Abstract
Breastfeeding is an experience that only a mother and her infant(s) can share. Infants who can feed from the breast receive not only the best nutrition but also, due to the close physical contact between mother and child, it is the optimal nurturance they can receive from their mother. When breastfeeding is trouble free, maternal well-being is uniquely heightened. However, breastfeeding remains a challenge for many mother-infant dyads and more so for those whose infants are born prematurely. This article introduces a conceptual model of the breastfeeding challenges facing preterm mother-infant dyads. It distinguishes between a maternal caregiving and an infant growth/development components. Within the maternal component, two primary elements are considered, that is, maternal behavioral and nutritional care. The two primary elements within the infant component include infant non-nutritional and nutritional growth/development. It is proposed that an improved understanding of the factors associated with these four elements and how they interplay with each other within individual dyads will facilitate the identification of the breastfeeding challenges facing these mother-infant entities. Due to the intimate relationships existing between a mother and her infant(s), it is further advanced that breastfeeding studies would be optimized if mother-infant pairs are studied as one entity rather than mother and infant separately. It is proposed that this conceptual model will assist health professionals develop personalized breastfeeding management plans for individual preterm mother-infant dyads, while furthering the development of evidence-based interventions to optimize their breastfeeding experiences.
Keywords: : infant growth/development, infant oral feeding, stress, lactation, maternal behavior, mother-infant bonding
Introduction
The importance of breastfeeding has been advocated for more than two decades and is supported by increasing professional and public support throughout the world.1–9 Infants feeding from the breast receive not only the best nutrition but also, due to the close physical contact between mother and child, they are provided with the optimal nurturance from their mother.10–12 Breastfeeding initiation and maintenance, however, remain a challenge for many mother-infant dyads.2 It is an undertaking that does not involve only a mother and her infant, but often that of well-intentioned family members and friends, as well as health professionals when necessary. It may also be compounded by uncontrollable environmental and social factors such as family obligation and support, lifestyle, maternal characteristics, and health.13–15
For the majority of mothers whose infants are born term, this process evolves naturally. However, it is not so when mothers are stressed and/or their infants are sick. For instance, following a premature delivery with infants cared in neonatal intensive care units (NICU), the mother-preterm infant dyad is threatened by the fragility and immaturity of the baby and the unique stress that their mothers experience in the NICU environment.16,17
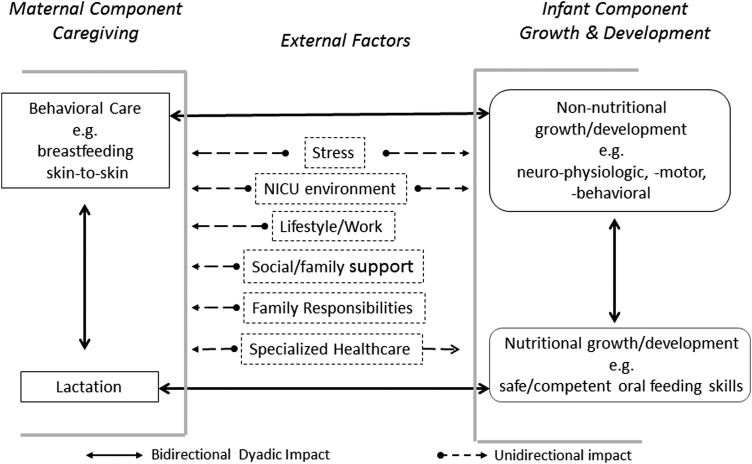
In an attempt to better understand the breastfeeding challenges facing preterm mother-infant dyads, a conceptual model is introduced that distinguishes between two components, that is, a maternal caregiving and an infant growth/development component (Fig. 1). In turn, the maternal caregiving component comprises two elements, maternal behavioral care and nutritional care/lactation, whereas the infant component distinguishes between their non-nutritional and nutritional growth/development. This model is based on the working premise that, during breastfeeding, these four elements are intimately connected and continually feedback positively or negatively on both partners of the dyad, modulating their interactions. It is advanced that in examining their individual interplays, a clearer understanding would be gained as to how a mother and her infant reciprocal responses impact on one another's actions.
FIG. 1.
Conceptual model of breastfeeding challenges facing preterm mother-infant dyad—A model presenting four major elements, maternal behavioral and nutritional care and infant non-nutritional and nutritional growth/development, making up the preterm mother-infant dyad with examples of external factors that may impact mother-infant breastfeeding interactions. The bilateral interactions (solid line arrows) between maternal and infant elements represent the reciprocal impact that each partner's actions may have on the other, be they beneficial or detrimental. The external factors (unidirectional dashed arrows) have a unidirectional impact on mother and/or infant as they are not controlled by the dyad, be they beneficial or detrimental
It is well recognized that there exists many more factors that can disrupt the equilibrium described in Figure 1 such as maternal health or attributes, infant's genetic makeup, cultural/societal dogmas, and/or governmental policies. However, it is advanced that the four elements presented in this model are the ones that have the most direct impact on breastfeeding performance. On this basis, one may consider these “disruptive” elements as stressors to the breastfeeding mother-infant dyad.
The Preterm Mother-Infant Dyad
“Breastfeeding” is defined herein as a maternal behavior insofar as it is a mother's prerogative to breastfeed that will allow her infant to feed at the breast rather than from a bottle. In turn, given such opportunity, infants can only breastfeed successfully if their oral feeding skills are sufficiently mature to allow them to remain latched on to the breast for the duration of a breastfeeding session.
The proposed conceptual framework is presented in Figure 1 with maternal behavior and nutritional care under the maternal component and infant non-nutritive and nutritive growth/development under the infant component. In addition, major “external factors,” for example, stress and NICU environment, which may support or hinder a mother and/or her preterm infant relationships are listed.
Maternal behavioral care defines the interactive actions initiated by mothers toward their infant(s), for example, willingness/interest in breastfeeding, milk expression if breastfeeding is not feasible, skin-to-skin holding, and daily routine care for the infant's welfare. Nutritional care (lactation) relates to mothers' ability to provide the appropriate supply of their own milk to meet their infants' nutritional needs. In turn, infants' non-nutritional growth/development addresses their neurophysiologic, neuromotor, and neurobehavioral development, whereas the nutritional element focuses on infants' ability to breastfeed safely and competently to grow. Within the dyad, the relationship that mothers develop with their infant(s) at the time of birth is essential in optimizing the proper establishment of maternal caregiving and infant growth/development.
Mother-infant interactions implicate a continual bidirectional feedback system as their respective actions reciprocally impact on one another, be they beneficial or not. This relationship would be optimized if it is mutualistic, that is, a reciprocal “give and take” interaction as both partners benefit from one another's sensory and behavioral exchanges, thereby optimizing the development of the mother-infant dyad.18,19 The external factors predominantly have a unidirectional impact on these four dyadic elements and are relatively “uncontrollable,” that is, their actions being imposed by environmental and social conditions and/or well-intentioned care providers. It is the complexity of all these interactions that often challenges the ability of mothers and infants to succeed in breastfeeding.
The interactive “give and take” that develops between the partners of the dyad may be “balanced” as often observed between a mother and her healthy infant or “imbalanced” when one and/or both partners encounter difficulties of their own.20,21 Following a premature delivery, the “imbalanced exchanges” primarily arise from infants' immaturity, fragility, and unstable medical status that require the care of neonatal specialists. Along with prolonged hospitalization in the stark environment of an NICU, the normal maternal caregiving is disrupted with breastfeeding initiation and maintenance frequently deferred. It is well acknowledged that these unexpected detrimental events become significant stressors for these mothers.22–28
Mother caregiving
Maternal behavioral care
Within the animal kingdom, humans are considered “semialtricial” as newborns are relatively helpless and must rely on mother for nurturance, nutrition, and locomotion.19,29 Mother can be viewed as the active partner of the dyad, the initiator who nurtures and feeds her child. Although infants may appear to be passive recipients, their responses to maternal investment are essential for maintaining the quality of maternal care as both partners' reciprocal feedback is required to ensure the dyad's integrity.19,30–32 Studies have speculated on the genetics of maternal behavior (motherhood) and their potential in affecting maternal caregiving through neurohormonal involvement and activation of specific areas of the central nervous system (CNS), for example, hypothalamus, amygdala, and medial preoptic area.29,31,33–35
Maternal behavioral care includes functions such as nurturing, maintaining close physical contact with the infant through skin-to-skin holding, attachment/bonding, appropriate responsiveness to infant's cues to meet their needs, and provision of needed care that the infant cannot perform.21,36–42 Over time, “balanced” mutualistic exchanges would be achieved if maternal and infant behaviors are synchronized with both partners continually adjusting to their reciprocal growing needs and development.40,43,44 Breastfeeding and skin-to-skin holding strengthen such mother-infant communications.37,45–47
However, a mother-infant dyad can also be tainted by maternal characteristics, stress, and environmental factors, for example, responsibilities toward other children, occupation, and marital relationship.13–15,40 If maternal behavioral care implicates hormonal stimulation, for example, prolactin and oxytocin, in return, its maintenance necessitates the continued presence and feedback of the young by mother's side.33,48–50 Attachment between mother and infant will be “secure” if mother is responsive, protective, and sensitive to her infant's emotional and physical needs, and “insecure” or “anxious” if mother is unpredictable, distant, and neglectful. Such early patterns of “attachment security” can have a long-lasting positive or negative impact on both mother and child.42,49,51–59
Nutritional care
To safeguard lactation, it is necessary to have a good understanding of both the physiology of lactation and maternal interest in lactating.2,60 Under normal circumstances, lactation performance is a function of “supply and demand,” namely, the greater an infant's nutritional need, the greater a mother's milk production/ejection.
Neville early on differentiated between two stages of lactation, namely Lactogenesis I consisting of the mammary glandular and ductal development, that is, their cellular and enzymatic differentiation, followed by Lactogenesis II pertaining to milk synthesis and ejection.61 It is important to recognize that milk synthesis is dependent upon the adequate presence of lactogenic hormones, for example, prolactin, leptin, opiates, and insulin, while milk ejection from the breast is primarily dependent upon the release of oxytocin from the posterior pituitary. As such, milk synthesis and ejection are regulated by two separate neuroendocrine functions that singly or together can affect the overall lactation performance of a mother (Stress and lactation section below).
Following the normal gestational period and birth of a term infant, it is generally expected that proper lactation will occur as a result of the normal anatomical and physiologic development of the mammary function. However, it remains uncertain whether following a shortened gestation due to a premature delivery, Lactogenesis I and II have sufficiently advanced to allow for proper milk synthesis and ejection. Understandably, the functionality of Lactogenesis I has been difficult to study at the cellular/molecular level. In regard to Lactogenesis II, results have been inconclusive. As the release of appropriate lactogenic hormones necessary for milk synthesis and ejection is a two-step neuroendocrine reflex, it is dependent upon the proper development of neural networks originating from the mammary sensory receptors to the CNS. Again, following a premature delivery and depending on the shortened gestation period involved, it is uncertain the extent to which such development has occurred.2,62,63
Indeed, if this reflex has not “fully” developed, one may speculate that maternal neurosensory and/or neuroendocrine responsiveness to breastfeeding would be hindered resulting in a decrease in milk synthesis and/or ejection. Peripheral factors such as mother's nipple shape and degree of elasticity and protractility may impede breastfeeding as they can play a determining role in the infant's ability to latch onto the nipple-areola complex.2 As lactation is a function of supply and demand, any decrease in infant's demand, whether due to infant's inability to latch on, immature nutritive sucking skills, poor endurance, and/or disinterest in breastfeeding would lead to decreased milk availability.
Infant growth/development
Non-nutritional growth/development
The non-nutritional benefits offered by breastfeeding to the preterm dyad relate to the impact that the frequent and close physical contact between mother and infant has in stimulating infant growth/development. This is substantiated by a number of studies in animals and humans showing the benefits of tactile stimulation.46,64–69 Infant growth/development can be measured by outcomes such as weight gain and maturation of physiological and neural functions. However, motor movement is one of the most examined areas of development because it is a resultant of the maturation of combined anatomical, peripheral, and central neurophysiologic and neuromotor functions. In addition, its ease of observation facilitates the identification of objective measures to follow its evolution.
Thelen70 has proposed that motor behavior or movements are observable “outputs” that emerge in a “self-organizing manner,” be it appropriate or not, from interactions between diverse subsystems, for example, physiological, biomechanical, and psychological.71 Under such definition, infants' ability to safely and competently breastfeed may be considered one of the earliest markers of non-nutritional growth/development.
It is also believed that brain plasticity reorganizes sensorimotor areas in response to repetitive beneficial or detrimental practices.72,73 If such “practice makes perfect” approach is constructive, preterm infants' physical isolation that limits sensory stimulations during their NICU stay would put them at a disadvantage. From the infants' standpoint, their direct physical/sensory contact with mother would be crucial for their growth/development.74 Therefore, the multisensory exchanges between mother and infant play an essential part in the safeguard of a close-knit relationship, proper development of maternal behavioral care, and infant growth/development.
Nutritional growth/development
Preterm infants' inability to transition readily from tube to independent breastfeeding or bottle feeding is an example of the motor developmental model discussed above. Unlike term infants, preterm infants have less endurance and may not have yet acquired the mature nutritive sucking skills that allow them to feed by mouth efficiently and safely.75,76 This is due to their immature nutritive sucking skills and their inability to coordinate suck-swallow-respiration-esophageal function.76–79 Indeed, with nutritive sucking occurring at 1 suck/sec,80 accumulation of milk in the oral cavity due to any delay in bolus formation and/or transport down to the stomach through the pharynx and esophagus would increase risks of adverse events, for example, choking, regurgitation, oxygen desaturation, and penetration/aspiration into the lungs.
Caregivers do not have a means to assess the maturity level of their infants' nutritive skills and often have taken to accelerating the advancement of daily oral feedings without necessarily evaluating its appropriateness for their individual patients. This, not only puts infants at risk of adverse events but also raises the risk of long-term oral feeding aversion.2,81 Therefore, one may speculate that oral feeding experience will be successful and safe, if sucking, swallowing, respiration, esophageal activity, their coordinated activities, infant behavioral states, and feeding positions are appropriate.82–84
Preterm mother-infant dyad interplay
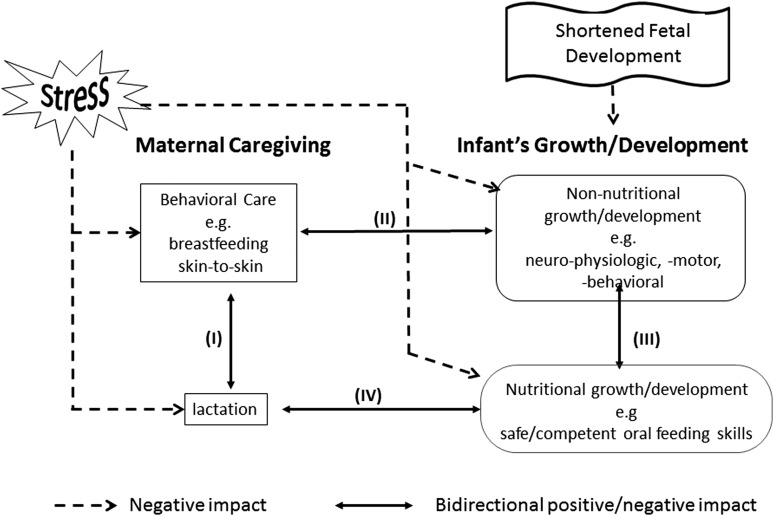
Figure 2 shows how the four elements presented in this conceptual model are so intricately linked that alterations in any one of them can readily lead to an unsafe disequilibrium between mother and infant. Such imbalance, if remained undetected, can lead to a multilevel downward spiral, not only threatening breastfeeding success but also the integrity of the whole dyad. This scenario does not pertain only to mother-preterm infants, but any high-risk infant with chronic conditions, for example, bronchopulmonary dysplasia and cardiac or congenital anomalies.
FIG. 2.
Mother-infant dyad interplays and the negative impact of prematurity and stress—schematic presenting the four interactive pathways (I, II, III, and IV) between mother and infant, be they beneficial or detrimental, and how stress may singly or together impact negatively the four elements of the mother-infant dyad.
The proposed four elements highlight four interactive pathways between and within mother and infant components that can challenge the breastfeeding performance of the dyad. It was mentioned earlier that the quality of these interplays are dependent upon the reciprocal feedbacks between mother and child as they can be positive/beneficial or negative/detrimental depending upon the partners' respective circumstances.
Pathway I relates to the interaction between maternal behavior and lactation. Indeed, the greater a mother's drive to breastfeed, the more likely her milk availability will increase,85 increasing the probability of her infant being exclusively breastfed or receiving only mother's milk. In reverse, poor lactation may negatively feedback to mother, potentially leading to a decreased drive in breastfeeding and/or interest in expressing milk,30 simultaneously increasing maternal stress. Pathway II shows the interactions between maternal behavior and infant non-nutritional growth/development that importantly relate to nurturing and bonding. If maternal behavior is inadequate, nurturing and caring/holding one's infant close would decrease affecting infant's growth/development. In return, if infant non-nutritional growth/development was delayed due to a shortened fetal development or infant postnatal fragility, mother-infant contact would be reduced along with increased maternal stress, overall leading to a decrease in maternal-infant interaction. Pathway III presents the interactions between an infant's non-nutritive and nutritive growth/development. With a delayed non-nutritive growth/development, an infant's neurophysiologic and neuromotor maturation would likely impact on his/her oral feeding skills and ability to breastfeed safely and competently. In return, such difficulty would reduce the nutritional and nurturing benefits that breastfeeding offers, further hindering the infant's overall growth/development. Pathway IV relates to the feedback between infant breastfeeding aptitude and lactation.
Safe and competent neonatal oral feeding is a complex dynamic system that relies on the coordination of multiple physiologic functions, behavioral state dynamics, and social interactions.86 If an infant cannot latch on and remain latched onto the breast due to immature nutritive sucking skills and/or poor endurance, lactation over time will decrease and eventually cease. This may result from various causes, for example, the “supply and demand” principle mentioned earlier, as a decreased demand from the part of the infant will reduce milk supply; infant's inability to remain on the breast will likely increase maternal stress, leading to a decrease in milk production and/or maternal drive to breastfeeding; and with poor lactation, baby in turn will lose interest in breastfeeding and turn to bottle feeding.
Stress impact on the preterm mother-infant dyad
Figure 2 also illustrates how stress may differentially impact on the four elements of the mother-infant dyad depending on the nature of the stressor. Stress may be generated separately from within the individual, mother or child, or both partners of the dyad, or arise from external factors.
Stress and maternal behavioral care
The birth of an infant engenders a certain level of maternal stress. It is not only due to concern over the well-being and caring of the newborn but also due to the adjustments mothers need to make in their own life. A broad range of factors can affect the ease with which women settle into their new role as mothers, for example, maternal characteristics, coping skills, depression, anxiety, personal health, socioeconomic status, family/social support, life style, and/or work.13,14,17,60,87–90 All these factors potentially may hamper women's transition to motherhood. As such, it is understandable that maternal stress may worsen with the birth of a premature high-risk infant. It is now well recognized that mothers' inability to act as a parent in the stark and high-pressured environment of an NICU is a definite stressor.16,17,91
Animal studies have reported that a 4-hour separation between a dam and her pups increases the anxiety-generated c-fos activity in specific brain regions of postpartum rats.92 In an earlier study, we observed that dams' corticosterone stress response to various stressors is dampened when compared to virgin rats, but is significantly heightened when the stressor threatens their young.93 A number of studies conducted on breastfeeding women and animals are supportive of such an altered effect of stress during lactation, suggesting that involvement of oxytocin, prolactin, brain CRF-binding protein, and opiates, and activation of the hypothalamo-pituitary-adrenocortical axis are likely implicated.93–98
Over the last two decades, the recognition of oxytocin as a “social” hormone and its importance in the protection of the mother-infant dyad does not relate only to its lactogenic properties, but just as importantly to its social/behavioral impact on motherhood.31,51,54,99–106 The intimate relationship existing between a mother-infant dyad106 is exemplified by a number of studies demonstrating that maternal well-being or lack thereof is echoed in her child. Maternal increase in circulating cortisol levels during stress has been associated with corresponding changes in their infant.106 During times of “balanced” interplays, heart rhythms are coordinated between mother and infant.107
Studies have reported 34% to 40% of mothers of very low birth weight infants were significantly depressed compared to 8–15% of mothers of healthy term infant.17,106,108–110 On the other hand, a meta-analysis study found that “parents of preterm-born children experience only slightly more stress than parents of term-born children, with small effect sizes”.111 This may be explained by the resilience of mothers whose infants are in an NICU as they find themselves in a situation that they cannot control.112 However, in an earlier study focused on mothers of infants born between 26 and 29 weeks gestation, we noted that mother's responses to self-reported questionnaires pertaining to depression and parental stress in the NICU were significantly correlated to the individual subjects' social desirability trait. Social desirability bias is one's tendency to overreport or underreport good or bad behaviors to be regarded more favorably by others.17 Thus, interpretation of self-reported data needs to be more closely scrutinized to ensure the truthfulness of subjects' responses.
Maternal depression has been linked to decreased quality of mother-infant interaction and attachment, more specifically, decreased positive affective involvement and communication as well as breastfeeding performance.17,113,114 More severe and longer lasting psychological conditions such as posttraumatic stress situation have been reported in mothers of preterm infants.115–118 With a high-risk infant, this dyadic interaction may be further affected by maternal feelings of disappointment, guilt, insecurity, and choice not “to engage actively in mothering” for fear that one's infant may die.16,119–121 Therefore, the ability of a mother to compensate for her infant's difficulties through her behavior and traits, for example, resilience, ability to cope, and sensitivity to her infant's cues, is a strong determinant of their dyadic outcome.122–124 In a study conducted on physiotherapists' perceptions about the major obstacles to successful breastfeeding, three categories were identified: maternal obstacles, health professionals, and society. Maternal obstacles comprised lack of motivation, insufficient knowledge, anxiety, and work. Health professionals' obstacles included lack of support, inappropriate lactation management, lack of knowledge/conflicting advice, negative attitudes, and staff shortages. Societal obstacles consisted primarily of lack of social support and lifestyles. However, for these mothers, the most important methods of motivation to maintain breastfeeding pertained to increased information/education and contact with other breastfeeders,87 an observation also supported by other studies.125–127
Stress and lactation
Based on human and animal studies, the suppressive effect of stress on lactation is generally well recognized. However, the research literature does not support such a consistent outcome. This is likely due to the wide variations in study designs, methodologies, as well as subjects' characteristics/traits, particularly as it relates to clinical studies.93,128,129 Nevertheless, such apparent inconsistencies only emphasize the importance of not “rushing” into generalizing that a particular stressor may be beneficial, detrimental, or have no effect insofar as maternal responses may also be influenced at the same time by the presence of additional environmental and physiologic factors, or their infant feedback to breastfeeding.
From animal research, we have a good physiologic understanding of how and why stress can inhibit lactation at the level of the CNS and peripherally on milk synthesis and ejection. Breastfeeding stimulates the release of the lactogenic hormones in the CNS, for example, prolactin from the anterior pituitary for milk synthesis and oxytocin from the posterior pituitary for milk ejection. There is anatomical evidence of interconnections between the neuroendocrine hypothalamus and the central autonomic system that can directly alter lactogenic hormones at the hypothalamic level or indirectly through catecholaminergic and peptidergic neural networks.130–133 Peripheral inhibition of milk synthesis and ejection may occur as stimulation of the central and/or peripheral autonomic systems can lead to vasoconstriction resulting in decreased hormonal delivery to the mammary alveoli and myoepithelium, respectively.60,134–136 The time delay between lactogenic hormone release and the resulting milk synthesized in the mammary alveoli would appear prolonged hours.137 This contrasts with the release of milk stored in the mammary alveoli that occurs immediately in response to oxytocin. As such, depending upon the duration of a stressor, acute or prolonged, suppression of lactation may result from a decrease in milk synthesis and/or ejection. Consequently, when mother's milk availability decreases under stress, it becomes difficult to determine whether it is a result of a decrease in milk synthesis and/or milk release.
Stress and infant growth/development
Being born prematurely is a major stressor that infants encounter. Nevertheless, with immature neurophysiologic functions and underdeveloped organs, they must adapt to their ex-utero environment to survive. Consequently, during their time in the NICU, infants are faced with a variety of stressors that they have no control over. As safe oral feeding is one of the last milestones they need to attain before hospital discharge, some of these infants may not have reached the developmental stage that allows them to readily feed by mouth. Under such circumstances, breastfeeding and bottle feeding can become an additional daily struggle that we know can lead to unsafe and inefficient feeding along with long-term feeding aversion.2 Although safe and competent oral feeding require mature skills, it is important to remember that oral feeding difficulties can also be of nonoral origins, for example, infant's clinical status at feeding time and during the feeding (e.g., cardiorespiratory status and fatigue), the NICU environment (e.g., light and noise level), infant's own behavioral states (e.g., sleepy, quiet alert, or crying), and/or organization (e.g., calm or agitated).138–140
The NICU Setting
The caring of preterm infants, due to their fragility, rests initially and understandably in the hands of the neonatal medical team in NICUs. Such setting drastically restricts opportunities for mother-infant direct contact. In addition, after overcoming the immediate life-threatening and damaging consequences of chronic conditions such as intraventricular hemorrhage and necrotizing enterocolitis, these infants' hospital discharge is often delayed by their inability to feed by mouth as attainment of independent oral feeding is one of the criteria recommended by the AAP for hospital discharge.141 Consequently, the longer the transition from tube to independent oral feeding, the longer the hospitalization will be.142,143
Within a NICU, introduction and progression of oral feeding, be it breast or bottle, involve the input of the multidisciplinary medical team caring for the infant that includes, not only neonatologists, neonatal nurse practitioners, and neonatal nurses but often feeding specialists, for example, occupational therapists, speech pathologists, neonatal nutritionists, and lactation consultants when breastfeeding is concerned. Under such circumstances, mother and baby receive recommendations from all members of the team. If these messages are not consistent, confusion will likely arise with mothers uncertain about which feeding recommendation is best and infant needing to “adapt” to everyone's differing feeding approaches. This is a good example of an “uncontrollable” external factor mentioned earlier. Therefore, having one feeding plan agreed upon by all team members would be critical from the time a mother and her infant are introduced to oral feeding. Indeed, proper communication, relationship, and performance are important factors that will allow all players to work together effectively and successfully, just as it should be within a mother-infant dyad. At this time, this is unfortunately not common practice, but awareness that multidisciplinary interventions can be beneficial in the care of pediatric feeding disorders is growing.144
Conclusion
Due to the intimate interactive exchanges existing between a mother and her infant(s) and the knowledge that stress may impact mother and infant differentially and at the same time, it is clear that studying mother and infant independently from one another, as customarily done, is not productive. Studies relating to maternal stress following a premature delivery primarily focus on mothers or infants separately without considering how importantly one partner can affect the other. Thus, to begin deciphering the mother-preterm infant “breastfeeding puzzle”, it would be more relevant to consider the mother-infant pair as one entity rather than two separate entities, that is, mother and infant.
The four elements defined in this model could be readily monitored. For instance, maternal behavioral care may be evaluated by the frequency of maternal NICU visits and skin-to-skin holding; lactation by the frequency of breastfeeding events and milk volume collected during milk expression; infants' non-nutritional growth/development by their overall medical status; and infants'nutritional growth/development by their daily weight gain and oral feeding performance.
As such, there is a unique opportunity to determine within a dyad at risk, the elements within each partner that may benefit from support. In addition, in evaluating the reciprocal effects of mother-infant interplays, this approach would ensure that any clinical management plan developed to assist one partner will, at the least, not be detrimental to the other. As such, our working model may be envisaged as a potential “diagnostic” tool. In summary, this working concept would not only facilitate the selection of relevant interventions that could be offered but also importantly determine whether any ensuing benefits to one partner have relevance for the other.
Acknowledgments
The author wishes to thank her many collaborators who have supported her work over the years and for support from the National Institutes of Health (R01-HD-28140; HD 044469; MO1RR000188). The contents of this publication are solely the responsibility of the author and do not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
Disclosure Statement
The author had the sole responsibility for all parts of the article and has no conflict of interests to disclose.
References
- 1.Gartner LM, Eaton AP, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics 1997;100:1035–10399411381 [Google Scholar]
- 2.Lau C, Hurst N. Oral feeding in infants. Curr Probl Pediatr 1999;29:105–124 [DOI] [PubMed] [Google Scholar]
- 3.Victora CG, Barros FC, Horta BL, et al. Breastfeeding and school achievement in Brazilian adolescents. Acta Paediatr 2005;94:1656–1660 [DOI] [PubMed] [Google Scholar]
- 4.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007;153:1–186 [PMC free article] [PubMed] [Google Scholar]
- 5.Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol 2009;2:222–231 [PMC free article] [PubMed] [Google Scholar]
- 6.Renfrew MJ, McCormick FM, Wade A, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2012;5:CD001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horta BL, Loret de MC, Victora CG. Breastfeeding and intelligence: A systematic review and meta-analysis. Acta Paediatr 2015;104:14–19 [DOI] [PubMed] [Google Scholar]
- 8.Rollins NC, Bhandari N, Hajeebhoy N, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016;387:491–504 [DOI] [PubMed] [Google Scholar]
- 9.Feldman-Winter L. The AAP updates its policy on breastfeeding and reaches consensus on recommended duration of exclusive breastfeeding. J Hum Lact 2012;28:116–117 [DOI] [PubMed] [Google Scholar]
- 10.Smith JP, Forrester R. Maternal time use and nurturing: Analysis of the association between breastfeeding practice and time spent interacting with baby. Breastfeed Med 2017;12:269–278 [DOI] [PubMed] [Google Scholar]
- 11.Strathearn L, Mamun AA, Najman JM, et al. Does breastfeeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatrics 2009;123:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev Psychobiol 2003;43:109–119 [DOI] [PubMed] [Google Scholar]
- 13.Shin H, Park YJ, Kim MJ. Predictors of maternal sensitivity during the early postpartum period. J Adv Nurs 2006;55:425–434 [DOI] [PubMed] [Google Scholar]
- 14.Findler L, Taubman-Ben-Ari O, Jacob K. Internal and external contributors to maternal mental health and marital adaptation one year after birth: Comparisons of mothers of pre-term and full-term twins. Women Health 2007;46:39–60 [DOI] [PubMed] [Google Scholar]
- 15.Guendelman S, Kosa JL, Pearl M, et al. Juggling work and breastfeeding: Effects of maternity leave and occupational characteristics. Pediatrics 2009;123:e38–e46 [DOI] [PubMed] [Google Scholar]
- 16.Miles MS, Funk SG, Carlson J. Parental stressor scale: Neonatal intensive care unit. Nurs Res 1993;42:148–152 [PubMed] [Google Scholar]
- 17.Lau C, Hurst NM, Smith EO, et al. Ethnic/racial diversity, maternal stress, lactation and very low birthweight infants. J Perinatol 2007;27:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman R. Mutual influences between child emotion regulation and parent-child reciprocity support development across the first 10 years of life: Implications for developmental psychopathology. Dev Psychopathol 2015;27:1007–1023 [DOI] [PubMed] [Google Scholar]
- 19.Lau C, Henning SJ. Mutualism in mother-offspring interaction: Its importance in the regulation of milk release. In: Handbook of Human Growth and Developmental Biology, Meisami E, Timiras PS, eds. Boca Raton: CRC Press, Inc., 1990, pp. 195–216 [Google Scholar]
- 20.Lau C, Geddes D, Mizuno K, et al. The development of oral feeding skills in infants. Int J Pediatr 2012;2012:572341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman E, Lau C. The development of the mother-infant mutualistic screening scale. J Peds Mother Care 2017;2:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forcada-Guex M, Borghini A, Pierrehumbert B, et al. Prematurity, maternal posttraumatic stress and consequences on the mother-infant relationship. Early Hum Dev 2011;87:21–26 [DOI] [PubMed] [Google Scholar]
- 23.Yaari M, Millo I, Harel-Gadassi A, et al. Maternal resolution of preterm birth from 1 to 18 months. Attach Hum Dev 2017;19:487–503 [DOI] [PubMed] [Google Scholar]
- 24.Beck CT, Harrison L. Posttraumatic stress in mothers related to giving birth prematurely: A mixed research synthesis. J Am Psychiatr Nurses Assoc 2017;23:241–257 [DOI] [PubMed] [Google Scholar]
- 25.Ionio C, Colombo C, Brazzoduro V, et al. Mothers and fathers in NICU: The impact of preterm birth on parental distress. Eur J Psychol 2016;12:604–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietze TR, Rose FF, Moore TA. Maternal variables associated with physiologic stress and perinatal complications in preterm infants. J Neonatal Perinatal Med 2016;9:271–277 [DOI] [PubMed] [Google Scholar]
- 27.Holditch-Davis D, Santos H, Levy J, et al. Patterns of psychological distress in mothers of preterm infants. Infant Behav Dev 2015;41:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heydarpour S, Keshavarz Z, Bakhtiari M. Factors affecting adaptation to the role of motherhood in mothers of preterm infants admitted to the neonatal intensive care unit: A qualitative study. J Adv Nurs 2017;73:138–148 [DOI] [PubMed] [Google Scholar]
- 29.Numan M. Parental behavior. In: Reference Module in Neuroscience and Biobehavioral Psychology, Stein J, ed. Cambridge, MA: Elsevier, 2017, pp. 1–15 [Google Scholar]
- 30.Lau C. The effect of stress on lactation—its significance for the preterm infant. Adv Exp Med Biol 2002;503:91–97 [DOI] [PubMed] [Google Scholar]
- 31.Bridges RS. Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol 2015;36:178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bridges RS. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm Behav 2016;77:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridges RS. The genetics of motherhood. Nat Genet 1998;20:108–109 [DOI] [PubMed] [Google Scholar]
- 34.Kim P, Leckman JF, Mayes LC, et al. Perceived quality of maternal care in childhood and structure and function of mothers' brain. Dev Sci 2010;13:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim P, Leckman JF, Mayes LC, et al. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci 2010;124:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korja R, Maunu J, Kirjavainen J, et al. Mother-infant interaction is influenced by the amount of holding in preterm infants. Early Hum Dev 2008;84:257–267 [DOI] [PubMed] [Google Scholar]
- 37.Charpak N, Tessier R, Ruiz JG, et al. Twenty-year follow-up of kangaroo mother care versus traditional care. Pediatrics 2017;139: pii: [DOI] [PubMed] [Google Scholar]
- 38.Cho ES, Kim SJ, Kwon MS, et al. The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal-infant attachment, and maternal stress. J Pediatr Nurs 2016;31:430–438 [DOI] [PubMed] [Google Scholar]
- 39.Rosenblatt JS. Outline of the evolution of behavioral and nonbehavioral patterns of parental care among the vertebrates: Critical characteristics of mammalian and avian parental behavior. Scand J Psychol 2003;44:265–271 [DOI] [PubMed] [Google Scholar]
- 40.Swain JE, Lorberbaum JP, Kose S, et al. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry 2007;48:262–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guedeney N, Lamas C, Bekhechi V, et al. [Attachment process between an infant and his/her mother: The first year]. Arch Pediatr 2008;15 Suppl 1:S12–S19 [DOI] [PubMed] [Google Scholar]
- 42.Ferber SG, Feldman R, Makhoul IR. The development of maternal touch across the first year of life. Early Hum Dev 2008;84:363–370 [DOI] [PubMed] [Google Scholar]
- 43.Atzil S, Hendler T, Feldman R. The brain basis of social synchrony. Soc Cogn Affect Neurosci 2014;9:1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pratt M, Singer M, Kanat-Maymon Y, et al. Infant negative reactivity defines the effects of parent-child synchrony on physiological and behavioral regulation of social stress. Dev Psychopathol 2015;27:1191–1204 [DOI] [PubMed] [Google Scholar]
- 45.Kim P, Feldman R, Mayes LC, et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry 2011;52:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry 2014;75:56–64 [DOI] [PubMed] [Google Scholar]
- 47.Tessier R, Cristo M, Velez S, et al. Kangaroo mother care and the bonding hypothesis. Pediatrics 1998;102:e17. [DOI] [PubMed] [Google Scholar]
- 48.Brazelton TB, Tronick E, Adamson L, et al. Early mother-infant reciprocity. Ciba Foundation Symposium 1975;33:137–154 [DOI] [PubMed] [Google Scholar]
- 49.Bowlby J. A Secure Base: Parent-Child Attachment and Healthy Human Development. New York: Basic Books, 1988 [Google Scholar]
- 50.Li J, Kendall GE, Henderson S, et al. Maternal psychosocial well-being in pregnancy and breastfeeding duration. Acta Paediatr 2008;97:221–225 [DOI] [PubMed] [Google Scholar]
- 51.Apter-Levi Y, Zagoory-Sharon O, Feldman R. Oxytocin and vasopressin support distinct configurations of social synchrony. Brain Res 2014;1580:124–132 [DOI] [PubMed] [Google Scholar]
- 52.Torner L, Toschi N, Nava G, et al. Increased hypothalamic expression of prolactin in lactation: Involvement in behavioural and neuroendocrine stress responses. Eur J Neurosci 2002;15:1381–1389 [DOI] [PubMed] [Google Scholar]
- 53.Leuner B, Shors TJ. Learning during motherhood: A resistance to stress. Horm Behav 2006;50:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tops M, van Peer JM, Korf J, et al. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 2007;44:444–449 [DOI] [PubMed] [Google Scholar]
- 55.Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 2011;36:1249–1256 [DOI] [PubMed] [Google Scholar]
- 56.DeWitt SJ, Sparks JW, Swank PB, et al. Physical growth of low birthweight infants in the first year of life: Impact of maternal behaviors. Early Hum Dev 1997;47:19–34 [DOI] [PubMed] [Google Scholar]
- 57.Bergmeier H, Aksan N, McPhie S, et al. Mutually responsive orientation: A novel observational assessment of mother-child mealtime interactions. Appetite 2016;105:400–409 [DOI] [PubMed] [Google Scholar]
- 58.Leclere C, Viaux S, Avril M, et al. Why synchrony matters during mother-child interactions: A systematic review. PLoS One 2014;9:e113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantymaa M, Puura K, Luoma I, et al. Shared pleasure in early mother-infant interaction: Predicting lower levels of emotional and behavioral problems in the child and protecting against the influence of parental psychopathology. Infant Ment Health J 2015;36:223–237 [DOI] [PubMed] [Google Scholar]
- 60.Lau C. Effects of stress on lactation. Pediatr Clin North Am 2001;48:221–234 [DOI] [PubMed] [Google Scholar]
- 61.Neville MC, Morton J, Umemura S. Lactogenesis. The transition from pregnancy to lactation. Pediatr Clin North Am 2001;48:35–52 [DOI] [PubMed] [Google Scholar]
- 62.Hartmann P, Cregan M. Lactogenesis and the effets of insulin-dependent diabetes mellitus and prematurity. J Nutr 2001;131:3016S–3020S [DOI] [PubMed] [Google Scholar]
- 63.Parker LA, Sullivan S, Krueger C, et al. Association of timing of initiation of breastmilk expression on milk volume and timing of lactogenesis stage II among mothers of very low-birth-weight infants. Breastfeed Med 2015;10:84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Field T, Schanberg SM, Scafidi F, et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics 1986;77:654–658 [PubMed] [Google Scholar]
- 65.Field T, Scafidi F, Schanberg S. Massage of preterm newborns to improve growth and development. Pediatr Nurs 1987;13:385–386 [Google Scholar]
- 66.Tessier R, Charpak N, Giron M, et al. Kangaroo Mother Care, home environment and father involvement in the first year of life: A randomized controlled study. Acta Paediatr 2009;98:1444–1450 [DOI] [PubMed] [Google Scholar]
- 67.Field T, Diego M, Hernandez-Reif M. Preterm infant massage therapy research: A review. Infant Behav Dev 2010;33:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau C, Fucile S, Gisel EG. Impact of nonnutritive oral motor stimulation and infant massage therapy on oral feeding skills of preterm infants. J Neonat Perinat Med 2012;5:311–315 [Google Scholar]
- 69.Moore ER, Bergman N, Anderson GC, et al. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev 2016;11:CD003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thelen E. Motor development. A new synthesis. Am Psychol 1995;50:79–95 [DOI] [PubMed] [Google Scholar]
- 71.Newell KM, Corcos DM. Issues in variability and motor control. In: Variability and Motor Control, Newell KM. and Corcos DM, eds. Champaign IL: Human Kinetics Publisher, 1993, pp. 1–12 [Google Scholar]
- 72.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996;272:1791–1794 [DOI] [PubMed] [Google Scholar]
- 73.Byl NN, Merzenich MM, Cheung S, et al. A primate model for studying focal dystonia and repetitive strain injury: Effects on the primary somatosensory cortex. Phys Ther 1997;77:269–284 [DOI] [PubMed] [Google Scholar]
- 74.Munoz-Hoyos A, Molina-Carballo A, Augustin-Morales M, et al. Psychosocial dwarfism: Psychopathological aspects and putative neuroendocrine markers. Psychiatry Res 2011;188:96–101 [DOI] [PubMed] [Google Scholar]
- 75.Lau C. Development of suck and swallow mechanisms in infants. Ann Nutr Metab 2015;66 Suppl 5:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lau C. Development of infant oral feeding skills: What do we know? Am J Clin Nutr 2016;103:616S–621S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lau C, Alagugurusamy R, Schanler RJ, et al. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr 2000;89:846–852 [PubMed] [Google Scholar]
- 78.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow-respiration in preterm infants. Acta Paediatr 2003;92:721–727 [PubMed] [Google Scholar]
- 79.Amaizu N, Shulman R, Schanler R, et al. Maturation of oral feeding skills in preterm infants. Acta Paediatr 2008;97:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolff PH. The serial organization of sucking in the young infant. Pediatrics 1968;42:943–956 [PubMed] [Google Scholar]
- 81.Jadcherla SR, Peng J, Moore R, et al. Impact of personalized feeding program in 100 NICU infants: A novel pathophysiology-based approach for better outcomes. J Pediatr Gastroenterol Nutr 2012;54:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf LS, Glass RP. Feeding and Swallowing Disorders in Infancy: Assessment and Management. Tucson: Therapy Skills Builders, 1992 [Google Scholar]
- 83.White-Traut RC, Nelson MN, Silvestri JM, et al. Effect of auditory, tactile, visual, and vestibular intervention on length of stay, alertness, and feeding progression in preterm infants. Dev Med Child Neurol 2002;44:91–97 [DOI] [PubMed] [Google Scholar]
- 84.Lau C. Development of oral feeding skills in the preterm infant. In: The Handbook of Growth and Growth Monitoring in Health and Disease, Pt 3, Preedy VR, ed. New York, NY: Springer, 2012, pp. 499–512 [Google Scholar]
- 85.Hopkinson JM, Schanler RJ, Garza C. Milk production by mothers of premature infants. Pediatrics 1988;81:815–820 [PubMed] [Google Scholar]
- 86.Goldfield EC, Perez J, Engstler K. Neonatal feeding behavior as a complex dynamical system. Semin Speech Lang 2017;38:77–86 [DOI] [PubMed] [Google Scholar]
- 87.Bergh AM. Obstacles to and motivation for successful breast-feeding. Curationis 1993;16:24–29 [DOI] [PubMed] [Google Scholar]
- 88.Meyer EC, Garcia Coll CT, Seifer R, et al. Psychological distress in mothers of preterm infants. J Dev Behav Pediatr 1995;16:412–417 [PubMed] [Google Scholar]
- 89.Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosom Med 2006;68:938–946 [DOI] [PubMed] [Google Scholar]
- 90.Hildingsson I, Tingvall M, Rubertsson C. Partner support in the childbearing period—a follow up study. Women Birth 2008;21:141–148 [DOI] [PubMed] [Google Scholar]
- 91.Melnyk BM, Crean HF, Feinstein NF, et al. Maternal anxiety and depression after a premature infant's discharge from the neonatal intensive care unit: Explanatory effects of the creating opportunities for parent empowerment program. Nurs Res 2008;57:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith CD, Lonstein JS. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res 2008;190:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lau C. Effects of various stressors on milk release in the rat. Physiol Behav 1992;51:1157–1163 [DOI] [PubMed] [Google Scholar]
- 94.Asher I, Kaplan B, Modai I, et al. Mood and hormonal changes during late pregnancy and puerperium. Clin Exp Obstet Gynecol 1995;22:321–325 [PubMed] [Google Scholar]
- 95.Heinrichs M, Meinlschmidt G, Wippich W, et al. Selective amnesic effects of oxytocin on human memory. Physiol Behav 2004;83:31–38 [DOI] [PubMed] [Google Scholar]
- 96.Cox EQ, Stuebe A, Pearson B, et al. Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology 2015;55:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klampfl SM, Schramm MM, Stinnett GS, et al. Brain CRF-binding protein modulates aspects of maternal behavior under stressful conditions and supports a hypo-anxious state in lactating rats. Horm Behav 2016;84:136–144 [DOI] [PubMed] [Google Scholar]
- 98.Ralph CR, Tilbrook AJ. The hypothalamo-pituitary-adrenal (HPA) axis in sheep is attenuated during lactation in response to psychosocial and predator stress. Domest Anim Endocrinol 2016;55:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol 2000;85 Spec No:111S–124S [DOI] [PubMed] [Google Scholar]
- 100.Strathearn L, Fonagy P, Amico J, et al. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 2009;34:2655–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gordon I, Zagoory-Sharon O, Leckman JF, et al. Oxytocin and the development of parenting in humans. Biol Psychiatry 2010;68:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen FS, Kumsta R, von DB, et al. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci U S A 2011;108:19937–19942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mogi K, Nagasawa M, Kikusui T. Developmental consequences and biological significance of mother-infant bonding. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1232–1241 [DOI] [PubMed] [Google Scholar]
- 104.Strathearn L, Iyengar U, Fonagy P, et al. Maternal oxytocin response during mother-infant interaction: Associations with adult temperament. Horm Behav 2012;61:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feldman R, Monakhov M, Pratt M, et al. Oxytocin pathway genes: Evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry 2016;79:174–184 [DOI] [PubMed] [Google Scholar]
- 106.Spratt EG, Marsh C, Wahlquist AE, et al. Biologic effects of stress and bonding in mother-infant pairs. Int J Psychiatry Med 2016;51:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feldman R, Magori-Cohen R, Galili G, et al. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav Dev 2011;34:569–577 [DOI] [PubMed] [Google Scholar]
- 108.O'Hara MW, Swain AM. Rates and risk of postpartum depression – a meta-analysis. Intl Rev Psychiatry 1996;8:37 [Google Scholar]
- 109.Ramsay M, Gisel EG, McCusker J, et al. Infant sucking ability, non-organic failure to thrive, maternal characteristics, and feeding practices: A prospective cohort study. Dev Med Child Neurol 2002;44:405–414 [DOI] [PubMed] [Google Scholar]
- 110.Park J, Thoyre S, Estrem H, et al. Mothers' psychological distress and feeding of their preterm infants. MCN Am J Matern Child Nurs 2016;41:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schappin R, Wijnroks L, Uniken Venema MM, et al. Rethinking stress in parents of preterm infants: A meta-analysis. PLoS One 2013;8:e54992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rossman B, Greene MM, Kratovil AL, et al. Resilience in mothers of very-low-birth-weight infants hospitalized in the NICU. J Obstet Gynecol Neonatal Nurs 2017;46:434–445 [DOI] [PubMed] [Google Scholar]
- 113.Klaus MH, Jerauld R, Kreger NC, et al. Maternal attachment. Importance of the first post-partum days. N Engl J Med 1972;286:460–463 [DOI] [PubMed] [Google Scholar]
- 114.Korja R, Savonlahti E, hlqvist-Bjorkroth S, et al. Maternal depression is associated with mother-infant interaction in preterm infants. Acta Paediatr 2008;97:724–730 [DOI] [PubMed] [Google Scholar]
- 115.Holditch-Davis D, Bartlett TR, Blickman AL, et al. Posttraumatic stress symptoms in mothers of premature infants. J Obstet Gynecol Neonatal Nurs 2003;32:161–171 [DOI] [PubMed] [Google Scholar]
- 116.Kersting A, Dorsch M, Wesselmann U, et al. Maternal posttraumatic stress response after the birth of a very low-birth-weight infant. J Psychosom Res 2004;57:473–476 [DOI] [PubMed] [Google Scholar]
- 117.Vanderbilt D, Bushley T, Young R, et al. Acute posttraumatic stress symptoms among urban mothers with newborns in the neonatal intensive care unit: A preliminary study. J Dev Behav Pediatr 2009;30:50–56 [DOI] [PubMed] [Google Scholar]
- 118.Jubinville J, Newburn-Cook C, Hegadoren K, et al. Symptoms of acute stress disorder in mothers of premature infants. Adv Neonatal Care 2012;12:246–253 [DOI] [PubMed] [Google Scholar]
- 119.Reid T. Maternal identity in preterm birth. J Child Health Care 2000;4:23–29 [DOI] [PubMed] [Google Scholar]
- 120.Shin H, White-Traut R. The conceptual structure of transition to motherhood in the neonatal intensive care unit. J Adv Nurs 2007;58:90–98 [DOI] [PubMed] [Google Scholar]
- 121.Lindberg B, Ohrling K. Experiences of having a prematurely born infant from the perspective of mothers in northern Sweden. Int J Circumpolar Health 2008;67:461–471 [DOI] [PubMed] [Google Scholar]
- 122.Fuertes M, Santos PL, Beeghly M, et al. More than maternal sensitivity shapes attachment: Infant coping and temperament. Ann N Y Acad Sci 2006;1094:292–296 [DOI] [PubMed] [Google Scholar]
- 123.Coppola G, Cassibba R, Costantini A. What can make the difference? Premature birth and maternal sensitivity at 3 months of age: The role of attachment organization, traumatic reaction and baby's medical risk. Infant Behav Dev 2007;30:679–684 [DOI] [PubMed] [Google Scholar]
- 124.Singer LT, Fulton S, Kirchner HL, et al. Parenting very low birth weight children at school age: Maternal stress and coping. J Pediatr 2007;151:463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meier PP, Engstrom JL, Mingolelli SS, et al. The rush mothers' milk club: Breastfeeding interventions for mothers with very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs 2004;33:164–174 [DOI] [PubMed] [Google Scholar]
- 126.Shakya P, Kunieda MK, Koyama M, et al. Effectiveness of community-based peer support for mothers to improve their breastfeeding practices: A systematic review and meta-analysis. PLoS One 2017;12:e0177434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burns E, Schmied V. “The right help at the right time”: Positive constructions of peer and professional support for breastfeeding. Women Birth 2017. [Epub ahead of print]; DOI: 10.1016/j.wombi.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 128.Dewey KG, McCrory MA. Effects of dieting and physical activity on pregnancy and lactation. Am J Clin Nutr 1994;59:446S–452S [DOI] [PubMed] [Google Scholar]
- 129.Heinrichs M, Meinlschmidt G, Neumann I, et al. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab 2001;86:4798–4804 [DOI] [PubMed] [Google Scholar]
- 130.Feldman S, Conforti N, Melamed E. Hypothalamic norepinephrine mediates limbic effects on adrenocortical secretion. Brain Res Bull 1988;21:587–590 [DOI] [PubMed] [Google Scholar]
- 131.Almeida OF, Yassouridis A, Forgas-Moya I. Reduced availability of milk after central injections of corticotropin-releasing hormone in lactating rats. Neuroendocrinology 1994;59:72–77 [DOI] [PubMed] [Google Scholar]
- 132.Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol 2008;586:377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Geoffrey Harris Memorial Lecture, Kitakyushu, Japan, October 1998. Front Neuroendocrinol 1999;20:270–295 [DOI] [PubMed] [Google Scholar]
- 134.Gorewit RC, Aromando MC. Mechanisms involved in the adrenaline-induced blockade of milk ejection in dairy cattle. Proc Soc Exp Biol Med 1985;180:340–347 [DOI] [PubMed] [Google Scholar]
- 135.Lau C, Henning SJ. Mammary resistance: A possible controlling factor in milk ejection. J Endocrinol 1987;112:379–385 [DOI] [PubMed] [Google Scholar]
- 136.Lefcourt AM, Paul G, Mayer H, et al. Response of catecholamines to manual teat stimulation or machine-milking of Lacaune and Friesen dairy ewes. J Dairy Sci 1997;80:3205–3211 [DOI] [PubMed] [Google Scholar]
- 137.Grosvenor CE, Mena F. Evidence for a time delay between prolactin release and the resulting rise in milk secretion rate in the rat. J Endocrinol 1973;58:31–39 [DOI] [PubMed] [Google Scholar]
- 138.Silberstein D, Geva R, Feldman R, et al. The transition to oral feeding in low-risk premature infants: Relation to infant neurobehavioral functioning and mother-infant feeding interaction. Early Hum Dev 2009;85:157–162 [DOI] [PubMed] [Google Scholar]
- 139.Ludington-Hoe SM, Abouelfettoh A. Light reduction capabilities of homemade and commercial incubator covers in NICU. ISRN Nurs 2013;2013:502393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Philbin MK, Gray L. Changing levels of quiet in an intensive care nursery. J Perinatol 2002;22:455–460 [DOI] [PubMed] [Google Scholar]
- 141.American Academy of Pediatrics. Hospital discharge of the high-risk neonate. Proposed guidelines. Pediatrics 1998;102:411–417 [PubMed] [Google Scholar]
- 142.Schanler RJ, Shulman RJ, Lau C, et al. Feeding strategies for premature infants: Randomized trial of gastrointestinal priming and tube-feeding method [see comments]. Pediatrics 1999;103:434–439 [DOI] [PubMed] [Google Scholar]
- 143.Eichenwald EC, Blackwell M, Lloyd JS, et al. Inter-neonatal intensive care unit variation in discharge timing: Influence of apnea and feeding management. Pediatrics 2001;108:928–933 [DOI] [PubMed] [Google Scholar]
- 144.Sharp WG, Volkert VM, Scahill L, et al. A systematic review and meta-analysis of intensive multidisciplinary intervention for pediatric feeding disorders: How standard is the standard of care? J Pediatr 2017;181:116–124 [DOI] [PubMed] [Google Scholar]