Abstract
Tiny but highly efficient, a light emitting diode (LED) can power a therapy device, such as a phototherapy device, and, at the same time, decrease the device’s size requirements. In this study, a LED phototherapy device was designed to investigate the possible impact on wound healing using a mouse model and a cell line exposed to red and blue light. To enhance wound phototherapy, a gelatin sponge was fabricated. Results showed that the red and blue lights promoted cell growth and wound healing, while the blue light with a gelatin sponge protected the wound from infection in the early stages of wound healing. The LED phototherapy device combined with the gelatin sponge, therefore, has potential significance in clinical application for wound healing.
Graphical abstract
A LED phototherapy device was designed to detect the possible effects for wound healing using a mouse model and a cell line exposed to red and blue light. To enhance the wound phototherapy, a gelatin sponge was fabricated and applied to the wound healing. Results showed that the red and blue light promoted cell growth and wound healing, while the blue light with a gelatin sponge protected the wound from infection in the early stages of wound healing. The LED phototherapy device combined with the gelatin sponge, therefore, has potential significance in clinical application for wound healing.
INTRODUCTION
Ultrasound, electrical stimulation, phototherapy, and negative pressure wound therapy are all believed to be beneficial for wound healing (1). The phototherapy technologies, especially laser therapy, that are delivered in hospital settings have positively affected the healing of wounds and proved to be conservatively effective at inducing chronic wound healing (2). Recently, LED phototherapy devices, which are simple and tiny light sources, have been applied widely in clinics.
Photobiomodulation, formerly known as Low Level Light/Laser Therapy (LLLT), is theorized to stimulate cells at a cellular level and may cause bacterial protein denaturation and affect the microbial membrane (3). Also, photobiomodulation may increase the immune expression of collagen type I during tissue repair and improve the quality of newly formed tissue in the presence of hypothyroidism (4, 5). Photobiomodulation may also promote cell metabolism; induce analgesia, anti-inflammatory action, tissue repair; and stimulate neovascularization and the early formation of collagen fibers (6, 7).
With appropriate wavelength and adequate power density, LED phototherapy will be at least partly if not significantly, effective (8). Light at 870 nm/930 nm and energy higher than 100 J/cm2 seem to be an efficacious therapeutic approach (3). Red or near infrared light promotes tissue repair, while blue light, such as that at 470 nm, is known to be antimicrobial, yet not impairing of wound healing with anti-inflammatory in vitro (9). Light at 660 nm could accelerate the oral mucosa wound-healing process with an energy density of 4 J/cm2 in comparison to 20 J/cm2 (7). Blue light at 415 nm with energy higher than 100 J/cm2 was shown to be efficacious in eradicating infection and increasing the survival rates of animal models with wound infections (3).
Apart from the effect of LED phototherapy alone on wound healing, a protector substance such as nanomaterial and biomaterial used together with LED has been reported to achieve better curative effects in recent years. Graphene nanomaterial showed the ability to enhance light absorption and improve phototherapy effect (10). A relatively novel material named photosensitive semiconducting polymer-incorporated nanofiber was verified to benefit skin wound regeneration (11). Furthermore, recent investigations showed that curcuma extract (12), gold nanoparticles associated with neutral red and mesoporous silica shell (13, 14) combined with LED phototherapy may improve the wound healing process. As for biomaterials, fabrics such as collagenous fibers and natural silks were frequently used as cell scaffolds to provide supporting structures and adaptive growth environments (15–17). However, they were relatively poor in penetrability and attachable ability (18, 19). Gelatin sponge is a kind of high polymer material that was usually used in clinics for hemostasis with disadvantage of strong water-absorbing ability, adequate softness and good biocompatibility. The loose and porous structure of gelatin sponge could make it easy to absorb the blood and therapeutic drugs, meanwhile providing a good supporting scaffold in favor of cell growth and migration. Since the gelatin was extracted from natural animal products, it was free of poisonousness and might offer a biological microenvironment beneficial for cell living and proliferation. Therefore, gelatin sponge has widely been applied in clinical practice. For instance, it was reported that blue-violet LED irradiation combined with gelatin sponge protection provided high-efficiency hemostasis during tooth extraction (20). The soft polyporous bibulous gelatin sponge material not only protected the wound from being infected but also absorbed other drugs like growth factors (21) and antibiotics (22) and released them in a sustainable way. It might be highly predictable that the gelatin sponge combined with LED phototherapy might jointly aid in wound healing.
In this study, a LED light device was designed to investigate the effects of blue and red light on wound healing in a mouse wound model. The gelatin sponge was also fabricated for wound phototherapy.
MATERIALS AND METHODS
Design of the phototherapy device
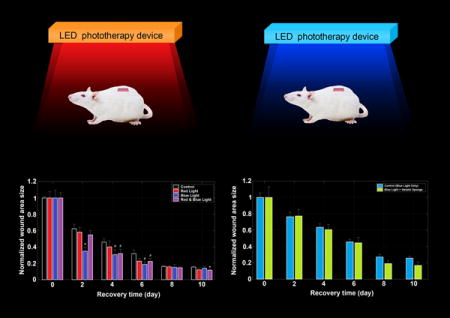
The LED phototherapy device (LPD) was designed with two LED arrays, red monochromatic LEDs (wavelength = 625 ± 5 nm) and blue monochromatic LEDs (wavelength = 465 ± 5 nm), installed on the same plate with partial overlapping. Each array consisted of 21 LEDs (3×7 array), of which every LED provided about 1 watt light power in total to all directions and the illumination was adequate to cover all the experimental area (about 15×30 cm). According to the measurement of light intensity with an optical power meter, each mouse was exposed to light irradiation of approximately 3 J/cm2.
Cell experiment using the scratch assay method
The human skin fibroblasts (HSF, cell density of 1×106/mL and tested negative in mycoplasma, bacteria and fungus test) were gifted from iCell Bioscience Inc (Shanghai). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 ug/ml streptomycin at 37°C, 5% CO2. The cell suspensions were transferred to 6-well microplates and incubated for 4 days for the cell experiments.
The scratch assay method was conducted according to reference (9). At the middle of the cell growth monolayer, the wound space was made using a sterile 1.0 mL pipette tip across the center of each well, creating a cell-free area under an inverted microscope (23–25). Cellular debris was washed off with DMEM, and cultured with fresh medium. Plates were then irradiated separately with red, blue and mixed (red and blue) light at 3 J/cm2 doses, while the control plates were not irradiated with light. Microscopic images of the cells including the cell-free area were acquired immediately after scratching (marked as 0-hour time point) and after a 24 hour incubation period (marked as 24-hour time point), by using an inverted microscope equipped with a camera. The scratch closure rate, embodied in the relative with of cell-free scratch, was determined by comparing the difference between the wound widths at the 0- and 24-hour time points.
Preparation of the gelatin sponge
Five grams of gelatin were dissolved in ultra-pure water at a volume of 100 mL, and, subsequently, 0.6 mL 30% formaldehyde solution was added. The mixed solution was kept stirring for about 20 minutes until it was bubbling completely. The bubbles were then placed immediately in −20 °C for freezing and solidification. After three days of cryopreservation, the solidified bubbles were desiccated under a vacuum until they were completely dry, and then incised into square pieces of 5×5×1 mm, after which time the gelatin sponge slices were ready for use in the experiments.
Animal wound models experiments
In total, 24 male BALB/c mice with an average age of six weeks, and weighing between 18~22 g, were purchased from Shanghai Slack Laboratory Animal Co., Ltd. The mice were kept in individual cages lined with wood chips and maintained at a temperature of 25°C in a natural day/night light cycle and supplied with adequate feed and water. After a regular quarantine and adaptation period, the mice were, at random, divided into control (CTR, no irradiation), red light (RL, λ=625±5 nm, 10 min, 3 J/cm2), blue light (BL, λ=465±5 nm, 10 min, 3 J/cm2), and red-blue-mixed light (RBL, λ=625±5 nm, 5 min, 1.5 J/cm2 and λ=465±5 nm, 5 min, 1.5 J/cm2) groups, with six mice in each group (Table 1).
Table 1.
Description of the irradiation conditions and protocols for each experimental group.
| Group | Irradiation Condition | Irradiation Protocol |
|---|---|---|
| CTR | Control | No irradiation |
| RL | Red light phototherapy | λ=625±5 nm, 10 min, 3 J/cm2 |
| BL | Blue light phototherapy | λ=465±5 nm, 10 min, 3 J/cm2 |
| RBL | Red-blue-mixed light phototherapy | λ=625±5 nm, 5 min, 1.5 J/cm2 and λ=465±5 nm, 5 min, 1.5 J/cm2 |
After intraperitoneal general anesthesia (chloral hydrate 10%, 4.5 ml/kg), the animals had their dorsa shaved and disinfected with 75% alcohol (Aladdin, SP). A square excisional wound (8×8 mm) was created with a scalpel in the mid-dorsal region of each rat and was left without suturing or dressing. Laser irradiation was started immediately after surgery and repeated every two days. Every time, the LED phototherapy device was placed above the animals at a distance of 15 cm so that the wounds could be irradiated with the corresponding wavelength in a vertical direction. All irradiation events was performed at 19:00 (nighttime Local Time) in darkness to avoid the interference of other light rays, including daylight. To investigate the process of wound healing in real time, we photographed each mouse after every irradiation with a straightedge as the reference object and calculated the wound size of each mouse by the method described below.
To determine the most suitable wavelength of light to use, we performed a pilot study as described above and found that blue light (λ=465±5 nm, 10 min, 3 J/cm2) had the most positive effects (Fig. 4). Moreover, the wavelength and dose were selected based on previous studies by Meireles et al. (26) and de Sousa et al. (27). Accordingly, we purchased another 12 male BALB/c mice and kept them in the same conditions as described above. The animals were randomly distributed into 2 groups of 6 mice each, according to whether the wound was covered or not: an uncovered control group (CTR-BL, λ=465±5 nm, 10 min, 3 J/cm2) and a gelatin sponge slice covered group (GS-BL, λ=465±5 nm, 10 min, 3 J/cm2) (Table 2).
Figure 4.
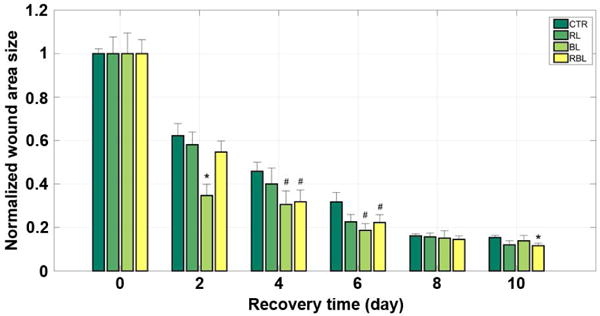
The mean wound area size of each of the four groups during the 10-day recovery period after surgery. *: p < 0.05 vs control and #: p < 0. 1 vs control; t-test. CTR, control group; BL, blue light group; RL, red light group; RBL, red-blue-mixed light group.
Table 2.
Description of experimental groups in the gelatin sponge slice test.
| Group | Condition | Irradiation Protocol |
|---|---|---|
| CTR-BL | Control | λ=465±5 nm, 10 min, 3 J/cm2 |
| GS-BL | Covered by gelatin sponge slice | λ=465±5 nm, 10 min, 3 J/cm2 |
The dorsal skin excision surgery was performed in the same way as described above, after which the mice in GS-BL group were covered and fitted with gelatin sponge slices while the CTR-BL group was kept with bare wounds. Next, the irradiation with blue light (λ=465±5 nm, 10 min, 3 J/cm2) alone proceeded using the same protocols as mentioned above.
Wound area size calculation
Since the straightedge was fixed at nearly the same vertical height to the wound of the mice during the photographing, the scale of the straightedge could be used to estimate the wound length and width. However, the irregular wound shape usually made it difficult to accurately estimate the wound area size by conventional measurement of directly reading scales. Therefore, we programmed an algorithm script using the build-in APIs of MatLab (MathWorks, USA) to recognize the outline and closed region of each wound. The brief protocol for wound area size calculation was as follows: by the reference of the straightedge scale, the original wound image was clipped to be a 10×10 mm mini square image that include the whole wound area; the resultant image was read into the memory by calling the function “imread”; the image object was mapped from RGB (red, green, blue) space to the HSV (hue, saturation, value) space by calling the function “rgb2hsv”; the v-channel, determining the brightness of color, was abstracted to be a density image and then was converted to be a binary image with pixels of either white or black, by calling the function “im2bw”; the abstracted binary image underwent morphological close operation and outliers removal by calling function “imclose” and “bwareaopen”, respectively, and finally we could obtain a clear black-white image with a main white area whose shape and boundary fitted the wound area well; according to the proportion of the white pixels (Pwhite) and the view scope size of the clipped image (Aimage), the wound area size (Awound) could be calculated out by their arithmetic product (Awound = Pwhite* Aimage (mm2)). As a matter of convenience, we developed a software tool based on the algorithm mentioned above that runs on the MatLab platform and provides a friendly GUI (graphical user interface) (see Supporting Information, Figure S1).
Histologic section and stain
The surgically separated skin tissues of mice were fixed using 30% sucrose solutions for 2 days and embedded by OCT-freeze medium for 2 hours before frozen section. The tissue was then sectioned to be 10-μm slices using a freezing microtome at −22°C. Subsequently, the tissue slice was transferred onto the clean glass slide pretreated with gelatin adhesive. Before H-E stain, the tissue slice was cleaned with water to remove the remaining embedded medium and then was stained by the haematoxylin for 8 min. The further brief protocol was as following: water cleaning for 1 minute; 1% HCl-alcohol infiltrating for 5 seconds; water cleaning for 1 minute; 1% NH3 solution infiltrating for 10 seconds; water cleaning for 2 minutes; eosin staining for 2 minutes; 2× 70% alcohol cleaning.
RESULTS AND DISCUSSION
Cell growth and migration with the LED light irradiation
The margins of the cell-free zone on monolayers of human skin fibroblasts were clearly demarcated right after the central scratch was inflicted and before the irradiation operation (Fig. 1, top panels, 0 h). A typical photograph taken 24 hours post-irradiation is shown for each group (CTR, BL, RL, RBL) in Fig. 1 (bottom panels) and shows the effects of irradiation using each wavelength treatment. The mean cell-free scratch widths were calculated with the cell images for each group (n=18). The 24 h mean scratch widths were normalized by the width data measured at 0 h to be the relative widths. The mean relative widths of cell-free scratches for the irradiated groups were less than that of the control group, and in particular, the red light group (RL) showed the smallest mean scratch width after 24 h compared with other groups, suggesting that the red light performed best in promoting the cell growth and migration (Fig. 1 and Fig. 2).
Figure 1.
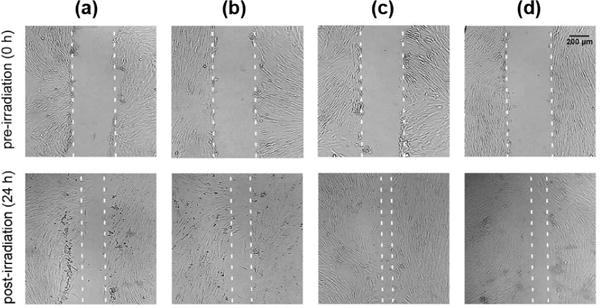
The phototherapy effects on the closure of cell-free scratch for human skin fibroblasts under different wavelength conditions. (a), (b), (c) and (d) represent the control (CTR), blue light (BL), red light (RL), red-blue-mixed light (RBL) groups, respectively. The dashed lines indicate the edges of cell-free scratch region.
Figure 2.
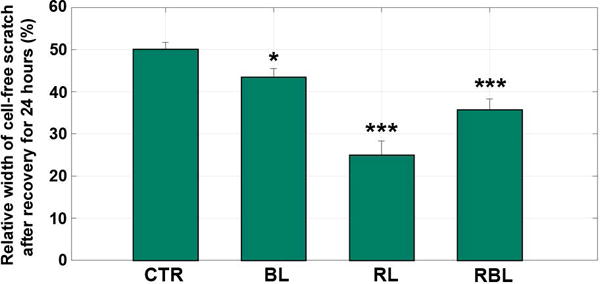
The relative width of cell-free scratch after recovery for 24 hours since irradiation compared with the width before irradiation (at the time point of 0 hour). Mean ± S.E.M. *: p < 0.05 vs control and ***: p < 0.001 vs control; t-test. CTR, control group; BL, blue light group; RL, red light group; RBL, red-blue-mixed light group.
Wound healing with irradiation
The wound area size data were acquired every two days for ten days (day 0, day 2, …) during the post-surgery period. In order to lower the individual differences and measurement errors as much as possible, we normalized the wound size data by dividing each mean wound size by the first measured size (on the first day after surgery, i.e. day 0) (Table 3). According to the measurement taken every two days, the average wound sizes of all groups began to decrease gradually and had declined by nearly half on the second day after surgery (CTR: ~38%; RL: ~42%; BL: ~65%; RBL: ~45%) (Fig. 3 and Fig. 4). Compared with the CTR group, the irradiated groups healed much faster, especially the BL group whose mean wound size reduced sharply by nearly 65% (p < 0.05 vs control). Additionally, in the following two measurements, days 4 and 6, the mean wound sizes of the BL group still ranked as the smallest sizes (4 days: ~31%, p < 0.1 vs control; 6 days: ~19%, p < 0.1 vs control), which showed that the blue light phototherapy performed best in wound healing promotion. Indeed, there was not much doubt that blue light promoted wound healing, given the great role played in antibiosis and disinfection, which was also illustrated in previous studies (9, 28).
Table 3.
Normalized wound sizes sampled for all groups at two-day intervals over a 10-day post-surgery period. CTR, RL, BL and RBL groups are defined in Table 1.
| Recovery Time (day) |
Normalized Wound Size (mm ± #####) | |||
|---|---|---|---|---|
| CTR | RL | BL | RBL | |
| 0 | 1 ± 0.02259 | 1 ± 0.07691 | 1 ± 0.09447 | 1 ± 0.06467 |
| 2 | 0.62204 ± 0.05633 | 0.58086 ± 0.05857 | 0.34703 ± 0.05216 | 0.54755 ± 0.05045 |
| 4 | 0.45881 ± 0.04120 | 0.40028 ± 0.07373 | 0.30578 ± 0.06278 | 0.31852 ± 0.05441 |
| 6 | 0.31757 ± 0.04361 | 0.22640 ± 0.03374 | 0.18684 ± 0.03089 | 0.22249 ± 0.03652 |
| 8 | 0.16175 ± 0.00956 | 0.15671 ± 0.01809 | 0.15160 ± 0.03369 | 0.14527 ± 0.01636 |
| 10 | 0.15412 ± 0.01018 | 0.12031 ± 0.01833 | 0.13873 ± 0.02481 | 0.11651 ± 0.01154 |
Figure 3.

The wound healing process during the 10-day recovery period after surgery, by tracking the typical experimental subjects in each group. (a), (b), (c) and (d) represent the control (CTR), red light (RL), blue light (BL), red-blue-mixed light (RBL) groups, respectively, over the 10-day period.
Moreover, although it has been well documented that red or infrared light irradiance with wavelengths in the range of 600 to 1000 nm promotes the repair process of skin in experimental animal and human wounds, also verified in our research wherein the RL group healed faster than the CTR group, red light was still far inferior to blue (2 days: RL, ~58% while BL, ~35%; 4 days: RL, ~40% while BL, ~31%; 6 days: RL, ~23% while BL, ~19%).
Since many investigations indicated that red or infrared light engendered faster tissue healing and blue light kept infections away, it was not difficult to imagine that combining them concurrently would promote wound healing and suppress infection simultaneously. However, inconsistent with our expectations, the results from our experiment showed that red light combined with blue light failed to significantly accelerate wound healing and did not even perform better than blue light alone (2 days: BL, ~35% better than RBL, ~55%; 4 days: BL, ~31% slightly better than RBL, ~32%; 6 days: BL, ~19% better than RBL, ~22%).
Note that the wound size data on days 8 and 10 have few reference values compared to that from days 2, 4 and 6 for the reason that the wound sizes were too small to estimate easily on the last two measurement days (days 8 and 10); the edge of the wound was very difficult to delineate clearly (Fig. 3).
Those two interesting findings, mentioned above, are pending further research. Nevertheless, it was clear that blue light irradiation was the most suitable wavelength for proceeding with the analysis using the gelatin sponge in this study.
Having performed the best among the other light treatments in the assay described above, blue light (λ=465 nm) was applied in a test to determine whether a gelatin sponge slice placed on the wound is beneficial to wound healing or not. Only two groups, the uncovered control (CTR-BL) and the gelatin sponge (GS-BL) groups, were tested using the same conditions and protocols described for the experiments described above.
On the basis of wound size data sampled along the 10 day recovery period (Fig. 5 and Fig. 6), it was a little surprising to find that the gelatin sponge slices did not function in a stable manner. More specifically, the sponge slices slightly slowed down the wound healing process in the first 4 days, but accelerated it in the last 6 days, when compared with the control group (2 days: CTR-BL, ~76% slightly better than GS-BL, ~78%; 4 days: CTR-BL, ~63% slightly better than GS-BL, ~61%; while 6 days: CTR-BL, ~46% worse than GS-BL, ~44%; 8 days: CTR-BL, ~27% worse than GS-BL, ~19%; 10 days: CTR-BL, ~26% worse than GS-BL, ~17%) (Table 4). In addition, we found that the gelatin sponge slices attached to the wounds were nearly absorbed by the 5th day. Therefore, we had evidence to conjecture that the gelatin sponge slices were probably shading the wounds from the blue light irradiation and thus blocking the phototherapy until the substance began to work by being completely absorbed around the 5th day. In addition to the wound area size comparison and analysis, the results of histological examinations showed that the cell density and tissue structure of the renascent skin in the GS group after 10 days of recovery were more similar to the original skin before surgery, compared to those in the control group, suggesting that gelatin sponge might benefit wound healing (Fig. 7).
Figure 5.

The wound healing process during the 10-day recovery period after surgery, by tracking the typical experimental subjects in each group. (a) and (b) represent the control (CTR-BL) and gelatin sponge (GS-BL) groups, respectively, over the 10-day period.
Figure 6.
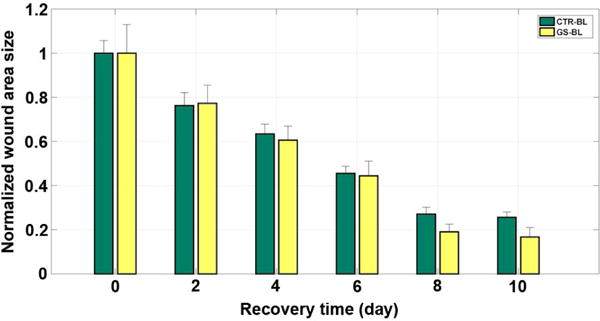
The mean wound area size of each group during the 10-day recovery period after surgery. CTR-BL, blue light irradiated control group; GS-BL, blue light irradiated and covered with gelatin sponge group.
Table 4.
Normalized wound sizes sampled for groups CTR-BL and GS-BL (defined in Table 2) at two-day intervals over a 10-day post-surgery period.
| Recovery Time (day) |
Normalized Wound Size (mm ± #####) | |
|---|---|---|
| CTR-BL | GS-BL | |
| 0 | 1 ± 0.05728 | 1 ± 0.12953 |
| 2 | 0.76271 ± 0.05843 | 0.77276 ± 0.08228 |
| 4 | 0.63431 ± 0.04385 | 0.60615 ± 0.06300 |
| 6 | 0.45524 ± 0.03216 | 0.44453 ± 0.06624 |
| 8 | 0.27072 ± 0.03148 | 0.19053 ± 0.03518 |
| 10 | 0.25681 ± 0.02390 | 0.16723 ± 0.04318 |
Figure 7.

The histological microscopic images of the original and renascent skin for mice (400×, H-E stain). (a) The original skin tissue without surgery and irradiation. (b) The renascent skin tissue with gelatin sponge slice covering at 10 days after surgery (GS-BL group). (c) The renascent skin tissue without gelatin sponge slice covering at 10 days after surgery (CTR-BL group).
Our results showed that both blue light at wavelength of 465 nm and red light at wavelength of 625 nm can promote the wound healing process. However, the two wavelengths produced different effects: blue light worked best in wound healing and in the repair process of mouse dorsal skin, while the red light had the most positive effect on cell growth and migration. Since mice were not kept in a sterile environment, they were at risk of being infected by harmful bacteria in the air, which could be a major factor hindering wound healing. Blue light has been well documented as a kind of bacteria killer with a powerful ability to resist infections. Therefore, it was reasonable that irradiation of blue light could accelerate wound healing in mice. With regard to the cell growth assay, infection was impossible due to our careful culturing in a bioclean system. In this assay, blue light seemed useless while red light’s vital role in cell growth was obvious. This might be one of the potential reasons why the red light performed best in the cell assay.
CONCLUSIONS
Phototherapy with red and blue light appeared to promote cell growth and, therefore, served as an effective treatment for wound healing. The light irradiation combined with a gelatin sponge might protect the wound from infection in the early stages of wound healing, with the gelatin sponge acting as a kind of biocompatible film support during wound healing.
Supplementary Material
Figure S1. The graphical user interface of the self-developed software for wound area size calculation.
Acknowledgments
This paper is supported in part by Beijing Advanced Innovation Center for Imaging Technology and in part by the Natural Science Foundation of China (NSFC) (61422507, 61475095, 61520106014). We are also grateful for the support of the Key Laboratory of Specialty Fiber Optics and Optical Access Networks (SKLSFO2015-06). The work of X. Xu was supported by NIH awards R01LM011415 and R01LM012434.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information can be found in the online version of this article:
References
- 1.Korzendorfer H, Hettrick H. Biophysical Technologies for Management of Wound Bioburden. Advances in wound care. 2014;3:733–741. doi: 10.1089/wound.2013.0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramalho KM, Luiz AC, De PEC, Tunér J, Magalhães RP, Gallottini MM. Use of laser phototherapy on a delayed wound healing of oral mucosa previously submitted to radiotherapy: case report. International Wound Journal. 2011;8:413–418. doi: 10.1111/j.1742-481X.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SL P, I F, G D. Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing. Future Microbiol. 2015;10:18. doi: 10.2217/fmb.14.109. [DOI] [PubMed] [Google Scholar]
- 4.Paraguassu GM, Xavier FC, Cangussu MC, Ramalho MJ, Cury PR, dos Santos JN, Pinheiro AL, Ramalho LM. Effect of laser phototherapy (lambda660 nm) on type I and III collagen expression during wound healing in hypothyroid rats: an immunohistochemical study in a rodent model. Photomed Laser Surg. 2014;32:281–288. doi: 10.1089/pho.2013.3604. [DOI] [PubMed] [Google Scholar]
- 5.Colombo F, Neto Ade A, Sousa AP, Marchionni AM, Pinheiro AL, Reis SR. Effect of low-level laser therapy (lambda660 nm) on angiogenesis in wound healing: a immunohistochemical study in a rodent model. Brazilian dental journal. 2013;24:308–312. doi: 10.1590/0103-6440201301867. [DOI] [PubMed] [Google Scholar]
- 6.Kilik R, Lakyova L, Sabo J, Kruzliak P, Lacjakova K, Vasilenko T, Vidova M, Longauer F, Radonak J. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and diabetic rats. BioMed research international. 2014;2014:269253. doi: 10.1155/2014/269253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner VP, Meurer L, Martins MA, Danilevicz CK, Magnusson AS, Marques MM, Filho MS, Squarize CH, Martins MD. Influence of different energy densities of laser phototherapy on oral wound healing. Journal of biomedical optics. 2013;18:128002. doi: 10.1117/1.JBO.18.12.128002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WS, Calderhead RG. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Therapy. 2011;20:205–215. doi: 10.5978/islsm.20.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masson-Meyers DS, Bumah VV, Enwemeka CS. Blue light does not impair wound healing in vitro. Journal of Photochemistry & Photobiology B Biology. 2016;160:53–60. doi: 10.1016/j.jphotobiol.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Wang Y, Liu J, Zhai G. Recent developments of phototherapy based on graphene family nanomaterials. Current Medicinal Chemistry. 2016;23 doi: 10.2174/0929867323666161019141817. [DOI] [PubMed] [Google Scholar]
- 11.Jin G, Li J, Li K. Photosensitive semiconducting polymer-incorporated nanofibers for promoting the regeneration of skin wound. Materials Science & Engineering C. 2016;70:1176–1181. doi: 10.1016/j.msec.2016.04.107. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez-Boscá A, Navarro-López V, Carrión-Gutiérrez M, Martínez-Andrés A, Vilata-Corell JJ, Asín-Llorca M, Jf HDLC, Bernd A. Efficiency and safety of a Curcuma extract combined with visible blue light phototherapy on adults with plaque psoriasis: A phase IV, randomized, open pilot clinical trial. Journal of Dermatology. 2016:1–2. doi: 10.1111/1346-8138.13668. [DOI] [PubMed] [Google Scholar]
- 13.Verissimo TV, Santos NT, Silva JR, Azevedo RB, Gomes AJ, Lunardi CN. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Materials Science & Engineering C. 2016;65:199. doi: 10.1016/j.msec.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Detrembleur C, De Pauw‐Gillet MC, Mornet S, Jérôme C, Duguet E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light‐Activated Release and Potential Phototherapy. Small. 2015;11:2323–2332. doi: 10.1002/smll.201402145. [DOI] [PubMed] [Google Scholar]
- 15.Altman GH, Horan RL, Lu HH, Moreau J, I M, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen JS, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67A:559–570. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- 17.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 18.Karamuk E, Mayer J, Wagner B, Bischoff B, Billia M, Seidl R, Wintermantel E. Embroidery technology for medical textiles. Proceedings of the Medical Textiles ‘99medical Textiles. 1999 in press. [Google Scholar]
- 19.Van Eijk F, Saris DBF, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJA. Tissue engineering of ligaments: A comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893–903. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Ishikawa I, Kumasaka A, Morita S, Katagiri S, Okano T, Ando T. Blue-violet light-emitting diode irradiation in combination with hemostatic gelatin sponge (Spongel) application ameliorates immediate socket bleeding in patients taking warfarin. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2014;117:170–177. doi: 10.1016/j.oooo.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Hiwatashi N, Hirano S, Mizuta M, Tateya I, Kanemaru SI, Nakamura T, Ito J, Kawai K, Suzuki S. Biocompatibility and Efficacy of Collagen/Gelatin Sponge Scaffold With Sustained Release of Basic Fibroblast Growth Factor on Vocal Fold Fibroblasts in 3-Dimensional Culture. Annals of Otology Rhinology & Laryngology. 2015;124:116. doi: 10.1177/0003489414546396. [DOI] [PubMed] [Google Scholar]
- 22.Drognitz O, Thorn D, Krüger T, Gatermann SG, Iven H, Bruch HP, Muhl E. Release of vancomycin and teicoplanin from a plasticized and resorbable gelatin sponge: in vitro investigation of a new antibiotic delivery system with glycopeptides. Infection. 2006;34:29–34. doi: 10.1007/s15010-006-1067-1. [DOI] [PubMed] [Google Scholar]
- 23.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocol. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 24.Basso FG, Pansani TN, Turrioni AP, Bagnato VS, Hebling J, Ca DSC. In vitro wound healing improvement by low-level laser therapy application in cultured gingival fibroblasts. International Journal of Dentistry. 2012;2012:719452–719452. doi: 10.1155/2012/719452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ab Rahman MR, Abdul RF, Mohd BM. Evaluation of Wound Closure Activity of Nigella sativa, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum Extracts on Scratched Monolayer of Human Gingival Fibroblasts. Evidence-based Complementary and Alternative Medicine. 2014;2014:190342–190342. doi: 10.1155/2014/190342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meireles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL. Effectiveness of laser photobiomodulation at 660 or 780 nanometers on the repair of third-degree burns in diabetic rats. Photomedicine & Laser Surgery. 2008;26:47–54. doi: 10.1089/pho.2007.2051. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa AP, RJ SJ, Jr, Ramos TA, De SJ, Cangussu MC, Pinheiro AL. Effect of LED phototherapy of three distinct wavelengths on fibroblasts on wound healing: a histological study in a rodent model. Photomedicine & Laser Surgery. 2010;28:547–552. doi: 10.1089/pho.2009.2605. [DOI] [PubMed] [Google Scholar]
- 28.Antoniou C, Dessinioti C, Sotiriadis D, Kalokasidis K, Kontochristopoulos G, Petridis A, Rigopoulos D, Vezina D, Nikolis A. A multicenter, randomized, split‐face clinical trial evaluating the efficacy and safety of chromophore gel‐assisted blue light phototherapy for the treatment of acne. International Journal of Dermatology. 2016;55:1321–1328. doi: 10.1111/ijd.13349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The graphical user interface of the self-developed software for wound area size calculation.


