Abstract
We hypothesized that the length of treatment-free survival following (a) initial diagnosis and (b) first-line treatment would be associated with improved subsequent five-year relative survival (RS5) in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL). 19,879 patients incident CLL/SLL cases (median age=76 years) were identified from SEER-Medicare. RS5 improved from 0.73 (95% CI: 0.72, 0.74) at diagnosis to 0.81 (95% CI: 0.80, 0.82) at year 1 and 0.89 (95% CI: 0.83, 0.96) at year 10 among those who had not received treatment. In our analysis of survival patterns following first-line treatment, RS5 improved from 0.55 (95% CI: 0.53, 0.57) at initiation of first-line treatment to 0.84 (95% CI: 0.75, 0.92) among patients who had not been retreated at year 5 following first-line therapy. Longer periods of treatment-free survival following initial diagnosis and first-line treatment were both predictive of meaningfully improved prognosis in CLL/SLL patients.
Keywords: Leukemia, lymphocytic, chronic, B-cell, lymphoma, non-Hodgkin, survival, prognosis, epidemiology, Medicare
Introduction
Conditional relative survival (RS) rates are used in cancer epidemiology to describe a patient’s future RS conditional on having survived a given number of years since diagnosis. Conditional RS is a clinically relevant metric that allows for estimated survival probabilities to be updated based on the initial course of disease [1]. For example, colorectal cancer patients have a five-year relative survival rate (RS5) of 63% at diagnosis, but for those who have survived five years after diagnosis, subsequent RS5 is ~95% [2]. Similar patterns have been described for many types of cancer [3].
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is an exception: RS5 is ~80% both at diagnosis and at year five following diagnosis [2]. This difference may be attributed to the lack of curative treatment for CLL/SLL, as well as variation in the natural history of the disease. While some patients present with advanced disease and are candidates for treatment at diagnosis, some early-stage CLL/SLL patients may live for years without significant disease progression or need for treatment [4-6]. For these reasons, length of treatment-free survival since diagnosis may provide more information about a patient’s subsequent prognosis than length of overall survival since diagnosis.
Similarly, length of progression-free survival following first-line treatment is recognized as an important prognostic marker in patients with CLL/SLL [7] and other forms of non-Hodgkin lymphoma [8,9]. However, while this relationship has been elucidated in a younger cohort of clinical trial participants who received first-line FCR [10], it has not been characterized quantitatively among older CLL/SLL patients in real-world settings.
In this study, we explored RS patterns in older CLL/SLL patients identified in the SEER-Medicare Linked Database. Following patients longitudinally after initial diagnosis, we updated patients’ RS5 estimates based on the length of treatment-free survival since (a) initial diagnosis and (b) first-line treatment for CLL/SLL. We hypothesized that the length of treatment-free survival following (a) and (b) would be positively associated with subsequent RS5.
Methods
Data sources
The study data were obtained from the 2014 SEER-Medicare linkage, from which we identified SEER cancer cases diagnosed in 1992–2011 and their Medicare records from 1991 to 2013. Within SEER registry catchment areas, 93% of patients aged 65+ years diagnosed with cancer have been linked to their Medicare claims data [11]. Participating registries collect data for all cancer patients diagnosed within their defined geographic area. Registry data include month and year of diagnosis, age at diagnosis, race, tumor stage, and histology. Medicare files from the Centers for Medicare and Medicaid Services (CMS) include demographic and enrollment information, date of death, and all bills submitted for inpatient hospital care, outpatient hospital care, physician services, and prescription fills. Normative mortality rates based on the U.S. general population were obtained from The Human Mortality Database [12], which assembles historical U.S. life tables based on data published by the U.S. Census Bureau [13] and National Center for Health Statistics [14].
Institutional Review Board review and research ethics
This research project was approved by SEER-Medicare Program staff and the University of Iowa Institutional Review Board (IRB). The University of Iowa IRB granted a waiver of informed consent because the project consisted of a secondary analysis of existing data. SEER-Medicare Program staff reviewed the manuscript to ensure that it met reporting requirements to protect patient confidentiality.
Cohort definitions: CLL/SLL inception cohort and first-line treatment cohort
Two cohorts were analyzed: (a) an inception cohort of newly diagnosed CLL/SLL patients followed from their diagnosis date and (b) a sub-cohort of patients who received first-line antineoplastic therapy at any time following diagnosis, followed from the end of first-line treatment (Figure 1). For the CLL/SLL inception cohort, our study population consisted of patients diagnosed with CLL/SLL in 1992–2011, and who were age 66+ years at the time of diagnosis. Cases were excluded if they had no specific diagnosis month recorded by SEER, had a prior malignancy, had inconsistent birth or death dates recorded by SEER and CMS, were diagnosed at death, did not have microscopic or laboratory diagnostic confirmation, were not enrolled in traditional fee-for-service Medicare Parts A and B at diagnosis and the prior 365 days, or had claims for antineoplastic therapy prior to the diagnosis date recorded by SEER (Figure 1). The age and Medicare enrollment restrictions ensured that we could use Medicare claims data to characterize patients’ antineoplastic treatments from diagnosis onward, as well as comorbidities and proxies for advanced CLL/SLL disease. Patients with a prior malignancy were excluded to ensure that we could infer that claims for antineoplastic therapies reflected treatment for CLL/SLL.
Figure 1.
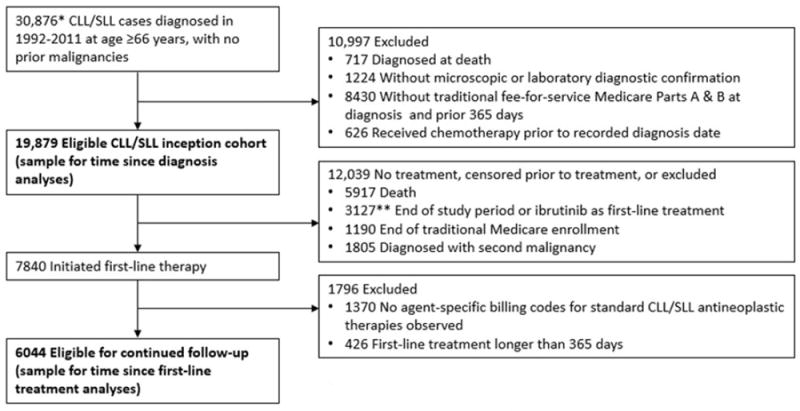
Flow diagram showing identification of CLL/SLL patients eligible for conditional relative survival analyses.
* Number also reflects the following preliminary eligibility requirements: specific month of diagnosis for CLL/SLL was recorded by SEER, and consistent birth and death dates were recorded by SEER and Medicare for the patient.
** Fewer than 11 patients received ibrutinib as first-line treatment. Combined with another exclusion category due to SEER-Medicare reporting requirements.
For the sub-cohort of patients who initiated firstline treatment, patients were excluded if prior to firstline treatment any of the following censoring events occurred: the end of the study period (31 December 2013), loss of traditional Medicare enrollment, diagnosis with a second primary malignancy, or initiation of ibrutinib (a novel Bruton’s tyrosine kinase inhibitor initially approved in 2013). Patients were also excluded if their first course of antineoplastic therapy was longer than 365 days. For most CLL/SLL chemotherapy regimens, the treatment plan would consist of six cycles of treatment at four-week intervals. Some patients may discontinue treatment early or take breaks between treatment cycles, and treatment with some agents (e.g. chlorambucil or rituximab monotherapy) may continue for 12 months.
Identification of antineoplastic treatment
Receipt of antineoplastic treatment was assessed using procedure, diagnosis and drug codes recorded in Medicare inpatient, outpatient, and prescription claims. See Appendix Tables A1 and A2 for details on the codes and code ranges that were used. Corticosteroids were not included in our study definition of antineoplastic therapy because they are frequently used for other indications, and rarely used as monotherapy for CLL/SLL. Drug-specific HCPCS and National Drug Codes (NDCs) were used to identify specific antineoplastic agents administered. Patients were classified as having received chemo-immunotherapy (CIT), chemotherapy alone, or immunotherapy alone. Table 1 describes how patients’ treatment regimens were classified. A prior chart validation study performed in lymphoma patients found that Medicare claims data generally provide reliable information on whether and when patients receive chemotherapy [15].
Table 1.
Characteristics of newly diagnosed CLL/SLL patients and subset of patients who initiated first-line antineoplastic treatment.
| Characteristic | Inception cohort of 19,879 newly diagnosed CLL/SLL patients N (%) | 6044 patients who initiated first-line treatment N (%) |
|---|---|---|
| Age at diagnosis in years | ||
| 66–74 | 8045 (40%) | 3009 (50%) |
| 75–79 | 4677 (24%) | 1513 (25%) |
| 80+ | 7157 (36%) | 1522 (25%) |
| Female sex | 9225 (46%) | 2644 (44%) |
| Diagnosis year | ||
| 1992–2000 | 5605 (28%) | 1046 (17%) |
| 2001–2005 | 6831 (34%) | 1947 (32%) |
| 2006–2013 | 7443 (37%) | 3051 (50%) |
| Indicators of advanced CLL/SLL | ||
| Anemia | 3479 (18%) | 2485 (41%) |
| Thrombocytopenia/coagulation disorder | 797 (4%) | 871 (14%) |
| Immune deficiency | 122 (1%) | 263 (4%) |
| Count of major comorbidities | ||
| None | 11,903 (60%) | 3081 (51%) |
| One | 4775 (24%) | 1695 (28%) |
| Two or more | 3201 (16%) | 1268 (21%) |
| Hospitalized in prior year | 4168 (21%) | 2531 (42%) |
| Type of first-line treatment | ||
| Chemo-immunotherapy | – | 2423 (40%) |
| Chemotherapy alone | – | 2236 (37%) |
| Immunotherapy alone | – | 1385 (23%) |
CLL: chronic lymphocytic leukemia; SLL: small lymphocytic lymphoma.
Outcome
The study endpoint was all-cause mortality. The SEERMedicare dataset includes each patient’s date of death as recorded in the Social Security Administration’s Death Master File. In the files used in the present study from the 2014 SEER-Medicare linkage, mortality data were available through the end of 2013. Patients who were alive on 31 December 2013, were right-censored in our survival analyses.
Covariates
A number of demographic and clinical characteristics were assessed in order to characterize our study sample (see Table 1). As a summary measure of comorbidity burden, we report a count of the following 12 major comorbidities included in the National Cancer Institute and Charlson comorbidity indices [16-18]: cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia/paraplegia, liver disease, myocardial infarction, peptic ulcer disease, renal disease, rheumatic disease, and human immunodeficiency virus (HIV) infection. In addition, we provide data on the frequency of several health conditions associated with advanced CLL/SLL disease, e.g. anemia and infection. Health conditions were assessed based on administrative diagnosis codes recorded during the year prior to retreatment. Following the approach of Klabunde et al. [18], a condition was considered present if a corresponding inpatient diagnosis code or two outpatient diagnosis codes 30+ days apart were observed. A higher standard was required for outpatient diagnosis codes, because they can sometimes reflect diagnoses that were ruled out or merely considered as part of a differential diagnosis.
Statistical methods
Five-year relative overall survival (RS5) was used to characterize CLL/SLL patients’ prognosis. RS5 – the ratio of five-year observed overall survival relative to overall survival in the general population after conditioning on age, sex and calendar year – was estimated with the Ederer II method [19-21]. RS measures are frequently used in cancer epidemiology as indirect estimates of the burden of cancer-specific mortality in defined patient populations. The statistical significance of differences and trends in RS across patient subgroups was assessed using the additive hazards model endorsed by Dickman et al. for analyses of RS data within a generalized linear models framework [20].
Results
We identified an inception cohort of 19,879 patients newly diagnosed with CLL/SLL who met inclusion criteria (Figure 1). At diagnosis, the median patient age was 76 years (interquartile range [IQR]: 71, 82); 46% were female. The majority of patients (71%) were diagnosed after 2000, in the rituximab era of CLL/SLL therapeutics, and the study period included eligible follow-up time through 2013, before the era of B-cell receptor or bcl-2 targeted therapeutics. Additional baseline characteristics are shown in Table 1. During the first year following diagnosis, 22% of patients initiated antineoplastic treatment, 10% died, 4% were censored, and 64% remained alive and untreated at the end of the year.
Five-year relative survival was 0.73 (95% CI: 0.72, 0.74) at diagnosis. Among patients who survived one year without treatment, RS5 increased to 0.81 (95% CI: 0.80, 0.82). As the length of treatment-free survival increased, RS5 improved modestly but steadily to 0.89 (95% CI: 0.83, 0.96) for patients at year 10 following diagnosis (Figure 2; test for trend: p<.001).
Figure 2.
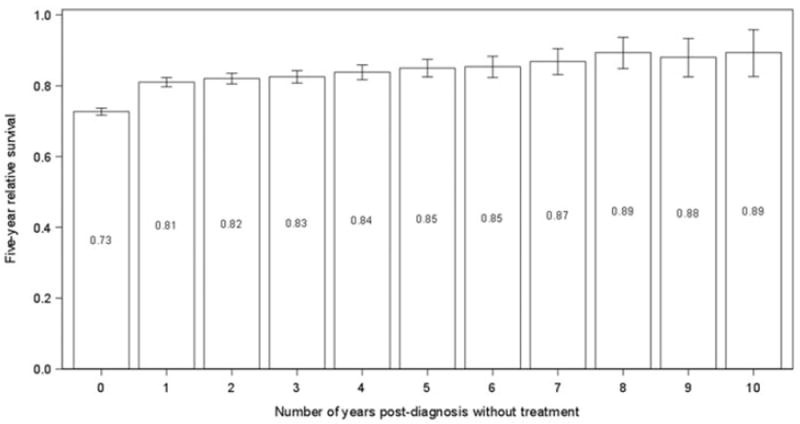
Estimated five-year relative survival (with 95% confidence intervals) conditional on the length of treatment-free survival since initial diagnosis with CLL/SLL.
A similar but more pronounced pattern was observed in patients following first-line therapy: a longer duration of treatment-free survival following firstline treatment was associated with meaningful incremental improvements in RS5. At initiation of first-line treatment, RS5 was 0.55 (95% CI: 0.53, 0.57). RS5 improved to 0.84 (95% CI: 0.75, 0.92) for patients who survived five years without retreatment following firstline therapy (Figure 3; test for trend: p<.001). After stratifying patients by the type of first-line antineoplastic treatment they received, this relationship was strongest among those who received CIT or immunotherapy as first-line treatment (Figure 3). At year five following first-line treatment, patients in these groups who had not been retreated were estimated to have survival similar to that of the general population.
Figure 3.
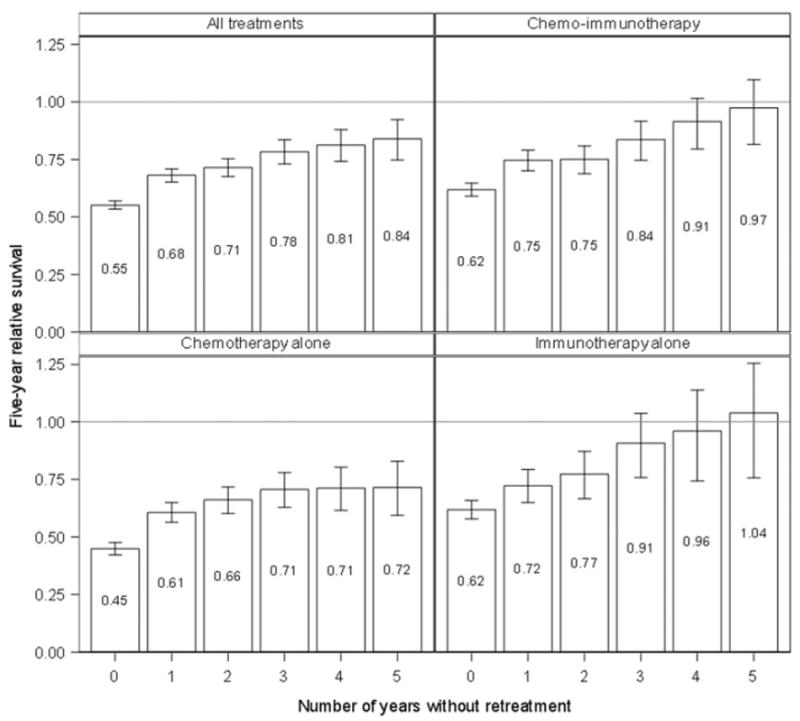
Estimated five-year relative survival (with 95% confidence intervals) conditional on the length of treatment-free survival since first-line treatment, stratified by type of first-line treatment.
Discussion
In this population-based study of older adults diagnosed with CLL/SLL in 1992–2011, longer intervals of treatment-free survival following initial diagnosis and first-line treatment were associated with improved subsequent survival outcomes. Among patients newly diagnosed with CLL/SLL, RS5 improved from 0.73 at diagnosis to 0.81 and 0.85 among patients who remained untreated at years one and five following diagnosis, respectively. Among patients who receiving first-line therapy, prognosis was considerably worse at the initiation of first-line therapy (RS5=0.55), but improved considerably among those with longer treatment- free intervals following first-line treatment. Subsequent RS5 was 0.78 and 0.84 among those who had not been retreated at years three and five, respectively, following first-line treatment. Among patients who received CIT or immunotherapy as firstline treatment, survival for five years without retreatment was associated with normal subsequent survival (RS5 ≈ 1).
Prior studies of conditional RS patterns in lymphoma patients have noted that in patients with indolent lymphomas such as CLL/SLL, RS5 is more or less unchanged after stratifying on time-since-diagnosis [22]. In contrast, the current study demonstrated that duration of treatment-free survival since diagnosis was predictive of modest but steady improvements in RS5. Differences in degree of disease progression at diagnosis are likely to be the primary explanation for the trend observed in our data. While some patients present with advanced disease and are candidates for treatment at diagnosis, some early-stage CLL/SLL patients may live for years without significant disease progression or need for treatment [4-6]. An important limitation of our study is that SEER-Medicare does not include staging information for patients with leukemias including CLL. Due to this limitation, our finding that subsequent survival improves gradually with longer treatment-free survival would be most relevant for updating prognostic estimates in patients who present with early-stage CLL/SLL and go for some time without needing treatment.
Among patients who have received first-line therapy for CLL/SLL, a longer interval of treatment-free survival is widely recognized as a favorable prognostic marker. Our data allow for a more precise quantification of this relationship in a large, unselected sample of older patients identified from SEER-Medicare. Our findings are broadly consistent with findings reported for follicular lymphoma, where two-year event-free survival following first-line treatment was associated with a normal subsequent life expectancy [8,9]. Among older patients with CLL/SLL who received CIT or immunotherapy as first-line treatment, five year treatment-free survival was associated with a similarly favorable prognosis. In interpreting these results, it is important to recognize that RS in CLL/SLL patients who received first-line therapy compared to the general population is a function of the severity and natural course of the patient’s disease and its responsiveness to treatment, as well as patient selection. Patients who received first-line therapy were deemed fit enough to receive chemotherapy and/or immunotherapy. For this reason, it is possible that our RS5 estimates may overestimate the degree to which their life expectancy has ‘normalized,’ since general population life tables are being used as a substitute for the comparator of true interest: the patients’ lifespans in the absence of CLL/SLL.
The favorable outcomes observed for patients who received immunotherapy alone as first-line treatment (Figure 3) is likely explained by the fact that immunotherapy alone may be considered for patients with less severe disease, particularly in community practice settings with lower thresholds for starting patients on anti-CD20 monoclonal antibody therapy. Consistent with this hypothesis, in an exploratory analysis we found that time to first-line treatment was shorter among patients diagnosed after the introduction of rituximab compared to earlier in our study period (see Appendix Figure A1).
This study had a number of other limitations. First, we lacked data on clinical prognostic markers, including initial staging, chromosomal abnormalities detected by fluorescence in situ hybridization (FISH), and functional status. Ideally, these measures would be combined with duration of treatment-free survival to develop a more strongly predictive and individualized prognostic score. However, after a patient has received first-line treatment, initial clinical prognostic markers may be less informative than the patient’s responsiveness to first-line treatment. In the follicular lymphoma research referenced earlier, the Follicular Lymphoma International Prognostic Index (FLIPI) evaluated at diagnosis did not predict subsequent survival outcomes after conditioning on age, sex, and duration of event-free survival following first-line treatment [9].
Another limitation was that we relied on administrative claims data records to determine whether and when patients received antineoplastic therapy. While chart confirmation of treatment would have been ideal and would have provided more accurate and detailed information about the type of treatment the patients received, prior research has found that Medicare claims data generally provide reliable information regarding chemotherapy in patients with lymphoma [15]. Finally, the conditional RS patterns we describe here are based on outcomes observed during the rituximab and pre-rituximab eras of CLL/SLL therapeutics, and will need to be reassessed once large-scale epidemiologic data are available for patients treated with novel targeted therapies.
Updating prognostic measures based on duration of treatment-free survival provides data that is clinically relevant to patients with CLL/SLL and their physicians. Although significant effort is devoted to calculation and communication of prognosis at time of initial consultation following diagnosis of CLL/SLL, the current study adds to available information highlighting the dynamic nature of prognosis over time in CLL/SLL patients. In addition, these data may be useful for interpreting the results from clinical trials of first-line therapies in patients with CLL/SLL. Since these trials typically have relatively short follow-up periods (e.g. one to three years), it is important to know the extent to which two- or three-year survival following first-line therapy is predictive of survival comparable to that expected in the general population.
Supplementary Material
Acknowledgments
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P50 CA097274, the University of Iowa Holden Comprehensive Cancer Center (HCCC) Population Research Core, which is supported in part by P30 CA086862, and by a Cancer & Aging Pilot Project Award from the University of Iowa HCCC and Center on Aging.
Footnotes
Geolocation information: Study data were obtained from patients residing in the United States.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1349905.
References
- 1.Shack L, Bryant H, Lockwood G, et al. Conditional relative survival: a different perspective to measuring cancer outcomes. Cancer Epidemiol. 2013;37:446–448. doi: 10.1016/j.canep.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Ellison LF, Bryant H, Lockwood G, et al. Conditional survival analyses across cancer sites. Health Rep. 2011;22:21–25. [PubMed] [Google Scholar]
- 3.Janssen-Heijnen ML, Gondos A, Bray F, et al. Clinical relevance of conditional survival of cancer patients in Europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010;28:2520–2528. doi: 10.1200/JCO.2009.25.9697. [DOI] [PubMed] [Google Scholar]
- 4.Boggs DR, Sofferman SA, Wintrobe MM, et al. Factors influencing the duration of survival of patients with chronic lymphocytic leukemia. Am J Med. 1966;40:243–254. doi: 10.1016/0002-9343(66)90105-7. [DOI] [PubMed] [Google Scholar]
- 5.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. [Google Scholar]
- 6.Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin N Am. 2004;18:795–805. doi: 10.1016/j.hoc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M. Chronic lymphocytic leukemia: 2015 Update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 8.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. JCO. 2015;33:2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer MJ, Bachy E, Ghesquieres H, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096–1101. doi: 10.1002/ajh.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam CS, O’Brien S, Plunkett W, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab) Blood. 2014;124:3059–3064. doi: 10.1182/blood-2014-06-583765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 12.Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany) 2016 Available from: www.mortality.org.
- 13.U.S. Census Bureau. Population Estimates and Projection, Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States. Washington (DC): various years. [Google Scholar]
- 14.National Center for Health Statistics. Vital statistics of the United States : mortality, Part A. II. Washington (DC): Government Printing Office; various years. [Google Scholar]
- 15.Chen-Hardee S, Chrischilles EA, Voelker MD, et al. Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin’s lymphoma (United States) Cancer Causes Control. 2006;17:647–654. doi: 10.1007/s10552-005-0502-4. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, Howlader N, Mariotto AB, et al. Technical report #2011-01: estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method. National Cancer Institute Surveillance Research Program. 2011 [Google Scholar]
- 20.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 21.Ederer F, Heise H. Methodological note no 10: instructions to IBM 650 programmers in processing survival computations. Bethesda (MD): National Cancer Institute End Results Evaluation Section; 1959. [Google Scholar]
- 22.van de Schans SA, van Steenbergen LN, Coebergh JW, et al. Actual prognosis during follow-up of survivors of B-cell non-Hodgkin lymphoma in the Netherlands. Haematologica. 2014;99:339–345. doi: 10.3324/haematol.2012.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


