Abstract
Background:
Widespread protein aggregation occurs in the living system under stress or during aging, owing to disturbance of endoplasmic reticulum (ER) proteostasis. Many neurodegenerative diseases may have a common mechanism: the failure of protein homeostasis. Perturbation of ER results in unfolded protein response (UPR). Prolonged chronical UPR may activate apoptotic pathways and cause cell death.
Methods:
Research articles on Sigma-1 receptor were reviewed.
Results:
ER is associated to mitochondria by the mitochondria-associated ER-membrane, MAM. The sigma-1 receptor (Sig-1R), a well-known ER-chaperone localizes in the MAM. It serves for Ca2+-signaling between the ER and mitochondria, involved in ion channel activities and especially important during neuronal differentiation. Sig-1R acts as central modulator in inter-organelle signaling. Sig-1R helps cell survival by attenuating ER-stress. According to sequence based predictions Sig-1R is a 223 amino acid protein with two transmembrane (2TM) domains. The X-ray structure of the Sig-1R [1] showed a membrane-bound trimeric assembly with one transmembrane (1TM) region. Despite the in vitro determined assembly, the results of in vivo studies are rather consistent with the 2TM structure. The receptor has unique and versatile pharmacological profile. Dimethyl tryptamine (DMT) and neuroactive steroids are endogenous ligands that activate Sig-1R. The receptor has a plethora of interacting client proteins. Sig-1R exists in oligomeric structures (dimer-trimer-octamer-multimer) and this fact may explain interaction with diverse proteins.
Conclusion:
Sig-1R agonists have been used in the treatment of different neurodegenerative diseases, e.g. Alzheimer’s and Parkinson’s diseases (AD and PD) and amyotrophic lateral sclerosis. Utilization of Sig-1R agents early in AD and similar other diseases has remained an overlooked therapeutic opportunity.
Keywords: Neurodegenerative diseases, sigma-1 receptor, pharmacology, agonist, DMT, antagonist, chaperone
1. Introduction: brief history of the sigma-1 receptor
Neurodegenerative diseases (NDDs) are described as disorders with progressive loss of neuronal integrity, both structural and functional. Abnormal protein folding is widely believed to be coupled with neurodegeneration. Alzheimer’s and Parkinson’s disease (AD and PD) are the most common forms of NDDs; Lewy body disease (DLB), Huntington disease (HD) and amyotrophic lateral sclerosis (ALS) are less frequent. Currently, only symptomatic treatments are available, which ameliorate the symptoms without halting or slowing the progression of disease. There is an urgent need to develop effective, disease modifying therapeutics to protect and restore neuronal integrity. The first step on this way is a better understanding of the pathomechanisms of NDDs for the selection of new drug targets. Among the putative targets for neuroprotection, recently the sigma-1 receptor (Sig-1R) has gained more and more attention.
The Sig-1R is an enigmatic protein with a unique amino acid sequence (homologous only to yeast sterol isomerases [2]), a unique pharmacological profile and a puzzling history. Not long time ago, it has been classified as a member of the orphan receptors for which no endogenous ligand was known until the recent discovery of the endogenous hallucinogen DMT (N,N-dimethyltryptamine) as its endogenous agonist [3]. Today, the Sig-1R is considered to be a non-G-protein coupled, non-ionotropic intracellular chaperone at the endoplasmic reticulum (ER) that modulates Ca2+-signaling.
It has been a long way to arrive to this conclusion. The history of discovery of Sig-1R has existed for 40 years. Originally, Sig-1R was thought to be a member of the opioid receptor family. In 1976, Martin reported that the psychotomimetic effects of SKF-10,047 (N-allylnormetazocine) in chronic spinal dogs do not originate from the µ and κ opioid receptors (named from the first letter of their selective ligands morphine and ketacozine, respectively). He proposed the introduction of a new type of the opioid receptors; sigma (from the first letter of SKF-10,047) [4]. This receptor was reported to be blocked by the universal opioid antagonist, naloxone. Later, Su identified a distinct binding site to mediate the psychotomimetic effect of SKF-10,047 that was, however, insensitive to naloxone and naltrexone [5]. Mistakenly, the binding site was named sigma/opioid receptor; then the name was changed to sigma receptor [6]. Sig-1R was also defined as an NMDA (N-methyl-d-aspartate) receptor because phencyclidine (PCP) binds to the sigma receptor and (+)SKF-10,047 to the PCP-site of the NMDA receptor [7]. Radioligand binding studies identified two populations of the sigma binding sites (Sig-1 and Sig-2) that differ in their ligand selectivity, tissue and subcellular distribution [8, 9]. In recent years, significant achievements have been made in our understanding of the molecular basis of the Sig-1 subtype in normal and pathological conditions. The sigma-2 subtype has remained more obscure due to the lack of suitable research tools (it has not yet been cloned). Sig-1 receptors (the original sigma receptors) display high (nanomolar) affinity for the ‘unnatural’ (+) stereoisomer of benzomorphans https://en.wikipedia.org/wiki/Benzomorphan (e.g. SKF-10,047, pentazocine) and only low to moderate affinities for (-) isomers. (+)-Pentacozine, the prototypic Sig-1R agonist binds with more than 100-fold selectivity to Sig-1 versus Sig-2 binding sites, while 1,3-di-o-tolylguanidine (DTG) binds nearly equally well at both populations [10]. DTG in the presence of (+) pentazocine (to mask Sig-1 binding) has been the ligand of choice to study the Sig-2 subtype. In this review, we will focus on the Sig-1R type. The pharmacology of the Sig-1R has been summarized in recent reviews [11-17].
2. The unique properties of Sig-1R: chaperone function, distribution, sub-cellular localization and transloca-tion
There are several properties of Sig-1R that make this protein unique among the receptors. The most important feature of Sig-1R: it is an intracellular receptor acting as a chaperone protein. The Sig-1R differs also structurally from the classical neurotransmitter receptors: although a transmembrane protein, it has only one transmembrane region [1]. (The results of in vivo studies are rather consistent with the 2 transmembrane structure of Sig-1R. Sig-1R shows no postnatal ontogeny and age-dependent increase of receptor density [18]. These properties and the widespread modulatory functions of Sig-1R underline the universal role of Sig-1R in cellular functions and cell survival. Alternative splicing results in the canonical isoform (223 amino acids) and 4 other transcript variants (UniProt identifiers: Q99720-1 to Q99720-5) [19].
Sig-1R has very versatile cellular function. Interestingly, the Sig-1R knock-out mice are viable and fertile, and do not show any apparent phenotype changes except for a reduced hypermotility response compared to wild-type mice [20]. Through regulation of lipid rafts, secretases, kinases, neuroreceptors and ion channels, the Sig-1R can influence signal transduction, citric acid cycle, oxidative stress, neuronal plasticity, etc.
The central nervous system (CNS) appears to be the primary site of Sig-1R activity and effects. Sig-1Rs are broadly spread in the CNS (e.g. hippocampus: highest density in dentate gyrus; hypothalamus, mesencephalon, olfactory bulb), in peripheral (abundantly in liver, kidney, lung, muscle), endocrine, immune and reproductive tissues [21]. At the cellular level, the Sig-1Rs are expressed in neurons, oligodendrocytes and ependymocytes. They are primarily found at the ER membranes that are physically associated with the mitochondria (MAM: mitochondria-associated ER membrane) [22] and are mainly associated with neuronal perikarya and dendrites [21, 23, 24].
It is also unique that Sig-1R is a transmembrane protein which can dynamically translocate inside cells. Sig-1R localizes mainly at the MAM in ceramide-enriched microdomains, apparently bound to ceramide [25]. Sig-1R also binds the binding immunoglobulin protein (BiP), another ER-chaperone that prevents the translocation of Sig-1R [23]. Translocation of Sig-1Rs from the ER to the plasma membrane (PM) has been reported [26], where Sig-1R interacts with many receptors, ion channels and kinases. Translocation occurs after stimulation of Sig-1R by agonists like cocaine: the receptor can dissociate from BiP and translocate to the PM, the nuclear envelope and can interact with cytosol proteins [27].
3. Structural features of the Sig-1R
The heterogeneity of the sigma receptors and the lack of selective sigma ligands have greatly contributed to the difficulties of the classification of this receptor type. The controversy of the nature of sigma receptors was finally solved in 1996 by the successful cloning of the Sig-1R gene from guinea pig liver [28]. It was demonstrated that the Sig-1R consists of 223 amino acids hypothetically with two putative transmembrane domains (TMDs) [29, 30]. The Sig-1R shares no sequence homology with any mammalian protein. However, it shares 60% amino acid identity to a yeast sterol isomerase, ERG2, although the mammalian Sig-1R does not have any enzyme activity [2, 31]. Subsequent cloning studies from a variety of tissues found 90% sequence conservation in mammalian species [32, 33]. The gene that encodes the human Sig-1R is located on chromosome 9p13 and contains four exons and three introns [34]. Hydropathy analysis of the Sig-1R described three hydrophobic regions [35]. Sequence-based algorithms that predict amino acid hydrophobicity (TMBase) have indicated two major hydrophobic helical transmembrane sequences (TMDI and TMDII) [29]. Former studies indicate that amino acid (AA) sequence 11-29 and 92-112 represent the TMDI and TMDII, respectively. It is proposed that AA 29-92 forms a cytosolic loop and both the C- and N-termini are localized in the ER lumen [23, 36, 37]. More recent predictions show that TMDI spams between AA 8 and 34, TMDII between AA 83 and 111 [38]. The Sig-1R orientation predicts that if the protein translocates into the plasma membrane, the C-and N-termini are located outside the cell. The so called steroid binding domain I and II (SBDL-I; SBDL-II) together with the TMDs and the C-terminus have been revealed to be essential for ligand binding [39-41]. (The SBDL regions are thus named because of sequence homology to yeast sterol isomerase.) Photolabeling studies of A. Pal, et al. suggest that SBDL-I and SBDL-II regions of Sig-1R may be involved in forming a Sig-1R ligand binding site [42]. The 50AA cytosolic loop between the TMDI and TMDII has been studied by NMR and proposed to contain 3 short helical regions [38]. A model of the key structural motifs of Sig-1R was published by Ortega-Roland as well as U.B. Chu and A.E. Ruoho [43]. An excellent review summarizes our present knowledge about the Sig-1R structure [44].
Very recently, the first crystal structure of the full-length human Sig-1R was reported in complex with two chemically divergent ligands, PD144418 (a high affinity and selective sigma-1 antagonist) and 4-IBP (agonist or inverse agonist) [1]. The structure reveals a triangular organization comprising three tightly associated protomers, each with a single transmembrane domain at the corner of a trimeric triangle. The C-terminal domain of the receptor shows an extensive, flat, hydrophobic membrane-proximal surface, the cytosolic surface is polar. Receptor binding would occur through a charge-charge interaction with the highly conserved Glu172, consistent with previous site-directed mutagenesis studies identifying this residue as essential for ligand binding [1, 45, 46]. However, this crystal structure of the Sig-1R was gained by an in vitro approach of embedding the receptor in lipid-containing nanodiscs. The discrepancy between the sequence-based prediction (two transmembrane) and X-ray (one transmembrane) structures suggests that the in vitro crystal structure not accurately represents the in vivo structure. The structure differences may indicate a potentially amorphous intrinsically disordered in vivo structure for the Sig-1R.
Previous biochemical and cellular studies suggest that oligomerization is a key functional property of this receptor and may be linked to ligand efficacy [1, 47, 48]. Sig-1R exists in rat liver membrane in oligomeric form [42]. Forster resonance energy transfer (FRET) analyses have shown that Sig-1R exists constitutively in monomeric and oligomeric (dimer-trimer-octamer-multimer) form [49]. Dimer and monomer forms of Sig-1R were favored in the presence of agonist (+)-pentazocine. Simultaneous studies demonstrated the presence of higher order oligomers favored by the Sig-1R antagonist haloperidol. A hypothetic mechanistic model of Sig-1R oligomerization proposes that agonists create functionally active dimers and/or monomers from higher order oligomers [48]. Putative antagonists (or inverse agonists) shift the equilibrium to the tetramer/oligomer forms, and thus higher oligomers might represent a reservoir for the active form.
The receptor as a chaperone protein is able to accommodate many client proteins (Tables 2 and 3), and tolerates very different structure of binding clients. The C-terminal tail (amino acid sequence 110-123) possesses chaperone functions [23, 50]. The mystery, why Sig-1R has so many interacting proteins (Tables 2 and 3) can be dissolved with the activity of alternately spliced variants of Sig-1 R or the interaction oligomeric structures of the receptor with proteins. The hypothetical IDP character of Sig-1R might explain the receptor structure stabilization by trimerization (in membrane environment) and interactions with the co-chaperone BiP [38].
Table 2.
Sig-1R interacting proteins (direct interactions) [14].
|
Plasma membrane proteins: ▪ Acid-sensing ion channels (ASICs) ▪ Dopamine receptors (D1R, D2R) ▪ Muscarinic acetylcholine receptor (mAchR) ▪ Mu-Opioid receptor (MoR) ▪ NMDA receptor (subunit1) ▪ Cannabinoid receptor1 (CB1R) ▪ Tropomyosin receptor kinase B (Trk B) ▪ Platelet-derived growth factor receptor (PDGFR) ▪ Integrin-D1 ▪ Voltage-gated potassium channels (Kv) ▪ Voltage-gated sodium channels (Na v) |
|
Cytosol proteins: ▪ Cell engulfment and motility domain (ELMOD) ▪ Ras-related C3 botulinum toxin substrate (Rac)-GTPase |
|
ER-mitochondrion interface (MAM) and mitochondrial proteins ▪ Binding immunoglobulin protein (BiP) ▪ Inositol 1,4,5-triphosphate receptor Type 3 (IP3R3) ▪ Ankyrin ▪ Voltage-dependent anion channels (VDAC) ▪ Inositol-requiring enzyme (IRE1) ▪ Insulin-induced gene (Insig) |
|
Nuclear envelope proteins ▪ Emerin ▪ Ran-binding protein (RanBP2) |
Table 3.
Proposed Sig-1R interacting proteins revealed by bioinformatics analyses [58].
|
Plasma membrane proteins ▪ PDZ-domain containing 11 (PDZD11) ▪ Transmembrane 7 superfamily member 2 (TM75F2) |
|
Citoplasm proteins ▪ Eukaryotic translation initiation factor 5A (EIF5A) ▪ Heat shock 70-kDa protein5 (HSPA5) ▪ Nascent polypeptide-associated complex α-subunit 2 (NACA2) ▪ RNA export 1 homolog (RAE1) ▪ Ribosomal protein S27a (RPS27A) ▪ Sec61 α-2 subunit (SEC61A2) ▪ Ubiquitin A52 residue ribosomal protein fusion product1 (UBA52) ▪ Ubiquitin C (UBC) ▪ Exportin 1, CRM1 homolog (XP01) ▪ Exportin (tRNA) (XPOT) |
|
ER membrane ▪ Ancient ubiquitous protein1 (AUP1) ▪ Chromosome 14 open reading frame (C14 orf1) ▪ Cytochrom P450, family 51 (CYP51A1) ▪ Eukaryotic translation initiation factor 5A (EIF5A) ▪ Glucosidase, α, neutral AB (GANAB) ▪ Hydroxysteroid (17-β) dehydrogenase 12 (HSD17B12) ▪ Nascent polypeptide-associated complex α-subunit 2 (NACA2) ▪ NAD(P) dependent steroid dehydrogenase-like (NSDHL) ▪ Retinol dehydrogenase 11 (RDH11) ▪ Ribophorin II (RPN2) ▪ Sterol-C4-methyl oxidase like (SC4MOL) ▪ Sec61 α-2 subunit (SEC61A2) ▪ Squalene epoxidase (SQLE) ▪ Surfeit 4 (SURF4) ▪ Transmembrane 7 superfamily member 2 (TM75F2) |
|
Mitochondria ▪ Cytochrome c-1 (inner membrane) (CYC1) ▪ Prohibitin (inner membrane) (PHB) ▪ Solute carrier family 25, member 39 (inner membrane) (SLC25A39) ▪ Solute carrier family 25, member 11 (inner membrane) (SLC25A11) ▪ Voltage dependent anion channel 2 (outer membrane) (VDAC2) |
|
Nucleus ▪ Exportin 1 (XP01) ▪ Exportin (tRNA) nuclear export receptor for tRNAs (XPOT) ▪ Cytochrom c-1 (CYC1) ▪ Eukaryotic translation initiation factor 5A (EIF5A) ▪ Lamin B receptor (LBR) ▪ Nascent polypeptide-associated complex α-subunit 2 (NACA2) ▪ Nucleoporin 205 kDa (NUP205) ▪ RNA export 1 homolog (RAE1) ▪ Ribosomal protein S27 (RPS27A) ▪ Ubiquitin A52 residue ribosomal protein fusion product1 (UBA52) ▪ Ubiquitin C (UBC) |
4. Sig-1R agonists, antagonists and allosteric modulators
An intriguing feature of the Sig-1R is that it binds ligands with very diverse structures. These include small molecules that also interact with other targets, such as haloperidol, dextrometorphan, metamphetamine, verapramil, chloroquine, fluoxetine, donepezil, etc. Specifically, a majority of antipsychotics possess high to moderate binding affinities to Sig-1R [51]. Based on extensive structure-activity relationship studies, Glennon et al. published a simple pharmacophore model for small molecule binding to the Sig-1R [52]. He postulated that the binding site consists of a basic amine site separated by two hydrophobic regions. The primary hydrophobic region is optimally an N-arylalkyl group and is situated at four-to six-carbons (6-10 Å), and a second hydrophobic domain 1- to 3-carbon apart (2.5-4 Å) from the obligate nitrogen. The unique hydrophobic nature of the ligand binding site enables hydrophobic moieties to associate with the binding site. Indeed, most Sig-1R ligands possess hydrophobic or amphipathic property (e.g., haloperidol and fluvoxamine) and are positively charged. The neuroactive steroids (e.g. DHEA, progesterone), that induce several of their physiological effects through sigma receptors and are hypothesized to be endogenous ligands for them, were shown to bind with moderate to weak binding affinities at this site [6]. Interestingly, this steroid does not have a nitrogen atom. The endogenous ligand for the Sig-1R has not been unequivocally identified yet, but the endohallucinogen DMT and sphingosine have also been postulated [3, 6, 53].
The molecular basis of ligand efficacy is yet to be solved. Different methods are used in Sig-1R binding studies and no standard method of characterization has been chosen. The efficacy (agonist, antagonist) is not an absolute property of the ligand, it is influenced by the methods and tissues of the measurements. By convention, Sig-1R ’agonists’ are those compounds that induce the typical Sig-1R effects in whole-animal physiologic studies or resemble the phenotypes of receptor overexpression. Selective Sig-1R agonists include: (+)-pentazocine, dehydroepiandrosterone, (+)-SKF10,047, PRE-084 (2-morpholin-4-ylethyl-1-phenyl- cyclohexane-1-carboxylate), and SA4503 (1-[2-(3,4-dimethoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazine). ’Antagonists’ are those compounds that generally have no effects on their own, but attenuate the effects of Sig-1 stimulation or resemble the results of the gene knock-down. Sig-1R antagonists that are commonly cited in the literature include: progesterone, haloperidol, BD1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine), BD1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine), and NE-100 (4-methoxy-3-(2-phenylethoxy)-N,N-dipropylbenzeneethan-amine). The involvement of the Sig-1 subtype in a given system requires careful analysis and verification using selective genetic and pharmacological tools. Several known drugs (e.g. SKF 83959, an atypical dopamine receptor-1 agonist) potentiate the binding of (+)-pentazocine to Sig-1R by allosteric manner [54]. SKF 83959 also inhibits microglia mediated inflammation [55]. SOMCL-668 (3-methyl-phenyl-2,3,4,5-tetrahydro-1H-benzo(d)azepin-7-ol), the latest identified novel and selective allosteric modulator of Sig-1R displays anti-seizure and antidepressant activity [54, 56].
A complete list of Sig-1R ligands is difficult to obtain, as many compounds only have been used in pharmaceutical research and are not available, due to drug development. A very useful summary of the pharmacological properties of Sig-1R ligands were published by E.J. Cobos et al. [57]. Table 1 shows the short list of the most important Sig-1R agonists, antagonists and allosteric modulators.
Table 1.
A short list of Sig-1R ligands (agonists and antagonists).
| Agonists | Refs. | Other Activities |
|---|---|---|
| N,N-Dimethyltryptamine (DMT) | [3, 172, 173] | 5HT2A receptor agonist |
| (+)-N-allylnormetazocine ((+)-SKF 10,047) | [51] | NMDA receptor ligand |
| 2-(4-morpholino)ethyl-1-phenylcyclohexane-1-carboxylate (PRE-084) | [23, 174] | – |
| 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl) piperazine (SA 4503, cutamesine) | [175] | – |
| 4-phenyl-1-(4-phenylbutyl)piperidine (PPBP) | [176] | nNOS inhibition |
| 1-(2,8-Dimethyl-1-thia-3,8-diazaspiro(4,5)dec-3-yl)-3-(1H-indol-3-yl)propan-1-one (AF710B) | [123] | allosteric M1 agonist |
| Dehydroepioandrosterone (DHEA) | [177] | neurosteroid, nuclear receptors |
| Donepezil | [128, 178, 179] | cholinesterase inhibitor |
| Amitriptyline | [180] | neurotransmitter reuptake blocker |
| Fluvoxamine | [23, 36, 181, 182] | 5HT reuptake inhibitor |
| Memantine | [183] | NMDAR antagonist |
| Ifenprodil | [184] | NMDAR antagonist, α-1 antagonist |
| (+)-Pentazocine | [23, 185, 186] | – |
| Cocaine | [23, 187] | neurotransmitter reuptake blocker |
| Dextromethorphan (DEX) | [30, 188] | NMDAR allosteric antagonist |
| Antagonists | ||
| N-(2-(3,4-dichlorophenyl)-N-methyl-2-(dimethylamino)ethylamine (BD 1047) | [189] | β-adrenoreceptor ligand |
| 1-(2-(3,4-dichlorophenyl)ethyl)-4-methylpiperazine (BD 1063) | [189] | – |
| 4-Methoxy-3-(2-phenylethoxy)- N,N-dipropylbenzeneethaneamine (NE-100) |
[23] | – |
| Progesterone | [23] | neurosteroid, progestogen |
| Haloperidol | [23] | D2 and D3 antagonist |
| Verapamil | [190] | Ca2+-antagonist, slow channel blocker |
| Sertraline | [60] | 5HT reuptake inhibitor |
5. Sig-1R interactions with proteins and lipids. Mechanisms of action
The chaperone activity and the specific 3D barrel structure [1] of Sig-1R may answer the question why the Sig-1R has so many client proteins. The lists of these proteins are given in Tables 2 and 3 [14, 58]. The Sig-1Rs have been shown to play a role in numerous phenomena including cardiovascular function, drug abuse, schizophrenia, clinical depression, cancer and modulate mood disorders, amnesic and cognitive deficits as well as potentiate analgesia [6, 11, 16, 59]. They are now considered as ligand-regulated molecular chaperones (for a review see [22, 60] of which the main physiological function is modulation of intracellular calcium signaling [23], inhibition of voltage-gated K+- channels [13, 30, 61], and release of various neurotransmitters including acetylcholine and glutamate [62]. Sig-1R is intimately involved with the glutamate system and seems to be an essential part of the excitotoxicity. Sig-1R is implicated in cellular differentiation, neuroplasticity, neuroprotection and cognitive functioning of the brain [11, 63]. The signal transduction pathways of these phenomena have not been clarified yet. Sig-1 antagonists, but not agonists were shown to display GTP- and suramine sensitive high affinity binding implying coupling of the receptor to G-proteins [64]. Early radioligand binding studies showed that the binding of Sig-1 ligands was altered by GTP and its analogues [65]. Contrary, evidence was published that the Sig-1R is not coupled to G-proteins [66]. Sig-1R has a high tendency to form heterodimers with other proteins, e.g. receptors, ion channels. The recent crystallization studies of the Sig-1R did not find any structure similarity of Sig-1R to the 7 transmembrane GPCRs [1]. The Sig-1R appears in a complex with voltage gated K+-channels (Kv1.4 and Kv1.5), leading to the idea that these receptors are auxiliary subunits [61]. There is evidence that Sig-1Rs are intracellular signal transduction amplifiers [67], regulators of inter-organelle communication [27, 68] or pluripotent modulators [14].
Sig-1R integrates many signaling pathways and in particular modulates Ca2+ signaling through the inositol triphosphate (IP3) receptor between the ER and mitochondria [23]. Its participation is important in other ion channel and related receptor (K+ channel, NMDA receptor) activities as well, in lipid transport and metabolism, especially during neuronal morphogenesis and differentiation. The ER-mitochondrion border at the MAM – where the Sig-1R resides – serves as an important subcellular interface in the regulation of cellular survival by enhancing the stress-response signaling [69]. Sig-1R protects the cells against reactive oxygen species (ROS) and activates the antioxidant response elements [70], therefore Sig-1R agonists may function as indirect antioxidants. The induction of Sig-1R can repress cell death signaling: up-regulation of Sig-1R suppresses the apoptosis caused by ER stress [71]. Tryptaminergic trace amines (like DMT) as well as neurosteroids (e.g. dehydroepiandrosterone, pregnenolon) are endogenous ligands that activate the Sig-1R [3] and are expected to elicit the described effects.
The ER chaperone function of the Sig-1R is essential for metabotropic receptor signaling and for survival against cellular (particularly ER) stress. Dysfunctional chaperones are responsible for numerous diseases [72], especially the neurodegenerative ones. Sig-1R has so far been implicated in illnesses like AD [73, 74], PD [75], cancer [76], cardiac conditions [77] retinal dysfunction, perinatal and traumatic brain injury, ALS, HIV-related dementia, major depression, and psychostimulant addiction [78]. In addition, Sig-1R mutations have been implicated in frontotemporal lobar degeneration (FTLD) and ALS [79]. How Sig-1R may relate to this plethora of diseases remains to be clarified but its protective influence has been verified on various aspects of cellular processes, such as Ca2+ signaling, mitochondrial functions, ER stress, apoptotic pathways, and tumor cell proliferation [12]. As Sig-1Rs are expressed in the immune system, immunomodulatory functions have also been reported in the literature [80, 81]. Although the interaction of Sig-1R in different tissues is very complex, apparently Sig-1R plays a central role in neurotransmission and apoptosis. The development of new knockout mice and transgenic lines will facilitate further research.
6. Sig-1R as a pluripotent modulator in cells
The ER-chaperone Sig-1R resides at then MAM where it supports survival of cells by controlling Ca-signaling from the ER to mitochondria via the IP3 receptor. Simultaneously, Sig-1R attenuates free radical damage via Nrf2 signaling. Sig-1R agonists facilitate receptor translocation from the MAM to the PM. The translocation of Sig-1R makes possible the interaction with (and regulation of) many other cytosolic or membrane-bound functional proteins, e.g. receptors, ion channels and kinases (Tables 2 and 3). Another translocation of Sig-1R from MAM to the nucleus membrane provides the possibility for interaction with chromatine-remodeling factors and thus transcriptional regulation of genes. Dysfunction of Sig-1R may have a role in disorders and thus the receptor provides novel therapeutic opportunities to treat these diseases.
Possessing both chaperone and receptor functions and the versatility of its targets the Sig-1R represents a pluripotent modulator in the living systems and is involved in the etiopathology of many diseases [14]. As a consequence, the actions of Sig-1R might be apparent only under abnormal state in the cells, e.g. at the beginning of a disease when big changes call Sig-1R to assist and maintain cell homeostasis. Sig-1R agonists function selectively only under pathological conditions thus limiting adverse side effects and have a favorable safety profile.
7. Common mechanisms of neurodegene-ration
NDDs as diverse as AD, PD, ALS-FTLD, HD, retinal degeneration and stroke share common cellular and molecular pathological mechanisms such as excitotoxicity, calcium dysregulation and oxidative stress, mitochondrial dysfunction and ER stress. Dysfunction of glial cells may also contribute to neurodegeneration.
Fundamental cellular functions demand regulated protein folding homeostasis. Formation of misfolded proteins that may associate to toxic assemblies is the common feature of neurodegenerative disease. ER is the subcellular organelle with the function of folding and modifying secretory and membrane proteins.
Formation of misfolded proteins and toxic aggregates point to the common pathway of NDDs: failure in normal protein folding [82]. Growing evidence suggests that (besides other cellular stress mechanisms) mainly ER-stress contributes to the development of diseased state of neurons. Chronic ER-stress and activation of unfolded protein response (UPR) may result in impaired Ca2+- and redox-homeostasis and oxidative stress disturbing vital mitochondrial functions. Intracellular Ca2+, released from ER, increases mitochondrial ROS production and further accumulation of ROS within ER and mitochondria, disturbing their vital functions. Chronic ER stress may also induce inflammatory processes through the UPR pathways. These events: ER-stress, oxidative stress triggered by ROS-production as well as mitochondrial dysfunction and inflammation together form a molecular web that is associated with a wide range of diseases including NDDs [83].
7.1. ER-stress and Unfolded Protein Response
ER function can be altered in different ways and ER stress can be caused by a defective (mutated) mitochondrion, hypoxia, hyperglycemia, hyperlipidemia, viral infections, disturbances in cellular Ca2+ levels, redox regulation, or by oxidative stress. These so-called stress signals exhaust the ER machinery and result in buildup of unfolded proteins. An adaptive process called UPR is triggered with an aim to restore ER homeostasis. Originally, UPR has a cell protective effect: it prevents overload of the ER lumen with newly synthesized proteins and activates degradation of misfolded proteins. However, if the stress signal is severe and/or prolonged, misfolded proteins enter the mitochondria and cause dysfunction in energy production [82, 84]. As a result, cell death pathways are triggered in the form of apoptotic and pro-inflammatory reactions [85], as it will be detailed below.
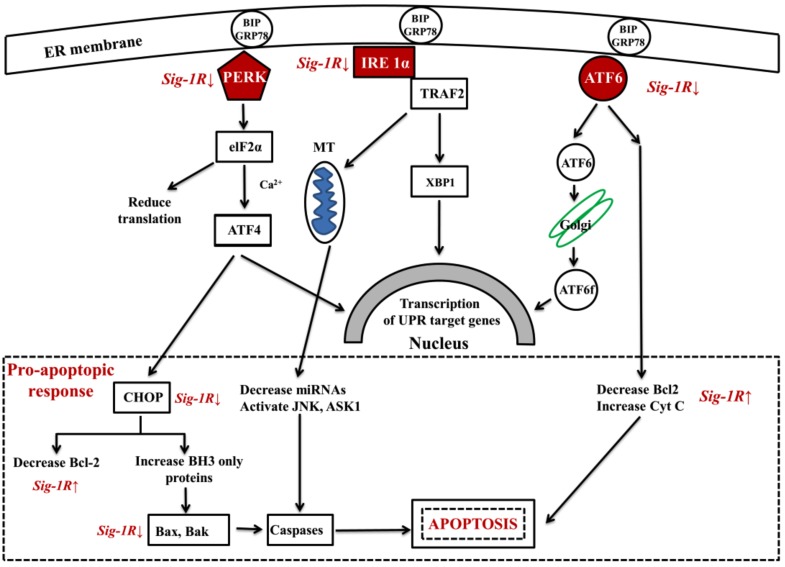
Fig. 1. shows that three major pathways mediate the UPR: protein kinase RNA-like kinase (PERK), inositol requiring enzyme 1 (IRE1a) and activating transcription factor 6 (ATF6). Sustained activation of PERK triggers a signalization cascade leading to C/EBP homologous protein (CHOP) upregulation, this process inhibits the expression of anti-apoptotic B-cell lymphoma 2 (Bcl-2) and upregulates the pro-apoptotic BH3-only proteins. These events result in triggering Bak- (Bcl-2 homologous antagonist killer) and Bax (Bcl-2 like protein 4)-dependent apoptosis [86].
Fig. (1).
Sig-1R regulation of the three signal pathways of ER-stress activated UPR. ER chaperone BiP/GRP78 under normal conditions binds all the three ER-stress sensors (PERK: protein kinase RNA like ER-kinase; IRE1α: inositol requiring enzyme 1α; ATF6: activating transcription factor 6). Under ER-stress BiP dissociates from sensors. PERK and IRE1α will be phosphorylated and oligomerized, ATF6 translocates to the Golgi.(Abbreviations: eIF2α: eukaryotic translation initiation factor 2α; XBP1: X-box binding protein 1 (spliced form); TRAF2: TNF-associated factor-2; ATF4: transcriptional activator factor-4; MT: mitochondrion; CHOP: c/EBF homologous protein; Bcl-2: B-cell lymphoma 2; Bax: Bcl-2 like protein 2; Bak: Bcl-2 homologous antagonist killer; JNK: c-Jun terminal amino kinase; ASK1: apoptosis signal regulating kinase). Sig-1R activation increases cell survival by decreasing the activation of PERK, ATF6 and IRE1α as well as lowering pro-apoptopic response (CHOP, Bax, Bak) and increases anti-apoptopic Bcl-2 activity.
IRE1a may induce another signalization cascade leading to activation of c-JUN amino terminal kinase (JNK) and apoptosis signal regulating kinase (ASK), both of them trigger caspase activation and apoptotic cell death.
ATF6 activation can also decrease Bcl-2 level and increase cytochrom-C release, contributing to the activation of the apoptotic cascade [87].
In summary, UPR is a bifunctional cellular response to protein misfolding, possessing both pro- and anti-survival effects. Basal activity of the UPR is beneficial by activating the ER-assisted degradation (ERAD) machinery for clearing misfolded proteins by the ubiquitin-proteasoma system and autophagy. However, sustained ER stress and chronic UPR could trigger rather a cell death than cell maintenance program [88]. Chronic ER-stress response might be linked to NDDs such as AD, PD and HD [86, 89].
Malfunction of the “ER stress – UPR – mitochondrial function - ROS production” web is central to a large diversity of illnesses. Growing evidences suggest ER stress with UPR as being a cardinal component in the development of many pathological conditions [90, 91]. Chronic UPR is thought to play a key role in neurodegenerative diseases and in many illnesses of civilization such as, diabetes, cancer, atherosclerosis, autoimmune, and cardiovascular disorders [83]. All of these disorders may have common pathological components: deficits in ER-proteostasis which lead to misfolded proteins accumulation characteristic of NDDs [86, 92]. Targeting Sig-1R by agonists may regulate ER stress and UPR, manage ER perturbations, regulate the formation of toxic misfolded proteins, and prevent the cell-killing apoptotic pathways (Fig. 1) [93]. Similar effects are expected from the endogenous Sig-1R ligands.
7.2. Mitochondrial Dysfunction and Ca2+-overload of Cells
Mitochondria play critical roles in neuronal maintenance by generating ATP and biosynthetic substrates, regulating Ca2+-homeostasis and initiating apoptosis. Mitochondrial dysfunction is associated with NDDs [94, 95]. According to the most recent models, ATP insufficiency and Ca2+-dysregulation are the initial triggers of the excitotoxic cascade.
Mitochondrial fission and fusion are parts of normal organellar maintenance and particularly significant in axons. ER was identified as a crucial component of the fission machinery [96]. Fragmented mitochondria and increased fission activity are associated with NDDs such as AD, PD and HD [95]. The common membrane site MAM is very important in lipid synthesis and trafficking, Ca2+-homeostasis and regulation of mitochondria-triggered apoptosis. Mitochondria-MAM dysregulation was proposed as one of the underlying causes of AD, contributing to neuronal loss [97, 98].
Sustained activation of NMDA receptors increases intracellular Ca2+ level, followed by failure in Ca2+-homeostasis. Activation of mitochondrial permeability transition pore opening is triggered by Ca2+-uptake into the mitochondrial matrix [99]. Continuous release of glutamate causes persistant activation of the NMDA receptors, leading to neuronal excitotoxicity. Excess of intracellular Ca2+ may contribute to neurodegeneration in many NDDs such as AD, PD and ALS [100-102].
7.3. Oxidative Stress
ROS are generated under different conditions, e.g. mitochondrial dysfunction and production by glial cells. Neurons are particularly vulnerable to glial stress [103]. Oxidative damage can affect proteins, nucleic acids and lipids. Detection of increased ROS production and oxidative damage in NDDs such as AD and PD is the best evidence for the causative effect of ROS [94, 103]. Oxidative stress also decreases the production of ATP and the antioxidant capacity (e.g. the level of reduced coenzymes) by impairing mitochondrial function [94].
7.4. Neuroinflammation, Reactive Gliosis
Reactive astrogliosis, the activation of astrocytes leads to increased expression of certain genes, e.g. glial fibrillary acidic protein (GFAP). Upregulation of GFAP causes astrocyte proliferation, then astrocyte migration and production of cytokines, chemokines and neurotrophic factors that aggravate brain damage.
Microglial cells are macrophage-derived resident immune cells of the CNS. As a result of dyshomeostasis in CNS, microglia is activated similarly to macrophages in the periphery showing pro-inflammatory (M1) and anti-inflammatory (M2) responses. Pro-inflammatory microglial response can cause damage to the CNS, through the release of ROS and pro-inflammatory cytokines [104]. Limiting of the inflammatory M1 and enhancing of reparative M2 responses are desired for neuroprotection.
8. Neuroprotective actions by Sig-1R ligands
8.1. Modulation of the ER and Mitochondrial Function
Sig-1R as a chaperone modulates UPR (Fig. 1). Under normal conditions Sig-1R is in dormant state in MAM forming a complex with another ER-chaperone BiP [25]. After ER stress or ligand stimulation Sig-1R dissociates from BiP and modulates the activities of the 3 major branches of UPR (PERK, IRE1a, ATF6). ER stress is also associated with the upregulation of Sig-1R expression.
Sig-1R overexpression increases cell survival by decreasing the activation of PERK and ATF6, probably with direct protein-protein interaction. Low activity of Sig-1R destabilizes the conformation of IRE1a and decreases cell survival [105].
Sig-1R ligands ameliorate mitochondrial function, e.g. the agonist BHDP protected rat liver cells. Another agonist, SA4503 protected cardiomyocytes from ischemic stress. Sig-1R agonists preserved mitochondrial respiration and ATP synthesis [106, 107]. Under ER-stress Sig-1R dissociates from the BiP complex, then interacts directly with IP3R3 and stabilizes its structure. IP3R3 in turn promote Ca2+-uptake and cell survival [23].
Sig-1R may also regulate the pro- and anti-apoptotic signals that target mitochondria: the receptor activation increases Bcl-2 expression, the elevated level of Bcl-2 interacts with IP3R thus increasing IP3R-mediated mitochondrial Ca2+-uptake and ATP production [108, 109].
8.2. Attenuation of ROS Production
Increased oxidative damage was demonstrated in the tissues of Sig-1 knockout or knockdown mice [70]. Activation of Sig-1R decreases ROS-accumulation probably via the modulation of ROS-neutralizing proteins, upregulation of antioxidant response elements (NAD(P):quinine oxidoreductase1 (NQO1), superoxide dismutase (SOD).
8.3. Ca2+-homeostasis and Glutamate Activity
Activation of Sig-1Rs may dampen the excitotoxic effect of glutamate (Glu). Excessive Glu effects can be pronounced during acute events such as ischemic stroke and trauma, or milder but prolonged in chronic NDDs such as AD, PD, HD and ALS [110].
The regulation of intracellular Ca2+ homeostasis is one of the major mechanisms by which Sig-1R ligands perform neuroptotection. (+)-Pentazocine modulates the sustained Ca2+-flux at toxic concentration of glutamate in primary rat cortical neurons [111]. Sig-1R agonists attenuate intracellular Ca2+ elevation in the Na-azide--glucose deprivation ischemia model [112]. Sig-1R interacts in cell survival by normalization of ER-Ca2+ concentrations by promoting Ca2+-entry into mitochondria through stabilization of IP3R3 receptors [23]. Activation of Sig-1Rs under certain conditions inhibits NMDA receptors, which are the principal mediators of excitotoxic damage [101, 113].
8.4. Modulation of Glial Activity
Sig-1R ligands may ameliorate reactive astrogliosis in rodent models of stroke and ALS [114, 115]. Similarly, Sig-1R ligands modulate microglial activity in animal models of PD and ALS, affecting both M1 and M2 type microglial responses [102, 115, 116]. Sig-1R elicits neuroprotective and neurorestorative effects by balancing the inflammatory and reparative microglial responses: treating an ALS model animal with PRE-084 increased the number of glial cells associated with neuronal repair [115].
9. Therapeutic application of Sig-1R modulation
A short list of preclinical studies and clinical trials of the cytoprotective effect of Sig-1R agonists are summarized in (Table 4).
Table 4.
A short list of preclinical and clinical trials with cytoprotective Sig-1R agonists.
| Disease/Drug Candidate | Drug Activities | Preclinical Studies | Refs. | Clinical Trials (Phase 1,2,3) | Refs. | |
|---|---|---|---|---|---|---|
| AD | Afobazole | Sig-1R agonist, melatonin (MT3) agonist | Neuron culture Mice, icv Aβ 25-35 (AD model) |
[59, 121, 122] | - | - |
| AF710B | Sig-1R agonist, M1 agonist |
AD mouse model | [123] | - | - | |
| Anavex 2-73 | Sig-1R agonist, M1 agonist |
Mice, icv Aβ 25-35 | [124] | - | - | |
| Anavex 2-73 | - | - | - | Phase 1 (max. dose 55mg) Phase 2a (oral, iv adm) |
www.anavex.com | |
| Anavex 2-73+ Donepezil | ChE-ase inhibitor | Mice, icv Aβ 25-35 (AD model) |
[126] | - | - | |
| Anavex Plus (combination of Anavex 2-73 plus Donepezil) | - | Synergistic effect in AD disease models | www.anavex.com | A patent application was filed in 2016 for the combination | www.anavex.com | |
| PD | PRE 084 | Sig-1R agonist | PD mouse model (6-OH DOPA lesion) |
[116] | - | - |
| ALS | Cutamesine (SA 4503) |
Sig-1R agonist | ALS mouse model SOD1 (G93A) model +NSC34motoneuron cells SOD1 (G93A) mouse |
[139] [140] [140] [102] | - | - |
| PRE-084 | Sig-1R agonist | wobbler mouse | [115] | - | - | |
| HD | PRE-084 | Sig-1R agonist | PC63 cells | [141, 142] | - | - |
| Pridopidine | Sig-1R agonist, DA stabilizer |
HD mouse model | [144, 145] | - | - | |
|
TBI
traumatic brain injury |
PPBP | Sig-1R agonist | TBI neonatal mouse model | [154, 155] | - | - |
| Retinal ganglion cell death | (+) Pentazocine | Sig-1R agonist | Murine retinal cells RGC-5 cells | [157, 158] | - | - |
| Ischemic stroke | Cutamesine (SA4503) | Sig-1R agonist | - | - | Phase-2 trial | [156] |
9.1. Alzheimer’s Disease
AD is a very complex, multifactorial disease of heterogeneous genetic and environmental background, characterized by severe cognitive impairment and memory loss [117]. The pharmacology of Sig-1R and its role in AD was widely reviewed in 2009 [11] and in 2015 [13]. Functional deficiency of Sig-1R plays an important role in the β-amyloid (Aβ) induced neuronal loss. Certain genetic combinations of Sig-1R and apolipoprotein E (apo-E) genotypes synergistically increase the risk of AD [118]. Sig-1R genotype is a determining risk factor in sporadic AD [73].
Sig-1R levels do not change considerably during normal ageing [62], however, in brains and postmortem tissues of AD patients decreased level of Sig-1R was demonstrated [74, 119, 120].
Drugs activating Sig-1R are considered to be antiamnestic and neuroprotective in NDDs such as AD. Several Sig-1R agonists (e.g. afobazole) protected cultured neurons against Aβ 25-35 toxicity [121, 122]. The same agonists attenuated learning and memory deficits observed in mice after intra-cerebroventricular injection of Aβ 25-35 [59].
Very recently, a mixed muscarinic-1 (M1) and Sig-1R agonist (AF710B, 1-(2,8-Dimethyl-1-thia-3,8-diazaspiro(4,5)dec-3-yl)-3-(1H-indol-3-yl)propan-1-one) has been used in AD mouse models. AF710B protects synapses via concomitant activation of the Sig-1R and a super-sensitive M1R, via a hypothetical complex of M1R-Sig-1R [123].
Anavex 2-73 (Tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine hydrochloride), an agonist of the intracellular Sig-1 chaperone protein is also a mixed ligand for sigma1/muscarinic receptors. It has been reported to bind to Sig-1 receptor in the high nanomolar and the muscarinic receptor in the low micromolar range. Anavex2-73 was shown to block tau-hyperphosphorylation and Aβ 1-42 generation in the Aβ 25-35 injection mouse model of AD [124] and protected mitochondria [125]. The latest studies in this mouse model demonstrated that the combination of Sig-1R agonists with donepezil showed a synergic protective effect [126], that is Sig-1R drugs offer a neuroprotective potential, also in combination with other therapeutic agents (e.g. with donepezil). Anavex Plus is a promising, potentially novel combination drug for AD, filed for patent application. It combines Anavex 2-73 Plus donepezil (Aricept), the best-selling AD drug. The combination drug produced up to 80% greater reversal of memory loss and neuroprotection compared to the administration of its individual counterparts. A patent application has been filed for Anavex Plus by the company.
According to its website (www.anavex.com), Anavex conducted a Phase 1 clinical trial in healthy men and Germany, which showed a maximum tolerated dose of 55 mg. The company completed a Phase 2a trial with Anavex 2-73 to compare oral and intravenous doses in people with mild to moderate AD at the end of 2016. Thereafter, the company plans to initiate a Phase 2/3 trial with the compound enrolling up to 300 mild to moderate AD patients.
The mechanism of Sig-1R mediated neuroprotective and anti-amnestic effects are not fully understood, but may include regulation of Ca2+ [121], modulation of Bcl-2 and caspase levels [127] and attenuation of oxidative stress [128].
The protective role of Sig-1R activation in AD is yet under discussion. In heterogeneous knock-out mice the Sig-1R deficiency reduced Aβ 25-35 induced hippocampal neuron death and cognitive deficits [129]. Sig-1R deficiency or blockade can reduce Aβ enhanced Ca2+-influx and prevent neurotoxicity [130]. The discrepancy in the scientific literature is hard to be reconciled, perhaps exact timing of Sig-1R activation might explain the contradictory effects. Although preclinical evidences suggest that Sig-1R agonists might be useful in treating AD, no selective Sig-1R agonist is currently available for clinical use.
9.2. Parkinson’s Disease
Likewise in Alzheimer’s disease [74] Sig-1Rs are downregulated in the brains of early stage PD patients [75, 131]. Recently, the pharmacological stimulation of the Sig-1R has shown some neurorestorative effects in experimental PD [116], the treatment of mice with the Sig-1R agonist PRE-084 caused both behavioral and histological improvements in the 6-hydroxydopamine-lesion mouse model of PD. Daily treatment of mice with 0.3 mg/kg PRE-084 during 5 weeks improved forelimb use, together with a modest recovery of dopamine levels. PRE-084 as Sig-1R agonist also upregulated the neurotrophic factors BDNF and GDNF and activated the trophic factor mediators ERK1 and ERK2 [116]. These results show that the restoration of synaptic connectivity correlates with the functional recovery in PD. As inflammation contributes to the pathophysiology of PD, the attenuation of M1 microglial response by PRE-084 treatment supports neuronal recovery in the PD mouse model. In vitro experiments where wild type and Sig-1R knock down CHO-cells were treated with dopamine suggest that Sig-1Rs are important endogenous substrates that counteract the dopamine cytotoxicity [132]. The results show that the Sig-1R-NF-κB-Bcl-2 pathway plays a crucial role against dopamine induced apoptosis.
9.3. Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Lobe Dementia (FTLD)
ALS is characterized by the progressive loss of motoneurons in the brainstem, motor cortex and spinal cord causing paralysis and death. Pathological hallmarks include calcium dysregulation and cytotoxicity, ER-stress and mitochondrial dysfunction [133], accumulation of misfolded proteins, neuro-inflammation and degradation of motoneurons [102]. It was demonstrated that a loss of Sig-1R is causative for a juvenile form of ALS (ALS16) and collapse of MAM is a common pathomechanism in both Sig-1R and SOD-1 linked ALS [134]. Mutations in the Sig-1R gene were found in patients with FTLD-ALS and a juvenile form of ALS [79, 135]. The receptor mutation lies very close to an area of chromosome 9, called the ALS-FTLD locus, which researchers have linked to ALS and FTLD [136]. A significant reduction of Sig-1R protein level was found in the lumbar spinal cord of ALS patients [137]. Aberrant modifications of Sig-1R ((abnormal accumulation in ER structures, enlarged C-terminals) were found in α motoneurons during the pathogenesis of ALS [137]. Since Sig-1R can regulate ERAD [138], this Sig-1R accumulation and co-localization with aberrant protein assemblies may reflect a compensatory effort by Sig-1R to clear misfolded proteins in ALS.
Attempts to target Sig-1Rs to treat ALS gave promising results in model experiments both in vitro and in vivo. The protective role of Sig-1R in motoneurons of an ALS mouse model has been confirmed [139]. SA4503, a Sig-1R agonist suppressed motor neuron damage in model experiments, both in vitro (NSC34 motor neuron cells) and in vivo (in the SOD1 (G93A) mouse model) [140]. PRE-084 treatment prolonged life span and slowed the progression of ALS symptoms in a SOD1 (G93A) mouse model of ALS [102]. Chronic (8 weeks) PRE-084 treatment (0.25 mg/kg, i.p., three times a week) improved motoneuron survival and locomotor performances in the wobbler mouse, an animal model of spontaneous motoneuron degeneration [115]. Experimental results suggest that targeting Sig-1R with agonists may help ameliorate protein aggregation and slow disease progression by enhancing the chaperone activity of Sig-1R.
9.4. Huntington Disease (HD)
HD is a “polyglutamine” (polyQ) disease, a genetic disorder: an autosomal dominant mutation in the huntingtin gene by a repetitive CAG region. HD is characterized by progressive motor and cognitive deficits resulted by selective neuronal loss.
Recent studies in the neuronal HD model (the cell line PC6.3) showed decreased Sig-1R levels in the presence of a mutant huntingtin protein expression (containing 120 glutamine repeats, 120 polyQ or 120Q huntingtin [141]. Administration of PRE-084 to the mutant PC6.3 cells restored the deficits in Sig-1R protein levels caused by the mutant huntingtin expression, probably by modulation of post-transcriptional processes [141]. PRE-084 also promoted cell viability and decreased caspase-3 and caspase-12 (an ER resident caspase) cleavage, probably by attenuation of ER stress through the activation of Sig-1R. PRE-084 can perform neuroprotection also by modulation of the calpastatin/calpain system [142] which positively affects NF-κB signaling to upregulate various cellular antioxidants and decrease ROS levels [141].
Sig-1R is involved in degradation of intranuclear inclusions in a cellular model of HD [143].
A registered drug, pridopidine, originally described as a dopamine stabilizer, improves HD motor symptoms as measured in clinical trials. Binding studies shows that Sig-1R is a high affinity receptor for pridopidine: the compound is also a Sig-1R agonist. HD mouse model experiments demonstrate that Sig-1R mediates the beneficial effect of pridopidine by preventing spine loss of striatal medium spiny neurons in HD [144]. Activation of Sig-1R and the subsequent upregulation of BDNF pathway represents the core component of the mode of action of pridopidine (promoting neuronal plasticity and survival) [145].
9.5. Traumatic Brain Injury
Traumatic brain injury (TBI) is one of the leading acute neurological disorders causing death and disability worldwide [146]. Very few effective treatments are available to mitigate neuronal damage and patients need long term care for physical and psychosocial rehabilitation. The outcome and costs of treatment after TBI are difficult to predict due to the physical disability combined the emotional and psychosocial consequences of the trauma [147]. The high rate of blast-related TBIs resulting from current combat operations profoundly affects the health and quality of life of service members and veterans during times of both war and peace.
Physical trauma to the brain initiates a series of inflammatory reactions, which includes release of intracellular products from damaged cells, microglia activation, cytokine production, and recruiting peripheral inflammatory cells into damaged areas. Microglia function is critical in response to brain injury [148]. Upon activation, microglia undergoes morphological transformation, secretes inflammatory mediators, and clears tissue debris by phagocytosis [149]. However, activated microglia can aggravate brain injury and impair its restoration [150]. A cumulative evidence suggests that activated microglia can release pro-inflammatory cytokines (e.g., TNF-α) and nitric oxide, which extend the neuronal injury after TBI [151]. Therefore, inhibition of microglial activation can protect brain tissue from the sequalae of TBI [152], and can be an important element of therapeutic approaches against TBI. Chronic microglial responses may be responsible for neurodegeneration in many neurological disorders and the role of Sig-1Rs might be to “switch-off” microglia after they responded to an injury allowing for microglial cells to leave site of damage [153].
Considering the presence of Sig-1R on microglial cells [11] and the effects Sig-1R agonists on reducing microglia activation [102], it was postulated and recently confirmed that activation of Sig-1R receptors after TBI can reduce lesion volume, lessen brain edema, and attenuate neurological deficits [154]. The Sig-1R agonist PPBP (4-phenyl-1-(4-phenylbutyl)piperidine) protects brain against newborn excitotoxic brain injury in an established neonatal mouse model by inhibiting microglial activation in vivo [155].
9.6. Other Diseases
Sig-1R agonists have been tried in animal models of different disorders and also used for the treatment of different diseases. Thus, cutamesine (SA4503) was used for recovery enhancement after ischemic stroke [156]. (+)-Pentazocine reduced NMDA-induced retinal ganglion cell death: recently Sig-1R is a promising therapeutic target for retinal neurodegenerative disorders [157, 158].
10. Dimethyltryptamine as a natural Sig-1R ligand
The discovery of the tryptamine analogue DMT as a natural ligand of the Sig-1R has moved the 40-year long fuzzy history of both of these molecules toward clarification. DMT was classified as an endogenous hallucinogen [159, 160] with exact physiological function unclear [161]. Ayahuasca, a psychoactive Amazonian ethnomedicinal brew, raised increased scientific and public interest during the last two decades. Ayahuasca is prepared from two admixture plants (Banisteriopsis caapi and Psychotria viridis) the latter provides the psychoactive alkaloid DMT [162, 163]. More than 40 years of research has proven to be insufficient to provide a proper neurobiological description for the role of DMT as endogenous hallucinogen. This was in part due to a paradigm problem in which DMT has been primarily studied in terms of being “hallucinogen” since it has been known to produce false perceptions. These effects presumably reflect action on serotonin (5-HT) receptors (5-HT1A, -2A and -2C) [164], and very probably involve the metabotropic glutamate receptors [165]. However, the Sig-1R action of DMT may turn out to be more revealing and more relevant for its physiological function. The main ingredients of the ayahuasca are DMT and the β-carboline derivative alkaloid harmine [166]. It is reasonable to suppose that DMT from this exogenous source can exert Sig-1R agonist effect and mitigate a wide variety of problems related to the disorganized web of mitochondrial dysfunction, ER stress, and UPR.
DMT is considered as a natural ligand of the Sig-1R [3]. It is assumed that the Sig-1R might be involved in the DMT-induced psychedelic effects [167]; however, this is probably not so, since many drugs—typically non-hallucinogens—bind to the Sig-1R with higher affinity than DMT. Another vista has been opening up instead. If the Sig-1R mitigates ER stress [71], promotes neuronal survival against oxidative stress [70], and regulates immune processes [168], one may attribute the same role to DMT [169]. Since the Sig-1R is also known to regulate morphogenesis of neuronal cells, such as neurite outgrowth, synaptogenesis, and myelination [170]; neurorestorative effects are reasonably expected from DMT. In a recent paper [169] Frecska et al. concluded that the function of DMT may extend central nervous activity and involve a more universal role in cellular protective mechanisms. These authors provided converging evidence that the role of DMT can be conceptualized along the line of adaptive mechanisms like neuroprotection, and immunity. The theoretical work was followed by experimental studies and Sig-1R mediated anti-inflammatory [80] and anti-hypoxia effects [171] of DMT were verified in vitro.
Conclusion
Sig-1R is an intracellular receptor acting as ER-chaperone (residing in the MAM) with unique pharmacological profile. It has also a unique amino acid sequence and steric structure among the receptor proteins. DMT is the endogenous ligand of Sig-1R. Oligomerization and translocation from the MAM to different biological membranes are key functional properties . The receptor has versatile cellular function and binds ligands of very diverse structure. Sig-1R is a ligand-regulated molecular chaperone which is not coupled to G-proteins. The main physiological functions (modulation of Ca2+ signaling, inhibition of voltage-dependent K+ channels, release of various neurotransmitters, immunomodulation) are known, however, the signal transduction pathways are not yet clarified.
Sig-1R is a pluripotent modulator of cellular events, regulating the inter-organelle communication and cell survival by increasing stress-response signaling. The receptor can interact with many proteins (with diverse structure and function) and can form oligomeric assemblies and heterodimers with other proteins. The existence of different oligomeric forms might provide a wide adaptability, which enables Sig-1R to interact with structurally different substrates.
Many marketed psychotropic drugs (fluvoxamine, donepezil) have affinity on Sig-1R, however, the mechanism of action is not yet clear. Receptor binding affinity and pharmacological activities frequently do not show good correlation, the molecular basis of ligand efficacy is to be discovered. Although Sig-1R agonists show neuroprotective effect in cellular and animal models, no effective registered neuroprotective drugs are on the market. Sig-1R agonists would be ideal drug candidates for translational medicine, because the known ligands have limited side effects: their modulatory action starts only under pathological conditions. A further work is required for clarifying the cellular mechanisms and molecular targets of Sig-1R. Application of Sig-1R agonists alone, very probably, will not be sufficient for the treatment of neurodegenerative diseases.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
This work was supported by the grants KTIA_13_NAP-A-III/7, KTIA_13_NAP-A-II/7 and GINOP-2.3.2-15-2016-00060.
LIST OF ABBREVIATIONS
- AChE
Acetylcholine esterase
- AD
Alzheimer’s disease
- ALS
Amyotrophic lateral sclerosis
- ASK
Apoptosis signal-regulating kinase
- ATF6
Activating transcription factor 6
- ATP
Adenosine triphosphate
- BACE
Beta-site APP cleaving enzyme
- Bak
Bcl-2 homologous antagonist killer
- Bax
Bcl-2 like protein 4
- Bcl-2
B-cell lymphoma 2
- BiP
Binding immunoglobulin protein
- BDNF
Brain derived neurotrophic factor
- CNS
Central nervous system
- DMT
Dimethyltryptamine
- DTG
1,3-Di-o.tolyl guanidine
- ER
Endoplasmic reticulum
- ERAD
ER-associated degradation
- ERG2
Yeast sterol isomerase
- ERK
Extracellular signal regulated kinases
- FTLD
Frontotemporal lobar degeneration
- FRET
Forster resonance energy transfer
- GDNF
Glial cell-derived neurotrophic factor
- GFAP
Glial fibrillary acidic protein
- GPCR
G-protein coupled receptors
- GSK3B
Glycogen synthase kinase 3b
- GTP
Guanosine triphosphate
- HD
Huntington disease
- IRE1
Inositol requiring enzyme 1
- IP3R
Inositol-triphosphate receptor
- ID
Intrinsically disordered
- JNK
c-Jun amino terminal kinase
- MAM
Mitochondrion associated ER-membrane
- M1R
Muscarinic-1 receptor
- NDD
Neurodegenerative diseases
- NFkB
Nuclear factor-kappa B
- NMDA
N-methyl-D-aspartate
- Nrf2
NF-E2-related nuclear factor 2
- PCP
Phencyclidine
- PD
Parkinson’s disease
- PERK
Protein kinase RNA like ER-kinase
- PM
Plasma membrane
- ROS
Reactive oxygene species
- Sig-1R
Sigma-1 receptor
- Sig-2R
Sigma-2 receptor
- SOD
Superoxide dismutase
- TBI
Traumatic brain injury
- TNFa
Tumor necrosis factor a
- UPR
Unfolded protein response
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REfERENCES
- 1.Schmidt H.R., Zheng S., Gurpinar E., Koehl A., Manglik A., Kruse A.C. Crystal structure of the human σ1 receptor. Nature. 2016;532(7600):527–530. doi: 10.1038/nature17391. [http://dx.doi.org/10.1038/nature17391]. [PMID: 27042935]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moebius F.F., Reiter R.J., Hanner M., Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: Evidence for a pharmacological relationship with the yeast sterol C8-C7 isomerase. Br. J. Pharmacol. 1997;121(1):1–6. doi: 10.1038/sj.bjp.0701079. [http://dx.doi. org/10.1038/sj.bjp.0701079]. [PMID: 9146879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontanilla D., Johannessen M., Hajipour A.R., Cozzi N.V., Jackson M.B., Ruoho A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323(5916):934–937. doi: 10.1126/science.1166127. [http://dx.doi.org/ 10.1126/science.1166127]. [PMID: 19213917]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin W.R., Eades C.G., Thompson J.A., Huppler R.E., Gilbert P.E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197(3):517–532. [PMID: 945347]. [PubMed] [Google Scholar]
- 5.Su T.P. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223(2):284–290. [PMID: 6290634]. [PubMed] [Google Scholar]
- 6.Su T.P., London E.D., Jaffe J.H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240(4849):219–221. doi: 10.1126/science.2832949. [http://dx.doi.org/10. 1126/science.2832949]. [PMID: 2832949]. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel D.B. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983;92(3-4):269–274. doi: 10.1016/0014-2999(83)90297-2. [http://dx.doi.org/10.1016/0014-2999(83)90297-2]. [PMID: 6313397]. [DOI] [PubMed] [Google Scholar]
- 8.Hellewell S.B., Bruce A., Feinstein G., Orringer J., Williams W., Bowen W.D. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994;268(1):9–18. doi: 10.1016/0922-4106(94)90115-5. [http:// dx.doi.org/10.1016/0922-4106(94)90115-5]. [PMID: 7925616]. [DOI] [PubMed] [Google Scholar]
- 9.Bowen W.D., Hellewell S.B., McGarry K.A. Evidence for a multi-site model of the rat brain sigma receptor. Eur. J. Pharmacol. 1989;163(2-3):309–318. doi: 10.1016/0014-2999(89)90200-8. [http://dx.doi.org/10.1016/0014-2999(89)90200-8]. [PMID: 2542066]. [DOI] [PubMed] [Google Scholar]
- 10.Glennon R.A., Ablordeppey S.Y., Ismaiel A.M., el-Ashmawy M.B., Fischer J.B., Howie K.B. Structural features important for sigma 1 receptor binding. J. Med. Chem. 1994;37(8):1214–1219. doi: 10.1021/jm00034a020. [http://dx.doi.org/10.1021/jm00034a020]. [PMID: 8164264]. [DOI] [PubMed] [Google Scholar]
- 11.Maurice T., Su T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [http://dx.doi.org/10. 1016/j.pharmthera.2009.07.001]. [PMID: 19619582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai S.Y., Pokrass M.J., Klauer N.R., De Credico N.E., Su T.P. Sigma-1 receptor chaperones in neurodegenerative and psychiatric disorders. Expert Opin. Ther. Targets. 2014;18(12):1461–1476. doi: 10.1517/14728222.2014.972939. [PMID: 25331742]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen L., Lucke-Wold B.P., Mookerjee S.A., Cavendish J.Z., Robson M.J., Scandinaro A.L., Matsumoto R.R. Role of sigma-1 receptors in neurodegenerative diseases. J. Pharmacol. Sci. 2015;127(1):17–29. doi: 10.1016/j.jphs.2014.12.005. [http://dx.doi.org/10.1016/j.jphs.2014.12.005]. [PMID: 25704014]. [DOI] [PubMed] [Google Scholar]
- 14.Su T.P., Su T.C., Nakamura Y., Tsai S.Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci. 2016;37(4):262–278. doi: 10.1016/j.tips.2016.01.003. [http://dx.doi.org/10.1016/j.tips.2016. 01.003]. [PMID: 26869505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin J.L., Fang M., Zhao Y.X., Liu X.Y. Roles of sigma-1 receptors in Alzheimer’s disease. Int. J. Clin. Exp. Med. 2015;8(4):4808–4820. [PMID: 26131055]. [PMC free article] [PubMed] [Google Scholar]
- 16.Rousseaux C.G., Greene S.F. Sigma receptors [σRs]: biology in normal and diseased states. J. Recept. Signal Transduct. Res. 2016;36(4):327–388. doi: 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S.B., Su T.P. Sigma Receptors: Their Role in Disease and as Therapeutic Targets. 2017. [DOI] [PubMed]
- 18.Ishiwata K., Kobayashi T., Kawamura K., Matsuno K. Age-related changes of the binding of [3h]SA4503 to sigma1 receptors in the rat brain. Ann. Nucl. Med. 2003;17(1):73–77. doi: 10.1007/BF02988264. [http://dx.doi. org/10.1007/BF02988264]. [PMID: 12691135]. [DOI] [PubMed] [Google Scholar]
- 19.Pundir S., Martin M.J., O’Donovan C. UniProt Protein Knowledgebase. In: Wu C., Arighi C., Ross K., editors. Protein Bioinformatics. Methods in Molecular Biology. Vol. 1558. New York, NY: Humana Press; 2017. pp. 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langa F., Codony X., Tovar V., Lavado A., Giménez E., Cozar P., Cantero M., Dordal A., Hernández E., Pérez R., Monroy X., Zamanillo D., Guitart X., Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur. J. Neurosci. 2003;18(8):2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [http://dx.doi.org/10.1046/ j.1460-9568.2003.02950.x]. [PMID: 14622179]. [DOI] [PubMed] [Google Scholar]
- 21.Pabba M. The essential roles of protein-protein interaction in sigma-1 receptor functions. Front. Cell. Neurosci. 2013;7:50. doi: 10.3389/fncel.2013.00050. [http://dx.doi.org/10.3389/fncel.2013.00050]. [PMID: 23630466]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T., Tsai S.Y., Mori T., Fujimoto M., Su T.P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets. 2011;15(5):557–577. doi: 10.1517/14728222.2011.560837. [http://dx.doi.org/10.1517/14728222.2011.560837]. [PMID: 21375464]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131(3):596–610. doi: 10.1016/j.cell.2007.08.036. [http://dx.doi.org/10.1016/ j.cell.2007.08.036]. [PMID: 17981125]. [DOI] [PubMed] [Google Scholar]
- 24.Mavlyutov T.A., Ruoho A.E. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J. Mol. Signal. 2007;2:8. doi: 10.1186/1750-2187-2-8. [http://dx.doi.org/10.1186/1750-2187-2-8]. [PMID: 17883859]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi T., Su T.P. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell. Biochem. 2010;51:381–398. doi: 10.1007/978-90-481-8622-8_13. [http:// dx.doi.org/10.1007/978-90-481-8622-8_13]. [PMID: 20213551]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin-Surun M.P., Collin T., Denavit-Saubié M., Baulieu E.E., Monnet F.P. Intracellular σ1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc. Natl. Acad. Sci. USA. 1999;96(14):8196–8199. doi: 10.1073/pnas.96.14.8196. [http://dx.doi.org/10.1073/ pnas.96.14.8196]. [PMID: 10393971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su T.P., Hayashi T., Maurice T., Buch S., Ruoho A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010;31(12):557–566. doi: 10.1016/j.tips.2010.08.007. [http://dx.doi. org/10.1016/j.tips.2010.08.007]. [PMID: 20869780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanner M., Moebius F.F., Flandorfer A., Knaus H.G., Striessnig J., Kempner E., Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA. 1996;93(15):8072–8077. doi: 10.1073/pnas.93.15.8072. [http://dx.doi.org/ 10.1073/pnas.93.15.8072]. [PMID: 8755605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aydar E., Palmer C.P., Klyachko V.A., Jackson M.B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34(3):399–410. doi: 10.1016/s0896-6273(02)00677-3. [http://dx.doi.org/10.1016/S0896-6273(02)00677-3]. [PMID: 11988171]. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T., Su T. The sigma receptor: Evolution of the concept in neuropsychopharmacology. Curr. Neuropharmacol. 2005;3(4):267–280. doi: 10.2174/157015905774322516. [http://dx.doi.org/10.2174/157015905774322516]. [PMID: 18369400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moebius F.F., Bermoser K., Reiter R.J., Hanner M., Glossmann H. Yeast sterol C8-C7 isomerase: Identification and characterization of a high-affinity binding site for enzyme inhibitors. Biochemistry. 1996;35(51):16871–16878. doi: 10.1021/bi961996m. [http://dx.doi.org/10.1021/ bi961996m]. [PMID: 8988026]. [DOI] [PubMed] [Google Scholar]
- 32.Seth P., Fei Y.J., Li H.W., Huang W., Leibach F.H., Ganapathy V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998;70(3):922–931. doi: 10.1046/j.1471-4159.1998.70030922.x. [http://dx.doi. org/10.1046/j.1471-4159.1998.70030922.x]. [PMID: 9489711]. [DOI] [PubMed] [Google Scholar]
- 33.Mei J., Pasternak G.W. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem. Pharmacol. 2001;62(3):349–355. doi: 10.1016/s0006-2952(01)00666-9. [http://dx.doi.org/10.1016/S0006-2952(01) 00666-9]. [PMID: 11434908]. [DOI] [PubMed] [Google Scholar]
- 34.Prasad P.D., Li H.W., Fei Y.J., Ganapathy M.E., Fujita T., Plumley L.H., Yang-Feng T.L., Leibach F.H., Ganapathy V. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J. Neurochem. 1998;70(2):443–451. doi: 10.1046/j.1471-4159.1998.70020443.x. [http://dx.doi.org/10.1046/j.1471-4159.1998.70020443.x]. [PMID: 9453537]. [DOI] [PubMed] [Google Scholar]
- 35.Moebius F.F., Striessnig J., Glossmann H. The mysteries of sigma receptors: new family members reveal a role in cholesterol synthesis. Trends Pharmacol. Sci. 1997;18(3):67–70. doi: 10.1016/s0165-6147(96)01037-1. [http://dx. doi.org/10.1016/S0165-6147(96)01037-1]. [PMID: 9133773]. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi T., Su T.P. An update on the development of drugs for neuropsychiatric disorders: Focusing on the sigma 1 receptor ligand. Expert Opin. Ther. Targets. 2008;12(1):45–58. doi: 10.1517/14728222.12.1.45. [http://dx. doi.org/10.1517/14728222.12.1.45]. [PMID: 18076369]. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T., Stahl S.M. The sigma-1 (delta-1) receptor and its role in the treament of mood disorders. Drugs Future. 2009;34(2):137. [Google Scholar]
- 38.Ortega-Roldan J.L., Ossa F., Amin N.T., Schnell J.R. Solution NMR studies reveal the location of the second transmembrane domain of the human sigma-1 receptor. FEBS Lett. 2015;589(5):659–665. doi: 10.1016/j.febslet.2015.01.033. [http://dx.doi.org/10.1016/j.febslet.2015.01.033]. [PMID: 25647032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganapathy M.E., Prasad P.D., Huang W., Seth P., Leibach F.H., Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J. Pharmacol. Exp. Ther. 1999;289(1):251–260. [PMID: 10087012]. [PubMed] [Google Scholar]
- 40.Manohar M., Banister S.D., Beinat C., O’Brien-Brown J., Kassiou M. Recent advances in the development of sigma-1 receptor ligands. Aust. J. Chem. 2015;68(4):600–609. [http://dx.doi.org/ 10.1071/CH14590]. [Google Scholar]
- 41.Pal A., Chu U.B., Ramachandran S., Grawoig D., Guo L.W., Hajipour A.R., Ruoho A.E. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J. Biol. Chem. 2008;283(28):19646–19656. doi: 10.1074/jbc.M802192200. [http://dx.doi.org/10.1074/jbc.M802192200]. [PMID: 18467334]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal A., Hajipour A.R., Fontanilla D., Ramachandran S., Chu U.B., Mavlyutov T., Ruoho A.E. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol. Pharmacol. 2007;72(4):921–933. doi: 10.1124/mol.107.038307. [http://dx.doi.org/10.1124/ mol.107.038307]. [PMID: 17622576]. [DOI] [PubMed] [Google Scholar]
- 43.Chu U.B., Ruoho A.E. Biochemical Pharmacology of the Sigma-1 Receptor. Mol. Pharmacol. 2016;89(1):142–153. doi: 10.1124/mol.115.101170. [http://dx.doi. org/10.1124/mol.115.101170]. [PMID: 26560551]. [DOI] [PubMed] [Google Scholar]
- 44.Ossa F., Schnell J.R., Ortega-Roldan J.L. A Review of the Human Sigma-1 Receptor Structure. In: Smith S.B., Su T.P., editors. Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Springer; 2017. p. 312. [http://dx.doi.org/10.1007/978-3-319-50174-1_3] [Google Scholar]
- 45.Seth P., Ganapathy M.E., Conway S.J., Bridges C.D., Smith S.B., Casellas P., Ganapathy V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim. Biophys. Acta. 2001;1540(1):59–67. doi: 10.1016/s0167-4889(01)00117-3. [http://dx.doi.org/ 10.1016/S0167-4889(01)00117-3]. [PMID: 11476895]. [DOI] [PubMed] [Google Scholar]
- 46.Brune S., Schepmann D., Klempnauer K.H., Marson D., Dal Col V., Laurini E., Fermeglia M., Wünsch B., Pricl S. The sigma enigma: In vitro/in silico site-directed mutagenesis studies unveil σ1 receptor ligand binding. Biochemistry. 2014;53(18):2993–3003. doi: 10.1021/bi401575g. [http://dx.doi.org/10.1021/bi401575g]. [PMID: 24766040]. [DOI] [PubMed] [Google Scholar]
- 47.Gromek K.A., Suchy F.P., Meddaugh H.R., Wrobel R.L., LaPointe L.M., Chu U.B., Primm J.G., Ruoho A.E., Senes A., Fox B.G. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J. Biol. Chem. 2014;289(29):20333–20344. doi: 10.1074/jbc.M113.537993. [http://dx.doi.org/10.1074/jbc.M113.537993]. [PMID: 24847081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu U.B., Ruoho A.E. Biochemical pharmacology of the sigma-1 receptor. Mol. Pharmacol. 2016;89(1):142–153. doi: 10.1124/mol.115.101170. [http://dx.doi. org/10.1124/mol.115.101170]. [PMID: 26560551]. [DOI] [PubMed] [Google Scholar]
- 49.Mishra A.K., Mavlyutov T., Singh D.R., Biener G., Yang J., Oliver J.A., Ruoho A., Raicu V. The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. Biochem. J. 2015;466(2):263–271. doi: 10.1042/BJ20141321. [http://dx.doi.org/10.1042/BJ20141321]. [PMID: 25510962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z., Bowen W.D. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: Constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008;283(42):28198–28215. doi: 10.1074/jbc.M802099200. [http://dx.doi.org/10.1074/ jbc.M802099200]. [PMID: 18539593]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi T., Su T.P. Sigma-1 receptor ligands: Potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18(5):269–284. doi: 10.2165/00023210-200418050-00001. [http://dx.doi.org/10.2165/00023210-200418050-00001]. [PMID: 15089113]. [DOI] [PubMed] [Google Scholar]
- 52.Glennon R.A. Pharmacophore identification for sigma-1 (sigma1) receptor binding: application of the “deconstruction-reconstruction-elaboration” approach. Mini Rev. Med. Chem. 2005;5(10):927–940. doi: 10.2174/138955705774329519. [http://dx.doi.org/10.2174/138955705774329519]. [PMID: 16250835]. [DOI] [PubMed] [Google Scholar]
- 53.Ruoho A.E., Chu U.B., Ramachandran S., Fontanilla D., Mavlyutov T., Hajipour A.R. The ligand binding region of the sigma-1 receptor: studies utilizing photoaffinity probes, sphingosine and N-alkylamines. Curr. Pharm. Des. 2012;18(7):920–929. doi: 10.2174/138161212799436584. [http://dx. doi.org/10.2174/138161212799436584]. [PMID: 22288412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo L., Zhao J., Jin G., Zhao B., Wang G., Zhang A., Zhen X. SKF83959 is a potent allosteric modulator of sigma-1 receptor. Mol. Pharmacol. 2013;83(3):577–586. doi: 10.1124/mol.112.083840. [http://dx.doi.org/10.1124/ mol.112.083840]. [PMID: 23295385]. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z., Li L., Zheng L.T., Xu Z., Guo L., Zhen X. Allosteric modulation of sigma-1 receptors by SKF83959 inhibits microglia-mediated inflammation. J. Neurochem. 2015;134(5):904–914. doi: 10.1111/jnc.13182. [http://dx.doi.org/10.1111/jnc.13182]. [PMID: 26031312]. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Guo L., Jiang H.F., Zheng L.T., Zhang A., Zhen X.C. Allosteric modulation of sigma-1 receptors elicits rapid antidepressant activity. CNS Neurosci. Ther. 2016;22(5):368–377. doi: 10.1111/cns.12502. [http://dx.doi.org/10.1111/cns.12502]. [PMID: 26854125]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cobos E.J., Entrena J.M., Nieto F.R., Cendán C.M., Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008;6(4):344–366. doi: 10.2174/157015908787386113. [http://dx. doi.org/10.2174/157015908787386113]. [PMID: 19587856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitt T., Ogris C., Sonnhammer E.L. FunCoup 3.0: database of genome-wide functional coupling networks. Nucleic Acids Res. 2014;42(Database issue):D380–D388. doi: 10.1093/nar/gkt984. [http://dx.doi.org/10.1093/ nar/gkt984]. [PMID: 24185702]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurice T., Su T.P., Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83(2):413–428. doi: 10.1016/s0306-4522(97)00405-3. [http://dx.doi.org/10.1016/S0306-4522 (97)00405-3]. [PMID: 9460750]. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J. Pharmacol. Sci. 2015;127(1):6–9. doi: 10.1016/j.jphs.2014.11.010. [http://dx.doi.org/10.1016/ j.jphs.2014.11.010]. [PMID: 25704012]. [DOI] [PubMed] [Google Scholar]
- 61.Lupardus P.J., Wilke R.A., Aydar E., Palmer C.P., Chen Y., Ruoho A.E., Jackson M.B. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J. Physiol. 2000;526(Pt 3):527–539. doi: 10.1111/j.1469-7793.2000.00527.x. [http://dx.doi.org/10.1111/j.1469-7793.2000. 00527.x]. [PMID: 10922005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Waarde A., Ramakrishnan N.K., Rybczynska A.A., Elsinga P.H., Ishiwata K., Nijholt I.M., Luiten P.G., Dierckx R.A. The cholinergic system, sigma-1 receptors and cognition. Behav. Brain Res. 2011;221(2):543–554. doi: 10.1016/j.bbr.2009.12.043. [http://dx.doi.org/10.1016/j.bbr.2009. 12.043]. [PMID: 20060423]. [DOI] [PubMed] [Google Scholar]
- 63.Niitsu T., Iyo M., Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr. Pharm. Des. 2012;18(7):875–883. doi: 10.2174/138161212799436476. [http://dx.doi.org/ 10.2174/138161212799436476]. [PMID: 22288409]. [DOI] [PubMed] [Google Scholar]
- 64.Brimson J.M., Brown C.A., Safrany S.T. Antagonists show GTP-sensitive high-affinity binding to the sigma-1 receptor. Br. J. Pharmacol. 2011;164(2b):772–780. doi: 10.1111/j.1476-5381.2011.01417.x. [http://dx.doi.org/10.1111/ j.1476-5381.2011.01417.x]. [PMID: 21486275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itzhak Y. Multiple affinity binding states of the sigma receptor: effect of GTP-binding protein-modifying agents. Mol. Pharmacol. 1989;36(4):512–517. [PMID: 2554109]. [PubMed] [Google Scholar]
- 66.Hong W., Werling L.L. Evidence that the sigma(1) receptor is not directly coupled to G proteins. Eur. J. Pharmacol. 2000;408(2):117–125. doi: 10.1016/s0014-2999(00)00774-3. [http://dx.doi.org/10.1016/S0014-2999(00)00774-3]. [PMID: 11080517]. [DOI] [PubMed] [Google Scholar]
- 67.Su T.P., Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003;10(20):2073–2080. doi: 10.2174/0929867033456783. [http://dx.doi.org/10.2174/0929867033 456783]. [PMID: 12871086]. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi T. Sigma-1 receptor: the novel intracellular target of neuropsychotherapeutic drugs. J. Pharmacol. Sci. 2015;127(1):2–5. doi: 10.1016/j.jphs.2014.07.001. [http://dx.doi.org/10.1016/j.jphs.2014.07.001]. [PMID: 25704011]. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi T. Conversion of psychological stress into cellular stress response: roles of the sigma-1 receptor in the process. Psychiatry Clin. Neurosci. 2015;69(4):179–191. doi: 10.1111/pcn.12262. [http://dx.doi.org/10.1111/ pcn.12262]. [PMID: 25495202]. [DOI] [PubMed] [Google Scholar]
- 70.Pal A., Fontanilla D., Gopalakrishnan A., Chae Y.K., Markley J.L., Ruoho A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012;682(1-3):12–20. doi: 10.1016/j.ejphar.2012.01.030. [http://dx.doi.org/10.1016/ j.ejphar.2012.01.030]. [PMID: 22381068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omi T., Tanimukai H., Kanayama D., Sakagami Y., Tagami S., Okochi M., Morihara T., Sato M., Yanagida K., Kitasyoji A., Hara H., Imaizumi K., Maurice T., Chevallier N., Marchal S., Takeda M., Kudo T. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014;5:e1332. doi: 10.1038/cddis.2014.301. [http://dx.doi.org/10.1038/cddis.2014.301]. [PMID: 25032855]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai S.Y., Hayashi T., Mori T., Su T.P. Sigma-1 receptor chaperones and diseases. Cent. Nerv. Syst. Agents Med. Chem. 2009;9(3):184–189. doi: 10.2174/1871524910909030184. [http://dx.doi.org/10.2174/1871524910909030184]. [PMID: 20021352]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fehér Á., Juhász A., László A., Kálmán J., Jr, Pákáski M., Kálmán J., Janka Z. Association between a variant of the sigma-1 receptor gene and Alzheimer’s disease. Neurosci. Lett. 2012;517(2):136–139. doi: 10.1016/j.neulet.2012.04.046. [http://dx.doi.org/10.1016/j.neulet.2012.04.046]. [PMID: 22561649]. [DOI] [PubMed] [Google Scholar]
- 74.Mishina M., Ohyama M., Ishii K., Kitamura S., Kimura Y., Oda K., Kawamura K., Sasaki T., Kobayashi S., Katayama Y., Ishiwata K. Low density of sigma1 receptors in early Alzheimer’s disease. Ann. Nucl. Med. 2008;22(3):151–156. doi: 10.1007/s12149-007-0094-z. [http://dx.doi. org/10.1007/s12149-007-0094-z]. [PMID: 18498028]. [DOI] [PubMed] [Google Scholar]
- 75.Konitsiotis S. Novel pharmacological strategies for motor complications in Parkinson’s disease. Expert Opin. Investig. Drugs. 2005;14(4):377–392. doi: 10.1517/13543784.14.4.377. [http://dx.doi.org/10.1517/13543784.14.4.377]. [PMID: 15882115]. [DOI] [PubMed] [Google Scholar]
- 76.Vilner B.J., de Costa B.R., Bowen W.D. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J. Neurosci. 1995;15(1 Pt 1):117–134. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [PMID: 7823122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Novakova M., Bruderova V., Sulova Z., Kopacek J., Lacinova L., Kvetnansky R., Vasku A., Kaplan P., Krizanova O., Jurkovicova D. Modulation of expression of the sigma receptors in the heart of rat and mouse in normal and pathological conditions. Gen. Physiol. Biophys. 2007;26(2):110–117. [PMID: 17660585]. [PubMed] [Google Scholar]
- 78.Liu X.Y., Li H.L., Su J.B., Ding F.H., Zhao J.J., Chai F., Li Y.X., Cui S.C., Sun F.Y., Wu Z.Y., Xu P., Chen X.H. Regulation of RAGE splicing by hnRNP A1 and Tra2β-1 and its potential role in AD pathogenesis. J. Neurochem. 2015;133(2):187–198. doi: 10.1111/jnc.13069. [http://dx.doi.org/10.1111/jnc.13069]. [PMID: 25689357]. [DOI] [PubMed] [Google Scholar]
- 79.Luty A.A., Kwok J.B., Dobson-Stone C., Loy C.T., Coupland K.G., Karlström H., Sobow T., Tchorzewska J., Maruszak A., Barcikowska M., Panegyres P.K., Zekanowski C., Brooks W.S., Williams K.L., Blair I.P., Mather K.A., Sachdev P.S., Halliday G.M., Schofield P.R. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann. Neurol. 2010;68(5):639–649. doi: 10.1002/ana.22274. [http://dx.doi.org/10. 1002/ana.22274]. [PMID: 21031579]. [DOI] [PubMed] [Google Scholar]
- 80.Szabo A., Kovacs A., Frecska E., Rajnavolgyi E., Psychedelic N. N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS One. 2014;9(8):1–12. doi: 10.1371/journal.pone.0106533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong H., Ma Y., Ren Z., Xu B., Zhang Y., Chen J., Yang B. Sigma-1 receptor modulates neuroinflammation after traumatic brain injury. Cell. Mol. Neurobiol. 2016;36(5):639–645. doi: 10.1007/s10571-015-0244-0. [http://dx.doi.org/10.1007/s10571-015-0244-0]. [PMID: 26228028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volgyi K., Juhász G., Kovacs Z., Penke B. Dysfunction of endoplasmic reticulum (ER) and mitochondria (MT) in alzheimer’s disease: The role of the ER-MT cross-talk. Curr. Alzheimer Res. 2015;12(7):655–672. doi: 10.2174/1567205012666150710095035. [http://dx.doi.org/10.2174/1567205012666 150710095035]. [PMID: 26159206]. [DOI] [PubMed] [Google Scholar]
- 83.Chaudhari N., Talwar P., Parimisetty A., d’Hellencourt C.L., Ravanan P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014;8:1–15. doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schon E.A., Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [http://dx.doi.org/10.1016/j.mcn.2012.07.011]. [PMID: 22922446]. [DOI] [PubMed] [Google Scholar]
- 85.Hiramatsu N., Chiang W.C., Kurt T.D., Sigurdson C.J., Lin J.H. Multiple mechanisms of unfolded protein response-induced cell death. Am. J. Pathol. 2015;185(7):1800–1808. doi: 10.1016/j.ajpath.2015.03.009. [http://dx.doi.org/ 10.1016/j.ajpath.2015.03.009]. [PMID: 25956028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hetz C., Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014;15(4):233–249. doi: 10.1038/nrn3689. [http://dx.doi.org/10.1038/nrn3689]. [PMID: 24619348]. [DOI] [PubMed] [Google Scholar]
- 87.Sovolyova N., Healy S., Samali A., Logue S.E. Stressed to death - mechanisms of ER stress-induced cell death. Biol. Chem. 2014;395(1):1–13. doi: 10.1515/hsz-2013-0174. [http://dx.doi.org/10.1515/hsz-2013-0174]. [PMID: 24002662]. [DOI] [PubMed] [Google Scholar]
- 88.Safra M., Ben-Hamo S., Kenyon C., Henis-Korenblit S. The ire-1 ER stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J. Cell Sci. 2013;126(Pt 18):4136–4146. doi: 10.1242/jcs.123000. [http://dx.doi.org/10.1242/jcs.123000]. [PMID: 23843615]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halliday M., Mallucci G.R. Targeting the unfolded protein response in neurodegeneration: A new approach to therapy. 2014. http://dx.doi.org/10 [DOI] [PubMed]
- 90.Díaz-Villanueva J.F., Díaz-Molina R., García-González V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015;16(8):17193–17230. doi: 10.3390/ijms160817193. [http://dx.doi.org/10.3390/ijms160817193]. [PMID: 26225966]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Srivastava K.K., Kumar R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015;30(1):3–10. doi: 10.1007/s12291-014-0441-5. [http://dx.doi.org/ 10.1007/s12291-014-0441-5]. [PMID: 25646036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plácido A.I., Pereira C.M., Duarte A.I., Candeias E., Correia S.C., Santos R.X., Carvalho C., Cardoso S., Oliveira C.R., Moreira P.I. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842(9):1444–1453. doi: 10.1016/j.bbadis.2014.05.003. [http:// dx.doi.org/10.1016/j.bbadis.2014.05.003]. [PMID: 24832819]. [DOI] [PubMed] [Google Scholar]
- 93.Rivas A., Vidal R.L., Hetz C. Targeting the unfolded protein response for disease intervention. Expert Opin. Ther. Targets. 2015;19(9):1203–1218. doi: 10.1517/14728222.2015.1053869. [http://dx.doi.org/10.1517/14728222.2015. 1053869]. [PMID: 26166159]. [DOI] [PubMed] [Google Scholar]
- 94.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [http://dx.doi.org/10.1038/nature05292]. [PMID: 17051205]. [DOI] [PubMed] [Google Scholar]
- 95.Chaturvedi R.K., Flint Beal M. Mitochondrial diseases of the brain. Free Radic. Biol. Med. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [http://dx.doi.org/ 10.1016/j.freeradbiomed.2013.03.018]. [PMID: 23567191]. [DOI] [PubMed] [Google Scholar]
- 96.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER tubules mark sites of mitochondrial division. Science. 2011;334(6054):358–362. doi: 10.1126/science.1207385. [http://dx.doi.org/10. 1126/science.1207385]. [PMID: 21885730]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schon E.A., Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [http://dx.doi.org/10.1016/j.mcn.2012.07.011]. [PMID: 22922446]. [DOI] [PubMed] [Google Scholar]
- 98.Vance J.E. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim. Biophys. Acta. 2014;1841(4):595–609. doi: 10.1016/j.bbalip.2013.11.014. [http://dx.doi.org/10.1016/j.bbalip.2013.11.014]. [PMID: 24316057]. [DOI] [PubMed] [Google Scholar]
- 99.Choi I.Y., Lim J.H., Kim C., Song H.Y., Ju C., Kim W.K. 4-hydroxy-2(E)-Nonenal facilitates NMDA-induced neurotoxicity via triggering mitochondrial permeability transition pore opening and mitochondrial calcium overload. Exp. Neurobiol. 2013;22(3):200–207. doi: 10.5607/en.2013.22.3.200. [http://dx.doi.org/10.5607/en.2013.22.3.200]. [PMID: 24167414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [http:// dx.doi.org/10.1007/s00424-010-0809-1]. [PMID: 20229265]. [DOI] [PubMed] [Google Scholar]
- 101.Hazell A.S. Excitotoxic mechanisms in stroke: An update of concepts and treatment strategies. Neurochem. Int. 2007;50(7-8):941–953. doi: 10.1016/j.neuint.2007.04.026. [http://dx.doi.org/10.1016/j.neuint.2007.04.026]. [PMID: 17576023]. [DOI] [PubMed] [Google Scholar]
- 102.Mancuso R., Oliván S., Rando A., Casas C., Osta R., Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics. 2012;9(4):814–826. doi: 10.1007/s13311-012-0140-y. [http://dx.doi.org/10.1007/s13311-012-0140-y]. [PMID: 22935988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds A., Laurie C., Mosley R.L., Gendelman H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [http://dx.doi.org/10.1016/ S0074-7742(07)82016-2]. [PMID: 17678968]. [DOI] [PubMed] [Google Scholar]
- 104.Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [http://dx. doi.org/10.1038/nrneurol.2010.17]. [PMID: 20234358]. [DOI] [PubMed] [Google Scholar]
- 105.Mori T., Hayashi T., Hayashi E., Su T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8(10):1–13. doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klouz A., Saïd D.B., Ferchichi H., Kourda N., Ouanes L., Lakhal M., Tillement J.P., Morin D. Protection of cellular and mitochondrial functions against liver ischemia by N-benzyl-N'-(2-hydroxy-3,4-dimethoxybenzyl)-piperazine (BHDP), a sigma1 ligand. Eur. J. Pharmacol. 2008;578(2-3):292–299. doi: 10.1016/j.ejphar.2007.09.038. [http://dx.doi. org/10.1016/j.ejphar.2007.09.038]. [PMID: 17964567]. [DOI] [PubMed] [Google Scholar]
- 107.Tagashira H., Zhang C., Lu Y.M., Hasegawa H., Kanai H., Han F., Fukunaga K. Stimulation of σ1-receptor restores abnormal mitochondrial Ca2+ mobilization and ATP production following cardiac hypertrophy. Biochim. Biophys. Acta. 2013;1830(4):3082–3094. doi: 10.1016/j.bbagen.2012.12.029. [http://dx.doi.org/10.1016/j.bbagen.2012.12.029]. [PMID: 23298811]. [DOI] [PubMed] [Google Scholar]
- 108.Yang S., Bhardwaj A., Cheng J., Alkayed N.J., Hurn P.D., Kirsch J.R. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007;104(5):1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [http://dx.doi.org/10.1213/01.ane.0000260267.71185.73]. [PMID: 17456670]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A. ; De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R. ; Zecchini E., Pinton P. Ca2+ transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [http://dx.doi.org/10.1016/j.bbabio.2009.03.015]. [PMID: 19341702]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rousseaux C.G., Greene F.S. Sigma receptors [σRs]: biology in normal and diseased states. J. Recept. Signal Transduct. Res. 2016;36(4):327–388. doi: 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klette K.L., DeCoster M.A., Moreton J.E., Tortella F.C. Role of calcium in sigma-mediated neuroprotection in rat primary cortical neurons. Brain Res. 1995;704(1):31–41. doi: 10.1016/0006-8993(95)01103-x. [http://dx.doi.org/10. 1016/0006-8993(95)01103-X]. [PMID: 8750959]. [DOI] [PubMed] [Google Scholar]
- 112.Katnik C., Guerrero W.R., Pennypacker K.R., Herrera Y., Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J. Pharmacol. Exp. Ther. 2006;319(3):1355–1365. doi: 10.1124/jpet.106.107557. [http://dx. doi.org/10.1124/jpet.106.107557]. [PMID: 16988055]. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X.J., Liu L.L., Jiang S.X., Zhong Y.M., Yang X.L. Activation of the ζ receptor 1 suppresses NMDA responses in rat retinal ganglion cells. Neuroscience. 2011;177:12–22. doi: 10.1016/j.neuroscience.2010.12.064. [http://dx.doi.org/ 10.1016/j.neuroscience.2010.12.064]. [PMID: 21211548]. [DOI] [PubMed] [Google Scholar]
- 114.Ajmo C.T., Jr, Vernon D.O., Collier L., Pennypacker K.R., Cuevas J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr. Neurovasc. Res. 2006;3(2):89–98. doi: 10.2174/156720206776875849. [http://dx.doi.org/ 10.2174/156720206776875849]. [PMID: 16719792]. [DOI] [PubMed] [Google Scholar]
- 115.Peviani M., Salvaneschi E., Bontempi L., Petese A., Manzo A., Rossi D., Salmona M., Collina S., Bigini P., Curti D. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol. Dis. 2014;62:218–232. doi: 10.1016/j.nbd.2013.10.010. [http://dx.doi.org/10. 1016/j.nbd.2013.10.010]. [PMID: 24141020]. [DOI] [PubMed] [Google Scholar]
- 116.Francardo V., Bez F., Wieloch T., Nissbrandt H., Ruscher K., Cenci M.A. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137(Pt 7):1998–2014. doi: 10.1093/brain/awu107. [http://dx.doi.org/10.1093/brain/awu107]. [PMID: 24755275]. [DOI] [PubMed] [Google Scholar]
- 117.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77(1):43–51. doi: 10.1016/j.biopsych.2014.05.006. [http://dx.doi.org/10.1016/j.biopsych.2014.05.006]. [PMID: 24951455]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang Y., Zheng L., Halliday G., Dobson-Stone C., Wang Y., Tang H.D., Cao L., Deng Y.L., Wang G., Zhang Y.M., Wang J.H., Hallupp M., Kwok J., Chen S.D. Genetic polymorphisms in sigma-1 receptor and apolipoprotein E interact to influence the severity of Alzheimer’s disease. Curr. Alzheimer Res. 2011;8(7):765–770. doi: 10.2174/156720511797633232. [http://dx.doi.org/10.2174/156720511797633232]. [PMID: 21605063]. [DOI] [PubMed] [Google Scholar]
- 119.Hedskog L., Pinho C.M., Filadi R., Rönnbäck A., Hertwig L., Wiehager B., Larssen P., Gellhaar S., Sandebring A., Westerlund M., Graff C., Winblad B., Galter D., Behbahani H., Pizzo P., Glaser E., Ankarcrona M. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc. Natl. Acad. Sci. USA. 2013;110(19):7916–7921. doi: 10.1073/pnas.1300677110. [http://dx.doi.org/10.1073/pnas.1300677110]. [PMID: 23620518]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jansen K.L., Faull R.L., Storey P., Leslie R.A. Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer’s disease correlates with CA1 pyramidal cell loss. Brain Res. 1993;623(2):299–302. doi: 10.1016/0006-8993(93)91441-t. [http://dx.doi.org/10.1016/0006-8993(93)91441-T]. [PMID: 8221112]. [DOI] [PubMed] [Google Scholar]
- 121.Behensky A.A., Yasny I.E., Shuster A.M., Seredenin S.B., Petrov A.V., Cuevas J. Afobazole activation of σ-1 receptors modulates neuronal responses to amyloid-β25-35. J. Pharmacol. Exp. Ther. 2013;347(2):468–477. doi: 10.1124/jpet.113.208330. [http://dx.doi.org/10.1124/jpet.113. 208330]. [PMID: 24006338]. [DOI] [PubMed] [Google Scholar]
- 122.Marrazzo A., Caraci F., Salinaro E.T., Su T.P., Copani A., Ronsisvalle G. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16(11):1223–1226. doi: 10.1097/00001756-200508010-00018. [http://dx.doi.org/10.1097/00001756-200508010-00018]. [PMID: 16012353]. [DOI] [PubMed] [Google Scholar]
- 123.Fisher A., Bezprozvanny I., Wu L., Ryskamp D.A., Bar-Ner N., Natan N., Brandeis R., Elkon H., Nahum V., Gershonov E., LaFerla F.M., Medeiros R. AF710B, a novel M1/σ1 agonist with therapeutic efficacy in animal models of alzheimer’s disease. Neurodegener. Dis. 2016;16(1-2):95–110. doi: 10.1159/000440864. [http://dx.doi.org/ 10.1159/000440864]. [PMID: 26606130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lahmy V., Meunier J., Malmström S., Naert G., Givalois L., Kim S.H., Villard V., Vamvakides A., Maurice T. Blockade of Tau hyperphosphorylation and Aβ1−42 generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ1 receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38(9):1706–1723. doi: 10.1038/npp.2013.70. [http://dx.doi.org/10.1038/npp.2013.70]. [PMID: 23493042]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lahmy V., Long R., Morin D., Villard V., Maurice T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front. Cell. Neurosci. 2015;8:463. doi: 10.3389/fncel.2014.00463. [http://dx.doi.org/10.3389/fncel.2014. 00463]. [PMID: 25653589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maurice T. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behav. Brain Res. 2016;296:270–278. doi: 10.1016/j.bbr.2015.09.020. [http://dx.doi.org/10.1016/j.bbr.2015.09.020]. [PMID: 26386305]. [DOI] [PubMed] [Google Scholar]
- 127.Villard V., Espallergues J., Keller E., Alkam T., Nitta A., Yamada K., Nabeshima T., Vamvakides A., Maurice T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta(25-35)-induced toxicity in mice. Neuropsychopharmacology. 2009;34(6):1552–1566. doi: 10.1038/npp.2008.212. [http://dx.doi.org/10.1038/npp.2008.212]. [PMID: 19052542]. [DOI] [PubMed] [Google Scholar]
- 128.Meunier J., Ieni J., Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006;149(8):998–1012. doi: 10.1038/sj.bjp.0706927. [http://dx.doi. org/10.1038/sj.bjp.0706927]. [PMID: 17057756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin J., Sha S., Chen T., Wang C., Hong J., Jie P., Zhou R., Li L., Sokabe M., Chen L. Sigma-1 (σ1) receptor deficiency reduces β-amyloid(25-35)-induced hippocampal neuronal cell death and cognitive deficits through suppressing phosphorylation of the NMDA receptor NR2B. Neuropharmacology. 2015;89:215–224. doi: 10.1016/j.neuropharm.2014.09.027. [http://dx.doi.org/10.1016/j.neuropharm.2014.09.027]. [PMID: 25286118]. [DOI] [PubMed] [Google Scholar]
- 130.Sha S., Qu W.J., Li L., Lu Z.H., Chen L., Yu W.F., Chen L. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci. Ther. 2013;19(9):705–713. doi: 10.1111/cns.12129. [http://dx.doi.org/10. 1111/cns.12129]. [PMID: 23745740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mishina M., Ishiwata K., Ishii K., Kitamura S., Kimura Y., Kawamura K., Oda K., Sasaki T., Sakayori O., Hamamoto M., Kobayashi S., Katayama Y. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol. Scand. 2005;112(2):103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [http://dx.doi.org/10.1111/j.1600-0404.2005.00432.x]. [PMID: 16008536]. [DOI] [PubMed] [Google Scholar]
- 132.Mori T., Hayashi T., Su T.P. Compromising σ-1 receptors at the endoplasmic reticulum render cytotoxicity to physiologically relevant concentrations of dopamine in a nuclear factor-κB/Bcl-2-dependent mechanism: Potential relevance to Parkinson’s disease. J. Pharmacol. Exp. Ther. 2012;341(3):663–671. doi: 10.1124/jpet.111.190868. [http://dx.doi. org/10.1124/jpet.111.190868]. [PMID: 22399814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fukunaga K., Shinoda Y., Tagashira H. The role of SIGMAR1 gene mutation and mitochondrial dysfunction in amyotrophic lateral sclerosis. J. Pharmacol. Sci. 2015;127(1):36–41. doi: 10.1016/j.jphs.2014.12.012. [http://dx. doi.org/10.1016/j.jphs.2014.12.012]. [PMID: 25704016]. [DOI] [PubMed] [Google Scholar]
- 134.Watanabe S., Ilieva H., Tamada H., Nomura H., Komine O., Endo F., Jin S., Mancias P., Kiyama H., Yamanaka K. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 2016;8(12):1421–1437. doi: 10.15252/emmm.201606403. [http://dx.doi.org/10.15252/emmm. 201606403]. [PMID: 27821430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Al-Saif A., Al-Mohanna F., Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann. Neurol. 2011;70(6):913–919. doi: 10.1002/ana.22534. [http://dx.doi.org/10.1002/ana.22534]. [PMID: 21842496]. [DOI] [PubMed] [Google Scholar]
- 136.Mok K., Traynor B.J., Schymick J., Tienari P.J., Laaksovirta H., Peuralinna T., Myllykangas L., Chiò A., Shatunov A., Boeve B.F., Boxer A.L., DeJesus-Hernandez M., Mackenzie I.R., Waite A., Williams N., Morris H.R., Simón-Sánchez J., van Swieten J.C., Heutink P., Restagno G., Mora G., Morrison K.E., Shaw P.J., Rollinson P.S., Al-Chalabi A., Rademakers R., Pickering-Brown S., Orrell R.W., Nalls M.A., Hardy J. Chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol. Aging. 2012;33(1):209.e3–209.e8. doi: 10.1016/j.neurobiolaging.2011.08.005. [http://dx.doi.org/ 10.1016/j.neurobiolaging.2011.08.005]. [PMID: 21925771]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prause J., Goswami A., Katona I., Roos A., Schnizler M., Bushuven E., Dreier A., Buchkremer S., Johann S., Beyer C., Deschauer M., Troost D., Weis J. Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2013;22(8):1581–1600. doi: 10.1093/hmg/ddt008. [http://dx.doi.org/10.1093/hmg/ddt008]. [PMID: 23314020]. [DOI] [PubMed] [Google Scholar]
- 138.Hayashi T., Hayashi E., Fujimoto M., Sprong H., Su T.P. The lifetime of UDP-galactose:ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones. J. Biol. Chem. 2012;287(51):43156–43169. doi: 10.1074/jbc.M112.380444. [http://dx.doi.org/10.1074/jbc. M112.380444]. [PMID: 23105111]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mavlyutov T.A., Guo L.W., Epstein M.L., Ruoho A.E. Role of the sigma-1 receptor in amyotrophic lateral sclerosis (ALS). J. Pharmacol. Sci. 2015;127(1):10–16. doi: 10.1016/j.jphs.2014.12.013. [http://dx.doi.org/10. 1016/j.jphs.2014.12.013]. [PMID: 25704013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ono Y., Tanaka H., Takata M., Nagahara Y., Noda Y., Tsuruma K., Shimazawa M., Hozumi I., Hara H. SA4503, a sigma-1 receptor agonist, suppresses motor neuron damage in in vitro and in vivo amyotrophic lateral sclerosis models. Neurosci. Lett. 2014;559:174–178. doi: 10.1016/j.neulet.2013.12.005. [http://dx.doi.org/10.1016/j.neulet.2013.12.005]. [PMID: 24334165]. [DOI] [PubMed] [Google Scholar]
- 141.Hyrskyluoto A., Pulli I., Törnqvist K., Ho T.H., Korhonen L., Lindholm D. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: Involvement of calpastatin and the NF-κB pathway. Cell Death Dis. 2013;4:e646. doi: 10.1038/cddis.2013.170. [http://dx.doi.org/10.1038/cddis.2013.170]. [PMID: 23703391]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Z.Q., Kunimatsu M., Yang J.P., Ozaki Y., Sasaki M., Okamoto T. Proteolytic processing of nuclear factor kappa B by calpain in vitro. FEBS Lett. 1996;385(1-2):109–113. doi: 10.1016/0014-5793(96)00360-2. [http://dx.doi. org/10.1016/0014-5793(96)00360-2]. [PMID: 8641452]. [DOI] [PubMed] [Google Scholar]
- 143.Miki Y., Tanji K., Mori F., Wakabayashi K. Sigma-1 receptor is involved in degradation of intranuclear inclusions in a cellular model of Huntington’s disease. Neurobiol. Dis. 2015;74:25–31. doi: 10.1016/j.nbd.2014.11.005. [http://dx.doi.org/10.1016/j.nbd.2014.11.005]. [PMID: 25449906]. [DOI] [PubMed] [Google Scholar]
- 144.Ryskamp D., Wu J., Geva M., Kusko R., Grossman I., Hayden M., Bezprozvanny I. The sigma-1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease. 2017. [DOI] [PMC free article] [PubMed]
- 145.Geva M., Kusko R., Soares H., Fowler K.D., Birnberg T., Barash S., Wagner A.M., Fine T., Lysaght A., Weiner B., Cha Y., Kolitz S., Towfic F., Orbach A., Laufer R., Zeskind B., Grossman I., Hayden M.R. Pridopidine activates neuroprotective pathways impaired in Huntington disease. Hum. Mol. Genet. 2016;25(18):3975–3987. doi: 10.1093/hmg/ddw238. [http://dx.doi.org/10.1093/hmg/ddw238]. [PMID: 27466197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao J., Ha Y., Liou G.I., Gonsalvez G.B., Smith S.B., Bollinger K.E. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest. Ophthalmol. Vis. Sci. 2014;55(6):3375–3384. doi: 10.1167/iovs.13-12823. [http://dx.doi.org/10.1167/iovs.13-12823]. [PMID: 24812552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Opara J.A., Małecka E., Szczygiel J. Clinimetric measurement in traumatic brain injuries. J. Med. Life. 2014;7(2):124–127. [PMID: 25408714]. [PMC free article] [PubMed] [Google Scholar]
- 148.Aguzzi A., Barres B.A., Bennett M.L. Microglia: Scapegoat, saboteur, or something else? Science. 2013;339(6116):156–161. doi: 10.1126/science.1227901. [http://dx.doi.org/10.1126/science.1227901]. [PMID: 23307732]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gyoneva S., Ransohoff R.M. Inflammatory reaction after traumatic brain injury: Therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 2015;36(7):471–480. doi: 10.1016/j.tips.2015.04.003. [http://dx.doi.org/10.1016/j.tips.2015.04.003]. [PMID: 25979813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nat. Rev. Neurol. 2015;11(1):56–64. doi: 10.1038/nrneurol.2014.207. [http://dx.doi.org/ 10.1038/nrneurol.2014.207]. [PMID: 25385337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yuan Y., Zhu F., Pu Y., Wang D., Huang A., Hu X., Qin S., Sun X., Su Z., He C. Neuroprotective effects of nitidine against traumatic CNS injury via inhibiting microglia activation. Brain Behav. Immun. 2015;48:287–300. doi: 10.1016/j.bbi.2015.04.008. [http://dx.doi.org/10.1016/ j.bbi.2015.04.008]. [PMID: 25900440]. [DOI] [PubMed] [Google Scholar]
- 152.Festoff B.W., Ameenuddin S., Arnold P.M., Wong A., Santacruz K.S., Citron B.A. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J. Neurochem. 2006;97(5):1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [http:// dx.doi.org/10.1111/j.1471-4159.2006.03799.x]. [PMID: 16638021]. [DOI] [PubMed] [Google Scholar]
- 153.Moritz C., Berardi F., Abate C., Peri F. Live imaging reveals a new role for the sigma-1 (σ1) receptor in allowing microglia to leave brain injuries. Neurosci. Lett. 2015;591:13–18. doi: 10.1016/j.neulet.2015.02.004. [http://dx. doi.org/10.1016/j.neulet.2015.02.004]. [PMID: 25666889]. [DOI] [PubMed] [Google Scholar]
- 154.Dong H., Ma Y., Ren Z., Xu B., Zhang Y., Chen J., Yang B. Sigma-1 Receptor Modulates Neuroinflammation After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2016;36(5):639–645. doi: 10.1007/s10571-015-0244-0. [http://dx.doi.org/10.1007/s10571-015-0244-0]. [PMID: 26228028]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wegleiter K., Hermann M., Posod A., Wechselberger K., Stanika R.I., Obermair G.J., Kiechl-Kohlendorfer U., Urbanek M., Griesmaier E. The sigma-1 receptor agonist 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) protects against newborn excitotoxic brain injury by stabilizing the mitochondrial membrane potential in vitro and inhibiting microglial activation in vivo. Exp. Neurol. 2014;261:501–509. doi: 10.1016/j.expneurol.2014.07.022. [http://dx.doi.org/10.1016/j.expneurol.2014.07.022]. [PMID: 25111531]. [DOI] [PubMed] [Google Scholar]
- 156.Urfer R., Moebius H.J., Skoloudik D., Santamarina E., Sato W., Mita S., Muir K.W., Study C.S., Phase I.I. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke. 2014;45(11):3304–3310. doi: 10.1161/STROKEAHA.114.005835. [http://dx.doi.org/10.1161/STROKEAHA.114.005835]. [PMID: 25270629]. [DOI] [PubMed] [Google Scholar]
- 157.Zhao J., Mysona B.A., Qureshi A., Kim L., Fields T., Gonsalvez G.B., Smith S.B., Bollinger K.E. (+)-Pentazocine reduces NMDA-induced murine retinal ganglion cell death through a sigma R1-dependent mechanism. Invest. Ophthalmol. Vis. Sci. 2016;57(2):453–461. doi: 10.1167/iovs.15-18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tchedre K.T., Yorio T. sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest. Ophthalmol. Vis. Sci. 2008;49(6):2577–2588. doi: 10.1167/iovs.07-1101. [http://dx.doi.org/10.1167/iovs.07-1101]. [PMID: 18296662]. [DOI] [PubMed] [Google Scholar]
- 159.Christian S.T., Harrison R., Quayle E., Pagel J., Monti J. The in vitro identification of dimethyltryptamine (DMT) in mammalian brain and its characterization as a possible endogenous neuroregulatory agent. Biochem. Med. 1977;18(2):164–183. doi: 10.1016/0006-2944(77)90088-6. [http://dx. doi.org/10.1016/0006-2944(77)90088-6]. [PMID: 20877]. [DOI] [PubMed] [Google Scholar]
- 160.Hollister L.E. Some general thoughts about endogenous psychotogens. In: Usdin E., Hamburg D.A., Barchas J.D., editors. Neuroregulators and Psychiatric Disorders. New York, NY: Oxford University Press; 1977. pp. 550–556. [Google Scholar]
- 161.Barker S.A., McIlhenny E.H., Strassman R. A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010. Drug Test. Anal. 2012;4(7-8):617–635. doi: 10.1002/dta.422. [http://dx.doi.org/10.1002/dta.422]. [PMID: 22371425]. [DOI] [PubMed] [Google Scholar]
- 162.McKenna D.J. Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004;102(2):111–129. doi: 10.1016/j.pharmthera.2004.03.002. [http://dx.doi.org/10.1016/j.pharmthera. 2004.03.002]. [PMID: 15163593]. [DOI] [PubMed] [Google Scholar]
- 163.Szára S. DMT at fifty. Neuropsychopharmacol. Hung. 2007;9(4):201–205. [PMID: 18510265]. [PubMed] [Google Scholar]
- 164.Wallach J.V. Endogenous hallucinogens as ligands of the trace amine receptors: A possible role in sensory perception. Med. Hypotheses. 2009;72(1):91–94. doi: 10.1016/j.mehy.2008.07.052. [http://dx.doi.org/10.1016/j.mehy. 2008.07.052]. [PMID: 18805646]. [DOI] [PubMed] [Google Scholar]
- 165.Moreno J.L., Holloway T., Albizu L., Sealfon S.C., González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 2011;493(3):76–79. doi: 10.1016/j.neulet.2011.01.046. [http://dx.doi.org/10.1016/j.neulet.2011.01.046]. [PMID: 21276828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Callaway J.C., Raymon L.P., Hearn W.L., McKenna D.J., Grob C.S., Brito G.S., Mash D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996;20(6):492–497. doi: 10.1093/jat/20.6.492. [http://dx.doi.org/10.1093/jat/20.6.492]. [PMID: 8889686]. [DOI] [PubMed] [Google Scholar]
- 167.Su T.P., Hayashi T., Vaupel D.B. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci. Signal. 2009;2(61):1–7. doi: 10.1126/scisignal.261pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jarrott B., Williams S.J. Chronic brain inflammation: The neurochemical basis for drugs to reduce inflammation. Neurochem. Res. 2016;41(3):523–533. doi: 10.1007/s11064-015-1661-7. [http://dx.doi.org/10.1007/s11064-015-1661-7]. [PMID: 26177578]. [DOI] [PubMed] [Google Scholar]
- 169.Frecska E., Szabo A., Winkelman M.J., Luna L.E., McKenna D.J. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J. Neural Transm. (Vienna) 2013;120(9):1295–1303. doi: 10.1007/s00702-013-1024-y. [http://dx.doi.org/ 10.1007/s00702-013-1024-y]. [PMID: 23619992]. [DOI] [PubMed] [Google Scholar]
- 170.Ruscher K., Wieloch T. The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration. J. Pharmacol. Sci. 2015;127(1):30–35. doi: 10.1016/j.jphs.2014.11.011. [http://dx.doi.org/10.1016/j.jphs.2014.11.011]. [PMID: 25704015]. [DOI] [PubMed] [Google Scholar]
- 171.Szabo A., Kovacs A., Riba J., Djurovic S., Rajnavolgyi E., Frecska E. The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. 2016;10:1–11. doi: 10.3389/fnins.2016.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Smith R.L., Canton H., Barrett R.J., Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol. Biochem. Behav. 1998;61(3):323–330. doi: 10.1016/s0091-3057(98)00110-5. [http://dx.doi.org/10.1016/S0091-3057(98)00110-5]. [PMID: 9768567]. [DOI] [PubMed] [Google Scholar]
- 173.Su T.P., Hayashi T., Vaupel D.B. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci. Signal. 2009;2(61):pe12. doi: 10.1126/scisignal.261pe12. [http://dx.doi.org/10.1126/ scisignal.261pe12]. [PMID: 19278957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maurice T., Phan V.L., Urani A., Kamei H., Noda Y., Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: Pharmacological evidence and therapeutic opportunities. Jpn. J. Pharmacol. 1999;81(2):125–155. doi: 10.1254/jjp.81.125. [http://dx.doi.org/10.1254/jjp.81.125]. [PMID: 10591471]. [DOI] [PubMed] [Google Scholar]
- 175.Matsuno K., Nakazawa M., Okamoto K., Kawashima Y., Mita S. Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur. J. Pharmacol. 1996;306(1-3):271–279. doi: 10.1016/0014-2999(96)00201-4. [http:// dx.doi.org/10.1016/0014-2999(96)00201-4]. [PMID: 8813641]. [DOI] [PubMed] [Google Scholar]
- 176.Harukuni I., Bhardwaj A., Shaivitz A.B., DeVries A.C., London E.D., Hurn P.D., Traystman R.J., Kirsch J.R., Faraci F.M. sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine affords neuroprotection from focal ischemia with prolonged reperfusion. Stroke. 2000;31(4):976–982. doi: 10.1161/01.str.31.4.976. [http://dx.doi.org/10.1161/ 01.STR.31.4.976]. [PMID: 10754008]. [DOI] [PubMed] [Google Scholar]
- 177.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [http://dx.doi. org/10.1016/j.pneurobio.2012.09.001]. [PMID: 23044468]. [DOI] [PubMed] [Google Scholar]
- 178.Ishima T., Nishimura T., Iyo M., Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: Role of sigma-1 receptors and IP3 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(7):1656–1659. doi: 10.1016/j.pnpbp.2008.06.011. [http://dx.doi.org/10.1016/j.pnpbp.2008.06.011]. [PMID: 18647636]. [DOI] [PubMed] [Google Scholar]
- 179.Maurice T., Meunier J., Feng B., Ieni J., Monaghan D.T. Interaction with sigma(1) protein, but not N-methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J. Pharmacol. Exp. Ther. 2006;317(2):606–614. doi: 10.1124/jpet.105.097394. [http://dx.doi.org/ 10.1124/jpet.105.097394]. [PMID: 16397090]. [DOI] [PubMed] [Google Scholar]
- 180.Villard V., Meunier J., Chevallier N., Maurice T. Pharmacological interaction with the sigma1 (σ1)-receptor in the acute behavioral effects of antidepressants. J. Pharmacol. Sci. 2011;115(3):279–292. doi: 10.1254/jphs.10191fp. [http://dx.doi.org/10.1254/jphs.10191FP]. [PMID: 21427517]. [DOI] [PubMed] [Google Scholar]
- 181.Narita N., Hashimoto K., Tomitaka S., Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol. 1996;307(1):117–119. doi: 10.1016/0014-2999(96)00254-3. [http://dx.doi.org/10.1016/0014-2999(96)00254-3]. [PMID: 8831113]. [DOI] [PubMed] [Google Scholar]
- 182.Bhuiyan M.S., Tagashira H., Fukunaga K. Sigma-1 receptor stimulation with fluvoxamine activates Akt-eNOS signaling in the thoracic aorta of ovariectomized rats with abdominal aortic banding. Eur. J. Pharmacol. 2011;650(2-3):621–628. doi: 10.1016/j.ejphar.2010.10.055. [http://dx.doi. org/10.1016/j.ejphar.2010.10.055]. [PMID: 21044620]. [DOI] [PubMed] [Google Scholar]
- 183.Peeters M., Romieu P., Maurice T., Su T.P., Maloteaux J.M., Hermans E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci. 2004;19(8):2212–2220. doi: 10.1111/j.0953-816X.2004.03297.x. [http://dx.doi.org/10.1111/j.0953-816X.2004.03297.x]. [PMID: 15090047]. [DOI] [PubMed] [Google Scholar]
- 184.Ishima T., Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by ifenprodil: The role of sigma-1 and IP3 receptors. PLoS One. 2012;7(5):e37989. doi: 10.1371/journal.pone.0037989. [http:// dx.doi.org/10.1371/journal.pone.0037989]. [PMID: 22655093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McCann D.J., Weissman A.D., Su T.P. Sigma-1 and sigma-2 sites in rat brain: Comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17(3):182–189. doi: 10.1002/syn.890170307. [http://dx.doi.org/ 10.1002/syn.890170307]. [PMID: 7974201]. [DOI] [PubMed] [Google Scholar]
- 186.Vilner B.J., John C.S., Bowen W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55(2):408–413. [PMID: 7812973]. [PubMed] [Google Scholar]
- 187.Sharkey J., Glen K.A., Wolfe S., Kuhar M.J. Cocaine binding at sigma receptors. Eur. J. Pharmacol. 1988;149(1-2):171–174. doi: 10.1016/0014-2999(88)90058-1. [http:// dx.doi.org/10.1016/0014-2999(88)90058-1]. [PMID: 2840298]. [DOI] [PubMed] [Google Scholar]
- 188.Nguyen L., Robson M.J., Healy J.R., Scandinaro A.L., Matsumoto R.R. Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. PLoS One. 2014;9(2):e89985. doi: 10.1371/journal.pone.0089985. [http://dx.doi.org/10.1371/journal.pone.0089985]. [PMID: 24587167]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Matsumoto R.R., Bowen W.D., Tom M.A., Vo V.N., Truong D.D., De Costa B.R. Characterization of two novel sigma receptor ligands: Antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharmacol. 1995;280(3):301–310. doi: 10.1016/0014-2999(95)00208-3. [http://dx. doi.org/10.1016/0014-2999(95)00208-3]. [PMID: 8566098]. [DOI] [PubMed] [Google Scholar]
- 190.Cagnotto A., Bastone A., Mennini T. [3H](+)-pentazocine binding to rat brain sigma 1 receptors. Eur. J. Pharmacol. 1994;266(2):131–138. doi: 10.1016/0922-4106(94)90102-3. [http://dx.doi.org/10.1016/0922-4106(94)90102-3]. [PMID: 8157067]. [DOI] [PubMed] [Google Scholar]