Abstract
Individuals differ greatly in their sensitivity to rewards and punishments. In the extreme these differences are implicated in a range of psychiatric disorders from addiction to depression. However, it is unclear how these differences influence the recruitment of attention, working memory, and long-term memory when responding to potential rewards. Here, we used a rewarded memory-guided visual search task and event-related potentials (ERPs) to examine the influence of individual differences in self-reported reward/punishment sensitivity, as measured by the Behavioral Inhibition System (BIS) / Behavioral Activation System (BAS) Scales, on the recruitment of cognitive mechanisms in conditions of potential reward. Select subscales of the BAS, including the Fun Seeking and Reward Responsiveness scales, showed unique relationships with context updating to reward cues and working memory maintenance of potentially rewarded stimuli. In contrast, BIS scores showed unique relationships with deployment of attention at different points in the task. These results suggest that sensitivity to rewards (i.e. BAS) and to punishment (i.e. BIS) may play an important role in the recruitment of specific and distinct cognitive mechanisms in conditions of potential rewards.
Introduction
Rewards are essential for motivating goal directed behavior and increasing the likelihood that we will act to fulfill our basic needs (Berridge & Robinson, 1998). Certain natural stimuli such as food, sex, and social interactions are inherently rewarding because they are necessary for individual and group survival. Other stimuli such as money or drugs may become rewarding through learned associations (Rescorla, Wagner, Black, & Prokasy, 1972; Corr, 2004; Pickering & Gray, 2001). The opportunity to obtain rewards frequently influences goal-directed behavior aimed at increasing the likelihood of obtaining the reward. This effect is seen across a range of contexts and tasks that require the use of different cognitive mechanisms, including working memory, long-term memory, and attention (Berridge, 1996; Berridge & Kringelbach, 2008; Berridge & Robinson, 1998; Clark, 2012; O’Doherty, 2004; Schultz, 2006).
Recruitment of Cognitive Mechanisms
Existing evidence suggests that potential rewards influence the recruitment of multiple cognitive mechanisms. In general, potential rewards induce widespread activity in brain areas involved in many basic cognitive functions across a variety of different tasks (Berridge, 1996; Berridge & Kringelbach, 2008; Berridge & Robinson, 1998; Clark, 2012; O’Doherty, 2004; Schultz, 2006; Vickery, Chun, & Lee, 2011). Stimuli associated with high rewards have been shown to be particularly effective at attracting attention (Hickey, Chelazzi, & Theeuwes, 2010a, 2011; Qi, Zeng, Ding, & Li, 2013), and increasing the strength of working memory representations of those stimuli (Reinhart & Woodman, 2014) when compared to low reward stimuli. The increased recruitment of these mechanisms enhances one’s ability to quickly locate and identify stimuli relevant to potential high rewards. The results of Reinhart and Woodman (2014) are particularly interesting in that they suggest some level of specificity in the recruitment of cognitive mechanisms in conditions of reward by demonstrating that, in contrast to working memory, an ERP index of long-term memory did not change as a function of reward magnitude. However, these previous studies have yet to fully separate the influence of potential rewards on attention, working memory, and long-term memory by investigating them in a single paradigm. Additionally, because these studies have not included a no-reward condition, the effect of potential reward versus reward magnitude on the recruitment of these mechanisms remains unclear.
Event-Related potentials (ERPs) are an excellent way to index the recruitment of these mechanisms for two reasons. First, the millisecond-by-millisecond timescale of ERPs allows for the measurement of sensory, attentional, and working/long-term memory responses to stimuli as they develop over time. Second, previous research has identified unique ERP components that reliably index the recruitment of specific cognitive mechanisms. In the current study we will focus on four specific ERP components to index four specific cognitive mechanisms. Those ERP components are the P3 as an index of stimulus evaluation/context updating, the N2 posterior-contralateral (N2pc) as an index of attentional deployment, the contralateral delay activity (CDA) and an index of working memory maintenance, and the P170 as an index of long-term memory activation.
The P3 ERP component has been shown to index stimulus evaluation and context updating such that larger P3 amplitudes are seen following more salient or otherwise task relevant stimuli (Cohen & Polich, 1997; Kok, 2001). The P3 is also sensitive to relevant individual differences in personality correlates of impulsivity and reward seeking, as well as overall risk for externalizing psychopathology (Baskin-Sommers et al., 2014; Carlson & Thái, 2010; Iacono, Carlson, Malone, & McGue, 2002). The N2pc component is an index of selective attention that is measured from occipital areas as the difference in amplitude between activity contralateral versus ipsilateral to an attended stimulus with greater contralateral activity suggesting greater deployment of attention (Eimer, 1996; Woodman & Arita, 2011; Woodman & Luck, 1999). The N2pc has also shown relevant associations with trait level individual differences as well as sensitivity to stimulus salience (Della Libera & Chelazzi, 2006; Eimer & Kiss, 2007). The CDA is a negative going sustained component measured from occipital areas as the activity contralateral versus ipsilateral to a remembered stimulus. The CDA has been linked to maintenance and manipulation of information in working memory such that, as the amount of information held in working memory increases, so does the amplitude of the CDA (Vogel, McCollough, & Machizawa, 2005; Vogel & Machizawa, 2004; Woodman & Vogel, 2008). The P170 (also referred to as the anterior P1) is a frontocentral positivity indexing the recruitment of long-term memory due to familiarity (Duarte, Ranganath, Winward, Hayward, & Knight, 2004; Friedman, 2004; Voss, Schendan, & Paller, 2010), or the initiation of memory search (Diana, Vilberg, & Reder, 2005). Although the P170 is a positive going wave, the amplitude of the P170 is more negative when a stimulus in long-term memory is correctly recognized (Tsivilis, Otten, & Rugg, 2001) and becomes reliably more negative as it is seen multiple times over repeated trials (Reinhart & Woodman, 2014). This increasing negativity is believed to indicate an increase in the strength of stimulus representations in long-term memory as a stimulus is seen multiple times.
Individual Differences in Reward Sensitivity
Sensitivity to reward is not a unitary construct, and individuals can vary greatly in different aspects of reward sensitivity. These different aspects of reward sensitivity are related to different personality traits, behaviors, physiological responses and forms of psychopathology (Balconi, Falbo, & Conte, 2012; Segarra, Poy, López, & Moltó, 2014; Voigt et al., 2009). Two neurobehavioral networks have been argued to underlie and balance differences in sensitivity to reward and loss as a means of motivating physiological and behavioral responses (Gray, 1972,1981). The Behavioral Activation System (BAS) controls appetitive motivation and responds to cues of impending reward or non-punishment. Activity in this system initiates movement towards goals, as well as positive feelings in response to rewarding stimuli. The BAS is also comprised of sub-components related to desire for reward and pleasure when obtaining rewards, which may have different underlying neural processes (Berridge, 1996; Gard, Gard, Kring, & John, 2006). The Behavioral Inhibition System (BIS), as originally described by Gray (1972, 1981), controls aversive motivation and responds to cues of punishment, loss, and reward failure. Activity in this system inhibits behavioral responses that might lead to negative outcomes and induces negative feelings in response to punishment and reward-failure. It has been proposed more recently that BIS underlies a broader range of responses including action uncertainty or goal conflict, even if no clearly aversive stimuli are present (Corr, 2008; Gray & McNaughton, 2000). In this case, BIS stays active until the goal is reached or the conflict is resolved, and may increase one’s capacity to react to uncertain stimuli (Corr & Matthews, 2009). Differences in the activity of these systems have also been proposed to underlie many trait level individual characteristics, including reward seeking, impulsivity, and anxiety (Gray, 1972, 1981). Imbalances in BIS and BAS have also been implicated in various psychiatric disorders, including addiction (Volkow et al., 2010), psychopathy (Buckholtz et al., 2010), and depression (Treadway & Zald, 2011).
Growing evidence suggests that these individual differences may influence the recruitment of cognitive mechanisms. Specifically, higher levels of self-reported BAS have been shown to be related to increased attention to potentially rewarded targets (Hickey, Chelazzi, & Theeuwes, 2010b) and previously rewarded distractor stimuli (Qi et al., 2013). There is also evidence that individuals who are more sensitive to losses or reward omissions, such as those with high BIS sensitivity, show greater processing of loss feedback and cues that signal potential reward omissions (Foti, Kotov, Klein, & Hajcak, 2011; Van den Berg, Franken, & Muris, 2011). Individual motivation more broadly has also been shown to influence top-down control processes that can influence selection of visual stimuli (Padmala & Pessoa, 2011). Furthermore, distinct brain areas including fronto-parietal regions (Engelmann, Damaraju, Padmala, & Pessoa, 2009), the dorsolateral prefrontal cortex (Savine & Braver, 2010), and the orbitofrontal cortex (Locke & Braver, 2008) have shown associations with motivation related personality traits. However, these previous studies have focused primarily on attention to or processing of cues and feedback leaving the relationship between these individual differences in sensitivity and cognitive mechanisms such as working and long-term memory still in need of investigation. Additionally, there remains a need to clarify the specificity of relationships between different aspects of reward sensitivity (e.g. BIS vs. BAS) and different cognitive mechanisms.
Current Study
The current study was intended to accomplish two primary aims. Our first aim was to replicate and extend the results of Reinhart and Woodman (2014) showing selective recruitment of working memory, but not long-term memory, in conditions of high reward. We did so by utilizing a similar paradigm and adding two key elements to our investigation. One, the current study includes the investigation of attention, in addition to working memory and long-term memory, as a potential mechanism of action in conditions of reward that was not investigated by Reinhart and Woodman. Two, the current study includes a no reward condition to allow for the investigation of not only reward magnitude effects, but also effect of the presence versus absence of reward. Our second aim was to go beyond the investigation of these basic cognitive mechanisms on a group level, and to determine the specificity with which individual differences in sensitivity to rewards may change the recruitment of some or all of these mechanisms. Based on these aims we tested the following competing hypotheses.
First, the broad influence of reward in the brain and across tasks suggests that reward (i.e. the within subjects effect of reward condition) may broadly influence the recruitment of multiple cognitive mechanisms (e.g. attention, working memory, and long-term memory). Alternatively, potential rewards may selectively influence the recruitment of specific cognitive mechanisms, such as working memory, but not others such as long-term memory. Second, individual differences reward sensitivity may broadly influence the recruitment of multiple cognitive mechanisms with those more sensitive to rewards showing additional recruitment of all of those mechanisms. Alternatively, the heterogeneous nature of reward sensitivity suggests that distinct aspects of these individual differences in reward sensitivity (i.e. desire for rewards versus pleasure when receiving rewards) may influence the recruitment of specific cognitive mechanisms. For example, high reward responsiveness may be selectively related to increased processing of initial reward cues, whereas high desire for rewards may be selectively related to the recruitment of working memory when anticipating a potentially rewarded target.
Method
Participants and Procedure
Undergraduate and community participants (N = 80) were recruited using the Vanderbilt psychology online research sign up system. Participants were screened for normal color vision and either normal or corrected to normal visual acuity, as well as history of head injury or neurological disorders. Participants completed a four-hour experimental session including the rewarded visual search task with ERP recording and self-report measures of reward sensitivity. Participants also completed a short behavioral change detection task to measure visual working memory capacity (Fukuda, Awh, & Vogel, 2010). Fifteen participants were excluded because of contaminated ERP data (i.e. more than 30% of trials with eye movements, blinks, or recording errors) leaving a final sample of 65 participants.
Change Detection Task
In the change detection task (Figure S1), participants were presented with and were required to remember arrays of two, four, six, or eight colored squares. After a delay participants were required to indicate whether the color of a single square matched or did not match the color of the square in that location in the original array. Thus participants were required to remember both the color and location of all squares in the memory array. Varying the set size (i.e. the number of squares to be remembered) from two to eight allowed for an estimation of participants’ visual working memory capacity (i.e. how many squares could be accurately held in memory at one time). Participants completed one practice block and four test blocks, consisting of 40 trials each. See supplemental materials for additional details regarding this task and the stimuli used.
Rewarded Visual Search Task
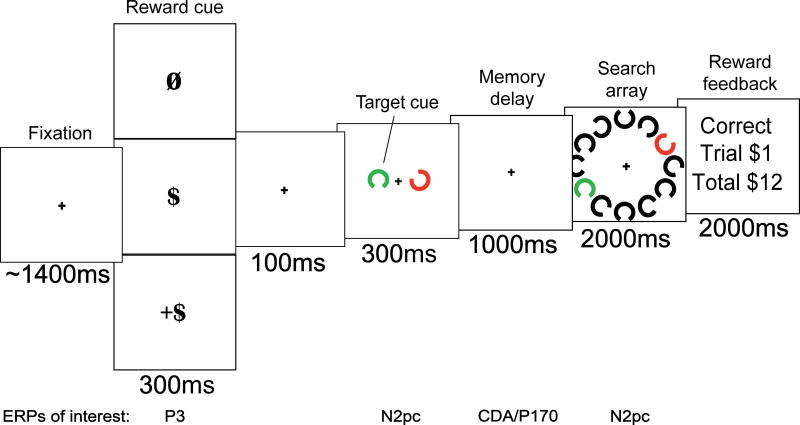
A rewarded, memory-guided visual search task (Figure 1) included multiple stages that were designed to involve specific cognitive mechanisms including attention, working memory, and long-term memory, as well as context updating following the reward cue. The potential for reward, as well as reward magnitude (no, low, or high) was manipulated across trials and cued at the start of each trial. Low reward trials resulted in $1 earned and high reward trials resulted in $5 earned for a correct response. Participants completed a total of 840 trials (120 rewarded). To increase the salience of the reward, participants received the money earned on four randomly selected trials (two low reward trials and two high reward trials). Participants were told about this bonus prior to completing the task.
Figure 1.
Rewarded visual search task. A single trial of the rewarded visual search task is shown. The to be remembered target is a match to the search array and feedback is for a correct response on a low reward trial.
Each trial began with a randomly jittered fixation cross (1200ms −1600ms) followed by a reward cue (300ms). The cue was either the null symbol (Ø) for no reward, a dollar sign ($) for low reward, or a dollar sign with a plus sign (+$) for high reward. The reward cue was followed by a second fixation cross (100ms) before the presentation of the target. Targets (300ms) were red or green Landolt-Cs in one of eight possible orientations. Target color was determined prior to the task, remained the same on all trials, and was counterbalanced across participants. Participants had to remember the orientation of the target stimuli. A shape in the non-target color was presented contralateral to the target shape on each trial to minimize physical stimulus confounds in the ERP recordings. The orientation of the target remained the same for runs of seven trials. After a run of seven trials, a new run of seven trials started with the target in a new orientation. Searching for the same target for a series of trials allows for the transfer of target representations from working memory to long-term memory (Reinhart & Woodman, 2014). This design allowed us to investigate the effect of reward on ERP components that track this transfer of information. During 95% of trial runs, trials one through four were no reward, trial five was rewarded (equal numbers of low and high reward trials), and trials six and seven were, again, no reward. Having the rewarded trial occur in the middle of a run of seven trials allowed us to investigate reward-modulated re-instantiations of target representations into working memory (Reinhart & Woodman, 2014). A memory delay period (1000ms) followed the target during which only a fixation cross was presented. The search array (2000ms) included 10 black shapes, one red shape, and one green shape arranged in a circular pattern. The position of the target shape within the array was randomly determined prior to the start of each trial. Participants were required to indicate if the orientation of the shape in the target color was a match or non-match to the previously presented target as quickly and accurately as possible by pressing one of two buttons on a gamepad. Responses had to occur within 2000ms to be correct. Each trial ended with a feedback display that conveyed the accuracy of the response, the amount earned on that trial, and the total amount earned.
Event-Related Potentials
Participants’ raw electroencephalograms (EEG) were recorded from 21 tin (Sn) electrodes placed using the International 10–20 System. Scalp sites included 3 midline (Fz, Cz, and Pz) sites, 7 lateral pairs (F3/F4, C3/C4, P3/P4, PO3/PO4, T3/T4, T5/T6, and O1/O2), and 2 non-standard electrodes (OL, halfway between O1 and T5; and OR halfway between O2 and T6; Carlisle, Arita, Pardo, & Woodman, 2011). Electrodes were held on the scalp with an elastic cap (Electro-cap International, Eaton, OH, USA). Raw EEG signal was amplified (bandpass of 0.01 – 100 Hz) using an SA Instrumentation Amplifier and digitized at 250 Hz. The right mastoid electrode was used as the online reference. Raw EEG was re-referenced offline to the average of the right and left mastoids. Peri-orbital electrodes placed 1 cm lateral to the external canthi were used to detect eye movement and shifts from fixation. An electrode beneath the left eye was used to measure vertical eye movements and blinks. We used a 2-step ocular artifact rejection method (Woodman and Luck 2003) to remove eye movements and blinks. Trials accompanied by incorrect behavioral responses or ocular or myogenic artifacts were excluded from the averages. Participants with greater than 30% of trials rejected for artifacts or incorrect behavioral responses (i.e. < 84 trials remaining at each serial position in the no reward condition, and < 35 trials remaining in each of the low and high reward conditions) were excluded from all analyses. Data preprocessing, artifact rejection, and ERP averaging were completed in ERPSS and EEGLAB toolbox for MATLAB. All data were filtered after artifact rejection using a .1Hz highpass filter and a 30Hz lowpass filter. Four event-related potentials (ERPs) were derived from the raw EEG to index specific cognitive processes as they unfolded on a millisecond-by-millisecond time scale. A 200ms pre-stimulus baseline was used for all ERP components.
The P3 was measured as the mean ERP amplitude at electrode site Pz between 400ms - 600ms post reward cue onset. The N2pc was measured as the difference in mean amplitude between activity contralateral and ipsilateral to the target at electrode sites OL/OR between 200ms – 300ms post target/array onset. The CDA was measured as the difference in mean amplitude between activity contralateral and ipsilateral to the target at electrode sites OL/OR between 300–1000ms following the presentation of the target. The P170 was measured as the mean amplitude at electrode site Fz between 150ms – 300ms post target onset.
Reward Sensitivity Measure
We used the BIS/BAS scales (Carver & White, 1994) to measure trait level sensitivity to reward and punishment. The BIS/BAS scales were developed as a self-report questionnaire to measure individual differences in BIS and BAS sensitivity as defined by Gray (1972, 1981). There are three BAS-related subscales assessing different aspects of behavioral approach motivation, including general reward responsiveness, drive for reward, and fun/sensation seeking. The Reward Responsiveness subscale measures positive responses to the anticipation of reward and rewarding stimuli. The Drive subscale measures the persistent pursuit of goals and internal drive to obtain rewards. The Fun Seeking subscale measures the desire for novel rewards and the willingness to engage in an activity “on a whim” if it might be enjoyable. We used the BAS Drive and BAS Reward Responsiveness subscales to assess trait level desire for reward and experience of pleasure from receiving a reward, respectively. The BAS Fun Seeking scale was included to measure the tendency toward a more impulsive (i.e. “on a whim”) type of reward seeking behavior. Although the task used did not have explicit losses, the BIS Total score was used as a control measure to assess participant’s sensitivity more broadly to uncertainty and potential reward failure. These scales have shown good reliability with Chronbach’s Alpha reliabilities for the BAS Drive, Reward Responsiveness, Fun Seeking, and BIS scales being reported as .76, .73, .66, and .74 respectively (Carver & White, 1994). By comparison, Alpha values for the same scales in the current sample were .65, .68, .70, and .75 respectively.
Analysis
Only correct trials were included in all analyses. Additionally, trials with reaction times faster than 250ms or more than 3sd from the mean were excluded. To obtain a single no reward value, we computed the average of no reward trials at serial positions 4 and 6 from each run of seven trials for each of our dependent variables. This was done to minimize serial position effects and the effect of reward anticipation or post reward differences. We also computed the averages of trials 2–3, 3–4, and 6–7 to assess for serial position effects within the no reward condition. These averages, as well as comparisons with the average of 4 and 6, can be seen in Table S1. The average of trials 4 and 6 did not significantly differ from the averages of other serial positions for all dependent variables except reaction time. For reaction time, the average of trials 3–4 was significantly faster than the average of 4 and 6. However, nearly identical results were obtained for subsequent reaction time analyses using the average of 3–4. Therefore, the average of 4 and 6 was used for consistency with other dependent measures. The averages for each variable at individual serial positions can be seen in Figure S2 to allow for a more direct comparison with Reinhart & Woodman (2014).
We used paired samples t-tests to test the difference between both low and high reward from no reward, and between low and high reward conditions for accuracy, reaction time, and for each of the ERP components described above. Difference scores were then computed for each measure to isolate the reward-induced change and control for individual differences in baseline responses. For example, high reward related change in reaction time (RT) was computed as ΔRT = High-Reward RT – No-Reward RT. These difference scores were used in all subsequent analyses to test for relationships with BIS/BAS scores. Correlations were computed between RT difference scores and BIS/BAS scores to assess the relationship between reward sensitivity and reward-induced change in behavioral performance. Correlations were not computed for accuracy because the lack of variability in accuracy would render those correlations uninterpretable. Stepwise multiple regression analyses were used to evaluate which ERP responses’ reward related amplitude change best predicted BIS/BAS scores. Separate stepwise linear regressions were computed for each BAS subscale and BIS Total scores, with reward related change (i.e. difference scores) in the ERP measures of interest as independent variables. Criteria for inclusion in the model at each step were p-in < .05, p-out > .10.
Results
Participants
The final sample consisted of 65 participants, 47% women, with a mean (sd) age of 23.5 (0.58). The mean (sd) scores for the BAS Drive, BAS Reward Responsiveness, BAS Fun Seeking, and BIS Total scales were 11.98 (2.09), 17.66 (2.11), 12.29 (2.43), and 21.25 (3.73) respectively. One participant’s score on the BAS Reward Responsiveness scale was more than three standard deviations from the mean and therefore was removed as an outlier. Analyses with BAS Reward Responsiveness therefore include 64 participants. The average working memory capacity calculated from the change detection task in the current sample was 3.26 (1.00) items. Healthy adults typically have a working memory capacity of around three items on this and similar tasks (Fukuda et al., 2010). Participants’ average working memory capacity was also significantly correlated with BIS Total scores (r = .28, p = .03), but not any of the BAS subscale scores (rs < .15, ps > .14).
Effects of Reward
Table 1 shows means and significant differences by reward level for reaction time, accuracy, and each of the ERP measures of interest (t values and effect sizes can be seen in table S1). Behaviorally, there were significant effects of reward on reaction time, and on accuracy. Participants responded faster on both low and high reward trials compared to no reward. There was no reaction time difference between low and high reward trials. Participants were also more accurate on high reward trials than both no and low reward trials despite accuracy being near ceiling across all reward levels. None of the individual difference measures were significantly correlated with reward related change in reaction time (|rs| < .14, ps > .27).
Table 1.
Behavioral and ERP Measures by Reward Level
| Means (sd)
|
|||
|---|---|---|---|
| No reward | Low Reward | High Reward | |
| Reaction Time (ms) | 665 (88)a | 647 (85)b | 650 (98)b |
| Accuracy (%) | 97.78 (2.41)a | 97.95 (3.32)a | 98.50 (2.55)b |
| Cue P3 (µV) | 0.01 (2.23)a | 7.64 (6.31)b | 12.12 (7.71)c |
| Target N2pc (µV) | −0.62 (0.72)a | −0.95 (1.16)b | −0.62 (1.19)a,b |
| Delay CDA (µV) | −0.64 (0.63)a | −0.96 (1.13)b | −0.60 (1.12)a |
| Delay P170 (µV) | 3.11 (3.07)a | 2.97 (4.09)a | 3.21 (3.08)a |
| Array N2pc (µV) | −0.89 (1.07)a | −1.14 (1.42)a | −1.07 (1.41)a |
Note: Mean values within each reward level. Means for the no reward trials are the average of serial positions 4 & 6. Means with different superscripts are significantly different at p < .05 (e.g. all reward levels are significantly different from each other for the Cue P3 component).
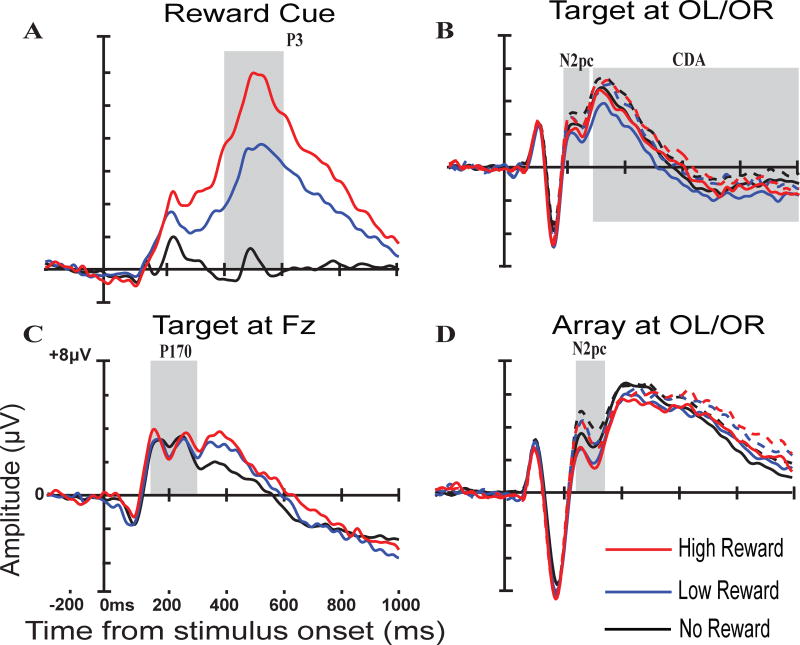
The recruitment of select cognitive mechanisms also differed by reward level. Potential rewards influenced the extent to which participants engaged in context updating following the reward cue in the expected way, with a significant effect for both overall reward and reward magnitude. Larger P3 amplitudes were observed following high reward cues than low reward cues and following both high and low reward cues compared to no reward cues. Potential rewards also influenced the deployment of attention to the initial target (N2pc) and the maintenance of the target in working memory (CDA). However, for the sample as a whole, the pattern of these effects was in the opposite direction as expected. Both the N2pc to the target and the CDA during the memory delay showed significantly larger (i.e. more negative) amplitudes on low reward, but not high reward, trials compared to no reward trials. CDA amplitude was also significantly more negative on low reward trials than high reward trials. N2pc amplitude on low reward trials was more negative than on high reward trials at a trend level (t = −1.79, p = .08). Consistent with Reinhart & Woodman (2014), the amplitude of the P170 indexing the recruitment of long-term memory did not change as a function of reward level. The N2pc indexing deployment of attention to the search array did not change as a function of reward level. Grand-average ERP waveforms by reward level can be seen in Figure 21.
Figure 2.
ERP indices of the recruitment of cognitive mechanisms by reward level. A) P3 following the reward cue. B) N2pc & CDA following target onset. C) P170 following target onset. The late positive peak (300ms – 450ms) in this waveform was analyzed post-hoc using a paired samples t-test. There was no significant effect of reward (ts < 1.2, ps > .24). D) N2pc following onset of the search array. No reward trials (average of serial positions 4 & 6) shown in black, low reward trials in blue, and high reward trials in red. For the N2pc and CDA dashed lines are ipsilateral to the target, solid lines are contralateral.
Reward Sensitivity
Regression analyses showed that BIS/BAS scores could be significantly predicted by reward related change in ERP amplitudes. Furthermore, BIS Total scores and the BAS subscale scores were predicted by different ERP components indexing unique cognitive mechanisms (see Table 2). In general, BAS subscale scores were predicted by ERPs indexing working memory related mechanisms, whereas BIS scores were predicted by ERPs indexing attention mechanisms.
Table 2.
Stepwise linear regression models predicting BIS/BAS scores from reward related change in ERP amplitude.
| Predictors | B | SE(B) | β | Adj. R2 |
|---|---|---|---|---|
| BAS Fun Seeking | ||||
| Δ Cue P3 High - Low | 0.26 | 0.09 | 0.33 | .10 |
| BAS Reward Responsiveness | ||||
| Δ Cue P3 High - Low | −0.37 | 0.17 | −0.26 | |
| Δ CDA High - Low | −0.37 | 0.17 | −0.26 | .13 |
| BIS Total (Model 1) | ||||
| Δ Target N2pc Low - No | 1.04 | 0.35 | 0.34 | |
| Δ Array N2pc High - Low | 0.7 | 0.33 | 0.25 | .19 |
| BIS Total (Model 2) | ||||
| Δ Target N2pc Low - No | 1.05 | 0.36 | 0.34 | |
| Average WM Capacity | 0.92 | 0.44 | 0.24 | .16 |
Note: Only predictors included in the final model (i.e. those that contribute unique variance) are shown. All difference scores for each ERP measure of interest (n = 15) were included as possible predictors in the initial model. BAS Drive scores were not significantly predicted by reward related change in any ERP measures. For BIS Total scores, Model 1 included only ERP difference scores (i.e. the same as the models for BAS Fun Seeking and BAS Reward Responsiveness). Model 2 is a second regression analysis that included average working memory as an additional possible predictor because of the significant correlation between working memory capacity and BIS scores.
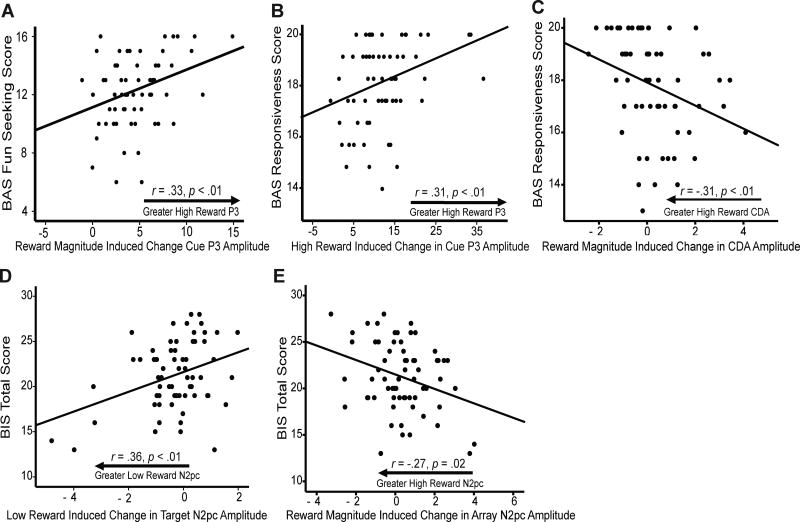
More specifically, only reward magnitude related change in P3 amplitude (i.e. the difference between low and high reward P3) following the initial reward cue significantly predicted BAS Fun Seeking scores. This reward magnitude related change in P3 amplitude explained 9.5% (Adj. R2 = .095) of the variance in BAS Fun Seeking scores. BAS Reward Responsiveness was significantly predicted by high reward change in P3 amplitude (i.e. high – no) to the reward cue and reward magnitude related change in CDA amplitude. Together, reward related change in P3 and CDA amplitudes explained 13.5% (Adj. R2 = .135) of the variance in BAS Reward Responsiveness scores. BAS Drive scores were not significantly predicted by any ERP measures.
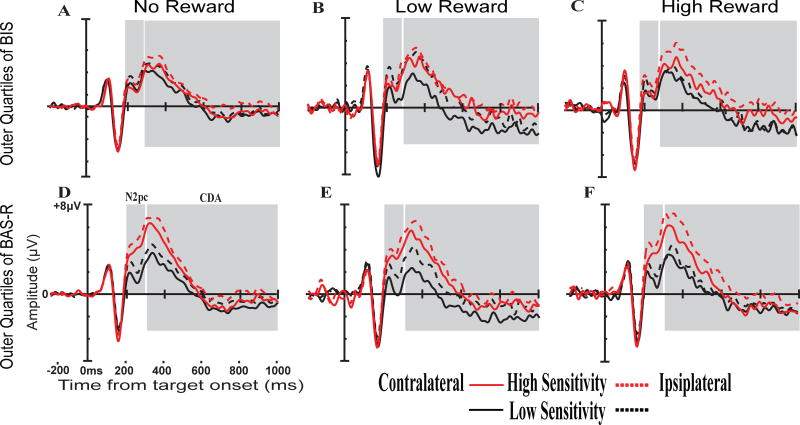
BIS Total scores were significantly predicted by low reward related change in N2pc amplitude to the initial target and reward magnitude related change in N2pc amplitude to the search array. Together, these changes in N2pc amplitude predicted 16.2% (Adj. R2 = .162) of the variance in BIS Total scores. Because BIS Total scores were also correlated with average working memory capacity, a separate stepwise linear regression was performed with average working memory capacity added to the original model. In this case, reward magnitude related change in N2pc amplitude to the search array no longer significantly predicted BIS Total scores. Instead, low reward related change in N2pc to the initial target and average working memory capacity together predicted 16.6% (Adj. R2 = .166) of the variance in BIS Total scores2. N2pc and CDA waveforms as a function of BAS Reward Responsiveness and BIS Total scores by reward level can be seen in Figure 3. Additionally, scatterplots of the relationships between BIS/BAS scores and the significant predictors from each regression model are presented in Figure 4.
Figure 3.
N2pc and CDA waveforms following target onset by reward level and individual differences in reward sensitivity. Waveforms shown are for the upper (red) and lower (black) quartiles of BIS and BAS reward-responsiveness scores. A) No reward trials by BIS scores. B) Low reward trials by BIS. C) High Reward trials by BIS. D) No reward trials by BAS reward-responsiveness. E) Low reward trials by BAS reward-responsiveness. F) High reward trials by BAS reward-responsiveness.
Figure 4.
Scatterplots of BIS/BAS scores and the ERP measures that were significant predictors of those scores in regression models. ERP amplitudes in microvolts (µV). A) BAS Fun Seeking score by reward magnitude (i.e. high – low) induced change in P3 to the reward cue. B) BAS Reward Responsiveness score by high reward induced change in P3 to the reward cue. C) BAS Reward Responsiveness score by reward magnitude induced change in CDA. D) BIS score by low reward induced change in N2pc to the target. E) BIS score by reward magnitude induced change in N2pc to the search array.
Discussion
Reward has been shown to enhance the recruitment of cognitive mechanisms such as attention and working memory (Hickey, Chelazzi, & Theeuwes, 2010a; Reinhart & Woodman, 2014). However, not all individuals seek rewards or respond to being rewarded in the same way (Balconi et al., 2012; Erdle & Rushton, 2010). Despite this, our understanding of how individual differences in reward sensitivity influence the recruitment of specific cognitive mechanisms in conditions of reward had been limited. Therefore, the current study tested two sets of competing hypotheses regarding the influence of potential rewards on the recruitment of cognitive mechanisms and the role of individual differences in reward sensitivity. First, we examined whether potential rewards would broadly influence the recruitment of multiple cognitive mechanisms, or whether potential rewards would have a selective effect of the recruitment of specific cognitive mechanisms. Secondly, we examined whether individual differences in reward sensitivity in general would broadly influence the recruitment of these cognitive mechanisms or whether different aspects of reward sensitivity would influence the recruitment of specific mechanisms.
Cognitive Mechanisms
The results of this study support the hypothesis that potential rewards influence the recruitment of select cognitive mechanisms, but not others. Specifically, we replicated the findings of Reinhart & Woodman (2014) showing that potential rewards influence the recruitment of working memory but not long-term memory. We also found that potential rewards influence the deployment of attention to targets at their initial presentation, but not to targets within the search array. This difference may be due to the overall greater selective attention required to identify the target within the more complex search array as evidenced by larger N2pc amplitudes across reward conditions for the search array compared to the initial target. Therefore, the effect of reward may have been smaller relative to the overall attention deployed to targets in the search array. Finally, we found evidence that differences in initial context updating from reward cues were related to both the potential for reward and reward magnitude as evidenced by the significant differences in P3 amplitude across conditions. These results suggest that potential rewards selectively influence context updating, working memory, and the deployment of attention, but not the recruitment of long-term memory. Additionally, context updating also appears to be sensitive to reward magnitude, and that potential rewards appear to influence the deployment of attention in some situations (i.e. for initial targets) and not others (i.e. targets in the search array).
However, there is one key difference between the results of this study and those of Reinhart and Woodman regarding the recruitment of working memory. Reinhart and Woodman found greater CDA amplitude on high reward trials than on low reward trials, which the current study did not. The current study found greater CDA (and N2pc) amplitude on low reward trials compared to no reward and high reward trials. We propose two possible explanations for this difference, the inclusion of no reward trials in the current study, and the use of more salient and face valid reward cues. The inclusion of no reward trials, and therefore a manipulation of reward magnitude, may have altered how attention and working memory were recruited to perform the task. Additionally, the use of more salient reward cues compared to the colored circles used by Reinhart and Woodman may have, on high reward trials in particular, led to increased recruitment of cognitive resources immediately following the reward cue. This increased recruitment of resources in the form of context updating is demonstrated by the increased P3 amplitude on high reward trials compared to no and low reward trials. This may have subsequently reduced the need for increased attention and working memory recruitment following the target. This greater early recruitment of resources on high reward trials compared to low reward trials and subsequent lack of additional attentional and working memory resources needed, would account for the smaller N2pc and CDA amplitudes observed on high reward trials compared to low reward trials. Although these results are the opposite of what was initially expected, the amplitude of the CDA has been shown to decrease with reductions in cognitive demands (Clark, Appelbaum, van den Berg, Mitroff, & Woldorff, 2015), which may be what was observed here.
Reward Sensitivity
The results of the current study also support the hypothesis that individual differences in specific aspects of reward and punishment sensitivity influence the recruitment of select cognitive mechanisms in conditions of potential reward. Although reward related change in ERP responses did not significantly predict BAS Drive scores, there were unique components that showed predictive utility for BAS Fun Seeking and BAS Reward Responsiveness scores. Specifically, reward magnitude related change in P3 amplitude to the initial reward cue predicted BAS Fun Seeking scores such that individuals who showed a greater P3 response to high reward cues relative to low reward cues had higher BAS Fun Seeking Scores. This suggests that individuals who tend to engage in more impulsive, “on a whim”, type reward seeking behavior, as is measured by the BAS Fun Seeking, scale may find high reward cues more salient, and may devote greater cognitive resources to recognizing the potential for high rewards compared to low rewards to update their mental context accordingly. Reward related change in P3 amplitude, in this case high reward induced change (compared to no reward), also significantly predicted BAS Reward Responsiveness scores suggesting a similar salience of high reward cues. However, BAS Reward Responsiveness scores were also related to the maintenance of potentially rewarded targets in working memory. Here, greater high reward CDA amplitude compared to low reward predicted higher BAS Reward Responsiveness scores. This suggests that individuals high in BAS Reward Responsiveness may devote more cognitive resources to both context updating when presented with high reward cues, and greater maintenance of stimuli in working memory, if those stimuli are relevant to earning high rewards. Neither reward induced changes in the deployment of attention or the recruitment of long term memory were predictive of BAS Fun Seeking scores or BAS Reward Responsiveness scores suggesting a specific relationship between the traits measured by BAS subscales and the recruitment of working memory/context updating in conditions of reward.
In contrast to the BAS subscales, BIS Total scores were not significantly predicted by ERP amplitudes indexing context updating or working memory. Instead, reward induced changes in deployment of attention to both the initial target and the search array together significantly predicted participants’ BIS Total scores. Notably, participants with high BIS scores showed the opposite pattern of ERP responses indexing the deployment of attention compared to those with low BIS scores and the sample as a whole. ERP indices for individuals low in BIS are consistent with greater deployment of attention to targets (i.e. more negative N2pc amplitude) on low reward trials, which is similar to the sample as a whole. In contrast, ERP indices for individuals high in BIS suggest less recruitment of attentional resources on low reward trials and greater recruitment or attention on high reward trials. These results suggest that, despite any increased recruitment of resources following the reward cue as discussed above, those highest in BIS sensitivity still recruited additional resources to deploy attention to high reward targets.
The finding that high BIS was specifically related to increased recruitment of attention in high reward conditions suggests that individuals with high BIS sensitivity may have conceptualized an incorrect response on high reward trials as potential reward failure and therefore recruited additional resources to avoid this “loss”. Participants high in BIS may have also been more sensitive to the uncertainty regarding the response required at the search array (i.e. target match vs. target non-match) and therefore recruited more resources to attend to the initial target.
These results suggest that the motivation for performance on the task (i.e. earn rewards vs avoid reward failures) may impact which cognitive mechanisms are recruited but individuals who strongly hole those motivations. Whereas those high in BIS would be motivated by relative loss avoidance, the motivation for those high in BAS-responsiveness is to ensure earning a reward. It is possible that individuals high in BAS Reward Responsiveness experienced the high reward targets as rewarding in and of themselves because they had developed learned associations through the repetition of the target in the first four trials of that run. In this case the rewarding nature of the target led to increased recruitment of working memory to ensure the earning of a reward. In regards to individuals high in BAS Fun Seeking, these results suggest an initial greater salience of high reward cues that could potentially signal a sense of excitement at the prospect of a high reward, but no sustained increases in the recruitment of other cognitive mechanisms.
Limitations
There are several key limitations to the current study including the lack of explicit loss trials in the task, the near ceiling accuracy making it impossible to analyze error trials because of their limited numbers, and the potential for ERP component overlap influencing the amplitude of the N2pc and CDA components. First, the BIS is most sensitive to punishment and loss. Although BIS is also sensitive to non-rewards and even response uncertainty, the lack of overt loss trials limits the generalizability of the current results. Furthermore, the limited number of incorrect trials, which would have constituted a failure to earn a reward, also limits the conclusions that can be drawn from the current data. We can say that individuals high in BIS sensitivity appeared to be proactively recruiting more attentional resources as a way to ensure that they would avoid reward failure, but without loss, or at least more failed reward trials, we can not conclusively state that this is the case. Tasks that include overt losses instead of only non-rewards would allow for the investigation of reward expectancies and whether the same mechanisms are involved in true loss avoidance as in reward seeking.
ERP component overlap between the P3 to the reward cue and the N2pc and CDA following the reward cue may also be at least partially responsible for the finding that high reward trials had smaller N2pc and CDA amplitudes than low reward trials. The time window for the P3 ERP component overlapped fully with the N2pc time window and by 200ms with the CDA time window following the target. The amplitude of the N2pc and CDA on high reward trials may have been reduced (i.e. made less negative) by the overlapping, large, and positive going P3 response to high reward cues. To reduce this component overlap future work should increase the time between the reward cue presentation and the target as well as possibly jittering this time window to help any remaining component overlap be removed during the averaging process. Although the time between trials was jittered in the current study, the time between the reward cue and target was not. Finally, as is true with many ERP studies, the interpretation of ERP components depends upon the reverse inference that the waveforms reflect a specific cognitive process. Although we have evidence that each of these components is related to the cognitive mechanism it was used to measure, the ERP component and the cognitive mechanism should not be conflated.
Conclusions
The results of the current study support the hypotheses that potential rewards influence the recruitment of select cognitive mechanisms, specifically attention and working memory, and that different aspects of reward sensitivity, BIS and BAS Reward Responsiveness respectively, are related to greater recruitment of these mechanisms. Despite not having overt losses it appears that sensitivity to potentially failed rewards or response uncertainty can influence the recruitment of attention when there is the potential for a high reward. Additionally BAS Reward Responsiveness appears to selectively influence the recruitment of working memory for potential high reward targets. Despite the limitations discussed, the results of this study provide some initial insight into how individual differences in reward sensitivity influence the recruitment of cognitive mechanisms in conditions of potential reward.
Supplementary Material
Acknowledgments
Funding: National Institute of Mental Health.
Footnotes
The authors are aware that ERP waveforms have been, by convention, typically shown with negative amplitude plotted up. However, for consistency with the first author’s previous work and for ease of interpretation by a wider audience we chose to plot positive amplitude up. Not all authors were in agreement with this decision.
Regression analyses were also computed for each of the BAS subscale scores with average working memory capacity included in the model. The inclusion of average working memory capacity did not change the predictors included in the final model for any of the BAS subscales.
References
- Balconi M, Falbo L, Conte VA. BIS and BAS correlates with psychophysiological and cortical response systems during aversive and appetitive emotional stimuli processing. Motivation and Emotion. 2012;36(2):218–231. http://dx.doi.org/10.1007/s11031-011-9244-7. [Google Scholar]
- Baskin-Sommers AR, Krusemark EA, Curtin JJ, Lee C, Vujnovich A, Newman JP. The impact of cognitive control, incentives, and working memory load on the P3 responses of externalizing prisoners. Biological Psychology. 2014;96:86–93. doi: 10.1016/j.biopsycho.2013.12.005. http://dx.doi.org/10.1016/j.biopsycho.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience & Biobehavioral Reviews. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. http://dx.doi.org/10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. https://doi.org/10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Carlisle NB, Arita JT, Pardo D, Woodman GF. Attentional Templates in Visual Working Memory. Journal of Neuroscience. 2011;31(25):9315–9322. doi: 10.1523/JNEUROSCI.1097-11.2011. https://doi.org/10.1523/JNEUROSCI.1097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Thái S. ERPs on a continuous performance task and self-reported psychopathic traits: P3 and CNV augmentation are associated with Fearless Dominance. Biological Psychology. 2010;85(2):318–330. doi: 10.1016/j.biopsycho.2010.08.002. 85 http://dx.doi.org/10.1016/j.biopsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319. http://dx.doi.org/10.1037/0022-3514.67.2.319. [Google Scholar]
- Clark K, Appelbaum LG, van den Berg B, Mitroff SR, Woldorff MG. Improvement in Visual Search with Practice: Mapping Learning-Related Changes in Neurocognitive Stages of Processing. The Journal of Neuroscience. 2015;35(13):5351–5359. doi: 10.1523/JNEUROSCI.1152-14.2015. http://dx.doi.org/10.1523/JNEUROSCI.1152-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Polich J. On the number of trials needed for P300. International Journal of Psychophysiology. 1997;25(3):249–255. doi: 10.1016/s0167-8760(96)00743-x. https://doi.org/10.1016/S0167-8760(96)00743-X. [DOI] [PubMed] [Google Scholar]
- Corr PJ. Reinforcement sensitivity theory and personality. Neuroscience & Biobehavioral Reviews. 2004;28(3):317–332. doi: 10.1016/j.neubiorev.2004.01.005. http://dx.doi.org/10.1016/j.neubiorev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychological Science. 2006;17(3):222–227. doi: 10.1111/j.1467-9280.2006.01689.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Diana RA, Vilberg KL, Reder LM. Identifying the ERP correlate of a recognition memory search attempt. Brain Research. Cognitive Brain Research. 2005;24(3):674–684. doi: 10.1016/j.cogbrainres.2005.04.001. https://doi.org/10.1016/j.cogbrainres.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research. 2004;18(3):255–272. doi: 10.1016/j.cogbrainres.2003.10.010. https://doi.org/10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology. 1996;99(3):225–234. doi: 10.1016/0013-4694(96)95711-9. http://dx.doi.org/10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology. 2007;74(1):108–112. doi: 10.1016/j.biopsycho.2006.06.008. http://dx.doi.org/10.1016/j.biopsycho.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined Effects of Attention and Motivation on Visual Task Performance: Transient and Sustained Motivational Effects. Frontiers in Human Neuroscience. 2009:3. doi: 10.3389/neuro.09.004.2009. https://doi.org/10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed]
- Erdle S, Rushton JP. The general factor of personality, BIS-BAS, expectancies of reward and punishment, self-esteem, and positive and negative affect. Personality and Individual Differences. 2010;48(6):762–766. https://doi.org/10.1016/j.paid.2010.01.025. [Google Scholar]
- Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of Abnormal Child Psychology. 2011;39(7):913–924. doi: 10.1007/s10802-011-9503-9. http://dx.doi.org/10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Awh E, Vogel EK. Discrete capacity limits in visual working memory. Current Opinion in Neurobiology. 2010;20(2):177–182. doi: 10.1016/j.conb.2010.03.005. http://dx.doi.org/10.1016/j.conb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. Journal of Research in Personality. 2006;40(6):1086–1102. http://dx.doi.org/10.1016/j.jrp.2005.11.001. [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward changes salience in human vision via the anterior cingulate. The Journal of Neuroscience. 2010a;30(33):11096–11103. doi: 10.1523/JNEUROSCI.1026-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.1026-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward guides vision when it’s your thing: trait reward-seeking in reward-mediated visual priming. PloS One. 2010b;5(11):e14087. doi: 10.1371/journal.pone.0014087. http://dx.doi.org/10.1371/journal.pone.0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward has a residual impact on target selection in visual search, but not on the suppression of distractors. Visual Cognition. 2011;19(1):117–128. http://dx.doi.org/10.1080/13506285.2010.503946. [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59(8):750–757. doi: 10.1001/archpsyc.59.8.750. http://dx.doi.org/10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38(3):557–577. doi: 10.1017/s0048577201990559. http://dx.doi.org/10.1017/S0048577201990559. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8(1):99–112. doi: 10.3758/cabn.8.1.99. http://dx.doi.org/10.3758/CABN.8.1.99. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Reward Reduces Conflict by Enhancing Attentional Control and Biasing Visual Cortical Processing. Journal of Cognitive Neuroscience. 2011;23(11):3419–3432. doi: 10.1162/jocn_a_00011. https://doi.org/10.1162/jocn_a_00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering A, Gray JA, et al. Dopamine, appetitive reinforcement, and the neuropsychology of human learning: An individual differences approach. Advances in Individual Differences Research. 2001:113–149. [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. Neural correlates of reward-driven attentional capture in visual search. Brain Research. 2013;1532:32–43. doi: 10.1016/j.brainres.2013.07.044. https://doi.org/10.1016/j.brainres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Reinhart RM, Woodman GF. High stakes trigger the use of multiple memories to enhance the control of attention. Cerebral Cortex. 2014;24(8):2022–2035. doi: 10.1093/cercor/bht057. http://dx.doi.org/10.1093/cercor/bht057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR, Black AH, Prokasy WF. Classical conditioning II: Current research and theory. Black, AH. 1972:64–99. [Google Scholar]
- Savine AC, Braver TS. Motivated Cognitive Control: Reward Incentives Modulate Preparatory Neural Activity during Task-Switching. Journal of Neuroscience. 2010;30(31):10294–10305. doi: 10.1523/JNEUROSCI.2052-10.2010. https://doi.org/10.1523/JNEUROSCI.2052-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Segarra P, Poy R, López R, Moltó J. Characterizing Carver and White’s BIS/BAS subscales using the Five Factor Model of personality. Personality and Individual Differences. 2014;61:18–23. http://dx.doi.org/10.1016/j.paid.2013.12.027. [Google Scholar]
- Tsivilis D, Otten LJ, Rugg MD. Context effects on the neural correlates of recognition memory: an electrophysiological study. Neuron. 2001;31(3):497–505. doi: 10.1016/s0896-6273(01)00376-2. http://dx.doi.org/10.1016/S0896-6273(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Van den Berg I, Franken IHA, Muris P. Individual differences in sensitivity to reward: Association with electrophysiological responses to monetary gains and losses. Journal of Psychophysiology. 2011;25(2):81–86. https://doi.org/10.1027/0269-8803/a000032. [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. doi: 10.1038/nature02447. https://doi.org/10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. doi: 10.1038/nature04171. http://dx.doi.org/10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Voigt DC, Dillard JP, Braddock KH, Anderson JW, Sopory P, Stephenson MT. BIS/BAS scales and their relationship to risky health behaviours. Personality and Individual Differences. 2009;47(2):89–93. http://dx.doi.org/10.1016/j.paid.2009.02.003. [Google Scholar]
- Voss JL, Schendan HE, Paller KA. Finding meaning in novel geometric shapes influences electrophysiological correlates of repetition and dissociates perceptual and conceptual priming. Neuroimage. 2010;49(3):2879–2889. doi: 10.1016/j.neuroimage.2009.09.012. http://dx.doi.org/10.1016/j.neuroimage.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Arita JT. Direct electrophysiological measurement of attentional templates in visual working memory. Psychological Science. 2011;22(2):212–215. doi: 10.1177/0956797610395395. http://dx.doi.org/10.1177/0956797610395395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400(6747):867–869. doi: 10.1038/23698. http://dx.doi.org.proxy.library.vanderbilt.edu/10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK. Selective storage and maintenance of an object’s features in visual working memory. Psychonomic Bulletin & Review. 2008;15(1):223–229. doi: 10.3758/pbr.15.1.223. http://dx.doi.org/10.3758/PBR.15.1.223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.