Abstract
Bipolar disorder is characterized by a host of sleep-wake abnormalities that suggests that the reticular activating system (RAS) is involved in these symptoms. One of the signs of the disease is a decrease in high frequency gamma band activity, which accounts for a number of additional deficits. Bipolar disorder has also been found to overexpress neuronal calcium sensor protein 1 (NCS-1). Recent studies showed that elements in the RAS generate gamma band activity that is mediated by high threshold calcium (Ca2+) channels. This mini-review provides a description of recent findings on the role of Ca2+ and Ca2+ channels in bipolar disorder, emphasizing the involvement of arousal-related systems in the manifestation of many of the disease symptoms. This will hopefully bring attention to a much-needed area of research and provide novel avenues for therapeutic development.
Keywords: Arousal, Ca2+ channels, gamma oscillations, lithium, N-type, P/Q-type, cAMP/PKA, CaMKII
Graphical Abstract
Bipolar disorder has sleep-wake abnormalities, decreased gamma, and overexpressed NCS-1. The reticular activating system (RAS) generates gamma via Ca2+ channels, pointing to roles for RAS and Ca2+ in the disease.
Introduction
Bipolar disorder patients manifest a number of sleep-wake abnormalities that indicate that the reticular activating system (RAS) is involved in the sleep and arousal-related symptoms of the disease. One of the signs of the disease is a decrease in high frequency gamma band activity, which accounts for a number of additional deficits. Recent studies showed that elements in the RAS generate gamma band activity that is mediated by high threshold calcium (Ca2+) channels. This review provides a description of recent findings on the role of Ca2+, Ca2+ channels, and neuronal calcium sensor protein 1 (NCS-1) in bipolar disorder, emphasizing the involvement of arousal-related systems in the manifestation of many of the disease symptoms. This will hopefully bring attention to a much-needed area of research and provide novel avenues for therapeutic development.
A. Bipolar Disorder
A. 1. Symptoms
Bipolar disorder, originally called manic-depressive illness, is a highly heritable psychiatric disorder [1], characterized by recurrent episodes of elevated mood or mania and depression, which correspond to changes in activity or energy and are related to characteristic physical, cognitive, and behavioral symptoms [2]. The mean age of symptom onset is ~22 years of age [3,4]. Hypomania is a less severe form of mania, which is usually brief and lacking major social disturbances. Symptoms associated with the manic state are the defining feature of bipolar disorder, ranging from impaired judgment [5] to psychosis or a break with reality [6]. The symptoms involved in mania nearly always have a significant negative impact on function at work, interpersonal relationships, and leisure activities [7], which, if left untreated, can be long lasting and devastating [8]. Manic or depressive episode onset is often foreshadowed by sleep disturbances [9].
Depressive episodes are the most frequent clinical presentation nearly 40% of the time; when patients are symptomatic they are depressed [10]. Depression may display marked seasonality, occurring most often during winter months [11]. While depressed, bipolar disorder patients suffer from “inverse” neuro-vegetative symptoms (hypersomnia), weight gain, increased appetite, psychotic episodes [3], and cognitive impairments [12,13]. Further, depressive episodes may be marked by mood reactivity rather than simply feelings of sadness [3,14] and, in fact, symptoms associated with mania or hypermania may co-occur [15], giving rise to increased motor activity, rapid or pressured speech, and grandiose delusions [16].
Mixed states are considered to be high risk for suicidal behavior [17,18] and deliberate self-harm [19], while comorbid anxiety disorders occur more frequently in these states. Bipolar disorder patients also show comorbid psychiatric disorders such as personality disorders, attention deficit hyperactivity disorder (ADHD), and schizophrenia [2,20]. Substance misuse is evident in over a third of cases and is more likely with early onset of the disorder. There is a partial overlap with schizophrenic symptoms [21–23], with approximately 50% of patients exhibiting hallucinations, delusions, and/or disorganized thoughts during manic states [24–28], and there is also partial overlap in genetic basis across schizophrenia and bipolar disorder [29,30].
A. 2. Etiology
The phenotypic expression of bipolar disorder arises from both genetic and environmental influences. Bipolar disorder has a heritability of roughly 75% explained mainly by common variant alleles [31,32], which overlap with genetic components of schizophrenia [33]. First degree-relatives of those affected with bipolar disorder have nearly a ten-fold increased risk for developing the disorder compared to the general population [16]. Environmental factors such as physical or sexual abuse in childhood is almost twice as common in bipolar disorder patients and is associated with earlier onset, a higher rate of suicide attempts, and more co-occurring disorders [34,35]. Negative life events and stress, in the form of childhood adversity or highly conflicted families, is higher among adults with bipolar disorder [36].
A. 3. Electroencephalogram (EEG) and oscillations
Sleep-wake disturbances usually occur at the onset of a manic or depressive episode [37]. The patterns seen in the EEG of bipolar disorder patients include fragmented sleep, increased rapid eye movement (REM) sleep drive, decreased slow wave sleep (SWS), insomnia during the manic state and hypersomnia during the depressive state [38,39]. These patients show reduced sleep time and longer sleep onset during manic episodes, which include decreased REM sleep latency and increased REM sleep duration, while during depression they manifest insomnia or hypersomnia, which also includes decreased REM sleep latency and increased REM sleep density [37–41]. Two meta-analyses described the main sleep-wake symptoms in bipolar disorder as fragmented sleep (including increased sleep latency, waking after sleep onset, and overall sleep efficiency) [42,43]. Additionally, bipolar disorder patients display similar pathophysiological symptoms as seen in schizophrenic patients: dysregulation of blink reflexes [44], decreased habituation of the P50 potential in the paired stimulus paradigm [45.46], and an exaggerated startle response [47]. Taken together, these results strongly suggest that disturbances in the function of the reticular activating system (RAS) are involved in the arousal-related symptoms of bipolar disorder.
Significantly, aberrant gamma band activity, similar to that seen in schizophrenia, has been reported in bipolar disorder patients [48]. In the manic or mixed state, bipolar disorder patients display reduced auditory EEG synchronization in the beta/gamma band (20–50 Hz) during a click train paradigm [49]. Gamma-band activity is associated with cognitive and executive functions [50–53]. Gamma band oscillations are essential to information processing during states of arousal such as sensory perception, motor behavior, and memory formation [54]. Therefore, decreased gamma band activity may underlie many of the symptoms in this disorder such as sleep disturbances, emotional dysregulation, attention and memory deficits, and impairments in fine motor skills [55]. Furthermore, human postmortem studies reported increased expression of neuronal calcium sensor protein 1 (NCS-1) in the brains of some bipolar disorder and schizophrenic patients [56]. That is, over expression of NCS-1 is present in the same disorders that show disrupted gamma band activity [57]. Recent results described below show that gamma band activity related to arousal is mediated by Ca2+ channels. Since NCS-1 modulates Ca2+ channels, its over expression may be responsible for the decrease in gamma band activity present in at least some bipolar disorder patients.
A. 4. Treatment
Lithium (Li+) has been shown to effectively treat the mood disturbances seen in bipolar disorder, while limited by its side effects [58]. It has also been shown to have neuroprotective properties. Li+ appears to act by inhibiting the interaction between NCS-1 and inositol 1,4,5-triphosphate receptor protein (InsP3R) [59]. NCS-1 is known to amplify the Ca2+ signal through the enhancement of InsP3Rs [60], which are known to be present within the RAS [61]. In fact, this interaction could explain the hyperarousal of the waking system seen in this disorder and the ability of Li+ to restore the imbalance. For example, a common symptom seen during the manic state of bipolar disorder is insomnia [62], which has been proposed as an overactive waking system intruding into sleep time [63]. Insomnia seen in bipolar disorder could be a result of increased levels of NCS-1, which lead to increases in high-frequency activity and ultimately, hyperactivation of the waking system. Li+ may be able to alleviate the hyperarousal by downregulating the interaction between NCS-1 and InsP3R, thus decreasing high frequency activity. This theory is supported by evidence that Li+ treatment has been seen to increase SWS and reduce REM sleep [64–68], as well as increase REM sleep latency [69]. The down regulation by Li+ of NCS-1 interactions with InsP3R could reduce the abnormal high-frequency activity and restore proper rhythms to these systems.
B. Reticular Activating System (RAS)
B. 1. Sleep and waking
Sleep and waking are controlled by the reticular activating system (RAS). The pedunculopontine nucleus (PPN) is that part of the RAS that is active during waking and REM sleep, while the catecholaminergic cell groups (which are the locus coeruleus and raphe) are active during waking and SWS, but not REM sleep [70]. The PPN, locus coeruleus, and raphe nuclei are located in the posterior midbrain on either side of the ventral portion of the central gray. PPN neurons are known to oscillate at beta/gamma frequencies in vivo during waking and REM sleep, but not during SWS [71–76]. Brainstem transections anterior to the PPN prevented the expression of gamma frequencies in the EEG, while lesions posterior to the PPN allowed the manifestation of cortical gamma activity, and stimulation of the PPN led to gamma band frequencies on the cortical EEG [77–82]. That is, PPN activity is reflected in the cortical EEG, although partial lesions of the PPN do not significantly alter sleep and waking architecture [83]. This may be because there are other areas modulating sleep and waking homeostasis, including hypothalamic and basal forebrain regions. However, stimulation of hypothalamic and basal forebrain regions must be applied for much longer periods (10–20 sec) [84,85] compared to RAS stimulation (1–2 sec) to induce waking [63,79,84]. In addition, optogenetic studies have found that induction of waking by stimulation of orexin neurons, for example, is blocked by inactivation of the locus coeruleus in the RAS [84]. That is, the RAS may be the final output for the arousal induced by some of these more anterior modulatory regions [63].
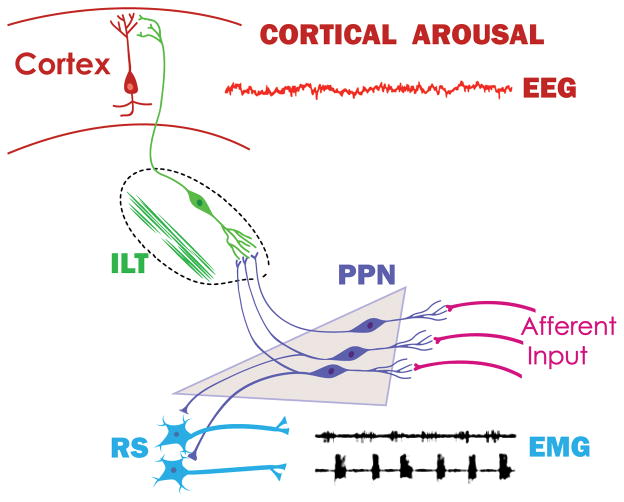
Figure 1 provides a diagram of the anatomical connections of the PPN, the cholinergic arm of the RAS, and how it drives the intralaminar thalamus to induce cortical arousal and simultaneously the reticulospinal system to modulate posture and locomotion.
Figure 1. Ascending and descending projections of the PPN.
The RAS is comprised of three specific nuclei, the noradrenergic locus coeruleus, the serotonergic raphe nucleus, and the PPN [63]. The PPN is active during waking and REM sleep, that is, during states of high frequency EEG activity in the gamma range. The locus coeruleus and raphe are active during waking and during slow wave sleep, but not during REM sleep. That is, the only part of the RAS active during arousal states (waking and REM sleep) is the PPN. The PPN is made up of cholinergic, glutamatergic, and GABAergic neurons. The PPN receives afferent sensory information from all of the sensory pathways in parallel to the primary sensory pathways (aff. input). The main projections of the PPN are to the intralaminar thalamus (ILT), especially the parafascicular nucleus, that in turn projects to the cortex. Activation of sensory afferents or stimulation of the PPN induces acetylcholine release in the thalamus, leading to cortical arousal manifested in high frequency activity in the electroencephalogram (EEG). Simultaneously, descending projections of the PPN to reticulospinal (RS) pathways modulate posture and locomotion, manifested by alternation of electromyograms (EMG) of agonist muscles in left and right legs. Not shown are descending projections of the PPN to the SubCD that modulate REM sleep [63,70].
B. 2. Gamma band activity in the RAS
Gamma band activity is a hallmark of the activated, high frequency states of waking and REM sleep, but it is not present during slow wave sleep. Gamma band activity has been reported in the PPN of the mouse in vitro [86], in the REM sleep-induction region of the rat to which the PPN projects [87], in the cat in vivo [81], and in the region of the PPN in humans during stepping [88]. A recent study described firing patterns of neurons in the region of the PPN in the monkey trained to walk on a treadmill [89]. PPN neurons during standing fired at low rates but then fired at beta/gamma frequencies during locomotion. Significantly, the same neurons also fired at low frequencies during sleep, but increased their firing rates to beta/gamma frequencies upon waking, showing that the same cells participate in both locomotion and arousal. Thus, there is ample evidence for gamma band activity in this region in vitro, in vivo, and across species, including man. The PPN, in which every cell manifests gamma band activity (see Section B.3), then becomes a gamma-making machine. We speculate that it is the continued activation of the RAS during waking that allows the maintenance of the background of gamma activity necessary to support the state capable of reliably assessing the world around us on a continuous basis - preconscious awareness [63,90].
As far as the cortex is concerned, the difference between gamma band activity during waking vs REM sleep appears to be a lack of coherence [91]. That is, brainstem driving of gamma band activity during waking carries with it coherence across distant cortical regions, while driving of gamma band activity during REM sleep does not include coherence across distant regions [91,92]. Also, carbachol-induced REM sleep with cataplexy is characterized by decreased gamma band coherence in the cortex [89 93]. These results suggest that, a) brainstem centers drive gamma band activity that is manifested in the cortical EEG, b) during waking, brainstem-thalamic projections include coherence across regions, and c) during REM sleep, which is controlled by the subcoeruleus dorsalis (SubCD) region (lesion of this region eliminates REM sleep, while injection of carbachol induces REM sleep signs), drives cortical EEG rhythms without coherence.
As far as locomotion is concerned, stimulation of the PPN at 40–60 Hz (gamma band) was found to elicit locomotion on a treadmill in the decerebrate animal (reviewed in [94], accounting for the effects of stimulating the so-called “mesencephalic locomotor region” (MLR) [95]. The PPN, however, as part of the RAS, is known to modulate posture and locomotion, so that the assignation of the PPN as part of the MLR is inaccurate. A nearby structure, the cuneiform nucleus, was also invoked as the MLR, but recent studies using deep brain stimulation (DBS) of the PPN for Parkinson’s disease were found to induce ameliorative effects on posture and locomotion [94], but, DBS of the cuneiform nucleus does not produce ameliorative effects on posture or locomotion [96]. It should be noted that the optimal frequencies of DBS of the human PPN were higher than 25 Hz, mostly in the 40–60 Hz range, that is, in the beta/gamma band [94,96]. Frequencies of PPN DBS in the 10–20 Hz range [97] or above 100 Hz simply are not effective in Parkinson’s patients [94,96]. DBS must be at the natural frequencies likely to activate these cells maximally, i.e. beta/gamma.
As far as sleep/wake cycles are concerned, PPN neurons increase firing rates during REM sleep (“REM-on”), or both waking and REM sleep (“Wake/REM-on”), and some cells fire only during waking (“Wake-on”) [72,75,76], suggestive of increased excitation only during activated states. These findings confirm the fact that PPN neurons are mainly active during states marked by high frequency EEG activity such as waking and REM sleep. Injections of glutamate into the PPN of the rat were found to increase both waking and REM sleep, but injections of NMDA increased only waking, while injections of kainic acid (KA) increased only REM sleep [98–101]. Thus, the two states appear to be independently activated by NMDA vs KA receptors. Moreover, the intracellular pathways mediating the two states are different. For example, the CaMKII activation inhibitor, KN-93, microinjected into the PPN of freely moving rats resulted in decreased waking but not REM sleep [102]. We showed that beta/gamma band oscillations in PPN neurons recorded in vitro were blocked by superfusion of KN-93 [103], suggesting that some cells manifest their oscillations via the CaMKII pathway. Moreover, the effects of the stimulant modafinil, which are mediated by increased electrical coupling, are modulated by the CaMKII pathway since KN-93 inhibits the action of modafinil [104–106].
Conversely, increased extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in the PPN is associated with maintenance of sleep via suppression of waking [107], while activation of intracellular protein kinase A (PKA) in the PPN instead contributed to REM sleep recovery following REM sleep deprivation [108]. Moreover, during REM sleep, pCREB activation in PPN cholinergic neurons was induced by REM sleep, and PPN intracellular PKA activation is mediated by a transcriptional cascade involving pCREB [109]. These findings suggest that waking in vivo may be modulated by the CaMKII pathway, while REM sleep may be modulated by the cAMP/PK pathway, in the PPN [90,103]. In addition, it appears that the cAMP-dependent pathway phosphorylates N-type calcium channels [110], while CaMKII regulates P/Q-type calcium channels [111]. Therefore, the presence of P/Q-type calcium channels is related to CaMKII and waking, while the presence of N-type calcium channels is more related to cAMP and REM sleep [90,103].
N- and P/Q-type calcium channel subtypes both are linked to rapid release of synaptic vesicles [112,113], but knockout models manifest markedly different phenotypes [114]. P/Q-type (Cav2.1) knockout animals have deficient gamma band activity in the EEG, abnormal sleep-wake states, ataxia, are prone to seizures (low frequency synchrony), and die by 3 weeks of age [115,116]. On the other hand, N-type (Cav2.2) knockout animals show few sleep-wake abnormalities but exhibit decreased nociceptive responses, and are otherwise normal [114]. While the two types of receptors are modulated by G-protein coupled receptors, they require different G-protein subunits [117]. Intracellularly, protein kinase C (PKC) enhances N-type channel activity but has no effect on P/Q-type channel function [118], while CaMKII was shown to modulate P/Q-type channel function [119]. That is, the two calcium channel subtypes are modulated by different intracellular pathways, N-type by the cAMP/PK pathway, and P/Q-type via the CaMKII pathway. The implication from all of these results is that there is a “waking” pathway mediated by CaMKII and P/Q-type channels, and a “REM sleep” pathway mediated by cAMP/PK and N-type channels.
B. 3. Calcium channels in the PPN
We found that all rat PPN cell types manifested beta/gamma oscillations in the presence of synaptic blockers and tetrodotoxin when the membrane potential was depolarized using current ramps [120]. In fact, intrinsic gamma oscillations are the only property present in every PPN neuron, whether of type I, II, or III, or transmitter type, cholinergic, glutamatergic or GABAergic, while cells around the PPN do not share that property [120]. More recent studies discovered that in some PPN cells (50%), the N-type calcium channel blocker ω-conotoxin-GVIA (ω-CgTx) reduced gamma oscillation amplitude, while subsequent addition of the P/Q-type blocker ω-agatoxin-IVA (ω-Aga) blocked the remaining oscillations. That is, these cells had both N- and P/Q-type channels. In other cells (20%), gamma oscillations were not affected by ω-CgTx, however, ω-Aga blocked the oscillations, suggesting that these cells had only P/Q-type channels. In the rest of the cells (30%), ω-Aga had no effect on gamma oscillations, while ω-CgTx blocked them, suggesting these had only N-type channels. Similar results were found during recordings of voltage-dependent calcium currents. These results confirm the presence of cells in the PPN that manifest gamma band oscillations through only N-type (30%), only P/Q-type (20%), and both N- and P/Q-type (50%) calcium channels [121,122].
This new cell type classification proposes that some PPN neurons fire only during REM sleep (“REM-on”, N-type only), only during waking (“Wake-on”, P/Q-type only), or during both waking and REM sleep (“Wake/REM-on”, N-type + P/Q-type) [121,122]. Armed with this information, we could now address the role of NCS-1 and lithium in the function of gamma band activity mediated by PPN neurons.
B. 4. NCS-1
Our findings demonstrated that intracellular NCS-1 can exert a concentration-dependent biphasic effect on gamma band oscillatory activity of PPN neurons [123]. That is, low concentrations promoted, while high concentrations of NCS-1 blocked, gamma band oscillations. Lower NCS-1 concentrations such as 1 μM enhanced oscillation frequency and amplitude only after 20 min, 10 μM had a very early (within 5–15 min) enhancing effect of oscillation amplitude, followed by an inhibitory effect, ultimately reducing the amplitude and frequency of gamma oscillations by 30 min. Moreover, low intracellular NCS-1 concentrations (both 0.5 and 1 μM) were able to reduce the amplitude of PPN calcium currents within 5–15 min. Strikingly, the time course of the calcium current block (~5–15 min) was similar to the 10 μM NCS-1 effect on oscillation amplitude (~5–15 min), but both of these effects were faster than the one observed for the enhancement of gamma band amplitude and gamma frequency of oscillations (>20 min), suggesting that multiple intracellular mechanisms may mediate the NCS-1 effects on PPN oscillations.
For example, NCS-1 appears to facilitate P/Q-type calcium channel currents [124], and also regulate InsP3 activity [60]. We assume that activation of muscarinic cholinergic receptors will trigger a G protein coupled pathway [125] that releases InsP3, which acts with cytoplasmic NCS-1 to bind to the InsP3 receptor in the endoplasmic reticulum to release intracellular calcium. Consequently, it is that release that potentiates calcium channel mediated currents. Much research is required to substantiate these suggestions.
B. 5. NCS-1 and intracellular [Ca2+] dynamics
Previous in vitro experiments determined that NCS-1 activated two calmodulin-dependent enzymes [126]. These authors found that 1 μM NCS-1 was maximally effective, while higher concentrations were less potent in activating these enzymes. This study also found that 1 μM NCS-1 potentiated nitric oxide synthase (NOS) 2–4 times, which was an effect that saturated at 10 μM NCS-1. They concluded that NCS-1 represents an ideal switch for neurons to respond rapidly to slight variations in internal calcium concentrations. We tested 1 μM and 10 μM NCS-1 for our initial studies as a result of these findings. Based on the human postmortem results showing over expression of NCS-1 in the brains of schizophrenic and bipolar disorder patients, but not in normal controls or major depression patients [56], we addressed the role of NCS-1 in the PPN. While the mean increase in NCS-1 was in the order of 50%, the individual levels between the lowest level in a control subject and the highest level in a schizophrenic or bipolar disorder patient was in the order of 5- to 8-fold (see Figure 2 in [56]). Therefore, we expected that, if 1 μM was an optimal concentration for the normal effect of NCS-1, then a 10-fold increase, or 10 μM NCS-1, would be an excessive level in keeping with significant overexpression. Our results indeed showed that NCS-1 at 1 μM promoted gamma band oscillations in PPN neurons (tripled amplitude, increased power at all frequencies), while 10 μM NCS-1 at first potentiated (quadrupled amplitude, no effect on frequencies), but soon significantly reduced, the oscillations. To some degree, the results on frequency and amplitude also suggest that there is an early effect that is different from the later effect, as we observed in the calcium current results. As far as gamma band frequencies are concerned, only 0.5 μM and 1 μM NCS-1 increased peak power, while higher concentrations (5 μM and 10 μM) failed to increase peak power at gamma band.
Calcium participates in a myriad of neuronal processes and its metabolism is tightly controlled. For example, neurite outgrowth does not occur if there is too little calcium and growth stops if there is too much, suggesting that a narrow window is essential for neurite outgrowth [127]. NCS-1 is known to modulate the optimal level of calcium necessary for neurite outgrowth [128]. Therefore, we speculate that NCS-1 at optimal concentrations will help maintain gamma band oscillations dependent on P/Q-type calcium channels, but too little or too much will lead to a decrease or interrupted pattern of gamma band activity. The use of ω -Aga blocked ramp-induced oscillations when using 1 μM NCS-1, suggesting that indeed P/Q-type calcium channels are responsible for these oscillations. Our previous studies found that P/Q-type calcium channels were essential for gamma oscillations in the PPN, while N-type channels were permissive [120]. Since NCS-1 is known to down regulate N-type calcium channels [129], we assume that these are the channels being blocked when input resistance increased.
Biphasic effects on intracellular [Ca2+] have been previously described to affect the time course of calcium channel inactivation [130]. In addition, two separate [Ca2+] transients (both from intracellular stores and present throughout membrane calcium channels) were observed when muscarinic, but not nicotinic, receptors were activated [131]. A significant increase in the formation of intracellular inositol phosphate (InsP) occurred during intracellular [Ca2+] transients. In cortical neurons, glutamate-dependent membrane depolarization affected intracellular [Ca2+] in a biphasic manner, which was found to modulate ERK1/2 signaling as well as cAMP responsive element binding protein (CREB) phosphorylation, and increased gene expression of brain-derived neurotrophic factor (BDNF) [132]. Furthermore, Na+ and Ca2+ influx triggered by membrane depolarization has been found to mediate a biphasic stimulatory effect followed by an inhibitory regulation of adenylyl cyclase in cerebellar granule cells [133]. The biphasic effects described here could be mediated by a rapid recruitment of calcium channels and its known downstream intracellular modulatory machinery (e.g. intracellular G-protein modulation [125]) by NCS-1 at 1 μM, while a more extreme [Ca2+]-buffering by 5–10 μM NCS-1 could interfere with downstream elements. Future experiments are needed to further confirm this hypothesis.
We assume that the reason why 1 μM NCS-1 increased oscillation amplitude was because an intracellular pathway was recruited that helps maintain oscillations for prolonged periods, perhaps through the mechanisms described above. The increase observed with 10 μM may indicate a response to a bolus of NCS-1 that soon (within 5–15 min), saturated the substrate available for its action and ultimately led to a decrease in oscillation amplitude (>30 min). The effects of 5 μM NCS-1 would be expected to cause changes both early and late, but do not, suggesting that this concentration rapidly saturates two different intracellular mechanisms.
B. 6. Lithium
Briefly, our results show that, a) Li+ caused a decrease in the amplitude and frequency of Ca2+ channel-mediated, high frequency oscillations in PPN neurons, b) Li+ significantly reduced the enhancing effect of NCS-1 on these oscillations, c) Li+ significantly down regulated Ca2+ channel activity regardless of channel type, while d) the presence of NCS-1 reduced the effect of Li+ on Ca2+ currents, and e) an intracellular mechanism involving voltage-independent activation of G-proteins mediated these effects [134].
Regardless of concentration used, Li+ reduced the amplitude and frequency of gamma band oscillations in PPN neurons. From a clinical standpoint, then, use of this agent would tend, in a normal individual manifesting high frequency oscillations during the alerted states of waking and REM sleep, to reduce arousal or REM sleep drive. Considering this, a common symptom present in bipolar disorder is insomnia [62], which is an overactive waking system intruding into sleep time [63]. Insomnia seen in bipolar disorder could be a result of increased levels of NCS-1 that lead to increased high-frequency activity and ultimately, hyperarousal of the waking system. These findings all point to increased arousal and REM sleep drive due to the hyperactivity of the PPN, yet it is overactive in a specific manner. The presence of increased responses to repetitive stimuli and exaggerated reflexes suggest that phasic responses to brief stimuli are dysregulated. Conversely, reduced and aberrant gamma band activity reflects the improper maintenance of gamma band activity on a tonic basis. The effects on tonic processes may disturb processes that rely on continuous gamma oscillations, resulting in dysfunctions in sensory perception, problem solving and memory.
Lithium may be able to alleviate the hyperarousal by downregulating the interaction between NCS-1 and InsP3, thus decreasing high frequency activity. This theory is supported by evidence that lithium treatment has been seen to increase slow wave sleep and reduce REM sleep [64–68], as well as increase REM sleep latency [69]. The downregulation by lithium of NCS-1 interactions with InsP3 reduces the abnormal high-frequency activity and restores proper rhythms to these systems. Therefore, we speculate that Li+ at optimal, therapeutic levels will restore proper rhythmicity by inhibiting the effects of NCS-1 InsP3 however, too much will result in dysfunctional activity.
In addition, Li+ blunted NCS-1-mediated enhancement of high frequency oscillations such as those previously described in PPN neurons [134]. This suggests that, in individuals over expressing NCS-1, Li+ would down regulate NCS-1 and perhaps restore gamma band oscillations. Although others have described how therapeutic levels of Li+ can inhibit the intracellular interaction between NCS-1 and InsP3R [55], our study identifies one mechanism of Li+ action as a key regulator of neuronal high frequency rhythmicity. Indeed, both gamma band oscillations and total Ca2+ current (ICa) steady-state reductions were only counteracted by increasing free intracellular NCS-1, suggesting some overlapping effects. To date, Li+ is the only agent that has been studied in an attempt to reduce the physiological effects of over expressed NCS-1. However, given the multiple roles of NCS-1 intracellularly, there may be other agents that can also modulate its effects of over expressed.
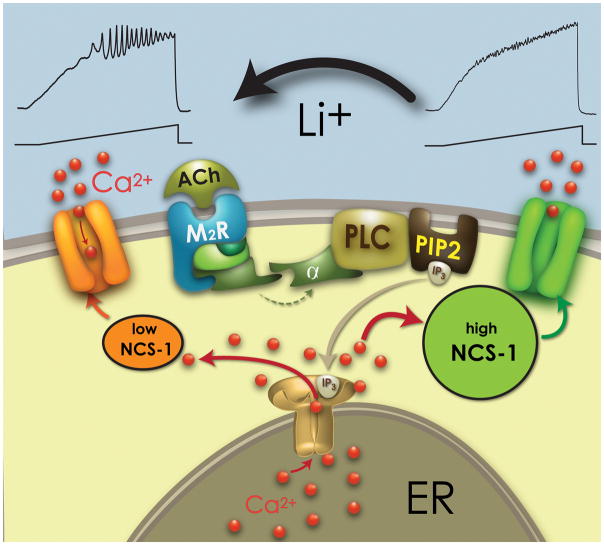
Figure 2 shows the proposed mechanism by which Li+ reduces the inhibitory effects of over expressed NCS-1 on gamma band oscillations, thereby restoring an appropriate level of gamma activity. As the results described above suggest, the concentration of Li+ is critical since too low would not sufficiently restore oscillations and too high would block oscillations directly via other intracellular pathways. Both P/Q and N-type Ca2+ channel modulation by G-proteins can involve either voltage-dependent or independent mechanisms [135–137]. In PPN neurons, M2 muscarinic receptors mediated a voltage-dependent inhibition of Ca2+ currents [125,138]. However, the present results showed a G-protein mediated voltage-independent inhibition that was mediated by Li+ that further reduced I2/I1 ICa ratio values. Such an effect on G-protein dependent Ca2+ channel modulation may be mediated by a Li+ blockade of Gα subunits GTPase activity [139]. Voltage-independent G-protein inhibition of P/Q-type channels has been found to require NCS-1 (without altering channel opening kinetics [140]), suggesting how increasing NCS-1 intracellular concentration was able to prevent the full magnitude of Li+ effects on “P/Q only” and “N+P/Q” cells, but only partially in “N only” PPN cells. Finally, I2/I1 ratio values were not significantly altered by NCS-1, suggesting that the Li+/NCS-1 intracellular pathway only affected the basal activity of Ca2+ channels, as previously described for inward rectifying channels [141].
Figure 2. Role of Li+ in restoring high threshold Ca2+ channel-mediated gamma band oscillations.
Representative cholinergic receptor (ACh) binding to a muscarinic receptor (M2R) which leads to G protein coupling to phospholipase C (PLC), that in turn cleaves phospholipid phosphatidylinositol biphosphate (PIP2) into inositol triphosphate (IP3). IP3 is released and binds to IP3 receptors in the endoplasmic reticulum (ER) to release calcium (Ca2+). NCS-1 at low levels (left side, orange sphere) promotes oscillations through Ca2+ channels (top left), while high levels of NCS-1 (right side, green sphere) inhibits gamma band oscillations. The presence of Li+ at low concentrations (top) reduces the effects of NCS-1 and is permissive to oscillations.
In summary, Li+ treatment would compensate for the effects of over expression of NCS-1 [56] and of the reduced gamma [48] observed in some bipolar disorder patients, perhaps by partially preventing the action of excessive NCS-1 and restoring intracellular pathways mediating normal gamma band activity. Figure 2 summarizes these results. While low levels of NCS-1 (left side) allow high threshold Ca2+ channels to mediate intrinsic membrane oscillations, high levels of NCS-1 (right side), in keeping with over expression, inhibit oscillations. The presence of optimal concentrations of Li+ (top) reduced the effects of NCS-1 over expression and were permissive to the manifestation of gamma band oscillations. We should also emphasize that the PPN is the source of the P50 potential [142], which undergoes decreased habituation in bipolar disorder [45,46], lending further support for the involvement of the PPN in this disease. The P50 potential is a state-dependent midlatency auditory evoked response that is present during waking and REM sleep, but absent during slow wave sleep [142]. That is, it is an arousal-related waveform and its habituation provides a measure of sensory gating. Thus, if the response to the second stimulus of a pair does not habituate normally, it is indicative of a lack of sensory afferent control, permitting excessive afferent signals from reaching cortical levels, as is present in bipolar disorder.
C. Clinical Implications
C.1. Ca2+ Homeostasis
It is hopefully clear how disturbances in RAS functions can lead to such symptoms of bipolar disorder as hypervigilance (increased phasic and/or tonic output from the RAS), impaired perception (due to lack of maintained gamma band activity), psychosis (again due to impaired perceptual processing), and cyclic alternation between mania and depression, which could be due to fluctuating levels of NCS-1 in at times decreasing, and at other times boosting, gamma oscillations. The links between these specific symptoms and Ca2+ homeostasis and NCS-1 remain to be investigated. However, it would seem that agents that can modulate NCS-1 expression may regulate its effects on Ca2+ channels and their effects on gamma oscillations.
Although the physiological roles of Ca2+ sensors in various cellular functions is becoming more clear, their involvement in the physiopathology of neuropsychiatric disorders remain less well characterized. On the other hand, the association between dysregulated Ca2+ signaling and neurodegenerative diseases is well established. A failure in Ca2+ homeostasis can produce high cytosolic free Ca2+ and result in neuronal excitotoxicity, culminating in cell death. Further, prolonged Ca2+ accumulation and sustained deviations from set homeostatic Ca2+ levels can rapidly result in cellular impairment. For example, overactivation of glutamate receptors impairs cellular Ca2+ homeostasis and the associated excessive rises in cytosolic Ca2+ leads to the activation of enzymes that degrade proteins, membranes and nucleic acids. In fact, abnormal glutamatergic neurotransmission has been implicated in the pathophysiology of bipolar disorder. Given the importance of Ca2+ sensors in cellular mechanisms, these proteins could provide the missing link between Ca2+ dysregulation and synaptic impairment in disease pathology. For example, we do not know how the various intracellular pathways that modulate the specific Ca2+ channels in question, or their actions on G proteins, are modulated or disturbed in bipolar disorder. Given the results described above, this remains a wide open area of potential research, with attractive possibilities for novel therapeutic interventions.
C.2. The Ca2+ Hypothesis
Ca2+ is central in the regulation of many different events over a wide range of time courses, from less than milliseconds to hours, days or even weeks [143]. More importantly, intracellular Ca2+ concentrations in the resting state are very low (10–100 nM) and strictly regulated in order to achieve a proper regulative function. The Ca2+ hypothesis posits that as the brain ages, the average level of intracellular free Ca2+ concentration increase, thus resulting in the disruption of intracellular Ca2+ regulation and homeostasis, which leads to the neuronal deterioration in Alzheimer’s as well as in normal aging brain [144–146]. It is likely that acquired neurodegenerative changes are involved in neuropsychiatric diseases as well, due to the evidence of dysregulation of Ca2+ signaling and homeostasis. Increased intracellular free Ca2+ can result in numerous catastrophic outcomes for neurons: production of superoxide radicals, with subsequent cellular toxicity; phospholipase C activation, which activates PKC, ultimately resulting in enhanced Ca2+ channel activity, InsP3R generation and additional Ca2+ release from internal stores; activation of CaMKII, which leads to further glutamate release; activation of endonucleases, which fragment DNA; production of nitric oxide synthase, which inhibits mitochondrial respiration, with citric acid cycle enzyme aconitase and DNA synthesis. It is clear that even small disruptions of Ca2+ homeostasis can have devastating consequences; what is more, sustained disruptions of Ca2+ homeostasis have devastating consequences. Given the importance of Ca2+ signaling in cellular mechanisms, disruptions in this regulation, whether local or global, severe or minor, transient or acute, all lead to altered neuronal function.
Even though great effort has gone into uncovering the molecular mechanisms involved in bipolar disorder, the true underlying molecular abnormalities remain elusive. Actually, the enormous amount of data that has been generated identifies a seemingly bewildering array of molecules involved in virtually all aspects of neuronal intracellular chemistry as potentially altered. This has led some researchers to postulate that these disorders are likely to have a multifactorial origin [147,148]. The commonality between these neurochemical abnormalities may lie in their association with potential modifications in Ca2+ signaling and homeostasis.
Neurochemical studies have shown that various neurotransmitters, including serotonin, dopamine, glutamate, and GABA, may be involved in the pathophysiology of both diseases [149–152], suggesting that they may be united by common underlying neurochemical abnormalities in synaptic signaling processes. Neuropathological studies have provided evidence that suggest dysfunction of glutamate- and GABA-mediated neurotransmission is involved in the pathophysiology of schizophrenia and bipolar disorder [153]. More recent evidence has uncovered a possible role of the hippocampus, linking it to memory deficits in the course of these diseases [154]. Ca2+-binding proteins involved in the G-protein InsP3R-Ca2+ signaling cascade [155] appear to play a role in the pathogenesis [156] and, accordingly, levels of NCS-1 show significant alterations in schizophrenic and bipolar disorder patients [56].
Conclusion
In summary, the role of calcium channels in the etiology of bipolar disorder has been established in at least some patients. Recent discoveries on the control of high threshold calcium channels provide a number of novel directions for the development of new therapeutic strategies.
Acknowledgments
Supported by NIH award P30 GM110702 from the IDeA program at NIGMS to the CTN, allowing the center to generate over $100 million in grant support for its members over the last 15 years. EGR would also like to express profound gratitude to all of the Federal funding agencies, especially NIH and NSF that have continuously funded his lab for the last 40 years.
Footnotes
Conflict of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- 1.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60(5):497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 2.Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ [Internet] 2012 Dec;:345. doi: 10.1136/bmj.e8508. [cited 2016 Nov 17] Available from: https://www.ncbi.nlm.nih.gov/pubmed/23271744. [DOI] [PubMed]
- 3.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 4.Zisook S, Lesser I, Steward JW, et al. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164(10):1539–46. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
- 5.Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania—a systematic review and meta-analysis. J Affect Disord. 2011;134(1–3):14–9. doi: 10.1016/j.jad.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Beentjes TA, Goossens PJ, Poslawsky IE. Caregiver burden in bipolar hypomania and mania: a systematic review. Perspect Psychiatr Care. 2012;48(4):187–97. doi: 10.1111/j.1744-6163.2012.00328.x. [DOI] [PubMed] [Google Scholar]
- 7.Elgie R, Morselli PL. Social functioning in bipolar patients: the perception and perspective of patients, relatives and advocacy organizations - a review. Bipolar Disord. 2007;9(1–2):144–57. doi: 10.1111/j.1399-5618.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Titmarsh S. Characteristics and duration of mania: implications for continuation treatment. Prog Neurol Psychiatry. 2013;17:26–7. [Google Scholar]
- 9.McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev. 2012;32(7):650–63. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–37. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord. 2014;169(Supp1):S12–6. doi: 10.1016/S0165-0327(14)70004-7. [DOI] [PubMed] [Google Scholar]
- 12.Borkowska A, Rybakowski JK. Neuropsychological frontal lobe tests indicate that bipolar depressed patients are more impaired than unipolar. Bipolar Disord. 2011;3(2):88–94. doi: 10.1034/j.1399-5618.2001.030207.x. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe J, Granholm E, Butters N, Sounders E, Janowsky D. Verbal memory deficits associated with major affective disorders: a comparison of unipolar and bipolar patients. J Affect Disord. 1987;13(1):83–92. doi: 10.1016/0165-0327(87)90077-2. [DOI] [PubMed] [Google Scholar]
- 14.Kolodziej ME, Griffin ML, Bender R, Weiss RD. Assessment of depressive symptom severity among patienst with co-occurring bipolar disorder and substance dependence. J Affect Disord. 2008;106(1–2):83–9. doi: 10.1016/j.jad.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SL, Morriss R, Scott J, et al. Depressive maniac symptoms are not opposite poles in bipolar disorder. Acta Psychiatr Scand. 2011;123(3):206–10. doi: 10.1111/j.1600-0447.2010.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164(1):331–43. doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muneer A. Treatment of the depressive phase of bipolar affective disorder: a review. J Pak Med Assoc. 2013;63(6):763–9. [PubMed] [Google Scholar]
- 18.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Antisocial personality disorder and borderline symptoms are differentially related to impulsivity and course of illness in bipolar disorder. J Affect Disord. 2013;148(2–3):384–90. doi: 10.1016/j.jad.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novick DM, Swartz HA, Frank E. Suicide attempts in bipolar I and bipolar II disorder: a review and meta-analysis of the evidence. Bipolar Disord. 2010;12(1):1–9. doi: 10.1111/j.1399-5618.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donfrancesco R, Miano S, Martines F, Ferrante L, Melegari MG, Masi G. Bipolar disorder co-morbidity in children with attention deficit hyperactivity disorder. Psychiat Res. 2011;186(2–3):333–7. doi: 10.1016/j.psychres.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowski KE, Cohen BM, Keshavan MS, Ongur D. Relationship of neuro-cognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res. 2011;133(1–3):212–7. doi: 10.1016/j.schres.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen C, Grossman LS, Harrow M, Bonner-Jackson A, Faull R. Diagnostic and prognostic significance of Schneiderian first-rank symptoms: a 20-year longitudinal study of schizophrenia and bipolar disorder. Compr Psychiatry. 2011;52(2):126–31. doi: 10.1016/j.comppsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson S, Sponheim SR. Dimensions underlying psychotic and manic symptomatology: extending normal-range personality traits to schizophrenia and bipolar spectra. Compr Psychiatry. 2014;55(8):1809–19. doi: 10.1016/j.comppsych.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Basso MR, Lowery N, Chormley C, et al. Neuropsychological impairment and psychosis in mania. J Clin Exp Neuropsychol. 2009;31(5):523–32. doi: 10.1080/13803390802304516. [DOI] [PubMed] [Google Scholar]
- 25.Coryell W, Leon AC, Turvey C, Akiskal HS, Meuller T, Edicott J. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J Affect Disord. 2001;67(1–3):79–88. doi: 10.1016/s0165-0327(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 26.Keck PE, Jr, McElroy SL, Havens JR, et al. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr Psychiatry. 2003;44(4):263–9. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- 27.Lindenmayer JP, Bossie CA, Kujawa M, Zhu Y, Canuso CM. Dimensions of psychosis in patients with bipolar mania as measured by the positive and negative syndrome scale. Psychopathology. 2008;41(4):264–70. doi: 10.1159/000128325. [DOI] [PubMed] [Google Scholar]
- 28.Ostergaard SD, Bertelsen A, Nielson J, Mors O, Petrides G. The association between psychotic mania, psychotic depression and mixed affective episodes among 14,529 patients with bipolar disorder. J Affect Disord. 2013;147(1–3):44–50. doi: 10.1016/j.jad.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014;40(3):504–15. doi: 10.1093/schbul/sbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari AK, Zai CC, Muller DJ, Kennedy JL. Genetics in schizophrenia: where are we and what next? Dialogues Clin Neurosci. 2010;12(3):289–303. doi: 10.31887/DCNS.2010.12.3/atiwari. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014;7:33–42. doi: 10.2147/TACG.S39297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60(5):497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan PF, Daly MJ, O’Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–51. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brietzke E, Kauer Sant’anna M, Jackowski A, et al. Impact of childhood stress on psychopathology. Rev Bras Psiquiatr. 2012;34(4):480–8. doi: 10.1016/j.rbp.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Etain B, Henry C, Bellivier F, Mathieu F, Leboyer M. Beyond Genetics: childhood affectibe trauma in bipolar disorder. Bipolar Disord. 2008;10(8):867–76. doi: 10.1111/j.1399-5618.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 36.Miklowitz DJ, Chang KD. Prevention of bipolar disorder in at-risk children: Theoretical assumptions and empirical foundations. Dev Psychopathol. 2008;20(3):881–97. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna BS, Eyler LT. Overlapping prefrontal systems involved in cognitive and emotional processing in euthymic bipolar disorder and following sleep deprivation: a review of functional neuroimaging studies. Clin Psychol Rev. 2012;32(7):650–63. doi: 10.1016/j.cpr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadrmas A, Winokur G. Manic depressive illness and EEG abnormalities. J Clin Psychiatry. 1979;40(7):306–7. [PubMed] [Google Scholar]
- 39.Kupfer DJ, Foster FG, Coble P, McPartland RJ, Ulrich RF. The application of EEG sleep for the differential diagnosis of affective disorders. Am J Psychiatry. 1978;135(1):69–74. doi: 10.1176/ajp.135.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan KA, Williams R. Hypersomnia: an overlooked, but not overestimated, sleep disturbance in bipolar disorder. Evid Based Mental Health. 2017;20:59. doi: 10.1136/eb-2016-102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. 2014;44:45–57. doi: 10.1016/j.neubiorev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. Sleep Med Rev. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Geoffroy PA, Scott J, Boudebesse C, Lajnef M, Henry C, Leboyer M, Belliver F, Etain B. Sleep in patients with remitted bipolar disorders; a meta-analysis of actigraphy studies. Acta Psychiat Scand. 2015;131:89–99. doi: 10.1111/acps.12367. [DOI] [PubMed] [Google Scholar]
- 44.Depue RA, Arbisi P, Krauss S, et al. Seasonal independence of low prolactin concentration and high spontaneous blink rates in unipolar and bipolar II seasonal affective disorder. Arch Gen Psychiatry. 1990;47(4):356–64. doi: 10.1001/archpsyc.1990.01810160056009. [DOI] [PubMed] [Google Scholar]
- 45.Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry. 2005;162(1):43–9. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 46.Schulze KK, Hall MH, McDonald C, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiatry. 2007;62(2):121–8. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50(6):418–24. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- 48.Ozerdem A, Guntenkin B, Atagun I, Turp B, Başar E. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132(3):325–32. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15(8):1369–72. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- 50.Eckhorn R, Bauer R, Jordan W, et al. Coherent oscillations: a mechanism of feature linking in the visual system? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–30. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 51.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86(5):1698–702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philips S, Takeda Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient than efficient visual search in human EEG. Int J Psychophysiol. 2009;73(3):350–54. doi: 10.1016/j.ijpsycho.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–74. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 54.Kann O, Papageorgiou IE, Draguhn A. (2014): Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34(8):1270–82. doi: 10.1038/jcbfm.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldberg JF, Chenappa KN. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11(Suppl 2):123–37. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 56.Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic P, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci U S A. 2003;100(1):313–7. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leicht G, Vauth S, Polomac N, et al. EEG-Informed fMRI Reveals a Disturbed Gamma-Band-Specific Network in Subjects at High Risk for Psychosis. Schizophr Bull. 2016;42(1):239–49. doi: 10.1093/schbul/sbv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown KM, Tracy DK. Lithium: the pharmacodynamics actions of the amazing ion. Ther Adv Psychopharmacol. 2012;3(3):163–76. doi: 10.1177/2045125312471963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlecker C, Boehmerle W, Jeromin A, et al. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of Li+ J Clin Invest. 2006;116(6):1668–74. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasri NN, Holmes AM, Bultynck G, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23(2):312–21. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodrigo J, Suburo AM, Bentura ML, et al. Distribution of the inositol 1,4,5-trisphosphate receptor, P400, in adult rat brain. J Comp Neurol. 1993;337(3):493–517. doi: 10.1002/cne.903370311. [DOI] [PubMed] [Google Scholar]
- 62.Berger M, van Calker D, Riemann D. Sleep and manipulations of the sleep-wake rhythm in depression. Acta Psychiatr Scand Suppl. 2003;418:83–91. doi: 10.1034/j.1600-0447.108.s418.17.x. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Rill E. Waking and the Reticular Activating System. New York: Academic Press; 2015. p. 330. [Google Scholar]
- 64.Friston KJ, Sharpley AL, Solomon RA, Cowen PJ. Lithium increases slow wave sleep: possible mediation by brain 5-HT2 receptors? Psychopharmacology (Berl) 1989;98(1):139–40. doi: 10.1007/BF00442020. [DOI] [PubMed] [Google Scholar]
- 65.Jones CA, Perez E, Amici R, et al. Lithium affects REM sleep occurrence, autonomic activity and brain second messengers in the rat. Behav Brain Res. 2008;187(2):254–61. doi: 10.1016/j.bbr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Ota SM, Moreira KD, Suchecki D, Oliveira MG, Tiba PA. Lithium prevents REM sleep deprivation-induced impairments on memory consolidation. Sleep. 2013;36(11):1677–84. doi: 10.5665/sleep.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi A, Jr, Lee-Chiong T. Medications and their effects on sleep. Med Clin N Am. 2004;88(3):751–66. doi: 10.1016/j.mcna.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Zamboni G, Perez E, Amici R, Jones CA, Parmeggiani PL. Control of REM sleep: an aspect of the regulation of physiological homeostasis. Arch Ital Biol. 1999;137(4):249–62. [PubMed] [Google Scholar]
- 69.Campbell SS, Gillin JC, Kripke DF, Janowsky DS, Risch SC. Lithium delays circadian phase of temperature and REM sleep in a bipolar depressive: a case report. Psychiatry Res. 1989;27(1):23–9. doi: 10.1016/0165-1781(89)90005-x. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Rill E. Sleep and arousal states: reticular activating system. In: Squire LR, Bloom F, Spitzer N, Gage F, Albright T, editors. New Encyclopedia of Neuroscience. Vol. 8. Oxford, England: Elsevier; 2009. pp. 137–43. [Google Scholar]
- 71.Boucetta S, Cissé Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34(13):4708–27. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70(4):611–21. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 73.Datta S, Siwek DF, Huang MP. Improvement of two-way active avoidance memory requires protein kinase a activation and brain-derived neurotrophic factor expression in the dorsal hippocampus. J Mol Neurosci. 2009;38(3):257–64. doi: 10.1007/s12031-009-9206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kayama Y, Ohta M, Jodo E. Firing of “possibly” cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569(2):210–20. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 75.Sakai K, el Mansari M, Jouvet M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527(2):213–23. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- 76.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10(8):2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol. 1949;1(4):475–86. [PubMed] [Google Scholar]
- 78.Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 79.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–73. [PubMed] [Google Scholar]
- 80.Steriade M, Constantinescu E, Apostol V. Correlations between alterations of the cortical transaminase activity and EEG patterns of sleep and wakefulness induced by brain-stem transections. Brain Res. 1969;13(1):177–80. doi: 10.1016/0006-8993(69)90152-8. [DOI] [PubMed] [Google Scholar]
- 81.Steriade M, Pare D, Datta S, Oakson G, Curro Dossi RC. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10(8):2560–79. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steriade M, Curro Dossi RC, Pare D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991;88(10):4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral ponto-mesencephalic tegmentum cholinergic area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458(2):285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 84.Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanisms of hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A [Internet] 2012 Sep;109(39):E2635–44. doi: 10.1073/pnas.1202526109. [cited 2016 Nov 17] Available from: https://www.ncbi.nlm.nih.gov//pmc/articles/PMC3465396/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han Y, Shi Y, Xi W, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24(6):693–8. doi: 10.1016/j.cub.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Ishibashi M, Gumenchuk I, Kang B, et al. Orexin Receptor Activation Generates Gamma Band Input to Cholinergic and Serotonergic Arousal System Neurons and Drives an Intrinsic Ca(2+)-Dependent Resonance in LDT and PPT Cholinergic Neurons. Front Neurol [Internet] 2015 Jun;6:120. doi: 10.3389/fneur.2015.00120. [cited 2016 Nov 17] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4451588/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electro-physiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143(3):739–55. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraix V, Bastin J, David O, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS One [Internet] 2013 Dec;8(12):e83919. doi: 10.1371/journal.pone.0083919. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3875496/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goetz L, Piallat B, Bhattacharjee M, et al. The primate pedunculopontine nucleus region: towards a dual role in locomotion and waking state. J Neural Transm (Vienna) 2016;123(7):667–78. doi: 10.1007/s00702-016-1577-7. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17(3):227–38. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castro S, Falconi A, Chase M, Torterolo P. Coherent neocortical 40-Hz oscillations are not present during REM sleep. Eur J Neurosci. 2013;37(8):1330–9. doi: 10.1111/ejn.12143. [DOI] [PubMed] [Google Scholar]
- 92.Cavelli M, Castro S, Schwartzkopf N, Chase M, Falconi A, Torterolo P. Coherent cortical oscillations decrease during REM sleep in the rat. Behav Brain Res. 2015;281:318–25. doi: 10.1016/j.bbr.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 93.Torterolo P, Castro-Zaballa S, Cavelli M, Chase M, Falconi A. Neocortical 40 Hz oscillations during carbachol-induced rapid eye movement sleep and cataplexy. Eur J Neurosci. 2016;43(4):580–9. doi: 10.1111/ejn.13151. [DOI] [PubMed] [Google Scholar]
- 94.Garcia-Rill E, Hyde J, Kezunovic N, Urbano FJ, Petersen E. The physiology of the pedunculopontine nucleus- implications for deep brain stimulation. J Neural Transm (Vienna) 2014;122(2):225–35. doi: 10.1007/s00702-014-1243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11(4):659–66. [PubMed] [Google Scholar]
- 96.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130(Pt 6):1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- 97.Noga BR, Sanchez FJ, Villamil LM, O’Toole C, Kasicki S, Olszewski M, Cabaj AM, Majczynski H, Stawinska U, Jordan LM. Mesencephalic locomotor region during voluntary locomotion. Frontiers Neural Circ. 2017;11:34. doi: 10.3389/fncir.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87(4):1790–8. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 99.Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77(6):2975–88. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- 100.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopotine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001;66(1):109–16. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 101.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rate. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):752–9. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 102.Datta S, O’Malley MW, Patterson EH. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J Neurosci. 2011;31(47):17007–16. doi: 10.1523/JNEUROSCI.3981-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Rill E, Kezunovic N, D’Onofrio S, et al. Gamma band activity in the RAS- intracellular mechanisms. Exptl Brain Res. 2014;232(5):1509–22. doi: 10.1007/s00221-013-3794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30(11):1405–14. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S. Arousal, motor control, and Parkinson’s disease. Transl Neurosci [Internet] 2015 Oct;6(1):198–207. doi: 10.1515/tnsci-2015-0021. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936629/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Urbano FJ, Leznik E, Llinás R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104(30):12554–9. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Desarnaud F, Macone BW, Datta S. Activation of extracellular signal-regulated kinase signaling in the pedunculopontine tegmental cells is involved in the maintenance of sleep in rats. J Neurochem. 2011;116(4):577–87. doi: 10.1111/j.1471-4159.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Datta S, Desarnaud F. Protein kinase A in the pedunculopontine tegmental nucleus of rat contributes to regulation of rapid eye movement sleep. J Neurosci. 2010;30(37):12263–73. doi: 10.1523/JNEUROSCI.1563-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Datta S. Regulation of neuronal activities within REM sleep-sign generators. Sleep. 2009;32(9):1113–4. doi: 10.1093/sleep/32.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic Ca2+ channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J. 1995;14(13):3036–44. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jenkins MA, Christel CJ, Jiao X, et al. Ca2+-dependent facilitation of Cav1. 3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30(15):5125–35. doi: 10.1523/JNEUROSCI.4367-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568(Pt 1):199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reid MS, Prichep L, O’Leary S, Ciplet D, Tom ML, Howard B. Quantitative electroencephalographic studies of cue-induced cocaine craving. Clin Electroencephalogr. 2003;34(3):110–23. doi: 10.1177/155005940303400305. [DOI] [PubMed] [Google Scholar]
- 114.Pietrobon D. Function and dysfunction of synaptic calcium channels: insights from mouse models. Curr Opin Neurobiol. 2005;15(3):257–65. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Jun K, Piedras-Renteria ES, Smith SM, et al. Albation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha (1A)-subunit. Proc Natl Acad Sci U S A. 1999;96(26):15245–50. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Llinás RR, Choi S, Urbano FJ, Shin HS. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci U S A. 2007;104(45):17819–24. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agler HL, Evans J, Colecraft HM, Yue DT. Custom distinctions in the interaction of G-protein b subunits with N-type (Cav2.2) versus P/Q-type (Cav2. 1) Ca2+ channels. J Gen Physiol. 2003;121(6):495–510. doi: 10.1085/jgp.200208770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stea A, Soomg TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal Ca2+ channels. Neuron. 1995;15(4):929–40. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 119.Jiang X, Lautermilch NJ, Watari H, et al. Modulation of Cav2.1 channels by Ca+/calmodulin-dependent kinase II bound to the C-terminal domain. Proc Nat Acad Sci U S A. 2008;105(1):341–6. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kezunovic N, Urbano FJ, Simon C, Hyde J, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34(3):404–15. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luster B, D’Onofrio S, Urbano F, Garcia-Rill E. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep [Internet] 2015 Jun;3(6):e12431. doi: 10.14814/phy2.12431. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4510632/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luster B, Urbano FJ, Garcia-Rill E. Intracellular mechanisms modulating gamma band activity in the pedunculopontine nucleus (PPN) Physiol Rep [Internet] 2016 Jun;4(12):e12787. doi: 10.14814/phy2.12787. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923228/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.D’Onofrio S, Kezunovic N, Hyde JR, et al. Modulation of gamma oscillations in the pedunculopontine nucleus (PPN) by neuronal calcium sensor protein-1 (NCS-1): relevance to schizophrenia and bipolar disorder. J Neurophysiol. 2015;113(3):709–19. doi: 10.1152/jn.00828.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295(5563):2276–79. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 125.Kezunovic N, Hyde J, Goitia B, Bisagno V, Urbano FJ, Garcia-Rill E. Muscarinic modulation of high frequency activity in the pedunculopontine nucleus (PPN) Front Neurol [Internet] 2013 Nov;4:176. doi: 10.3389/fneur.2013.00176. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3818577/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schaad NC, De Castro E, Nef S, et al. Direct modulation of calmodulin targets by the neuronal calcium sensor NCS-1. Proc Natl Acad Sci U S A. 1996;93(17):9253–8. doi: 10.1073/pnas.93.17.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11(4):891–9. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hui K, Fei GH, Saab BJ, Su J, Roder JC, Feng ZP. Neuronal calcium sensor-1 modulation of optimal calcium for neurite outgrowth. Development. 2007;134(24):4479–89. doi: 10.1242/dev.008979. [DOI] [PubMed] [Google Scholar]
- 129.Handley MT, Lian LY, Haynes LP, Burgoyne RD. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PLoS One [Internet] 2010 May;5(5):e10534. doi: 10.1371/journal.pone.0010534. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2866544/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cox DH, Dunlap K. Inactivation of N-type calcium current in chick sensory neurons: calcium and voltage dependence. J Gen Physiol. 1994;104(2):311–36. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Forsythe ID, Lambert DG, Nahorski SR, Lindsdell P. Elevation of cytosolic calcium by cholinoceptor agonists in SH-SY5Y human neuroblastoma cells: estimation of the contribution of voltage-dependent currents. Br J Pharmacol. 1992;107(1):207–14. doi: 10.1111/j.1476-5381.1992.tb14488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dravid SM, Baden DG, Murray TF. Brevetoxin activation of voltage-gated sodium channels regulates Ca dynamics and ERK1/2 phosphorylation in murine neocortical neurons. J Neurochem. 2004;89(3):739–49. doi: 10.1111/j.1471-4159.2004.02407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cooper DM, Schell MJ, Thorn P, Irvine RF. Regulation of adenylyl cyclase by membrane potential. J Biol Chem. 1998;273(42):27703–7. doi: 10.1074/jbc.273.42.27703. [DOI] [PubMed] [Google Scholar]
- 134.D’Onofrio S, Urbano FJ, Mesias E, Garcia-Rill E. Lithium decreases the effects of neuronal calcium sensor protein 1 in pedunculopontine neurons. Physiol Rep [Internet] 2016;4(6):e12740. doi: 10.14814/phy2.12740. [cited 2016 Nov 16] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4814880/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380(6571):258–62. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 136.Kammermeier PJ, Ruiz-Velasco V, Ikeda SR. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Galpha q/11 and Gbeta gamma. J Neurosci. 2000;20(15):5623–9. doi: 10.1523/JNEUROSCI.20-15-05623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuo CC, Bean BP. G-protein modulation of ion permeation through N-type calcium channels. Nature. 1993;365(6443):258–62. doi: 10.1038/365258a0. [DOI] [PubMed] [Google Scholar]
- 138.Beck P, Mahaffey S, Urbano FJ, Garcia-Rill E. Role of G-proteins in the effects of leptin on pedunculopontine nucleus (PPN) J Neurochem. 2013;126(6):705–14. doi: 10.1111/jnc.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Avissar S, Schreiber G, Danon A, Belmaker RH. Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature. 1988;331(6155):440–2. doi: 10.1038/331440a0. [DOI] [PubMed] [Google Scholar]
- 140.Weiss JL, Burgoyne RD. Voltage-independent inhibition of P/Q-type Ca2+ channels in adrenal chromaffin cells via a neuronal Ca2+ sensor-1-dependent pathway involves Src family tyrosine kinase. J Biol Chem. 2001;276(48):44804–11. doi: 10.1074/jbc.M103262200. [DOI] [PubMed] [Google Scholar]
- 141.Farhy Tselnicker I, Tsemakhovich V, Rishal I, Kahanovitch U, Dessauer CW, Dascal N. Dual regulation of G proteins and the G-protein-activated K+ channels by lithium. Proc Natl Acad Sci U S A. 2015;111(13):5018–23. doi: 10.1073/pnas.1316425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garcia-Rill E, Skinner RD. The sleep state-dependent P50 midlatency auditory evoked potential. In: Lee-Chiong TL, Carskadon MA, Sateia MJ, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 697–704. [Google Scholar]
- 143.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40(2):331–46. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 144.Disterhoft JF, Thompson LT, Weiss C, et al. The calcium hypothesis for Alzheimer’s disease: Insights from animal and human studies. Neurosci Res Commun. 1995;17(2):121–31. [Google Scholar]
- 145.Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano) 1989;1(1):17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 146.Landfield PW. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging. 1987;8(4):346–7. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- 147.Goodman AB, Pardee AB. Meeting report; “Molecular neurobiological mechanisms in schizophrenia: seeking a synthesis,” April 11–14, 1999. Biol Psychiatry. 2000;48(3):173–83. doi: 10.1016/s0006-3223(00)00904-5. [DOI] [PubMed] [Google Scholar]
- 148.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296(5568):692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 149.Austin J. Schizophrenia: an update and review. J Genet Couns. 2005;14(5):329–40. doi: 10.1007/s10897-005-1622-4. [DOI] [PubMed] [Google Scholar]
- 150.Schiffer HH. Glutamate receptor genes: susceptibility factors in schizophrenia and depressive disorders? Mol Neurobiol. 2002;25(2):191–212. doi: 10.1385/MN:25:2:191. [DOI] [PubMed] [Google Scholar]
- 151.Squires RF, Saederup E. A review of evidence for GABergic predominance/glutamatergic deficit as a common etiological factor in both schizophrenia and affective psychoses: more support for a continuum hypothesis of “functional” psychosis. Neurochem Res. 1991;16(10):1099–111. doi: 10.1007/BF00966587. [DOI] [PubMed] [Google Scholar]
- 152.Van Den Bogaert A, Del-Favero J, Van Broeckhoven C. Major affective disorders and schizophrenia: a common molecular signature? Hum Mutat. 2006;27(9):833–53. doi: 10.1002/humu.20369. [DOI] [PubMed] [Google Scholar]
- 153.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33(6):523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 154.Braunewell KH. The darker side of Ca2+ signaling by neuronal Ca2+-sensor proteins: from Alzheimer’s disease to cancer. Trends Pharmacol Sci. 2005;26(7):345–51. doi: 10.1016/j.tips.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 155.Gomperts BD, Tatham PER, Kramer IM. Signal Transduction. San Diego: Academic Press; 2002. [Google Scholar]
- 156.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43(1):70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]