Abstract
Purpose
To examine and compare risks of serious hypoglycemia among antidiabetic monotherapy-treated adults receiving metformin, a sulfonylurea, a meglitinide, or a thiazolidinedione
Methods
We performed a retrospective cohort study of apparently new users of monotherapy with metformin, glimepiride, glipizide, glyburide, pioglitazone, rosiglitazone, nateglinide, or repaglinide within a dataset of Medicaid beneficiaries from California, Florida, New York, Ohio, and Pennsylvania. We did not include users of dipeptidyl peptidase-4 inhibitors, glucagonlike peptide 1 agonists, or sodium-glucose co-transporter 2 inhibitors. We identified serious hypoglycemia outcomes within 180 days following new use using a validated, diagnosis-based algorithm. We calculated age- and sex-standardized outcome occurrence rates for each drug and generated propensity score-adjusted hazard ratios vs. metformin using Cox proportional hazards regression.
Results
The ranking of standardized occurrence rates of serious hypoglycemia was glyburide > glimepiride > glipizide > repaglinide > nateglinide > rosiglitazone > pioglitazone > metformin. Rates were increased for all study drugs at higher average daily doses. Adjusted hazard ratios (95% confidence intervals) vs. metformin were 3.95 (3.66, 4.26) for glyburide, 3.28 (2.98, 3.62) for glimepiride, 2.57 (2.38, 2.78) for glipizide, 2.03 (1.64, 2.52) for repaglinide, 1.21 (0.89, 1.66) for nateglinide, 0.90 (0.75, 1.07) for rosiglitazone, and 0.80 (0.68, 0.93) for pioglitazone.
Conclusions
Sulfonylureas were associated with the highest rates of serious hypoglycemia. Among all study drugs, the highest rate was seen with glyburide. Pioglitazone was associated with a lower adjusted hazard for serious hypoglycemia vs. metformin, while rosiglitazone and nateglinide had hazards similar to that of metformin.
Keywords: hypoglycemia, metformin, sulfonylurea compounds, thiazolidinedione, meglitinide
Introduction
Nearly all individuals with type 2 diabetes mellitus (T2DM) will eventually need drug therapy to manage their disease.1 Monotherapy with an antidiabetic agent is recommended when lifestyle changes alone cannot achieve or maintain glycemic goals.2 Metformin is widely regarded as the preferred first-line medication in patients without a contraindication (e.g., hypersensitivity, severe renal dysfunction) and in whom it is tolerated.2–4 For patients who cannot (or do not) receive metformin, American Diabetes Association and European Association for the Study of Diabetes guidelines recommend use of a second-line antidiabetic agent, such as a sulfonylurea (SU; including glimepiride, glipizide, or glyburide), meglitinide (nateglinide or repaglinide), or thiazolidinedione (TZD; pioglitazone or rosiglitazone), among others.2,3 In 2012, these oral antidiabetic medications together accounted for ~100 million prescriptions to over 13 million T2DM patients in the United States (US).5
Hypoglycemia, a commonly-occurring and potentially life-threatening sequela of antidiabetic therapy, was named as one of three high-priority adverse drug events targeted by the National Action Plan for Adverse Drug Event Prevention issued in 2014 by the US Department of Health and Human Services.6 Hypoglycemia caused by antidiabetic drugs can result in coma or seizure, and is associated with latent complications including myocardial ischemic injury, dementia, and increased mortality.7
With an increasing number of oral therapies for T2DM, the comparative safety of therapeutic alternatives is an important consideration when choosing the best therapy for a particular patient. However, few studies have compared antidiabetic agents with respect to hypoglycemia risk, and cross-study comparisons are hindered by differences in study populations and inconsistently defined outcomes. Randomized controlled trials in particular do not use consistent outcome definitions or necessarily reflect real-world drug effects. Many studies treat all members of a given drug class as identical, an assumption that is seldom justified, and also neglect dose-response assessment. Moreover, serious hypoglycemia has been investigated in relatively few studies of SUs and has not been examined carefully for either meglitinides or TZDs. Recognizing this knowledge gap, the US National Action Plan for Adverse Drug Event Prevention calls for research to “identify rates of serious hypoglycemia in ambulatory care settings” among patients receiving antidiabetic therapies.6 We therefore examined rates of serious hypoglycemia (i.e., leading to an emergency department [ED] visit or hospitalization) among individuals treated with monotherapies of metformin, a SU, a meglitinide, and a TZD.
Patients and Methods
Overview and study population
We conducted a new user cohort study to examine associations between oral antidiabetic monotherapy regimens and serious hypoglycemia. The study cohort consisted exclusively of person-time exposed to monotherapy with metformin, glimepiride, glipizide, glyburide, pioglitazone, rosiglitazone, nateglinide, or repaglinide. Users of dipeptidyl peptidase-4 inhibitors, glucagonlike peptide 1 agonists, or sodium-glucose co-transporter 2 inhibitors were not included. Data included enrollment and healthcare claims from US Medicaid enrollees aged 18–100 years from California, Florida, New York, Ohio, and Pennsylvania during 1999–2010. These states have five of the largest Medicaid programs in the US, with a prevalent population of ~26 million (~38% of the entire US Medicaid population).8 Because a large proportion of Medicaid beneficiaries are co-enrolled in Medicare,9 we also obtained and utilized Medicare claims to ascertain a more complete picture of enrollees’ healthcare.10–12 The work described herein was approved by the institutional review board of the University of Pennsylvania.
Defining the study cohort
We defined apparently new users as individuals with ≥183 days of Medicaid enrollment before their first prescription for a study drug of interest; the date on which this prescription was dispensed defined cohort entry. The 183-day period immediately preceding cohort entry served as the baseline period. We did not require cohort members to meet a claims-based operational definition for T2DM during baseline or on the cohort entry date, since off-label use of these drugs would be rare and we aimed to elucidate serious hypoglycemia risk representative of real-world use. Women with a pregnancy diagnosis during the baseline period were excluded from study. The rationale for this exclusion was to avoid areas of non-overlap in the propensity score13 (PS), since pregnant women treated with a SU receive glyburide almost exclusively.14 As claims based-approaches to identifying new users are imperfect,15 we recognized that a small proportion of subjects might actually be prevalent antidiabetic drug users. Persons could enter the cohort more than once if they re-met inclusion criteria after a censoring event (described below).
Follow-up began on cohort entry and continued until the first occurrence of the following: 1) death, as ascertained from linkage to the Social Security Administration Death Master File; 2) the 181st day; 3) >15 day gap in treatment with the study drug defining cohort entry; 4) a prescription for any other antidiabetic medication; 5) loss of Medicaid eligibility; or 6) the end of the dataset. For the proportional hazards regression analysis described below, the occurrence of an outcome of interest also served as a censoring event. Hospitalization did not serve as a censoring event, but periods of hospitalization were excluded from follow-up time to minimize immeasurable time bias.16
Ascertainment of exposure and dose
Exposure was defined by the antidiabetic drug dispensed on the day of cohort entry. Drug exposure periods were determined by: 1) the days’ supply field on Medicaid prescription claims from California, Florida, New York, and Pennsylvania; 2) an imputed 30-day duration for Medicaid prescription claims from Ohio, as this state’s Medicaid data does not include days’ supply; and 3) the days’ supply field on Medicare prescription claims from California, Florida, New York, Ohio, and Pennsylvania. Prescription claims for which days’ supply was missing and not otherwise imputed were excluded from study; this was a very rare occurrence.
We calculated average daily dose as [dispensed quantity × strength of the dosage form]/[days’ supply value], assuming that the prescription was consumed over the days’ supply. Prescriptions with an average daily dose exceeding twice the recommended maximum daily dose (Supplemental Table 1) were assumed to have recording errors and were excluded from dose analyses. Medicaid claims from Ohio were also excluded from dose analyses since we imputed their days’ supply values. For each study drug, we dichotomized average daily dose as high vs. low—defined by ≥ mode dose and < mode dose, respectively. We chose the mode as a measure of central tendency to minimize the impact of extreme dose values on the selection of a cutpoint.
Ascertainment of covariates
As described below, a multinomial PS model was fit that included covariates (Table 1) from the following categories: 1) demographic factors; 2) healthcare utilization intensity measures; 3) chronic diseases known to affect glucose homeostasis; and 4) current drugs that can affect the level of blood glucose (Supplemental Table 2). All covariates were ascertained during the 183-day baseline period, except for current drugs affecting blood glucose which were defined as a prescription in the 30 days (7 days for anti-infectives) prior to cohort entry. International Classification of Diseases 9th Revision Clinical Modification (ICD-9-CM) codes and National Drug Codes were used to identify these covariates. Calendar year of cohort entry was not included in the PS; this avoided a distortion in the PS distribution driven by very low prescribing of rosiglitazone during 2008–2010. We therefore included calendar year of cohort entry as a covariate in the proportional hazards regression model.
Ascertainment of outcome
The outcome of interest was serious hypoglycemia, operationally defined by one of the following ICD-9-CM discharge diagnosis codes in any position on an ED claim or the principal position on an inpatient claim: 1) 251.0 (hypoglycemic coma); 2) 251.1 (other specific hypoglycemia); 3) 251.2 (hypoglycemia, unspecified); or 4) 250.8X (diabetes with other specified manifestations), as long as not co-occurring with ≥1 exclusionary diagnosis suggesting manifestations other than hypoglycemia (Supplemental Table 3). This algorithm, which we have used previously,17–20 has a positive predictive value of 89% for the ED component21 and 78% for the inpatient component.17 These performance measures were derived from validation studies within Emergency Medicine Network data and Centers for Medicare and Medicaid Services data, respectively, using medical records as the gold standard.
Statistical analysis
We first calculated descriptive statistics for baseline covariates and calculated crude overall and average daily dose-stratified occurrence rates. An occurrence rate includes both incident and recurrent events, which is different from an incidence rate that only includes incident events. We then identified all T2DM patients aged 18–100 years with any prescription for any antidiabetic drug during 2010. This population served as the reference population for direct standardization of occurrence rates. Ninety-five percent confidence intervals (95% CIs) were calculated assuming a Poisson distribution.
Direct standardization adjusts for age and sex, but does not account for other factors related to both exposure and outcome. We therefore used PSs to more completely adjust for confounding. We calculated standardized mean differences (SMDs) for baseline covariates to examine potential differences between users of each antidiabetic drug of interest vs. metformin. A SMD is the difference in means between two exposure groups divided by the pooled standard deviation.22 A multinomial PS was generated using logistic regression. We assessed the goodness-of-fit of the PS model by examining the PS distribution of each antidiabetic drug of interest vs. metformin. We also examined the balance between exposure groups using weighted conditional standardized differences (WCSDs). A WCSD is the conditional difference in the mean of a covariate between two exposure groups in the units of the pooled standard deviation integrated over the distribution of the PS.23 It allows one to compare the difference in means of baseline covariates between two exposure groups in subjects with the same PS. Lastly, we used Cox proportional hazards regression24 to estimate hazard ratios (HRs) and 95% CIs for the associations between each study drug and the outcome, using metformin as the referent. We selected metformin as the referent because it is known to have a low risk of hypoglycemia and is commonly used.25 Proportional hazards assumptions were examined via inclusion of an interaction term of exposure by survival time. Two pre-specified secondary analyses were performed: 1) excluding persons enrolled in managed care plans, as Centers of Medicare and Medicaid Services claims may be incomplete for such persons;26 and 2) excluding person-time following second and later cohort entries per person, to minimize bias from depletion of susceptibles. Analyses were performed using SAS v9.4 (SAS Institute Inc.: Cary, North Carolina, US).
Results
We identified 971,792 incident users of the antidiabetic monotherapies under study. Five, 17,233, and 29,369 users were excluded due to missing sex, missing birth date, and pregnancy, respectively. The final cohort included 925,185 individuals, 63.7% of whom were female. The median age at cohort entry was 59.9 years. Users contributed 228,017 person-years (p-y) of observation during which 6,406 serious hypoglycemia events occurred, resulting in a crude occurrence rate of 28.1 per 1,000 p-y. Characteristics of users by exposure group are shown in Table 1 (Panels A & B). Standardized occurrence rates of serious hypoglycemia are presented in Table 2. The ranking of overall standardized occurrence rates was glyburide > glimepiride > glipizide > repaglinide > nateglinide > rosiglitazone > pioglitazone > metformin. When stratified by average daily dose, standardized occurrence rates were higher in the high dose group vs. low dose group for all study drugs.
Table 1, Panel A.
Characteristics of monotherapy new users of sulfonylureas vs. metformin
| Metformin N=489,979 |
glimepiride N=50,022 |
glipizide N=149,949 |
glyburide N=109,681 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years of follow-up | 122,694 | 12,510 | 36,279 | 26,277 | |||||||
| Outcomes within 180 days of cohort entry | 1,557 | 714 | 1,789 | 1,834 | |||||||
| Average daily dose (milligrams/day) | Median (Q1–Q3) | 1,000 (1,000–2,000) | 4.0 (2.0–8.0) | 10.0 (5.0–20.0) | 10.0 (5.0–15.0) | ||||||
| Mode | 1,000 | 4.0 | 10.0 | 10.0 | |||||||
| Demographics | Group | %* | %* | SMD | WCSD | %* | SMD | WCSD | %* | SMD | WCSD |
| Age at cohort entry, continuous, in years** | Median (Q1–Q3) | 55.6 (43.7–68.5) | 66.5 (52.7–76.5) | 0.53 | 0.19 | 64.2 (50.4–75.2) | 0.43 | 0.15 | 64.4 (50.4–75.2) | 0.43 | 0.11 |
| Sex** | Female | 65.7 | 62.2 | 0.07 | 0.11 | 60.7 | 0.10 | 0.08 | 60.5 | 0.11 | 0.06 |
| Race** | White | 39.4 | 44.7 | 0.11 | 0.19 | 36.7 | 0.05 | 0.16 | 36.9 | 0.05 | 0.12 |
| Black | 16.4 | 13.1 | 0.10 | 0.11 | 18.8 | 0.06 | 0.11 | 16.4 | 0.00 | 0.09 | |
| Hispanic/Latino | 21.2 | 15.8 | 0.14 | 0.07 | 22.0 | 0.02 | 0.12 | 22.5 | 0.03 | 0.07 | |
| Other/Unknown | 23.0 | 26.4 | 0.08 | 0.16 | 22.5 | 0.01 | 0.09 | 24.2 | 0.03 | 0.08 | |
| State of residence** | CA | 43.0 | 38.6 | 0.09 | 0.04 | 42.0 | 0.02 | 0.04 | 52.5 | 0.19 | 0.03 |
| FL | 11.2 | 11.9 | 0.02 | 0.07 | 15.5 | 0.13 | 0.08 | 10.7 | 0.02 | 0.06 | |
| NY | 29.5 | 27.3 | 0.05 | 0.16 | 26.6 | 0.07 | 0.12 | 23.4 | 0.14 | 0.12 | |
| OH | 9.2 | 12.9 | 0.12 | 0.07 | 7.1 | 0.08 | 0.08 | 7.5 | 0.06 | 0.07 | |
| PA | 7.1 | 9.4 | 0.08 | 0.04 | 8.9 | 0.07 | 0.05 | 6.0 | 0.04 | 0.03 | |
| Calendar year of cohort entry† | 1999–2001 | 10.8 | 21.0 | 0.28 | 0.27 | 26.6 | 0.41 | 0.41 | 32.1 | 0.54 | 0.51 |
| 2002 | 5.6 | 8.9 | 0.13 | 0.12 | 10.2 | 0.17 | 0.16 | 10.9 | 0.19 | 0.17 | |
| 2003 | 6.4 | 9.2 | 0.10 | 0.10 | 9.0 | 0.10 | 0.10 | 9.4 | 0.11 | 0.10 | |
| 2004 | 6.7 | 8.3 | 0.06 | 0.06 | 7.4 | 0.03 | 0.04 | 7.8 | 0.04 | 0.04 | |
| 2005 | 8.8 | 8.9 | 0.01 | 0.04 | 8.4 | 0.01 | 0.02 | 8.2 | 0.02 | 0.03 | |
| 2006 | 12.6 | 12.6 | 0.00 | 0.05 | 14.6 | 0.06 | 0.05 | 13.0 | 0.01 | 0.06 | |
| 2007 | 10.3 | 7.9 | 0.08 | 0.11 | 6.8 | 0.12 | 0.14 | 5.7 | 0.17 | 0.17 | |
| 2008 | 10.4 | 7.2 | 0.11 | 0.14 | 5.6 | 0.18 | 0.21 | 4.4 | 0.23 | 0.25 | |
| 2009 | 13.2 | 8.2 | 0.16 | 0.22 | 6.1 | 0.24 | 0.30 | 4.3 | 0.32 | 0.36 | |
| 2010 | 15.1 | 7.8 | 0.23 | 0.28 | 5.2 | 0.33 | 0.39 | 4.2 | 0.38 | 0.41 | |
| Co-coverage by Medicare** | Yes | 46.5 | 63.5 | 0.35 | 0.10 | 58.7 | 0.25 | 0.10 | 57.2 | 0.22 | 0.10 |
| Healthcare utilization covariates in baseline period** | Group | %* | %* | SMD | WCSD | %* | SMD* | WCSD | %* | SMD | WCSD |
| Nursing home residence, ever | Yes | 4.1 | 10.0 | 0.23 | 0.23 | 11.6 | 0.28 | 0.05 | 9.8 | 0.22 | 0.03 |
| # Hospitalizations (continuous) | Median (Q1–Q3) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.22 | 0.22 | 0.0 (0.0–0.0) | 0.26 | 0.07 | 0.0 (0.0–0.0) | 0.23 | 0.08 |
| # Unique drugs dispensed (continuous) | Median (Q1–Q3) | 6.0 (2.0–10.0) | 7.0 (2.0–12.0) | 0.17 | 0.17 | 5.0 (1.0–10.0) | 0.05 | 0.15 | 5.0 (1.0–10.0) | 0.10 | 0.17 |
| # Ambulatory care visits without a hypoglycemia diagnosis (continuous) | Median (Q1–Q3) | 4.0 (1.0–10.0) | 5.0 (2.0–11.0) | 0.07 | 0.07 | 4.0 (1.0–10.0) | 0.04 | 0.12 | 4.0 (1.0–9.0) | 0.06 | 0.11 |
| Diseases in baseline period** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Alcohol abuse | Yes | 1.9 | 2.2 | 0.02 | 0.04 | 2.7 | 0.06 | 0.04 | 2.6 | 0.05 | 0.03 |
| Cancer | Yes | 5.1 | 8.5 | 0.14 | 0.04 | 7.6 | 0.10 | 0.03 | 7.4 | 0.09 | 0.03 |
| Dementia | Yes | 2.2 | 5.6 | 0.17 | 0.03 | 5.7 | 0.18 | 0.04 | 4.8 | 0.14 | 0.03 |
| Hypoglycemia, ambulatory care visit | Yes | 0.9 | 1.1 | 0.02 | 0.02 | 1.0 | 0.01 | 0.03 | 0.9 | 0.00 | 0.03 |
| Hypoglycemia, serious | Yes | 0.4 | 0.7 | 0.03 | 0.03 | 0.9 | 0.05 | 0.02 | 0.9 | 0.06 | 0.02 |
| Kidney diseases | Yes | 5.3 | 14.5 | 0.31 | 0.04 | 13.8 | 0.29 | 0.07 | 10.9 | 0.21 | 0.06 |
| Liver diseases | Yes | 6.4 | 9.6 | 0.12 | 0.08 | 8.2 | 0.07 | 0.04 | 7.9 | 0.06 | 0.04 |
| Obesity | Yes | 10.0 | 5.9 | 0.16 | 0.09 | 5.4 | 0.17 | 0.08 | 4.7 | 0.20 | 0.08 |
| Drugs that can affect blood glucose, in 7 days prior to cohort entry** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Co-trimoxazole | Yes | 0.4 | 0.4 | 0.00 | 0.02 | 0.5 | 0.02 | 0.02 | 0.5 | 0.01 | 0.02 |
| Quinolones | Yes | 0.8 | 1.4 | 0.05 | 0.03 | 1.2 | 0.03 | 0.02 | 1.1 | 0.02 | 0.01 |
| Drugs that can affect blood glucose, in 30 days prior to cohort entry** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Angiotensin converting enzyme inhibitors | Yes | 11.3 | 11.7 | 0.01 | 0.04 | 11.2 | 0.00 | 0.04 | 10.7 | 0.02 | 0.04 |
| Angiotensin II receptor antagonists | Yes | 4.0 | 5.4 | 0.07 | 0.06 | 3.4 | 0.03 | 0.03 | 3.0 | 0.05 | 0.03 |
| Antipsychotics, atypical | Yes | 8.4 | 6.5 | 0.07 | 0.06 | 6.6 | 0.07 | 0.07 | 5.8 | 0.10 | 0.07 |
| Beta blockers | Yes | 13.2 | 15.9 | 0.08 | 0.12 | 13.0 | 0.01 | 0.07 | 11.4 | 0.06 | 0.07 |
| Calcineurin inhibitors | Yes | 0.1 | 0.3 | 0.05 | 0.01 | 0.5 | 0.08 | 0.01 | 0.3 | 0.05 | 0.01 |
| Corticosteroids | Yes | 3.1 | 4.7 | 0.08 | 0.08 | 4.4 | 0.07 | 0.07 | 4.1 | 0.05 | 0.05 |
| Diuretics, thiazide | Yes | 6.3 | 4.4 | 0.08 | 0.03 | 4.3 | 0.09 | 0.03 | 3.9 | 0.11 | 0.04 |
| Haloperidol | Yes | 0.5 | 0.4 | 0.01 | 0.01 | 0.5 | 0.01 | 0.02 | 0.5 | 0.00 | 0.01 |
| Protease inhibitors | Yes | 0.4 | 0.3 | 0.03 | 0.01 | 0.6 | 0.02 | 0.00 | 0.6 | 0.02 | 0.00 |
| Quinine | Yes | 0.3 | 0.6 | 0.05 | 0.01 | 0.4 | 0.03 | 0.01 | 0.5 | 0.04 | 0.01 |
| Salicylates | Yes | 4.1 | 4.7 | 0.03 | 0.03 | 3.9 | 0.01 | 0.04 | 3.8 | 0.01 | 0.03 |
Q = quartile; SMD = standardized mean difference (vs. metformin); WCSD = weighted conditional standardized difference (vs. metformin)
unless otherwise noted
included in propensity score
included as a covariate in Cox proportional hazards model
Table 1, Panel B.
Characteristics of monotherapy new users of meglitinides and thiazolidinediones vs. metformin
| metformin N=489,979 |
nateglinide N=8,129 |
repaglinide N=10,107 |
pioglitazone N=62,775 |
rosiglitazone N=44,543 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person-years of follow-up | 122,694 | 1,721 | 2,168 | 15,594 | 10,774 | |||||||||
| Outcomes within 180 days of cohort entry | 1,557 | 42 | 100 | 212 | 158 | |||||||||
| Average daily dose (milligrams/day) | Median (Q1–Q3) | 1,000 (1,000–2000) | 360 (186–360) | 3.0 (1.5–6.0) | 30.0 (15.0–45.0) | 4.1 (4.0–8.0) | ||||||||
| Mode | 1000 | 360 | 6.0 | 30.0 | 8.0 | |||||||||
| Demographics | Group | %* | %* | SMD | WCSD | %* | SMD | WCSD | %* | SMD | WCSD | %* | SMD | WCSD |
| Age at cohort entry, continuous, in years** | Median (Q1–Q3) | 55.6 (43.7–68.5) | 68.9 (56.0–78.4) | 0.67 | 0.26 | 70.2 (58.1–79.5) | 0.75 | 0.31 | 64.8 (51.3–74.6) | 0.43 | 0.18 | 65.7 (52.1–75.2) | 0.47 | 0.21 |
| Sex** | Female | 65.7 | 64.7 | 0.02 | 0.14 | 65.0 | 0.02 | 0.18 | 62.1 | 0.08 | 0.07 | 63.4 | 0.05 | 0.09 |
| Race** | White | 39.4 | 47.2 | 0.16 | 0.28 | 44.8 | 0.11 | 0.28 | 38.6 | 0.02 | 0.13 | 40.5 | 0.02 | 0.16 |
| Black | 16.4 | 12.4 | 0.12 | 0.14 | 15.8 | 0.02 | 0.18 | 15.1 | 0.04 | 0.08 | 15.6 | 0.02 | 0.09 | |
| Hispanic/Latino | 21.2 | 15.3 | 0.15 | 0.17 | 13.5 | 0.20 | 0.21 | 21.4 | 0.00 | 0.12 | 18.6 | 0.07 | 0.11 | |
| Other/Unknown | 23.0 | 25.2 | 0.05 | 0.21 | 25.9 | 0.07 | 0.20 | 25.0 | 0.05 | 0.13 | 25.4 | 0.06 | 0.10 | |
| State of residence** | CA | 43.0 | 44.8 | 0.04 | 0.03 | 32.7 | 0.21 | 0.07 | 51.5 | 0.17 | 0.02 | 40.7 | 0.05 | 0.02 |
| FL | 11.2 | 12.2 | 0.03 | 0.09 | 13.5 | 0.07 | 0.16 | 12.4 | 0.04 | 0.04 | 16.9 | 0.16 | 0.05 | |
| NY | 29.5 | 24.8 | 0.11 | 0.21 | 30.0 | 0.01 | 0.27 | 20.7 | 0.20 | 0.10 | 26.5 | 0.07 | 0.14 | |
| OH | 9.2 | 10.3 | 0.04 | 0.15 | 5.1 | 0.16 | 0.11 | 9.4 | 0.01 | 0.05 | 8.0 | 0.04 | 0.04 | |
| PA | 7.1 | 7.8 | 0.03 | 0.05 | 18.6 | 0.35 | 0.13 | 6.0 | 0.05 | 0.02 | 7.9 | 0.03 | 0.02 | |
| Calendar year of cohort entry† | 1999–2001 | 10.8 | 12.1 | 0.04 | 0.15 | 30.0 | 0.49 | 0.64 | 11.9 | 0.03 | 0.06 | 19.3 | 0.24 | 0.21 |
| 2002 | 5.6 | 15.9 | 0.34 | 0.39 | 9.8 | 0.16 | 0.15 | 8.0 | 0.09 | 0.08 | 11.8 | 0.22 | 0.21 | |
| 2003 | 6.4 | 12.1 | 0.20 | 0.21 | 8.0 | 0.06 | 0.06 | 9.8 | 0.12 | 0.11 | 12.6 | 0.21 | 0.22 | |
| 2004 | 6.7 | 10.3 | 0.13 | 0.13 | 6.6 | 0.00 | 0.03 | 9.7 | 0.11 | 0.10 | 11.9 | 0.18 | 0.20 | |
| 2005 | 8.8 | 14.5 | 0.18 | 0.18 | 5.9 | 0.11 | 0.09 | 13.2 | 0.14 | 0.15 | 13.5 | 0.15 | 0.17 | |
| 2006 | 12.6 | 14.0 | 0.04 | 0.05 | 14.4 | 0.05 | 0.08 | 15.2 | 0.07 | 0.07 | 19.8 | 0.20 | 0.15 | |
| 2007 | 10.3 | 8.2 | 0.07 | 0.12 | 7.7 | 0.09 | 0.14 | 9.4 | 0.03 | 0.04 | 6.8 | 0.12 | 0.14 | |
| 2008 | 10.4 | 5.7 | 0.17 | 0.24 | 7.2 | 0.11 | 0.19 | 7.7 | 0.09 | 0.12 | 1.9 | 0.36 | 0.36 | |
| 2009 | 13.2 | 3.9 | 0.34 | 0.41 | 5.9 | 0.25 | 0.34 | 7.9 | 0.18 | 0.22 | 1.6 | 0.45 | 0.48 | |
| 2010 | 15.1 | 3.4 | 0.41 | 0.49 | 4.4 | 0.37 | 0.46 | 7.2 | 0.25 | 0.31 | 0.7 | 0.56 | 0.59 | |
| Co-coverage by Medicare** | Yes | 46.5 | 70.6 | 0.51 | 0.14 | 75.3 | 0.62 | 0.16 | 60.6 | 0.29 | 0.05 | 64.0 | 0.36 | 0.09 |
| Healthcare utilization covariates in baseline period** | Group | %* | %* | SMD | WCSD | %* | SMD | WCSD | %* | SMD | WCSD | %* | SMD | WCSD |
| Nursing home residence, ever | Yes | 4.1 | 10.9 | 0.26 | 0.08 | 15.3 | 0.38 | 0.08 | 6.4 | 0.10 | 0.02 | 8.8 | 0.19 | 0.03 |
| # Hospitalizations (continuous) | Median (Q1–Q3) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.38 | 0.18 | 0.0 (0.0–1.0) | 0.62 | 0.26 | 0.0 (0.0–0.0) | 0.08 | 0.08 | 0.0 (0.0–0.0) | 0.14 | 0.08 |
| # Unique drugs dispensed (continuous) | Median (Q1–Q3) | 6.0 (2.0–10.0) | 9.0 (5.0–14.0) | 0.48 | 0.47 | 8.0 (3.0–13.0) | 0.32 | 0.39 | 7.0 (3.0–12.0) | 0.17 | 0.21 | 6.0 (2.0–11.0) | 0.10 | 0.20 |
| # Ambulatory care visits without a hypoglycemia diagnosis (continuous) | Median (Q1–Q3) | 4.0 (1.0–10.0) | 7.0 (3.0–14.0) | 0.25 | 0.13 | 7.0 (2.0–13.0) | 0.15 | 0.18 | 6.0 (2.0–11.0) | 0.10 | 0.07 | 6.0 (2.0–12.0) | 0.10 | 0.07 |
| Diseases in baseline period** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Alcohol abuse | Yes | 1.9 | 2.1 | 0.01 | 0.08 | 2.5 | 0.04 | 0.10 | 1.7 | 0.01 | 0.03 | 1.7 | 0.01 | 0.04 |
| Cancer | Yes | 5.1 | 9.4 | 0.17 | 0.08 | 11.0 | 0.22 | 0.10 | 6.7 | 0.07 | 0.03 | 7.4 | 0.09 | 0.03 |
| Dementia | Yes | 2.2 | 6.3 | 0.20 | 0.08 | 8.1 | 0.27 | 0.07 | 4.0 | 0.10 | 0.03 | 4.8 | 0.14 | 0.04 |
| Hypoglycemia, ambulatory care visit | Yes | 0.9 | 1.6 | 0.06 | 0.06 | 2.0 | 0.09 | 0.05 | 1.3 | 0.04 | 0.03 | 1.5 | 0.05 | 0.04 |
| Hypoglycemia, serious | Yes | 0.4 | 1.2 | 0.08 | 0.04 | 2.0 | 0.14 | 0.04 | 0.6 | 0.03 | 0.01 | 0.8 | 0.05 | 0.01 |
| Kidney diseases | Yes | 5.3 | 22.3 | 0.51 | 0.04 | 25.5 | 0.58 | 0.05 | 13.4 | 0.28 | 0.05 | 13.8 | 0.29 | 0.04 |
| Liver diseases | Yes | 6.4 | 11.1 | 0.17 | 0.10 | 11.6 | 0.18 | 0.10 | 8.4 | 0.08 | 0.03 | 7.8 | 0.06 | 0.04 |
| Obesity | Yes | 10.0 | 6.7 | 0.12 | 0.14 | 6.3 | 0.14 | 0.16 | 6.2 | 0.14 | 0.06 | 6.1 | 0.14 | 0.07 |
| Drugs can affect blood glucose, in 7 days prior to cohort entry** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Co-trimoxazole | Yes | 0.4 | 0.6 | 0.03 | 0.04 | 0.7 | 0.05 | 0.04 | 0.3 | 0.01 | 0.02 | 0.4 | 0.00 | 0.02 |
| Quinolones | Yes | 0.8 | 1.7 | 0.08 | 0.06 | 1.5 | 0.06 | 0.03 | 1.1 | 0.02 | 0.02 | 1.1 | 0.03 | 0.02 |
| Drugs can affect blood glucose, in 30 days prior to cohort entry** | Group | % | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD | % | SMD | WCSD |
| Angiotensin converting enzyme inhibitors | Yes | 11.3 | 11.6 | 0.01 | 0.04 | 12.3 | 0.03 | 0.10 | 10.8 | 0.02 | 0.04 | 10.6 | 0.02 | 0.04 |
| Angiotensin II receptor antagonists | Yes | 4.0 | 8.2 | 0.18 | 0.12 | 6.1 | 0.10 | 0.08 | 5.9 | 0.09 | 0.05 | 5.0 | 0.05 | 0.05 |
| Antipsychotics, atypical | Yes | 8.4 | 6.9 | 0.06 | 0.09 | 6.0 | 0.09 | 0.08 | 7.1 | 0.05 | 0.05 | 6.4 | 0.08 | 0.07 |
| Beta blockers | Yes | 13.2 | 18.9 | 0.15 | 0.17 | 16.5 | 0.09 | 0.11 | 14.1 | 0.02 | 0.09 | 13.1 | 0.00 | 0.09 |
| Calcineurin inhibitors | Yes | 0.1 | 0.6 | 0.09 | 0.02 | 0.9 | 0.12 | 0.02 | 0.2 | 0.04 | 0.01 | 0.3 | 0.05 | 0.01 |
| Corticosteroids | Yes | 3.1 | 5.4 | 0.11 | 0.13 | 5.9 | 0.14 | 0.13 | 2.9 | 0.01 | 0.05 | 2.9 | 0.01 | 0.06 |
| Diuretics, thiazide | Yes | 6.3 | 4.2 | 0.09 | 0.04 | 4.0 | 0.10 | 0.03 | 4.3 | 0.09 | 0.02 | 4.3 | 0.09 | 0.03 |
| Haloperidol | Yes | 0.5 | 0.4 | 0.01 | 0.03 | 0.5 | 0.00 | 0.04 | 0.4 | 0.02 | 0.01 | 0.4 | 0.02 | 0.01 |
| Protease inhibitors | Yes | 0.4 | 0.2 | 0.05 | 0.00 | 0.4 | 0.01 | 0.02 | 0.6 | 0.02 | 0.01 | 0.9 | 0.06 | 0.00 |
| Quinine | Yes | 0.3 | 0.9 | 0.09 | 0.04 | 0.6 | 0.05 | 0.02 | 0.6 | 0.05 | 0.01 | 0.7 | 0.06 | 0.01 |
| Salicylates | Yes | 4.1 | 6.6 | 0.11 | 0.09 | 4.8 | 0.04 | 0.05 | 5.4 | 0.06 | 0.04 | 5.1 | 0.05 | 0.04 |
Q = quartile; SMD = standardized mean difference (vs. metformin); WCSD = weighted conditional standardized difference (vs. metformin)
unless otherwise noted
included in propensity score
included as a covariate in Cox proportional hazards model
Table 2.
Standardized occurrence rates (95 % confidence intervals) of serious hypoglycemia per 1,000 person-years of follow-up, by exposure group—overall and stratified by average daily dose
| Overall | Low dose | High dose | |
|---|---|---|---|
| glyburide | 68.0 (64.9, 71.2) | 64.5 (60.8, 68.2) | 74.3 (67.1, 81.4) |
| glimepiride | 52.9 (48.9, 56.9) | 50.3 (45.2, 55.4) | 58.8 (50.3, 67.2) |
| glipizide | 49.6 (47.2, 51.9) | 42.5 (39.6, 45.4) | 59.4 (55.1, 63.8) |
| repaglinide | 44.4 (34.7, 54.1) | 37.2 (27.8, 46.7) | 66.8 (33.8, 99.9) |
| nateglinide | 23.2 (15.6, 30.7) | 19.1 (8.9, 29.3) | 28.2 (15.7, 40.7) |
| rosiglitazone | 14.6 (12.3, 17) | 14.5 (11.7, 17.4) | 17.2 (11.7, 22.8) |
| pioglitazone | 13.8 (11.9, 15.7) | 8.5 (6.3, 10.7) | 18.1 (14.8, 21.4) |
| metformin | 11.9 (11.3, 12.5) | 8.8 (7.9, 9.7) | 13.4 (12.6, 14.3) |
Unadjusted HRs (95% CIs) vs. metformin were 5.07 (4.72, 5.45) for glyburide, 4.14 (3.77, 4.56) for glimepiride, 3.35 (3.12, 3.61) for glipizide, 3.30 (2.67, 4.08) for repaglinide, 1.80 (1.32, 2.47) for nateglinide, 1.11 (0.94, 1.32) for rosiglitazone, and 0.99 (0.85, 1.15) for pioglitazone. In general, baseline characteristics were well balanced between each antidiabetic vs. metformin after conditioning on PS. PS-adjusted HRs vs. metformin are shown in Figure 1. PS-adjusted HRs for glimepiride and glyburide, each vs. glipizide, were 1.28 (1.16, 1.40) and 1.53 (1.43, 1.65) respectively. The PS-adjusted HR for nateglinide vs. repaglinide was 0.60 (0.41, 0.87). The PS-adjusted HR for rosiglitazone vs. pioglitazone was 1.12 (0.90, 1.40). Findings from pre-specified secondary analyses (data not shown) were similar to the primary analysis.
Figure 1.
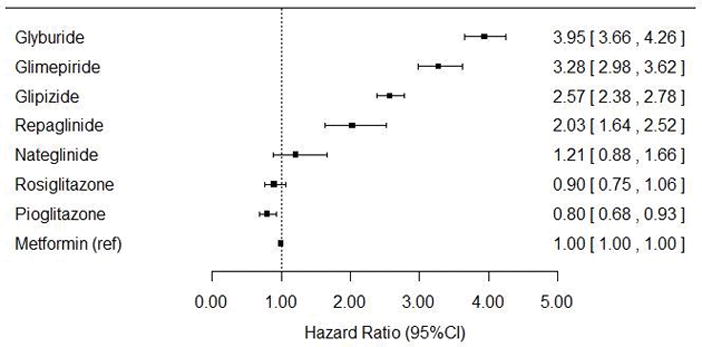
Propensity score-adjusted hazard ratios for serious hypoglycemia, using metformin as the referent
Discussion
To our knowledge, this is the first study to examine and compare the rate of serious hypoglycemia among individuals receiving antidiabetic monotherapy with metformin, a SU, a meglitinide, or a TZD in a real-world setting. It is also unique as it was sufficiently powered to make meaningful comparisons among individual drugs, rather than assume that all agents of a given pharmacologic class carry the same risk.
Our study confirms that SUs are associated with higher rates of hypoglycemia than either metformin or TZDs. Bennett et al.25 performed a meta-analysis of randomized trials that compared the risk of having at least one mild or moderate hypoglycemic event among individuals receiving various antidiabetic therapies. The pooled odds ratios (ORs) were 4.59 for SUs vs. metformin and 3.88 for SUs vs. TZDs—consistent with our findings. Our study also confirms that among individual SUs, glyburide is associated with a higher rate of hypoglycemia compared to glipizide and glimepiride. This is consistent with the pooled absolute risk difference of 0.03 (0, 0.05) comparing glyburide with other second generation SUs reported in a meta-analysis.27
Less is known about the risk of hypoglycemia associated with meglitinides. Bennett et al.25 reported a pooled OR of 3.01 for meglitinides vs. metformin and a statistically non-significant difference between SUs and meglitinides. In another meta-analysis, repaglinide was found to have a similar incidence of any hypoglycemia (i.e., not limited to serious events) as SUs.27 We found that repaglinide was associated with a rate of hypoglycemia slightly lower than that of SUs, whereas the rate for nateglinide was substantially lower and similar to metformin.
Although few studies have examined hypoglycemia among TZDs users, a prior study reported a similar risk of any hypoglycemia between TZDs and metformin.27 We found that the rate of serious hypoglycemia in pioglitazone users was lower than that in metformin users. Although rosiglitazone had a higher standardized occurrence rate than metformin, the difference was not statistically significant after adjusting for PS. This is concordant with the finding from A Diabetes Outcome Progression Trial,28 which found no significant difference in the number of self-reported hypoglycemic events between individuals receiving rosiglitazone and those receiving metformin.25
The standardized occurrence rates of serious hypoglycemia reported in our study were higher than crude incidence rates reported previously both for SUs (49.6–68.0 vs. 10–30 per 1,000 p-y29–31) and metformin (11.9 vs. 0.6 per 1,000 p-y32). This is not surprising since an occurrence rate includes both initial and recurrent events. Furthermore, the follow-up period in our study was 180 days from initiation of an antidiabetic therapy, during which the risk of hypoglycemia is typically highest. In addition, our study population and the reference population used for standardization were Medicaid enrollees who have relatively high burdens of disability and chronic conditions and thus may be more vulnerable to serious hypoglycemia.
We found that the occurrence rate of serious hypoglycemia was dose-dependent for all study drugs. This was not surprising for SUs and meglitinides since they stimulate insulin secretion in a dose-dependent manner.33 Further, among patients treated with the same antidiabetic agent, those receiving higher doses usually have more serious disease and are at a higher risk of fluctuating glycemic control than those treated at lower doses. This potential confounding-by-severity among doses may be in part responsible for the observed dose-response relationships.
Our study has a number of strengths. We avoided conflating associations between concomitant antidiabetic drugs and serious hypoglycemia by restricting the study to monotherapy-treated patients. We minimized confounding between exposure groups by using direct standardization and PS adjustment techniques and examining dose-response relationships. We utilized a very large database to permit the study of less commonly-used antidiabetic drugs. Finally, we used an algorithm with a high positive predictive value to identify serious hypoglycemia, thereby minimizing outcome misclassification.
Our study also has limitations. First, we probably underestimated the rate of serious hypoglycemia as defined by the American Diabetes Association—an event requiring assistance of another person to actively administer carbohydrates, glucagon, or take other corrective actions.2 Our outcome of serious hypoglycemia required ED presentation and/or inpatient hospitalization. Second, we may have misclassified some prevalent users of an antidiabetic drug or drugs as new users of monotherapy;15 upon cohort entry, these persons may have been less susceptible to the hypoglycemic effects of the exposure of interest vs. “true” new users. This misclassification could occur if a physician provided a drug sample (most applicable to a patented branded product) or the patient made a cash payment for a generic drug dispensed at a retail pharmacy (for which claims are often not transmitted to the insurer34), particularly via a generic prescription drug discount program. Third, we could not adjust for potential confounders not recorded in administrative claims data, e.g., irregular meal schedule and exercise. Fourth, the available sample size for nateglinide was relatively small by pharmacoepidemiologic standards, which limited our ability to generate numerically precise estimates for this drug. Fifth, we did not study newer oral antidiabetic drugs such as dipeptidyl peptidase-4 inhibitors, glucagonlike peptide 1 agonists, and sodium-glucose co-transporter 2 inhibitors. Finally, our study population consisted of Medicaid enrollees and this may limit the generalizability of the findings. Nevertheless, because the Medicaid population is a large and vulnerable one, it is important to study.
Serious hypoglycemia caused by antidiabetic agents is widely recognized as a major clinical and public health problem, and an important consideration when choosing antidiabetic therapy. Our findings support existing evidence that SUs have the highest rates of serious hypoglycemia among oral antidiabetic drugs. However, there may be clinically meaningful differences in hypoglycemia risk with different sulfonylureas. These data may help prescribers consider serious hypoglycemia risk when choosing an oral antidiabetic agent for Medicaid beneficiaries.
Supplementary Material
Key points.
Hypoglycemia is a common and potentially life-threatening adverse effect of antidiabetic agents, yet few studies comparing antidiabetic monotherapies with respect to hypoglycemia risk have been performed.
The ranking of crude standardized occurrence rates of serious hypoglycemia was glyburide > glimepiride > glipizide > repaglinide > nateglinide > rosiglitazone > pioglitazone > metformin.
The rate of serious hypoglycemia increased with average daily dose for all study drugs.
After adjusting for confounding, the ranking of hazard ratios for serious hypoglycemia was glyburide > glimepiride > glipizide > repaglinide > nateglinide ≈ rosiglitazone ≈ metformin (referent) > pioglitazone.
Acknowledgments
Sources of support: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK102694) and the National Institute on Aging (R01 AG025152).
The authors wish to thank Ms. Qing Liu and Ms. Min Du from the University of Pennsylvania for their statistical programming support.
Footnotes
Previous presentation: This work has not been presented elsewhere in whole or in part.
Conflicts of interest: Dr. Leonard, Dr. Han, Dr. Bilker, Dr. Cardillo, Dr. Flory, and Ms. Brensinger have no conflicts of interest to disclose. Dr. Hennessy has received salary support through his employer for research funded by Bristol-Myers Squibb, directs a pharmacoepidemiology training program that receives support from Pfizer Inc and Sanofi US, and has consulted for GlaxoSmithKline, Merck Sharp & Dohme Corp, Novo Nordisk Pharmaceuticals Inc, and Sanofi US, all unrelated to this research.
References
- 1.Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;369(3):287–288. doi: 10.1056/NEJMc1306652. [DOI] [PubMed] [Google Scholar]
- 2.Standards of medical care in diabetes-2017: Summary of revisions. Diabetes Care. 2017;40(Suppl 1):S4–S5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: A patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 4.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract. 2017;23(2):207–238. doi: 10.4158/EP161682.CS. [DOI] [PubMed] [Google Scholar]
- 5.Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003–2012. Diabetes Care. 2014;37(5):1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services’ Office of Disease Prevention and Health Promotion. [Last accessed: 07/20/2017];National action plan for adverse drug event prevention. 2014 Available at: https://health.gov/hcq/ade-action-plan.asp. Last updated: 07/20/2017.
- 7.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser Family Foundation. Medicaid enrollment: June 2010 data snapshot. [Last accessed: 07/20/2017];Kaiser Commission on Medicaid Facts. 2011 Publication #8050-03. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8050-03.pdf.
- 9.Kaiser Family Foundation. Dual eligible beneficiaries by race/ethnicity. [Last accessed: 07/20/2017];State Health Facts. Available at: http://kff.org/other/state-indicator/dual-eligible-beneficiaries-by-re/
- 10.Hennessy S, Bilker WB, Weber A, Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol Drug Saf. 2003;12(2):103–111. doi: 10.1002/pds.765. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid services (CMS) Med Care. 2007;45(12):1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 12.Leonard CE, Brensinger CM, Nam YH, et al. The quality of Medicaid and Medicare data obtained from CMS and its contractors: Implications for pharmacoepidemiology. BMC Health Serv Res. 2017;17(1) doi: 10.1186/s12913-017-2247-7. 304-017-2247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Wang SV, Leonard CE, et al. Pilot test of the Sentinel modular program for propensity score-matched cohort analyses: Application to glyburide, glipizide, and serious hypoglycemia. Epidemiology. 2017 doi: 10.1097/EDE.0000000000000709. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson W, Bolen S, Witkop CT, Neale D, Wilson L, Bass E. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: A systematic review. Obstet Gynecol. 2009;113(1):193–205. doi: 10.1097/AOG.0b013e318190a459. [DOI] [PubMed] [Google Scholar]
- 15.Cepeda MS, Fife D, Denarie M, Bradford D, Roy S, Yuan Y. Quantification of missing prescriptions in commercial claims databases: Results of a cohort study. Pharmacoepidemiol Drug Saf. 2017;26(4):386–392. doi: 10.1002/pds.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168(3):329–335. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- 17.Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88(2):214–222. doi: 10.1038/clpt.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard CE, Bilker WB, Brensinger CM, et al. Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clin Pharmacol Ther. 2016;99(5):538–547. doi: 10.1002/cpt.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard CE, Han X, Bilker WB, et al. Comparative risk of severe hypoglycemia among concomitant users of thiazolidinedione antidiabetic agents and antihyperlipidemics. Diabetes Res Clin Pract. 2016;115:60–67. doi: 10.1016/j.diabres.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Chiang CW, Leonard CE, Bilker WB, Brensinger CM, Hennessy S. Biomedical informatics approaches to identifying drug-drug interactions: Application to insulin secretagogues. Epidemiology. 2017;28(3):459–468. doi: 10.1097/EDE.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginde AA, Blanc PG, Lieberman RM, Camargo CA., Jr Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. The American Statistician. 1986;40(3):249–251. [Google Scholar]
- 23.Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf. 2008;17(12):1202–1217. doi: 10.1002/pds.1673. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The robust inference for the cox proportional hazards model. JASA. 1989;84(408):1074–1078. [Google Scholar]
- 25.Bennett WL, Wilson LM, Bolen S, et al. Oral diabetes medications for adults with type 2 diabetes: An update. Agency for Healthcare Research and Quality; 2011. Report No.: 11-EHC038-EF. [PubMed] [Google Scholar]
- 26.Byrd VL, Dodd AH. Assessing the usability of MAX 2008 encounter data for comprehensive managed care. Medicare Medicaid Res Rev. 2013;3(1) doi: 10.5600/mmrr.003.01.b01. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolen S, Wilson L, Vassy J, et al. Comparative effectiveness and safety of oral diabetes medications for adults with type 2 diabetes [internet] Agency for Healthcare Research and Quality; 2007. Report No.: 07-EHC010-EF. [PubMed] [Google Scholar]
- 28.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 29.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161(13):1653–1659. doi: 10.1001/archinte.161.13.1653. [DOI] [PubMed] [Google Scholar]
- 30.Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157(15):1681–1686. [PubMed] [Google Scholar]
- 31.van Staa T, Abenhaim L, Monette J. Rates of hypoglycemia in users of sulfonylureas. J Clin Epidemiol. 1997;50(6):735–741. doi: 10.1016/s0895-4356(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 32.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care. 2008;31(11):2086–2091. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groop LC, Barzilai N, Ratheiser K, et al. Dose-dependent effects of glyburide on insulin secretion and glucose uptake in humans. Diabetes Care. 1991;14(8):724–727. doi: 10.2337/diacare.14.8.724. [DOI] [PubMed] [Google Scholar]
- 34.Tungol A, Starner CI, Gunderson BW, Schafer JA, Qiu Y, Gleason PP. Generic drug discount programs: Are prescriptions being submitted for pharmacy benefit adjudication? J Manag Care Pharm. 2012;18(9):690–700. doi: 10.18553/jmcp.2012.18.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


