Abstract
D'Arcy Thompson was a proponent of applying mathematical and physical principles to biological systems, an approach that is becoming increasingly common in developmental biology. Indeed, the recent integration of quantitative experimental data, force measurements and mathematical modeling has changed our understanding of morphogenesis – the shaping of an organism during development. Emerging evidence suggests that the subcellular organization of contractile cytoskeletal networks plays a key role in force generation, while on the tissue level the spatial organization of forces determines the morphogenetic output. Inspired by D'Arcy Thompson's On Growth and Form, we review our current understanding of how biological forms are created and maintained by the generation and organization of contractile forces at the cell and tissue levels. We focus on recent advances in our understanding of how cells actively sculpt tissues and how forces are involved in specific morphogenetic processes.
KEY WORDS: Actin, Contractility, Morphogenesis, Myosin, Tension
Summary: This Review emphasizes the role of the actomyosin meshwork in determining the forces that operate within and between cells and their environment to shape and organize cells and tissues.
Introduction
In On Growth and Form, D'Arcy Thompson proposed that an organism's form is a ʻdiagram of the forces' that have acted and continue to act upon it (Thompson, 1917). At the time of its publication, what those forces were and how they were generated was a mystery. However, 100 years later, a number of cellular forces driving organism shape during embryonic development have been or are currently being described. The discovery of the types of forces influencing morphogenesis has relied on the inference of force from individual cell shapes or from direct measurements of force through manipulation of the tissue. A number of factors have been implicated in generating these types of forces. In particular, genetic screens identifying mutations that disrupt morphogenesis have revealed that a key driver of morphogenesis is the actomyosin cytoskeleton, a network composed of actin (which can polymerize into filaments) and myosin (a molecular motor) that can generate contractile force (Quintin et al., 2008).
In this Review we discuss how forces generated at the cell level are able to generate distinct tissue shapes. For simplicity, we focus on force generation by the actomyosin cytoskeleton and consider developmental examples in which actomyosin activity plays a role. First, we discuss how contractile forces are generated, from the molecular to the tissue level. In the second section, we discuss examples of tissue morphogenesis that illustrate how patterns of contractility sculpt tissues, from single cell ingression to compartment boundary maintenance. Finally, even 100 years after Thompson wrote his seminal book, we are still a long way from being able to look at an organism and understand every force required to shape its final form, so we will end by discussing future research opportunities in this field.
Contractile force generation: from molecules to tissues
While D'Arcy Thompson dismissed ʻthe many theories and speculations which would connect the phenomena of surface-tension with contractility [and] muscular movement' (p. 210, Thompson, 1917), we now understand that this connection is paramount. However, the tension at cell surfaces is not driven by surface tension, as Thompson understood it, but by tension in the cortical actin layer (Box 1). Below, we begin with an overview of the molecular components of contractile networks that underlie this tension. We then discuss advances in our understanding of the subcellular organization of these networks and how they generate contractile force, thus increasing cortical tension. Next, we discuss how these networks are connected between cells and the role that these networks play in shaping the mechanical properties of the tissue.
Box 1. A note about surface tension.
When D'Arcy Thompson referred to ‘surface tension' at cell boundaries he equated it to the surface tension at an air-liquid or liquid-liquid interface. This surface tension is driven by the minimization of the interface to realize the most energetically preferable arrangement of molecules in a liquid drop. While the plasma membrane does have an inherent surface tension, the forces we discuss in this Review are primarily, although not exclusively, the result of forces generated by an actomyosin network in the cell cortex underlying the plasma membrane. We refer to this form of ‘effective surface tension' as cortical tension. This is also consistent with work showing that the contribution of membrane tension to the effective surface tension is often negligible (Fischer-Friedrich et al., 2014). Cortical tension can be influenced by osmotic pressure, cell-cell adhesion, and myosin activity (Krens et al., 2017; Maitre et al., 2012; Steinberg, 1963). In the systems that we discuss, relative cortical tension can be measured without determining the source of the stress in the tissue and often without generating an absolute measurement of force. Because cortical tension and surface tension drive similar changes in shape, many of the in silico models discussed here model cortical tension as surface or line tension.
To illustrate the fundamental mechanisms of actomyosin-based contractility, we draw on data from multiple systems but focus on two well-studied model systems that have been instrumental to our understanding of these mechanisms. The first is fission yeast cytokinesis, in which a contractile actomyosin ring assembles at the equator of the yeast cell and constricts, separating the daughter cells. The fission yeast contractile ring is arguably the best-understood model of non-muscle actomyosin-based contractile force generation. The second is ventral furrow formation in the early Drosophila embryo, a system that involves tissue folding and has been studied from the molecular to the tissue level. In this example, actomyosin contractility at the apical surface of epithelial cells causes cells to change from columnar to wedge shaped, which results in a tissue fold or furrow.
Molecular level: the importance of myosin motor function
Our understanding of the role of physical forces in generating organism and tissue form is predicated on our understanding of the molecular mechanisms that convert chemical energy to kinetic energy. There are many mechanisms for biological systems to generate force, including more than one mechanism to generate contractile force (Vale and Milligan, 2000). Thus, the mechanism of contractility in each morphogenetic system must be tested individually. Actin networks are regulated by proteins that control the rate of polymerization and depolymerization of individual actin filaments (F-actin). In addition, a host of other proteins control actin network architecture by crosslinking F-actin together, by bundling actin filaments into cables, and by regulating the stability or formation of dendritic branches that are formed by the Arp2/3 complex (Pollard, 2007). Myosin (non-muscle myosin 2 in this case) is primarily regulated through phosphorylation of the myosin regulatory light chain, which controls motor activity and the formation of bipolar filaments important for contractile function (Heissler and Sellers, 2016). Note that some myosin 2 proteins, such as that in fission yeast, do not form a typical minifilament, but form other types of oligomers (Laplante et al., 2016). Regulation of cell contractility through transcriptional regulation of myosin and actin regulators has also been observed (Calvo et al., 2013; Pollard, 2007). Mechanisms of actomyosin-based contraction can be roughly classified as dependent on, or independent of, myosin 2 motor activity. Evidence suggests that both modes of contractility exist in cells (Ma et al., 2012; Vicente-Manzanares et al., 2007). Thus, one or both modes could be important for tissue-level forces.
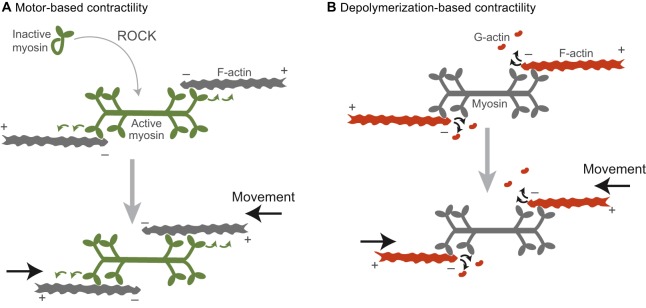
The classical model of contractile force generation has myosin 2 functioning as a motor, converting energy from ATP hydrolysis into directed motion and cytoskeletal network contraction. This contractile model relies on active myosin 2 forming higher-order structures, or oligomers (Fenix et al., 2016; Laplante et al., 2016). The most common type of oligomer is a bipolar myosin filament, the formation of which is regulated by phosphorylation of the myosin 2 regulatory light chain by various kinases (e.g. Rho kinase, ROCK and Citron kinase) (Fig. 1A) (Amano et al., 1996; Heissler and Sellers, 2014; Yamashiro et al., 2003). In bipolar myosin filaments, motor heads at both ends of the myosin filament are able to interact with and walk along distinct actin filaments (F-actin) towards the barbed or plus end (Fig. 1A, green arrows). In striated muscle, a stereotyped version of this interaction slides antiparallel F-actin networks together (Huxley and Hanson, 1954). One prediction of this model is that the speed of contraction is correlated with the ATPase activity of the motor (and more specifically ADP release), which has been observed experimentally for both muscle contraction rates and in in vitro motility assays (Barany, 1967; Yengo et al., 2012). This prediction is important for distinguishing between the motor-dependent and motor-independent models of contractility.
Fig. 1.
Two models by which networks of actin and myosin can generate contractile forces. (A) Myosin is activated by ROCK and polymerizes into a bipolar filament (green). Contractility (black arrows) is generated by the motor activity of myosin as it walks along antiparallel actin filaments (green arrows). (B) Contractility (black arrows) is driven by F-actin depolymerization into G-actin. In this case, myosin would act as one of potentially many crosslinkers between neighboring filaments. The plus (barbed) and minus (pointed) ends of the F-actin filaments are denoted. Note that here, for illustrative purposes, we denote actin subunits depolymerizing from the filament end, but in reality proteins that mediate depolymerization (i.e. cofilin) cause filament severing (Andrianantoandro and Pollard, 2006). Colors indicate the force generator in each model.
Alternatively, myosin 2 and other proteins can function as crosslinkers, allowing depolymerization of actin filaments to drive contraction (Fig. 1B) (Sun et al., 2010). This type of myosin motor-independent contraction has been shown to operate in ring closure for cytokinetic events in several organisms (Ma et al., 2012; Mendes Pinto et al., 2012; Xue and Sokac, 2016). In the early Drosophila embryo, there is a mass ring closure event during which all cells are separated from a central yolk compartment. Interestingly, this process occurs in two stages, one of which depends on myosin motor activity, and another that is myosin independent and depends on actin depolymerization (Xue and Sokac, 2016). This suggests that these two mechanisms could be used individually or in combination to effect contraction.
It is difficult to determine whether contractile events in vivo depend on the motor function of myosin 2, as this is difficult to distinguish from a dependence on its crosslinking function. Common methods to inhibit myosin 2, such as Rho kinase (ROCK) inhibition, disrupt both motor and crosslinking functions because they prevent phosphorylation and thus the conformational changes that are required for bipolar filament assembly (Craig et al., 1983). Another myosin inhibitor, blebbistatin, locks the actin-binding domain of myosin 2 in a weak actin-binding state, which has similar affinity for actin as unphosphorylated myosin even though the myosin is filamentous (Kovács et al., 2004; Ramamurthy et al., 2004). Therefore, testing for myosin motor activity requires mutants that only disrupt this activity. For example, mutants of the myosin heavy chain that are known to disrupt motor activity have been used for this purpose (Ma et al., 2012; Vicente-Manzanares et al., 2007). In addition, some mutations in the Drosophila myosin regulatory light chain decrease motor activity without overtly affecting bipolar filament formation (Vasquez et al., 2016).
Cell level: the importance of actomyosin network organization
In striated muscle, contraction relies on a stereotypic antiparallel organization of F-actin arrays within a sarcomere, with bipolar myosin filaments in the center (Fig. 1A). However, models of contraction with purified components demonstrate that unstructured networks can also contract, and this is thought to correspond to the situation in the cortex of smooth muscle and non-muscle cells (Koenderink et al., 2009; Murrell and Gardel, 2012; Soares e Silva et al., 2011). Here we discuss findings that demonstrate the importance of network organization for contracting cells, even in what were previously considered unstructured networks.
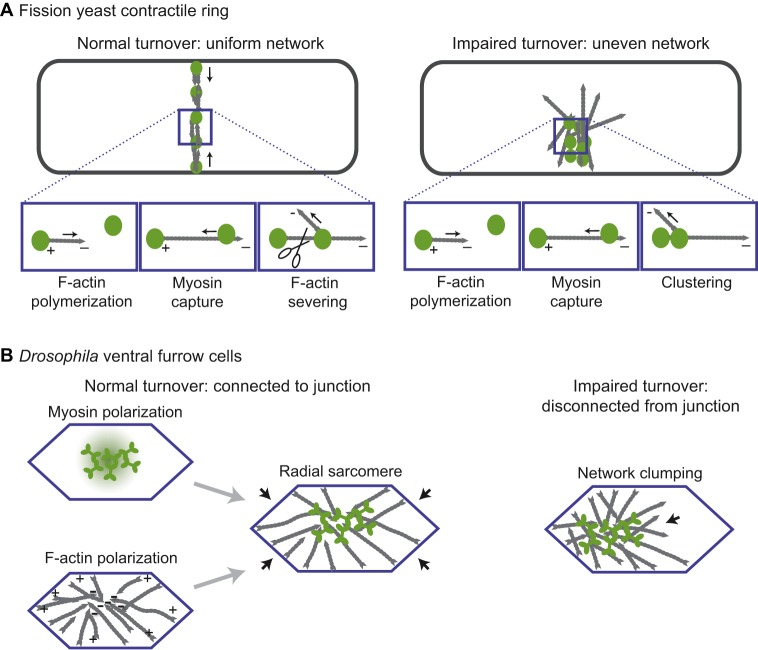
The reconstitution of contractility with purified components and defined F-actin architectures has shown that maximizing the antiparallel organization of actin networks enhances myosin-based constriction velocity (Reymann et al., 2012). This suggests that antiparallel F-actin networks are more efficient at generating contractile forces. Further supporting the importance of actin network organization in producing contractile force, modeling of fission yeast cytokinesis reveals that actomyosin rings in which myosin is oriented nearer to F-actin minus ends – a more sarcomere-like organization – are able to generate higher tension (Fig. 2A) (Stachowiak et al., 2014). Although the organization of the actomyosin network is important for contractile force generation in these in vitro and in silico experiments, it is often unclear whether such organization exists and how it is generated in non-muscle tissues.
Fig. 2.
Actomyosin network organization in the fission yeast contractile ring and in Drosophila ventral furrow cells. (A) (Left) Organization of a normal contractile ring in fission yeast. The panels beneath show actin polymerizing from a node structure that contains myosin and an F-actin elongating formin, capturing a second myosin node, and being severed to maintain the correct density of actin. F-actin is in gray and myosin oligomers are in green. There is not a net polarity to this network, but within the network myosin captures polymerizing F-actin near the minus end and pulls on it. (Right) A contractile ring with actin turnover inhibited. Myosin nodes aggregate because they are never detached from each other. (B) (Left) The actomyosin network in a normal Drosophila ventral furrow cell is organized in a manner that resembles a sarcomere, but is radially arranged. The myosin (green) is activated at the center of the apical cell surface. Actin organization is depicted in gray. The organization of each component is depicted separately and together. (Right) The actin network in a Drosophila ventral furrow cell that has impaired turnover. The network aggregates and separates from junctions (blue) on one side of the cell (arrow).
Epithelia have properties that might enable organized actomyosin networks to form at a cellular level, as has been found to be the case in the Drosophila embryo. Adherens junctions around the apical circumference of epithelial cells serve as platforms in which F-actin is both assembled and anchored (Michael and Yap, 2013). In ventral furrow cells of the Drosophila embryo, F-actin plus ends are enriched at the adherens junctions at the boundaries of the apical surface and F-actin minus ends are enriched in the apical center (Fig. 2B) (Coravos and Martin, 2016). In many contracting epithelial cells, including salivary gland cells, amnioserosa and follicle cells, active RhoA, ROCK and myosin have been found to accumulate at the center of the apical or basal cortex (referred to as medial myosin accumulation) (Chung et al., 2017; Coravos and Martin, 2016; Mason et al., 2013, 2016; Qin et al., 2017). Thus, ventral furrow cells, and possibly these other cell types, have a cytoskeletal organization that resembles a muscle sarcomere, except that it is radially arranged. Disrupting this organized structure by activating myosin across the entire apical surface inhibits apical constriction, suggesting that this network organization is crucial for contraction (Coravos and Martin, 2016). Consistent with a sarcomere-like (i.e. motor-dependent) mode of constriction in ventral furrow cells, mutants in the myosin regulatory light chain that decrease myosin motor/ATPase activity result in a proportionate decrease in apical constriction and tissue folding rate (Vasquez et al., 2016). Thus, in some cases, non-muscle cells require spatially organized myosin motor activity to contract cells and tissues. Future research is needed to determine whether mammalian non-muscle cell types also have this type of cytoskeletal organization or if this is an adaptation to support the rapid embryonic development of insects or other invertebrates.
Transmitting force between cells: the importance of actin network turnover
The creation of many biological forms requires the propagation of forces across tissues. This is seen in the morphogenesis of the Drosophila pupal wing, the folding of the neural tube, and the formation of the Drosophila ventral furrow. In the pupal wing, the tissue must be anchored on the distal end to the extracellular matrix (ECM) and on the other to the wing hinge, which contracts, for the epithelium to expand correctly (Aigouy et al., 2010). This suggests that forces are propagated across the full length of the wing as hinge contraction stretches the wing into shape (Etournay et al., 2015; Ray et al., 2015). Recent work on mammalian neural tube closure has also shown that forces are propagated across the neuroepithelium as it folds (Galea et al., 2017). This propagation involves supracellular actomyosin cables, which in vertebrate gastrulation form across the cell surface and in neurulation at cell-cell interfaces. These supracellular cables are linked through intercellular adhesions to form a larger network (Galea et al., 2017; Pfister et al., 2016). The folding of the neural tube is a particularly important example given that defects in neural tube closure result in a serious and relatively common birth defect, spina bifida (Wallingford et al., 2013). We speculate that the following discussion will be relevant to understanding the causes of spina bifida.
For tissues to transmit forces, the individual cells that make up the tissue must be mechanically linked. In epithelial tissues, this often occurs at adherens junctions, which contain the self-binding adhesion receptor E-cadherin (Lecuit and Yap, 2015). However, cells in some tissue types can be connected through an integrin-ECM-integrin attachment (e.g. a myotendinous junction) (Goody et al., 2015). In order to transmit force and maintain tissue integrity, the junctional proteins also need to be robustly coupled to the actomyosin cortex as the cortex constricts and/or remodels (Roh-Johnson et al., 2012). In the case of myosin motor-driven contraction, this attachment is thought to depend on actin-adhesion receptor interactions through adaptor proteins such as α-catenin and β-catenin (Ladoux et al., 2015). In addition, other F-actin-binding proteins, such as vinculin and afadin, can be recruited to intercellular junctions and are important for junction functionality (Choi et al., 2016; Huveneers et al., 2012). Interestingly, in less complex metazoans such as the sea anemone, the interaction between α-catenin and F-actin is constitutive (Clarke et al., 2016). However, in mammals, strong binding between α-catenin and F-actin depends on applied force, suggesting a catch-bond-like behavior (Buckley et al., 2014). Interestingly, actin binding by vinculin responds asymmetrically to applied force (i.e. force towards the F-actin minus end results in a maximally stable bond) (Huang et al., 2017). Such an asymmetry could result in a long-range polarity in the actin cytoskeletal network. Intriguingly, this asymmetry could help drive the sarcomere-like configuration of the actin network observed in ventral furrow cells (Fig. 2B). The additional layer of feedback in the mammalian system impacts the ability of cells to transmit forces and should be taken into account when analyzing these systems.
One lesson about the mechanism of intercellular force transmission comes, paradoxically, from studies of unicellular fission yeast. This concerns the importance of actin turnover (i.e. actin filament assembly and disassembly) in mediating the coupling between the actomyosin cortex and the adherens junction. Models of contractile ring formation in fission yeast have shown that actin disassembly and remodeling are required to generate a uniform contractile ring network. Actin turnover counterbalances the clustering/aggregation of the network that occurs when it is contracted by the myosin motor (Fig. 2A) (Stachowiak et al., 2014; Vavylonis et al., 2008). Disrupting actin turnover causes actomyosin to aggregate unevenly, not only in the contractile ring but also in contractile ventral furrow cells (Fig. 2A,B) (Chen and Pollard, 2011; Jodoin et al., 2015). Continuous actin turnover during contraction explains why the density of the actin network is constant as the network contracts in both the fission yeast contractile ring and cells of the Drosophila ventral furrow (Mason et al., 2013; Wu and Pollard, 2005). In addition, early work on sea urchin cytokinesis has demonstrated that the volume of the contractile ring decreases with constriction, suggesting that ring components are disassembled during contraction (Schroeder, 1972). In the ventral furrow, F-actin disassembly and the renewal of the apical actin meshwork are important for the stable coupling of that apical network to the adherens junctions; in the absence of robust actin turnover, actomyosin aggregates and separates from the junction (Fig. 2B) (Jodoin et al., 2015).
Coming back to neural tube closure, we hypothesize that this mechanism of propagating forces across a tissue by actin turnover could possibly explain the role of cofilin, an actin depolymerase, in neural tube defects. Single-nucleotide polymorphisms (SNPs) in the CFL1 gene, which encodes the non-muscle cofilin (cofilin 1), are associated with human spina bifida, although it is not clear whether or how these SNPs impact cofilin function (Zhu et al., 2007). In addition, cofilin mutants result in neural tube defects in mice (Escuin et al., 2015; Gurniak et al., 2005). Disruption of actin turnover also affects the establishment of planar cell polarity, and this role for actin turnover might also play a role in the neural tube defect (Mahaffey et al., 2013). Although it is still debated whether neural tube closure is driven by myosin 2 motor-dependent contractility (Escuin et al., 2015), it is possible that actin turnover contributes to morphogenesis by enabling stable connections of actomyosin networks to intercellular junctions. For future studies of neural tube closure, it will be important to use live imaging to determine whether actin networks aggregate and separate from junctions in cofilin mutants.
Actomyosin contractile systems regulate tissue stiffness
In addition to generating contractility, actomyosin networks contribute to the material properties of cells and tissues, which impacts how tissues respond to contractile forces during development. The details of actin network mechanics have been reviewed elsewhere (Gardel et al., 2008). Briefly, the elastic properties of actin networks depend on the actin and crosslinker concentration and on the amount of network stress, all of which are tightly regulated in the cell (Gardel et al., 2004; Xu et al., 2000). In actomyosin networks reconstituted from purified proteins, the effect of myosin activity on the properties of the actin network can vary. In the case of high ATP levels, myosin can fluidize the network; however, myosin can stiffen the network at low ATP levels, where the myosin motor heads will remain bound to F-actin for longer (Gardel et al., 2008).
Actomyosin contractility is also important for regulating tissue stiffness in developing organisms. This was observed in the context of axis elongation in the Xenopus embryo, where tissue stiffness was decreased when ROCK was inhibited (Zhou et al., 2009). The stiffness may be important for development as it increases over the course of axis elongation. One role for the increase in stiffness might be to allow the elongating tissue to push against neighboring tissues. In tissue explants of the Xenopus neural plate, actomyosin contractility was required for elongation when the tissue was cultured in agarose, which provides resistance to elongation, but not when the explant was cultured in liquid medium (Zhou et al., 2015). Additionally, during elongation of the C. elegans embryo, the actomyosin cortex plays a role in maintaining the anisotropic stiffness that is thought to drive tissue elongation (Vuong-Brender et al., 2017). In this sense, regulation of myosin activity and the organization of the actin network can affect morphogenesis without necessarily generating tissue contraction. The implication of this dual role for actomyosin networks in generating contractility and also regulating the mechanical properties of the same or neighboring tissues is that inhibiting myosin and F-actin may simultaneously block contractility and change the mechanical properties of the surrounding tissue, making it difficult to interpret resulting mutant phenotypes.
Relaxation of cortical tension through the negative regulation of myosin also plays an important role in certain morphogenetic processes, including the expansion of the ventricle lumen in the zebrafish hindbrain and processes of tissue elongation that include zebrafish epiboly and Drosophila dorsal closure. The expansion or inflation of the zebrafish hindbrain requires the epithelium surrounding the ventricle lumen to relax through the activity of myosin phosphatase, an inhibitor of myosin activation (Gutzman and Sive, 2010). Mutation of myosin phosphatase causes an abnormally small ventricle, presumably because the surrounding epithelium has high cortical tension or stiffness and cannot deform. Recently, it was shown that an Arf guanine nucleotide exchange factor, cytohesin, is required to downregulate actomyosin contractility to enable tissue elongation in both zebrafish and Drosophila (West et al., 2017). Thus, the regulation of stiffness through modulation of actomyosin contractility appears to be a strategy by which organisms restrict or permit tissue deformations.
Tissue-level organization of contractility and form
We next discuss how different tissue-level spatial organizations of cell contractility create different forms. In the spirit of On Growth and Form, we will focus on systems for which there is a significant understanding of physical forces driving the processes described. In many of the cases that we have chosen, our understanding of the process has been enhanced with the help of minimal mechanical models. Thompson described several phenomena in terms of surface energy or surface tension. Although surface tension and energy minimization in the manner of soap bubbles is not consistent with our current understanding of the energy-consuming mechanisms governing cell shape, energy models, such as vertex models, are often useful for modeling morphogenetic systems (Farhadifar et al., 2007; Fletcher et al., 2014). Furthermore, the investigation of how contractility drives morphogenesis is often accompanied by measurements of cortical tension. However, it should be noted that, in addition to actomyosin-based contractility, tissue surface tension (Graner et al., 2017) is influenced by cell-cell adhesion and cell osmotic pressure (Krens et al., 2017; Maître et al., 2012; Steinberg, 1963).
The case studies we have chosen focus on how different spatial organizations of contractility and cortical tension drive different morphogenetic processes. We will start with relatively simple examples in which individual cells have elevated levels of cortical tension with respect to their neighbors. From there, we then discuss how the number and arrangement of tension-generating cells sculpt tissues in processes ranging from tissue folding to elongation. In general, these systems all use a conserved force-generating module, the actomyosin cytoskeleton. In many cases, the exact nature and organization of the actomyosin cytoskeleton has yet to be established. However, the arrangement of contracting cells influences where forces are balanced, which ultimately affects the resulting form.
Differential tension driving epithelial cell ingression
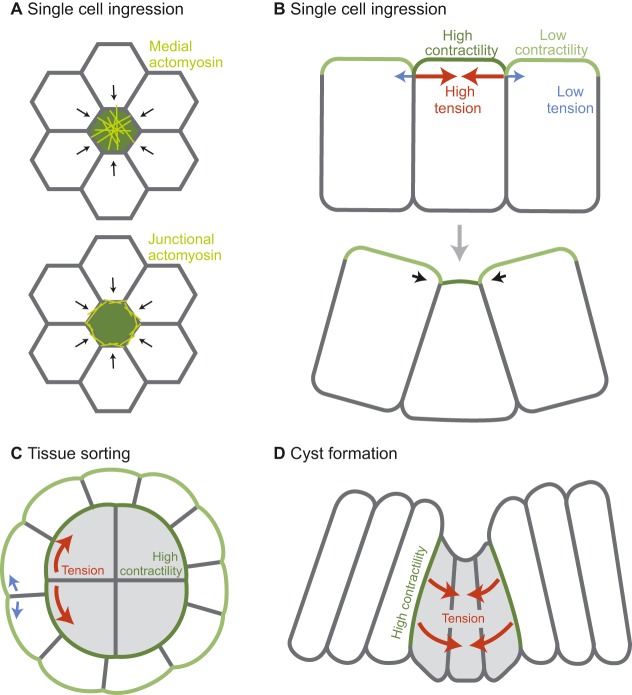
Cell ingression is the process by which single or multiple cells leave an epithelial layer. Cell ingression from an epithelium is important for regulating growth and homeostasis and is also important for tissue architecture. In numerous examples, from C. elegans to the mouse, it has been shown that cell ingression is associated with elevated contractility and cortical tension in the ingressing cell. The tension differential between ingressing and neighboring cells during the ingression processes is often dependent on actomyosin accumulation in the ingressing cell or at the boundary between the ingressing and non-ingressing cells (Fig. 3A,B).
Fig. 3.
Differential contractility can cause ingression, cell sorting, and morphological changes. (A) An en face view of an epithelial cell experiencing higher cortical tension levels than neighboring cells, illustrating the difference between medial localization (top) and junctional localization (bottom) of the actomyosin network (yellow). (B) Apical-basal transverse section of a cell before (top) and during (bottom) ingression. Relative cortical tension is represented in green, with darker green indicating higher contractility. (C) Sorting between two cell types: white cells (lower contractility) and gray cells (higher contractility). (D) Cyst formation due to high tension at clone boundaries and the resulting inward pressure. (A-D) Red arrows denote the direction of high tension force and blue arrows denote direction of low tension force. Black arrows denote movement.
In early development, cell ingression is important to physically separate cells with different fates. During C. elegans gastrulation, two of the cells fated to be endoderm ingress from the surface layer (Lee and Goldstein, 2003). In mouse, the cells that will form the fetus come from the inner cell mass, a collection of cells that are internalized early in development (Johnson and Ziomek, 1981). Cell ingression has recently been shown to drive internalization for those cells that are not internalized by asymmetric cell division (Maître et al., 2016; Samarage et al., 2015). In both the C. elegans and mouse examples, cells gradually reduce their surface area (in both cases an apical surface) until they are completely enveloped by the remaining cells of the embryo. Laser ablations have shown that internalizing cells have higher cortical tension at the apical surface than do the neighboring cells, and that the higher levels of tension depend on actomyosin contractility (Roh-Johnson et al., 2012; Samarage et al., 2015). The imbalance in cortical tension between neighboring cells is likely to be crucial for driving ingression. For instance, prior to inner cell mass ingression in the mouse embryo there is a universal increase in cortical tension at the embryo surface, which does not result in ingression but rather compaction of the embryo, such that cells become tightly pressed together (Maître et al., 2015). Compaction also depends on the extension of filopodia from some of the cells onto neighboring cells (Fierro-González et al., 2013). These filopodia may also be required to generate cortical tension (Fierro-González et al., 2013).
It is interesting to note that actomyosin exhibits different organizations in these different systems. In C. elegans, an apical actomyosin cortex contracts centripetally to generate tension and reduce apical surface area (Roh-Johnson et al., 2012). By contrast, ingressing inner cell mass cells have a prominent actomyosin belt at intercellular junctions that appears to drive ingression (Samarage et al., 2015). During compaction, the surface actomyosin cortex undergoes pulsatile or wave-like contractions (Maître et al., 2015). When Drosophila neuroblasts delaminate from the surface ectoderm, another example of cell ingression, both junctional actomyosin accumulation and pulses of apical myosin accompany ingression (An et al., 2017; Simões et al., 2017). Disruption of the myosin pulsing disrupted invagination, although myosin depletion experiments have not indicated an essential role for myosin and it is possible that other processes are also crucial for neuroblast ingression (An et al., 2017; Simões et al., 2017). Despite the different spatiotemporal organizations of myosin in these divergent systems, overall it appears that elevated cortical tension in and around a single cell can drive cell ingression.
In general, the model that cell ingression is driven by differential regulation of cortical tension through actomyosin contractility and thus, force imbalance, seems to hold for a variety of systems. Apoptotic cells are extruded from epithelia in a system that parallels cell ingression. This process also depends on increased cortical tension and actomyosin contractility, but in both the apoptotic cell and neighboring cells (Kuipers et al., 2014; Rosenblatt et al., 2001; Slattum et al., 2009; Toyama et al., 2008). It seems clear that high cortical tension is driven by actomyosin activity, but it is also clear that there are a diversity of ways in which actomyosin can produce force (i.e. medial versus junctional and autonomous versus non-autonomous). In addition, other mechanisms, such as changes in basolateral contractility, could also drive ingression (Jodoin and Martin, 2016; Wu et al., 2014). An interesting future avenue of research is to address why apical constriction sometimes results in ingression and other times does not.
Tension between different populations of cells
Cell types can exhibit intrinsic differences in the levels of cortical tension, and it has been shown that cortical tension can be regulated at the boundaries of different cell types (Bielmeier et al., 2016). As we discussed previously, cortical tension is also often differentially regulated at cell-medium (often apical) versus cell-cell (often basolateral) interfaces. These imbalances can lead to cell sorting behavior (Fig. 3C), as well as to changes to tissue shape (Fig. 3D) (Harris, 1976; Krieg et al., 2008).
Cultured aggregates of zebrafish endoderm, mesoderm and ectoderm cells sort into clusters of distinct cell types due to difference in cortical tension between cell types (Krieg et al., 2008). The cells with the higher cortical tension will cluster and the cell type with lower tension will envelop them (Fig. 3C). This event depends on myosin activity as well as low osmotic pressure to create the difference in cortical tension between groups of cells (Krens et al., 2017; Krieg et al., 2008). This cell sorting can be explained using a cellular Potts model, which predicts that correct cell sorting depends not only on a difference in cortical tension between the two cell populations, but also on a difference in cortical tension between the cortex at the cell-medium interface and the cortex at the cell-cell interface (Krieg et al., 2008; Maître et al., 2012). There are three levels of cortical tension in this system and both the subcellular restriction of high tension to the tissue surface and the tension differential between the two cell types are required for generating a multilayered structure with high-tension cells at the interior.
Another example of cell sorting is the formation of cysts in epithelial tissues (Fig. 3D). It has been demonstrated that cancer cells are able to form cysts that separate them from non-cancerous cells (Cortina et al., 2007). In addition, cysts containing cells of one cell fate have been shown to separate from background cells of a different fate. An example of this has been observed in the Drosophila wing imaginal disc; cysts of wild-type cells develop in the wing epithelium where the majority of cells are misexpressing a cell fate-specifying transcription factor (Bielmeier et al., 2016). The formation of these cysts is yet another example of a mechanism whereby contraction drives tissue shape change. Clones of cells that differentially express a gene regulating cell fate accumulate myosin and F-actin along basolateral cell surfaces contacting the neighboring wild-type tissue, suggesting activation of the actomyosin contractile cortex (Bielmeier et al., 2016). No tension measurements were made in this system, but an in silico vertex model that assumed increased cortical tension at the contact boundaries between different cell types was able to recapitulate bending of the clone into a cyst-like bulge.
Both of the previous examples involve increased cortical tension at the boundaries of the two cell types, but different shapes emerge. The commonality is that differential cortical tension at an interface results in physical separation of cell types. In each of the systems discussed, the cells are able to respond to increases in tension without clear directional constraints. We next discuss the role of tissue stiffness and resistance in modifying the effects of contractility on tissue shape.
Resistance: the interplay of tension and stiffness
There are a number of examples of morphogenesis in which an initially isotropic cortical contractility at the cell level results in anisotropic forces and movements at the tissue level. In each of the cases that we discuss below, cell or tissue movement is blocked or force is balanced along one axis, but unbalanced along the other axis, resulting in an anisotropic shape change. This suggests that the organization of contractile forces can be shaped by overall tissue architecture as well as by actomyosin networks or other structural proteins, resulting in anisotropic tissue movement.
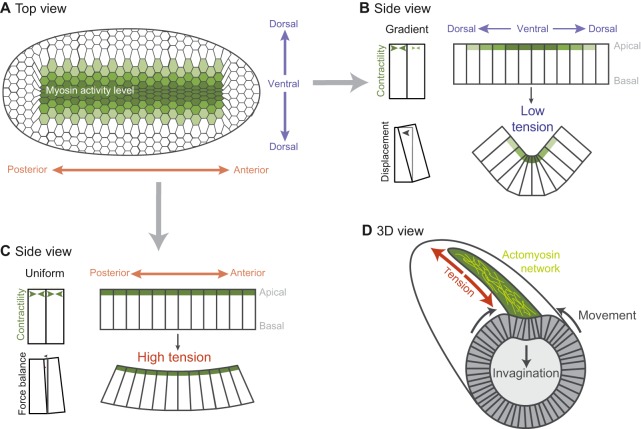
In addition to driving cell shape change, actomyosin-based cortical tension, when balanced, can resist deformation from neighboring cells. By doing so, patterned contractility can provide directionality to tissue curvature. During the formation of the Drosophila ventral furrow, the ellipsoid shape of the embryo and the differing distribution of myosin activation along the anterior-posterior versus ventral-dorsal axes establishes an inherent asymmetry that orients tissue curvature along the ventral-dorsal axis, forming a long, narrow furrow (Fig. 4A). In this system, cells initially have a radially organized cytoskeleton and, in the absence of surrounding forces, contract isotropically (Fig. 2B) (Chanet et al., 2017; Coravos and Martin, 2016; Martin et al., 2010). However, the domain of cells expressing the transcription factors that promote contractility is rectangular (∼70 cells long and 18 cells wide), and there is a ventral-dorsal gradient in the transcription of genes that promote contractility (Fig. 4B) (Heer et al., 2017; Lim et al., 2017). A consequence of this gradient being along the dorsal-ventral axis is that there is essentially uniform contractility and force balance along the anterior-posterior axis (Fig. 4C). The balanced contractile forces between neighboring cells prevents robust apical constriction along the anterior-posterior axis and leads to tension along that axis (Martin et al., 2010; Sweeton et al., 1991). By contrast, the gradient and resulting imbalance in contractility along the ventral-dorsal axis provides less resistance to cell constriction. This allows cells to mostly constrict along the ventral-dorsal axis and prevents the buildup of cortical tension along this axis (Chanet et al., 2017; Heer et al., 2017). The result is the formation of the wedge-shaped cells that fold the tissue along the ventral-dorsal axis (Fig. 4B versus C). The formation of wedge-shaped cells in the Drosophila ventral furrow is also regulated by basolateral cortical tension that initially resists tissue deformation. Basal expansion of the ventral cells is associated with a decrease in basal myosin levels, and vertex modeling predicts that this is important for the formation of wedge-shaped cells (Polyakov et al., 2014). Thus, the organization of the region of active contractility and actomyosin-based resistance from neighboring contractile cells, in combination with resistance within the cell, affects both individual cell shape changes as well as overall tissue architecture.
Fig. 4.
A spatial pattern of contractility results in Drosophila ventral furrow formation. (A) Ventral surface of the embryo. The distribution and magnitude of myosin activity is represented in green (darker green represents more myosin activity). (B) An apical contractility gradient causes a force imbalance that allows cells to constrict and the tissue to bend along the dorsal-ventral axis. (C) Uniform apical contractility along the anterior-posterior axis promotes balanced forces and tension, which prevents tissue bending along this axis. (D) An embryo in the process of folding, with the orientation of actomyosin fibers illustrated in yellow.
In addition to the pre-pattern in gene expression (Heer et al., 2017; Lim et al., 2017), mechanical feedback influences cytoskeletal organization and, presumably, force generation (Chanet et al., 2017). In both experiment and theory, actomyosin meshworks orient along the axis that is most resistant to constriction, possibly allowing greater tension to accumulate along that axis (Fig. 4D, yellow fibers). A remarkable illustration of this feedback loop and of the integrated nature of cell force generation and organism shape is that converting Drosophila embryos from an ellipsoid to a round shape disrupts the organization of the actomyosin meshworks in cells and the anisotropic tension in the tissue (Chanet et al., 2017). Although it is still necessary to find a way to specifically perturb this feedback loop, it is possible that this mechanism is important to reinforce cues provided by the pre-pattern in gene expression.
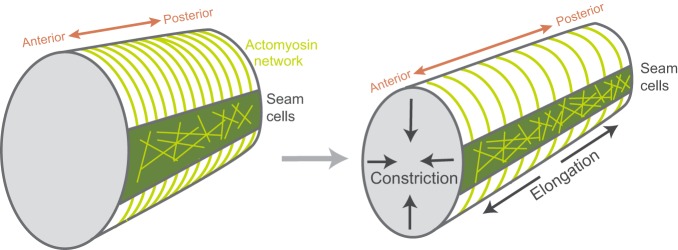
Like tissue folding in Drosophila, body extension in C. elegans involves anisotropic stress resulting in a directional deformation. Worms, such as C. elegans, are longer than they are wide, but they do not start that way. During C. elegans development, the cells of the epidermis initiate axis elongation, elongating the worm's body twofold. In this process, contractility is localized to cells, called seam cells, that run the length of the embryo (Gally et al., 2009) (Fig. 5). The neighboring ventral and dorsal epidermal cells have circumferentially oriented F-actin cables, but actomyosin contractility is suppressed in these cells (Diogon et al., 2007). The seam cells do not have a polarized actomyosin structure and yet they drive elongation along a single axis of the embryo. This is the result of inherent stress asymmetry due to the ellipsoidal shape of the embryo head, as well as anisotropic stiffness in neighboring cells (Vuong-Brender et al., 2017) that results from parallel bundles of actin cables that are oriented around the embryo circumference in cells neighboring the seam cells (Fig. 5) (Priess and Hirsh, 1986; Vuong-Brender et al., 2017). Contractility in the seam cells coupled with the stiff circumferential actin cables results in a circumferential squeeze. This causes cells and the embryo to extend along their anterior-posterior axis (Fig. 5). Thus, the circumferential actin cables function like a stiff corset to resist circumferential expansion and limit expansion to the anterior posterior axis. In sum, the forces that shape C. elegans are generated by contractility, but the directionality of tissue movements and the resulting organism shape are the result of asymmetric constraints in the embryo and anisotropic stiffness in the tissue. A similar corset-like mechanism also constrains growth of the Drosophila oocyte, although there is no contractile squeeze in this system. However, in the case of Drosophila, there is circumferential deposition of basal ECM fibers that constrains oocyte growth to the anterior-posterior axis (Haigo and Bilder, 2011).
Fig. 5.
Body axis elongation in the C. elegans embryo. Contractile seam cells are depicted in green with a non-polarized actomyosin network (yellow). The circumferential actin cables outside of the seam cells serve as a corset to restrict embryo lengthening to the anterior-posterior axis, and the radius of the animal shrinks.
Balance versus imbalance at intercellular junctions
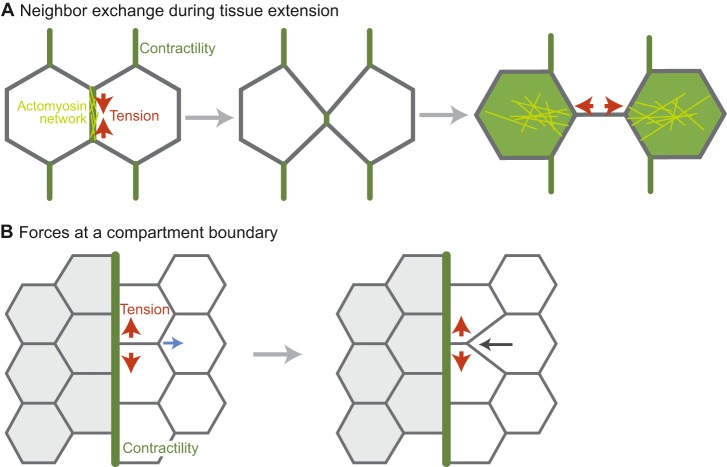
Whether contractile forces are organized such that they are balanced or not at junctions is also important for tissue shape and organization. During tissue extension, actomyosin cables can be enriched along specific intercellular junctions due to planar cell polarity (Bertet et al., 2004; Shindo and Wallingford, 2014; Zallen and Wieschaus, 2004). Actomyosin enrichment at junctions in these cases is associated with tension and junctional shrinkage, suggesting that there is unbalanced contractile force (Fig. 6A) (Fernandez-Gonzalez et al., 2009; Rauzi et al., 2008). This anisotropic tension operates in tandem with polarized basal cell migration to promote axis extension (Sun et al., 2017). Medial, not junctional, actomyosin contractility appears to elongate new cell contacts after the old contact has disappeared (Fig. 6A) (Collinet et al., 2015; Yu and Fernandez-Gonzalez, 2016). The consequence of polarized actomyosin contractility and cell crawling is that cells converge along the dorsal-ventral axis, resulting in an anterior-posterior extension.
Fig. 6.
Polarized junctional actomyosin contractility during tissue extension and at compartment boundaries. (A) Neighbor exchange during tissue extension. Actomyosin is planar polarized to the vertical junction. This actomyosin network leads to apical junction shrinkage either through contraction or by directionally stabilizing fluctuations in junction length (Rauzi et al., 2010). The junction is then expanded in the horizontal direction by medial actomyosin contractility (in the green cells). (B) The forces at a compartment boundary that inhibit cell rearrangement. When a rearrangement occurs near the boundary, the boundary resists deformation. For example, the horizontal junction shrinks from one end so that the boundary remains straight. Regions of contractility are depicted in green, with network organization in yellow. Red arrows denote the direction of contractile tension. The blue arrow denotes the direction of low tension and the black arrow denotes the direction of movement as a result of the low tension.
Alternatively, balanced forces along junctional interfaces have been shown to resist cell movement and mixing. In both development and homeostasis, different cell types are segregated from each other in what are known as compartments (Batlle and Wilkinson, 2012). Recent studies have shown that actomyosin contractility is often activated at cell interfaces of the compartment boundary (Landsberg et al., 2009; Major and Irvine, 2006; Monier et al., 2010), in a similar manner to the process described for cyst formation in the Drosophila wing (Bielmeier et al., 2016). In this case, balanced myosin contractility, and the resulting high tension, maintains a straight compartment boundary and prevents cell mixing between compartments (Fig. 6B). Activation of myosin has also been shown to inhibit cell mixing and maintain boundaries between different cell types in Xenopus (Fagotto et al., 2013). In Drosophila epithelia, high tension at boundaries resists the local deformations that result from cell division, as well as acting to bias cell intercalation so as to prevent cell movement across the boundary (Fig. 6B) and the mixing of cells of different fates (Monier et al., 2010; Umetsu et al., 2014). Thus, similar to examples of apical actomyosin cortex contraction, unbalanced junctional tension drives tissue shape change whereas balanced forces resist movement.
In conclusion, these examples illustrate the variety of ways in which different patterns of contractility and resulting tensions can sculpt the myriad of animal tissue shapes observed in nature. They also illustrate the importance of considering tissue context and force balance when thinking about how local forces drive a morphogenetic event. While we have focused on systems where there is knowledge of ‘active’ molecular mechanisms that underlie the resulting forces and/or material properties, there are many other systems yet to be characterized in this context, the analysis of which is likely to unearth new principles by which cells and tissues regulate their growth and form.
Perspectives: the next 100 years
Looking forward, there are still many open questions about how forces are generated and organized to form organs and organisms. One important avenue that we have highlighted is the use of quantitative models to test hypotheses of how mechanical forces change tissue form (Box 2). A key experimental limitation lies in our ability to measure mechanical properties and forces in biological systems. Laser ablation has been widely adopted to determine relative cortical tension, but this approach is a force inference method and does not provide an absolute force measurement (Hutson et al., 2003). Others have used micro-aspiration to measure cortical tension at the surface of organisms (Maître et al., 2015). This is a useful approach, but not all systems are accessible to a pipette tip. Recently, exciting new methods have been developed to measure and apply forces to tissues, such as optical tweezers, magnetic ferrofluid, and oil microdroplets (Bambardekar et al., 2015; Mitrossilis et al., 2017; Serwane et al., 2017; Sugimura et al., 2016). A better understanding of the relationship between forces and movement in cells and tissues will greatly advance our understanding of a wide range of morphogenetic systems.
Box 2. A note on models.
As we seek to understand increasingly complex morphogenetic processes, quantitative models are needed because our intuition does not adequately predict outcomes of complex phenomena. In this case, quantitative models are crucial for creating, validating and rejecting hypotheses. At the molecular level, agent-based models have been useful for understanding the collective behavior of cytoskeletal components (i.e. filaments and motors) and how they generate force (Borau et al., 2012; Chanet et al., 2017; Nedelec and Foethke, 2007). Modeling at larger length scales has also been used to understand cell-cell interactions (cell-based modeling) (Farhadifar et al., 2007; Krieg et al., 2008; Rauzi et al., 2008) and tissue morphogenesis (continuum and finite element models) (Conte et al., 2008; Heer et al., 2017; Savin et al., 2011; Vuong-Brender et al., 2017). For a more in-depth discussion of modeling approaches we point readers to Brodland (2015). It should be noted that not all models need be computational and the use of rubber bands and pegs to recapitulate tissue folding (Lewis, 1947) and rubber tubing with an elastic sheet to recapitulate the looping of the developing mouse gut (Savin et al., 2011) are elegant examples of how simple physical models can provide insight into such phenomena. Both computational and physical models are useful checks on the minimal set of requirements that reproduce biological systems and often suggest key experiments that can be used to either prove or disprove a model.
An intriguing, but also exasperating, question is the role of feedback in shaping tissues. Feedback is confounding because it makes it difficult to determine what is cause and what is effect. An important tool for deciphering feedback will be the use of computational models to distinguish between different signaling network organizations (Box 2) (Sharpe, 2017). Owing to excellent reviews of the molecular mechanisms of mechanical feedback and the emergent properties of active matter (Howard et al., 2011; Petridou et al., 2017), we will not discuss the details of the different feedback mechanisms. Suffice it to say, mechanical forces influence a range of cell behaviors, such as cell division, force generation and adhesion. Mechanical models that incorporate this feedback will be necessary for a full understanding of tissue form.
Another exciting area of future research is the role of heterogeneity in morphogenesis. In most multicellular systems, cells exhibit slight differences in behavior, but still robustly alter tissue shape. This aspect of development had often been ignored, but recent advances in quantitative live-cell imaging and the participation of physicists in the field have brought this phenomenon to the forefront. We expect that the next 100 years will bring significant insight into the roles that cell heterogeneity and stochasticity play in morphogenetic processes and a better understanding of the mechanisms that either suppress or enhance heterogeneity.
In summary, while the field of morphogenesis has made great strides towards understanding tissue shape using quantitative analyses, a full characterization of the emergence of tissue form is likely to await the discovery of as yet unappreciated fundamental principles.
Acknowledgements
We thank members of the A.C.M. lab and the J. Dunkel group at MIT for helpful discussion on systems of contractility in morphogenesis as well as feedback on this Review; L. Davidson for generous feedback; and the anonymous reviewers and the editor for their helpful comments.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors are funded by the National Institutes of Health (R01GM105984) and the American Cancer Society (125792-RSG-14-039-01-CSM). N.C.H. was also supported by a National Institutes of Health Pre-Doctoral Training Grant (T32GM007287). Deposited in PMC for release after 12 months.
References
- Aigouy B., Farhadifar R., Staple D. B., Sagner A., Röper J.-C., Jülicher F. and Eaton S. (2010). Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773-786. 10.1016/j.cell.2010.07.042 [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y. and Kaibuchi K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246-20249. 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- An Y., Xue G., Shaobo Y., Mingxi D., Zhou X., Yu W., Ishibashi T., Zhang L. and Yan Y. (2017). Apical constriction is driven by a pulsatile apical myosin network in delaminating Drosophila neuroblasts. Development 144, 2153-2164. 10.1242/dev.150763 [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E. and Pollard T. D. (2006). Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13-23. 10.1016/j.molcel.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Bambardekar K., Clément R., Blanc O., Chardès C. and Lenne P.-F. (2015). Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc. Natl. Acad. Sci. USA 112, 1416-1421. 10.1073/pnas.1418732112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany M. (1967). ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50 Suppl., 197-218. 10.1085/jgp.50.6.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E. and Wilkinson D. G. (2012). Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 4, a008227 10.1101/cshperspect.a008227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C., Sulak L. and Lecuit T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671. 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]
- Bielmeier C., Alt S., Weichselberger V., La Fortezza M., Harz H., Jülicher F., Salbreux G. and Classen A.-K. (2016). Interface contractility between differently fated cells drives cell elimination and cyst formation. Curr. Biol. 26, 563-574. 10.1016/j.cub.2015.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borau C., Kim T., Bidone T., Garcia-Aznar J. M. and Kamm R. D. (2012). Dynamic mechanisms of cell rigidity sensing: insights from a computational model of actomyosin networks. PLoS ONE 7, e49174 10.1371/journal.pone.0049174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodland G. W. (2015). How computational models can help unlock biological systems. Semin. Cell Dev. Biol. 47-48, 62-73. 10.1016/j.semcdb.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Buckley C. D., Tan J., Anderson K. L., Hanein D., Volkmann N., Weis W. I., Nelson W. J. and Dunn A. R. (2014). Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346, 1254211 10.1126/science.1254211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R. P., Chaudhry S. I., Harrington K., Williamson P., Moeendarbary E., Charras G. et al. (2013). Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 15, 637-646. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet S., Miller C. J., Vaishnav E. D., Ermentrout B., Davidson L. A. and Martin A. C. (2017). Actomyosin meshwork mechanosensing enables tissue shape to orient cell force. Nat. Commun. 8, 15014 10.1038/ncomms15014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. and Pollard T. D. (2011). Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol. 195, 485-498. 10.1083/jcb.201103067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Acharya B. R., Peyret G., Fardin M.-A., Mège R.-M., Ladoux B., Yap A. S., Fanning A. S. and Peifer M. (2016). Remodeling the zonula adherens in response to tension and the role of afadin in this response. J. Cell Biol. 213, 243-260. 10.1083/jcb.201506115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Kim S. and Andrew D. J. (2017). Uncoupling apical constriction from tissue invagination. Elife 6, e22235 10.7554/eLife.22235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. N., Miller P. W., Lowe C. J., Weis W. I. and Nelson W. J. (2016). Characterization of the cadherin-catenin complex of the sea anemone nematostella vectensis and implications for the evolution of metazoan cell-cell adhesion. Mol. Biol. Evol. 33, 2016-2029. 10.1093/molbev/msw084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C., Rauzi M., Lenne P.-F. and Lecuit T. (2015). Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol. 17, 1247-1258. 10.1038/ncb3226 [DOI] [PubMed] [Google Scholar]
- Conte V., Munoz J. J. and Miodownik M. (2008). A 3D finite element model of ventral furrow invagination in the Drosophila melanogaster embryo. J. Mech. Behav. Biomed. Mater. 1, 188-198. 10.1016/j.jmbbm.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Coravos J. S. and Martin A. C. (2016). Apical sarcomere-like actomyosin contracts nonmuscle Drosophila epithelial cells. Dev. Cell 39, 346-358. 10.1016/j.devcel.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina C., Palomo-Ponce S., Iglesias M., Fernández-Masip J. L., Vivancos A., Whissell G., Humà M., Peiró N., Gallego L., Jonkheer S. et al. (2007). EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 39, 1376-1383. 10.1038/ng.2007.11 [DOI] [PubMed] [Google Scholar]
- Craig R., Smith R. and Kendrick-Jones J. (1983). Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature 302, 436-439. 10.1038/302436a0 [DOI] [PubMed] [Google Scholar]
- Diogon M., Wissler F., Quintin S., Nagamatsu Y., Sookhareea S., Landmann F., Hutter H., Vitale N. and Labouesse M. (2007). The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development 134, 2469-2479. 10.1242/dev.005074 [DOI] [PubMed] [Google Scholar]
- Escuin S., Vernay B., Savery D., Gurniak C. B., Witke W., Greene N. D. E. and Copp A. J. (2015). Rho-kinase-dependent actin turnover and actomyosin disassembly are necessary for mouse spinal neural tube closure. J. Cell Sci. 128, 2468-2481. 10.1242/jcs.164574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etournay R., Popović M., Merkel M., Nandi A., Blasse C., Aigouy B., Brandl H., Myers G., Salbreux G., Jülicher F. et al. (2015). Interplay of cell dynamics and epithelial tension during morphogenesis of the Drosophila pupal wing. Elife 4, e07090 10.7554/eLife.07090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Rohani N., Touret A.-S. and Li R. (2013). A molecular base for cell sorting at embryonic boundaries: contact inhibition of cadherin adhesion by ephrin/Eph-dependent contractility. Dev. Cell 27, 72-87. 10.1016/j.devcel.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Farhadifar R., Röper J.-C., Aigouy B., Eaton S. and Jülicher F. (2007). The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095-2104. 10.1016/j.cub.2007.11.049 [DOI] [PubMed] [Google Scholar]
- Fenix A. M., Taneja N., Buttler C. A., Lewis J., Van Engelenburg S. B., Ohi R. and Burnette D. T. (2016). Expansion and concatenation of non-muscle myosin IIA filaments drive cellular contractile system formation during interphase and mitosis. Mol. Biol. Cell 27, 1465-1478. 10.1091/mbc.E15-10-0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R., Simoes S. M., Röper J.-C., Eaton S. and Zallen J. A. (2009). Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736-743. 10.1016/j.devcel.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-González J. C., White M. D., Silva J. C. and Plachta N. (2013). Cadherin-dependent filopodia control preimplantation embryo compaction. Nat. Cell Biol. 15, 1424-1433. 10.1038/ncb2875 [DOI] [PubMed] [Google Scholar]
- Fischer-Friedrich E., Hyman A. A., Jülicher F., Müller D. J. and Helenius J. (2014). Quantification of surface tension and internal pressure generated by single mitotic cells. Sci. Rep. 4, 6213 10.1038/srep06213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A. G., Osterfield M., Baker R. E. and Shvartsman S. Y. (2014). Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291-2304. 10.1016/j.bpj.2013.11.4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea G. L., Cho Y.-J., Galea G., Molè M. A., Rolo A., Savery D., Moulding D., Culshaw L. H., Nikolopoulou E., Greene N. D. E. et al. (2017). Biomechanical coupling facilitates spinal neural tube closure in mouse embryos. Proc. Natl. Acad. Sci. USA 114, E5177-E5186. 10.1073/pnas.1700934114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C., Wissler F., Zahreddine H., Quintin S., Landmann F. and Labouesse M. (2009). Myosin II regulation during C. elegans embryonic elongation: LET-502/ROCK, MRCK-1 and PAK-1, three kinases with different roles. Development 136, 3109-3119. 10.1242/dev.039412 [DOI] [PubMed] [Google Scholar]
- Gardel M. L., Shin J. H., MacKintosh F. C., Mahadevan L., Matsudaira P. and Weitz D. A. (2004). Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301-1305. 10.1126/science.1095087 [DOI] [PubMed] [Google Scholar]
- Gardel M. L., Kasza K. E., Brangwynne C. P., Liu J. and Weitz D. A. (2008). Chapter 19: mechanical response of cytoskeletal networks. Methods Cell Biol. 89, 487-519. 10.1016/S0091-679X(08)00619-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody M. F., Sher R. B. and Henry C. A. (2015). Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev. Biol. 401, 75-91. 10.1016/j.ydbio.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner F. and Riveline A. D. (2017). ‘The forms of tissues, or cell-aggregates’: D'Arcy Thompson's influence and its limits. Development 144, 4226-4237. [DOI] [PubMed] [Google Scholar]
- Gurniak C. B., Perlas E. and Witke W. (2005). The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev. Biol. 278, 231-241. 10.1016/j.ydbio.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Gutzman J. H. and Sive H. (2010). Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development 137, 795-804. 10.1242/dev.042705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S. L. and Bilder D. (2011). Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071-1074. 10.1126/science.1199424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K. (1976). Is cell sorting caused by differences in the work of intercellular adhesion? A critique of the Steinberg hypothesis. J. Theor. Biol. 61, 267-285. 10.1016/0022-5193(76)90019-9 [DOI] [PubMed] [Google Scholar]
- Heer N. C., Miller P. W., Chanet S., Stoop N., Dunkel J. and Martin A. C. (2017). Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 144, 1876-1886. 10.1242/dev.146761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler S. M. and Sellers J. R. (2014). Myosin light chains: teaching old dogs new tricks. Bioarchitecture 4, 169-188. 10.1080/19490992.2015.1054092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler S. M. and Sellers J. R. (2016). Various themes of myosin regulation. J. Mol. Biol. 428, 1927-1946. 10.1016/j.jmb.2016.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Grill S. W. and Bois J. S. (2011). Turing's next steps: the mechanochemical basis of morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 392-398. 10.1038/nrm3120 [DOI] [PubMed] [Google Scholar]
- Huang D. L., Bax N. A., Buckley C. D., Weis W. I. and Dunn A. R. (2017). Vinculin forms a directionally asymmetric catch bond with F-actin. Science 357, 703-706. 10.1126/science.aan2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson M. S., Tokutake Y., Chang M. S., Bloor J. W., Venakides S., Kiehart D. P. and Edwards G. S. (2003). Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145-149. 10.1126/science.1079552 [DOI] [PubMed] [Google Scholar]
- Huveneers S., Oldenburg J., Spanjaard E., van der Krogt G., Grigoriev I., Akhmanova A., Rehmann H. and de Rooij J. (2012). Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 196, 641-652. 10.1083/jcb.201108120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. and Hanson J. (1954). Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173, 973-976. 10.1038/173973a0 [DOI] [PubMed] [Google Scholar]
- Jodoin J. N. and Martin A. C. (2016). Abl suppresses cell extrusion and intercalation during epithelium folding. Mol. Biol. Cell 27, 2822-2832. 10.1091/mbc.E16-05-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodoin J. N., Coravos J. S., Chanet S., Vasquez C. G., Tworoger M., Kingston E. R., Perkins L. A., Perrimon N. and Martin A. C. (2015). Stable force balance between epithelial cells arises from F-actin turnover. Dev. Cell 35, 685-697. 10.1016/j.devcel.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. H. and Ziomek C. A. (1981). The foundation of two distinct cell lineages within the mouse morula. Cell 24, 71-80. 10.1016/0092-8674(81)90502-X [DOI] [PubMed] [Google Scholar]
- Koenderink G. H., Dogic Z., Nakamura F., Bendix P. M., MacKintosh F. C., Hartwig J. H., Stossel T. P. and Weitz D. A. (2009). An active biopolymer network controlled by molecular motors. Proc. Natl. Acad. Sci. USA 106, 15192-15197. 10.1073/pnas.0903974106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács M., Tóth J., Hetényi C., Málnási-Csizmadia A. and Sellers J. R. (2004). Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35557-35563. 10.1074/jbc.M405319200 [DOI] [PubMed] [Google Scholar]
- Krens S. F. G., Veldhuis J. H., Barone V., Čapek D., Maître J.-L., Brodland G. W. and Heisenberg C.-P. (2017). Interstitial fluid osmolarity modulates the action of differential tissue surface tension in progenitor cell segregation during gastrulation. Development 144, 1798-1806. 10.1242/dev.144964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M., Arboleda-Estudillo Y., Puech P.-H., Käfer J., Graner F., Müller D. J. and Heisenberg C.-P. (2008). Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429-436. 10.1038/ncb1705 [DOI] [PubMed] [Google Scholar]
- Kuipers D., Mehonic A., Kajita M., Peter L., Fujita Y., Duke T., Charras G. and Gale J. E. (2014). Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J. Cell Sci. 127, 1229-1241. 10.1242/jcs.138289 [DOI] [PubMed] [Google Scholar]
- Ladoux B., Nelson W. J., Yan J. and Mège R. M. (2015). The mechanotransduction machinery at work at adherens junctions. Integr. Biol. 7, 1109-1119. 10.1039/C5IB00070J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg K. P., Farhadifar R., Ranft J., Umetsu D., Widmann T. J., Bittig T., Said A., Jülicher F. and Dahmann C. (2009). Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950-1955. 10.1016/j.cub.2009.10.021 [DOI] [PubMed] [Google Scholar]
- Laplante C., Huang F., Tebbs I. R., Bewersdorf J. and Pollard T. D. (2016). Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. Proc. Natl. Acad. Sci. USA 113, E5876-E5885. 10.1073/pnas.1608252113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T. and Yap A. S. (2015). E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533-539. 10.1038/ncb3136 [DOI] [PubMed] [Google Scholar]
- Lee J.-Y. and Goldstein B. (2003). Mechanisms of cell positioning during C. elegans gastrulation. Development 130, 307-320. 10.1242/dev.00211 [DOI] [PubMed] [Google Scholar]
- Lewis W. H. (1947). Mechanics of invagination. Anat. Rec. 97, 139-156. 10.1002/ar.1090970203 [DOI] [PubMed] [Google Scholar]
- Lim B., Levine M. and Yamazaki Y. (2017). Transcriptional pre-patterning of Drosophila gastrulation. Curr. Biol. 27, 286-290. 10.1016/j.cub.2016.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Kovacs M., Conti M. A., Wang A., Zhang Y., Sellers J. R. and Adelstein R. S. (2012). Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc. Natl. Acad. Sci. USA 109, 4509-4514. 10.1073/pnas.1116268109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey J. P., Grego-Bessa J., Liem K. F. Jr. and Anderson K. V. (2013). Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development 140, 1262-1271. 10.1242/dev.085316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maître J.-L., Berthoumieux H., Krens S. F. G., Salbreux G., Julicher F., Paluch E. and Heisenberg C.-P. (2012). Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253-256. 10.1126/science.1225399 [DOI] [PubMed] [Google Scholar]
- Maître J.-L., Niwayama R., Turlier H., Nédélec F. and Hiiragi T. (2015). Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol. 17, 849-855. 10.1038/ncb3185 [DOI] [PubMed] [Google Scholar]
- Maître J.-L., Turlier H., Illukkumbura R., Eismann B., Niwayama R., Nédélec F. and Hiiragi T. (2016). Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 536, 344-348. 10.1038/nature18958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major R. J. and Irvine K. D. (2006). Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev. Dyn. 235, 3051-3058. 10.1002/dvdy.20966 [DOI] [PubMed] [Google Scholar]
- Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M. and Wieschaus E. F. (2010). Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735-749. 10.1083/jcb.200910099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F. M., Tworoger M. and Martin A. C. (2013). Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 15, 926-936. 10.1038/ncb2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason F. M., Xie S., Vasquez C. G., Tworoger M. and Martin A. C. (2016). RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214, 603-617. 10.1083/jcb.201603077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes Pinto I., Rubinstein B., Kucharavy A., Unruh J. R. and Li R. (2012). Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell 22, 1247-1260. 10.1016/j.devcel.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M. and Yap A. S. (2013). The regulation and functional impact of actin assembly at cadherin cell-cell adhesions. Semin. Cell Dev. Biol. 24, 298-307. 10.1016/j.semcdb.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Mitrossilis D., Röper J.-C., Le Roy D., Driquez B., Michel A., Ménager C., Shaw G., Le Denmat S., Ranno L., Dumas-Bouchiat F. et al. (2017). Mechanotransductive cascade of Myo-II-dependent mesoderm and endoderm invaginations in embryo gastrulation. Nat. Commun. 8, 13883 10.1038/ncomms13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier B., Pelissier-Monier A., Brand A. H. and Sanson B. (2010). An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 60-69. 10.1038/ncb2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell M. P. and Gardel M. L. (2012). F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl. Acad. Sci. USA 109, 20820-20825. 10.1073/pnas.1214753109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec F. and Foethke D. (2007). Collective Langevin dynamics of flexible cytoskeletal fibers. New J. Phys. 9, 427 10.1088/1367-2630/9/11/427 [DOI] [Google Scholar]
- Petridou N. I., Spiró Z. and Heisenberg C.-P. (2017). Multiscale force sensing in development. Nat. Cell Biol. 19, 581-588. 10.1038/ncb3524 [DOI] [PubMed] [Google Scholar]
- Pfister K., Shook D. R., Chang C., Keller R. and Skoglund P. (2016). Molecular model for force production and transmission during vertebrate gastrulation. Development 143, 715-727. 10.1242/dev.128090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. (2007). Regulation of actin filament assembly by Arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 36, 451-477. 10.1146/annurev.biophys.35.040405.101936 [DOI] [PubMed] [Google Scholar]
- Polyakov O., He B., Swan M., Shaevitz J. W., Kaschube M. and Wieschaus E. (2014). Passive mechanical forces control cell-shape change during Drosophila ventral furrow formation. Biophys. J. 107, 998-1010. 10.1016/j.bpj.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess J. R. and Hirsh D. I. (1986). Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117, 156-173. 10.1016/0012-1606(86)90358-1 [DOI] [PubMed] [Google Scholar]
- Qin X., Park B. O., Liu J., Chen B., Choesmel-Cadamuro V., Belguise K., Heo W. D. and Wang X. (2017). Cell-matrix adhesion and cell-cell adhesion differentially control basal myosin oscillation and Drosophila egg chamber elongation. Nat. Commun. 8, 14708 10.1038/ncomms14708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S., Gally C. and Labouesse M. (2008). Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 24, 221-230. 10.1016/j.tig.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Ramamurthy B., Yengo C. M., Straight A. F., Mitchison T. J. and Sweeney H. L. (2004). Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin IIb. Biochemistry 43, 14832-14839. 10.1021/bi0490284 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Verant P., Lecuit T. and Lenne P.-F. (2008). Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401-1410. 10.1038/ncb1798 [DOI] [PubMed] [Google Scholar]
- Rauzi M., Lenne P.-F. and Lecuit T. (2010). Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110-1114. 10.1038/nature09566 [DOI] [PubMed] [Google Scholar]
- Ray R. P., Matamoro-Vidal A., Ribeiro P. S., Tapon N., Houle D., Salazar-Ciudad I. and Thompson B. J. (2015). Patterned anchorage to the apical extracellular matrix defines tissue shape in the developing appendages of Drosophila. Dev. Cell 34, 310-322. 10.1016/j.devcel.2015.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann A.-C., Boujemaa-Paterski R., Martiel J.-L., Guerin C., Cao W., Chin H. F., De La Cruz E. M., Thery M. and Blanchoin L. (2012). Actin network architecture can determine myosin motor activity. Science 336, 1310-1314. 10.1126/science.1221708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh-Johnson M., Shemer G., Higgins C. D., McClellan J. H., Werts A. D., Tulu U. S., Gao L., Betzig E., Kiehart D. P. and Goldstein B. (2012). Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science 335, 1232-1235. 10.1126/science.1217869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J., Raff M. C. and Cramer L. P. (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847-1857. 10.1016/S0960-9822(01)00587-5 [DOI] [PubMed] [Google Scholar]
- Samarage C. R., White M. D., Álvarez Y. D., Fierro-González J. C., Henon Y., Jesudason E. C., Bissiere S., Fouras A. and Plachta N. (2015). Cortical tension allocates the first inner cells of the mammalian embryo. Dev. Cell 34, 435-447. 10.1016/j.devcel.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Savin T., Kurpios N. A., Shyer A. E., Florescu P., Liang H., Mahadevan L. and Tabin C. J. (2011). On the growth and form of the gut. Nature 476, 57-62. 10.1038/nature10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. (1972). The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J. Cell Biol. 53, 419-434. 10.1083/jcb.53.2.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwane F., Mongera A., Rowghanian P., Kealhofer D. A., Lucio A. A., Hockenbery Z. M. and Campàs O. (2017). In vivo quantification of spatially varying mechanical properties in developing tissues. Nat. Methods 14, 181-186. 10.1038/nmeth.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J. (2017). Computer modeling in developmental biology: growing today, essential tomorrow. Development 144, 4214-4225. [DOI] [PubMed] [Google Scholar]
- Shindo A. and Wallingford J. B. (2014). PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649-652. 10.1126/science.1243126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões S., Oh Y., Wang M. F. Z., Fernandez-Gonzalez R. and Tepass U. (2017). Myosin II promotes the anisotropic loss of the apical domain during Drosophila neuroblast ingression. J. Cell Biol. 216, 1387-1404. 10.1083/jcb.201608038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattum G., McGee K. M. and Rosenblatt J. (2009). P115 RhoGEF and microtubules decide the direction apoptotic cells extrude from an epithelium. J. Cell Biol. 186, 693-702. 10.1083/jcb.200903079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares e Silva M., Depken M., Stuhrmann B., Korsten M., MacKintosh F. C. and Koenderink G. H. (2011). Active multistage coarsening of actin networks driven by myosin motors. Proc. Natl. Acad. Sci. USA 108, 9408-9413. 10.1073/pnas.1016616108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak M. R., Laplante C., Chin H. F., Guirao B., Karatekin E., Pollard T. D. and O'Shaughnessy B. (2014). Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev. Cell 29, 547-561. 10.1016/j.devcel.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. (1963). Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401-408. 10.1126/science.141.3579.401 [DOI] [PubMed] [Google Scholar]
- Sugimura K., Lenne P.-F. and Graner F. (2016). Measuring forces and stresses in situ in living tissues. Development 143, 186-196. 10.1242/dev.119776 [DOI] [PubMed] [Google Scholar]
- Sun S. X., Walcott S. and Wolgemuth C. W. (2010). Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr. Biol. 20, R649-R654. 10.1016/j.cub.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Amourda C., Shagirov M., Hara Y., Saunders T. E. and Toyama Y. (2017). Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol. 19, 375-383. 10.1038/ncb3497 [DOI] [PubMed] [Google Scholar]
- Sweeton D., Parks S., Costa M. and Wieschaus E. (1991). Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112, 775-789. [DOI] [PubMed] [Google Scholar]
- Thompson D. W. (1917). On Growth and Form, 1st edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Toyama Y., Peralta X. G., Wells A. R., Kiehart D. P. and Edwards G. S. (2008). Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science 321, 1683-1686. 10.1126/science.1157052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D., Aigouy B., Aliee M., Sui L., Eaton S., Jülicher F. and Dahmann C. (2014). Local increases in mechanical tension shape compartment boundaries by biasing cell intercalations. Curr. Biol. 24, 1798-1805. 10.1016/j.cub.2014.06.052 [DOI] [PubMed] [Google Scholar]
- Vale R. D. and Milligan R. A. (2000). The way things move: looking under the hood of molecular motor proteins. Science 288, 88-95. 10.1126/science.288.5463.88 [DOI] [PubMed] [Google Scholar]
- Vasquez C. G., Heissler S. M., Billington N., Sellers J. R. and Martin A. C. (2016). Drosophila non-muscle myosin II motor activity determines the rate of tissue folding. Elife 5, e20828 10.7554/eLife.20828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D., Wu J.-Q., Hao S., O'Shaughnessy B. and Pollard T. D. (2008). Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319, 97-100. 10.1126/science.1151086 [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Zareno J., Whitmore L., Choi C. K. and Horwitz A. F. (2007). Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 176, 573-580. 10.1083/jcb.200612043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong-Brender T. T., Ben Amar M., Pontabry J. and Labouesse M. (2017). The interplay of stiffness and force anisotropies drives embryo elongation. Elife 6, e23866 10.7554/eLife.23866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J. B., Niswander L. A., Shaw G. M. and Finnell R. H. (2013). The continuing challenge of understanding, preventing, and treating neural tube defects. Science 339, 1222002 10.1126/science.1222002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. J., Zulueta-Coarasa T., Maier J. A., Lee D. M., Bruce A. E. E., Fernandez-Gonzalez R. and Harris T. J. C. (2017). An actomyosin-Arf-GEF negative feedback loop for tissue elongation under stress. Curr. Biol. 27, 2260-2270.e2265. 10.1016/j.cub.2017.06.038 [DOI] [PubMed] [Google Scholar]
- Wu J.-Q. and Pollard T. D. (2005). Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310-314. 10.1126/science.1113230 [DOI] [PubMed] [Google Scholar]
- Wu S. K., Gomez G. A., Michael M., Verma S., Cox H. L., Lefevre J. G., Parton R. G., Hamilton N. A., Neufeld Z. and Yap A. S. (2014). Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat. Cell Biol. 16, 167-178. 10.1038/ncb2900 [DOI] [PubMed] [Google Scholar]
- Xu J., Tseng Y. and Wirtz D. (2000). Strain hardening of actin filament networks. Regulation by the dynamic cross-linking protein alpha-actinin. J. Biol. Chem. 275, 35886-35892. 10.1074/jbc.M002377200 [DOI] [PubMed] [Google Scholar]
- Xue Z. and Sokac A. M. (2016). Back-to-back mechanisms drive actomyosin ring closure during Drosophila embryo cleavage. J. Cell Biol. 215, 335-344. 10.1083/jcb.201608025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S., Totsukawa G., Yamakita Y., Sasaki Y., Madaule P., Ishizaki T., Narumiya S. and Matsumura F. (2003). Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell 14, 1745-1756. 10.1091/mbc.E02-07-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yengo C. M., Takagi Y. and Sellers J. R. (2012). Temperature dependent measurements reveal similarities between muscle and non-muscle myosin motility. J. Muscle Res. Cell Motil. 33, 385-394. 10.1007/s10974-012-9316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. C. and Fernandez-Gonzalez R. (2016). Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. Elife 5, e10757 10.7554/eLife.10757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A. and Wieschaus E. (2004). Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343-355. 10.1016/S1534-5807(04)00060-7 [DOI] [PubMed] [Google Scholar]
- Zhou J., Kim H. Y. and Davidson L. A. (2009). Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136, 677-688. 10.1242/dev.026211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Pal S., Maiti S. and Davidson L. A. (2015). Force production and mechanical accommodation during convergent extension. Development 142, 692-701. 10.1242/dev.116533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Enaw J. O. E., Ma C., Shaw G. M., Lammer E. J. and Finnell R. H. (2007). Association between CFL1 gene polymorphisms and spina bifida risk in a California population. BMC Med. Genet. 8, 12 10.1186/1471-2350-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]