Abstract
Objective
Generation of an immunosuppressive microenvironment may enable a persistent Human Papillomavirus (HPV) infection in the setting of an otherwise normal immune system. We hypothesized that expression of the T-lymphocyte co-inhibitory receptor Programmed Death 1 (PD-1) and its ligand PD-L1 would be increased in the Recurrent Respiratory Papillomatosis (RRP) microenvironment compared to normal controls.
Study Design
Case-control study
Methods
Formalin-fixed paraffin embedded (FFPE) respiratory papilloma and normal controls were obtained under IRB approval, stained for CD4, CD8, FoxP3, and PD-1 and scored by automated cell count. PD-L1 staining was scored by a blinded pathologist using an Adjusted Inflammation Score (AIS) that accounted for epithelial and immune infiltrate.
Results
39 RRP cases and 7 controls were studied. All immunologic markers demonstrated significantly increased staining in RRP specimens compared to normal controls (all p<0.01). PD-1 correlated with both CD4 (p<0.0001) and CD8 (p<0.001) cell counts. Epithelial staining for PD-L1 (68%) and PD-L1+ infiltrating immune cells (76%) were observed in the majority of papilloma samples. The strongest staining for PD-L1 was usually observed in the basal papilloma layer adjacent to the immunologic infiltrate in the vascular core. Disease severity inversely correlated with CD8 cell counts (p=0.01). A correlation between disease severity and other immunologic markers was not observed.
Conclusions
Most RRP specimens demonstrate PD-1 T-lymphocyte infiltration and PD-L1 expression on both papilloma and infiltrating immune cells. This study suggests that this checkpoint pathway may be contributing to local immunosuppression in RRP, and opens the door for clinical trials utilizing PD-blocking monoclonal antibodies.
Keywords: Recurrent Respiratory Papillomatosis, RRP, laryngeal papilloma, Human Papillomavirus, HPV, Programmed Death, PD-1, PD-L1
Introduction
Recurrent respiratory papillomatosis (RRP) is a chronic viral infection of the aerodigestive tract that causes a debilitating, chronic disease in both pediatric and adult populations1. Several lines of evidence highlight the importance of the immune system in controlling HPV infection and its associated neoplasms, including RRP2. Despite the fact that five percent of the population shows evidence of laryngeal HPV infection3, only a very small percentage develop RRP. Patients with RRP have a normally functioning systemic immune system by overall measures of immune function4, and do appear to mount a peripheral antibody response to the viral proteins5. However, the local cytokine and immune cell environment established by the papilloma is Th2-biased6, polarizing effector T-lymphocytes away from a Th1-type cytotoxic CD8+ T-cell response (CTL) that could clear virally infected cells.
One mechanism of CTL inhibition within papillomas is inadequate IL-2/IFN-γ expression and high IL-4/IL-10 expression that maintains maturation failure of CD8+ T cells 6. Cytokine barriers prevent ingress of CTLs7 and there is an enriched population of immune suppressive FoxP3+ T cells8. Expression of programmed cell death-1 (PD-1) and its ligand programmed cell death ligand-1 (PD-L1) is another hypothesized immunosuppressive pathway in RRP 8. PD-1 is a T cell receptor that when activated provides a negative feedback signal for T cell function9 and is a marker for CD8+ T cell exhaustion10. PD-L1 is more highly expressed in active papilloma than clinically normal epithelium in RRP patients, and a large population of PD-1+CD4+ T cells may function to stabilize the Treg population11. However the cell type(s) expressing PD-L1 and their location are not currently known in RRP, co-localization of PD-1 and PD-L1 has not been demonstrated, and there is no information on the relationship between PD-1 pathway component expression and clinical outcomes or patient demographics in RRP.
We therefore sought to identify PD-1 and PD-L1 expression in papilloma samples using immunohistochemistry, describe differences in their expression compared to normal controls, and correlate their expression to patient demographics and disease severity.
Materials and Methods
Case Materials
Formalin fixed paraffin-embedded (FFPE) papilloma tissue was obtained through the archives of Surgical Pathology at the Johns Hopkins Hospital according to IRB-approved protocols. Supraglottic mucosa collected under IRB approval from patients undergoing phonosurgery for benign vocal fold pathology served as normal controls, and all normal controls were non-smokers. Demographic and clinicopathologic data was obtained from patient electronic medical records. Disease severity was scored as a linear variable by taking the number of laryngeal subsites involved with papilloma12 divided by the number of days between surgeries, averaged over the previous five surgeries to minimize variability. Subsites affected by papilloma were extracted from operative notes and archived clinical images from clinical examinations prior to surgery. This score incorporates disease extent as well as recurrence, and replicates retrospectively the prospective severity scoring system of Abramson, et al13, which has been used for many studies of immune function in RRP.
Immunohistochemistry
The CD4, CD8, and FoxP3 immunohistochemistry (IHC) was performed by the Pathology laboratories of the Johns Hopkins Hospital. PD-1 and PD-L1 staining was performed manually, with tissue sections deparaffinized and heat-induced antigen retrieval performed in Tris-EDTA buffer. After blocking, the sections were incubated with PD-1 (315M-96: Cell Marque) or PD-L1 (SP142: Spring Bioscience) anti-human polyclonal antibodies followed by a secondary biotinylated anti-goat IgG (Jackson Immuno Research). For detection, ABC-HRP (Vector Laboratories) was used, and sections visualized with the substrate diaminobenzidine (DAB: Vector Laboratories), and counterstained with hematoxylin.
Immunohistochemistry Scoring
To minimize scoring variability and allow for quantitative analysis, markers with intracellular expression (CD4, CD8, FoxP3, and PD-1) were scored using automated scanning (Aperio Imagescop, Leica) to collect 10 random high power fields (HPF) per slide and count positive cells (Aperio Scanscope software). As PD-L1 exhibited a more complex staining pattern, including membranous staining, these were scored by a blinded pathologist (J.A.B.). PD-L1 staining was scored using an Adjusted Inflammation Score (AIS), which takes into account both membranous staining of tumor tissue as well as the degree of PD-L1 expression on infiltrating cells14. The final AIS score is obtained by multiplying the % of PD-L1 positive tumors cells (<5, 5, 10, 20, 30, etc) by the intensity of intratumoral inflammation including TILs and histiocytes (graded 0 to 3).
Statistical analysis
Sample size was estimated for dichotomous variables of staining intensity and disease severity with a Fisher’s exact test and a 2×2 contingency table. Using a two-sided alpha of 0.05 and beta of 0.2, and given equal distribution between severe and mild disease, we estimated it would require a sample size of 20 patients in each group to detect a 40% difference in expression of markers between the two groups, within the range previously report for PD-1 expression as assessed by IHC. Clinical demographics were summarized using descriptive statistics. Mean values of positive cells/HPF were compared using student’s T test. Correlations between disease severity and immunologic markers was performed by calculating Pearson correlation coefficients. Significance was attributed to a p < 05.
Results
Of the known cohort of 212 Johns Hopkins RRP patients, thirty nine RRP patients were selected for this study, and their demographic data is summarized in Table 1. These patients were selected based upon the availability of tissue for analysis, in addition to surgery and follow-up at Johns Hopkins Hospital that allowed for estimation of disease severity. Attention was also paid to maintain a balance between age, male/female patients, juvenile/adult onset, and disease severity to ensure our selected cases were representative of RRP without a priori assumptions about expression patterns.
Table 1.
Clinical Demographics
| Variable | Patients, n = 39 |
|---|---|
| Age (years) | |
| Mean (SD) | 36 (22) |
| Range | 3 – 84 |
| Gender | |
| Male (%) | 22 (56%) |
| Female (%) | 17 (44%) |
| Disease Onset | |
| Juvenile | 26 (67%) |
| Adult | 13 (33% |
| Disease Severity | |
| Mild/Moderate | 26 (67%) |
| Severe | 13 (33%) |
Abbreviations: SD – Standard Deviation
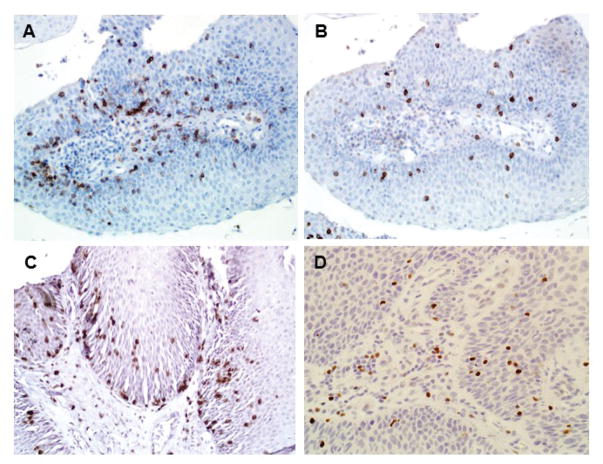
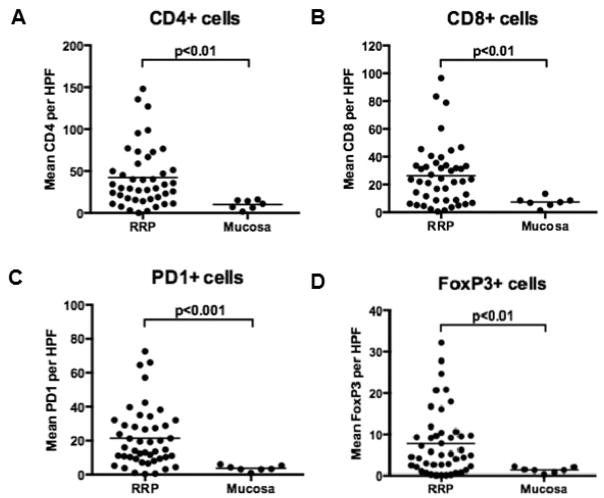
Representative photomicrographs for the IHC performed in this study are shown in Figure 1. Tumor infiltrating lymphocytes (TILs) were variably positive for CD4, CD8, FoxP3 and PD-1. Consistent with recruitment of inflammatory cells, TIL markers were variably positive within papillomas and cumulatively demonstrated significantly greater TIL marker positivity than normal squamous epithelium (Figure 2; p < 0.01 for all markers). There is a range of positivity between the RRP samples, with some showing little TIL marker staining and others showing robust TIL marker positivity. Using PD-1 as an example, papilloma samples ranged in positivity from 0 to 73 (mean = 21) PD-1+ cells/HPF compared to 1 to 6 (mean = 4) PD-1+ cells/HPF in normal controls. There was a strong relationship between CD4+ and CD8+ staining (r = 0.26; p < 0.001), CD4+ and PD-1+ (r = 0.37, p < 0.0001), CD8+ and PD-1+ (r = 0.27, p < 0.001), and CD4+ and FoxP3+ (r = 0.34, p < 0.001). There was no significant correlation between CD8+ and FoxP3+ staining (r = 0.02, p = 0.3).
Figure 1.
Tumor-infiltrating Lymphocytes in RRP. Presence of TILs confirmed in papilloma by immunohistochemistry for CD4 (A), CD8 (B), PD-1 (C), and FoxP3 (D). Magnification, 200x.
Figure 2.
Squamous epithelium in papilloma (n = 39) contains a significantly greater number of CD4 (A), CD8 (B), PD-1 (C), and FoxP3 (D) cells than normal laryngeal epithelium (n = 7). All p <0.01.
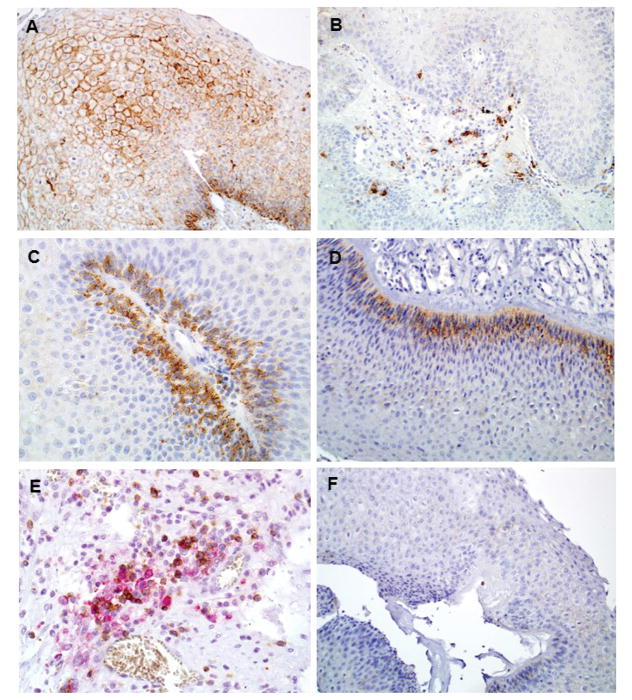
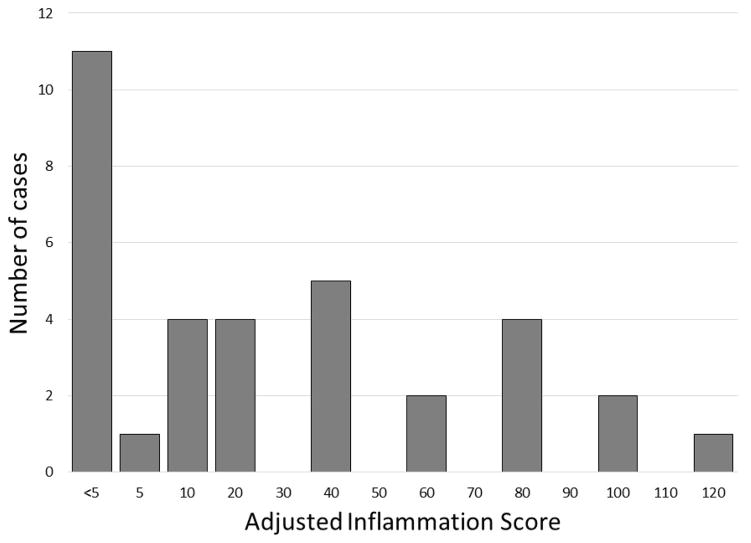
Thirty-four of the thirty-nine cases had adequate tissue available for PD-L1 staining, and representative photomicrographs are shown in Figure 3. Two main types of staining patterns were seen – membranous staining on the squamous papilloma itself (Figure 3A) and staining of infiltrating immune cells (Figure 3B). This staining pattern is similar to that observed in melanoma14 and head and neck cancer15. The strongest PD-L1 staining often occurred on the basal layer of the papilloma, directly adjacent to the vascular core that contains circulating immune cells (Figure 3C and 3D). Using dual-color IHC, co-localization of PD-1 and PD-L1 staining was seen in the vascular core of the papilloma (Figure 3E). Evaluated in a binary fashion as positive or negative staining, 68% (23/34) of papillomas exhibited some degree of positive epithelial cell staining, and 76% (26/34) demonstrated PD-L1+ infiltrating immune cells. Staining on less than 5% of papilloma cells was defined as negative (Figure 3F), to maintain consistency with previous studies14,16. No staining was seen on control samples. Papilloma PD-L1 positivity was further assessed via calculating of an Adjusted Inflammation Score (AIS) (Figure 4) that accounts for both epithelial and infiltrating immune cell staining. This scoring system quantifies PD-L1 positivity as a linear variable, and demonstrates the wide range of PD-L1 expression that exists within RRP samples.
Figure 3.
PD-L1 expression in RRP. Two main patterns of PD-L1 staining seen, either membranous staining of squamous papilloma itself (A), or mononuclear inflammatory cells (B). In multiple cases the basal layer of papilloma, adjacent to the vascular core, was the strongest site of membranous staining (C, D). Dual color IHC (E) demonstrates that PD-1 (brown) and PD-L1 (red) expression co-localizes in the vascular core of RRP. Background staining in cases with no PD-L1 expression was minimal (F). Magnification 200x.
Figure 4.
Histogram of Adjusted Inflammation Score for PD-L1 staining. This scoring scale incorporates both membranous staining of PD-L1 on the papilloma as well as the degree of PD-L1 positive infiltrating immune cells.
Finally, we correlated the disease severity score with TIL marker staining (Table 2). Consistent with others studies, there was a negative correlation between CD8 staining and disease severity (r = −0.35; p = 0.01)6. CD4, FoxP3, PD-1, PD-L1 staining and AIS did not correlate with disease severity.
Table 2.
Disease Severity Correlations
| Marker | Correlation with Disease Severity | p value |
|---|---|---|
| CD4 | 0.16 | 0.27 |
| CD8 | −0.35 | 0.01 |
| PD-1 | 0.15 | 0.30 |
| Fox P3 | −0.01 | 0.93 |
| PD-L1 | 0.18 | 0.26 |
| AIS | 0.10 | 0.49 |
Abbreviations: AIS – Adjusted Inflammation Score
Discussion
This study demonstrates at the protein level that the majority of RRP samples demonstrate infiltration of PD-1 positive immune cells and PD-L1 expression on both epithelial and infiltrating immune cells. Because anti-PD-1/PD-L1 monoclonal antibodies are now FDA-approved for selected advanced and metastatic malignancies including head and neck squamous cell carcinoma17,18, evidence suggesting that this pathway may be playing a role in local immune suppression within RRP samples has important translational research implications.
Our findings support the only previous study to demonstrate PD-1 expression in RRP samples8. Because we evaluated inflammatory markers by IHC retrospectively in a large sample of patients rather than by flow cytometry, dual staining was not performed to specifically characterize the PD-1+ TIL population. Nevertheless, the strong correlation between CD4+ and PD-1+ infiltrating immune cells very likely represents the same CD4+PD-1+ T-lymphocyte population previously described. Similarly, we observed correlation between CD4+ and FoxP3+ staining, consistent with the known enhancement of a CD4+FoxP3+ Treg population in papilloma compared to blood from the same patients8. A significant correlation between PD-1 and CD8 staining likely identifies an exhausted CD8+ T-lymphocyte population in RRP, often present in other solid tumors.8,19,20 The range of PD-1 positivity and variability amongst samples is interesting to note, including some samples which show no increased expression compared to normal controls. This suggests that the immunologic phenotype may vary considerably between patients, temporal changes may exist in expression patterns of PD-1 not captured on a single biopsy, or potential heterogeneity in the immune microenvironment even within a single patient’s papilloma are all possibilities for future investigation.
Furthermore, we demonstrate that a high percentage of papilloma samples exhibit PD-L1 expression on the squamous papilloma itself, often in the basal layer of the epithelium, adjacent to where interferon-producing immune cells would encounter papilloma cells. This same pattern of co-localization between PD-1+ immune cells and PD-L1+ epithelial and immune cells has been described in HPV-associated oropharyngeal squamous cell carcinoma, at the tumor-host interface21 as well in HPV-associated cervical cancer22 and suggests that PD-pathway-mediated adaptive immune resistance may indeed be playing a role in local immune suppression within the RRP tumor microenvironment23. Infiltrating immune cell types expressing PD-L1 in these RRP samples is poorly defined but may include both T-lymphocytes and antigen-presenting cells such as dendritic cells24.
The correlation between PD-1 and PD-L1 expression and clinical outcomes is complicated by the numerous cell populations that can express these markers. Acknowledging the limitations of a retrospective analysis of disease severity using averaged intersurgical interval and disease distribution, we did not observe a significant correlation between our scoring of disease severity and PD-1 or PD-L1 expression, although we did identify an inverse relationship between higher CD8+ staining and decreased disease severity, a finding that collaborates the work of Bonagura et al.6 The presence of CD8+ infiltrating immune cells, likely representing CD8+ T-lymphocytes, in the majority of RRP samples suggests the presence of one or mechanisms of suppression of T-lymphocyte function within these lesions. The strong correlation between CD8 and PD-1 staining suggests that a significant subset of RRP-infiltrating CD8+ T-lymphocytes may be susceptible to PD-mediated inhibition in the presence of PD-L1. In HPV-associated oropharyngeal squamous cell carcinoma, high PD-1 (CD4+PD-1+ and CD8+PD-1+) infiltration in the tumor was associated with improved overall survival23. The opposite effect is seen in a number of other diseases, such as renal cell carcinoma25 or nasopharyngeal cancer26, where PD-1 expression is associated with a poor outcome. Tim-3 co-expression, which in combination with PD-1 is a marker of T cell exhaustion, may differentiate between functionally exhausted or activated T-lymphocytes27 and could possibly clarify these discrepancies.
Taken together, these results provide evidence that the PD-1 and PD-L1 pathway may be playing a role in local immune suppression in RRP. The presence of PD-pathway receptors and ligands in the majority of analyzed samples provides a strong rationale for investigating the role of PD-1 or PD-L1 checkpoint inhibitors in patients with RRP not well controlled with standard of care surgical interventions. Separate clinical trials investigating the clinical effect of the PD-1 mAb Pembrolizumab and PD-L1 mAb Avelumab are currently underway, and given the high rates of PD-1 and PD-L1 expression in the RRP population, many patients may be eligible for these trials. Furthermore, as expression of PD-L1 has been shown to be a predictor of disease response to checkpoint inhibitor therapies in certain tumor types28, correlation between baseline or induced PD-L1 expression and clinical response to these checkpoint inhibition may define the RRP patient population most likely to benefit from these immunotherapies.
Conclusions
The majority of RRP specimens demonstrate PD-1 positive infiltrating immune cells and PD-L1 expression on both squamous papilloma and infiltrating immune cells. This suggests that the PD-immune checkpoint pathway may be contributing to local immunosuppression in RRP microenvironment, and provides a strong rationale for the execution of clinical trials utilizing PD-pathway blocking monoclonal antibodies in patients with aggressive RRP.
Acknowledgments
Financial Support for this study was provided by the American Society of Pediatric Otolaryngology Dustin Micah Harper RRP Research Grant (S. Best), the NIDCD Mentored Patient-Oriented Research Career Development Award 1K23DC014758 (S. Best) and the Intramural Research Program of the National Institutes of Health, NIDCD, project number ZIA-DC000087 (C. Allen). The authors thank Nae-Yuh Wang, PhD, for his assistance with statistical analysis.
Footnotes
Level of Evidence: NA
The authors have no conflict of interest to disclose.
Presented at the 138th Annual Meeting of the American Laryngological Association, San Diego, CA, USA; April 26–28, 2017
References
- 1.Gallagher TQ, Derkay CS. Recurrent respiratory papillomatosis: update 2008. Curr Opin Otolaryngol Head Neck Surg. 2008;16(6):536–542. doi: 10.1097/MOO.0b013e328316930e. [DOI] [PubMed] [Google Scholar]
- 2.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118(6–7):455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope. 1987;97(6):678–685. doi: 10.1288/00005537-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bonagura VR, Siegal FP, Abramson AL, et al. Enriched HLA-DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol. 1994;1(3):357–360. doi: 10.1128/cdli.1.3.357-360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnez W, Kashima HK, Leventhal B, et al. Antibody response to human papillomavirus (HPV) type 11 in children with juvenile-onset recurrent respiratory papillomatosis (RRP) Virology. 1992;188(1):384–387. doi: 10.1016/0042-6822(92)90770-p. [DOI] [PubMed] [Google Scholar]
- 6.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93(3):302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal DW, DeVoti JA, Steinberg BM, Abramson AL, Bonagura VR. T(H)2-like chemokine patterns correlate with disease severity in patients with recurrent respiratory papillomatosis. Mol Med. 2012;18:1338–1345. doi: 10.2119/molmed.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatam LJ, Devoti JA, Rosenthal DW, et al. Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD-1/PD-L1/L2 expression. Clin Cancer Res. 2012;18(7):1925–1935. doi: 10.1158/1078-0432.CCR-11-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 11.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derkay CS, Malis DJ, Zalzal G, Wiatrak BJ, Kashima HK, Coltrera MD. A staging system for assessing severity of disease and response to therapy in recurrent respiratory papillomatosis. Laryngoscope. 1998;108(6):935–937. doi: 10.1097/00005537-199806000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Abramson AL, Shikowitz MJ, Mullooly VM, Steinberg BM, Amella CA, Rothstein HR. Clinical effects of photodynamic therapy on recurrent laryngeal papillomas. Arch Otolaryngol Head Neck Surg. 1992;118(1):25–29. doi: 10.1001/archotol.1992.01880010029011. [DOI] [PubMed] [Google Scholar]
- 14.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourcade J, Sun Z, Benallaoua M, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severson JJ, Serracino HS, Mateescu V, et al. PD-1+Tim-3+ CD8+ T Lymphocytes Display Varied Degrees of Functional Exhaustion in Patients with Regionally Metastatic Differentiated Thyroid Cancer. Cancer Immunol Res. 2015;3(6):620–630. doi: 10.1158/2326-6066.CIR-14-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. 2016;29(7):753–763. doi: 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 24.Yang W, Song Y, Lu YL, Sun JZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Fang W, Qin T, et al. Co-expression of PD-1 and PD-L1 predicts poor outcome in nasopharyngeal carcinoma. Med Oncol. 2015;32(3):86. doi: 10.1007/s12032-015-0501-6. [DOI] [PubMed] [Google Scholar]
- 27.Granier C, Dariane C, Combe P, et al. Tim-3 expression on tumor-infiltrating PD-1+CD8+T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-0274. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]