Abstract
Objective
Mild parkinsonian signs (MPS) are an underappreciated neurologic condition in older adults; we assessed associations of MPS with measures of dopaminergic (catechol-O-methyltransferase [COMT] genotype, an indicator of synaptic dopamine levels) and vascular (white matter hyperintensities [WMH], an indicator of cerebral small vessel disease) factors.
Methods
In a cohort of older adults (mean age 82.6 years [SD 2.6]; 58.0% female; 38.8% black), we assessed cross-sectional associations of WMH volume and COMT Val158Met (rs4680) genotype (n = 35 Met/Met, n = 180 Val carriers) with MPS by regression models adjusted for demographic and health characteristics. Interactions between WMH and COMT were assessed and analyses were repeated stratified by COMT genotype (Met/Met related to higher synaptic dopamine vs Val carriers related to lower synaptic dopamine).
Results
MPS was present in 42.3% of our sample. WMH (odds ratio [OR] 1.16, confidence interval [CI] 1.05–1.27) but not COMT (Met/Met compared to Val carrier: OR 0.62, CI 0.27–1.42) was related to MPS. There was a significant interaction between WMH and COMT (p = 0.03). Stratified analyses reveled a strong association between WMH and MPS among COMT Val carriers (OR 1.23, CI 1.09–1.38), but not for Met/Met (OR 0.68, CI 0.45–1.02), independent of covariates.
Conclusions
WMH had a direct relation with MPS. In contrast, COMT was not associated with MPS, but it did modify the effect of WMH on MPS. The dopaminergic system may provide compensation for the effects of WMH on MPS. These findings suggest that MPS has a vascular rather than dopaminergic origin in older adults, but both factors are important in MPS manifestation.
Mild parkinsonian signs (MPS) are common in older adults without overt neurologic disorders and their presence is associated with poorer health outcomes, including cognitive impairment, disability, and premature death.1 Despite their high prevalence, MPS are largely underappreciated in clinical practice and research. This may be because the origins of MPS are not clear, though cerebral small vessel disease, identified by white matter hyperintensities (WMH), may be a contributor.1–3
Dopaminergic contributions to MPS are less well-studied, despite the role of striatal dopamine in related Parkinson disease. Dopamine levels are related to gait speed, a prominent component of MPS, in older adults without neurologic conditions.4 Catechol-O-methyltransferase (COMT) is an enzyme involved in the metabolic degradation of catecholamines, including dopamine, and is particularly important for clearance of synaptic dopamine levels following neurotransmitter release in the prefrontal cortex (PFC) due to low concentration of dopamine reuptake transporters in the PFC. COMT genotype is associated with Parkinson disease but its relation with MPS is unknown.5
We assessed the association of WMH volume and COMT genotype with presence of MPS and the interactions between WMH and COMT in relation with MPS. As bradykinesia is a prominent MPS with clear implications for the health and well-being of older adults6 and with an established neurologic contribution,7 we also assessed gait speed alone. We hypothesized that both WMH and COMT would be related to MPS and slower gait speed and that relation for one neurologic marker would depend on the presence or absence of the other.
Methods
Sample
Cross-sectional data from the Health Aging and Body Composition (Health ABC) ancillary Healthy Brain Project (HBP) were used. Participants at the Pittsburgh site were asked to participate in the HBP between 2006 and 2008 if they were free from neurologic disease and could walk 20 meters. Of the 1,527 participants enrolled in the Health ABC in 1997–1998 at the Pittsburgh site, 819 were alive and contacted to participate in the HBP. Among these, 315 received a 3T brain MRI. We excluded those with a self-reported stroke (n = 25), cognitive impairment indicated by a Modified Mini-Mental State Examination (3 MS)8 score below 80 (n = 22), or missing genotype, gait speed, MPS, MRI, or covariate data (n = 63; not mutually exclusive), resulting in an analytic sample of 215 participants. Those who were excluded were older (p < 0.001) and more likely to have cardiovascular disease (p < 0.001) than our analytic sample but did not differ on other demographic or health characteristics. Excluded individuals also had slower gait speed (p = 0.01) and higher WMH volume (p = 0.005) than included individuals, but these differences were related to exclusions on stroke and cognitive impairment and not to missing data.
Standard protocol approvals, registrations, and patient consents
Institutional review boards at participating institutions approved this study and all participants provided informed consent.
COMT genotyping
Single nucleotide polymorphisms of the COMT Val158Met (rs4680) gene determine levels of the COMT enzyme and, therefore, synaptic dopamine levels.9 The Met allele results in lower levels of COMT, and consequently slower clearance and higher synaptic levels of dopamine. In contrast, those with the Val allele have faster clearance and lower synaptic levels of dopamine. Therefore, individuals with the Met/Met genotype have the highest synaptic levels of dopamine.9
COMT genotyping has been described in detail elsewhere.10,11 Genomic DNA was extracted from EDTA anticoagulated whole blood and PCR-based genotyping used flanking primers COMTF: 5_-CACATCACCATCGAGATCAACA-3_ and COMTR: 5_GATGACCCTGGT GATAGTGG-3_. The 210-bp fragment flanking the Val158Met polymorphism (dbSNP, rs4680) was digested with 1.5 units of NlaIII, and resolved on 1% agarose gel. Genotypes were compared to sequence-verified control samples on the same gel. A 5% sample of blind duplicates was included for quality control with complete concordance for genotypes. Hardy-Weinberg equilibrium for genotype distribution was assessed.
Neuroimaging
Image acquisition
Details of the image acquisition protocol have been published previously.12 Images were obtained with a Siemens (Munich, Germany) 12-channel head coil and 3T Siemens Tim Trio MRI scanner at the Magnetic Resonance Research Center, University of Pittsburgh. Magnetization-prepared rapid gradient echo T1-weighted images were acquired in the axial plane: repetition time (TR) 2,300 ms; echo time (TE) 3.43 ms; inversion time (TI) 900 ms; flip angle 9; slice thickness 1 mm; field of view (FOV) 256 × 224 mm; voxel size 1 × 1 mm; matrix size 256 × 224; number of slices 176. Fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial plane: TR 9,160 ms; TE 89 ms; TI 2,500 ms; flip angle 150; FOV 256 × 212 mm; slice thickness 3 mm; matrix size 256 × 240; number of slices 48; and voxel size 1 × 1 mm. A neuroradiologist examined each MRI for neurologic abnormalities.
Image processing
Volumes for gray matter (GM), white matter (WM), and CSF were calculated by segmenting the skull-stripped T1-weighted image in native anatomical space. WMH volume was obtained from T2-weighted FLAIR image using a semiautomated method and was normalized to brain volume. A fuzzy connected algorithm with automated seed selection was used.13 The spatial distribution of WM tracts was obtained using the JHU atlas.14 GM volumes (GMV) for the left and right dorsolateral PFC (dlPFC) were estimated in cubic millimeters by summing tissue-specific voxels. Labeling of regions of interest followed previously published methods.15 Total intracranial volume was computed as the volume contained within the inner skull. GMV for the left and right dlPFC are reported as adjusted for intracranial volume by the formula 1 − GMV/intracranial volume.
Mild parkinsonian signs
The Unified Parkinson's Disease Rating Scale motor component (part III) was used to assess parkinsonian signs, including bradykinesia, tremor, rigidity, and gait disturbances. MPS was defined as (1) 2 or more items with a score of 1, indicating mild symptoms, (2) 1 item with a score of at least 2, indicating moderate to severe symptoms, or (3) a rest tremor score of 1 without meeting diagnostic criteria for Parkinson disease.16
Gait speed
Gait speed was assessed at usual pace on the GaitMat II, an instrumented 8-meter walkway. The first 2 and last 2 meters were inactive to allow acceleration and deceleration. Gait speed was calculated as the distance between the first switch closure of the first and last steps divided by the time to walk, using meters per second.
Covariates
Variables related to motor limitations were measured. Age, sex, race, and highest education level were self-reported at the baseline Health ABC visit. Prevalent diabetes mellitus, cardiovascular disease, and hypertension at time of MRI were recorded. Diabetes status was determined by self-report, use of diabetes medication, a fasting glucose of ≥126 mg/dL, or a 2-hour glucose tolerance test >200 mg/dL. Body mass index (BMI) was calculated by the standard formula (weight in kilograms)/(height in meters)2 and obesity was defined by the WHO as ≥30.17 Muscle strength was measured as the peak torque from isokinetic knee extension on a dynamometer (model 125 AP, Kin-Com, Chattanooga, TN). The right leg was measured unless counterindicated due to prior surgery, injury, or pain.
Global cognitive function was tested by the 3 MS.8 Processing speed, a behavioral marker of dlPFC integrity, was assessed by the Digit Symbol Substitution Test (DSST).18
Statistical analyses
Associations between demographic, health, and MRI variables with presence or absence of MPS were determined by χ2 for categorical variables and by t tests for continuous ones. Descriptive statistics by COMT genotype were calculated by χ2 statistics for categorical variables and by analysis of variance for continuous ones.
Logistic regression tested associations between WMH and MPS. Linear regression was used to assess associations between WMH and gait speed. As COMT was not associated with MPS or gait speed in univariate analyses, regression analyses were not conducted. Interaction terms of COMT genotype with WMH were tested and regression analyses were repeated stratified by COMT. Regression results were similar for Val/Met and Val/Val genotypes; therefore, analyses were conducted comparing Met/Met genotype to Val carriers in order to maximize sample sizes. Due to the relatively small sample size within the Met/Met stratum, stepwise backward elimination with exclusion of covariates at p > 0.05 was used. Final adjusted models for MPS included age and BMI. Final adjusted models for gait speed included age, race, diabetes, hypertension, BMI, and quadriceps strength.
Model fit was assessed and due to the skewed distribution of WMH, visual assessment of outliers was conducted. Fit was good for all models and all residuals were normally distributed. Two outliers in WMH volume were identified; analyses repeated without these outliers were identical to those reported here. Due to dlPFC associations with motor control, COMT function, and WMH, we accounted for integrity of this region with further adjustment by either DSST or dlPFC GMV to determine whether the associations were attenuated by processing speed or brain structure. Models were also repeated to assess whether COMT interactions were significant for WMH in specific WM tracts. Tracts assessed were identified a priori and included the anterior thalamic radiation, cingulum, frontal corpus callosum, and occipital corpus callosum in order to assess whether associations differed for frontal and nonfrontal tracts.
Results
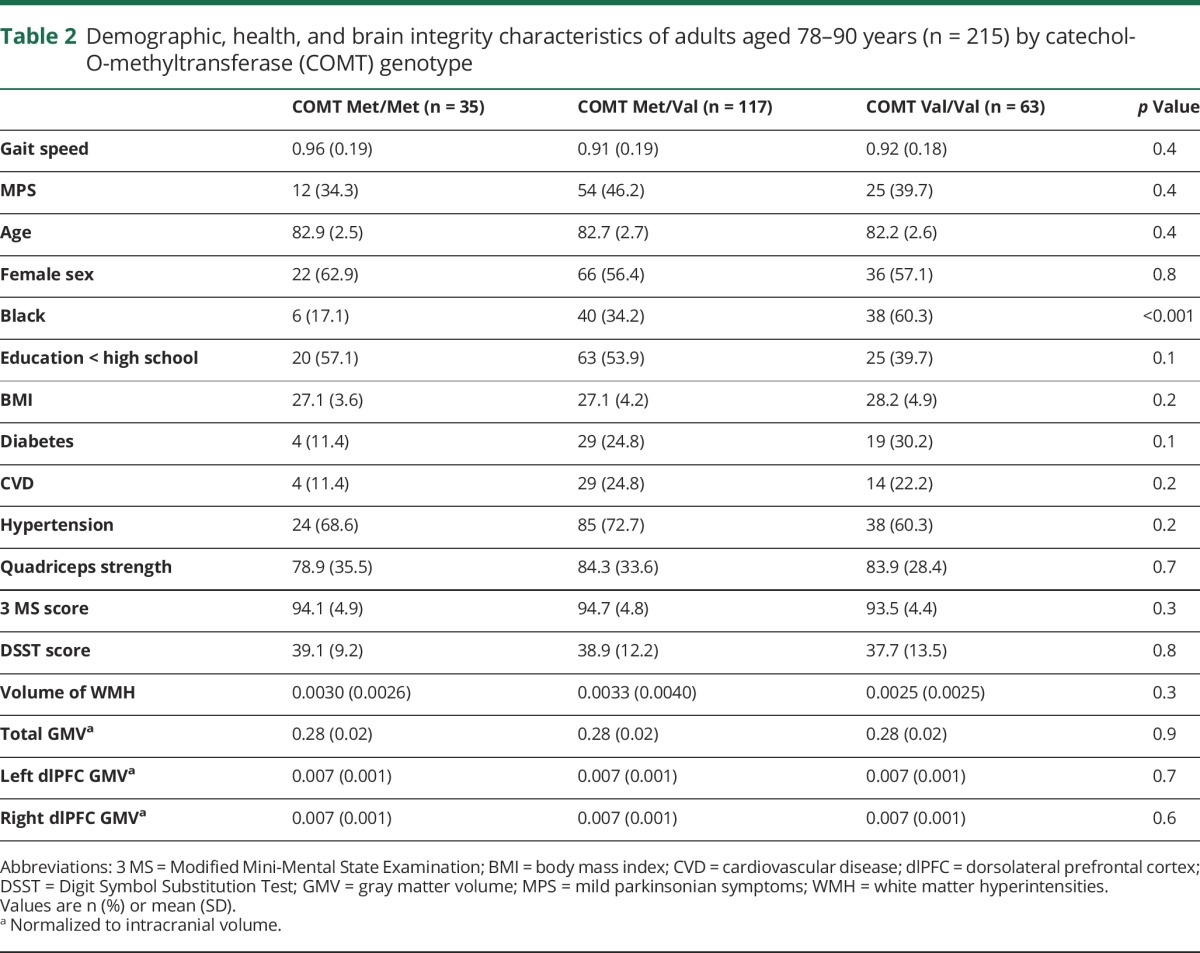
The analytic sample had an average age of 82.6 years (SD 2.6) and was 57.7% female and 39.1% black. Nearly half the sample (42.3%) had MPS and mean gait speed was 0.92 m/s (SD 0.19). Individuals with MPS were older and more likely to have hypertension; they also had lower 3 MS and DSST scores and slower gait (table 1). Having MPS was significantly associated with higher volume of WMH, but not with COMT genotype (table 1). Thirty-five participants (16.1%) had the Met/Met COMT genotype. Black race (p ≤ 0.001) was less common in those with the Met/Met COMT genotype compared to Val carriers (table 2). No other demographic, health, or MRI measures were significantly associated with COMT genotype (table 2). WMH was associated with gait speed (β = −12.7, confidence interval [CI] −19.7 to −5.6) in unadjusted analyses, but COMT was not (table 2).
Table 1.
Unadjusted associations of demographic, health, and brain integrity characteristics with mild parkinsonian symptoms (MPS) in adults aged 78–90 years (n = 215)
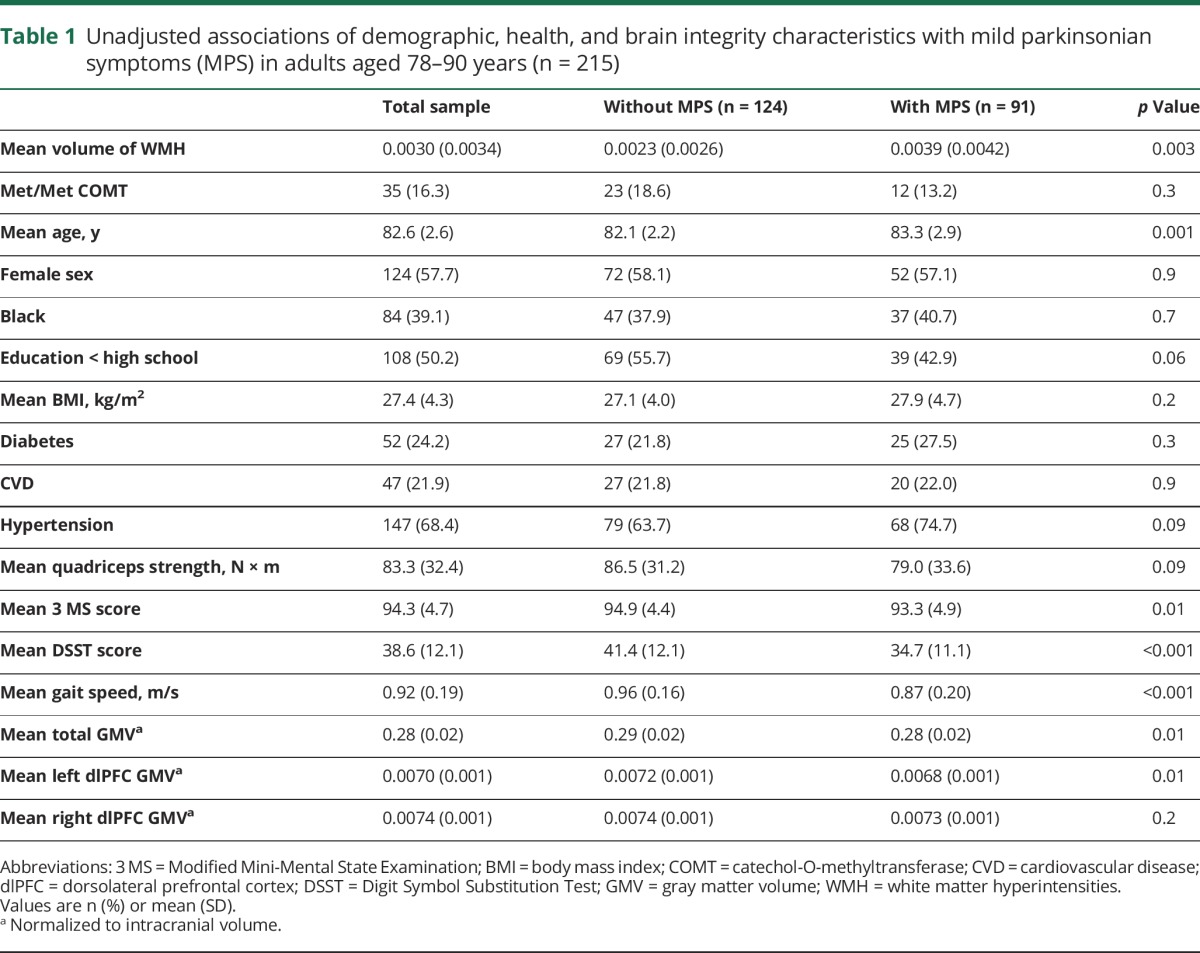
Table 2.
Demographic, health, and brain integrity characteristics of adults aged 78–90 years (n = 215) by catechol-O-methyltransferase (COMT) genotype
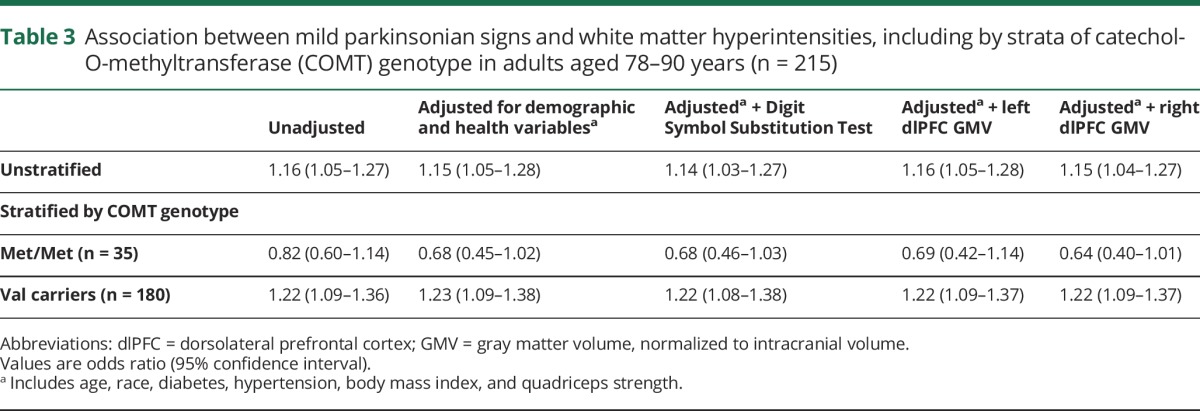
Greater volume of WMH was associated with significantly greater odds of having MPS after adjustment for age, race, diabetes, hypertension, BMI, and quadriceps strength (odds ratio [OR] 1.15, CI 1.05–1.28; table 3). There was a significant interaction between WMH and COMT genotype both before (β = −391.0, p = 0.03) and after adjustment (β = −428.2, p = 0.02), indicating that the association between WMH and MPS differed by genotype. Analyses stratified by COMT genotype (table 3) indicated an association between WMH and gait speed in adjusted models among those who were COMT Val carriers (OR 1.23, CI 1.09–1.38). In contrast, there was no association between WMH and gait speed in adjusted models among those with the COMT Met/Met genotype (OR 0.68, CI 0.45–1.02).
Table 3.
Association between mild parkinsonian signs and white matter hyperintensities, including by strata of catechol-O-methyltransferase (COMT) genotype in adults aged 78–90 years (n = 215)
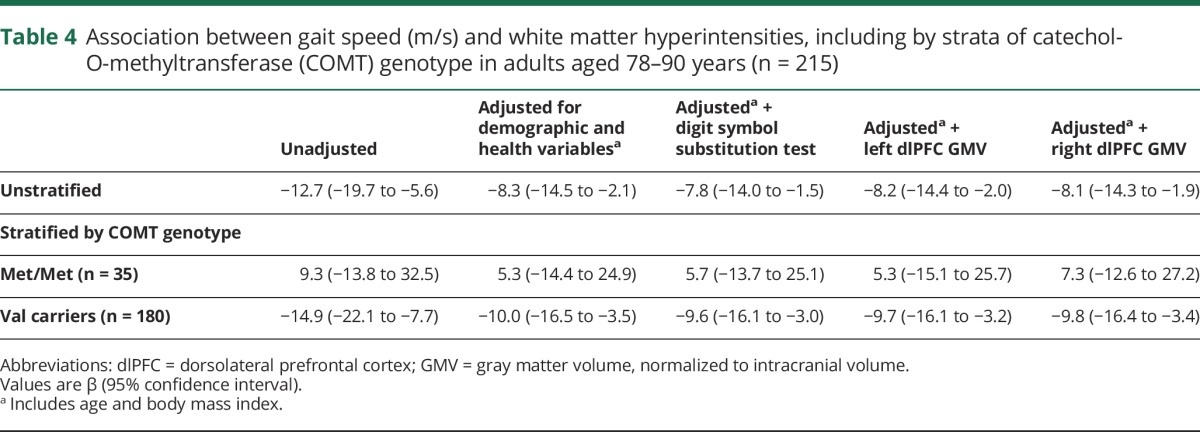
Greater volume of WMH was significantly associated with slower gait speed after adjustment for age, race, diabetes, hypertension, BMI, and quadriceps strength (β = −8.3, CI −14.5 to −2.1; table 4). The interaction between WMH and COMT genotype was significant (β = 24.2, p = 0.046) in unadjusted analyses, indicating that the association between WMH and gait speed differed by genotype. The interaction was somewhat attenuated but still strong after adjustment for health and demographic variables (β = 16.6, p = 0.1). Analyses stratified by COMT genotype (table 4) indicated a strong association between WMH and gait speed in adjusted models among those who were COMT Val carriers (β = −10.0, CI −16.5 to −3.5). In contrast, there was no association between WMH and gait speed in adjusted models among those with the COMT Met/Met genotype (β = 5.3, CI −14.4 to 24.9).
Table 4.
Association between gait speed (m/s) and white matter hyperintensities, including by strata of catechol-O-methyltransferase (COMT) genotype in adults aged 78–90 years (n = 215)
Addition of MPS to the gait speed models or gait speed to the MPS models did not change the results (data not shown). In all analyses, adjustment by DSST, left dlPFC GMV, or right dlPFC GMV did not attenuate the results (tables 3 and 4). In addition, results did not qualitatively differ when WMH in specific tracts was assessed (data not shown). WMH in individual tracts were highly correlated with total WMH volume (r range 0.68–0.89, all p < 0.001).
Discussion
In this sample of adults aged 78–90 years, we found that MPS were associated with WMH but not with COMT genotype. However, COMT genotype did modify the association of WMH with MPS, indicating a possible compensatory role of dopamine in the presence of cerebral small vessel disease. There was a strong association between greater WMH and MPS among COMT Val allele carriers, indicative of lower synaptic dopamine levels and lower PFC efficiency,19 whereas there were no associations in those with the COMT Met/Met genotype. The associations did not differ after adjustment for cognitive and structural markers of dlPFC integrity. Similar results were observed for gait speed.
We found no direct association of COMT genotype and MPS or gait speed in our sample. No previously reported studies have assessed associations of COMT genotype and MPS. A previous study found that those with the Met/Val COMT genotype had a faster gait speed cross-sectionally compared to the 2 homozygotes.20 It is unclear why our results differ as the samples were similar in age and other characteristics, but the previous study did not account for the known differences in COMT allele distribution by race,21 whereas our analyses were adjusted for race. A previous analysis of the full Health ABC cohort confirms our findings here of a lack of a cross-sectional association between COMT and gait speed.22 That study did find that individuals with the Met/Val genotype maintained gait speed during 10 years of follow-up better than individuals with either of the homozygote genotypes.22
While we did not find an association between COMT and MPS or gait speed, we did find that the association of WMH with MPS or gait speed differed by COMT genotype, suggesting that the link between COMT and motor impairments may not be direct. The previous studies that demonstrated an association of COMT with faster gait20 or slower gait decline22 found the Met/Val genotype to be most beneficial. In contrast, we found that the Met/Met genotype provided the greatest compensation for gait speed in the presence of WMH. Dopamine function is hypothesized to follow a U-shaped pattern, with levels that are too high or too low leading to poorer functional outcomes.23 Thus, in the previous studies, the Met/Val group was thought to have the optimal dopaminergic levels leading to preserved gait speed. However, in the presence of pathology such as WMH, it may be that higher levels of dopamine, indicated by the Met/Met COMT genotype, are necessary. It is also possible that we did not find an association between COMT and MPS or gait speed because the gene is an indirect measure of dopaminergic levels. Future studies should directly measure PFC dopamine levels through PET scans.
WMH are well-established as a risk factor for MPS and slow gait in older adults.2,3,24,25 However, not all older adults with WMH have motor impairments. Our results indicate that the relative functional capacity of the dopaminergic system might provide compensation in the presence of WMH. In a large, well-characterized cohort of older adults, no significant interactions were observed between WMH and risk factors for slow gait from peripheral systems (joint pain, muscle strength, vision impairment, lung capacity, obesity, or peripheral vascular disease).24 Those results suggest that integrity of the peripheral systems is not sufficient to compensate for the effects of WMH on gait in older adults. In contrast, compensatory factors related to WM microstructural integrity have been previously identified that may limit the effect of WMH on gait speed.26,27 The dopaminergic system is vital for gait performance4 and dopamine levels decline with age28 but with significant interindividual variation. The COMT enzyme regulates synaptic dopaminergic levels in the brain, with particular importance for clearance of dopamine in the PFC.9 The Met allele of the COMT Val158Met gene is associated with greater synaptic dopamine availability,9 higher levels of executive function,28,29 and more efficient dlPFC function.19 Any of these mechanisms might explain the apparent compensatory effect of the COMT Met/Met genotype for WMH in relation to MPS and gait speed in older adults. We did not find differences in the associations of WMH between frontal and nonfrontal tracts. This may be due to the fact that in this sample of adults in their 80s, WMH are already widespread, affecting all tracts approximately equally. This is evident by the strong correlation of tract-specific WMH with total WMH in our sample.
This study had several limitations of note. First, we had no direct measures of cortical dopamine levels. However, the role of COMT genotype is well-described in relation to its role on synaptic dopamine levels.9 In addition, we had small numbers of participants in individual strata, particularly for those with the Met/Met genotype, which limited power and the precision of our estimates. Despite these limitations, we were able to detect a difference in the association of WMH with MPS and gait speed by COMT genotype in this well-characterized cohort of older adults.
These findings suggest that MPS has a vascular rather than dopaminergic origin in older adults, but both factors are important in MPS manifestation. Our results indicate a compensatory role for the dopaminergic system in maintenance of motor function in the presence of age-related WMH. Results were not attenuated by addition of DSST or dlPFC GMV to the model, indicating that these associations are independent of processing speed and structural integrity of the dlPFC. Results were also not attenuated when adjusting for MPS in gait speed models or gait speed in MPS models, indicating that these are likely independent processes. Future studies should directly assess cortical dopamine levels to determine their role in maintaining motor function of older adults with WMH. Interventions that target the dopaminergic system may be important for increasing brain resilience and enhancing motor and functional outcomes in older adults.
Glossary
- 3 MS
Modified Mini-Mental State Examination
- BMI
body mass index
- CI
confidence interval
- COMT
catechol-O-methyltransferase
- dlPFC
dorsolateral prefrontal cortex
- DSST
Digit Symbol Substitution Test
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- GM
gray matter
- GMV
gray matter volume
- HBP
Healthy Brain Project
- Health ABC
Health Aging and Body Composition
- MPS
mild parkinsonian signs
- OR
odds ratio
- PFC
prefrontal cortex
- TE
echo time
- TI
inversion time
- TR
repetition time
- WM
white matter
- WMH
white matter hyperintensities
Author contributions
Andrea L. Rosso: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content. Nicolaas Bohnen: interpretation of data, critical revision of manuscript for intellectual content. Lenore Launer: interpretation of data, critical revision of manuscript for intellectual content. Howard J. Aizenstein: study supervision, critical revision of manuscript for intellectual content. Kristine Yaffe: study supervision, critical revision of manuscript for intellectual content. Caterina Rosano: study concept and design, interpretation of data, critical revision of manuscript for intellectual content, study supervision.
Study funding
Health ABC was supported by National Institute on Aging (NIA) contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106), NIA grant R01-AG-028050, and NINR grant R01-NR-012459. The Healthy Brain Project was supported in part by the NIA (K23-AG-028966, R01-AG-029232) and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827-07. These analyses were supported by the University of Pittsburgh Clinical and Translational Science Institute (KL2 TR000146) and the NIA (1 K01 AG053431-01).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in older adults and its association with adverse health outcomes and neuropathology. J Gerontol A Biol Sci Med Sci 2016;71:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatate J, Miwa K, Matsumoto M, et al. Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat Disord 2016;26:29–34. [DOI] [PubMed] [Google Scholar]
- 3.de Laat KF, van Norden AG, Gons RA, et al. Cerebral white matter lesions and lacunar infarcts contribute to the presence of mild parkinsonian signs. Stroke 2012;43:2574–2579. [DOI] [PubMed] [Google Scholar]
- 4.Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res 2008;185:391–398. [DOI] [PubMed] [Google Scholar]
- 5.Cacabelos R. Parkinson's disease: from pathogenesis to pharmacogenomics. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 7.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 2013;68:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 9.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 2004;29:1943–1961. [DOI] [PubMed] [Google Scholar]
- 10.Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry 1999;46:557–567. [DOI] [PubMed] [Google Scholar]
- 11.Fiocco AJ, Lindquist K, Ferrell R, et al. COMT genotype and cognitive function: an 8-year longitudinal study in white and black elders. Neurology 2010;74:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. NeuroImage 2012;62:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 2008;40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol 2004;61:1273–1276. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452. [PubMed] [Google Scholar]
- 18.Wechsler D. Manual for the Wechsler Adult Intelligence Scale: Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 19.Nyberg L, Andersson M, Kauppi K, et al. Age-related and genetic modulation of frontal cortex efficiency. J Cogn Neurosci 2014;26:746–754. [DOI] [PubMed] [Google Scholar]
- 20.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging 2010;31:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeMille MM, Kidd JR, Ruggeri V, et al. Population variation in linkage disequilibrium across the COMT gene considering promoter region and coding region variation. Hum Genet 2002;111:521–537. [DOI] [PubMed] [Google Scholar]
- 22.Metti AL, Rosano C, Boudreau R, et al. COMT genotype and gait speed changes over ten years in older adults. J Am Geriatr Soc 2017;65:2016–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte AV, Floel A. Effects of COMT polymorphisms on brain function and behavior in health and disease. Brain Res Bull 2012;88:418–428. [DOI] [PubMed] [Google Scholar]
- 24.Rosso AL, Studenski SA, Longstreth WT Jr, Brach JS, Boudreau RM, Rosano C. Contributors to poor mobility in older adults: integrating white matter hyperintensities and conditions affecting other systems. J Gerontol A Biol Sci Med Sci 2017;72:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005;53:649–654. [DOI] [PubMed] [Google Scholar]
- 26.Rosario BL, Rosso AL, Aizenstein HJ, et al. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci 2016;71:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2011;134:73–83. [DOI] [PubMed] [Google Scholar]
- 28.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 2006;30:791–807. [DOI] [PubMed] [Google Scholar]
- 29.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev 2010;34:670–677. [DOI] [PubMed] [Google Scholar]


