulation of LHR.
Following ovulation, LHR is drastically induced again toward luteinization. The mechanism underlying this step has not been fully understood. However, partly, a positive correlation with progesterone is suggested.
The expression of these gonadotropin receptors is controlled by sophisticated and complicated systems. A breakdown of this system can lead to ovulatory disorders, including polycystic ovary syndrome (PCOS). In patients with PCOS, an excess of androgens, LH, IGF‐1,67 and IL‐668, 69 has been reported. Further analysis is desired in order to elucidate the mechanisms that are involved in these diseases and to improve ovulation induction therapy.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any studies with human or animal subjects performed by any of the authors.
Kishi H, Kitahara Y, Imai F, Nakao K, Suwa H. Expression of the gonadotropin receptors during follicular development. Reprod Med Biol. 2018;17:11–19. https://doi.org/10.1002/rmb2.12075
REFERENCES
- 1. Abdallah MA, Lei ZM, Li X, et al. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J Clin Endocrinol Metab. 2004;89:952‐956. [DOI] [PubMed] [Google Scholar]
- 2. Apaja PM, Harju KT, Aatsinki JT, Petaja‐Repo UE, Rajaniemi HJ. Identification and structural characterization of the neuronal luteinizing hormone receptor associated with sensory systems. J Biol Chem. 2004;279:1899‐1906. [DOI] [PubMed] [Google Scholar]
- 3. Fields MJ, Shemesh M. Extragonadal luteinizing hormone receptors in the reproductive tract of domestic animals. Biol Reprod. 2004;71:1412‐1418. [DOI] [PubMed] [Google Scholar]
- 4. Saner‐Amigh K, Mayhew BA, Mantero F, et al. Elevated expression of luteinizing hormone receptor in aldosterone‐producing adenomas. J Clin Endocrinol Metab. 2006;91:1136‐1142. [DOI] [PubMed] [Google Scholar]
- 5. Kasahara Y, Kitahara Y, Nakamura K, Minegishi T. Downregulation of LH receptor mRNA in the rat uterus. Mol Med Rep. 2012;5:1146‐1150. [DOI] [PubMed] [Google Scholar]
- 6. Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60:461‐496. [DOI] [PubMed] [Google Scholar]
- 7. Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141‐174. [DOI] [PubMed] [Google Scholar]
- 8. Minegishi T, Nakamura K, Takakura Y, Ibuki Y, Igarashi M. Cloning and sequencing of human FSH receptor cDNA. Biochem Biophys Res Commun. 1991;175:1125‐1130. [DOI] [PubMed] [Google Scholar]
- 9. Minegishi T, Nakamura K, Takakura Y, et al. Cloning and sequencing of human LH/hCG receptor cDNA. Biochem Biophys Res Commun. 1990;172:1049‐1054. [DOI] [PubMed] [Google Scholar]
- 10. Yamoto M, Shima K, Nakano R. Gonadotropin receptors in human ovarian follicles and corpora lutea throughout the menstrual cycle. Horm Res. 1992;37(Suppl 1):5‐11. [DOI] [PubMed] [Google Scholar]
- 11. Dierich A, Sairam MR, Monaco L, et al. Impairing follicle‐stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612‐13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle‐stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84:2951‐2956. [DOI] [PubMed] [Google Scholar]
- 14. Sen A, Prizant H, Light A, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA‐125b expression. Proc Natl Acad Sci U S A. 2014;111:3008‐3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeppesen JV, Kristensen SG, Nielsen ME, et al. LH‐receptor gene expression in human granulosa and cumulus cells from antral and preovulatory follicles. J Clin Endocrinol Metab. 2012;97:E1524‐E1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orisaka M, Jiang JY, Orisaka S, Kotsuji F, Tsang BK. Growth differentiation factor 9 promotes rat preantral follicle growth by up‐regulating follicular androgen biosynthesis. Endocrinology. 2009;150:2740‐2748. [DOI] [PubMed] [Google Scholar]
- 17. Orisaka M, Orisaka S, Jiang JY, et al. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol. 2006;20:2456‐2468. [DOI] [PubMed] [Google Scholar]
- 18. Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121‐155. [DOI] [PubMed] [Google Scholar]
- 19. Bukovsky A, Chen TT, Wimalasena J, Caudle MR. Cellular localization of luteinizing hormone receptor immunoreactivity in the ovaries of immature, gonadotropin‐primed and normal cycling rats. Biol Reprod. 1993;48:1367‐1382. [DOI] [PubMed] [Google Scholar]
- 20. Zhang M, Shi H, Segaloff DL, van Voorhis BJ. Expression and localization of luteinizing hormone receptor in the female mouse reproductive tract. Biol Reprod. 2001;64:179‐187. [DOI] [PubMed] [Google Scholar]
- 21. Yung Y, Aviel‐Ronen S, Maman E, et al. Localization of luteinizing hormone receptor protein in the human ovary. Mol Hum Reprod. 2014;20:844‐849. [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa Y, Miyamoto K, Abe Y, et al. Induction of follicle stimulating hormone receptor by erythroid differentiation factor on rat granulosa cell. Biochem Biophys Res Commun. 1988;156:668‐674. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura M, Minegishi T, Hasegawa Y, et al. Effect of an activin A on follicle‐stimulating hormone (FSH) receptor messenger ribonucleic acid levels and FSH receptor expressions in cultured rat granulosa cells. Endocrinology. 1993;133:538‐544. [DOI] [PubMed] [Google Scholar]
- 24. Miro F, Smyth CD, Hillier SG. Development‐related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology. 1991;129:3388‐3394. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura K, Nakamura M, Igarashi S, et al. Effect of activin on luteinizing hormone–human chorionic gonadotropin receptor messenger ribonucleic acid in granulosa cells. Endocrinology. 1994;134:2329‐2335. [DOI] [PubMed] [Google Scholar]
- 26. Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab. 1991;72:1206‐1211. [DOI] [PubMed] [Google Scholar]
- 27. Tano M, Minegishi T, Nakamura K, et al. Regulation of follistatin messenger ribonucleic acid in cultured rat granulosa cells. Mol Cell Endocrinol. 1995;109:167‐174. [DOI] [PubMed] [Google Scholar]
- 28. Kishi H, Minegishi T, Tano M, Kameda T, Ibuki Y, Miyamoto K. The effect of activin and FSH on the differentiation of rat granulosa cells. FEBS Lett. 1998;422:274‐278. [DOI] [PubMed] [Google Scholar]
- 29. Jeppesen JV, Nielsen ME, Kristensen SG, Yding AC. Concentration of activin A and follistatin in follicular fluid from human small antral follicles associated to gene expression of the corresponding granulosa cells. Mol Cell Endocrinol. 2012;356:48‐54. [DOI] [PubMed] [Google Scholar]
- 30. Oliver JE, Aitman TJ, Powell JF, Wilson CA, Clayton RN. Insulin‐like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinology. 1989;124:2671‐2679. [DOI] [PubMed] [Google Scholar]
- 31. Zhou J, Kumar TR, Matzuk MM, Bondy C. Insulin‐like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol Endocrinol. 1997;11:1924‐1933. [DOI] [PubMed] [Google Scholar]
- 32. Adashi EY, Resnick CE, Svoboda ME, van Wyk JJ. Somatomedin‐C enhances induction of luteinizing hormone receptors by follicle‐stimulating hormone in cultured rat granulosa cells. Endocrinology. 1985;116:2369‐2375. [DOI] [PubMed] [Google Scholar]
- 33. Kanzaki M, Hattori MA, Horiuchi R, Kojima I. Co‐ordinate actions of FSH and insulin‐like growth factor‐I on LH receptor expression in rat granulosa cells. J Endocrinol. 1994;141:301‐308. [DOI] [PubMed] [Google Scholar]
- 34. Hirakawa T, Minegishi T, Abe K, Kishi H, Ibuki Y, Miyamoto K. A role of insulin‐like growth factor I in luteinizing hormone receptor expression in granulosa cells. Endocrinology. 1999;140:4965‐4971. [DOI] [PubMed] [Google Scholar]
- 35. Minegishi T, Hirakawa T, Kishi H, et al. A role of insulin‐like growth factor I for follicle‐stimulating hormone receptor expression in rat granulosa cells. Biol Reprod. 2000;62:325‐333. [DOI] [PubMed] [Google Scholar]
- 36. Pencharz RI. Effect of estrogens and androgens alone and in combination with chorionic gonadotropin on the ovary of the hypophysectomized rat. Science. 1940;91:554‐555. [DOI] [PubMed] [Google Scholar]
- 37. Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor‐beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247‐3262. [DOI] [PubMed] [Google Scholar]
- 38. Ikeda S, Nakamura K, Kogure K, et al. Effect of estrogen on the expression of luteinizing hormone–human chorionic gonadotropin receptor messenger ribonucleic acid in cultured rat granulosa cells. Endocrinology. 2008;149:1524‐1533. [DOI] [PubMed] [Google Scholar]
- 39. Menon KM, Menon B. Regulation of luteinizing hormone receptor expression by an RNA binding protein: role of ERK signaling. Indian J Med Res. 2014;140(Suppl):S112‐S119. [PMC free article] [PubMed] [Google Scholar]
- 40. Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin‐6 family of cytokines and gp130. Blood. 1995;86:1243‐1254. [PubMed] [Google Scholar]
- 41. Gorospe WC, Hughes FM Jr, Spangelo BL. Interleukin‐6: effects on and production by rat granulosa cells in vitro . Endocrinology. 1992;130:1750‐1752. [DOI] [PubMed] [Google Scholar]
- 42. Shimada M, Yanai Y, Okazaki T, et al. Synaptosomal‐associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21:2487‐2502. [DOI] [PubMed] [Google Scholar]
- 43. Gorospe WC, Spangelo BL. Interleukin‐6 production by rat granulosa cells in vitro: effects of cytokines, follicle‐stimulating hormone, and cyclic 3’,5’‐adenosine monophosphate. Biol Reprod. 1993;48:538‐543. [DOI] [PubMed] [Google Scholar]
- 44. Tamura K, Kawaguchi T, Kogo H. Interleukin‐6 inhibits the expression of luteinizing hormone receptor mRNA during the maturation of cultured rat granulosa cells. J Endocrinol. 2001;170:121‐127. [DOI] [PubMed] [Google Scholar]
- 45. Machelon V, Nome F, Salesse R. Comparative IL‐6 effects on FSH and hCG‐induced functions in porcine granulosa cell cultures. Cell Mol Biol (Noisy‐le‐grand). 1994; 40: 373‐380. [PubMed] [Google Scholar]
- 46. Alpizar E, Spicer LJ. Effects of interleukin‐6 on proliferation and follicle‐stimulating hormone‐induced estradiol production by bovine granulosa cells in vitro: dependence on size of follicle. Biol Reprod. 1994;50:38‐43. [DOI] [PubMed] [Google Scholar]
- 47. Tamura K, Kawaguchi T, Hara T, et al. Interleukin‐6 decreases estrogen production and messenger ribonucleic acid expression encoding aromatase during in vitro cytodifferentiation of rat granulosa cell. Mol Cell Endocrinol. 2000;170:103‐111. [DOI] [PubMed] [Google Scholar]
- 48. Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. Interleukin‐6: an autocrine regulator of the mouse cumulus cell–oocyte complex expansion process. Endocrinology. 2009;150:3360‐3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Imai F, Kishi H, Nakao K, Nishimura T, Minegishi T. IL‐6 up‐regulates the expression of rat LH receptors during granulosa cell differentiation. Endocrinology. 2014;155:1436‐1444. [DOI] [PubMed] [Google Scholar]
- 50. Hu ZZ, Tsai‐Morris CH, Buczko E, Dufau ML. Hormonal regulation of LH receptor mRNA and expression in the rat ovary. FEBS Lett. 1990;274:181‐184. [DOI] [PubMed] [Google Scholar]
- 51. LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin‐induced up‐ and down‐regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277‐3279. [DOI] [PubMed] [Google Scholar]
- 52. Nakamura K, Minegishi T, Takakura Y, et al. Hormonal regulation of gonadotropin receptor mRNA in rat ovary during follicular growth and luteinization. Mol Cell Endocrinol. 1991;82:259‐263. [DOI] [PubMed] [Google Scholar]
- 53. Kishi H, Minegishi T, Tano M, Abe Y, Ibuki Y, Miyamoto K. Down‐regulation of LH/hCG receptor in rat cultured granulosa cells. FEBS Lett. 1997;402:198‐202. [DOI] [PubMed] [Google Scholar]
- 54. Kitahara Y, Nakamura K, Kogure K, Minegishi T. Role of microRNA‐136‐3p on the expression of luteinizing hormone–human chorionic gonadotropin receptor mRNA in rat ovaries. Biol Reprod. 2013;89:114. [DOI] [PubMed] [Google Scholar]
- 55. Minegishi T, Tano M, Abe Y, Nakamura K, Ibuki Y, Miyamoto K. Expression of luteinizing hormone/human chorionic gonadotrophin (LH/HCG) receptor mRNA in the human ovary. Mol Hum Reprod. 1997;3:101‐107. [DOI] [PubMed] [Google Scholar]
- 56. Yung Y, Maman E, Ophir L, et al. Progesterone antagonist, RU486, represses LHCGR expression and LH/hCG signaling in cultured luteinized human mural granulosa cells. Gynecol Endocrinol. 2014;30:42‐47. [DOI] [PubMed] [Google Scholar]
- 57. Sairam MR, Krishnamurthy H. The role of follicle‐stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res. 2001;32:601‐608. [DOI] [PubMed] [Google Scholar]
- 58. Krishnamurthy H, Babu PS, Morales CR, Sairam MR. Delay in sexual maturity of the follicle‐stimulating hormone receptor knockout male mouse. Biol Reprod. 2001;65:522‐531. [DOI] [PubMed] [Google Scholar]
- 59. Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle‐stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62:1146‐1159. [DOI] [PubMed] [Google Scholar]
- 60. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172‐183. [DOI] [PubMed] [Google Scholar]
- 61. Lei ZM, Mishra S, Zou W, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184‐200. [DOI] [PubMed] [Google Scholar]
- 62. Brune M, Adams C, Gromoll J. Primate FSH‐receptor promoter nucleotide sequence heterogeneity affects FSH‐receptor transcription. Mol Cell Endocrinol. 2010;317:90‐98. [DOI] [PubMed] [Google Scholar]
- 63. Hermann BP, Heckert LL. Transcriptional regulation of the FSH receptor: new perspectives. Mol Cell Endocrinol. 2007;260–262:100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen S, Liu X, Segaloff DL. A novel cyclic adenosine 3’,5’‐monophosphate‐responsive element involved in the transcriptional regulation of the lutropin receptor gene in granulosa cells. Mol Endocrinol. 2000;14:1498‐1508. [DOI] [PubMed] [Google Scholar]
- 65. Yoshino M, Mizutani T, Yamada K, et al. Early growth response gene‐1 regulates the expression of the rat luteinizing hormone receptor gene. Biol Reprod. 2002;66:1813‐1819. [DOI] [PubMed] [Google Scholar]
- 66. Nakao K, Kishi H, Imai F, Suwa H, Hirakawa T, Minegishi T. TNF‐alpha suppressed FSH‐induced LH receptor expression through transcriptional regulation in rat granulosa cells. Endocrinology. 2015;156:3192‐3202. [DOI] [PubMed] [Google Scholar]
- 67. Stubbs SA, Webber LJ, Stark J, et al. Role of insulin‐like growth factors in initiation of follicle growth in normal and polycystic human ovaries. J Clin Endocrinol Metab. 2013;98:3298‐3305. [DOI] [PubMed] [Google Scholar]
- 68. Tarkun I, Cetinarslan B, Turemen E, Canturk Z, Biyikli M. Association between circulating tumor necrosis factor‐alpha, interleukin‐6, and insulin resistance in normal‐weight women with polycystic ovary syndrome. Metab Syndr Relat Disord. 2006;4:122‐128. [DOI] [PubMed] [Google Scholar]
- 69. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin‐6 levels in women with polycystic ovary syndrome: a systematic review and meta‐analysis. PLoS ONE. 2016;11:e0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]


