ABSTRACT
Polymicrobial intra-abdominal infections (IAIs) are clinically prevalent and cause significant morbidity and mortality, especially those involving fungi. Our laboratory developed a mouse model of IAI and demonstrated that intraperitoneal inoculation with Candida albicans or other virulent non-albicans Candida (NAC) species plus Staphylococcus aureus resulted in 70 to 80% mortality in 48 to 72 h due to robust local and systemic inflammation (sepsis). Surprisingly, inoculation with Candida dubliniensis or Candida glabrata with S. aureus resulted in minimal mortality, and rechallenge of these mice with lethal C. albicans/S. aureus (i.e., coninfection) resulted in >90% protection. The purpose of this study was to define requirements for C. dubliniensis/S. aureus-mediated protection and interrogate the mechanism of the protective response. Protection was conferred by C. dubliniensis alone or by killed C. dubliniensis plus live S. aureus. S. aureus alone was not protective, and killed S. aureus compromised C. dubliniensis-induced protection. C. dubliniensis/S. aureus also protected against lethal challenge by NAC plus S. aureus and could protect for a long-term duration (60 days between primary challenge and C. albicans/S. aureus rechallenge). Unexpectedly, mice deficient in T and B cells (Rag-1 knockouts [KO]) survived both the initial C. dubliniensis/S. aureus challenge and the C. albicans/S. aureus rechallenge, indicating that adaptive immunity did not play a role. Similarly, mice depleted of macrophages prior to rechallenge were also protected. In contrast, protection was associated with high numbers of Gr-1hi polymorphonuclear leukocytes (PMNLs) in peritoneal lavage fluid within 4 h of rechallenge, and in vivo depletion of Gr-1+ cells prior to rechallenge abrogated protection. These results suggest that Candida species can induce protection against a lethal C. albicans/S. aureus IAI that is mediated by PMNLs and postulated to be a unique form of trained innate immunity.
KEYWORDS: Candida albicans, immune protection, Staphylococcus aureus, innate immunity, intra-abdominal infection
IMPORTANCE
Polymicrobial intra-abdominal infections are clinically devastating infections with high mortality rates, particularly those involving fungal pathogens, including Candida species. Even in patients receiving aggressive antimicrobial therapy, mortality rates remain unacceptably high. There are no available vaccines against IAI, which is complicated by the polymicrobial nature of the infection. IAI leads to lethal systemic inflammation (sepsis), which is difficult to target pharmacologically, as components of the inflammatory response are also needed to control the infection. Our studies demonstrate that prior inoculation with low-virulence Candida species provides strong protection against subsequent lethal infection with C. albicans and S. aureus. Surprisingly, protection is long-lived but not mediated by adaptive (specific) immunity. Instead, protection is dependent on cells of the innate immune system (nonspecific immunity) and provides protection against other virulent Candida species. This discovery implies that a form of trained innate immunity may be clinically effective against polymicrobial IAI.
INTRODUCTION
Intra-abdominal infections (IAIs) are caused by the invasion and replication of microbes in the abdominal cavity (1, 2). Severe IAI can occur as a result of bowel perforation, laparotomy surgery, intestinal hernias, or insertion of medical devices, such as peritoneal catheters (3). If these infections are left untreated or misdiagnosed, microorganisms can migrate into the bloodstream, causing sepsis and leading to significant morbidity and mortality (4–6). IAIs are often polymicrobial, and infections involving both bacterial and fungal pathogens, such as Candida albicans, result in significantly higher mortality rates than infections involving bacterial species only (7–13). Bacterial coinfection during intra-abdominal candidiasis is common (up to 67%) (14). Along with Gram-negative enteric bacteria, Gram-positive species, including Staphylococcus aureus, are also frequently coisolated pathogens, particularly with nosocomial infections (15–20). Pathogenesis is not well understood, although inflammatory responses leading to sepsis are hypothesized to play a major role.
Our laboratory has been studying polymicrobial IAIs by using an experimental mouse model of C. albicans/S. aureus IAI (i.e., coinfection) which results in 70 to 80% mortality by 48 to 72 h postinoculation (21–23). Characterization of polymicrobial C. albicans/S. aureus IAI indicated that robust local and systemic inflammation is associated with mortality, as demonstrated by dramatically elevated levels of proinflammatory cytokines (interleukin-6, tumor necrosis factor alpha, and interleukin-1β) both locally and systemically despite a similar microbial burden and dissemination in monomicrobial infections (23). Treatment with indomethacin, a nonsteroidal anti-inflammatory drug (NSAID), prevented mortality, demonstrating a key role for inflammation in lethality (21).
One question arising from these studies was whether lethality was unique to C. albicans or whether other fungal species are also synergistically lethal with S. aureus. Subsequent studies using non-albicans Candida (NAC) species or non-Candida fungal species resulted in various levels of mortality. Coinfections with S. aureus plus Candida glabrata or Saccharomyces cerevisiae, both of which are avirulent in mouse models of systemic infection (22), resulted in no mortality. Coinfection with S. aureus plus Candida krusei or Candida tropicalis resulted in 80 to 90% mortality. However, Candida dubliniensis, a close phylogenetic relative of C. albicans, resulted in little to no mortality during coinfection, although infected mice showed some level of morbidity for a short time (22). In all cases, monomicrobial infections with NAC species, S. cerevisiae, or S. aureus alone were not lethal (22).
To investigate whether an avirulent coinfection could confer any protection against the lethal C. albicans/S. aureus coinfection, C. dubliniensis/S. aureus-infected mice were subsequently challenged 14 days after primary infection with a lethal dose of C. albicans plus S. aureus. Surprisingly, the C. dubliniensis/S. aureus primary challenge led to 70 to 80% protection (E. Nash and M. C. Noverr, unpublished results). The purpose of the present study was to define the requirements for inducing protective immunity and to identify the cellular mechanisms involved.
RESULTS
Requirements for protection against lethal polymicrobial IAI. (i) Role of NAC species.
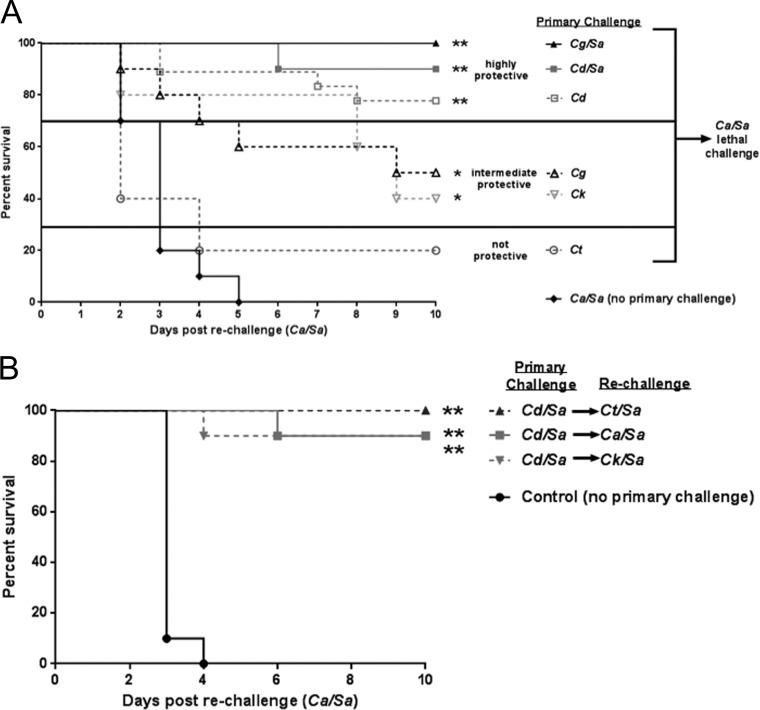
To build upon the initial observation that C. dubliniensis/S. aureus primary challenge confers protection against C. albicans/S. aureus coinfection, we sought to determine whether other NAC species could also confer protection with or without S. aureus. For this, groups of mice were inoculated with either a monomicrobial primary challenge of one of several NAC species (C. krusei, C. tropicalis, C. dubliniensis, or C. glabrata) or a polymicrobial challenge with S. aureus. Table 1 summarizes survival after the primary challenge. Monomicrobial infections resulted in 100% survival, consistent with previous reports (21, 22). Coinoculation with C. dubliniensis/S. aureus and C. glabrata/S. aureus resulted in 90 and 100% survival, respectively. C. krusei/S. aureus and C. tropicalis/S. aureus, on the other hand, showed reduced survival (50 and 40%, respectively), also consistent with previous reports, and animals were not rechallenged, in order to avoid survivor selection bias (22). All other groups were rechallenged after 14 days with a lethal inoculum of C. albicans/S. aureus and monitored for survival (Fig. 1A). A monomicrobial primary challenge with C. krusei or C. glabrata provided an intermediate level of protection upon rechallenge with C. albicans/S. aureus (40 to 50% survival) (P < 0.05). Primary challenge with C. dubliniensis conferred 80% protection (P < 0.0001). Primary challenge with C. tropicalis did not confer any level of protection. The addition of S. aureus in the primary challenge with either C. glabrata or C. dubliniensis increased survival following rechallenge (C. glabrata, 50%, and C. glabrata/S. aureus, 100%; C. dubliniensis, 80%, and C. dubliniensis/S. aureus, 90%).
TABLE 1 .
NAC species (with or without S. aureus coinfection) primary challenge survival
| Primary challenge |
% survivalc after primary challenge |
MTDd (days) | |
|---|---|---|---|
| NAC speciesa | S. aureusb | ||
| C. dubliniensis | − | 100 | NA |
| C. glabrata | − | 100 | NA |
| C. krusei | − | 100 | NA |
| C. tropicalis | − | 100 | NA |
| C. dubliniensis | + | 90 | 3 |
| C. glabrata | + | 100 | NA |
| C. krusei | + | 50 | 4 |
| C. tropicalis | + | 40 | 6 |
Inoculum of 1.75 × 107 live Candida sp. cells injected i.p.
Inoculum of 8 × 107 live S. aureus cells injected i.p.
Results are cumulative from 5 studies with a 14-day observation period.
MTD, median time to death of mice that succumbed to infection. NA, not applicable.
FIG 1 .
Role of NAC species in protection against lethal polymicrobial IAI. Mice (n = 10/group) were injected i.p. with 3.5 × 107 CFU of C. dubliniensis (Cd) C. glabrata (Cg), C. tropicalis (Ct), or C. krusei (Ck) alone (standard inocula) or in combination with 8 × 107 CFU of S. aureus (Sa) (standard inocula) as a primary challenge, and then rechallenged with C. albicans/S. aureus after 14 days (A) or injected i.p. with C. dubliniensis/S. aureus as the primary challenge and rechallenged with C. albicans (Ca), C. tropicalis, or C. krusei in combination with S. aureus after 14 days (standard inocula) (B). Animals receiving no primary challenge served as the positive (lethal) control. Mice were monitored for 10 days post-rechallenge. Data are representative of 2 separate experiments. *, P < 0.05; **, P < 0.0001 (significantly different from control by log rank Mantel-Cox test).
Recognizing that coinfection with C. krusei/S. aureus or C. tropicalis/S. aureus results in ~50% mortality, we also tested whether primary challenge with C. dubliniensis/S. aureus conferred any level of protection against rechallenge with C. krusei/S. aureus or C. tropicalis/S. aureus (cross-protection). Interestingly, primary challenge with C. dubliniensis/S. aureus conferred 90 to 100% protection upon rechallenge with either C. krusei/S. aureus or C. tropicalis/S. aureus compared with naive mice (P < 0.0001) (Fig. 1B).
(ii) Limits of C. dubliniensis-mediated protection.
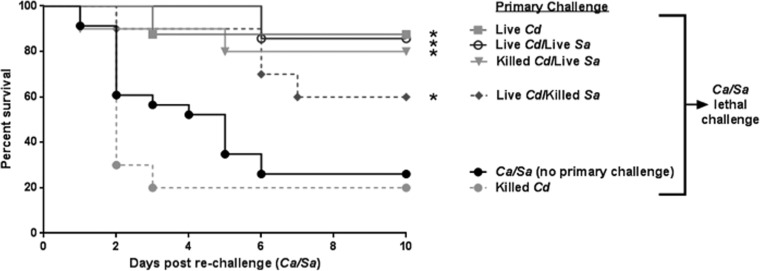
We next sought to determine the requirements for protection by C. dubliniensis against C. albicans/S. aureus IAI. For this experiment, different permutations of viable and nonviable C. dubliniensis with or without S. aureus were given as the primary challenge, followed by rechallenge with C. albicans/S. aureus. Viable C. dubliniensis alone and nonviable C. dubliniensis plus live S. aureus both provided a high level of protection (80%) upon C. albicans/S. aureus rechallenge, compared to animals that received no primary challenge (P < 0.01) (Fig. 2). Interestingly, incorporating killed S. aureus into the primary challenge compromised live C. dubliniensis-induced protection by ~30%. Killed C. dubliniensis alone did not provide protection against subsequent C. albicans/S. aureus IAI (Fig. 2).
FIG 2 .
Limits of C. dubliniensis-mediated protection against lethal polymicrobial IAI. Mice (n = 10/group) were injected i.p. with different permutations of viable and nonviable C. dubliniensis and S. aureus as the primary challenge followed by rechallenge with C. albicans/S. aureus (standard inocula). Animals receiving no primary challenge served as the positive (lethal) control. Mice were monitored for 10 days post-rechallenge. Data are representative of 3 separate experiments. *, significantly different from control (P < 0.05) by log rank Mantel-Cox test.
(iii) Limits of C. albicans-mediated protection.
Because C. dubliniensis, a phylogenetically close relative of C. albicans, provided such a high level of protection alone against rechallenge with C. albicans/S. aureus, we tested whether C. albicans alone conferred similar protection. Similar to the C. dubliniensis studies, different permutations of viable and nonviable C. albicans and/or S. aureus were given as the primary challenge, followed by lethal C. albicans/S. aureus coinfection; animals were monitored for survival (Table 2). Surprisingly, monomicrobial primary challenge with live or killed C. albicans or S. aureus did not provide significant protection against C. albicans/S. aureus rechallenge. While a minority of mice (40%) given the initial C. albicans primary challenge survived, only 50% of the surviving mice were protected against the subsequent rechallenge with C. albicans/S. aureus. Reductions in the primary challenge inocula increased survival (to 90%) but failed to enhance protection beyond 70% (see Fig. S1A and B in the supplemental material). In contrast, primary challenge with killed C. albicans and live S. aureus resulted in 90% survival following rechallenge with C. albicans/S. aureus. The reverse combination (live C. albicans/killed S. aureus) failed to provide any level of protection, with a primary challenge mortality rate to similar to that with live C. albicans alone.
TABLE 2 .
Limits of C. albicans-mediated protection
| Primary challenge |
% survival after: |
||
|---|---|---|---|
| C. albicansa | S. aureusb | Primary challengec |
Rechallenge with C. albicans/S. aureus |
| − | − | NA | 25 |
| Live C. albicans | − | 40 (9) | 50 |
| Killed C. albicans | − | 100 | 0 |
| − | Live S. aureus | 100 | 25 |
| − | Killed C. albicans | 100 | 20 |
| Live C. albicans | Killed S. aureus | 60 (5) | 15 |
| Killed C. albicans | Live S. aureus | 100 | 90 |
| Killed C. albicans | Killed S. aureus | 100 | 0 |
Inoculum of 1.75 × 107 live or killed C. albicans was injected i.p.
Inoculum of 8 × 107 live or killed S. aureus was injected i.p.
Results are cumulative from 6 studies with a 14-day observation period. NA, not applicable. Values in parentheses indicate the median time to death of mice that succumbed to infection.
Limits of C. albicans-mediated protection. Mice (n = 10/group) were injected i.p. with the standard 2.5× inocula of viable Ca (1.75 × 107), a 1× inocula (7 × 106), or a 0.14× inocula of 1 × 106 as the primary challenge followed by re-challenge with Ca/Sa (standard inocula) after 14 days. Animals receiving no primary challenge served as the positive (lethal) control. Mice were monitored for 10 days post re-challenge. Data are representative of 4 separate experiments. (A) Survival of animals in the 14-day primary challenge. (B) Survival of animals after re-challenge with Ca/Sa. Download FIG S1, PDF file, 0.1 MB (61.2KB, pdf) .
Copyright © 2018 Lilly et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(iv) C. dubliniensis induces long-term protection against polymicrobial IAI.
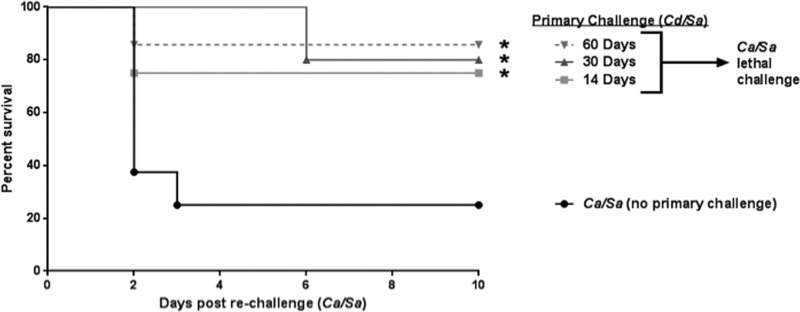
To determine if the protection conferred by C. dubliniensis/S. aureus would extend beyond the 14-day postchallenge period, mice were rechallenged with lethal C. albicans/S. aureus either 30 or 60 days following primary challenge. Consistent with previous results, at 14 days high-level protection (75 to 90% survival) (P < 0.05) was observed in animals rechallenged up to 60 days after the primary challenge compared to naive animals (Fig. 3).
FIG 3 .
C. dubliniensis induces long term protection against polymicrobial IAI. Mice (n = 10/group) were injected i.p. with C. dubliniensis and S. aureus as the primary challenge 14, 30, and 60 days prior to rechallenge with C. albicans / S. aureus (standard inocula). Animals receiving no primary challenge served as the positive (lethal) control. Mice were monitored for 10 days post-rechallenge. *, significantly different from control (P < 0.05) by log rank Mantel-Cox test.
Mechanisms involved in C. dubliniensis-induced protection. (i) Role of T and B cells.
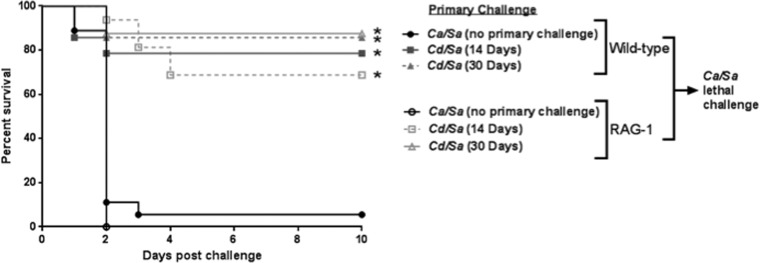
To determine the role of adaptive immunity in mediating protection against lethal IAI, rag-1 knockout (KO) mice, which lack T and B cells, were used in primary and secondary challenge experiments. Both wild-type (C57BL/6J) and rag-1 KO mice survived primary challenge with C. dubliniensis/S. aureus (data not shown). Unexpectedly, high-level protection was observed in both wild-type (80 to 90% survival) and rag-1 KO mice (70 to 90% survival; P < 0.001) following lethal rechallenge with C. albicans/S. aureus, compared with naive mice, which succumbed to the lethal challenge within 2 days (Fig. 4).
FIG 4 .
Role of T and B cells in C. dubliniensis-induced protection. RAG mice (deficient in T and B cells) (n = 10) and the background congenic strain, C57BL/6J mice (n = 10) were given the primary challenge of C. dubliniensis and S. aureus 30 days or 14 days prior to rechallenge with C. albicans/S. aureus (standard inocula). Animals receiving no primary challenge served as the positive (lethal) controls. Mice were monitored for 10 days post-rechallenge. *, significantly different from control (P < 0.05) by log rank Mantel-Cox test.
(ii) Role of macrophages.
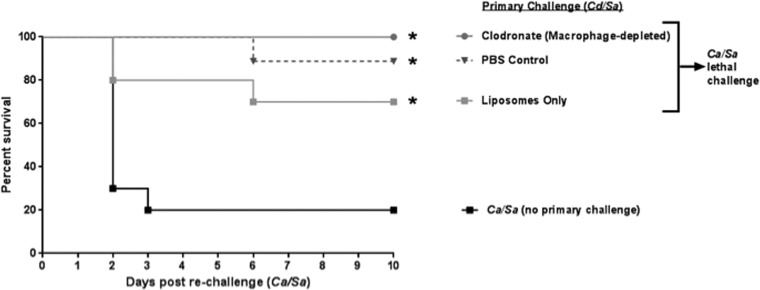
To evaluate the role of resident peritoneal macrophages in mediating protection in C. dubliniensis/S. aureus-infected mice, liposome-encapsulated clodronate was injected intraperitoneally (i.p.) 1 day prior to rechallenge with C. albicans/S. aureus, which resulted in ~90% depletion of peritoneal macrophages (Fig. S2A). Empty liposomes or phosphate-buffered saline (PBS) alone were administered to control animals. All treated animals given the primary C. dubliniensis/S. aureus challenge showed high-level protection (75 to 100%) upon rechallenge compared to the control group, which received no primary challenge (P < 0.02) (Fig. 5).
FIG 5 .
Role of macrophages in C. dubliniensis-induced protection. Mice (n = 10/group) previously given the primary challenge of C. dubliniensis/S. aureus (14 days prior) were injected i.p. with liposome-encapsulated clodronate (which results in ~90% depletion of resident peritoneal macrophages), liposomes only, or PBS 1 day prior to rechallenge with C. albicans/S. aureus. Animals receiving no primary challenge also served as the positive (lethal) controls. Mice were monitored for 10 days post-rechallenge. *, significantly different from control (P < 0.02) by log rank Mantel-Cox test.
Confirmation of macrophage and PMNL depletion. (A) Cd/Sa mice (n = 10/group) were injected i.p. with liposome-encapsulated clodronate one day prior to re-challenge with Ca/Sa to deplete macrophages. Empty liposomes or PBS alone were administered to control animals. Peritoneal lavage fluid was analyzed by flow cytometry to confirm depletion with red arrows indicating F4/80+ cells (macrophages). (B) Mice (n = 10/group) given the primary challenge of Cd/Sa were injected i.p. with 200 μg anti-Gr-1 (Ly6G/C) antibodies to deplete PMNLs or isotype control antibodies 48 h prior to and 2 h after re-challenge with Ca/Sa. Peritoneal lavage fluid was analyzed by flow cytometry to confirm depletion just prior to re-challenge with Ca/Sa. PMNLs are shown within the red encircled areas. Download FIG S2, PDF file, 0.1 MB (110.9KB, pdf) .
Copyright © 2018 Lilly et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(iii) Role of polymorphonuclear leukocytes
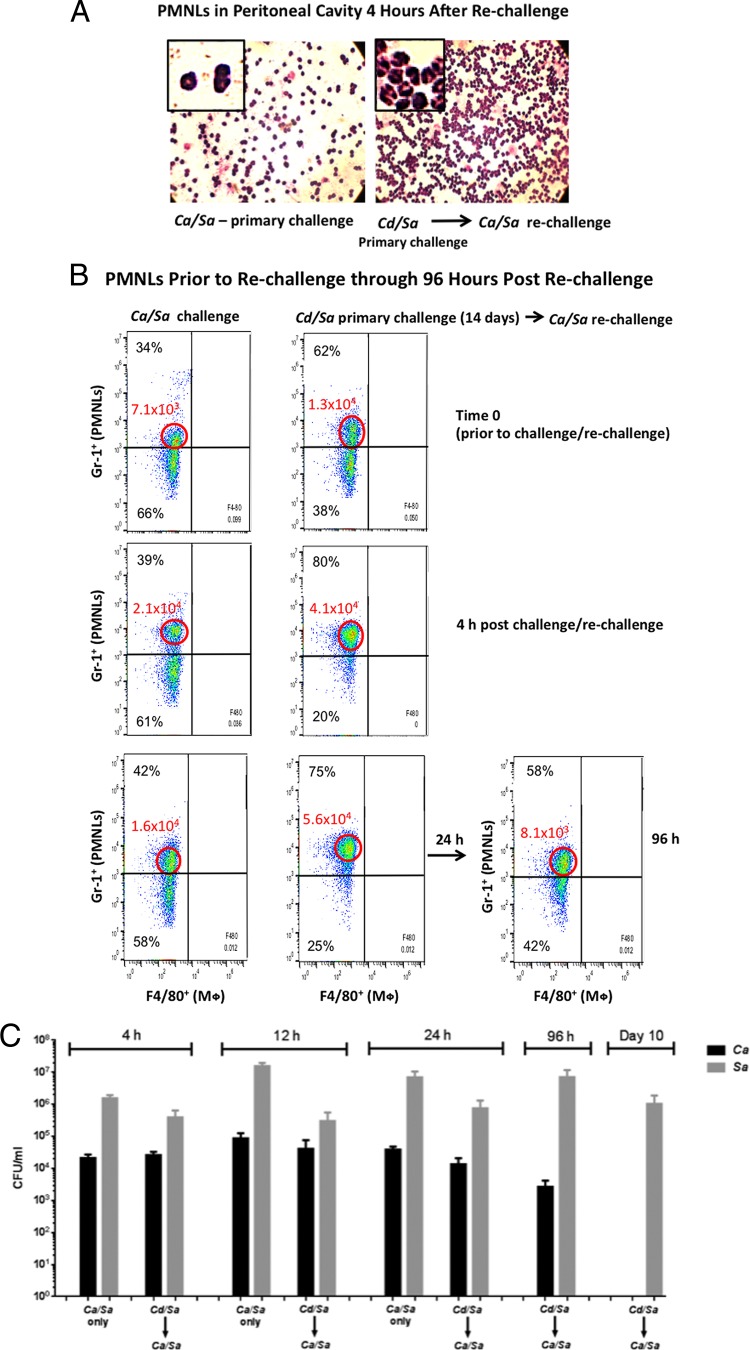
Our previous studies demonstrated that both lethal polymicrobial and nonlethal monomicrobial primary infections are associated with a significant influx of polymorphonuclear leukocytes (PMNLs) into the peritoneal cavity. Therefore, we investigated whether similar PMNL recruitment occurs following C. albicans/S. aureus rechallenge of C. dubliniensis/S. aureus-infected animals. Hematoxylin and eosin (H&E) staining of peritoneal lavage fluid showed substantially higher PMNL levels in the peritoneal cavity, as early as 4 h after rechallenge, compared to control animals given the lethal challenge alone (Fig. 6A). These observations were confirmed and extended quantitatively by flow cytometry using Gr-1 antibody, which recognizes both Ly6G and Ly6C markers (Fig. 6B). Not only were Gr-1hi cells present in the peritoneal lavage fluid at ~2-fold-higher levels in the rechallenged animals than in naive challenged mice from the time of inoculation (time zero) through 24 h postinoculation, but also the median fluorescence intensity (MFI) of the Gr-1hi cells was 2- to 3-fold higher in rechallenged mice than in the naive challenged mice through the 24-h period. In rechallenged mice, the MFI of the Gr-1hi cells increased 3- to 4-fold over the 24-h period, whereas a similar increase in the MFI was observed at 4 h in naive challenged mice and was followed by a reduction at 24 h. By 96 h in surviving rechallenged mice, Gr-1hi cells returned to baseline levels, similar to levels in naive mice at time zero. Analysis of microbial burdens in peritoneal lavage fluid from animals at 4, 12, and 24 h post-lethal challenge showed considerable levels of both S. aureus (105 to 106 CFU/ml) and C. albicans (104 to 105 CFU/ml) in both primary infection and rechallenged mice, with no significant differences between the groups at early time points. By 96 h postchallenge, S. aureus remained high in the protected rechallenged animals, while C. albicans was reduced ~1 log (all naive primary challenged animals had succumbed to the infection). At the end of the observation period (10 days), S. aureus CFU remained high in the protected rechallenged mice (90% of which were still alive), while C. albicans CFU had been cleared (Fig. 6C).
FIG 6 .
Presence of PMNLs in C. albicans/S. aureus rechallenged, protected animals. Mice (n = 10/group) were given the primary challenge of C. dubliniensis/S. aureus and rechallenged with C. albicans/S. aureus 14 days later. Control mice (n = 10) received no primary challenge. (A) H&E-stained smears of PMNLs from peritoneal lavage fluid collected 4 h after rechallenge. The illustration is representative of several individual mice evaluated. (B) Flow cytometry analysis results of PMNLs from peritoneal lavage fluid prior to rechallenge through 96 h post-rechallenge with C. albicans/S. aureus. Percentages indicate proportions of Gr-1hi PMNLs present in the total cell population. MFI of Gr-1hi PMNLs within the encircled areas are shown in red. The illustration is representative of results for several individual mice evaluated. (C) Microbial burden (C. albicans and S. aureus) in peritoneal lavage fluid of mice 4 h post-rechallenge through 10 days post-rechallenge with C. albicans/S. aureus in those that remained alive. Data are cumulative for all animals from each group. Mφ, macrophage(s).
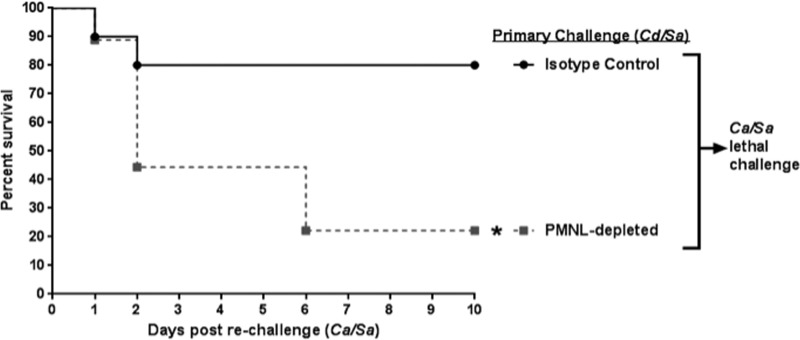
To confirm a role for PMNLs in protection, mice inoculated with the primary challenge of C. dubliniensis/S. aureus were injected i.p. with anti-Gr-1 antibodies to deplete PMNLs, or with isotype control antibodies, 48 h prior to and 2 h after rechallenge with C. albicans/S. aureus. Antibodies were given every 2 days thereafter to the remaining live animals for the duration of the study (10 days). As confirmation of PMNL depletion, flow cytometry analysis of peritoneal lavage fluid just prior to rechallenge with C. albicans/S. aureus showed ~60% reduction of Gr-1hi PMNLs (of animals that received a single injection of anti-Gr-1 antibody), in comparison with control animals that were administered isotype antibodies (Fig. S2B). Significantly reduced survival (20%) was observed in mice, with an ~60% reduction in PMNLs upon rechallenge with C. albicans/S. aureus, similar to negative-control animals who did not receive the primary challenge (naive mice) (Fig. 7). Positive-control animals receiving the isotype antibodies showed significant protection, with 80% survival (P = 0.02).
FIG 7 .
PMNL depletion abrogates protection. Mice (n = 10/group) given the primary challenge of C. dubliniensis/S. aureus were injected i.p. with 200 µg anti-Gr-1 (Ly6G/C) antibodies to deplete PMNLs or isotype control antibodies 48 h prior to and 2 h after rechallenge with C. albicans/S. aureus. Antibodies were given every 2 days to the remaining live animals for the duration of the study. Mice were monitored for 10 days post-rechallenge. *, significantly different from control (P = 0.02) by log rank Mantel-Cox test.
DISCUSSION
Our previous studies demonstrated wide variability in the ability of NAC species to induce synergistic lethality with S. aureus during polymicrobial IAI. An interesting follow-up study demonstrated that mice that survived a relatively avirulent polymicrobial challenge of C. dubliniensis and S. aureus exhibited high-level protection (80 to 90% survival) against a lethal C. albicans/S. aureus challenge. Here, we showed that protection was observed in rechallenged animals given the C. dubliniensis/S. aureus primary challenge up to 60 days prior to lethal challenge. While the long-term protection was suggestive of a role for adaptive immunity, protection was similarly observed in rag-1 KO mice, which are deficient in both T and B cells, suggesting a possible role for innate immunity. While immunologic memory is a key feature of adaptive immunity, more recently the term “trained innate immunity” has been used to describe innate immune cells, primarily monocytes and macrophages, that exhibit enhanced responsiveness upon reinfection (24). This memory-like phenotype is mediated by epigenetic modifications and metabolic changes after initial pathogen exposure, resulting in a reprogramed or trained innate immune cells (24) capable of responding more productively to a secondary exposure. However, the high level of protection that remained in macrophage-depleted animals compared to PMNL-depleted animals reduced the likelihood for a role for macrophage-mediated trained innate immunity and instead suggested a role for PMNL-mediated trained innate immunity.
The presence of visibly increased numbers of PMNLs in peritoneal lavage samples as early as 4 h post-lethal challenge in rechallenged mice compared to naive challenged mice was the first evidence that suggested a role for PMNLs in protection. Further analysis by flow cytometry revealed a distinct population of Gr-1hi PMNLs present in C. dubliniensis/S. aureus primary challenged mice at the time of lethal rechallenge, with increasing intensity of Gr-1hi over the following the 24 h. While a similar Gr-1+ cell population was observed and was increased in naive control animals that received the lethal challenge only, the cell-associated intensity of Gr-1hi cells never reached that observed in protected rechallenged mice. In addition, the Gr-1 intensity continued to decline at 24 h postinoculation in naive challenged mice. These results suggested that the presence of the Gr-1hi cells in the peritoneal cavity at the time of challenge is important for protection and that further migration of Gr-1hi PMNLs over a 24-h period promotes survival.
Protection by PMNLs was confirmed using antibody depletion (anti-Ly6G/C) with antibody-treated mice that exhibited significantly reduced survival compared to isotype-treated mice (~20% versus ~80% survival). Interestingly, while Gr-1+ cellular depletion was not 100% effective (~60%), the reduction was clearly sufficient during the 24-h period post-lethal challenge to reduce/eliminate protection. Incomplete antibody-mediated depletion of Gr-1+ cells has been previously reported, and it is possibly due to the presence of resistant cells residing in tissues (25). Interestingly, peritoneal microbial burden was very similar in both naive and protected mice challenged with lethal C. albicans/S. aureus coinfection at 4, 12, and 24 h postchallenge. This indicates that the protective response may act by controlling lethal inflammation rather than promoting antimicrobial activities. This is in agreement with our previous studies showing significant protection during IAI with NSAID treatment (21).
We hypothesize that the trained innate immunity conferred by Gr-1+ cells acts to reduce or control local and/or systemic inflammation to sublethal or subseptic levels. This controlled inflammatory response eventually leads to fungal clearance by day 10 postchallenge, and the residual bacterial burden is tolerated. Fungal clearance may be mediated by the same PMNLs or, alternatively, by other Gr-1+ PMNLs. It is unclear why S. aureus CFU counts remained high throughout the infection until it was eventually cleared. In addition, S. aureus is often still detected in animals given a primary challenge at the time of rechallenge (14 days), albeit at considerably lower levels (data not shown). Future studies can address these interesting monomicrobial conditions as well as the anti-Candida response in protected animals.
This PMNL-mediated protection is the first report of trained innate immunity mediated by Gr-1+ cells. Moreover, the protection we demonstrated was long-lived (up to 60 days postchallenge). This is particularly surprising, considering that the major population of Gr-1+ PMNLs are neutrophils, which are short-lived cells. A major question then arises: are these Gr-1+ PMNLs neutrophils or another type of polymorphonuclear leucocyte? Interestingly, myeloid-derived suppressor cells (MDSCs) are phenotypically similar to neutrophils (they are Gr-1+ and exhibit a polymorphonuclear granulocytic phenotype) with a similar lineage, arising from myeloid precursors in the bone marrow (26, 27). Known for their immunosuppressive properties, MDSCs infiltrate cancer tissues to regulate other immune cells, and they are much longer lived than neutrophils (26–28). High levels of MDSCs at these sites are associated with poor patient prognosis, making them a key therapeutic target for cancer treatment (28). In relation to our model, one may postulate that the inflammatory insult (infection) in the peritoneal cavity results in mobilization and expansion of MDSCs in the bone marrow.
Recruited MDSCs may act by reducing or controlling the inflammatory response, which prevents lethal sepsis. It is known that MDSCs exert direct antimicrobial activity, including activity against Candida species, and they may also directly participate in reductions in the microbial burden in protected animals (29, 30). In support of this hypothesis, murine MDSCs, which are heterogeneous, express high levels of Gr-1 (Ly6G and/or Ly6C), and depletion of these populations via an anti-Gr-1 antibody abrogated protection in our model. Initial attempts at depletion using only antibodies against Ly6G, which is predominantly expressed on neutrophils and subsets of MDSCs, failed to abrogate protection, possibly due to an inability to target all MDSC populations (data not shown). Further studies will interrogate the role for MDSC subsets as trained innate immune cell populations involved in protection against C. albicans/S. aureus IAI.
We also tested the ability of several other NAC species in a primary challenge with or without S. aureus to induce protection against lethal C. albicans/S. aureus rechallenge. Interestingly, a monomicrobial primary challenge of C. krusei or C. glabrata provided an intermediate level of protection, along with C. krusei/S. aureus and C. tropicalis/S. aureus coinfections, while C. glabrata/S. aureus and C. dubliniensis/S. aureus coinfections conferred the strongest protection. Like the lethal outcome from Candida/S. aureus challenge (21–23), protection against C. albicans/S. aureus lethal challenge was species specific and unrelated to morphology. C. glabrata only grows in the yeast form, and C. dubliniensis grows as both yeast and hyphae. Taking into account these results, we chose to focus specifically on C. dubliniensis due to the fact it is a close phylogenetic relative of C. albicans but exhibits low virulence in most animal models and is relatively rare clinically as an etiologic agent of infection (31–33). Although C. glabrata also exhibits low virulence in animal models (34, 35) and provided protection in our model, C. glabrata was not a good candidate for further study because it is a common etiologic pathogen in clinical situations and exhibits considerable innate antifungal resistance (36, 37). Furthermore, C. dubliniensis conferred high-level protection even in the absence of S. aureus, whereas S. aureus was required with C. glabrata to confer a similar level of protection, providing further support for our focus on C. dubliniensis.
In subsequent studies, killed C. dubliniensis/live S. aureus and live C. dubliniensis/killed S. aureus also conferred protection, but killed C. dubliniensis/killed S. aureus was not protective (data not shown). Of note, although live C. dubliniensis/live S. aureus-challenged mice exhibited 90% survival (Table 1), all showed initial signs of morbidity, as previously reported (22), with low mortality (10%), usually within 72 h postinoculation. This was unchanged even at 5-fold-higher inoculum levels (data not shown). Overall, these results suggest that several species of Candida can induce protection against the lethal C. albicans/S. aureus challenge, that S. aureus is usually required, and at least one of the two organisms must be viable. It is interesting that C. dubliniensis can induce high-level protection in the absence of S. aureus (and with no signs of morbidity). This may be due to the genetic relatedness of C. dubliniensis and C. albicans, such that the initial interactions with host cells mimic C. albicans but with reduced virulence or host damage. The genetic similarities and ability to induce cross-protection against other NAC species raise the question of whether C. dubliniensis vaccination also induces antigen-specific responses against proteins common to all Candida species. For example, the C. albicans Als3 vaccine is protective against intravenous infection with either C. albicans or S. aureus, due to antigenic similarity with a bacterial surface adhesin called clumping factor (38, 39). It is tempting to speculate that C. dubliniensis induces a similar response; however, the ALS3 gene is absent in C. dubliniensis and could not support induction of antigen-specific responses against the protein, so the mechanisms involved are clearly distinct. Equally interesting is that C. dubliniensis can provide protection against C. tropicalis/S. aureus and C. krusei/S. aureus coinfections, which are otherwise as lethal as C. albicans/S. aureus coinfection. Hence, the protection has a broad-spectrum nature toward several Candida species.
Recognizing the efficacy of protection provided by C. dubliniensis, it was surprising that C. albicans alone (monomicrobial challenge) could not confer a similar level of protection. However, the combination of killed C. albicans/live S. aureus primary challenge conferred a high level of protection similar to that of killed C. dubliniensis/live S. aureus primary challenge. A possible reason for the lack of protection from a live C. albicans primary challenge is that it creates considerable damage in the host, even in the monomicrobial setting, that is difficult to overcome even if a protective response is generated. Of note, the monomicrobial live C. albicans-challenged mice given the standard inocula had an ~60% mortality rate, which was inconsistent with our previous report for monomicrobial infection (21). However, the previous report entailed outcomes at 5 days postinfection. The mice in this study had a median time to death of 9 days. Yet, even with a 2.5-fold and 17.5-fold reduction in the monomicrobial challenge that increased survival to 70 to 90%, protection was still modest compared to that from a C. dubliniensis primary challenge (50 to 70%). Hence, C. dubliniensis is clearly superior to any other Candida species for induction of protection.
As for the role of S. aureus in protection, viable S. aureus is required when killed C. dubliniensis or C. albicans is given in the primary challenge. The addition of S. aureus in the primary challenge can also moderately enhance protection when given with live C. glabrata or C. dubliniensis, but with no ability to induce the protective response alone. Interestingly, inclusion of killed S. aureus with live Candida (C. dubliniensis or C. albicans) compromised any protection provided, suggestive of an enhanced inflammatory response in the host from killed S. aureus components, which in turn promoted sepsis, presumably independently, that dampened the protective effects of Candida. This phenomenon is similar to another model of lethal fungal IAI that entails live C. albicans with sterile feces (including killed bacteria) (40). Together, these results suggest that the Candida component of the primary challenge is the key driving force behind the protective response and that S. aureus is more or less a contributing factor or provides an adjuvant-like effect.
In summary, C. dubliniensis is a viable vaccine candidate or therapeutic agent to protect against lethal polymicrobial IAI involving virulent Candida species. This protection is mediated by a specific population of Gr-1+ PMNLs that are phenotypically similar to neutrophils but that could potentially be MDSCs, and these cells provide long-lived protection by a trained innate immune mechanism never before reported. Future studies will be focused on prospects of using C. dubliniensis as a vaccine candidate and characterizing the specific pathways and training mechanisms involved in induction of the protective PMNLs and the subsequent effector mechanisms that are required for mediating protection against lethal polymicrobial IAI.
MATERIALS AND METHODS
Mice.
For most experiments, female Swiss Webster mice, 5 to 7 weeks of age, were purchased from Charles River Laboratories, Inc. Additional studies used female RAG-1 KO and C57BL/6J mice (Jackson Laboratories). Animals were housed and handled according to institutionally recommended guidelines. All experiments involving animals were approved by the Louisiana State University Health Sciences Centre (LSUHSC) Institutional Animal Care and Use Committee.
Strains and growth conditions.
C. albicans strain DAY185, a prototrophic derivative of SC5314, was a gift from Aaron Mitchell (Carnegie Mellon University, Pittsburgh, PA). All other Candida species, with the exception of C. krusei, were provided by Jack Sobel (Wayne State University, Detroit, MI). C. krusei was obtained from the Fidel laboratory bank of isolates (LSUHSC, New Orleans, LA.) Frozen stocks were maintained at −80°C and streaked onto yeast extract-peptone-dextrose (YPD) agar prior to use. A single colony was transferred to 10 ml of YPD broth and the culture was shaken at 30°C for 12 to 18 h. The methicillin-resistant S. aureus strain NRS383 used in all experiments was obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) data bank. Frozen stocks were maintained at −80°C and streaked onto Trypticase soy agar (TSA) prior to use. A single colony was transferred to 10 ml of Trypticase soy broth (TSB) and shaken at 37°C overnight. On the following day, the overnight culture was diluted 1:100 in fresh TSB and shaken at 37°C for 3 h until the culture reached the log phase of growth. Prior to inoculation, cultures of both organisms were washed 3 times by centrifugation in sterile PBS (pH 7.4), counted using a hemocytometer, and diluted in sterile PBS to prepare standardized inocula. For experiments using UV irradiation-killed Candida species or S. aureus, cells were grown and washed as described above and then exposed in a thin liquid suspension to 4 doses of radiation (100 mJ/cm2) in a UV Stratalinker. Total killing was confirmed by plating 100-µl culture aliquots of UV-treated Candida (YPD agar) or S. aureus (TSA) and observing growth after incubation for 24 h at 30°C.
Murine model of fungal-bacterial intra-abdominal infection. (i) Primary challenge.
Groups (n = 10) of 6-week-old outbred Swiss Webster or inbred C57BL/6J and RAG-1 KO mice were injected i.p. with various Candida species (1.75 × 107/mouse) alone or in combination with S. aureus (8 × 107/mouse), live or killed, in a volume of 200 µl at 14 to 60 days prior to rechallenge.
(ii) Rechallenge.
For rechallenge, mice were injected i.p. with a lethal challenge of C. albicans, C. krusei, or C. tropicalis (1.75 × 107/mouse) and S. aureus (8 × 107/mouse) in a volume of 200 µl and observed for morbidity (hunched posture, inactivity, ruffled fur) and mortality up to 10 days after rechallenge. In some experiments, a subset of mice was sacrificed at earlier time points (4, 24, or 96 h) and peritoneal lavage fluid was collected for cellular analyses. For this, peritoneal cavities were injected with 2 ml of sterile saline followed by gentle massage of the peritoneal cavity. Peritoneal lavage fluid was then removed using a pipette inserted into a small incision in the abdominal cavity.
(iii) Macrophage depletion.
Liposome-encapsulated clodronate and liposome vehicle (1 mg/mouse; Encapsula NanoSciences) were injected i.p. in 200 µl 1 day prior to rechallenge of animals with C. albicans and S. aureus. Depletion was confirmed by flow cytometry.
(iv) Neutrophil depletion.
Mice were injected i.p. with either 200 μg rat anti-mouse Gr-1 (Ly6G/Ly6C) or rat IgG2A isotype control antibodies (Bio-X-Cell) in 200 µl sterile PBS to systemically deplete PMNLs 48 h prior to and 2 h after rechallenge with C. albicans and S. aureus. Injections were given every 2 days for the duration of the study. Depletion was confirmed by flow cytometry.
(v) Flow cytometry.
Cells isolated from peritoneal lavage fluid collected at the time of rechallenge (separate mice) and at 4, 24, and 96 h after rechallenge (2 mice/group) were incubated with fluorophore-conjugated anti-CD45 (leucocyte common antigen), anti-Ly6G/C (PMNLs), anti-F4/80 (macrophages), anti-CD3 (T cells), and isotype control antibodies (BD Biosciences). Unstained cells and cells stained with individual fluorophores were used as compensation controls. Expression was analyzed using the BD Accuri C6 Plus flow cytometer (BD Biosciences) and FlowJo software.
CFU analysis.
Microbial burdens in peritoneal lavage fluid were enumerated by serial dilution plating onto YPD agar containing 20 μg/ml nafcillin and 2 μg/ml vancomycin (for C. albicans enumeration) and TSA containing 20 μg/ml nafcillin and 2.5 μg/ml amphotericin B (for S. aureus enumeration) via the drop plate method (41). Plates were incubated overnight at 37°C. All CFU counts were expressed as the number of CFU per milliliter of peritoneal lavage fluid.
Histological analysis.
Cytological smears prepared from peritoneal lavage fluid were spray-fixed with CytoPrep (Fisher) and stained with H&E by using the Protocol Hema 3 Stat pack (Fisher) for visualization of neutrophils. Smears on slides were visualized by standard light microscopy.
Statistics.
Survival curves were compared using the log rank (Mantel-Cox) test. Significant differences were defined at a P level of <0.05. These statistical analyses were performed using Prism software (Graph Pad).
ACKNOWLEDGMENTS
We thank Aaron Mitchell (Carnegie Mellon University) for providing C. albicans strain DAY185 used in this study. We also thank Jack Sobel for contributing additional Candida species used in the study.
This study was funded by the National Institutes of Health, National Institute of Allergy and Infectious Diseases, grant R01-AI116025.
Footnotes
Citation Lilly EA, Ikeh M, Nash EE, Fidel PL, Noverr MC. 2018. Immune protection against lethal fungal-bacterial intra-abdominal infections. mBio 9:e01472-17. https://doi.org/10.1128/mBio.01472-17.
REFERENCES
- 1.Santos SG, Serufo JC, Silva RA, Marra BA, Reis CM, Hamdan JS, Nicoli JR, Carvalho MA, Farias LM. 2003. Microbiologic profile of intra-abdominal infections at Belo Horizonte, Brazil. Am J Infect Control 31:135–143. doi: 10.1067/mic.2003.54. [DOI] [PubMed] [Google Scholar]
- 2.Saini S, Gupta N, Aparna L, Lokveer MS, Griwan MS. 2004. Surgical infections: a microbiological study. Braz J Infect Dis 8:118–125. doi: 10.1590/S1413-86702004000200001. [DOI] [PubMed] [Google Scholar]
- 3.Heemken R, Gandawidjaja L, Hau T. 1997. Peritonitis: pathophysiology and local defense mechanisms. Hepatogastroenterology 44:927–936. [PubMed] [Google Scholar]
- 4.Cahill RA, Wang JH, Redmond HP. 2007. Enteric bacteria and their antigens may stimulate postoperative peritoneal adhesion formation. Surgery 141:403–410. doi: 10.1016/j.surg.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Karantonis FF, Nikiteas N, Perrea D, Vlachou A, Giamarellos-Bourboulis EJ, Tsigris C, Kostakis A. 2008. Evaluation of the effects of laparotomy and laparoscopy on the immune system in intra-abdominal sepsis—a review. J Invest Surg 21:330–339. doi: 10.1080/08941930802438914. [DOI] [PubMed] [Google Scholar]
- 6.Ozmen MM, Cöl C, Aksoy AM, Tekeli FA, Berberoglu M. 1999. Effect of CO(2) insufflation on bacteremia and bacterial translocation in an animal model of peritonitis. Surg Endosc 13:801–803. doi: 10.1007/s004649901103. [DOI] [PubMed] [Google Scholar]
- 7.Dupont H, Paugam-Burtz C, Muller-Serieys C, Fierobe L, Chosidow D, Marmuse JP, Mantz J, Desmonts JM. 2002. Predictive factors of mortality due to polymicrobial peritonitis with Candida isolation in peritoneal fluid in critically ill patients. Arch Surg 137:1341–1346. [DOI] [PubMed] [Google Scholar]
- 8.Montravers P, Dupont H, Gauzit R, Veber B, Auboyer C, Blin P, Hennequin C, Martin C. 2006. Candida as a risk factor for mortality in peritonitis. Crit Care Med 34:646–652. doi: 10.1097/01.CCM.0000201889.39443.D2. [DOI] [PubMed] [Google Scholar]
- 9.Montravers P, Gauzit R, Muller C, Marmuse JP, Fichelle A, Desmonts JM. 1996. Emergence of antibiotic-resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy. Clin Infect Dis 23:486–494. doi: 10.1093/clinids/23.3.486. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Bille J, Schneider R, Mosimann F, Francioli P. 1989. Clinical significance of Candida isolated from peritoneum in surgical patients. Lancet ii:1437–1440. doi: 10.1016/S0140-6736(89)92043-6. [DOI] [PubMed] [Google Scholar]
- 11.Blot SI, Vandewoude KH, De Waele JJ. 2007. Candida peritonitis. Curr Opin Crit Care 13:195–199. doi: 10.1097/MCC.0b013e328028fd92. [DOI] [PubMed] [Google Scholar]
- 12.Hwang SY, Yu SJ, Lee JH, Kim JS, Yoon JW, Kim YJ, Yoon JH, Kim EC, Lee HS. 2014. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis 33:259–264. doi: 10.1007/s10096-013-1953-2. [DOI] [PubMed] [Google Scholar]
- 13.Hassan EA, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. 2014. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis 23:69–74. doi: 10.1016/j.ijid.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Vergidis P, Clancy CJ, Shields RK, Park SY, Wildfeuer BN, Simmons RL, Nguyen MH. 2016. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS One 11:e0153247. doi: 10.1371/journal.pone.0153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ruiter J, Weel J, Manusama E, Kingma WP, van der Voort PH. 2009. The epidemiology of intra-abdominal flora in critically ill patients with secondary and tertiary abdominal sepsis. Infection 37:522–527. doi: 10.1007/s15010-009-8249-6. [DOI] [PubMed] [Google Scholar]
- 16.Hasibeder W, Halabi M. 2014. Candida peritonitis. Minerva Anestesiol 80:470–481. [PubMed] [Google Scholar]
- 17.Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW. 2009. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 76:622–628. doi: 10.1038/ki.2009.202. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti E, Gruessner AC, Troppmann C, Papalois BE, Sutherland DE, Dunn DL, Gruessner RW. 1996. Intra-abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg 183:307–316. [PubMed] [Google Scholar]
- 19.Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW. 2010. Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: predictors, treatment, and outcomes in 503 cases. Perit Dial Int 30:311–319. doi: 10.3747/pdi.2008.00258. [DOI] [PubMed] [Google Scholar]
- 20.Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, Buchman T, Dellinger EP, Jernigan J, Gorbach S, Chow AW, Bartlett J, Infectious Diseases Society of America . 2003. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis 37:997–1005. doi: 10.1086/378702. [DOI] [PubMed] [Google Scholar]
- 21.Peters BM, Noverr MC. 2013. Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect Immun 81:2178–2189. doi: 10.1128/IAI.00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash EE, Peters BM, Fidel PL, Noverr MC. 2015. Morphology-independent virulence of Candida species during polymicrobial intra-abdominal infections with Staphylococcus aureus. Infect Immun 84:90–98. doi: 10.1128/IAI.01059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash EE, Peters BM, Palmer GE, Fidel PL, Noverr MC. 2014. Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis. Infect Immun 82:3426–3435. doi: 10.1128/IAI.01746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moses K, Klein JC, Männ L, Klingberg A, Gunzer M, Brandau S. 2016. Survival of residual neutrophils and accelerated myelopoiesis limit the efficacy of antibody-mediated depletion of Ly-6G+ cells in tumor-bearing mice. J Leukoc Biol 99:811–823. doi: 10.1189/jlb.1HI0715-289R. [DOI] [PubMed] [Google Scholar]
- 26.Dai J, El Gazzar M, Li GY, Moorman JP, Yao ZQ. 2015. Myeloid-derived suppressor cells: paradoxical roles in infection and immunity. J Innate Immun 7:116–126. doi: 10.1159/000368233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabrilovich DI. 2017. Myeloid-derived suppressor cells. Cancer Immunol Res 5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Montero CM, Finke J, Montero AJ. 2014. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. 2011. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med 17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieber N, Singh A, Öz H, Carevic M, Bouzani M, Amich J, Ost M, Ye Z, Ballbach M, Schäfer I, Mezger M, Klimosch SN, Weber AN, Handgretinger R, Krappmann S, Liese J, Engeholm M, Schüle R, Salih HR, Marodi L, Speckmann C, Grimbacher B, Ruland J, Brown GD, Beilhack A, Loeffler J, Hartl D. 2015. Pathogenic fungi regulate immunity by inducing neutrophilic myeloid-derived suppressor cells. Cell Host Microbe 17:507–514. doi: 10.1016/j.chom.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparoto TH, Dionísio TJ, de Oliveira CE, Porto VC, Gelani V, Santos CF, Campanelli AP, Lara VS. 2009. Isolation of Candida dubliniensis from denture wearers. J Med Microbiol 58:959–962. doi: 10.1099/jmm.0.008391-0. [DOI] [PubMed] [Google Scholar]
- 32.Biasoli MS, Tosello ME, Luque AG, Magaró HM. 2010. Adherence, colonization and dissemination of Candida dubliniensis and other Candida species. Med Mycol 48:291–297. [DOI] [PubMed] [Google Scholar]
- 33.Shan Y, Fan S, Liu X, Li J. 2014. Prevalence of Candida albicans-closely related yeasts, Candida africana and Candida dubliniensis, in vulvovaginal candidiasis. Med Mycol 52:636–640. doi: 10.1093/mmy/myu003. [DOI] [PubMed] [Google Scholar]
- 34.Nash EE, Peters BM, Lilly EA, Noverr MC, Fidel PL Jr. 2016. A murine model of Candida glabrata vaginitis shows no evidence of an inflammatory immunopathogenic response. PLoS One 11:e0147969. doi: 10.1371/journal.pone.0147969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brieland J, Essig D, Jackson C, Frank D, Loebenberg D, Menzel F, Arnold B, DiDomenico B, Hare R. 2001. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect Immun 69:5046–5055. doi: 10.1128/IAI.69.8.5046-5055.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glöckner A, Cornely OA. 2015. Candida glabrata—unique features and challenges in the clinical management of invasive infections. Mycoses 58:445–450. doi: 10.1111/myc.12348. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues CF, Silva S, Henriques M. 2014. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis 33:673–688. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- 38.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, Bayer AS, Filler SG, Lipke P, Otoo H, Edwards JE Jr. 2008. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 76:4574–4580. doi: 10.1128/IAI.00700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE Jr. 2006. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 194:256–260. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 40.Cheng S, Clancy CJ, Xu W, Schneider F, Hao B, Mitchell AP, Nguyen MH. 2013. Profiling of Candida albicans gene expression during intra-abdominal candidiasis identifies biologic processes involved in pathogenesis. J Infect Dis 208:1529–1537. doi: 10.1093/infdis/jit335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donegan K, Matyac C, Seidler R, Porteous A. 1991. Evaluation of methods for sampling, recovery, and enumeration of bacteria applied to the phylloplane. Appl Environ Microbiol 57:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Limits of C. albicans-mediated protection. Mice (n = 10/group) were injected i.p. with the standard 2.5× inocula of viable Ca (1.75 × 107), a 1× inocula (7 × 106), or a 0.14× inocula of 1 × 106 as the primary challenge followed by re-challenge with Ca/Sa (standard inocula) after 14 days. Animals receiving no primary challenge served as the positive (lethal) control. Mice were monitored for 10 days post re-challenge. Data are representative of 4 separate experiments. (A) Survival of animals in the 14-day primary challenge. (B) Survival of animals after re-challenge with Ca/Sa. Download FIG S1, PDF file, 0.1 MB (61.2KB, pdf) .
Copyright © 2018 Lilly et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Confirmation of macrophage and PMNL depletion. (A) Cd/Sa mice (n = 10/group) were injected i.p. with liposome-encapsulated clodronate one day prior to re-challenge with Ca/Sa to deplete macrophages. Empty liposomes or PBS alone were administered to control animals. Peritoneal lavage fluid was analyzed by flow cytometry to confirm depletion with red arrows indicating F4/80+ cells (macrophages). (B) Mice (n = 10/group) given the primary challenge of Cd/Sa were injected i.p. with 200 μg anti-Gr-1 (Ly6G/C) antibodies to deplete PMNLs or isotype control antibodies 48 h prior to and 2 h after re-challenge with Ca/Sa. Peritoneal lavage fluid was analyzed by flow cytometry to confirm depletion just prior to re-challenge with Ca/Sa. PMNLs are shown within the red encircled areas. Download FIG S2, PDF file, 0.1 MB (110.9KB, pdf) .
Copyright © 2018 Lilly et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.