Abstract
Improving CD8+ T cell responses activated by subunit vaccination is crucial for improving vaccine efficacy and safety. Here we report a carrier-adjuvant system composed of self-assembling peptide nanofibers presenting an immunodominant antigen from herpes simplex virus (HSV) and toll-like receptor (TLR) agonists that induces robust effector and memory CD8+ T cell responses in mice. The effector function of vaccine-induced CD8+ T cells was influenced by the type of TLR agonist. The use of CpG (TLR9 agonist) resulted in significantly greater specific in vivo cytotoxicity and trended towards more cells producing both IFN-γ and TNF-α compared to gardiquimod (TLR7 agonist). Prime-boost immunization with peptide nanofibers combined with either adjuvant resulted in development of HSV-specific CD8+ memory T cells further demonstrating the capability of the carrier-adjuvant system to induce strong HSV-specific CD8+ T cell responses. Inclusion of peptide epitope-nanofibers in protein-based subunit vaccines should increase the functional spectrum of the vaccine-elicited immune response and protection.
Keywords: TLR agonist adjuvants, CD8+ T cell, HSV-2, Peptide nanofiber, Vaccine
1. Introduction
Immunization with subunit vaccines has the benefit of enhanced safety for use in at-risk populations such as immune compromised individuals. Most approved subunit vaccines induce vigorous antibody and CD4+ T cell responses, but are less effective at inducing strong CD8+ T cell responses. This is an important shortcoming because vaccines against many pathogens will need to induce a strong cell-mediated immune response to be effective. For example, approximately 17% of Americans between 15 and 49 years of age are infected with genital herpes for which there is no approved vaccine [1]. An effective HSV vaccine is urgently needed because genital HSV-2 infection increases the risk of acquisition of HIV and infection of newborns during vaginal delivery from mothers shedding HSV may result in severe neurological disease or death [2]. Vigorous HSV-specific B and T cell responses have been documented in animal models of genital HSV-2 infection [3–5] and while HSV-specific antibodies are involved in protection of animals [6–8], cell-mediated immunity is necessary for clearance of HSV-2 from peripheral sites [3,9].
Induction of pathogen-specific CD8+ T cell responses is easily achieved by immunization with live attenuated vaccines but is difficult with subunit vaccines. We previously reported that covalent linkage of a peptide antigen (MHC class I epitope from ovalbumin, SIINFEKL) to a self-assembling peptide domain (glutamine-rich sequence Q11, QQKFQFQFEQQ) resulted in strong antigen-specific effector and memory CD8+ T cell responses when injected into mice in the absence of added adjuvants [10]. In the current studies, we built on these results and demonstrate using a second self-assembling sequence KFE8 (FKFEFKFE) that immunization with peptide nanofibers bearing a CD8+ T cell epitope from HSV glycoprotein B (gB-NF) admixed with TLR agonists induces vigorous HSV-specific CD8+ T cell responses with protective cytokine and cytolytic functions and development of memory T cell responses.
2. Methods
2.1. Peptide synthesis and vaccine preparation
HSV gB peptide (SSIEFARL) was conjugated to the C-terminus of the self-assembling peptide domain KFE8 (FKFEFKFE) using a proteolytic spacer GGAAY using standard Fmoc Chemistry as reported previously [11–13]. To prepare nanofiber vaccine stock formulations, 5 mg of the fusion peptide KFE8-GGAAY-gB was dissolved in 50 µL of DMSO to disaggregate it and 450 µL of sterile water was added. To this, 200 µg of CpG (class B mouse TLR9 ligand, ODN 1826) or Gardiquimod (InvivoGen, San Diego, CA) dissolved in 500 µL of PBS was added and the mixture was vortexed prior to injection. Each dose contained 250 µg gB-NF and 10 µg TLR agonist.
2.2. Mice and immunization
Six week old C57BL/6 mice were purchased from Jackson Labs (Bar Harbor, ME) and all animal experiments were conducted under protocols approved by the UTMB Institutional Animal Care and Use Committee. Primary subcutaneous immunization of mice with peptide-NF and adjuvant was performed as described previously and quantitative assays performed at the height of the primary response on day 8 [10]. For recall experiments, mice were primed once subcutaneously with gB-NF and adjuvant and boosted with the same vaccine/adjuvant combination on day 28. Mice received an identical immunization on day 56 and the recall CD8+ T cell response was quantified three days later.
2.3. Flow cytometry
Lymphocytes were stained with anti-CD3, anti-CD8 (BD PharMingen) and tetramers composed of the immunodominant Kb-restricted HSV glycoprotein B peptide SSIEFARL (Medical and Biological Laboratories, Nagoya, Aichi, Japan). T lymphocytes were stained for intracellular cytokines as described previously [14]. Data were acquired on a BD LSRFortessa analyzer (BD Biosciences, San Jose, CA) at the University of Texas Medical Brach flow cytometry core laboratory and analyzed using FlowJo software (Tree Star, Ashland, OR).
2.4. In vivo cytotoxic T lymphocyte assay
The in vivo CTL assay was performed with modifications of a previously published method [14]. Briefly, equal numbers of 0.25 µM CFSE-labeled OVA peptide-pulsed splenocytes and 2.5 µM CFSE-labeled gB peptide-pulsed splenocytes were injected i.v. into non-immune or immune mice day eight after primary immunization with gB-NF/TLR agonist adjuvant. Splenocytes were harvested after 15 h and the percent specific lysis was calculated as described previously [14].
2.5. ELISPOT
ELISPOT assays for IFN-γ – secreting cells (IFN-γ SC) were performed as described previously [3]. HSV gB-specific IFN-γ SC were quantified using an ImmunoSpot reader and analyzed with ImmunoSpot software (Cellular Technology Ltd, Cleveland, OH).
2.6. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) on ranks (Kruskal-Wallis test) using GraphPad Prism (GraphPad Software, San Diego, CA).
3. Results and discussion
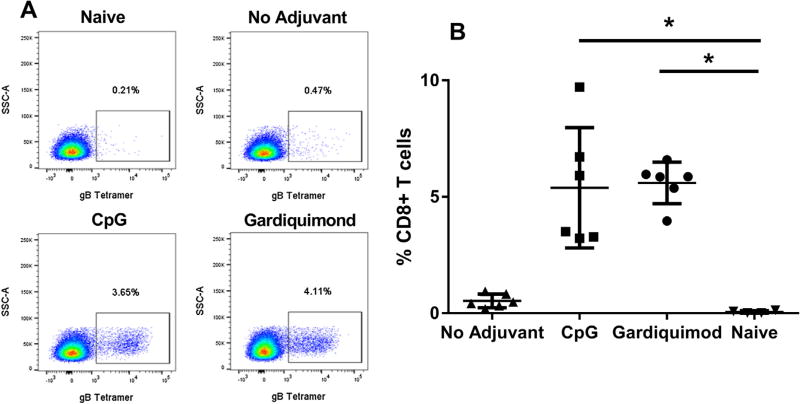
We showed previously that immunization of mice with peptide incorporated into nanofibers and given in adjuvant resulted in a vigorous CD8+ T cell response whereas immunization with free peptide and adjuvant did not [10]. In the current study, a significantly greater gB-specific CD8+ T cell response was obtained by immunization of mice with gB-NF and CpG (% gB tetramer + cells: 7.09 ± 0.76) compared to immunization with an identical amount of free gB peptide in CpG adjuvant (% gB tetramer + cells: 0.57 ± 0 .08; P < .05, Kruskal-Wallis test). We compared gB-specific CD8+ T cell responses that developed following immunization with gB-NF + CpG or Gardiquimod adjuvants. At the peak of the primary response following immunization [10], lymphocytes were collected from the draining lymph node and the number of gB-specific CD8+ T cells was determined by specific tetramer staining. As shown in Fig. 1, gB-specific responses were significantly increased (P < .05, one way ANOVA) in mice immunized with gB-NF and either CpG or Gardiquimod adjuvants compared to non-immune mice and were also consistently greater than mice immunized with gB-NF alone.
Fig. 1.
Activation of HSV-2 gB-specific CD8+ T cells by immunization with peptide-nanofiber vaccine adjuvanted with CpG or Gardiquimod. A. Detection of HSV-2 gB-specific CD8+ T cells by flow cytometry. B. HSV-2 gB-specific response in draining lymph nodes on day eight following primary peptide-nanofiber immunization. Results shown are pooled from two experiments of identical design. * P < .05 one way ANOVA.
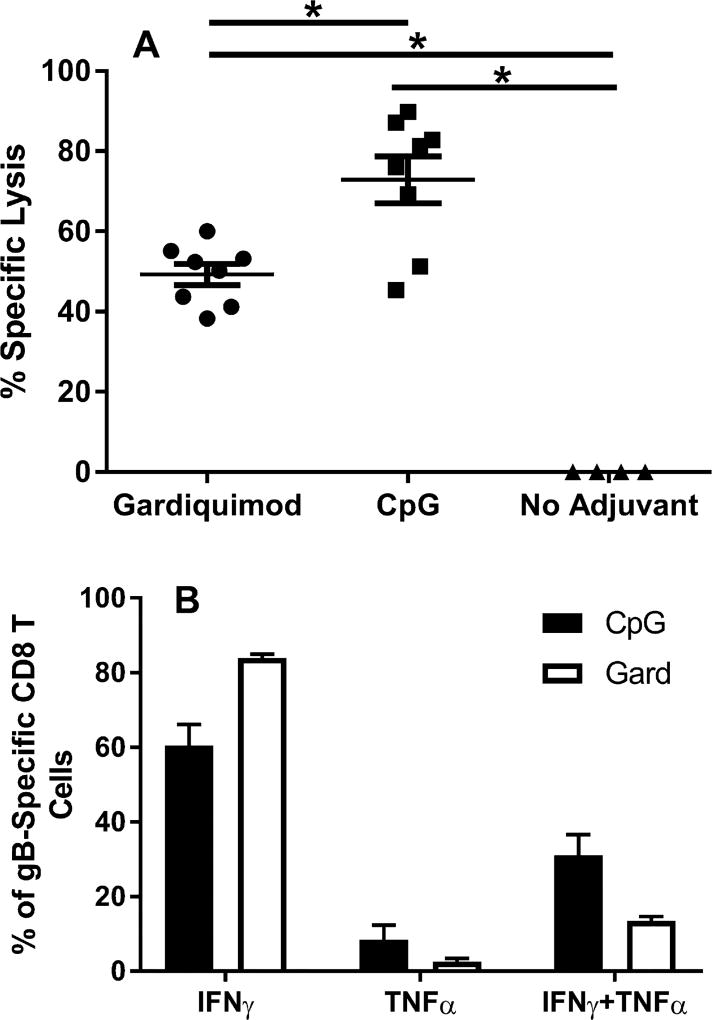
Clearance of HSV-2 by CD8+ T cells requires the expression of cytolytic activity and IFN-γ production [15]. A role for TNF-α in protection against HSV has also been documented in vivo [16] and may involve activation of cells with important roles in anti-HSV protection such as natural killer cells and macrophages [17,18]. We compared the expression of these cytokine and effector functions by gB-specific T cells from mice receiving gB-NF with either Gardiquimod or CpG adjuvant. The level of gB-specific cytotoxic T lymphocyte responses from immunized mice was determined using an in vivo assay. While the percent specific lysis in both the gB-NF + Gardiquimod and gB-NF + CpG immunized groups was significantly greater than in unimmunized mice (Fig. 2A), the percent specific lysis was significantly higher in the CpG immunization group compared to the Gardiquimod group (P < .05 one way ANOVA). This difference could potentially reflect higher numbers of gB-specific CD8+ T cells in the CpG immunization group. The production of cytokines known to be important for protection against HSV [15] differed somewhat among immunization groups. In both adjuvant groups and most dramatically in the gB-NF + Gardiquimod group, gB-specific T cells secreting only IFN-γ dominated the response (Fig. 2B). On an individual cell basis, the amount of IFN-γ production by gB-specific T cells from gB-NF + CpG and gB-NF + Gardiquimod groups was not different (MFI = 6543 ± 855 and 5519 ± 507, respectively). Immunization with gB-NF + CpG resulted in a higher percentage of CD8+ T cells producing both IFN-γ and TNF-α compared to immunization with gB-NF + Gardiquimod (30% vs 13.9%, respectively), although the difference was not statistically significant.
Fig. 2.
Expression of effector function of HSV-2 gB-specific T cells in mice following primary immunization with gB-NF vaccine given with CpG or Gardiquimod adjuvants. A. HSV-2 gB-specific cytolytic activity detected by in vivo CTL assay. Results shown are pooled from two experiments of identical design. * P < .05, one way ANOVA. B. Expression of cytokines following peptide stimulation of lymphocytes following primary immunization detected by intracellular cytokine staining. Results shown are pooled from two experiments of identical design.
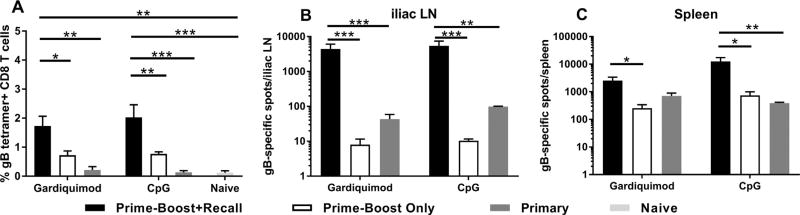
The induction of CD8+ memory T cell responses in the immunization groups was tested by priming with gB-NF adjuvanted with either CpG or Gardiquimod followed by boosting four weeks later by a second immunization with the same antigen/adjuvant combination. Four weeks after boosting, immune and non-immune animals received a recall immunization and the number of gB-specific CD8+ T cells was quantified three days later using specific tetramers and ELISPOT analysis. As shown in Fig. 3A, the number of gB-specific, CD8+ T cells in draining lymph nodes was significantly greater in gardiquimod prime-boost immunized mice that received the recall immunization (recall response) compared to non-immune mice receiving the identical immunization (day three primary response) or prime-boost only mice (no recall immunization) (P < .01 one-way ANOVA). Similarly, the recall response of CpG prime-boost mice receiving the recall immunization was significantly greater than in prime-boost only mice (P < .01, one-way ANOVA) or non-immune mice receiving the identical immunization (P < .001, one-way ANOVA). Further analysis demonstrated significantly higher numbers of gB-specific, IFN-γ secreting cells in the spleens and iliac lymph nodes of prime-boost immunized mice that received the recall immunization (Fig. 3B, C) compared to prime-boost only or non-immune mice that received the recall immunization (day three primary response only). Together, these results demonstrate a gB-specific CD8+ recall response at secondary lymphoid tissues in mice receiving a prime-boost immunization with either gB-NF + CpG or gB-NF + Gardiquimod.
Fig. 3.
HSV-2 gB-specific recall response of gB-NF immunized mice. A. Quantification of recall response by tetramer staining. Results shown are pooled from two experiments of identical design. Quantification of gB-specific IFN-γ secreting cells in the iliac LN (B) or spleen (C) by ELISPOT analysis. Results shown are from iliac LN and spleen lymphocytes of mice used for 3A. * P < .05, ** P < .01, *** P < .001, one way ANOVA.
In summary, the results of these studies demonstrate the ability of the novel peptide-nanofiber vaccine to elicit robust CD8+ T cell responses when used in conjunction with TLR agonist adjuvants. Importantly, different TLR-agonist adjuvants elicited T cells with important manifestations of effector function. CpG adjuvant vaccine recipients exhibited significantly greater specific cytotoxicity in vivo and trended towards higher IFN-γ and TNF-α co-production compared to gardiquimod vaccine recipients. Rapid clearance of virus by CD8+ T cells requires the production of IFN-γ and robust cytolytic activity [15], therefore the ability to tailor CD8+ T cell function by selection of appropriate adjuvant is an important attribute of this carrier-adjuvant vaccine. CD8+ T cell subsets are present near sites of previous epithelial HSV infection in humans [19] and viral clearance appears to correlate with the arrival of CD8+ T cells in the lesion [20]. Recently, human HSV-specific CD8+ T cells have been detected in close proximity to sensory nerve endings in HSV-infected individuals suggesting an important role for these cells in preventing or limiting virus shedding following reactivation from latency [21,22]. Future work with this vaccine should be aimed at eliciting tissue resident memory T cells at the sites of HSV latency and shedding. The ability of this novel synthetic nanofiber vaccine to induce antigen-specific CD8+ T cell responses has important safety benefits. Further, inclusion of the NF vaccine/adjuvant in non-infectious vaccine formulations has the potential to augment the vaccine-induced CD8+ T cell response against pathogens that require a multifaceted cellular immune response to achieve protection.
Acknowledgments
This study was supported by a grant from the National Institutes of Allergy and Infectious Disease (1R21AI117615-01). We thank Ms. Clarice Perry for excellent technical assistance.
Footnotes
Conflicts of interest
None.
References
- 1.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999–2010. J Infect Dis. 2014;209:325–33. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 2.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Newman LM, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5:e300–9. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–100. [PubMed] [Google Scholar]
- 4.Tang VA, Rosenthal KL. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol. 2010;87:39–44. doi: 10.1016/j.jri.2010.06.155. [DOI] [PubMed] [Google Scholar]
- 5.Xia J, Veselenak RL, Gorder SR, Bourne N, Milligan GN. Virus-specific immune memory at peripheral sites of herpes simplex virus type 2 (HSV-2) infection in guinea pigs. PLoS One. 2014;9:e114652. doi: 10.1371/journal.pone.0114652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourne N, Pyles RB, Bernstein DI, Stanberry LR. Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol. 2002;83:2797–801. doi: 10.1099/0022-1317-83-11-2797. [DOI] [PubMed] [Google Scholar]
- 7.Chu CF, Meador MG, Young CG, Strasser JE, Bourne N, Milligan GN. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J Reprod Immunol. 2008;78:58–67. doi: 10.1016/j.jri.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staats HF, Oakes JE, Lausch RN. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J Virol. 1991;65:6008–14. doi: 10.1128/jvi.65.11.6008-6014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parr MB, Parr EL. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology. 2000;101:126–31. doi: 10.1046/j.1365-2567.2000.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesson CB, Huelsmann EJ, Lacek AT, Kohlhapp FJ, Webb MF, Nabatiyan A, et al. Antigenic peptide nanofibers elicit adjuvant-free CD8(+) T cell responses. Vaccine. 2014;32:1174–80. doi: 10.1016/j.vaccine.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, et al. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–84. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–64. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–7. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson MH, Bird MD, Chu CF, Johnson AJ, Friedrich BM, Allman WR, et al. Rapid clearance of herpes simplex virus type 2 by CD8+ T cells requires high level expression of effector T cell functions. J Reprod Immunol. 2011;89:10–7. doi: 10.1016/j.jri.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–54. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossol-Voth R, Rossol S, Schutt KH, Corridori S, de Cian W, Falke D. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J Gen Virol. 1991;72(Pt 1):143–7. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- 17.Paludan SR, Malmgaard L, Ellermann-Eriksen S, Bosca L, Mogensen SC. Interferon (IFN)-gamma and Herpes simplex virus/tumor necrosis factor-alpha synergistically induce nitric oxide synthase 2 in macrophages through cooperative action of nuclear factor-kappa B and IFN regulatory factor-1. Eur Cytokine Netw. 2001;12:297–308. [PubMed] [Google Scholar]
- 18.Thapa M, Kuziel WA, Carr DJ. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J Virol. 2007;81:3704–13. doi: 10.1128/JVI.02626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–61. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 20.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–8. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol. 2012;86:10587–96. doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]