Abstract
Zonal organization plays an important role in cartilage structure and function, whereas most tissue-engineering strategies developed to date have only allowed the regeneration of cartilage with homogeneous biochemical and mechanical cues. To better restore tissue structure and function, there is a strong need to engineer materials with biomimetic gradient niche cues that recapitulate native tissue organization. To address this critical unmet need, in this study, we report a method for rapid formation of tissue-scale gradient hydrogels as a three-dimensional (3D) cell niche with tunable biochemical and physical properties. When encapsulated in stiffness gradient hydrogels, both chondrocytes and mesenchymal stem cells demonstrated zone-specific response and extracellular deposition that mimics zonal organization of articular cartilage. Blocking cell mechanosensing using blebbistatin abolished the zonal response of chondrocytes in 3D hydrogels with a stiffness gradient. Such tissue-scale gradient hydrogels can provide a 3D artificial cell niche to enable tissue engineering of various tissue types with zonal organizations or tissue interfaces.
Keywords: : gradient, hydrogels, zonal organization, cartilage, interfacial tissue engineering
Introduction
Despite the important role of zonal organization in cartilage structure and function, most tissue-engineering strategies developed to date have only allowed the regeneration of cartilage with homogeneous biochemical and mechanical cues.1–5 Cartilage injury is extremely common, yet cartilage has limited self-healing capacity due to its low cellularity and avascular nature.1 As articular cartilage transitions from the superficial zone to the deep zone, the extracellular matrix (ECM) of the cartilage is characterized by increased stiffness, higher amounts of ECM constituents such as glycoaminoglycans, and increased presence of a hypertrophic cartilage marker such as type X collagen.6
Various strategies have been developed to generate biomaterials with gradient cues as cell niches,7 such as electrospinning,3 modular hydrogel system,8 laser-scanning lithography,4 convection-based diffusion,9 microfluidics,10 and fusing prefabricated microspheres.11 However, most of these strategies often require lengthy fabrication processes or the use of reagents that are not cell friendly, which do not support the fabrication of tissue-scale gradient biomaterials that allow direct cell encapsulation in a homogenous manner. As such, how gradient niche cues influence cell-mediated tissue formation in three-dimensional (3D) cell niche remains largely unknown. In addition, although recent research has highlighted the role of mechanical signals, such as matrix stiffness, in modulating various cell fates, including cell proliferation and differentiation,12–14 how activating or blocking mechanosensing influences cell fate and ECM formation in 3D tissue remains to be examined.
To design zonal organization into engineered cartilage, one strategy is to spatially pattern chondrocytes from different zones with zone-specific phenotypes into stratified layers.15,16 Using sequential photopolymerization, multilayered hydrogels can be fabricated with chondrocyte subpopulations isolated from different zones and encapsulated in different layers to better mimic in vivo zonal organizations.2,17 Another strategy is to spatially pattern matrix cues using multilayered hydrogels with varying biochemical and mechanical properties using a single source of cells.18 Both biochemical and physical cues have been shown to play important roles in directing cell fates and tissue formation19–21; both mesenchymal stem cells (MSCs) and chondrocytes encapsulated in multilayered scaffolds with spatially varying matrix compositions and mechanical cues demonstrated zone-specific differentiation.18,22 While the above strategies have improved the zonal organization of engineered cartilage over that achieved with homogeneous hydrogels, the resulting tissues displayed distinctive layers, while native cartilage transition in a continuous gradient manner.23 Furthermore, the interfaces between layers are often weak and susceptible to delamination under mechanical stress due to discontinuities in hydrogel stiffness at the interfaces.24
In contrast to multilayered hydrogels, biomimetic gradient hydrogels present continuous changes in biochemical and/or mechanical cues, yielding more accurate mimicry of tissue interfaces and zonal organization in vivo. To induce zone-specific differentiation of MSCs in 3D to better mimic the bone–cartilage interface, hydrogels containing soluble factor gradients were fabricated by encapsulating microspheres with recombinant human bone morphogenic protein 2 (rhBMP-2) and insulin-like growth factor (rhIGF-I) in a gradient manner.25 Additional work has also been done to fabricate hydrogels with different matrix stiffness with developmental signaling approaches to induce zonal-dependent MSC differentiation.26 Attempts have also been made to fabricate hydrogels containing insoluble matrix gradients (for example, stiffness) using polyacrylamide (PAAM) hydrogels27,28 with biochemical cues.29 A microfluid-based method has also been explored to create dual-gradient polyethylene glycol (PEG) hydrogels containing both chemical and physical gradients,30 but such microscale platforms are not suitable for fabricating tissue-scale gradient hydrogels for repairing tissues with clinically relevant dimensions. While cells reside in a 3D niche in vivo, previous reports were largely limited to 2D studies due to the challenge of encapsulating cells in tissue-scale 3D gradient hydrogels in a rapid and cell-friendly manner. As such, there remains a critical need to develop a biomaterial platform that enables the investigation of cell behavior in tissue-scale gradient hydrogels in 3D. The goal of the present study is to develop a cell-friendly method for fabricating tissue-scale gradient hydrogels as a 3D cell niche to guide regeneration of cartilage with zonal organization. We hypothesize that hydrogels with stiffness gradient would induce zone-specific response of encapsulated cells in 3D, and the newly deposited tissues in gradient hydrogels mimic the zonal organization of native articular cartilage. To test this hypothesis, neonatal bovine chondrocytes or human MSCs were encapsulated in 3D gradient hydrogels for 7 or 21 days. Outcomes of newly formed tissues in different zones within gradient hydrogels were evaluated using mechanical testing, quantitative gene expressions, biochemical assays, and histology.
Materials and Methods
Chondrocyte isolation
Hyaline articular cartilage was dissected from the femoropatellar groove of two stifle joints from a 3-day-old calf (Research 87). The cartilage was sliced into thin pieces and digested in high-glucose Dulbecco's modified Eagle's medium (DMEM) containing1 mg/mL collagenase (type II and type IV [Worthington Biochemical]) and supplemented with 100 U/mL penicillin and 0.1-mg/mL streptomycin (Gibco, Invitrogen) for 24 h at 37°C. Digested suspension was filtered through a 70 μm nylon mesh, washed in Dulbecco's Phosphate-buffered Saline (DPBS) and centrifuged thrice at 460 g for 15 min. Primary chondrocytes without passage were used in later encapsulation in gradient hydrogels.
Synthesis of hydrogel precursors
Eight-arm PEG with norbornene end groups (PEGNB, MW 10 kDa) and linear PEG with thiol end groups (PEGSH, MW 1.5 kDa) were synthesized as previously reported.31,32 Chondroitin sulfate sodium salt was reacted with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) in an MES buffer for 5 min followed by the addition of 2-aminoethyl methacrylate (AEMA) at a molar ratio of 1:2:1. The solution was stirred for 24 h at room temperature, and then dialyzed against deionized (DI) water for 4 days using dialysis tubing (12–14 kDa MWCO). The purified solution was lyophilized and stored at −20°C until use.
Fabrication of 3D gradient hydrogels and cell encapsulation
The gradient maker (Hoefer SG-15; Amersham Biosciences) consists of interconnected vertical chambers filled with two hydrogel precursor solutions containing either 20% (w/v) or 2% (w/v) PEG, respectively. The stiffness of hydrogel is controlled by PEG, which is crosslinked by a mixture of PEGNB and PEGSH (6.9:3.1). For cell studies, the biochemical cue was maintained constant, with 3% (w/v) chondrointin added in both 20% and 2% PEG precursor solutions. Photoinitiator LAP (0.05% (w/v)) was used to induce crosslinking. Cells were homogeneously suspended with hydrogel precursor solutions in both chambers before mixing. For chondrocytes, cells were encapsulated at 15 M/mL and for hMSCs, cells were encapsulated at 10 M/mL. The effluent from the mixing chamber was pushed into a collecting mold by a peristaltic pump (MMP-100; C.B.S. Scientific) at 1 mL/min progressively, forming a continuous mechanical gradient precursor with homogeneous biochemical cues and cell suspension. The collecting mold is prepared from Teflon sheets and a glass slide and is optimized to the volume of gradient maker and width of the UV lamp for uniform curing of the gradient hydrogel for 5 min.
Cell culture
Chondrocytes were encapsulated within gradient hydrogels and cultured in chondrocyte growth media (high-glucose DMEM [Gibco] containing 10% fetal bovine serum [Gibco], 100 U/mL penicillin, and 0.1 mg/mL streptomycin [Gibco, Invitrogen], 50 μg/mL ascorbate-2-phosphate [Sigma-Aldrich], 40 μg/mL proline [Sigma-Aldrich], 100 μg/mL nonessential amino acid [Gibco], 100 μg/mL HEPES buffer [Sigma-Aldrich] for up to 3 weeks in 5% CO2 at 37°C. The medium was changed thrice per week. Selective group was treated with blebbistatin to examine the role of matrix stiffness sensing within gradient niche cues. Specifically, blebbistatin (EMD Biosciences) was added at a concentration of 50 μM with media change every 2 days for 1 week. Adult human mesenchymal stem cells (hMSCs) were purchased from the commercial company (Lonza) at passage 2 and were expanded for four passages in high-glucose DMEM (Gibco) supplemented with 5 ng/mL basic fibroblast growth factor, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Gibco, Invitrogen). hMSCs under chondrogenic differentiation were cultured in chondrogenic media as previously described,33 for 3 weeks in 5% CO2 at 37°C. The medium was changed thrice per week.
Fluorescence recovery after photobleaching diffusivity measurement
Hydrogel diffusivity was tested by fluorescence recovery after photobleaching (FRAP) of dextran (MW 20 kDa) conjugated with fluorescein isothiocyanate (FITC). Dextran was mixed with hydrogel solutions in gradient maker chamber at a final FITC concentration of 2 mg/mL, and individual gels were cut using biopsy (6 mm) punch. Photobleaching was done by exposing a 100 × 100 μm spot in the field of view to high-intensity laser light, and the total fluorescence intensity was visualized at 37°C using a Leica TCS SP5 confocal microscope at low light intensity. A series of images were taken every 1.365 s for 3 min to track the recovery of fluorescence. Control experiments were also performed using phosphate-buffered saline (PBS) as the diffusion media. The resulting recovery profiles were modeled by Fickian diffusion according to a previously reported method34 to calculate diffusivities of dextran in these hydrogels.
Mechanical testing
Unconfined compression tests were conducted and the compressive modulus was calculated using the linear curve fits of the stress versus strain curve for strain range of 10–20%. Acellular hydrogels were fabricated and swelled in PBS for 24 h at room temperature before mechanical testing. A preload of ∼2 mN was applied and the test was conducted at a rate of 1% strain s−1 to a maximum strain of 30%. The compressive modulus was then calculated by dividing stress over strain.
Gene expression analysis using real-time polymerase chain reaction
Total RNA was extracted from cells grown in 3D hydrogels (n = 3/group) after a 7-day culture. cDNA was synthesized from extracted RNA using the SuperScript III First-Strand Synthesis kit (Life Technologies) per manufacturer's instructions. Real-time polymerase chain reaction (RT-PCR) was then performed on an Applied Biosystems7900 Real-Time PCR system (Applied Biosystems, Life Technologies) using Power SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies). The relative expression levels of the genes of interest were determined using the comparative CT method (Qiagen), in which target gene expression was first normalized to that of the housekeeping gene (GAPDH), which was subsequently normalized to the gene expression level of the control group (day 1 chondrocytes or day 1 hMSCs).
Biochemical assay
Cell-laden hydrogels (n = 3) were digested in papainase solution (Worthington Biochemical) at 60°C for 16 h. DNA content was measured using the PicoGreen assay (Molecular Probes) using Lambda phage DNA as standard. Sulfated glycosaminoglycan (sGAG) content was quantified using the 1,9-dimenthylmethylene blue (DMMB) dye-binding assay with shark chondroitin sulfate (Sigma) as standard. Total collagen content was determined using acid hydrolysis followed by reaction with p-dimenthylaminobenzaldehyde and chloramine T (Sigma). Acellular controls for both day 1 and day 21 are included. All values reported in the study refer to the newly deposited sGAG and collagen, which have subtracted sGAG and collagen values from the acellular samples from the cellular samples.
Histological analysis and immunofluorescence staining
To visualize the ECM production and distribution, immunofluorescence staining of aggrecan and collagen I, II, and X were performed. Cell–hydrogel samples were harvested after 7 or 21 days for chondrocytes and 21 days for hMSCs, and fixed in 4% paraformaldehyde for 1 h at room temperature. The samples were transferred to a 30% sucrose solution (Sigma) overnight at 4°C before they were snap frozen in optimal cutting temperature compound (Sakura Tissue-Tek). Then, samples were cut using standard cryotome into 12-μm-thick sections. Rabbit polyclonal antibodies to aggrecan and collagen type I, II, and X (Abcam) were added to the samples separately. Sections were incubated with secondary antibody (Alexa Fluor 488 goat anti-rabbit) diluted 1:200 with Hoechst (2 μg/mL) for 1 h at room temperature. Samples were mounted with the mounting medium (Vectashield) and images were taken with a Zeiss fluorescence microscope.
Statistical test
All experiments were performed in triplicates. Results were reported as mean ± standard deviation. GraphPad Prism 6 (Graphpad Software) was used to perform statistical analysis. One-way analysis of variance and pairwise comparisons with Tukey's post hoc test were used to determine statistical significance (p < 0.05).
Results and Discussion
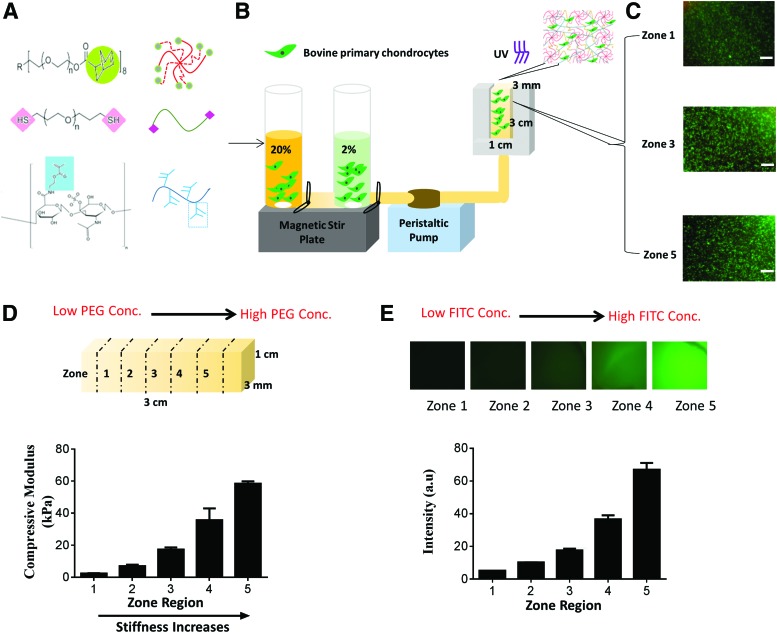
In this report, we present a facile method for the rapid formation of tissue-scale gradient hydrogels as 3D cell niche with tunable biochemical and biophysical properties. To provide the mechanical building blocks, a photocrosslinkable, multi-arm PEG hydrogel system was chosen as bioinert backbone (Fig. 1A). Previous studies have explored how gradients of soluble factors or small peptides such as arginylglycylaspartic acid (RGD)29,30,35,36 influence cell fates, yet how intact extracellular protein gradients influence cell fates remains unclear. To better mimic the biochemical composition of articular cartilage, chondroitin sulfate, a major glycosaminoglycan composition of cartilage ECM, was chemically incorporated through methacrylation (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/tea). This strategy allows more stable incorporation of large biological molecules in 3D. Chondroitin sulfate methacrylate (CS-MA) also enables enzymatic degradation and matrix turnover by cell-secreted chondroitinase.37 We employed a gradient maker-based platform that permitted continuous mixing of two cell-containing precursor solutions (containing PEG and CS-MA) into a mold to form continuous gradients (Supplementary Fig. S1B). Our method led to the rapid (∼2 min) formation of tissue-scale hydrogels (3 × 1 × 3 mm) with stiffness and/or ECM molecule gradient cues, while supporting homogeneous cell encapsulation in 3D. Upon exposure to light, the mixed cell-containing hydrogel solution quickly formed insoluble cell-laden gradient hydrogels (Fig. 1B). To facilitate analysis, we divided our gradient hydrogels evenly into five zones at the time of harvest, with zone 1 to zone 5 mimicking transitions from superficial to deep zones in articular cartilage. Critically, our platform is cell friendly, yielding high cell viability and homogeneous cell distribution throughout all zones in the gradient hydrogels (Fig. 1C), in contrast to previous hydrogels with continuous gradients that led to heterogeneous cell distribution and low cell viability.36
FIG. 1.
Gradient hydrogel fabrication and characterization. (A) Gradient hydrogel is composed of 8-arm-poly(ethylene glycol) (PEG)-norbornene (MW 10,000 g/mol), PEG-dithiol (MW 1500 g/mol), and 25% methacrylated chondroitin sulfate (CS-MA); (B) schematic representation of gradient maker assembly used to make gradient hydrogel; gradient hydrogel is bulk polymerized after the prepolymer solution is mixed with bovine primary chondrocytes. (C) Cell viability within selected zones of the gradient hydrogel on day 1 (scale bar = 200 μm). (D) Measurement of compressive modulus from zone 1 to zone 5 in gradient hydrogel. (E) Dual-gradient hydrogel with biochemical model protein (FITC tagged BSA) encapsulation can also be achieved. BSA, bovine serum albumin; FITC, fluorescein isothiocyanate. Color images available online at www.liebertpub.com/tea
Using two precursor solutions containing 2% (w/v) and 20% (w/v) PEG, we obtained gradient hydrogels with stiffness ranging from 2 to 60 kPa, as revealed by unconfined compressive testing (Fig. 1D). Since increasing hydrogel stiffness may lead to simultaneous changes in hydrogel diffusivity, we quantified zone-specific diffusivity of the gradient hydrogels through FRAP using fluorescently labeled dextran (MW 20 kDa) as a model biomolecule.34 We detected higher diffusivity in zone 1, but no significant difference in diffusivity was evident between zone 3 and zone 5 (Supplementary Fig. S1C, D). As a weight-bearing tissue, cartilage is particularly sensitive to mechanical stimulation. Both mechanical loading and soluble factors activate mechanotransduction signaling of chondrocytes,38–41 and cell fate can also be directly modulated by insoluble matrix cues such as stiffness.42,43 To mimic the increasing gradients of stiffness and glycosaminoglycan in cartilage from the superficial to the deep zone, we could also fabricate dual-gradient hydrogel with both stiffness and biochemical protein concentrations present in a gradient manner (Fig. 1D, E).
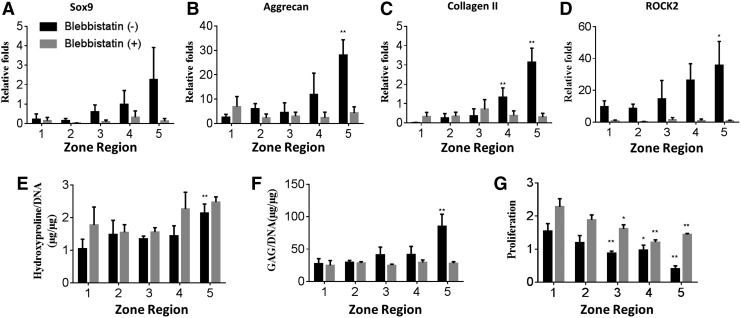
Next, to elucidate the effects of a gradient of matrix stiffness on cartilage regeneration, we encapsulated chondrocytes in 3D gradient hydrogels with a gradient of stiffness (5–60 kPa). Cell-laden gradient hydrogels were evenly divided into five zones (increasing stiffness from zone 1 to zone 5). Cell-containing gradient hydrogels were cultured in the growth medium for 7 days before the cells were harvested for analyses of gene expression, biochemical assays, and immunostainings. On day 7, quantitative PCR revealed a stiffness-dependent upregulation in the expression of cartilage markers, with increased expressions of the genes encoding Sox9, aggrecan, and collagen II (Fig. 2A–C). Expression of the genes encoding the mechanotransduction proteins ROCK2 (Fig. 2D) and nonmuscle myosin II (NMMIIB) (Supplementary Fig. S2) showed a similar trend, confirming that chondrocytes sensed the gradient of matrix stiffness in 3D. As expected, softer hydrogels generally improved cell survival and proliferation (Fig. 2G); the cell number in zone 1 was threefold higher than that in zone 5 after 7 days of culture (Fig. 2G). Consistent with the trend in gene expression, increasing matrix stiffness generally increased cellular production of collagen (Fig. 2E) and sGAG production (Fig. 2G), with significant differences between zone 5 and zone 1.
FIG. 2.
Quantitative gene expressions and production of cartilage matrix by chondrocytes encapsulated in different zones across 3D gradient hydrogels. All samples were cultured for 7 days in the chondrocyte medium with or without blebbistatin before analyses. (A–D) Normalized gene expressions of cartilage markers (Sox9, Col II, and Agg) by chondrocytes within each zone of the gradient hydrogels; (E, F) biochemical assays to quantify per cell production of cartilage matrix collagen (as shown by hydroxyproline /DNA) and sGAG; (G) fold of cell proliferation in different zones on day 7. (*p < 0.05; **p < 0.01 compared to zone 1 in each group). 3D, three dimensional; sGAG, sulfated glycosaminoglycan.
Blebbistatin is a small molecule that specifically inhibits myosin II and has been widely used to examine the effects of blocking mechanosensing on cellular response. We evaluated whether blocking mechanosensing abolished the zonal response of chondrocytes in 3D hydrogels with a stiffness gradient. Blebbistatin treatment minimized ROCK2 (Fig. 2D) and NMMIIB (Supplementary Fig. S2A) expression across all zones and abolished the zonal cartilage-specific expression of the genes encoding collagen II, aggrecan, and Sox9 (Fig. 2A). These results confirm that mechanosensing is required for chondrocytes to maintain their cartilage phenotype, and increasing the matrix stiffness did indeed result in the upregulation of cartilage markers in stiffer zones. While assays of gene expression reflect a cellular phenotype at one time point, biochemical assays quantify ECM deposition over time. Interestingly, blebbistatin treatment increased cell proliferation in all zones and only led to minor changes in collagen deposition (Fig. 2E). In contrast, blebbistatin treatment reduced sGAG deposition across all zones to a minimal level comparable to zone 1 (Fig. 2F). It is well documented that the transcriptional factor Sox9 plays an important role in regulating major cartilage ECM expression, including aggrecan and type II collagen.44,45 A previous 2D study has shown that Rho kinase (ROCK) causes a dose-dependent increase in Sox9 expression in response to transforming growth factor-β and mechanical compression.46 Since chondrocytes rapidly dedifferentiate and lose phenotype in 2D culture, these studies only offered limited insights on how mechanosensing influences chondrocyte phenotype. A previous 3D study on osteoarthritis chondrocyte has also shown zonally dependent biosynthesis behavior in response to mechanical compression.47 Our 3D gradient hydrogels, thus, are suitable for unraveling the interaction between ROCK and Sox9 in a physiologically relevant manner. Our results demonstrate that chondrocytes respond to matrix stiffness in 3D through the ROCK pathway (Fig. 2D and Supplementary Fig. S2), and ROCK expression correlates with cartilage matrix expression in a dose-dependent manner.
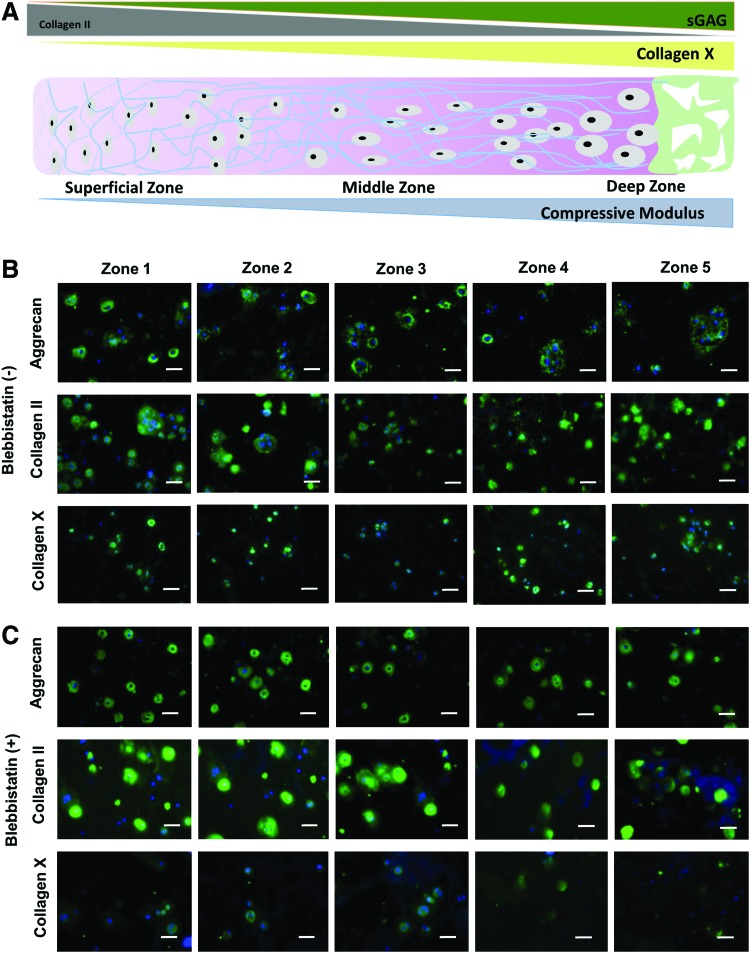
The size of sGAG nodules increases and the size of collagen II decreases as cartilage transitions from the superficial zone to the deep zone cartilage (Fig. 3A).48 We therefore examined how gradient hydrogels regulate the distribution of newly formed cartilage matrix by chondrocytes in 3D. Immunohistochemistry indicated that chondrocytes deposited substantial amount of cartilage matrix proteins after 7 days of culture in 3D gradient hydrogels (Fig. 3B). Importantly, chondrocytes encapsulated in gradient hydrogels exhibited an ECM distribution that mimics that of native articular cartilage, with larger nodules of type II collagen observed in zone 1 and larger sGAG nodules in zone 5. Collagen X is a hypertrophy marker for cartilage that is more prevalent in deep zone cartilage.49 Our results showed that as stiffness increases from zone 1 to zone 5, chondrocytes are more hypertrophic (Fig. 3B). These findings strongly suggest that chondrocytes sense mechanical gradients in 3D hydrogels and deposited ECM in a manner that mimics native cartilage zonal organization.
FIG. 3.
Immunostainings of cartilage markers by encapsulated chondrocytes in 3D gradient hydrogels on day 7. (A) Schematic illustration of native cartilage zonal organizations from superficial to deep zone for matrix compositions and mechanical properties. (B) Immunostaining of aggrecan, type II collagen, and type X collagen within each zone of (B) gradient hydrogel cultured without blebbistatin; and (C) gradient hydrogels cultured with blebbistatin treatment. (Scale bar = 50 μm). Color images available online at www.liebertpub.com/tea
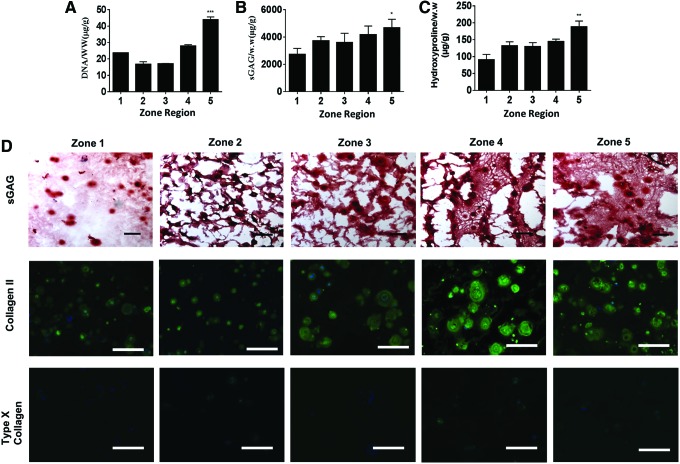
The function of engineered cartilage is largely determined by the amount and distribution of newly deposited cartilage matrix over time. To characterize the newly deposited cartilage matrix produced by chondrocytes in different zones, we measured cell proliferation and the accumulative amount of neocartilage matrix, including sGAG and collagen, after 21 days of culture. Increasing matrix stiffness from zone 1 to zone 5 led to increased chondrocyte proliferation, sGAG, and collagen (Fig. 4A–C). Interestingly, a reverse trend of proliferation was observed on day 21 (Fig. 4A) compared to that of day 7 (Fig. 2G). This suggests that cell proliferation was initially inhibited by the higher physical constraint of stiffer hydrogels. However, as chondrocytes degrade hydrogels over time to overcome such physical constraint, chondrocyte proliferation increased in stiffer zones. To visualize the distribution and phenotype of newly deposited matrix, histology and immunostaining were performed. Safranin-O staining of sGAG and immunostaining of type II collagen showed that increasing stiffness in gradient hydrogels led to larger nodules of neocartilage and more ECM matrix deposition after a 3-week culture (Fig. 4D). This trend agrees with biochemical assays (Fig. 4B, C) and gene expression observations (Fig. 2) Furthermore, chondrocytes in all zones produced minimal type I collagen (Supplementary Fig. S3), suggesting minimal undesirable fibrocartilage phenotype. Interestingly, type X collagen on day 21 (Fig. 4D) decreased significantly compared to day 7 (Fig. 3), indicating that gradient hydrogels downregulated hypertrophy phenotype. In addition, neonatal chondrocytes exhibit very high capability of degradation, but the deposition at 3 weeks has not yet caught up the degradation; therefore, we have observed a slight decrease in the mechanical property of gradient hydrogels after the 3-week cultivation (Supplementary Fig. S4). Together, these findings suggest that our gradient hydrogels provide a 3D niche for guiding chondrocytes to regenerate articular cartilage with desirable phenotype in a zonal organized manner. A previous study by Callahan et al. has employed gradient hydrogels to examine chondrocyte phenotype.50 Interestingly, they reported that increasing hydrogel stiffness led to a decreased cartilage matrix deposition, which is opposite to our observation. One of the major differences between their study and this study is the phenotype of cells. We used bovine neonatal chondrocytes, which are highly proliferative and possess strong cartilage-producing and matrix-remodeling capability. In contrast, Callahan study used human osteoarthritic chondrocytes, which have poor ability to proliferate, produce matrix, or degrade the hydrogel matrix. As such, although their gradient hydrogels show overlapping stiffness gradients as this study, the compromised phenotype of osteoarthritic chondrocytes did not lead to substantial cartilage formation throughout the stiffness range. Together, these results highlight the importance of choosing the right cell phenotype in combination with the gradient niche cues to achieve desirable new tissue formation with zone-specific responses.
FIG. 4.
Biochemical and immunostaining of cartilage markers of chondrocytes within gradient hydrogel. (A–C) Chondrocyte proliferation and extracellular protein deposition after the 3-week culture in gradient hydrogel (*p < 0.05; **p < 0.01; ***p < 0.001 compared to zone 1); (D) immunostaining of type II and type X collagen, and Safranin-O staining of sGAG proteins within gradient hydrogel. Type X collagen after 3 weeks of culture is minimal compared to that of type II collagen and sGAG, suggesting the hyaline cartilage formation instead of hypertrophic chondrocyte phenotypes. (Scale bar = 100 μm). Color images available online at www.liebertpub.com/tea
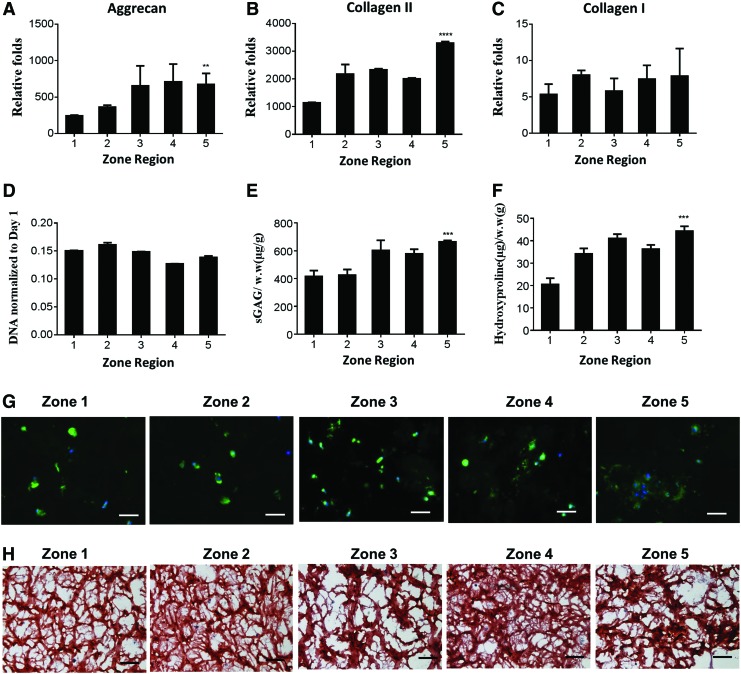
Bone marrow-derived MSCs are more abundantly available and expandable than chondrocytes, representing a promising autologous cell source for cartilage repair.51 We examined how gradients of matrix stiffness influence MSC chondrogenesis in 3D by encapsulating MSCs homogeneously in mechanical-gradient hydrogels. High cell viability was observed throughout all zones 24 h after encapsulation (Supplementary Fig. S5A) with comparable cell number across all zones (Supplementary Fig. S5B). Increasing matrix stiffness led to a stiffness-dependent increase in cartilage-specific gene expression by MSCs, with two to threefold higher expression of the genes encoding aggrecan (Fig. 5A) and type II collagen (Fig. 5B) in the stiffer zone (zone 5) compared to the softer zone (zone 1). Type I collagen expression did not significantly differ across stiffness zones (Fig. 5C). Unlike chondrocytes, MSCs have a limited capacity to degrade hydrogels in 3D and cell numbers often decline over time. Within the stiffness range tested in this study, MSCs exhibited decreases in DNA content in all zones (Fig. 5D). After the 3 week of culture, ECM (sGAG and collagen) deposition by MSCs increased in a stiffness-dependent manner (Fig. 5E, F), a trend that was confirmed by immunostaining of collagen II (Fig. 5G) and histological staining of sGAG (Fig. 5H). While the reported platform allows recreation of zonal organization of stiffness and sGAG transition consistent with native cartilage from superficial zone to deep zone, the trend of type II collagen transition is opposite to that of native cartilage. Specifically, increasing matrix stiffness led to increasing type II deposition, yet in native cartilage, less type II collagen was present in deep zone cartilage with higher stiffness. It is a challenge to decouple the production of sGAG and type II collagen production by encapsulated cells in 3D using this platform. Future studies may employ additional cues to induce opposite trend of type II collagen production that better mimics native cartilage organization
FIG. 5.
Quantitative gene expressions and biochemical assays of hMSCs in 3D gradient hydrogel. (A–C) Cartilage markers (Agg, Col II) and Collagen I gene expressions within each zone of the gradient hydrogel (day 7 expressions normalized to day 0 MSCs); (D) day 21 DNA normalized to day 1 DNA; (E, F) sGAG and hydroxyproline per wet weight measured within each zone of the gradient hydrogel. (**p < 0.01; ***p < 0.001; ****p < 0.0001 compared to zone 1); (G) Immunostaining of Collagen II protein within gradient hydrogel (scale bar = 50 μm); (H) Safranin-O staining of sGAG within each zone of gradient hydrogel is shown (scale bar = 100 μm). hMSC, adult human mesenchymal stem cell; MSC, mesenchymal stem cell. Color images available online at www.liebertpub.com/tea
Conclusions
In conclusion, in this study, we report a facile method for engineering tissue-scale gradient hydrogels for use as a 3D cell niche to better recapitulate tissue zonal organization. Compared to previous work on developing biomaterials to induce tissue zonal organization, the platform reported in this study is superior in the following aspects: (1) enabling fabricating clinically relevant scale tissues with gradient cues and (2) accelerating the speed of gradient hydrogel formation. These gradient hydrogels induced zone-appropriate behavior in encapsulated cells and prompted the deposition of new cartilage ECM that mimicked articular cartilage zonal structures. This platform can be easily adapted to create gradient hydrogels with tissue-specific biochemical and mechanical cues. While we focused on recreating articular cartilage zonal organization in this investigation, we expect that this platform will serve as a versatile tool for engineering other interfacial tissues such as ligament, tendon, and osteochondral interface. In addition, our platform facilitates high-throughput screening for elucidating complex stem cell–niche interactions across a range of biochemical/mechanical cues for studying cell types such as cancer cells.
Supplementary Material
Acknowledgments
This work was supported by the following grants: NIH R01DE024772 (F.Y.), NSF CAREER award (CBET-1351289) (F.Y.), and California Institute for Regenerative Medicine Tools and Technologies Award (RT3-07804) (F.Y.). The authors also acknowledge funding from the Stanford Chem-H Institute (F.Y.), Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), and Alliance for Cancer Gene Therapy Young Investigator award grant (F.Y.). D.Q.Z. would like to thank Stanford Graduate Fellowship and Stanford Bio-X Interdisciplinary Program SIGF Fellowship for support.
Disclosure Statement
No competing financial interest exists.
References
- 1.Tew S.R., et al. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum 43, 215, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Sharma B., et al. Designing zonal organization into tissue-engineered cartilage. Tissue Eng 13, 405, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Sundararaghavan H.G., and Burdick J.A. Gradients with depth in electrospun fibrous scaffolds for directed cell behavior. Biomacromolecules 12, 2344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon J.J., et al. Micropatterning of poly(ethylene glycol) diacrylate hydrogels with biomolecules to regulate and guide endothelial morphogenesis. Tissue Eng Part A 15, 579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant S.J., et al. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng 86, 747, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Gama C.I., et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2, 467, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Klein T.J., et al. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev 15, 143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein T.J., et al. Strategies for zonal cartilage repair using hydrogels. Macromol Biosci 9, 1049, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Du Y., et al. Convection-driven generation of long-range material gradients. Biomaterials 31, 2686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dormer N.H., et al. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A 100, 162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M., et al. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng Part C Methods 14, 299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martins R.P., et al. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng 14, 431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert P.M., et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammoto T., et al. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell 21, 758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T.K., et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage 11, 653, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Klein T.J., et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage 11, 595, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Schuurman W., et al. Cartilage regeneration using zonal chondrocyte subpopulations: a promising approach or an overcomplicated strategy? J Tissue Eng Regen Med 9, 669, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen L.H., et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 32, 6946, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kshitiz , et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol 4, 1008, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy W.L., McDevitt T.C., and Engler A.J. Materials as stem cell regulators. Nat Mater 13, 547, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsberg E., von Recum H.A., and Mahoney M.J. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin Biol Ther 6, 847, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Camarero-Espinosa S., et al. Directed cell growth in multi-zonal scaffolds for cartilage tissue engineering. Biomaterials 74, 42, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Spitters T.W., et al. Glucose gradients influence zonal matrix deposition in 3D cartilage constructs. Tissue Eng Part A 20, 3270, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harley B.A., et al. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A 92, 1078, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Wang X., et al. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release 134, 81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karimi T., et al. A developmentally inspired combined mechanical and biochemical signaling approach on zonal lineage commitment of mesenchymal stem cells in articular cartilage regeneration. Integr Biol (Camb) 7, 112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo C.M., et al. Cell movement is guided by the rigidity of the substrate. Biophys J 79, 144, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong J.Y., et al. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir 19, 1908, 2003 [Google Scholar]
- 29.He J., et al. Rapid generation of biologically relevant hydrogels containing long-range chemical gradients. Adv Funct Mater 20, 131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J.Y., et al. Simultaneous generation of chemical concentration and mechanical shear stress gradients using microfluidic osmotic flow comparable to interstitial flow. Lab Chip 9, 2194, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Fairbanks B.D., et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater 21, 5005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson S.B., et al. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials 32, 3564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai J.H., et al. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep 3, 3553, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai L., Dewi R.E., and Heilshorn S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Funct Mater 25, 1344, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon O., et al. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv Mater 25, 6366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karpiak J.V., Ner Y., and Almutairi A. Density gradient multilayer polymerization for creating complex tissue. Adv Mater 24, 1466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taipale J., and Keski-Oja J. Growth factors in the extracellular matrix. FASEB J 11, 51, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Millward-Sadler S.J., and Salter D.M. Integrin-dependent signal cascades in chondrocyte mechanotransduction. Ann Biomed Eng 32, 435, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury T.T., et al. Integrin-mediated mechanotransduction processes in TGFbeta-stimulated monolayer-expanded chondrocytes. Biochem Biophys Res Commun 318, 873, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Deschner J., et al. Signal transduction by mechanical strain in chondrocytes. Curr Opin Clin Nutr Metab Care 6, 289, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee D.A., et al. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology 37, 149, 2000 [PubMed] [Google Scholar]
- 42.Engler A.J., et al. Matrix elasticity directs stem cell lineage specification. Cell, 126, 677, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Saha K., et al. Substrate modulus directs neural stem cell behavior. Biophys J 95, 4426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furumatsu T., and Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama 64, 351, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Han Y., and Lefebvre V. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol Cell Biol 28, 4999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haudenschild D.R., et al. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum 62, 191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeon J.E., et al. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis Cartilage 20, 906–915, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Poole A.R., et al. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res (391 Suppl), S26, 2001 [DOI] [PubMed] [Google Scholar]
- 49.van der Kraan P.M., and van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage 20, 223, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Callahan L.A., et al. Primary human chondrocyte extracellular matrix formation and phenotype maintenance using RGD-derivatized PEGDM hydrogels possessing a continuous Young's modulus gradient. Acta Biomater 9, 6095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.