Abstract
Recently, there have been considerable advancements in cancer therapies thereby prolonging the life of cancer survivors. However, these recent advancements present new challenges in the management of bone disease in cancer survivors. Bone acts as a fertile soil for cancer seeding and bone health is often compromised because of increased inflammatory cytokines in cancer, direct cancer metastasis and toxic effects of anti-cancer therapies. This effect is more pronounced in elderly population who already have compromised bone mineral density leading to increased skeletal related events and bone pain. Timely diagnosis and effective interventions are essential for reducing bone-related morbidity in cancer survivors. Also, a complex interdependence exists between cancer related bone disease and tumor growth, creating a vicious circle of extensive bone destruction and cancer progression. Hence, maintenance of bone health and integrity plays a pivotal role in comprehensive cancer care. The bone-targeted treatments have been shown to preserve bone health, and modify the course of the underlying cancer. Management of long-term bone health requires a broad knowledge base that both endocrinologists, oncologists and other care team members should be aware of. The manuscript highlights the skeletal effects of cancer, adjuvant therapies used for hormone-responsive cancers, chemotherapy induced bone loss and steps for accurate diagnosis and management of bone disease in cancer survivors by bridging the gaps in the comprehensive cancer care.
Keywords: Bone, breast cancer, chemotherapy, multiple myeloma, prostate cancer, Denosumab, Zoledronic acid
1. Introduction
Cancer is a major risk factor for both generalized and local bone loss, with bone loss in cancer patients substantially greater than in the general population. The negative effects of cancer on bone health remains largely unrecognized, thus causing inadequate institution of preventive measures and causing delay in providing appropriate treatment. In cancer patients, there are several factors that may contribute to bone loss. These include hypogonadism (from chemotherapy, surgery, irradiation or gonadal infiltration by the tumor), pathologic fractures from metastatic invasion, immobilization, medications affecting bone metabolism such as glucocorticoids, chemotherapy drugs and opiates, increase in inflammatory markers and paraneoplastic hormonal secretion that promote bone resorption (1). This manuscript outlines the pathophysiology of bone disease in cancer survivors and helps in bridging the gaps between endocrinologists and oncologists in providing up-to-date comprehensive cancer care to prevent skeletal morbidity in this population.
2. Normal bone physiology
Bone remodeling is a dynamic process that repairs microfractures and replaces old bone with new bone. The normal bone remodeling process consists of five phases: the resting, activation, resorption, reversal, and formation phases (2).
In the activation phase, osteoclasts are recruited to the surface of the bone.
In the resorption phase (about 3–5 weeks), osteoclasts generate an acidic microenvironment between the cell and the surface of the bone, dissolving or resorbing the mineral content of the bone.
In the reversal phase, osteoclasts undergo apoptosis and osteoblasts are recruited to the bone surface.
In the formation phase (about 3–5 months), osteoblasts then deposit collagen; this is mineralized to form new bone.
Bone formation and resorption are closely coupled processes involving a balanced interplay between osteoblasts and osteoclasts. Osteoclasts are large multinucleate giant cells that are primarily involved in bone resorption. Osteoblasts make bone by producing a fibrous matrix/scaffolding that eventually gets mineralized by the deposition of calcium phosphate. Osteoclasts are regulated by osteoblasts through the expression of cytokines such as receptor activator of nuclear factor-κB ligand (RANKL), which activates osteoclast differentiation, and osteoprotegerin (OPG) that in turn inhibits RANKL. RANKL stimulates osteoclast activation by inducing secretion of lytic enzymes into a sealed resorption vacuole formed between the osteoclast and the bone surface. Acidification of this compartment by secretion of protons leads to the activation of tartrate-resistant acid phosphatase (TRAP) and cathepsin K, which are the two main enzymes responsible for the degradation of bone mineral and collagen matrices (2).
3.0 Pathophysiology of bone disease in cancer patients
3.1 Bone loss due to aging
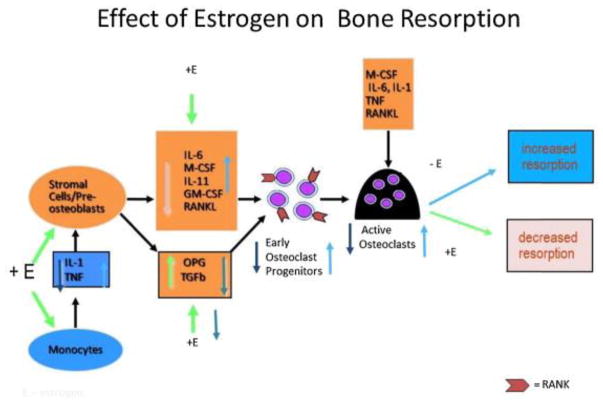
The advanced treatment regimens and comprehensive cancer care have significantly increased survival in cancer patients. With this increased life expectancy, the incidence and diagnosis of general aging diseases like osteoporosis has gone up. Moreover, cancer by itself and cancer therapies add “fuel to the fire” exacerbating the bone loss and fractures. In the aging population, physiologic decrease in estrogen level uncouples the “remodeling cycle” by increasing osteoclastic resorption activity without a corresponding increase in osteoblastic activity. Thus, normal aging tends to be associated with 0.5% – 1% bone loss per year in both men and women starting from middle age (3). The bone loss is more pronounced in immediate post-menopausal women due to a significant decrease in circulating estrogen levels (2, 3). Though men do not have this “accelerated phase” immediate post-menopausal bone loss, they are prone to “continuous phase” bone loss due to elevated PTH levels, decreased estrogen and testosterone levels by aging (4). There is substantial evidence that estrogen is at least as important as testosterone in determining bone mass in aging men and that estrogen deficiency plays a prominent role in continuous phase bone loss in men (4). In both genders, estrogen deficiency impairs the normal bone remodeling cycle by increasing osteoclastic resorption activity without a corresponding increase in osteoblastic activity. Hence, the amount of bone resorbed is greater than that of the amount deposited, leading to a net loss of bone. This process was originally described as ‘uncoupling’. The cellular changes that occur in estrogen deficiency are depicted in figure 1. In estrogen deficiency, there is an increased production of Tumor necrosis factor (TNFα) and cells of the stromal/osteoblastic lineage become more sensitive to interleukin-1 (IL-1). IL-1 and TNF stimulate stromal cells/pre-osteoblasts to release several cytokines- IL-6, macrophage colony stimulating factor (M-CSF), IL-11, granulocyte macrophage colony-stimulating factor (GM-CSF), and transforming growth factor (TGF). The final cytokine in the osteoclastogenesis cascade is receptor activator of nuclear factor B ligand (RANK ligand), which is produced by osteoblasts that binds to its receptor (RANK) on osteoclasts (2). RANKL has a natural antagonist osteoprotegerin (OPG) that is a soluble receptor secreted by the stromal osteoblast lineage cells (4). The important action of estrogen is to increase OPG secretion (2, 4) and decrease M-CSF (5) and RANK (6). In the presence of estrogen, RANK-RANKL interaction is inhibited there by inhibiting osteoclastogenesis cascade (4). In retrospect, we now realize that the uncoupling factor secreted by the osteoblasts is RANKL. In short, estrogen deficiency leads to increased levels of IL-6, M-CSF, IL-11 and RANK ligand (2) (Figure 1). Moreover, in aging men and women, there is a negative calcium balance in the body further affecting the bone mineralization.
Figure 1.
Cellular changes that occur with estrogen changes. +E depicts effects in presence of estrogen; E depicts effects in absence of estrogen. IL-1 is Interleukin 1, TNF – Tumor Necrosis Factor, OPG – Osteoprotegerin. Estrogen decreases osteoclastogenesis and increases osteoclast apoptosis. Estrogen reduces osteoclastogenesis by suppressing IL-1 and TNF and increasing the sensitivity of stromal cells/preosteoblasts to IL-1, thus suppressing M-CSF, RANKL, and perhaps most notably, IL-6. In addition, estrogen stimulates the production of OPG, the potent inhibitor of osteoclastogenesis. Estrogen also reduces the responsiveness of osteoclast precursors to RANKL. Estrogen also promotes osteoclastic apoptosis, thereby reducing osteoclast lifespan. This effect appears to be mediated by TGFβ. Adapted from Tella et al., (2) with permission.
3.2 Direct effects of cancer and bone loss
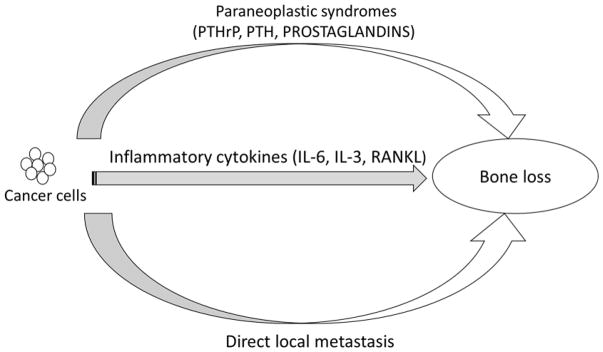
Bone loss in cancer survivors can be due to systemic inflammatory cytokines or hormones produced by the cancer cells and/or due to localized bone effects due to metastatic deposits. The most common cytokines involved in cancer-related bone loss are Receptor activator of nuclear factor B ligand (RANKL), interleukin-6 (IL-6), IL-3, tumor necrosis factor (TNF) alpha and beta. This explains how cancer causes accelerated bone turnover state, especially in the aging population who already has elevated cytokines as described in section 3.1 and figure 1. In addition, bone tissue is one of the organs that is considerably effected in malignancy due to ectopic secretion of hormones (PTH, PTHrP, 1, 25 (OH)2 vitamin D and prostaglandins) (1) or from immune cross-reactivity between cancer cells and bone tissue. The pathophysiology of cancer associated bone loss is detailed in Figure 2.
Figure 2.
Schematic diagram detailing the pathogenesis of cancer induced bone loss. PTHrP: Parathyroid Hormone related Peptide, PTH: Parathyroid hormone, IL-6: interleukin 6, IL-3: interleukin 3, RANKL: Receptor activator of nuclear factor B ligand.
3.2.1 Bone loss due to skeletal metastasis
Bone acts as a fertile soil for cancer cell seeding (1, 7, 8) and is a most common site of metastasis for many cancers especially breast, prostate, multiple myeloma, lung and renal cancers. In fact, about 75% of patients with multiple myeloma have bone pain due to skeletal events or generalized osteoporosis at the time of diagnosis. The skeletal metastasis can be osteolytic or osteoblastic (increased bone production). Osteolytic lesions are most commonly seen in various cancers whereas osteoblastic lesions are most often seen in prostate cancer and 10–20% of cases of breast cancer. In either case, the baseline mechanism of skeletal metastasis is the same as bone resorption and formation is coupled process and bone formation dominates in osteoblastic lesions whereas bone resorption gets dominated in osteolytic lesions and either of them can lead to skeletal related events. In prostate cancer, Prostate Specific Antigen (PSA) activates TGF- β, an osteoblast growth factor. Also, there is an increased production of local PTHrP that is thought to increase osteoblast progenitor cell proliferation and early osteoblast differentiation (9). These osteoblastic lesions are also associated with significant bone pain and increased fracture risk.
3.2.1.1 The “seeding” mechanism of skeletal metastasis
Tumor cells often break off from the primary tumor and penetrate through basement membrane of angiolymphatic system, disseminating to distant organs. Most of these “circulating tumor cells (CTCs)” die, but few of them are harbored in the bone marrow microenvironment due to the interaction of CXCR4 (a chemokine receptor expressed on CTC) and CXCL12 (a stromal derived factor -1 in bone). These “disseminated tumor cells (DTCs)” in the bone marrow microenvironment produce PTHrP, interleukin-6 (IL-6), prostaglandin E2 (PGE2), tumor necrosis factor (TNF), and macrophage colony-stimulating factor (M-CSF) that increases the production of RANK ligand. RANK ligand is a potent stimulator for osteoclasts thereby causing local bone resorption, which in turn increases the formation of transforming growth factor-beta (TGF-β). TGF- β stimulates the tumor cells to produce PTHrP, bone morphogenic proteins, insulin-like growth factors, fibroblast growth factors, platelet-derived growth factor that increase tumor growth. This vicious cycle further increases tumor growth and bone damage (10). Hematologic malignancies-myeloma and lymphomas not only enhance osteoblast action but also inhibit osteoblast bone formation by inhibiting Wnt signaling pathway. This osteoblast inhibition is mediated through Dickkopf WNT Signaling Pathway Inhibitor 1 (DKK1), and/or secreted frizzled-related protein 2 (sFRP 2) secreted by the tumor cells (11).
3.3 Chemotherapy induced bone loss
Chemotherapeutic agents have undergone tremendous advancement over the past decade. These advanced chemotherapeutic drugs may result in undesired long-term effects on bone health by acting through multiple different mechanisms as summarized in Table 1. In addition, chemotherapy leads to sarcopenia, decreased mobility there by leading to falls. Premature menopause secondary to chemotherapy increases the bone loss by almost 8-fold compared to that of bone loss seen in post-menopausal women (12).
Table 1.
Summary of chemotherapy agents and their effects on bone and mineral metabolism.
| Chemotherapy agent | Effect on bone and mineral metabolism |
|---|---|
| Cyclophosphamide | Arrests the cell division of preosteoblasts and osteoclasts, and Amenorrhea. |
| Cyclosporine | Stimulate osteoclasts, suppress osteoblasts, and inhibit mineral apposition. |
| Doxorubicin | Inhibits the proliferation and differentiation of osteoblasts, alters the interaction of PTH to its receptor on osteoblasts. |
| Estramustin | Hypophosphatemia, hypocalcemia→secondary hyperparathyroidism, osteomalacia |
| 5-Flurouracil + Leucovorin | Decrease in 1 alpha hydroxylase activity→decreased 1, 25 (OH)2 vitamin D levels. |
| Ifosfamide | Fanconi syndrome → renal phosphate wasting→ osteomalacia. |
| Methotrexate | Increased bone resorption, increased incidence of fractures. |
| Platinum based compounds | Hypomagnesemia |
3.4 Hormonal therapy induced bone loss
Therapy with tamoxifen, aromatase inhibitors, GnRH agonists and antagonists are the main stay of treatment in hormone responsive cancers- breast and prostate cancer. Aromatase inhibitors act by inhibiting the aromatization of androgens to estrogen there by reducing the estrogen levels by 96–99% (13). This considerable decrease in estrogen levels leads to rapid bone loss, which by far exceeds (almost double the risk) the normal post-menopausal bone loss (12, 13). With the use of aromatase inhibitors, there is a time and dose dependent increase risk of fractures (14). This effect is more pronounced in patients with low baseline BMD and serum estrogen concentrations (15). Androgen deprivation therapy with GnRH agonists and antagonists used in the management of breast and prostate malignancies also lead to bone loss by decreasing the estrogen levels. There is almost a 7-fold increase in bone loss at the end of 1 year when GnRH agonist/antagonist + aromatase inhibitor therapy is used in combination in pre-menopausal women. This corresponds to a fracture risk increase by 40–50% (16). Moreover, androgen deficiency leads to decrease in lean body mass, increase in fat mass and impaired muscular strength that may contribute to increased fracture risk (17).
4.0 Screening and diagnosis of bone loss in cancer survivors
Bone loss is diagnosed clinically when there is a presence of fragility fracture or by bone mineral density measured by bone densitometry (DXA) that is less than or equal to 2.5 standard deviations below that of a young adult reference population (T-Score). However, in patients with T-Score greater than −2.5 with risk factors for bone loss, fracture risk assessment (FRAX) is calculated to estimate the risk of major osteoporotic and hip fracture in 10 years. It is important to note that FRAX calculator should not be used in patients who are already on anti-resorptive medications. The measurement of BMD in cancer survivors can be challenging especially if there are metastasis in the region of interest. If spine and hip region cannot be included in region of interest, forearm can be used as alternate site to evaluate for bone loss (18). Moreover, compared to that of general population, the cancer survivors have many other risk factors that physician should evaluate in more detail. These risk factors include – estrogen/testosterone deficiency secondary to hypogonadism either through the direct effects of cancer or due to radiation/surgical or chemotherapy (19, 20) or hormonal treatment (detailed in section 3.4) leading to enhanced bone loss, local invasion of cancer tissue leading to pathological fractures, malnutrition following nausea and vomiting leading to deficiency in nutrients such as calcium and vitamin D, immobilization leading to muscle wasting and bone loss, narcotic pain medication such as morphine that increase fall risk (21) and hormonal or other activity of the tumor (e.g. production of cytokines, parathyroid hormone related peptide (PTHrP) (as detailed in section 3.2) perhaps leading to an increased bone loss. This comprehensive evaluation should include fall risk assessment, medication reconciliation, and physical exam to determine peripheral neuropathy. With all these risk factors, fracture risk assessment in cancer survivors is challenging and physician should look beyond the BMD measurements while making treatment decisions.
When to check bone mineral density (BMD)?
There are several guidelines that recommend that BMD should be evaluated in cancer survivors who are currently on aromatase inhibitors (AIs), premature gonadal failure secondary to cancer therapy (22–25). As per United Kingdom Expert Group, and international expert panel BMD should be measured within 3 to 6 months of initiating medical castration therapy with AIs, GnRH agonist/antagonists that decrease estrogen levels in all women < 75 years of age (22). However, DXA scans are not usually needed in less than 2–3-year time frame in patients who are already on anti-resorption medications unless clinically warranted.
5.0 Prevention of skeletal morbidity in comprehensive cancer care
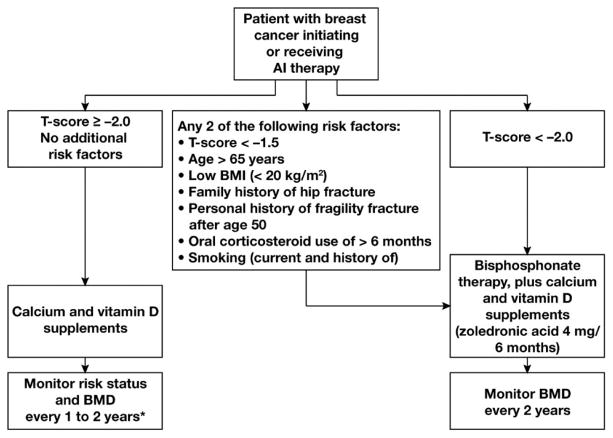
BMD measurement alone or combined with FRAX is usually used as a criterion for the management of osteoporosis in general population. Due to the presence of multiple risk factors in cancer survivors (as discussed in above sections), BMD and/or T-Scores should not be the only criterion for determining fracture risk in this patient population. Moreover, treatment indications are liberalized in the patients who are on hormone deprivation therapy. Annals of oncology clinical practice guideline recommend to treat all cancer survivors who are on chronic hormone deprivation therapy that potentiates bone loss with T-Score of less than 2.0 (25) (Figure 3). The guideline also recommends treating the patients who are on hormone deprivation therapy and have at least two of the following risk factors: age > 65 years, T-Score less than or equal to −1.5, current or history of smoking, glucocorticoid use for more than 6 months, body mass index (BMI) < 20 kg/m2, personal history of osteoporotic fracture above age 50 and family history of hip fracture. In all other patients who do not meet the above criteria, exercise and optimization of calcium and vitamin D intake is warranted. Sarcopenia and cachexia are usually seen in cancer survivors and it is the responsibility of the comprehensive care team to discuss about physical therapy for improving the muscle strength to limit falls. Moreover, the risk of falls is increased by high dose analgesics and sedatives that are used in cancer survivors to alleviate the pain.
Figure 3.
Recommended algorithm for managing bone health in women receiving aromatase inhibitor (AI) therapy for breast cancer. *If patients experience an annual decrease in bone mineral density (BMD) of ≥10% (using the same DXA absorptiometry machine), secondary causes of bone loss such as vitamin D deficiency should be evaluated and antiresorptive therapy initiated. Use lowest T-score from 3 sites.
In addition to these generalized measures, cancer specific treatment goals based on the evidence based medicine are discussed in respective sections below.
6.0 Breast cancer
Breast cancer is most common type of malignancy in women worldwide (26). Though the incidence of breast cancer is steadily increasing, early diagnosis and development of effective therapeutic regimens have improved patient survival.
Routine adjuvant therapy is given to prevent cancer recurrence and death. The type of adjuvant therapy used is determined by hormone receptor status and menopausal state (27). For example, treatment of premenopausal women with hormone receptor positive breast cancer is aimed at inhibiting the effect of estrogen on the breast and is usually achieved by estrogen receptor blockage (such as with tamoxifen), or ovarian function suppression with surgical oophorectomy or with the use of GnRH agonist/antagonist therapy (28). The superiority of aromatase inhibitors (AIs) over tamoxifen in hormone-receptor positive disease has been established in multiple trials (29, 30). Moreover, the side effects of uterine cancer and thromboembolic events seen with tamoxifen therapy are not observed in AI therapy, which might have led to the increased use of AI therapy in the last decade (27). On the other hand, AI therapy is associated with arthralgia, muscle pain and bone loss, which are not seen in tamoxifen therapy. In addition, the anti-estrogen therapy with AIs, GnRH agonist/antagonists and chemotherapy induced ovarian failure in premenopausal women increases the bone loss as high as 8-fold increase compared to that of post-menopausal female without cancer. Though tamoxifen therapy is associated with bone loss when used in pre-menopausal women, when used in post-menopausal women it has bone protective effects (28).
It is always prudent to get a detailed baseline laboratory testing to rule out secondary causes of osteoporosis and DXA screening in women with breast cancer prior to initiation of AI adjuvant therapy. The laboratory testing for secondary causes of osteoporosis includes (but not limited to) serum levels of calcium, phosphate, magnesium, 24-hour calcium and creatinine levels, 25-hydroxyvitamin D, parathyroid hormone, hemoglobin, C-reactive protein, ALP (bone-specific), thyroid-stimulating hormone, celiac disease antibodies and protein electrophoresis (serum and/or urine). These tests are to be tailored based on the risk factors and clinical manifestations. Immobility from cancer-associated muscle weakness and sarcopenia is also associated with an increased risk for bone loss and fractures. Such risk factors must also be considered in preventing and treating AI-induced bone loss. General guidelines mentioned in section 5 are to be followed in managing bone loss in breast cancer survivors. A special emphasis on anti-resorptive use in breast cancer survivors is detailly described in the present section.
6.1 Bisphosphonates
6.1.1 Prevention of AI-Induced Bone Loss
Bisphosphonates have been used in AI-treated patients for almost two decades (27). The earliest studies showed that administration of oral bisphosphonates resulted in significant BMD increase (32–34). Although the data from these studies are not as robust as for intravenous bisphosphonates, observational studies have reported prevention of AI-induced bone loss with oral bisphosphonate regimens that are typically used for postmenopausal osteoporosis (35).
Bisphosphonates have been shown to be effective in preventing anti-estrogen therapy induced bone loss in both pre- and post-menopausal women (32–42). The ABCSG-12 trial included 1,803 premenopausal women with hormone responsive early stage breast cancer treated with GnRH agonist randomized to tamoxifen versus anastrozole with or without zoledronic acid 4 mg IV every 6 months (36). Patients who were given zoledronic acid had significantly higher BMDs compared to the women who did not receive the bisphosphonate (36). In another study of premenopausal women, addition of zoledronic acid 4 mg every 3 months to adjuvant chemotherapy and/or endocrine therapy led to lumbar spine BMD increase by 3.14% versus 6.43% decrease in the placebo group in 24 months (P<0.0001) (37). In postmenopausal women, the Z-FAST and ZO-FAST trials assessed the efficacy of concomitant treatment with zoledronic acid 4 mg every 6 months with AI (upfront arm) versus delayed treatment arm (initiation of ZA when BMD T-score falls to <-2.0 or occurrence of a non-traumatic fracture) (32–35). Significant BMD increase at the lumbar spine and hip were noted in the upfront arms of both studies (38–41). A 5-year follow up study was done yielding the similar positive results in upfront arm (42). Of note, despite these great benefits in BMD, it is important to note that bisphosphonates resulted in modest reduction of fractures (RR 0·85, 95% CI 0·75–0·97; 2p=0·02) (43).
6.1.2 Adjuvant Bisphosphonate Therapy
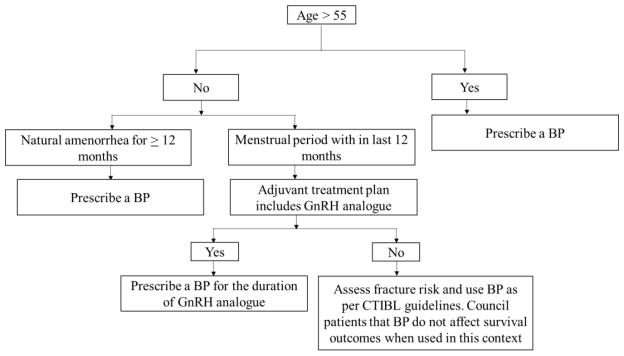
The interaction between cancer cells, bone cells and the bone microenvironment drive a vicious cycle that promotes malignant metastatic processes in cancer. Bisphosphonates’ role in the adjuvant setting by prevention of breast cancer recurrence and reduction of metastases has been evaluated by multiple studies. It is important to note that endocrine status (i.e, low vs high estrogen setting) may influence the effect of bisphosphonates on disease progression (27). A double-blind randomized trial involving 3,233 women with early stage breast cancer did not show any significant differences in terms of overall survival (OS) and disease-free survival (DFS) between the clodronate and placebo groups (44). Notably, longer recurrence-free interval (0.75 [0.57–0.99]; P=0.045), as well as bone and non-bone metastasis-free interval were observed when post-menopausal women aged more than 50 years were included (44). In the ABCSG-12 trial, addition of zoledronic acid to adjuvant therapy (tamoxifen or anastrozole) significantly increased DFS at 48 months (HR =0.64, p=0.01) and at 62 months (HR=0.68, p=0.009) (45, 46). Prolonged DFS and improved OS were confirmed after a median follow up of 95 months (46). In the Z-FAST and ZO-FAST trials, zoledronic acid significantly reduced disease recurrence in both upfront and delayed arms, though there was a significant DFS improvement in the upfront arm compared to that of delayed arm (HR=0.59, p=0.03) (39, 41). In a recent meta-analysis of 26 RCTs involving 18,766 patients, 10-year risk of bone recurrence was significantly reduced with the addition of adjuvant bisphosphonates. Moreover, there was an approximately one third reduction in bone metastases and one sixth reduction in breast cancer deaths in postmenopausal women (43). These data mean that many postmenopausal women will receive adjuvant bisphosphonates irrespective of BMD and fracture risk (47, 48) (Figure 4).
Figure 4.
Algorithm of using bisphosphonates as an adjuvant therapy. BP: bisphosphonate. Always use Include Vitamin D 1000–2000 international units and calcium 1000 mg/day and ensure vitamin D level is adequate (> 30 ng/ml) while initiating bisphosphonate. If vitamin D level is not in normal range, it must be corrected with higher doses of vitamin D to increase the level to > 30 ng/ml. Adapted from (48) with permission.
6.3 Denosumab
6.3.1 AI-Induced Bone Loss
Denosumab is a RANK ligand inhibitor and is most potent anti-resorptive till date. As detailed in section 3.2, RANKL is a key mediator of direct effects of cancer on bone health and metastatic bone resorption. Denosumab binds to RANKL and inhibits osteoclast formation, function and survival thereby reducing bone resorption. Ellis et al, randomized 252 women with early stage breast cancer undergoing adjuvant AI therapy to receive subcutaneous denosumab 60 mg or placebo every 6 months for 2 years (49, 50) Lumbar spine BMD significantly increased by 5.5% and 7.6% (P<0.0001 for both), after 12 and 24 months, respectively (49, 50). Similar improvement in BMD was also noted at the total hip, total body, femoral neck and distal one-third of the radius (49, 50). The ABCSG-18 trial randomized postmenopausal women with hormone-responsive early stage breast cancer receiving AI to denosumab 60 mg or placebo every 6 months (51) The primary endpoint was time to first clinical fracture and patients in the denosumab group had a significantly delayed time to fracture [HR=0.50 (95% CI 0.31–0.64), p<0.0001] (51). Moreover, at 36 months, BMD increase of 10% at the lumbar spine, 8% at the total hip and 6.5% at the femoral neck (all adjusted p values <0.0001) were observed in the denosumab group compared to that of placebo. Denosumab is the only anti-resorptive that has shown anti-fracture efficacy in cancer therapy induced bone loss.
7.0 Prostate cancer
Prostate cancer is second most common cancer in men with most affected above age of 70 years usually managed by either medical (ADT) and/or surgical castration. Given its age distribution, BMD is frequently low by the time of initial diagnosis as demonstrated in a study of 618 prostate cancer subjects in which only 20% had normal bone density, 39% had osteopenia and 41% had osteoporosis (52). None of these patients have retained their baseline bone densities at the end of 6 years of ADT. ADT decreases the estrogen and testosterone levels, dose dependently (53), further accelerating the bone loss. Moreover, prostate cancer frequently metastasizes to bone, especially pelvic and vertebral bones. These bone metastases on top of compromised bone mineral density lead to fractures and other SREs causing significant morbidity and an effect on overall survival. Maintenance of bone health in prostate cancer care is thus clearly warranted.
7.1 Maintenance of bone health
Advanced prostate cancer patients who are on ADT should be screened as per routine screening process detailed in section 4.0. As discussed in the breast cancer section (section 6), life style interventions like alcohol and smoking cessation, muscle strengthening exercises and adequate lighting at home to avoid falls play an important role in reducing the fractures there by reducing the morbidity and mortality. Pharmacological intervention includes optimization of vitamin D and dietary calcium intake of 1200 mg as per IOM guidelines (54).
7.1.1: Prevention of ADT induced bone loss
SERMs, bisphosphonates and denosumab were evaluated in the management of SREs and preservation of bone health in prostate cancer survivors. Toremifene (a SERM) has increased the BMD in men receiving ADT for prostate cancer (55), but it was not approved by the FDA as there was no adequate data supporting the fracture reduction, time to disease progression and overall survival. Along with life style modifications (smoking cessation, weight bearing exercise) and optimization of calcium and vitamin D intake as per institute of medicine (IOM) guidelines, osteoclast inhibition by bisphosphonates and denosumab is the current standard of care for maintaining bone health in prostate cancer patients who are on androgen deprivation therapy (ADT) (56–58).
7.1.2: Management of skeletal metastases and skeletal related events (SREs)
Though all bisphosphonates have shown benefit in improving BMD (56–58), only ZA has shown benefit in reducing SREs in castration resistant prostate cancer (59–61). However, this encouraging results are not demonstrated by ZA in castration sensitive prostate cancer (62). Moreover, ZA was shown to be ineffective in prevention of new metastasis (60, 61). Denosumab is shown more effective than ZA in reducing the SREs, fractures and in delaying the first occurrence of bone metastasis in castration resistant prostate cancer (63). In patients with castration resistant prostate cancer with raising PSA levels, denosumab has shown to be beneficial in bone-metastasis-free survival by a median of 4·2 months compared to that of placebo (median 29·5 [95% CI 25·4–33·3] vs 25·2 [22·2–29·5] months; hazard ratio [HR] 0·85, 95% CI 0·73–0·98, p=0·028). Denosumab has also shown to significantly delayed time to first bone metastasis (33·2 [95% CI 29·5–38·0] vs 29·5 [22·4–33·1] months; HR 0·84, 95% CI 0·71–0·98, p=0·032) (64). Though denosumab has shown to delay the time of first occurrence of bone metastasis, it failed to improve overall survival or overall progression-free survival (64). In short, despite benefits of preserving bone health and decreasing SREs, neither ZA nor Denosumab have shown overall survival benefit. Contrary to ZA and Denosumab, Docetaxel given as part of chemotherapy (65), and alpha particle emitting Radio-Pharmaceuticals-Radium-223 (66, 67) have shown overall survival benefit and in preventing bone metastasis when combined with ADT.
Bone pain secondary to bone metastasis can be effectively managed by single fraction of external beam radiation therapy using 8 Gy. Radium 223 is the only bone-targeted agent that has shown to prolong survival in prostate cancer with bone metastasis. Bisphosphonates and denosumab are not approved by FDA for the pain management in prostate cancer bone lesions, however, they can be used as an alternative or adjuvant to radio pharmacy and external beam radiation therapy. Other treatment modalities used in managing bone lesions in prostate cancer include magnetic resonance guided focused ultrasound (68), hemi body irradiation (for extensive metastasis), surgical removal of lesions, analgesics and of course, systemic chemotherapy.
8.0 Differentiated Thyroid cancer (DTC)
DTC is most common endocrine malignancy with a 10-year survival rate of 80–95%. Depending upon the cancer stage, thyroid cancer is usually managed by surgical excision of thyroid gland, I131 therapy and suppression of TSH with supra-physiological doses of levothyroxine. A nested case control study in Canada (69) demonstrated that high (> 93 mcg) and medium (44–93 mcg) cumulative doses of levothyroxine were associated with a higher risk for fractures compared with low cumulative doses (< 44 μg daily). All high-grade DTC patients are usually managed with 2.2 mcg/kg levothyroxine, which falls under this high levothyroxine dose category. Thyroid hormone increases bone resorption by acting through nuclear triiodothyronine (T3) receptors, predominantly thyroid receptor (TR)-alpha-1 thereby causing increased bone resorption there by leading to major osteoporotic fractures (70). This risk further increases in case of post-menopausal women. Maintenance of skeletal health in thyroid cancer survivors is thus clearly warranted, which involves life style interventions, optimal calcium/vitamin D supplementation and titrating the levothyroxine dose to lowest possible dose to maintain TSH levels as per ATA thyroid cancer management guidelines (71) depending up on the staging of the cancer. For example, a stage 3 papillary thyroid cancer patient who had total thyroidectomy done almost 5 years ago with no biochemical or anatomical evidence of disease on routine follow ups can have TSH in 0.1–0.5 mIU/ml rather than <0.1 mIU/ml. Bone mineral density should be closely monitored (once every two years) in these patients who are being treated with the TSH suppressive levothyroxine.
9.0 Multiple myeloma
Multiple myeloma (MM) is a clonal B-cell disorder that results from proliferation and accumulation of malignant plasma cells leading to elevated immunoglobulin levels with concomitant immune system deficiency, renal failure and osteolytic bone lesions. The skeletal involvement in the form of osteolytic lesions is a telltale sign of MM with the prevalence of 70–80% (72) in newly diagnosed cases. Given the median age of diagnosis being 65 years, osteoporosis is also common in this cohort further compromising the bone health. The clinical consequences of osteolytic lesions and osteoporosis (fractures and severe bone pain) worsen the quality of life and survival rate in this patient population (73). The bone disease in MM is primarily due to the interaction of myeloma cells to bone marrow stromal cells (BMSCs) mediated through vascular cell adhesion molecule-1 (VCAM-1), which favors the production of factors that promote osteoclastogenesis (74). BMSCs-MM cells interaction express IL-6, Macrophage inflammatory protein 1a (MIP 1a), RANKL and M-CSF that promote osteoclastogenesis. In addition, myeloma cells express decrease the expression of OPG, which inhibits RANK-RANKL interaction and myeloma cells are also known to secrete Dkk1 and sFRP-2, which inhibit osteoblast differentiation and function (75). Therefore, agents that act through osteoclast inhibition or osteoblast stimulation are the cornerstone in maintaining the bone health in MM.
Bisphosphonates and Denosumab are extensively evaluated for preserving bone health and in preventing SREs. Intravenous bisphosphonates (ZA and pamidronate) are the mainstay of therapy in MM and are known to be beneficial in reducing SREs (RR 0.80, 95% CI 0.72–0.89), better pain control (RR 0.75, 95% CI 0.60–0.95) and in preventing pathological vertebral fractures (RR 0.74, 95% CI 0.62–0.89) (76). As per a systemic review and meta-analysis (76), as a group, bisphosphonates showed positive effects in reducing SREs (RR:0.80; 0.72–0.89), vertebral fractures (RR:0.74; 0.62–0.89) failed to show overall survival benefit [HR] 0.96, 95% CI 0.82–1.13) and progression free survival (HR 0.71, 95% CI 0.41–1.19). However, in a subset analysis, improved survival was noted with ZA (HR 0.42, 95% CI 0.81–0.22). Moreover, in a largest head to head randomized control trial that compared oral (clodronate) and intravenous bisphosphonate (ZA), a superior efficacy in terms of SREs and progression free survival was demonstrated in patients who had bone disease at baseline (p<=0.0107, survival advantage of 10 months) (77, 78). Currently IV bisphosphonates, pamidronate and ZA are approved in USA for use in MM. It is important to note that renal insufficiency is one of the well noted complication of MM, which limits the use of ZA (especially when creatinine clearance is < 30). In such patients who have extensive renal and skeletal disease, pamidronate 90 mg can be infused over an extended period (4–6 h) (79). As per recent international myeloma working group recommendations (79), bisphosphonates are to be initiated along with the antimyeloma therapy regardless of skeletal metastasis but the benefit is of a question in non-skeletal MM disease. As in other cancers, Kyphoplasty and low dose radiation therapy along with bisphosphonate therapy can be used appropriately for symptomatic vertebral compression fractures and SREs (79). In case of long bone fractures and spinal cord related events, timely consultation to orthopedic surgeons is warranted.
Denosumab is another potent and efficacious anti-resorptive agent that theoretically should have better beneficial effects in MM patients due to its direct inhibition of RANKL and potential to use in renal insufficiency. Despite these theoretical benefits, the drug is not approved by FDA in the management of MM as there was a decrease in survival rate in ad-hoc analysis of a head to head comparison study with ZA (80). Sorscher et al., (81), propose that this improved survival of ZA may be due to its interference with the post-translational modification of oncogenic RAS in the myeloma cells. Of note, as per Liu eta al., (82) RAS mutation was seen in about 39% of multiple myeloma cases. However, in ASCO 2017, pioneering work of Raje et al (83)., have showed that denosumab was non-inferior to ZA in delaying time to first on study SRE in pts with newly diagnosed MM (p=0.01). In addition, the rate of renal complications in denosumab group were lower than that of ZA group. Denosumab is currently being evaluated in a head to head phase III randomized clinical trial (NCT01345019) and the results are expected in 2019 and until then denosumab is not indicated and approved in MM patients.
9.1 The antimyeloma agents
Immunomodulatory agents (thalidomide, and lenalidomide) are the mainstay of treatment in the newly diagnosed and relapsing cases of MM with or without bone disease (84, 85). In vitro studies have shown that these agents also act as potent anti-resorptives by blocking the RANK stimulated osteoclastogenesis. In addition, thalidomide is also known to alter the production of inflammatory cytokines further limiting the osteoclastogenesis. In a study (86) that evaluated the combination of thalidomide and dexamethasone, it was demonstrated that bone resorption markers (CTX and TRACP-5b) were significantly reduced at 3 and 6 months’ post treatment. Bortezomib, a proteasome inhibitor alters abnormal bone metabolism, both by reducing bone resorption and by enhancing bone formation, which is evident by decreased bone resorption markers and increased bone formation markers (bALP) (87, 88).
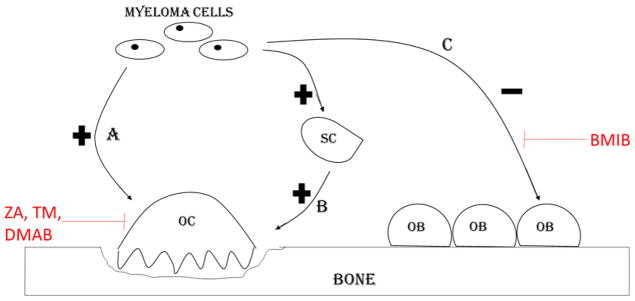
The pathogenesis of bone resorption, bone metastasis and agents used in management of the same in MM bone disease are summarized in figure 5.
Figure 5.
Myeloma bone disease. A. Myeloma cells secrete osteoclast activating factors (IL-6, IL-3) that stimulate osteoclast induced bone resorption. B. Stromal cells stimulate osteoclasts by increasing the RANK L production. C. Myeloma cells directly inhibit osteoblast differentiation by expressing osteoblast inhibitory factors (Dickkopf-1, secreted frizzled-related protein 2 (sFRP2)), which are blocked by anti-myeloma agent bortezomib (BMIB). Zoledronic acid (ZA), temozolomide (TM) and denosumab (DMAB) inhibit osteoclast bone resorption. OC=Osteoclast, OB=Osteoblast, SC=Stromal cell.
10.0 Bone health in paraneoplastic syndromes
Bone tissue is one of the organs that is considerably effected in malignancy due to ectopic secretion of hormones- parathyroid hormone (PTH), parathyroid hormone related peptide (PTHrP), 1, 25 (OH)2 vitamin D and prostaglandins (89) (Figure 2), cytokines (IL-6, TNF-alpha and beta), direct skeletal metastasis or from immune cross-reactivity between malignant and bone tissues thereby leading to hypercalcemia. Almost 80% of cases are caused by elevated PTHrP levels- most commonly known as humoral hypercalcemia of malignancy (HHM). Classical biochemical findings in HHM include elevated PTHrP, suppressed endogenous PTH secretion due to hypercalcemia mediated by PTHrP and low levels of active vitamin D. The PTHrP levels can also provide information about prognosis of cancer and likelihood of response of hypercalcemia from bisphosphonate therapy. Hypercalcemia in the setting of serum PTHrP concentration above 12 pmol/L is often associated with an inferior survival (90) and poor response to bisphosphonate therapy (91). In cases of elevated PTH or inappropriately normal PTH, one should consider concomitant presence of primary hyperparathyroidism or ectopic PTH secreting neoplasm. In the setting of hypercalcemia with normal PTH and PTHrP, next step is the measurement of active vitamin D, which is common in lymphomas and ovarian dysgerminomas (92). It is fascinating to note that metastatic breast cancer cells may produce PTHrP locally, without a major increase in serum PTHrP leading to hypercalcemia in the setting of low PTH, PTHrP and 1, 25 (OH)2 vitamin D levels (93, 94). Other causes of hypercalcemia like hyperthyroidism induced bone loss, medication induced (thiazides, lithium) should not be over looked in this patient population as they can exacerbate the degree of hypercalcemia.
Hypercalcemia of malignancy often causes considerable morbidity, and effective treatment can improve patient quality of life. Even long standing mild to moderate degree of hypercalcemia warrants treatment as it can lead to decrease bone quality due to demineralization of bone leading to fragility fractures thus increasing morbidity. In addition, poor nutritional status can exacerbate the hypercalcemia and poor bone health in cancer survivors. For example, dehydration can relatively increase the serum calcium levels, decrease calcium filtration from kidney. Hypercalcemia can also induce nephrogenic diabetes insipidus further exacerbating the fluid loss (94). Moreover, poor dietary intake of calcium can enhance increased calcium resorption from bones because of PTH. Hence, optimal management of hypercalcemia includes (but not limited to) fluid repletion and optimal nutritional intake. Aggressive hydration is recommended in patients who have no significant cardiac or renal disease. Intravenous bisphosphonates (Zoledronic acid and Pamidronate) are the current standard of treatment and usually take couple of days to show hypocalcemic effects. Concomitant use of calcitonin helps in lowering calcium levels before the action of bisphosphonates peak. Prednisone (dose of 20–40 mg/day) can be used in patients with an overproduction of 1,25-dihydroxycholecalciferol as it blocks 1 alpha hydroxylase activity in macrophages. Patients with refractory hypercalcemia should be considered for denosumab therapy and lastly for hemodialysis.
11.0 Complications of osteoclast inhibitors especially in the doses used in cancer and how to prevent them?
The osteoclast inhibitors described above thus play an important role in the management of bone disease in cancer patients. However, they are being infamous because of their rare but serious side effects of atypical femur fractures and osteonecrosis of jaw (ONJ), especially with the frequency and doses that are used in cancer patients. A randomized-placebo controlled trial (95) has evaluated the non-inferiority of less frequent dosing of zoledronic acid (every 3 months) versus standard dosing (every 4 weeks) in patients with metastatic breast, prostate cancer and multiple myeloma for a period of 2 years. The proportions of SREs did not differ significantly between the every 4-week dosing group vs the every 12-week dosing group for patients with breast cancer, prostate cancer, or multiple myeloma (p<0.001 for non-inferiority). The bone pain reduction effect, side effect profile in terms of renal damage and osteonecrosis of jaw were similar in both the groups. This study opens the doors for less frequent administration of these agents retaining their positive effects on both health.
The other common side effects seen with the use of these anti-resorptives are hypocalcemia, hypophosphatemia, acute phase reactions and skin infections (denosumab). The degree of hypocalcemia and hypophosphatemia is more pronounced with denosumab, which is a stronger anti-resorptive. These mineral metabolism side effects can be easily prevented by optimizing the calcium and vitamin D levels before initiating the therapy with these agents. Moreover, it is also important to check PTH levels before the use of these agents as they can unmask and exacerbate the mild hypoparathyroidism leading to severe hypocalcemia. It is important to note that bisphosphonates are renally cleared and infusion times less than 15 min for zoledronic acid should be avoided, and serum creatinine should be measured before each zoledronic acid infusion. Bisphosphonates should be withheld if renal deterioration is noted with their use, but can be resumed at the previous dose when serum creatinine returns to within 10 % of baseline. In addition, bisphosphonates should be avoided when creatinine clearance is less than 30 (79).
Denosumab can be used in patients with chronic kidney disease but the risk of hypocalcemia and hypophosphatemia is more pronounced in the presence of chronic kidney disease (CKD). Optimization of calcium, phosphorous and vitamin D levels is highly recommended when denosumab is used in CKD patients. Also, it is important to rule out adynamic bone disease before initiating denosumab in CKD patients. Though bone biopsy is the gold standard test to rule out adynamic bone disease, certain bone turnover markers (bone specific alkaline phosphatase, trimeric form of P1NP and tartarate acid phosphatase) can help in differentiating severe osteoporosis/bone loss and adynamic bone disease.
12.0 Conclusion
Bone health among cancer survivors is a critically important aspect of cancer care and requires special attention and detailed evaluation by the comprehensive care team. Endocrinologists should be involved in the care team early in the disease course, given their expertise in and understanding of hormone action, bone and mineral metabolism. In patients with a new diagnosis of cancer, priority should be given to assess skeletal status, optimize bone health, and prevent cancer therapy–induced bone loss and fractures. In patients with more advanced stage cancer, the goal of the care team should be avoiding and managing complications such as SREs, bone pain and fractures. The growing recognition of bone microenvironment as a reservoir of malignant cells favors the use of bone protective agents such as bisphosphonates to prevent skeletal metastases and skeletal related events. Bone health in cancer survivors is a vital area for both clinical and basic science research that can help preventing the morbidity and mortality in cancer survivors.
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, MD, 20892.
Abbreviations
Adapted from Hadji et al. (25) with permission
- AI
aromatase inhibitor
- BMD
bone mineral density
- BMI
body mass index
Footnotes
Authors have no conflict of interests, nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with different types of cancer. Acta Oncol. 2009;48(1):105–115. doi: 10.1080/02841860802167490. [DOI] [PubMed] [Google Scholar]
- 2.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. Journal of Steroid Biochemistry & Molecular Biology. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordin BE, Need AG, Steurer T, Morris HA, Chatterton BE, Horowitz M. Nutrition, osteoporosis, and aging. Ann N Y Acad Sci. 1998;854:336–351. doi: 10.1111/j.1749-6632.1998.tb09914.x. [DOI] [PubMed] [Google Scholar]
- 4.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 5.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 6.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1989;1:571. [PubMed] [Google Scholar]
- 8.Kaplan RN, Rafii S, Lyden D. Preparing the ‘soil’: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David Roodman G, Silbermann R. Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep. 2015 Oct 28;4:753. doi: 10.1038/bonekey.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappard D, Bouvard B, Basle MF, Legrand E, Audran M. Bone metastasis: histological changes and pathophysiological mechanisms in osteolytic or osteosclerotic localizations. A review. Morphologie. 2011;95:65–75. doi: 10.1016/j.morpho.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 12.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, et al. NCCN Task Force Report: Bone Health In Cancer Care. J Natl Compr Canc Netw. 2013 Aug;11(Suppl 3):S1–50. doi: 10.6004/jnccn.2013.0215. [DOI] [PubMed] [Google Scholar]
- 13.Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82. doi: 10.1016/j.critrevonc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Gibson K, O’Bryant CL. Screening and management of osteoporosis in breast cancer patients on aromatase inhibitors. J Oncol Pharm Pract. 2008;14:139–145. doi: 10.1177/1078155208091866. [DOI] [PubMed] [Google Scholar]
- 15.Bouvard B, Hoppe E, Soulie P, et al. High prevalence of vertebral fractures in women with breast cancer starting aromatase inhibitor therapy. Ann Oncol. 2012;23:1151–1156. doi: 10.1093/annonc/mdr356. [DOI] [PubMed] [Google Scholar]
- 16.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 17.Allain TJ. Prostate cancer, osteoporosis and fracture risk. Gerontology. 2006;52:107–110. doi: 10.1159/000090956. [DOI] [PubMed] [Google Scholar]
- 18.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012 Jun;97(6):1802–22. doi: 10.1210/jc.2011-3045. [DOI] [PubMed] [Google Scholar]
- 19.Theriault RL. Pathophysiology and implications of cancer treatment-induced bone loss. Oncology (Williston Park) 2004;18:11–15. [PubMed] [Google Scholar]
- 20.van Leeuwen BL, Kamps WA, Jansen HW, Hoekstra HJ. The effect of chemotherapy on the growing skeleton. Cancer Treat Rev. 2000;26:363–376. doi: 10.1053/ctrv.2000.0180. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 22.Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–18. doi: 10.1016/j.ctrv.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Body JJ, Bergmann P, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, et al. Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club. Osteoporos Int. 2007;18:1439–1450. doi: 10.1007/s00198-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 25.Hadji P, Body JJ, Aapro MS, Brufsky A, Coleman RE, Guise T, et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–1416. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 26.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 27.Tremollieres FA, Ceausu I, Depypere H, et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas. 2017;95:65–71. doi: 10.1016/j.maturitas.2016.10.007. DOI: http://dx.doi.org/10.1016/j.maturitas.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996 Jan;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Gradishar W, Salerno KE. NCCN guidelines update: Breast cancer. J Natl Compr Canc Netw. 2016;14(5 Suppl):641–644. doi: 10.6004/jnccn.2016.0181. [DOI] [PubMed] [Google Scholar]
- 30.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 31.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 32.Saarto T, Vehmanen L, Elomaa I, Valimaki M, Makela P, Blomqvist C. The effect of clodronate and antioestrogens on bone loss associated with oestrogen withdrawal in postmenopausal women with breast cancer. Br J Cancer. 2001;84(8):1047–1051. doi: 10.1054/bjoc.2001.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, et al. Prevention of aromatase inhibitor-induced bone loss using risedronate: The SABRE trial. J Clin Oncol. 2010;28(6):967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 34.Markopoulos C, Tzoracoleftherakis E, Polychronis A, et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: Results from the ARBI prospective clinical trial. Breast Cancer Res. 2010;12(2):R24. doi: 10.1186/bcr2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouvard B, Soulie P, Hoppe E, et al. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann Oncol. 2014;25(4):843–847. doi: 10.1093/annonc/mdu008. [DOI] [PubMed] [Google Scholar]
- 36.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 37.Hadji P, Kauka A, Ziller M, et al. Effects of zoledronic acid on bone mineral density in premenopausal women receiving neoadjuvant or adjuvant therapies for HR+ breast cancer: The ProBONE II study. Osteoporos Int. 2014;25(4):1369–1378. doi: 10.1007/s00198-013-2615-z. [DOI] [PubMed] [Google Scholar]
- 38.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25(7):829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 39.Brufsky AM, Harker WG, Beck JT, et al. Final 5-year results of Z-FAST trial: Adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 40.Bundred NJ, Campbell ID, Davidson N, et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer. 2008;112(5):1001–1010. doi: 10.1002/cncr.23259. [DOI] [PubMed] [Google Scholar]
- 41.Eidtmann H, de Boer R, Bundred N, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21(11):2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 42.Coleman R, de Boer R, Eidtmann H, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann Oncol. 2013;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 43.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Coleman R, Powles T, et al. Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 44.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (national surgical adjuvant breast and bowel project protocol B-34): A multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majithia N, Atherton PJ, Lafky JM, et al. Zoledronic acid for treatment of osteopenia and osteoporosis in women with primary breast cancer undergoing adjuvant aromatase inhibitor therapy: A 5-year follow-up. Support Care Cancer. 2016;24(3):1219–1226. doi: 10.1007/s00520-015-2915-2. [DOI] [PubMed] [Google Scholar]
- 46.Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: Final analysis of the austrian breast and colorectal cancer study group trial 12. Ann Oncol. 2015;26(2):313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 47.Hadji P, Aapro MS, Body JJ, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017 Mar 23;7:1–12. doi: 10.1016/j.jbo.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016:379–390. doi: 10.1093/annonc/mdv617. [DOI] [PubMed] [Google Scholar]
- 49.Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 50.Ellis GK, Bone HG, Chlebowski R, et al. Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: Subgroup analyses of a phase 3 study. Breast Cancer Res Treat. 2009;118(1):81–87. doi: 10.1007/s10549-009-0352-y. [DOI] [PubMed] [Google Scholar]
- 51.Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 52.Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104:800–805. doi: 10.1111/j.1464-410X.2009.08483.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fracture after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 54.Institute of Medicine. 2011 Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 55.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008 Jan;179(1):152–5. doi: 10.1200/JCO.2007.13.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klotz LH, McNeill IY, Kebabdjian M, et al. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur Urol. 2013;63:927. doi: 10.1016/j.eururo.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Choo R, Lukka H, Cheung P, et al. Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1239. doi: 10.1016/j.ijrobp.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 59.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 60.Kamba T, Kamoto T, Maruo S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. Int J Clin Oncol. 2017 Feb;22(1):166–173. doi: 10.1007/s10147-016-1037-2. [DOI] [PubMed] [Google Scholar]
- 61.Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS) Eur Urol. 2015 Mar;67(3):482–91. doi: 10.1016/j.eururo.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance) J Clin Oncol. 2014;32:1143. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomized, double-blind study. Lancet. 2011;377:813. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomized, placebo-controlled trial. Lancet. 2012;379:39. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016 Mar 19;387(10024):1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 67.Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 68.Hurwitz MD, Ghanouni P, Kanaev SV, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turner MR, Camacho X, Fischer HD, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ. 2011;342:d2238. doi: 10.1136/bmj.d2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Brix TH, Hegedüs L. Low serum thyrotropin level duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res. 2014 Sep;29(9):2040–50. doi: 10.1002/jbmr.2244. [DOI] [PubMed] [Google Scholar]
- 71.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines. Thyroid January. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 73.Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–8. doi: 10.1016/j.ejca.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Terpos E, Dimopoulos MA. Myeloma bone disease: pathophysiology and management. Ann Oncol. 2005;16:1223–31. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 75.Terpos E, Sezer O, Croucher C, Dimopoulos MA. Myeloma bone disease and proteasome inhibition therapies. Blood. 2007;110:1098–1104. doi: 10.1182/blood-2007-03-067710. [DOI] [PubMed] [Google Scholar]
- 76.Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, Kumar A, Djulbegovic B. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD003188.pub3. [DOI] [PubMed] [Google Scholar]
- 77.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–99. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgan GJ, Davies FE, Gregory WM, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: MRC Myeloma IX trial. Blood. 2012;119:5374–83. doi: 10.1182/blood-2011-11-392522. [DOI] [PubMed] [Google Scholar]
- 79.Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013 Jun 20;31(18):2347–57. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011 Mar 20;29(9):1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 81.Sorscher SM, Lockhart AC. Ras Inhibition and the Survival Benefit Favoring Zoledronic Acid Compared With Denosumab in Patients With Multiple Myeloma. Journal of Clinical Oncology. 29(19):2735–2736. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 82.Liu P, Leong T, Quam L, et al. Activating mutations of N- and K-ras in multiple myeloma show different clinical associations: Analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood. 1996;88:2699–2706. [PubMed] [Google Scholar]
- 83.Raje NS, Roodman GD, Willenbacher W, et al. Impact of denosumab (DMB) compared with zoledronic acid (ZA) on renal function in the treatment of myeloma bone disease. J Clin Oncol. 2017;35(suppl) abstr 8005. [Google Scholar]
- 84.Melchert M, List A. The thalidomide saga. Int J Biochem Cell Biol. 2007;39:1489–99. doi: 10.1016/j.biocel.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 85.Breitkreutz I, Raab MS, Vallet S, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–32. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 86.Terpos E, Mihou D, Szydlo R, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19:1969–76. doi: 10.1038/sj.leu.2403890. [DOI] [PubMed] [Google Scholar]
- 87.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–92. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 88.Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131:71–3. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 89.Seyberth HW, Segre GV, Morgan JL, Sweetman BJ, Potts J, Oates JA. Prostaglandins as Mediators of Hypercalcemia Associated with Certain Types of Cancer. N Engl J Med. 1975;293:1278–1283. doi: 10.1056/NEJM197512182932502. December 18, 1975. [DOI] [PubMed] [Google Scholar]
- 90.Pecherstorfer M, Schilling T, Blind E, et al. Parathyroid hormone-related protein and life expectancy in hypercalcemic cancer patients. J Clin Endocrinol Metab. 1994;78(5):1268. doi: 10.1210/jcem.78.5.8175989. [DOI] [PubMed] [Google Scholar]
- 91.Gurney H, Grill V, Martin TJ. Parathyroid hormone-related protein and response to pamidronate in tumour-induced hypercalcemia. Lancet. 1993;341(8861):1611. doi: 10.1016/0140-6736(93)90756-7. [DOI] [PubMed] [Google Scholar]
- 92.Stewart AF. Clinical practice Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–9. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 93.Akhtari M, Mansuri J, Newman KA, Guise TM, Seth P. Biology of breast cancer bone metastasis. Cancer Biol Ther. 2008;7:3–9. doi: 10.4161/cbt.7.1.5163. [DOI] [PubMed] [Google Scholar]
- 94.Mirrakhimov AE. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. N Am J Med Sci. 2015 Nov;7(11):483–493. doi: 10.4103/1947-2714.170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases. A Randomized Clinical Trial. JAMA. 2017;317(1):48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]