Abstract
Background
Substance use (SU) and sleep problems appear interrelated, but few studies have examined the influence of adolescent sleep patterns on development of SU disorders. This study prospectively examined the influence of sleep habits on subsequent SU in youth who later transitioned into heavy drinking.
Methods
At time 1 (T1), participants (N=95) were substance-naïve 12-14 year-olds. Path-analytic models examined whether the effects of T1 risk factors (familial SU disorder, inhibition control, and externalizing and internalizing traits) on time 3 (M =19.8 years old) tobacco, alcohol, and cannabis were mediated by time 2 (M =15.1 years old) sleep chronotype, daytime sleepiness, and erratic sleep/wake behaviors.
Results
Significant direct path effects of T1 risk factors and time 2 sleep behaviors on time 3 SU were found, ps<.05. In models that examined the effect of each individual sleep behavior separately on substance use, more erratic sleep/wake and greater daytime sleepiness predicted higher lifetime use events for all substances (ps<.01). Higher evening chronotype tendencies predicted lower tobacco, and higher alcohol and cannabis lifetime use events (ps<.01). Erratic sleep/wake behaviors mediated the effect of inhibitory control on subsequent SU; less erratic sleep/wake behaviors predicted better inhibition control ( =−.20, p<.05).
Conclusions
Early-mid adolescent psychiatric health and sleep behaviors prior to drinking onset predicted greater SU five years later. Participants were substance-naïve at baseline, allowing for the examination of temporal order in the relationship between sleep problems and alcohol use. Early adolescent sleep problems may be an important risk factor for SU in later life.
Keywords: adolescence, substance use, sleep
Introduction
Adolescence is a period characterized by marked physiological, social, and neurocognitive changes and maturation. During puberty, youth begin to display a preference for later sleep and wake times (Wolfson & Carskadon, 1998), even after accounting for the influence of other social factors (e.g., home illumination, extracurricular involvement; National Sleep Foundation, 2014). Similar findings in other countries suggest that this phenomenon is not culturally bound but may reflect underlying biological changes (Yang, Kim, Patel, & Lee, 2005). Shifts in sleep patterns during adolescence often negatively affect sleep duration and quality, leaving many adolescents sleep deprived. Between ages 13-19, there is an overall decrease in weekend and weekday sleep duration (Wolfson & Carskadon, 1998). The proportion of youth who get at least 8 hours of sleep decreases from 40% to 23% between 9th to 12th grade (Kann et al., 2014).
Concomitant with changes in sleep are increases in substance use (SU) during adolescence (McKnight-Eily et al., 2011). Alcohol, marijuana, and tobacco are the most commonly used substances among youth (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2016). There is a threefold increase in the prevalence of cigarette, smokeless tobacco, and cannabis use from eigth-grade (13%, 9%, and 16% respectively) to the end of high-school (Johnston et al., 2016). Teens 12-18 years-old who experience problems with falling or staying asleep are more likely to endorse SU (O’Brien & Mindell, 2005; Roane & Taylor, 2008). Among students, decreased school-night sleep duration, increased differences between weekday and weekend sleep onset time, and lowered sleep quality have been associated with increased SU and related consequences (O’Brien & Mindell, 2005). Sleep timing propensity, or chronotype, has been consistently associated with SU (Hasler, Sitnick, Shaw, & Forbes, 2013). Morning chronotypes tend to have an earlier sleep onset and offset (i.e., wake-up) time. In comparison, evening chronotypes are more active later in the day and have a later pattern of sleep onset and offset. Compared to their morning chronotype counterparts, evening chronotypes engage in significantly more SU behaviors (Pieters, Van Der Vorst, Burk, Wiers, & Engels, 2010).
It is important to understand the possible contributions of environmental and psychosocial influences on why sleep problems may precede SU. This will further aid in efforts to better identify sleep as a possible risk factor and address the alarming trend of using alcohol and tobacco as sleep aids among students (Noland, Price, Dake, & Telljohann, 2009). The relationship between SU and sleep has been well-studied in adults, and to a lesser extent, in adolescents. Two key criteria must be considered in understanding the effects of sleep problems on adolescent SU. First, to ascertain whether alcohol use precedes or follows sleep disturbance, it is important to examine the onset of sleep problems prior to SU initiation. To date, there have been few studies examining this issue longitudinally. Children with daytime over-tiredness and trouble sleeping are twice as likely to begin drinking in adolescence and experience alcohol-related consequences compared to same-aged peers without early life sleep problems (Wong, Brower, Nigg, & Zucker, 2010). Recently, further evidence emerged to suggest that sleep problems precede, and are predictive of, future adolescent substance use (Hasler, Kirisci, & Clark, 2016; Miller, Janssen, & Jackson, 2016). Importantly, youth were substance-naïve at baseline in these studies, decreasing possible confounding effects of concomitant alcohol use and sleep problems.
The relationship between sleep patterns and SU has yet to be characterized in the context of a broader array of other age-appropriate psychological and environmental factors associated with these constructs. Such factors include psychiatric functioning, impulsivity, and family history (FH) of substance use disorder (SUD). One study found that daytime sleepiness predicted alcohol use above and beyond internalizing and externalizing symptomology (Miller et al., 2016) while another reported that the association between insomnia and use initiation decreased after controlling for them (Johnson & Breslau, 2001). The effect of FH on the development and maintenance of SU has been well documented (Barnow, Schuckit, Lucht, John, & Freyberger, 2002). However, if and how familial SUD affects sleep patterns is less clear. Healthy adults with FH of parental alcohol use disorder (AUD) take longer to fall asleep than those without (Rupp, Acebo, Seifer, & Carskadon, 2007). Substance-naïve 9-10 year-olds with parental history of alcoholism displayed lower delta power during non-rapid eye movement sleep than in negative FH children (Tarokh & Carskadon, 2010). Poor response inhibition has been associated with increased SU and poor sleep in adolescents (Beebe, 2011). Together, these findings suggest that the relationship between sleep and SU in adolescents interacts with other psychological and environmental factors. Yet, few studies have examined them together in a comprehensive prospective model.
The present study utilized a longitudinal design with three time points spanning eight years to test whether the effects of environmental and psychiatric risk factors at project entry (i.e., time 1 [T1] inhibitory control, externalizing and internalizing traits, and SUD FH) on subsequent lifetime SU events measured at time 3 (i.e., cumulative use of alcohol, tobacco, and cannabis; T3) are influenced by sleep behaviors at time 2 (i.e., chronotype, daytime sleepiness, and erratic sleep/wake behaviors; T2) in adolescents. T1 factors were chosen due to associations with sleep and SU in prior studies. Importantly, participants had not initiated SU at the time their sleep behaviors were assessed, allowing for the question of whether sleep in mid-adolescence predicts subsequent SU quantity. Based on previously reported cross-sectional findings, it was hypothesized that youth with poorer inhibitory control, increased internalizing and externalizing symptomology, and greater SUD FH density at T1 will have greater lifetime use events of alcohol, tobacco, and marijuana by T3 (Johnson & Breslau, 2001). Further, we propose that this relationship between risk factors and SU is mediated by sleep patterns in mid-adolescence. Youth with more T1 risk factors will have greater T2 evening chronotype tendencies and T2 sleep problems (i.e., greater daytime sleepiness and more erratic sleep/wake behaviors), which will in turn lead to greater lifetime SU events at T3 (Nicholson, Turner, Stone, & Robson, 2004). It was theorized that sleep problems influence SU outcomes through negative effects on basic biological functions (i.e., autonomic nervous system and circadian rhythms) associated with decreased capacity for self-regulation and impulse control (Hasler & Clark, 2013; Hasler et al., 2016).
Materials and Methods
Participants
This study is part of an ongoing longitudinal neuroimaging project on adolescent SU (R01-AA13419). At T1, participants were healthy 12-14 year-old San Diego middle school students with minimal SU experience. Potential participants were recruited through fliers and screened by bachelor’s or master’s level psychometrists. Parents provided verbal consent for a telephone screening to assess inclusion and exclusion criteria. Eligible participants were assessed using in-person detailed youth screening; parents underwent a parallel parent screen. Youth and parents were then followed annually following T1 at approximately the same time each year (within three months). At each annual follow-up, youth were administered the same detailed interview and questionnaires described below. The study protocol was approved by the University of California San Diego Human Research Protections Program.
Ages 12-14 years are an important time for neurodevelopment. During this period, development of frontal brain regions intensifies but the brain is mature enough to allow for longitudinal comparisons without gross structural changes (Giedd, 2004). Rapid psychosocial developments also occur in this age range and are reflected in increased rates of SU. Fifteen to 26% of students try alcohol by eighth-grade (SAMHSA, 2013). Further, sleep changes generally emerge in adolescence around age 11-12 (Colrain, Nicholas, & Baker, 2014), in part due to puberty-related changes in circadian rhythms and environment (e.g., earlier school times). The 12-14 age range is early enough to capture SU initiation and sleep pattern changes, but structural neurodevelopment has stabilized to allow for the examination of long term changes throughout adolescence. To account for developmental variation within this age range, age and pubertal development were included as covariates in all analyses.
Exclusionary criteria at T1 included: prenatal alcohol (>2 drinks in a week) or illicit drug exposure; born before 35th gestational week; history of DSM-IV (American Psychiatric Association, 2000) Axis I disorder, traumatic brain injury or loss of consciousness (>2 min), neurological or chronic medical illness, learning disability or mental retardation, or use of psychoactive medications; significant experience with substances; inadequate comprehension of English; and non-correctable sensory problems.
The parent study enrolled a total of N=295 participants (6 withdrew from the study by T3). The Sleep Habits Questionnaire (SHQ) was added to the parent study protocol at a later time period, thus not all 295 participants were administered this measure prior to alcohol use initiation. For the present analysis, no participant had consumed a full drink of alcohol or tried any other drug at T1 or T2 or had a history of Axis I disorder at T3 (measured by annual follow-up diagnostic interview; N=95; see Table 1). The study took place over 8.2 years, such that T1 data were collected at project entry, T2 data were collected 1.5 (SD=0.7, range: 0.9-3.5) years later, and T3 data 4.6 (SD=0.5, range: 2.6-5.9) years after time 2. Participants were, on average 13.4 (SD=0.7), 15.1 (SD=0.9), and 19.8 (SD=0.9) years-old at T1, T2, and T3, respectively.
Table 1.
Sample characteristic at time 1 and follow-ups (N = 95)
| Baseline Time 1 |
Follow-up Time 2 |
Follow-up Time 3 |
|
|---|---|---|---|
| M (SD) or % | |||
|
| |||
| Age | 13.4 (0.7) | 15.1 (.9) | 19.8 (.9) |
| % Female | 47.4% | ||
| Hollingshead Index (SES) | 22.1 (12.7) | ||
| Family History | |||
| Negative | 43.6% | ||
| Mild | 34.0% | ||
| Positive | 22.3% | ||
| Race | |||
| Latino/a | 20.0% | ||
| Caucasian | 68.4% | ||
| African-American | 3.2% | ||
| Asian | 6.3% | ||
| Other | 5.3% | ||
| Multiple races | 16.8% | ||
| % Conduct disorder | 3.2% | ||
| Sleepiness a | 13.2 (2.9) | ||
| Erratic sleep/wake behaviors a | 14.6 (4.0) | ||
| Chronotype a | 28.2 (4.9) | ||
| % Lifetime tobacco users | 0% | 54.7% | |
| % Lifetime drinkers | 0% | 100% | |
| % Lifetime cannabis users | 0% | 57.9% | |
| % Other recreational drug users b | 0% | 22.1% | |
| Past year drinking days | 0 (0.0) | 43.6 (55.3) | |
| Past year HED days | 0 (0.0) | 17.9 (35.6) | |
| Average monthly drinking days * | 0 (0.0) | 3.6 (4.5) | |
Sleep Habits Questionnaire scale score, higher values indicate more sleepiness, more erratic sleep/wake behaviors, and greater morning chronotype tendency
Amphetamines, barbiturates, hallucinogens, cocaine, inhalants, opiates, benzodiazepines, ecstasy, ketamine, gamma-Hydroxybutyric acid [GHB], and Phencyclidine [PCP]
Past 3 months
At T3, youth must have at least initiated alcohol use and been between ages 17-21. This age range was selected for two reasons. It coincides with the social event of transitioning out of high-school and into college (all participants attended post high-school education). Alcohol and other SU behaviors also change at this time, with college students more likely to be engaged in risky drinking behaviors than high-school students. Epidemiologically, tobacco and illicit drug use peaks during this time (SAHMSA, 2013). Further, age 17-21 years marks a range and developmental period that is arguably distinct from younger (i.e., high-school students) and older (i.e., post-college young adults) ages. Thus, T3 outcome data was confined to this age range to decrease heterogeneity in the outcome variables of interest (i.e., substance use) due to environmental factors unrelated to T2 sleep.
Procedures
After initial screen at T1, eligible youth were administered a comprehensive interview assessing SUD FH and psychopathology, substance use, and general background information. A different psychometrist interviewed the parent on background and FH. Participants were assured that responses are not shared with parents or schools. To maintain a high follow-up rate (>95%), participants were contacted quarterly and with brief interviews, newsletters, and birthday cards (Twitchell, Hertzog, Klein, & Schuckit, 1992).
Measures
Structured clinical interview
The Structured Clinical Interview (Brown, Myers, Mott, & Vik, 1994) was administered by trained bachelor- and masters-level psychometricians to youth at baseline and annual follow-ups to assess academic functioning, major medical illnesses, and activities involved (e.g., extracurricular).
Sleep
At T2, prior to any SU, participants were administered the self-report SHQ, adapted from the School Sleep Habits Survey (Wolfson & Carskadon, 1998; http://www.sleepforscience.org). Three aspects of sleep were assessed. The Sleepiness Scale sums items asking about past two-week degree of daytime sleepiness (i.e., fought sleep) experienced in ten common situations; higher scores indicated greater sleepiness during wake-hour activities. The Sleep/Wake Problems Behavior Scale asked the past two-week frequency of erratic behaviors related to sleeping and waking (e.g., stayed up all night); higher scores indicated greater frequency of problematic behaviors due to poor sleep/wake habits. The Superscience Morningness/Eveningness Scale assessed chronotype; higher scores indicated a greater tendency towards a morning chronotype, and lower scores indicating a greater tendency towards an evening chronotype.
Substance use measures
The Customary Drinking and Drug Use Record (Brown et al., 1998), an interview-based assessment, was administered to assess the pattern and severity of SU for each year beginning at T1. Lifetime SU events were defined as the cumulative total use occasions for each substance after T2 up to, and including, T3. The total number of SU occasions in the past year was assessed annually, summed, and recoded by the psychometrician. Parent or other informant (sibling, friend, and roommate) report of youth SU was collected at approximately the same time to confirm youth reports. There were no gross discrepancies between the two sources in the current study (N=95).
T1 risk factors
At T1, prior to any SU, parents were administered the Child Behavior Checklist to obtain T-scores of internalizing and externalizing symptoms based on an age- and gender-normed national sample (Achenbach, 1991). Higher scores indicated greater symptomology. T1 inhibitory control was measured using the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Color-Word Interference Condition 3 (Inhibition) subtest raw scores (i.e., time to completion). To better understand the effect of inhibitory control on sleep and SU, independent of general processing speed, analyses were also conducted using the difference between Conditions 1 and 2 average (Word and Color Naming) and Color Word Interference Condition 3. Significance and directionality of the findings reported below were unchanged when using this index.
Pubertal development
The Pubertal Development Scale (Petersen et al. 1988), a valid and reliable self-assessment measure of pubertal maturational stage, was administered at T2 when sleep behaviors were assessed.
Demographics
The Hollingshead Index of Social Position score (Hollingshead, 1965), an index of socioeconomic status (SES), was calculated for each subject using parental socioeconomic information (i.e., educational attainment, occupation, and salary of each parent) to characterize the youth’s home environment. Higher values indicate lower SES. The Family History Assessment Module (Rice et al., 1995) was administered to youth and both parents to assess familial density of alcohol and other SUD. Familial density was calculated as the weighted sum of biological parents (weighted 0.5) and biological grandparents (weighted 0.25) who endorsed two or more SUD symptoms.
Data Analysis
A path-analytic model was tested in MPlus (Muthén & Muthén, 2012) to explore the mediator role of T2 sleep behaviors on the relationship between T1 risk factors and T3 lifetime SU events. Based on the literature, a comprehensive a priori model was conceptualized (Figure 1). Four psychiatric and environmental T1risk factors were examined: FH density of SUD, inhibitory control, and internalizing and externalizing traits. Three T2 sleep behaviors were examined as possible mediators: chronotype, daytime sleepiness, and erratic sleep/wake behaviors. Three T3 outcome measures of SU were examined: lifetime use events of alcohol, cannabis, and tobacco. In an initial model, all three sleep behavior predictors were entered into the same path-analytic model to understand the unique effects of each sleep behavior, controlling for the other two. Daytime sleepiness, erratic sleep/wake behaviors, and evening chronotype tendencies were significantly intercorrelated (r= −.51 to .40; ps<.05), and tests of multicollinearity showed minimal variance inflation. However, due to their shared variance, a single model with all three sleep behaviors entered simultaneously may overlook a common risk factor for substance use. Thus, follow-up analyses were conducted with only one sleep predictor examined at a time to better understand the effects of each on substance use outcome. Other recreational drugs were not examined due to small sample size (i.e., 21 participants have tried other drugs, of whom ten had one lifetime use event).
Figure 1.
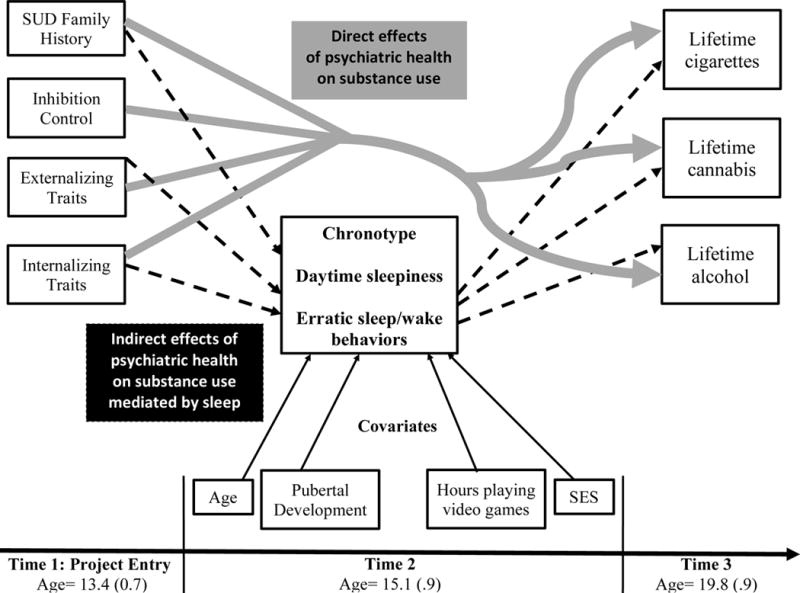
Conceptual path analytic model.
Paths indicate hypothesized direct effects of time 1 (T1) risk factors (substance use disorder family history, inhibition control, and internalizing and externalizing traits) to outcome substance use. Dashed black lines indicate hypothesized indirect effects of T1 risk factors, mediated by sleep behaviors (chronotype, daytime sleepiness, and erratic sleep/wake behaviors), on outcome substance use. Solid black lines indicate biological (age and pubertal development) and environmental (hours playing video games and SES) factors that may influence and covary with sleep behaviors. All T1 risk factors were assessed at time 1, project entry; all sleep behaviors and covariates were assessed at time 2, and all outcome substance use measures were assessed at time 3.
As expected, outcome SU variables were not normally distributed. Lifetime alcohol use events ranged from 1-2154 times (M=294.0, SD=379.4, Mdn=191, first quartile = 29, third quartile = 427, skewness = 2.4, kurtosis = 9.8). Lifetime tobacco use events ranged from 0-5010 (M=199.0, SD=641.8, Mdn=6, first quartile = 0, third quartile = 50, skewness = 5.3, kurtosis = 36.0). Lifetime marijuana use events ranged from 0-380 (M=37.6.0, SD=87.6, Mdn=2, first quartile = 0, third quartile = 23, skewness = 2.8, kurtosis = 9.5). A Poisson distribution was used for lifetime alcohol use events outcome. Other outcome measures were modeled using a zero-inflated Poisson distribution to more accurately capture excessive zeros in the data from youth who have not tried tobacco or marijuana.
Four biological and environmental aspects of adolescent development hypothesized to affect sleep and SU behaviors were tested as covariates in the model: SES, T2 age, pubertal development stage, and weekly hours playing video games. Higher SES youth may be at greater risk for developing substance use but decreased risk of tobacco use (Patrick, Wightman, Schoeni, & Schulenberg, 2012). Sleep patterns change as a function of age and pubertal stage throughout young adulthood, in which pubertal development may exert a positive indirect effect on alcohol use via sleep problems and chronotype (Pieters et al., 2010). Thus, age and pubertal developmental stage were included as covariates in the model to examine the relationship among T1 risk factors on T2 sleep, and in turn, T3 SU, independent of possible effects of age and maturation. The use of electronic media negatively impacts sleep (Cain & Gradisar, 2010), yet 24% of American adolescents play video games before bed (Calamaro, Mason, & Ratcliffe, 2009). Youth who play video games before bed tend to go to sleep later, have shorter sleep durations, and report more daytime tiredness and poorer sleep quality (Cain & Gradisar, 2010). Weekly number of hours spent playing videos was included as a covariate to reduce possible confounding effects of electronic games on sleep patterns. All reported path coefficients ( ) are standardized betas.
Results
Description of Sample
At T1 and T2, all participants (N=95) were substance-naïve. As the aim of this study was to understand the effects of biological and environmental risk factors and sleep behaviors on subsequent SU, only youth who at least tried alcohol by T3 were included in the analysis. Participants were 12-14 years-old at T1, 14-17 at T2, and 17-21 years-old at T3. Forty-seven percent of participants were female, and no significant differences in the distribution of gender were found among age groups. Further, youth did not differ in T1 familial density, internalizing and externalizing symptomology, and lifetime SU events by age. Unsurprisingly, older adolescents performed better on a measure of inhibitory control than younger adolescents. However, this is likely a reflection of enhanced neural maturation among older participants at T1 and that including age as a covariate in path models as described adequately accounted for this effect. Overall, these findings suggest that effects of the current study are unlikely attributable to participant age at T1. On average (SD), youth drank 3.6 days a month (4.5) and in total 44 days (SD=55.3, Mdn=24.1) in the past year. Sixty-percent initiated cannabis use, 55% tried tobacco products, and 22% tried other recreational drugs at least once in their lifetime. Transitioners (i.e., youth who have tried at least one standard alcoholic drink and any tobacco, cannabis, or other drug at least once in their lifetime) used tobacco products at least once in the past month (range: 1-30 days), cannabis on 27.5 days (85.7; range: 0-365), and other drugs on 29.7 days (71.8; range: 0-365) out of the year (Table 1). In the subsample of the parent study used for this analysis, no participant met DSM-5 (APA, 2013) diagnostic criteria for SUD at T3.
Path Analysis
Of the four covariates tested, only age was significantly associated with daytime sleepiness. As age increased, daytime sleepiness also increased, p<.05. Pubertal development, weekly video game hours, and SES were not associated with chronotype, daytime sleepiness, or erratic sleep/wake behaviors (p>.05) and were excluded from analyses.
Direct effects of T1 risk factors
The direct effects of T1 internalizing symptoms on lifetime use events of tobacco ( =.04), alcohol ( =−.35.), and cannabis ( =.36) were statistically significant, ps<.0001 (Figure 2). Those individuals who reported higher levels of T1 internalizing symptoms had greater lifetime events of tobacco and cannabis but lower lifetime alcohol use. The direct effects of T1 externalizing traits on subsequent SU were significant, higher levels of T1 externalizing traits predicted significantly lower lifetime events of tobacco use ( =−.05) but higher lifetime events of alcohol ( =.70), ps<.0001. The direct effects of T1 inhibitory control on subsequent SU were significant; better inhibition control predicted lower lifetime events of cigarette ( =−.70) and cannabis ( =−.64), ps<.0001. The direct effects of SUD FH density on subsequent SU were significant; higher SUD FH density predicted lower cigarettes ( =−.10) but higher alcohol ( =.57) and cannabis ( =.08) lifetime events, ps<.05.
Figure 2.
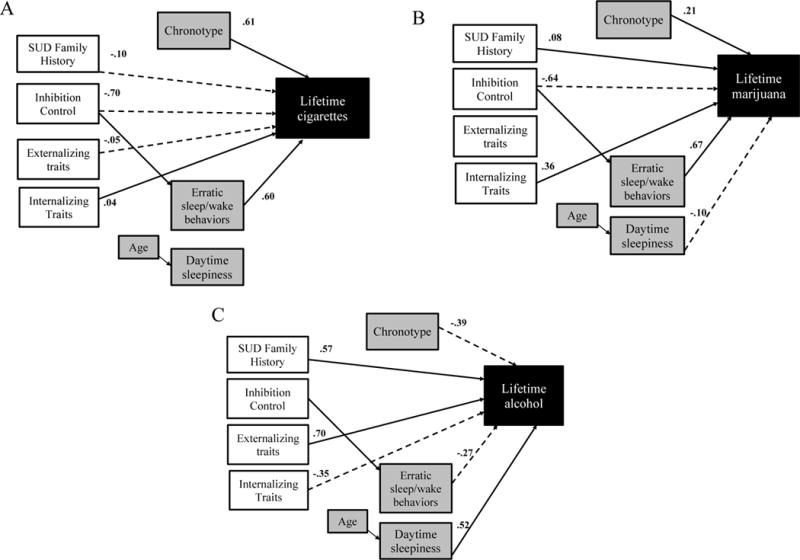
Results of path analytic model.
Note. SUD = Substance use disorder
All paths shown are significant effects, ps<.05. Solid lines indicate significant positive effects of time 1 (T1) risk factors and time 3 sleep behaviors on outcome lifetime substance use events and dashed lines indicate significant negative effects of T1 risk factors and time 3 sleep behaviors on T3 lifetime substance use events. Unshaded boxes are indices measured at time 1 (age=13.4 [0.7]) grey boxes are indices measured at time 2 (age=15.1 [.9]), and black-shaded boxes are indices measured at time 3 (19.8 [.9]). In all models, better inhibitory control (lower time to completion score) predicted less erratic sleep/wake behaviors ( =.20). Note that higher Chronotype score indicates greater morningness tendency and higher SUD Family History indicates greater familial density of SUD. Shown are standardized path coefficients.
Direct effects of T2 sleep behaviors
The direct effects of T2 erratic sleep/wake behaviors, daytime sleepiness, and chronotype on subsequent SU were significant. Controlling for daytime sleepiness and chronotype, more erratic sleep/wake behaviors predicted significantly higher tobacco ( =.60) and cannabis ( =.67), but lower alcohol ( =−.27) lifetime use events, ps<.0001. Controlling for erratic sleep/wake behaviors and chronotype, greater daytime sleepiness predicted significantly lower cannabis ( =−.10) but higher alcohol ( =.52; p<.0001) lifetime use events. Controlling for daytime sleepiness and erratic sleep/wake behaviors, higher evening chronotype tendencies predicted significantly lower tobacco ( =.61) and cannabis ( =.21), but higher alcohol ( =−.39) lifetime use events, ps<.0001.
In follow-up analyses that examined the effects of each sleep behavior in its own path-analytic model, more erratic sleep/wake behaviors predicted significantly higher tobacco ( =.41), cannabis ( =.54), and alcohol ( =.27) lifetime use events, ps<.0001. Greater daytime sleepiness predicted significantly higher tobacco ( =.22; p<.0001), cannabis ( =.12; p<.01), and alcohol ( =.57; p<.0001) lifetime use events. Higher evening chronotype tendencies predicted significantly lower tobacco ( =.28; p<.0001), but higher cannabis ( = −.12; p<.01) and alcohol ( = −.40; p<.0001) lifetime use events.
Mediation effects of T2 sleep behaviors
Erratic T2 sleep/wake behavior was a significant mediator of the effect of T1 inhibitory control on subsequent lifetime SU events. Better inhibitory control (i.e., lower time to completion score) predicted less erratic sleep/wake behaviors ( =.20) in all models, which, in follow-up analyses examining the effects of individual sleep behaviors in separate path-analytic models, in turn predicted lower lifetime tobacco ( =.41),cannabis ( =.54), and alcohol use ( =.27) events, ps<.05. No significant mediating effects of chronotype and daytime sleepiness were found.
Discussion
While SU and sleep behaviors have been examined extensively in adults (for review, see Colrain et al., 2014), their relationships in adolescents are less clear. Longitudinal designs, like that of the present study, allow for the consideration of temporal precedence, above and beyond a cross-sectional examination of the bidirectional association between sleep and alcohol use. We further extend previous findings with the inclusion of other substances commonly used by adolescents (e.g., cannabis). Results support the hypothesis that psychiatric and environmental risk factors and sleep behaviors in substance-naïve youth prospectively predict level of substance involvement in later life (Hasler et al., 2016; Miller et al., 2016; Roane & Taylor, 2008; Wong et al., 2010). T1 risk factors (i.e., internalizing and externalizing traits, inhibitory control, and familial density) and sleep behaviors (i.e., daytime sleepiness, erratic sleep/wake behaviors, and chronotype) were all unique predictors of subsequent alcohol, cannabis, and tobacco use. Further, erratic sleep/wake behavior was a significant mediator in the effect of T1 risk factors on lifetime SU events.
The relationship between SU and sleep is often described as bidirectional. Depending on the circadian phase during acute administration, some studies suggest that alcohol facilitates sleep onset, but interferes with sleep maintenance throughout the night (Van Reen, Rupp, Acebo, Seifer, & Carskadon, 2013). Alcohol’s effects on sleep appear to operate, in part, by enhancing the major inhibitory neurotransmitter gamma-aminobutyic acid and suppressing the activities of N-methyl-D-aspartate glutamate receptors, leading to easier sleep onset but less restful sleep (Koob et al., 1998). Moderate to high doses of alcohol delay the onset of, and decrease overall, rapid eye movement (REM) sleep (Ebrahim, Shapiro, Williams, & Fenwick, 2013; Pressman, 2012). On the other hand, nicotine binds to the nicotinic acetylcholine receptor neural pathways implicated in sleep regulation to produce the pleasurable effects reported by tobacco users (Jones, Sudweeks, & Yakel, 1999). Continued tobacco use leads to sleep disruption, manifested through increased sleep latency and REM sleep and decreased total sleep time (Jaehne, Loessl, Barkai, Riemann, & Hornyak, 2009). Δ-9-Tetrahydrocannabinol (THC), a psychotropically active cannabis compound, diffusely affects cannabinoid CB1-receptors in the brain throughout frontal, cerebellar, and basal ganglia regions. Acute administration of THC may decrease sleep latency, promote sleep onset, and decrease total REM sleep time (Nicholson et al., 2004). However, similar to nicotine and alcohol, prolonged cannabis use and withdrawal has been associated with increased dreams and sleep problems (Vandrey, Budney, Kamon, & Stanger, 2005).
Models showed significant main and mediating effects of T1 and T2 predictors on substance use outcome. Controlling for the other two sleep behaviors, each sleep behavior appeared to have an opposite effect on lifetime substance use events. For example, controlling for daytime sleepiness and chronotype, more erratic sleep/wake behaviors predicted higher tobacco and cannabis, but lower alcohol lifetime use events. In follow-up models examining each sleep behavior separately, the effects of sleep on substance use outcomes operated in a more consistent directionality, perhaps due to avoiding suppression effects. For example, greater early adolescent erratic sleep/wake behaviors predicted more use of all three substances by later adolescence. Since the three sleep behaviors were significantly and moderately intercorrelated, analyses that examined each sleep behavior while holding the other constant may decrease the influence of a common risk factor for substance use that daytime sleepiness, erratic sleep/wake behavior, and evening chronotype tendencies share. Thus, the results of follow-up analyses likely better reflect clinical observations of sleep behaviors in adolescents, since individuals with greater daytime sleepiness are also more likely to experience erratic sleep/wake behaviors and evening chronotype tendencies (Giannotti, Cortesi, Sebastiani, & Ottaviano, 2002).
In general, analyses modeling all sleep parameters simultaneously suggested that the influence of T1 risk factors on tobacco and marijuana use operated in the reverse direction as its effect on alcohol. For example, greater internalizing symptomology predicted increased T3 tobacco and marijuana but decreased alcohol use. The distinct psychopharmacological profiles of each substance may offer insight into a possible reason for the observed difference in directionality. Use patterns of alcohol (both a stimulant and relaxant) and cigarettes and marijuana (generally used as relaxants) may differ due to their psychopharmacological and social functions. Youth with high sensation seeking and low anxiety sensitivity prefer alcohol over marijuana and cigarettes (Comeau, Stewart, & Loba, 2001). Six percent of 12th graders reported using alcohol to sleep, while less than 1% reported the same reasons for marijuana (Terry-McElrath, O’Malley, & Johnston, 2009). The primary reason reported for alcohol use within this age group was “to have a good time” and “get intoxicated.” For marijuana, it was “to get high” and “relax” (Boys, Marsden, & Strang, 2001; Terry-McElrath et al., 2009). Examining reasons for use and how this may interact with the relationship between risk factors, sleep, and SU patterns is beyond the scope of the current study. Nevertheless, it is an important area to explore in future studies and may offer insight into mechanisms by which the current results operate.
There are several limitations in this study. Importantly, because T1 sleep data were not available, analyses did not control for prior sleep as a potential confounding factor, which limits the causal interpretations drawn regarding the relationship between inhibition control/others and sleep behaviors. Efforts were taken to account for other confounding factors often associated with SU (i.e., SES, conduct disorder, age), but environmental features that may affect sleep such as room ambience was not measured in this study. Another limitation of the study is the lack of power to examine T1 risk factors in 12, 13, and 14 year-olds separately. By examining all individuals in this age group together, it was assumed that age was not a significant moderator in the relationship among T1 risk factors, T2 sleep patterns, and T3 lifetime SU events. It is possible that future studies with larger sample sizes may be able to fill in this gap of the results reported here. Several unexpected results are of note. First, we tested biological and environmental factors as covariates in the model. Based on previous reports (Pieters et al., 2010) and results of large epidemiological surveys (National Sleep Foundation, 2014), we expected pubertal stage, age, increased video game playing, and lower SES to be associated with the sleep behaviors examined. However, only age was related to sleep (i.e., older youth reported more daytime sleepiness). Null findings for other covariates may be due to insufficient power to detect such effects.
Epidemiologically, a small proportion of teens with sleep problems use tobacco (6%) and alcohol (3%) as sleep aids (Noland et al., 2009). A possible reason for the onset of sleep problems in adolescence is the increased tendency for youth to go to sleep later, resulting in decreased total sleep time when coupled with earlier school hours (Owens, Belon, & Moss, 2010). Considering the high comorbidity between sleep problems and SUDs (Wong et al., 2015), targeting sleep improvements may be a useful tool in the prevention and treatment of SU. For example, later school start time has been recommended as one way to mitigate daytime sleepiness, fatigue, and negative mood (Owens et al., 2010). Adolescents in a SUD outpatient program who reported sleep disturbances showed significant improvements in sleep hygiene, daytime sleepiness, and rate of relapse after completing a multicomponent sleep treatment program, compared to noncompleters (Bootzin & Stevens, 2005). Nevertheless, further studies are needed to comprehensively assess healthy early adolescent sleep as protection from late adolescent SU.
In summary, by including only youth who have not initiated SU when psychiatric health and sleep behaviors were assessed, we were able to better understand temporal precedence in the interaction of these factors. Considering the multifaceted effects of tobacco, alcohol, and cannabis on sleep, a longitudinal examination of this relationship was necessary to ascertain whether problematic sleep behaviors precedes initiation of SU. However, as this study focused on SU in adolescents aged 17-24, it is unclear if and how results can be generalized to young adults and adults. Heavy alcohol, tobacco, and illicit drug use peak at 21-25, 21-34, and 18-25, respectively (SAMHSA, 2013). Thus, the examination of the interplay between sleep and SU in these older ages is an important topic of consideration in future directions.
Acknowledgments
The authors thank the participants, informants, participating San Diego schools and research assistants on this project. Portions of this study were presented at the 2015 meeting of Research Society on Alcoholism.
Funding: This study was supported by National Institute on Alcohol Abuse and Alcoholism grants R01 AA13419, U01 AA021692 (PI: Tapert), T32 AA013525 (PI: Riley), F31 AA024389 (PI: Nguyen-Louie), and the National Institute of Drug Abuse grant K12 DA031794 (PI: Squeglia).
Footnotes
Authors Contribution
TTN performed the analysis and interpretation of data, and drafting and revising of the manuscript for submission. IMC, GEM, and MJW provided consultations on data analysis and interpretation of findings. All authors provided critical revision of the manuscript for intellectual content and and approved the content of the final version for publication.
Contributor Information
Tam T. Nguyen-Louie, San Diego State University (SDSU)/UCSD Joint Doctoral Program, San Diego, California, USA
Ty Brumback, UCSD, La Jolla, California, USA
Matthew J. Worley, UCSD, La Jolla, California, USA
Ian M. Colrain, Center for Health Sciences, SRI International, Menlo Park, CA, USA; Melbourne School of Psychological Sciences, The University of Melbourne, Parkville, Vic., Australia
Georg E. Matt, SDSU, Department of Psychology, San Diego, California, USA
Lindsay M. Squeglia, Medical University of South Carolina, Department of Psychiatry and Behavioral Sciences, Charleston, South Carolina
Susan F. Tapert, University of California San Diego (UCSD), La Jolla, California, USA; 3350 La Jolla Village Drive, San Diego, CA 92161, US
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 profile. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Barnow S, Schuckit MA, Lucht M, John U, Freyberger HJ. The importance of a positive family history of alcoholism, parental rejection and emotional warmth, behavioral problems and peer substance use for alcohol problems in teenagers: a path analysis. Journal of Studies on Alcohol and Drugs. 2002;63(3):305–315. doi: 10.15288/jsa.2002.63.305. [DOI] [PubMed] [Google Scholar]
- Beebe DW. Cognitive, Behavioral, and Functional Consequences of Inadequate Sleep in Children and Adolescents. Pediatric Clinics of North America. 2011;58(3):649–665. doi: 10.1016/j.pcl.2011.03.002. http://dx.doi.org/10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review. 2005;25(5):629–644. doi: 10.1016/j.cpr.2005.04.007. http://dx.doi.org/10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Boys A, Marsden J, Strang J. Understanding reasons for drug use amongst young people: a functional perspective. Health Education Research. 2001;16(4):457–469. doi: 10.1093/her/16.4.457. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied and Preventive Psychology. 1994;3(2):61–73. http://dx.doi.org/10.1016/S0962-1849(05)80139-8. [Google Scholar]
- Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Medicine. 2010;11(8):735–742. doi: 10.1016/j.sleep.2010.02.006. http://dx.doi.org/10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Calamaro CJ, Mason TBA, Ratcliffe SJ. Adolescents Living the 24/7 Lifestyle: Effects of Caffeine and Technology on Sleep Duration and Daytime Functioning. Pediatrics. 2009;123(6):e1005–e1010. doi: 10.1542/peds.2008-3641. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Nicholas CL, Baker FC. Chapter 24 - Alcohol and the Sleeping Brain. In: Edith VS, Adolf P, editors. Handbook of Clinical Neurology. Vol. 125. Elsevier; 2014. pp. 415–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addictive Behaviors. 2001;26(6):803–825. doi: 10.1016/s0306-4603(01)00238-6. http://dx.doi.org/10.1016/S0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcoholism: Clinical and Experimental Research. 2013;37(4):539–549. doi: 10.1111/acer.12006. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals New York Academy of Sciences. 2004;102(1):77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. Circadian Misalignment, Reward-Related Brain Function, and Adolescent Alcohol Involvement. Alcoholism: Clinical and Experimental Research. 2013;37(4):558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Kirisci L, Clark DB. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and other drug involvement. Journal of Studies on Alcohol and Drugs. 2016;77(4):649–655. doi: 10.15288/jsad.2016.77.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214(3):357–364. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor Index of Social Position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Jaehne A, Loessl B, Barkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Medicine Reviews. 2009;13(5):363–377. doi: 10.1016/j.smrv.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Breslau N. Sleep problems and substance use in adolescence. Drug and Alcohol Dependence. 2001;64(1):1–7. doi: 10.1016/s0376-8716(00)00222-2. http://dx.doi.org/10.1016/S0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. 2015 Overview: Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research The University of Michigan; 2016. p. 85. [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends in Neurosciences. 1999;22(12):555–561. doi: 10.1016/S0166-2236(99)01471-X. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, Zaza S. Morbidity and mortality weekly report. Surveillance summaries. Suppl 4. Vol. 63. Washington, D.C.: 2014. Youth Risk Behavior Surveillance–United States, 2013; pp. 1–168. 2002. [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcoholism: Clinical and Experimental Research. 1998;22(1):3–9. doi: 10.1111/j.1530-0277.1998.tb03611.x. [DOI] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine. 2011;53(4–5):271–273. doi: 10.1016/j.ypmed.2011.06.020. http://dx.doi.org/10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Miller MB, Janssen T, Jackson KM. The prospective association between sleep and initiation of substance use in young adolescents. Journal of Adolescent Health. 2016 doi: 10.1016/j.jadohealth.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- National Sleep Foundation. Sleep in America Poll: Sleep in the Modern Family. Washington (DC): The Foundation; 2014. Mar, 2014. [Google Scholar]
- Nicholson AN, Turner C, Stone BM, Robson PJ. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. Journal of Clinical Psychopharmacology. 2004;24(3):305–313. doi: 10.1097/01.jcp.0000125688.05091.8f. [DOI] [PubMed] [Google Scholar]
- Noland H, Price JH, Dake J, Telljohann SK. Adolescents’ sleep behaviors and perceptions of sleep. Journal of School Health. 2009;79(5):224–230. doi: 10.1111/j.1746-1561.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behavioral Sleep Medicine. 2005;3(3):113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Archives of Pediatrics and Adolescent Medicine. 2010;164(7):608–614. doi: 10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Wightman P, Schoeni RF, Schulenberg JE. Socioeconomic status and substance use among young adults: A comparison across constructs and drugs. Journal of Studies on Alcohol and Drugs. 2012;73(5):772–782. doi: 10.15288/jsad.2012.73.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, Engels RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34(9):1512–1518. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- Pressman MR. Alcohol does not increase slow wave sleep. Alcoholism: Clinical and Experimental Research. 2012;36(8):1474. doi: 10.1111/j.1530-0277.2012.01746.x. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19(4):1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31(10):1351–1356. [PMC free article] [PubMed] [Google Scholar]
- Rupp TL, Acebo C, Seifer R, Carskadon MA. Effects of a moderate evening alcohol dose. II: performance. Alcoholism: Clinical and Experimental Research. 2007;31(8):1365–1371. doi: 10.1111/j.1530-0277.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. (NSDUH Series H-46, HHS Publication No. (SMA) 13-4795). [Google Scholar]
- Tarokh L, Carskadon MA. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. Journal of Sleep Research. 2010;19(1p2):165–174. doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM, Johnston LD. Reasons for drug use among american youth by consumption level, gender, and race/ethnicity: 1976–2005. Journal of Drug Issues. 2009;39(3):677–714. doi: 10.1177/002204260903900310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell G, Hertzog C, Klein J, Schuckit M. The anatomy of a follow-up. Addiction. 1992;87(9):1327–1333. doi: 10.1111/j.1360-0443.1992.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Van Reen E, Rupp TL, Acebo C, Seifer R, Carskadon MA. Biphasic effects of alcohol as a function of circadian phase. Sleep. 2013;36(1):137–145. doi: 10.5665/sleep.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug and Alcohol Dependence. 2005;78(2):205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69(4):875–887. doi: 10.1111/j.1467-8624.1998.tb06149.x. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcohol Clin Exp Res. 2010;34(6):1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Robertson GC, Dyson RB. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcoholism: Clinical and Experimental Research. 2015;39(2):355–362. doi: 10.1111/acer.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115(1 Suppl):250–256. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]


