Abstract
A 100% water-soluble surfactant polymer dressing (SPD) that is bio-compatible and non-ionic has been reported to improve wound closure in preliminary clinical studies. The mechanism of action of SPD in wound healing remains unclear. Biofilm infection is a significant problem that hinders proper wound closure. The objective of this study was to characterize the mechanism of action of SPD inhibition of bacterial biofilm development. Static biofilms (48 h) of the primary wound pathogens Pseudomonas aeruginosa (PA01), Staphylococcus aureus (USA300) were grown on polycarbonate membranes and treated with SPD with and without antibiotics for an additional 24 h. The standard antibiotics – tobramycin (10 μg/ml) for PA01 and rifampicin (10 μg/ml) for USA300, were used in these studies. Following 24 h treatment with and without antibiotics, the biofilms were characterized using scanning electron microscopy (SEM) structural imaging, in vitro imaging system (IVIS) proliferation imaging, colony forming units (CFU), viability assay, quantitative PCR (qPCR) for virulence gene expression. Because SPD is a surfactant based dressing, it potentially has a direct effect on Gram negative bacteria such as Pseudomonas primarily due to the lipid-based outer membrane of the bacteria. SPD is a surfactant based dressing that has potent anti-biofilm properties directly or in synergy with antibiotics.
Introduction
Chronic wounds represent a significant burden to patients, health care professionals, and the US health care system, affecting 5.7 million patients and costing an estimated 25 billion dollars annually1,2. Bioburden, particularly in the form of microbial ‘biofilms’, is a significant barrier to healing of chronic wounds3. By definition, a biofilm is an aggregate of microorganisms that are found to be associated with biotic or abiotic surfaces4. The aggregate is held together by polymeric matrix secreted by the bacteria themselves5. The self-produced matrix helps bacteria to adhere to each other and/or to the substrate surface and serves as a defensive barrier against the penetration of antimicrobial substances and antibodies6–11.
Wound debridement has been widely used to remove necrotic tissue from a wound to remove dead and infected tissue and promote healing12–15. Necrotic tissue prolongs the inflammatory stage and may serve as a reservoir for biofilm bacteria. Wound debridement may be performed in several different ways: surgical, autolytic, enzymatic, and mechanical15–18. Each of these has its own benefits and shortcomings, depending on the wound type and underlying patient health. Furthermore, wound cleansers are often used before or even alongside debridement agents to remove loosened tissue debris, bacteria, and other physicochemical contaminants that can seriously impede the wound healing process. Some dressings contain certain levels of metal elements (e.g., silver) as principal bactericides, while wound cleansers rely on the cleaning power of various surfactants to remove the debris from the wound bed. The use of surfactants for biofilm removal as part of chemical treatment has been proposed19. Most surfactants are primarily employed as wound scrubs and cleansing solutions20–26 and also as carriers for antibiotics and antimicrobials27. Poloxamer-based non-ionic surfactant gels have been used as delivery agents for antimicrobials. Compared to the standard silver sulfadiazine creams, surfactant gels are easily removed from wound surfaces28,29. Interestingly, poloxamer surfactants have been well tolerated by patients when topically applied20,21,30,31. It is claimed that poloxamer may have pro-healing effects on full-thickness skin wounds32.
The purpose of this current work was to evaluate the effect of a surfactant polymer dressing (SPD) on two primary wound pathogens - Pseudomonas aeruginosa PA01 and Staphylococcus aureus USA300. USA 300 is a methicillin resistant isolate. SPD is a burn and wound dressing that is 100% water-soluble, poloxamer-based and non-ionic. SPD is generally recognized as safe by the Food and Drug Administration and is used in clinic as clinic as a product that softens, loosens and traps debris and necrotic tissue. In addition to addressing the effect of SPD on PA01 and USA300 in their planktonic forms, this work investigates the potential effects of SPD on biofilm infection and related mechanisms.
Results
SPD exhibits anti-bacterial properties
SPD significantly decreased the growth rate of both Gram negative (P. aeruginosa PA01) and Gram positive (S. aureus USA300) bacteria grown planktonically in broth cultures. Optical density (OD600) measurements indicated slower growth kinetics in SPD treated compared to untreated broth cultures (Supplemental Fig. S1A,B). Viability analysis using CFU/ml calculations indicated significant decrease in SPD treated (106–108) compared to untreated (>1010) Gram positive and Gram negative bacterial strains. However, CFU/ml viability assay performed on cultures following 24 h of treatment suggested a bacteriostatic rather than bactericidal effect of SPD. Although viability was significantly decreased in SPD treated samples, the bacteria were still able to grow once the inhibitory effect of SPD was withdrawn (Supplemental Fig. S1C,D).
Rhl-regulated virulence factor, pyocyanin, inhibited by SPD
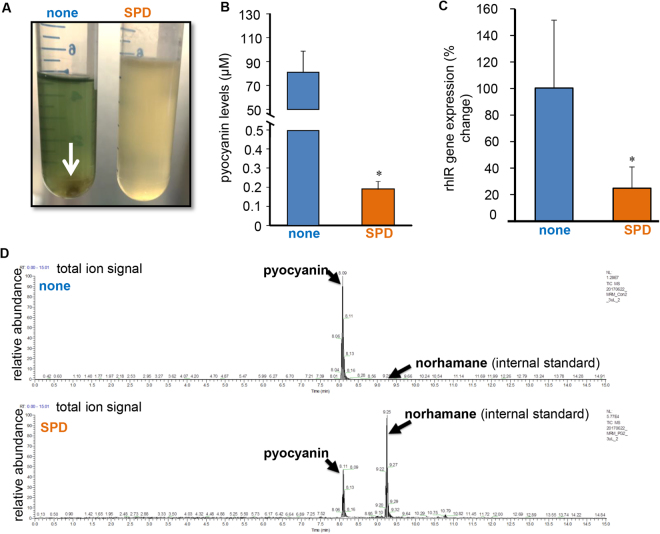
During growth curve studies it was observed that PA01 grown in the presence of SPD did not produce the characteristic green pigment pyocyanin after 48–72 h of treatment (Fig. 1A). Pyocyanin is a virulence factor produced by P. aeruginosa and is regulated by the rhl quorum sensing pathway. Liquid chromatography – mass spectrometry (LC-MS) analysis provided quantitative corroboration of low pyocyanin production in SPD treated samples (Fig. 1C). Furthermore, markedly lowered expression of rhlR was observed in SPD treated samples. 16 s rRNA was used as the housekeeping gene. Interestingly, untreated samples also showed characteristic aggregates of bacteria (Fig. 1A, white arrow) that were conspicuously absent in SPD treated cultures. The uniform turbidity of SPD treated cultures point towards the ability of SPD to inhibit aggregation of biofilm forming PA01.
Figure 1.
SPD inhibits Rhl regulated pyocyanin production by P. aeruginosa PA01. (A) Biofilm co-aggregation observed in the no treatment PA01 culture was not observed in SPD treated 48-72 hours cultures, n = 6. (B) Bar graph showing mean levels of pyocyanin in control and SPD treated samples. Data are shown mean ± SD, n = 6, *p < 0.05. (C) The total ion signal chromatograms of pyocyanin and internal standard norharmane produced by P. aeruginosa PA01 in normal condition (con) and in the presence of compound (SPD). The retention times for pyocyanin and norharmane with the solvent system used in this study were 8.1 min and 9.25 min, respectively. The total ion signal chromatograms of pyocyanin and norharmane (internal standard) produced by P. aeruginosa PA01 in normal condition (con) and in the presence of compound (SPD). The retention times for pyocyanin and norharmane with the solvent system used in this study were 8.1 min and 9.25 min, respectively. (D) P aeruginosa PA01 was either left untreated or treated for 24 h with SPD. mRNA was isolated and real-time PCR was performed to assess rhlR gene expression in PA01.
SPD disrupted biofilm matrix
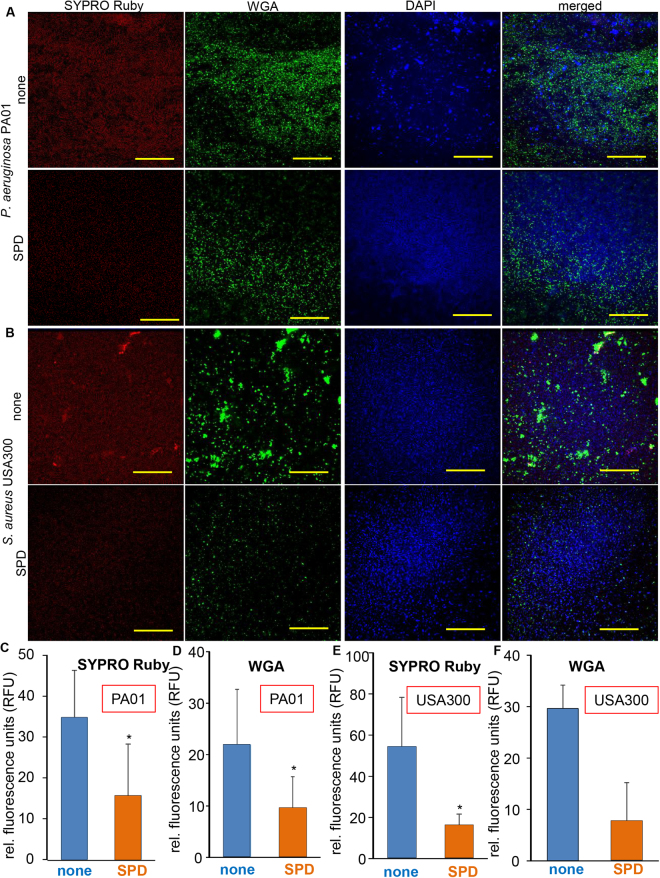
The observation that SPD inhibited PA01 aggregation in broth culture prompted our studies testing the effect of SPD on biofilm formation. We utilized the static biofilm model as previously published33. Bacteria in a biofilm matrix are encased within extrapolymeric substance (EPS) that is typically composed of nucleic acid, proteins and polysaccharides. Confocal microscopic analysis using matrix targeted stains such as SYPRO Ruby (protein components) and wheat germ agglutinin (WGA; cell wall components) indicated significant disruption of PA01 and USA300 biofilms by SPD (Fig. 2). This was visualized qualitatively as minimized positive staining in SYPRO Ruby (red), WGA (green) and DAPI (blue) channels in SPD treated compared to no treatment groups. Quantifications of SYPRO Ruby and WGA staining in PA01 and USA300 biofilms that were untreated or SPD treated indicated significant decrease in matrix components.
Figure 2.
SPD disrupts biofilm matrix in P. aeruginosa PA01 and S. aureus USA300. Triple staining with DAPI (DNA), SYPRO Ruby (biofilm matrices) and FITC-Wheat Germ Agglutinin (membrane glycoproteins) was performed for P. aeruginosa PA01. (A) Shown are representative images of PA01 biofilms that were untreated or SPD treated and stained with the respective dyes. Graphical representation of staining intensity for (C) SYPRO Ruby and (D) WGA are shown. Data are shown mean ± SD, n = 6. *p≤0.05. Scale bar = 40 µM. Triple staining with DAPI (DNA), SYPRO Ruby (biofilm matrices) and FITC-Wheat Germ Agglutinin (membrane glycoproteins) was performed for S. aureus USA300. (B) Shown are representative images of USA300 biofilms that were untreated or SPD treated and stained with the respective dyes. Graphical representation of staining intensity for (E) SYPRO Ruby and (F) WGA are shown. Data are shown mean ± SD, n = 6. *p≤0.05. Scale bar = 40 µM.
SPD inhibited metabolic activity of biofilm bacteria
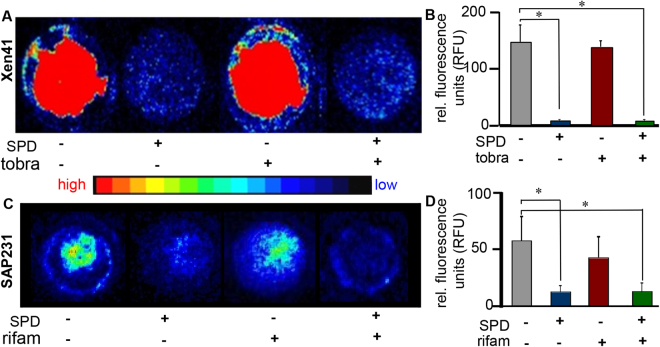
The effects of SPD on the metabolic activity of biofilm bacteria were investigated using bioluminescent strains of PA01 (Xen41) and USA300 (SAP231). Static biofilms of Xen41 and SAP231 were treated with SPD or relevant antibiotics alone or together for 24 h. Following 24 h treatment, IVIS imaging indicated that for both strains, SPD alone or in combination with antibiotic caused a significant decrease in bacterial metabolism within the biofilm (Fig. 3A,B) compared to no treatment. Biofilms treated with antibiotic alone (tobramycin for PA01 and rifampicin for USA300) showed metabolic activity similar to that of the control group. To test inhibition of metabolic activity a biochemical index, ADP/ATP ratio, was measured in PA01 (Supplemental Fig. S2). Elevated ADP/ATP in response to SPD indicated metabolic inhibition.
Figure 3.
SPD decreases metabolic activity of bacteria. Shown are representative IVIS images and corresponding graphical representations of intensity quantitation from 48 h biofilms of (A,B) P. aeruginosa Xen41 and (C,D) S. aureus SAP231 either left untreated, or treated for 24 h with antibiotic (tobramycin (tobra) or rifampicin (rifam) respectively) alone, SPD alone or a combination of SPD and antibiotic. Data are shown mean ± SD, n = 8, *p < 0.05. Also included is a heat map showing intensity ranges. The intensity of blue and red signals were quantified using ImageJ and shows that bacteria in both control and antibiotic treated group show higher metabolic activity whereas SPD and combination therapy resulted in decreased metabolic activity.
Synergistic function of SPD and antibiotics against biofilm
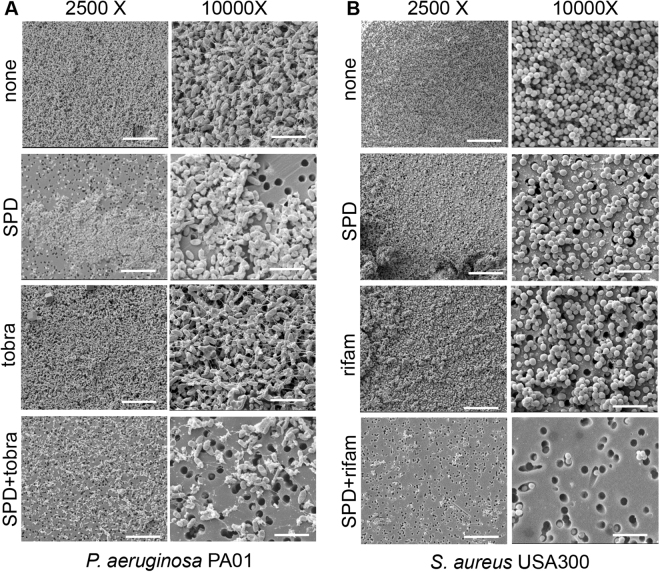
When SPD was applied to static biofilm of PA01 and USA300, impaired cell viability was observed. Such antimicrobial effect was further augmented in the presence of appropriate antibiotics (Fig. 4 and Supplemental Fig. S3). Compared to no-treatment and antibiotic alone, SPD treated PA01 (Supplemental Fig. S3A) and USA300 (Supplemental Fig. S3C) biofilms showed loss of bacteria viability as visualized by confocal microscopy. Such effect was further augmented in the SPD and antibiotic combination treatment group, indicating synergistic action between SPD and antibiotics (Supplemental Fig. S3B, D).
Figure 4.
SPD acts synergistically with antibiotics and disrupts biofilm structural integrity. 48 h static biofilms of (A) P. aeruginosa PA01 and (B) S. aureus USA300 were either left untreated or treated for 24 h with SPD or antibiotic (tobramycin (tobra) or rifampicin (rifam)) alone or in combination. Shown are representative scanning electron micrographs (SEM) at 2500× and 10000× magnifications. SPD alone or in combination with antibiotics showed fewer dense aggregates of bacteria with associated matrix. n = 6. Scale bar = 10 μm, 2500× magnification and 5 μm, 10000× magnification.
Static (48 h) biofilms of (A) P. aeruginosa PA01 and (B) S. aureus USA300 were either left untreated or treated for 24 h with SPD or antibiotic (tobramycin (tobra) or rifampicin (rifam) alone or in combination. Shown are representative scanning electron micrographs (SEM) at 2500× and 10000× magnifications. SPD alone or in combination with antibiotics showed fewer dense aggregates of bacteria with associated matrix. n = 6. Scale bar = 10 μm, 2500× magnification and 5 μm, 10000× magnification (Fig. 2).
Discussion
Anti-biofilm property of a clinically used wound debridement agent is of major significance in wound care. Surfactants are known to interact with cellular components such as proteins and lipids, resulting in inhibitory effects on the growth and viability of microbial cells34,35. Surfactants are also known to induce cell autolysis in bacterial strains such as Bacillus subtilis36–38 and inhibit respiration in P. aeruginosa39. Antibacterial agents can elicit both bacteriostatic and/or bactericidal effects. Bacteriostatic effects are reversible following the removal or neutralization of the antibacterial agent40. In contrast, bactericidal action results in irreversible or irreparable damage to a vital cell structure or function41. Earlier works have reported several anionic and nonionic detergents to have bacteriostatic, but not bactericidal activity42–47. In keeping with this, SPD, a surfactant, was found to exert a bacteriostatic effect on the bacterial strains studied. However, in the case of SPD the possibility of bacterial cell death may not be ruled out. Quorum-sensing (QS) is a social behavior exhibited by bacterial strains whereby chemical-based communication modulates behavior of the group/aggregates of bacteria. The production of several virulence factors by P. aeruginosa is controlled via threshold aggregation through two QS systems, las and rhl48. The rhl QS system has been shown to control the expression of the genes coding for pyocyanin, a toxic secondary metabolite, among other important virulence factors. PA01 grown in presence of SPD showed blunted pyocyanin production. It is widely accepted that the ability to communicate both within and between species is critical for bacterial survival and possibly pathogenicity49–51. The impact of quorum sensing on virulence, makes it an attractive target for the development of new therapeutic strategies52. Our observations are the first to describe a direct effect of SPD on QS. These findings could be attributed to a direct negative effect of SPD on bacterial cell viability and/or aggregation capacity. External application of surfactant disrupted the integrity of biological lipid membranes causing loss of bacterial cell viability. Furthermore, SPD mediated disruption of inter-bacterial physical interactions, could inhibit aggregation and formation of the bacterial quorum needed to activate QS pathways. Detergents are known to be capable of neutralizing adhesion of bacterial aggregates, a key process in biofilm formation53,54. Studies testing the effect of SPD on the expression of other rhl regulated virulence factors as well as other QS pathways such as las and PQS are warranted.
Findings of this work are consistent with previously reported observations claiming anti-biofilm effects of SPD in a porcine skin explant infected with P. aeruginosa55. A regimen of daily application of SPD resulted in elimination of P. aeruginosa biofilms within 3 days through ‘soft’ debridement via simple wiping off of the dressing. The use of SPD converted biofilm to a more susceptible planktonic-like phenotype. The ability of detergents to disperse biofilm has been previously reported56,57. Findings of this work demonstrating SPD-induced dissolution of biofilm matrix and dismantling of biofilm structure provide a mechanistic basis for the observation made in the pig skin explants study55. Bacteria that grow in biofilms have decreased antibiotic susceptibility that further promotes persistent infection in wounds or implanted devices58,59. Among the mechanisms responsible for such decreased susceptibility are retarded antibiotic penetration, altered microenvironment and slow growth and activation of protective stress responses59. Dispersion of biofilm structures, as evident in this work as well, has been recognized as an important factor that increases antibiotic susceptibility of detergent-treated biofilm bacteria60–62. The synergistic effect of combination therapies are rapidly becoming the new alternative strategy to increasing killing potency of antimicrobial agents and controlling bacterial infections63–65. Our observations are the first to describe a synergistic inhibition of biofilm viability by SPD with antibiotics. These findings could reflect improved antibiotic penetration and accessibility to bacterial targets within biofilm caused by SPD-mediated disruption of biofilm matrix structure.
In conclusion, SPD is a surfactant based wound dressing that possesses anti-biofilm properties directly or in synergy with antibiotics. Our studies provide initial insight into a possible role for SPD as an anti-biofilm agent. Taken together with its biocompatible and non-toxic nature, SPD may have promising applications in wound care. In vitro biofilm studies are useful tools to dissect mechanism of action of agents such as surfactant polymers but require supporting studies in relevant pre-clinical models and controlled clinical studies to ensure that these findings are translationally relevant.
Materials and Methods
Surfactant polymer dressing (SPD)
A non-ionic surfactant polymer dressing (SPD; PluroGel®, Medline Industries, Inc.) was used66. A 200 mg/ml final concentration was used for the experiments involving suspension culture. For non-suspension biofilm studies, a final concentration of 0.89 mg/mm2 of SPD was used.
Bacterial strains and growth conditions
The strains used for this study included Staphylococcus aureus (USA300LAC, the dominant clone of the CA-MRSA lineage) and Pseudomonas aeruginosa PA01 (wild-type strain; serotype O5)67,68. Additionally, for the IVIS experiments performed, bioluminescent strains of S. aureus (SAP231; base strain USA300) and P. aeruginosa (Xen41; base strain PA01) (PerkinElmer, USA) were used69. Both these strains contain an integrated plasmid carrying a luciferase gene that is constitutively expressed. S. aureus strains were cultured in tryptic soy broth (TSB) and P. aeruginosa strains were cultured in low salt Luria-Bertani LB broth for planktonic culture and the respective agar formats for biofilm studies (Thermo Fisher Scientific). Bioluminescent bacteria only produce light when they are alive and metabolically active. Thus, we used light emission as detected with the in vivo imaging system (IVIS) spectrum system (Perkin Elmer), as an indicator of bacterial activity.
Bacterial growth curves and CFU analyses
PA01 and USA300 were cultured in round bottom tubes in LB or TSB media, respectively, with continuous shaking @300 rpm at 37 °C. Absorbance was obtained using 10 mm optical path length quartz cuvettes in a UV-Vis spectrophotometer by measuring optical density (OD) at 600 nm over different time points. Colony forming units (CFU) are indicators of bacterial growth. Following a 24 h growth period with or without SPD, serial dilutions of the broth culture was performed using sterile 1× -phosphate buffered saline (PBS). A standard volume of select dilutions were plated on agar plates and incubated overnight at 37 °C. Manual colony count was performed and used to calculate total bacterial load per ml of broth (CFU/ml).
Bacterial growth following SPD withdrawal
To evaluate bacteriostatic properties of SPD, SPD was withdrawn to test whether bacterial growth recovers. PA01 and USA300 were cultured in round bottom tubes in LB or TSB media, respectively, with continuous shaking (300 rpm) at 37 °C for. Following a 10 h growth period with SPD, SPD was withdrawn from the growth media by centrifuging the broth and resuspending the bacterial cell pellet in fresh LB or TSB media. All three groups (broth without SPD, broth exposed to SPD for 10 h and broth treated with SPD for the entire 10 + 24 h duration) were then allowed to grow for an additional 24 h. Absorbance was obtained using 10 mm optical path length quartz cuvettes in a UV-Vis spectrophotometer by measuring optical density (OD) at 600 nm.
Mass spectrometric analysis of pyocyanin
To prepare P. aeruginosa conditioned medium, cultures were grown for 3 days to a high optical density (OD), at which quorum-sensing is activated in PA01, thus triggering the production of the cytotoxic exoproduct pyocyanin (PCN)70–72. PCN was extracted from cell-free filtrate using methanol (1:1) precipitation. Norharmane (NH) was used as internal standard. After centrifugation the supernatant was diluted 1:10 in 0.1% Formic Acid (v/v) in H2O and subjected to HPLC-MS analysis. Liquid chromatography and MS were performed by the Mass Spectrometry and Proteomics Facility (The Ohio State University) on a Dionex 3000 LC unit with a Thermo Quantiva triple quadrupole mass spectrometer equipped with an electrospray ionization source and run in the positive ion mode for detection. Briefly, an aliquot of the broth (fluid) following protein precipitation was added into an Agilent ZORBAX SB-Aq 3.5 µm column (150 × 3.0 mm). The mobile phase gradient was generated using H2O with 0.1% formic acid and 100% acetonitrile at a flow of 400 µL/min and a total run time of 15 min. The major PCN peak eluted at 8.1 min while NH eluted at 9.25 min and they were detected from using the following precursor to fragment transitions; for PCN 211.1/168.1, 211.1/183.1, and 211.1/196.1, while NH transitions were monitored at 169.1/115.0 and 169.1/142.1. With a series of standards from 0.001 to 50 µM PCN and an NH internal standard concentration of 0.28 µM the ratio of PCN to NH was determined for each to produce a calibration curve with an R2 of 0.9960 and a linear regression of (Area ratio = 1.892*conc in µM).
Aggregation assay
Multicell auto aggregation of planktonic PA01 cells is a phenotype observed in the initial phase of biofilm formation. Bacteria were grown in LB medium for overnight at 37 °C. Overnight culture was diluted in LB and incubated for 48 h in presence or absence of SPD under same culture conditions. Cells were gently inverted several times and placed at room temperature for 15 min73.
In vitro biofilm model
PA01 and USA300 biofilms were developed in vitro using a polycarbonate filter model. Grown overnight in LB and TSB media at 37 °C, bacteria were cultured on sterile polycarbonate membrane filters placed on LBA and TSA agar plates, respectively, and allowed to form mature biofilm for 48 h. The biofilms were then exposed to SPD alone, antibiotic (10 µg/ml each of tobramycin or rifampicin) alone and SPD in combination with antibiotic or left untreated for 24 h73.
Scanning electron microscopy (SEM)
Biofilm grown on polycarbonate membranes were fixed in 2% glutaraldehyde solution for 48 h at 4 °C, washed with phosphate-buffered saline (PBS), dehydrated in a graded ethanol series, washed with hexamethyldisilazane (HMDS, Ted Pella Inc., CA) and left to air-dry overnight. The samples were mounted and sputter-coated with a gold-palladium (Au-Pd) and imaged with the SEM operating at 5 kV in the secondary electron mode (XL 30 S; FEG, FEI Co., Hillsboro, OR)74.
Viability staining
The BacLight™ bacterial viability kit for microscopy and quantitative assays were used to monitor bacterial viability. Bacterial cells with a compromised membrane that are considered to be dead or dying stain red, whereas cells with an intact membrane stain green. The polycarbonate membrane discs were visualized using an Olympus Fluoview FV1000 spectral confocal microscope (Olympus FV1000 IX81, ON) under 40× magnification using an argon laser73.
Biofilm matrix staining
Bacterial biofilm were prepared under static conditions as described and stained with FilmTracer™ SYPRO® Ruby biofilm matrix stain (Molecular Probes) according to manufacturer’s instructions75. After 30 min incubation at room temperature, the fluorescent marker solution was removed. Biofilm were washed with water. Next, the biofilm discs were stained with wheat germ agglutinin, Alexa Fluor™ 488 conjugate (ThermoFisher Scientific, 5.0 µg/mL) for 10 minutes at room temperature and finally DAPI (Invitrogen 1:10,000) was used as a nuclear counterstain. Stained biofilms were visualized by confocal laser scanning microscopy (CLSM; Olympus FV1000 IX81, ON). Images were analyzed for their relative fluorescence units (RFU) using ImageJ, an application available from NIH at http://imagej.nih.gov/ij/download.html. After loading the image into ImageJ, the channels were split by running Image → Colour → Split and channel intensities were measured76.
Quantification of mRNA expression
Total RNA was isolated using Norgen™ RNA isolation kit, according to the manufacturer’s protocol. Gene expression levels were quantified with real-time PCR system and SYBR Green (Applied Biosystems) and normalized to 16 s rRNA as housekeeping genes. Expression levels were quantified employing the 2 (−ΔΔct) relative quantification methods. The following primer sets were used: P.aeruginosa 16 s rRNA (forward) 5′–GGG GGA TCT TCG GAC CTC A–3′, (reverse) 5′–TCC TTA GAG TGC CCA CCC G–3′, pmas_rhlR (forward) 5′–TGC CGT ATC GGC AAG GCT GC–3′, (reverse) 5′–TTC CAG GAC GGC GAA CAC GC–3′73.
ADP/ATP ratio assay
ADP/ATP was measured as marker of metabolic activity. Bacterial cells were collected by vigorously vortexing the polycarbonate membrane discs in assay buffer provided in the ADP/ATP Ratio Assay Kit (BioAssay Systems, Hayward, CA). Luminescence was measured according to the manufacturer’s instructions.
Statistics
Control and treated samples were compared using a paired t test. One-way ANOVA was used for all other comparison of differences between means of multiple samples in a group. p < 0.05 was considered significant. Data represented in bar graphs were plotted as mean ± SD. Replicates represent biological replicates.
Electronic supplementary material
Acknowledgements
We thank Matthew Bernier (Mass Spectrometry and Proteomics Facility, The Ohio State University) for his help in LC-MS. The financial support for the Quantiva QQQ instrument was provided by OSU and OSUCCC and the core was supported with NIH Award Number Grant P30 CA016058. We thank Drs. Mithun Sinha and Kanhaiya Singh for their assistance with pyocyanin studies. Wound care and biofilm research in the laboratory are funded by the NIH RO1 awards GM108014, GM077185, NR 015676, NR013898, and DK076566.
Author Contributions
Study conceived and designed: C.K.S., S.R. and P.D.G. Performed the experiments: P.D.G. and P.P. Analyzed the data: P.D.G. and S.S.M.S. Wrote the manuscript: P.D.G., S.S.M.S., S.R. and C.K.S.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19175-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Advances in wound care. 2015;4:560–582. doi: 10.1089/wound.2015.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CE, Kennedy JP. Treatment options to manage wound biofilm. Advances in wound care. 2012;1:120–126. doi: 10.1089/wound.2011.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vert M, et al. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012) Pure and Applied Chemistry. 2012;84:377–410. doi: 10.1351/PAC-REC-10-12-04. [DOI] [Google Scholar]
- 5.Flemming H-C, Wingender J. The biofilm matrix. Nature reviews. Microbiology. 2010;8:623. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 6.Dufour D, Leung V, Lévesque CM. Bacterial biofilm: structure, function, and antimicrobial resistance. Endodontic Topics. 2010;22:2–16. doi: 10.1111/j.1601-1546.2012.00277.x. [DOI] [Google Scholar]
- 7.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annual Reviews in Microbiology. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 8.Donné J, Dewilde S. Chapter Five-The Challenging World of Biofilm Physiology. Advances in microbial physiology. 2015;67:235–292. doi: 10.1016/bs.ampbs.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnsholt T, et al. The in vivo biofilm. Trends in microbiology. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. Journal of antimicrobial chemotherapy. 2010;65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 11.Cos P, Tote K, Horemans T, Maes L. Biofilms: an extra hurdle for effective antimicrobial therapy. Current pharmaceutical design. 2010;16:2279–2295. doi: 10.2174/138161210791792868. [DOI] [PubMed] [Google Scholar]
- 12.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. Journal of the American College of Surgeons. 1996;183:61–64. [PubMed] [Google Scholar]
- 13.Falanga V. Wound healing and its impairment in the diabetic foot. The Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 14.Steed DL. Debridement. The American journal of surgery. 2004;187:S71–S74. doi: 10.1016/S0002-9610(03)00307-6. [DOI] [Google Scholar]
- 15.Ayello EA, Cuddigan JE. Debridement: controlling the necrotic/cellular burden. Advances in skin & wound care. 2004;17:66–75. doi: 10.1097/00129334-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Ousey, K. & McIntosh, C. Understanding wound bed preparation and wound debridement. British journal of community nursing15 (2010). [DOI] [PubMed]
- 17.Stephen-Haynes, J. & Thompson, G. The different methods of wound debridement. British journal of community nursing12 (2007). [PubMed]
- 18.Falabella AF. Debridement and wound bed preparation. Dermatologic therapy. 2006;19:317–325. doi: 10.1111/j.1529-8019.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 19.Marberry KM, et al. Surfactant wound irrigation for the treatment of staphylococcal clinical isolates. Clinical orthopaedics and related research. 2002;403:73–79. doi: 10.1097/00003086-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Anglen J, Gainor B, Simpson W, Christensen G. The use of detergent irrigation for musculoskeletal wounds. International orthopaedics. 2003;27:40–46. doi: 10.1007/s00264-002-0398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant CA, et al. Search for a nontoxic surgical scrub solution for periorbital lacerations. Annals of emergency medicine. 1984;13:317–321. doi: 10.1016/S0196-0644(84)80113-4. [DOI] [PubMed] [Google Scholar]
- 22.Dire DJ, Welsh AP. A comparison of wound irrigation solutions used in the emergency department. Annals of emergency medicine. 1990;19:704–708. doi: 10.1016/S0196-0644(05)82484-9. [DOI] [PubMed] [Google Scholar]
- 23.Edlich RF, et al. Physical and chemical configuration of sutures in the development of surgical infection. Annals of surgery. 1973;177:679. doi: 10.1097/00000658-197306000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell JM, Bresnahan KA, Stair TO, Dhindsa HS, Edwards BA. Comparison of effects of suture and cyanoacrylate tissue adhesive on bacterial counts in contaminated lacerations. Antimicrobial agents and chemotherapy. 1995;39:559–560. doi: 10.1128/AAC.39.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodeheaver G. Controversies in topical wound management. Wounds. 1989;1:19–27. [Google Scholar]
- 26.Magee C, et al. Potentiation of wound infection by surgical drains. The American Journal of Surgery. 1976;131:547–549. doi: 10.1016/0002-9610(76)90007-6. [DOI] [PubMed] [Google Scholar]
- 27.Faulkner DM, et al. A new stable pluronic® F68 gel carrier for antibiotics in contaminated wound treatment. The American journal of emergency medicine. 1997;15:20–24. doi: 10.1016/S0735-6757(97)90041-3. [DOI] [PubMed] [Google Scholar]
- 28.Black JS, Drake DB. A prospective randomized trial comparing silver sulfadiazine cream with a water-soluble polyantimicrobial gel in partial-thickness burn wounds. Plastic Surgical Nursing. 2015;35:46–49. doi: 10.1097/PSN.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 29.Zölß C, Cech JD. Efficacy of a new multifunctional surfactant‐based biomaterial dressing with 1% silver sulphadiazine in chronic wounds. International wound journal. 2016;13:738–743. doi: 10.1111/iwj.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodeheaver GT, Smith SL, Thacker JG, Edgerton MT, Edlich RF. Mechanical cleansing of contaminated wounds with a surfactant. The American Journal of Surgery. 1975;129:241–245. doi: 10.1016/0002-9610(75)90231-7. [DOI] [PubMed] [Google Scholar]
- 31.Fowler EB, et al. Evaluation of pluronic polyols as carriers for grafting materials: study in rat calvaria defects. Journal of periodontology. 2002;73:191–197. doi: 10.1902/jop.2002.73.2.191. [DOI] [PubMed] [Google Scholar]
- 32.Kant V, et al. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochemica. 2014;116:5–13. doi: 10.1016/j.acthis.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Roy S, et al. Mixed‐species biofilm compromises wound healing by disrupting epidermal barrier function. The Journal of pathology. 2014;233:331–343. doi: 10.1002/path.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell, A. D. & Hugo, W. B. In Principles and Practice of Disinfection, Preservation and Sterilization (2nd edn) (eds Russell, A. D. Hugo, W. B. & Ayliffe, G. A. J.) 34–38 (Blackwell Scientific Publications, 1992).
- 35.Merianos. In Disinfection, Sterilization and Preservation (ed Block, S. S.) 225 (Lea & Febiger, 1991).
- 36.Cho H-Y, Tsuchido T, Ono H, Takano M. Cell death of Bacillus subtilis caused by surfactants at low concentrations results from induced cell autolysis. Journal of Fermentation and Bioengineering. 1990;70:11–14. doi: 10.1016/0922-338X(90)90022-O. [DOI] [Google Scholar]
- 37.Tsuchido T, Ahn Y-H, Takano M. Lysis of Bacillus subtilis cells by glycerol and sucrose esters of fatty acids. Applied and environmental microbiology. 1987;53:505–508. doi: 10.1128/aem.53.3.505-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchido T, Svarachorn A, Soga H, Takano M. Lysis and aberrant morphology of Bacillus subtilis cells caused by surfactants and their relation to autolysin activity. Antimicrobial agents and chemotherapy. 1990;34:781–785. doi: 10.1128/AAC.34.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majtan V, Majtanova L, Hostacka A, Hybenova D, Mlynarcik D. Effect of quaternary ammonium salts and amine oxides on Pseudomonas aeruginosa. Microbios. 1995;84:41–51. [PubMed] [Google Scholar]
- 40.Fitzgerald KA, Davies A, Russell AD. Uptake of 14C-chlorhexidine diacetate to Escherichia coli and Pseudomonas aeruginosa and its release by azolectin. FEMS Microbiol Lett. 1989;51:327–332. doi: 10.1111/j.1574-6968.1989.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 41.Denyer SP. Mechanisms of action of antibacterial biocides. International biodeterioration & biodegradation. 1995;36:227–245. doi: 10.1016/0964-8305(96)00015-7. [DOI] [Google Scholar]
- 42.Cowles PB. Alkyl sulfates: their selective bacteriostatic action. The Yale journal of biology and medicine. 1938;11:33. [PMC free article] [PubMed] [Google Scholar]
- 43.Birkeland JM, Steinhaus EA. Selective bacteriostatic action of sodium lauryl sulfate and of “Dreft. Proceedings of the Society for Experimental Biology and Medicine. 1939;40:86–88. doi: 10.3181/00379727-40-10314P. [DOI] [Google Scholar]
- 44.Baker Z, Harrison RW, Miller BF. & Technical Assistance of Robert, W. The Bactericidal Action of Synthetic Detergents. J Exp Med. 1941;74:611–620. doi: 10.1084/jem.74.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scales, F. & Kemp, M. A new group of sterilizing agents for the food industries and a treatment for chronic mastitis. (International Association of milk dealers, 1941).
- 46.Armstrong WM. Enhancement of the biological activity of sodium dodecyl sulphate by inorganic cations. Nature. 1957;179:780–781. doi: 10.1038/179780b0. [DOI] [PubMed] [Google Scholar]
- 47.Kabara JJ. Structure-function relationships of surfactants as antimicrobial agents. J Soc Cosmet Chem. 1978;29:733–741. [Google Scholar]
- 48.Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. Journal of bacteriology. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 50.Rutherford, S. T. & Bassler, B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med2, 10.1101/cshperspect.a012427 (2012). [DOI] [PMC free article] [PubMed]
- 51.de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/IAI.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antunes LC, et al. Inhibition of Salmonella host cell invasion by dimethyl sulfide. Appl Environ Microbiol. 2010;76:5300–5304. doi: 10.1128/AEM.00851-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Percival S, et al. Surfactants and their role in wound cleansing and biofilm management. Journal of Wound Care. 2017;26:680–690. doi: 10.12968/jowc.2017.26.11.680. [DOI] [PubMed] [Google Scholar]
- 54.Ganesh A, Nagendrababu V, John A, Deivanayagam K. The Effect of Addition of an EPS Degrading Enzyme with and without Detergent to 2% Chlorhexidine on Disruption of Enterococcus faecalis Biofilm: A Confocal Laser Scanning Microscopic Study. Journal of Clinical and Diagnostic Research: JCDR. 2015;9:ZC61. doi: 10.7860/JCDR/2015/14602.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips PL, et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. International wound journal. 2015;12:469–483. doi: 10.1111/iwj.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonnichsen L, et al. Lipopeptide biosurfactant viscosin enhances dispersal of Pseudomonas fluorescens SBW25 biofilms. Microbiology. 2015;161:2289–2297. doi: 10.1099/mic.0.000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parsek MR, Greenberg E. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends in microbiology. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 59.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. International Journal of Medical Microbiology. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 60.Hentzer M, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. The EMBO journal. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Solano C, Echeverz M, Lasa I. Biofilm dispersion and quorum sensing. Current opinion in microbiology. 2014;18:96–104. doi: 10.1016/j.mib.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Petrova OE, Sauer K. Escaping the biofilm in more than one way: desorption, detachment or dispersion. Current opinion in microbiology. 2016;30:67–78. doi: 10.1016/j.mib.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris TL, Worthington RJ, Melander C. Potent Small‐Molecule Suppression of Oxacillin Resistance in Methicillin‐Resistant Staphylococcus aureus. Angewandte Chemie International Edition. 2012;51:11254–11257. doi: 10.1002/anie.201206911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischbach MA. Combination therapies for combating antimicrobial resistance. Current opinion in microbiology. 2011;14:519–523. doi: 10.1016/j.mib.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clinical microbiology reviews. 2012;25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant‐based wound dressing can reduce bacterial biofilms in a porcine skin explant model. International wound journal. 2017;14:408–413. doi: 10.1111/iwj.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. The Lancet infectious diseases. 2009;9:384–392. doi: 10.1016/S1473-3099(09)70133-1. [DOI] [PubMed] [Google Scholar]
- 68.Baker P, et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Science advances. 2016;2:e1501632. doi: 10.1126/sciadv.1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dusane DH, et al. Effects of loading concentration, blood and synovial fluid on antibiotic release and anti-biofilm activity of bone cement beads. Journal of Controlled Release. 2017;248:24–32. doi: 10.1016/j.jconrel.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Das, T., Ibugo, A. I., Klare, W. & Manefield, M. In Microbial Biofilms-Importance and Applications (InTech, 2016).
- 71.Dietrich LE, Price‐Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Molecular microbiology. 2006;61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 72.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends in molecular medicine. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Banerjee J, et al. Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PloS one. 2015;10:e0119531. doi: 10.1371/journal.pone.0119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elgharably H, et al. First evidence of sternal wound biofilm following cardiac surgery. PloS one. 2013;8:e70360. doi: 10.1371/journal.pone.0070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arciola, C. R., Campoccia, D., Ravaioli, S. & Montanaro, L. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Frontiers in cellular and infection microbiology5 (2015). [DOI] [PMC free article] [PubMed]
- 76.Hartig, S. M. Basic image analysis and manipulation in ImageJ. Current protocols in molecular biology, 14.15. 11–14.15. 12 (2013). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.