Abstract
Although previous studies have shown that individuals with depressive tendencies have deficits in forgetting negative material, the detailed underlying neural mechanisms have not been elucidated. This study examined the intentional forgetting of negative and neutral material in individuals with depressive tendencies in two phases. In the study phase, the participants performed a directed forgetting task, where a total of 320 words were presented to them, each followed by an instructive cue to forget or remember the previously presented word. Subsequently, in the memory recognition test phase, the participants completed the “old or new discrimination task”. The results indicated that individuals with depressive tendencies had difficulties suppressing the memory encoding of negative words, while the suppression of memory encoding of neutral words was relatively intact. Moreover, individuals with depressive tendencies displayed enhanced word-evoked P2 and late positive potential for negative items, as well as enhanced cue-evoked P1 and N2 for the negative items that were required to be forgotten, as compared to individuals without depressive tendencies. Based on these results, we propose two mechanisms that may contribute to the failure of forgetting negative material in mild depression: (1) inefficient memory suppression and early selective attention, and (2) excessive preliminary processing.
Introduction
According to Beck’s cognitive model of depression, depressed individuals exhibit excessive negative biases in many aspects of cognitive processes, such as attention and memory, as compared to healthy controls1,2. This negative bias plays a critical role in the etiology as well as the maintenance of depressive symptoms (e.g. the ruminative thinking style)3–6. The majority of previous studies focused on the biased processing of emotional information in the aspects of perception, attention, memory and interpretation, neglecting the functions of inhibition and cognitive control in the depressed population2,7. Recent studies have demonstrated that depressed individuals are less successful in excluding unwanted thoughts and memories from awareness due to inhibitory deficits in the cognitive control system8–10. This impaired inhibitory function may consequently hinder recovery from depressive symptoms, and induce a vicious circle of a mood-congruent state and a persistent low mood7,11. Therefore, it is important to investigate the intentional forgetting and the associated neural mechanisms in the depressed population, which may broaden our understanding of the pathology of this disorder and aid in the development of more efficient treatments.
As reviewed by Anderson & Hanslmayr12, memory inhibition or suppression may occur during the stages of memory encoding and memory retrieval. The intentional forgetting at the stage of memory encoding can efficiently limit and disrupt the consolidation and further reflection of unwanted memories, which is the focus of this study. Memory inhibition during encoding is usually investigated using the directed forgetting (DF) paradigm13, which consists of two phases. In the study phase, participants are presented with a series of items, some of which are “to be remembered” (TBR) and others that are “to be forgotten” (TBF). When the “remember” cue is given, participants are required to remember or consolidate the previously presented item. On the contrary, when the “forget” cue is given, they should try their best to forget the previously presented item. In the memory recognition test phase, participants complete the “old or new discrimination task”, where the previously studied words are mixed with new words. The DF effect is defined as a higher recognition rate for TBR than for TBF items in the phase of memory recognition test12. There are two methods to perform the DF task: the item-method, and the list-method12,14. In the item-method, the “remember/forget” cue is presented after each item, whereas in the list-method, the cue is presented after a list of items. Both methods involve activation of the frontal regions, but their cognitive and neural mechanisms are quite different12. This study employed the item-method paradigm, since it provides an insight into how individuals with depressive tendencies control new, incoming information.
Previous DF studies have shown that, while the medial-temporal lobe, and especially the hippocampus, is significantly activated by the “remember” instruction, the prefrontal and parietal regions are activated by the “forget” instruction15–18. In particular, intentional forgetting recruits more executive control resources at the frontal lobe than incidental forgetting18. Furthermore, some studies demonstrated a negative correlation between activity in the dorsolateral prefrontal cortex and the hippocampus during successful forgetting17. Thus, the psychological mechanism of the DF task has been proposed as follows: items are tentatively kept in the working memory until the “remember/forget” instruction requires participants to selectively rehearse and elaborately encode the TBR items or to disrupt the encoding and suppress the consolidation of the TBF items using the inhibitory control mechanism15–24. In addition to selective rehearsal and inhibition, another three mechanisms have been proposed for the item-method DF, namely, set segregation (TBR and TBF items are organized into two groups so that the processing of the items of each group does not interfere with the other25), attentional withdrawal (attention is withdrawn from the task-irrelevant TBF items so that their representations are removed from the working memory26), and cognitive load (forgetting is more effective and easier when cognitive resources are allocated to the rehearsal of TBR items or exhausted by other processes27,28).
Behavioral studies have revealed that individuals with high levels of depression exhibit deficits in directed forgetting; depressed patients29 and individuals with depressive tendencies30 recognized more TBF words than nondepressed individuals, more specifically, they exhibit reduced forgetting of negative, compared to neutral, or positive TBF words31,32. However, to date, the neural mechanisms underlying the memory control deficits in the depressed population have only been explored in two empirical studies. In one study, Yang et al.9 found that depressed patients could not forget negative material and displayed stronger activation in their frontal and parietal regions (associated with inhibitory control function) when they attempted to forget negative TBF items. This abnormal activation indicated that even though more inhibitory control resources were recruited to terminate the rehearsal of negative TBF items, the intent failed due to excessive negative bias. Therefore, it was postulated that depressed patients are inefficient in forgetting negative material9. In the other study, Berman et al.8 demonstrated that patients with major depressive disorder have difficulty in forgetting negative words, using a DF procedure. They observed that when participants expelled negative information from their short-term memory, both major depressive patients and healthy controls robustly activated a network (including the inferior frontal gyrus) that has been implicated in memory selection and interference resolution; there was no difference in the magnitude of activation of the two groups. However, while the control group displayed focal activation in the left inferior frontal gyrus, the major depressive patients exhibited more diffuse activation (i.e., a larger spatial variability) in this region; this spatial variability was associated with the patients' greater behavioral variance in reaction times.
Although the two fMRI studies8,9 demonstrated that depressed patients have abnormal brain activation when they attempt to forget negative material, the detailed neural mechanisms associated with their failure in the DF task is still unclear. As introduced above, there are two procedures in the study phase of the DF paradigm: the participants first preliminarily encode the presented item and then implement the “remember/forget” cue to remember or forget the encoded item. However, when using the fMRI technique, it is difficult to discriminate these two quick procedures due to its low temporal resolution. In this context, the authors of the present study considered that the event-related potential (ERP) is a more suitable tool to separately investigate the neural deficits in the two procedures. Moreover, another catalyst for the execution of this study was to shed light on the previous DF studies in healthy subjects, which presented contrasting results associated with the memories of emotional vs. neutral material20,23,33,34, that may be due to individual differences in these studies. To examine the impact of individual differences on the performance in the DF task, this study included measures of individual differences in depressive tendency, trait anxiety, ruminative tendency, and approach/avoidance motivational system. Subsequently, the relations between individual differences and behavioral/ERP indexes were explored.
In accordance with previous DF studies in healthy subjects, the present work focused on three ERP components. The first one is the middle frontal N2, which reflects cognitive control and is usually observed in motor and memory inhibition tasks35–38. It has been established that the “forget” instructive cue evokes larger N2 amplitudes (more negative-going potentials) than the “remember” cue19,21,23,34. In some DF studies, the N2 (approximately 200 to 300 ms after cue onset) was also referred to as “the frontal positivity”, since this negative-going component actually had positive amplitude19,21,34. The second component of interest is the parietal P3. This component usually displays larger amplitudes following the “remember” cue compared to the “forget” cue, reflecting selective rehearsal of the TBR items19–23,33,34,39,40. In addition to the cue-evoked N2 and P3, the word-evoked ERPs are also examined. Specifically, previous studies indicated that the late positive potential (LPP) displays larger amplitudes for negative items than for neutral items, indicating more elaborate processing of negative material in healthy individuals20,23,33. It should be noted that, both P3 and LPP refer to large and slow positive potentials, which peak at the parietal electrodes later than 300 ms post-stimulus. In this study, the term “P3” is used to refer to the relatively earlier positive component (about 300 ms) with a sharper peak, and the term “LPP” to refer to the slower positive wave (after 500 ms) with a blunter peak41,42. In addition to the three ERP components that have been often mentioned in the DF paradigm, the present study investigated early components such as the occipital P1 and the frontal P2, which are well-known attention-related components43,44, but have not been explored in previous DF studies. Although both P1 and P2 are usually used as indexes of selective attention, P1 is more sensitive to stimulus features (feature-based attention45) and arousal levels46, while the anterior P2 is often associated with the motivational salience of a stimulus as determined by either task-relevant features or novelty47–51.
This study compared the behavioral and ERP data of individuals with and without depressive tendencies. It is hypothesized that individuals with depressive tendencies experience difficulty in forgetting negative material, which is due to deficits/dysfunctions in both procedures during the study phase of the DF task, i.e., the item encoding, and the implementation of the “remember/forget” cue. Accordingly, it is expected on the behavioral level that subjects with depressive tendencies will have a higher recognition rate for TBF negative words than nondepressed subjects. To examine the deficits during the encoding procedure, the word-evoked ERP components of occipital P1, frontal P2 and parietal LPP were investigated. While to examine the deficits in the implementation of the “remember/forget” cue, the cue-evoked ERP components of occipital P1, frontal N2 and parietal P3 were investigated. It was hypothesized that subjects with depressive tendencies would excessively encode and rehearse negative items compared with nondepressed subjects. Considering that the amplitude of word-evoked LPP is associated with elaborate encoding processing20,23,33, it was predicted that negative words may elicit larger word-evoked LPP in subjects with depressive tendencies than in nondepressed subjects. Furthermore, considering that the amplitude of cue-evoked P3 is associated with selective rehearsal19–23,33,34,39,40, it was predicted that negative TBR words may elicit larger cue-evoked P3 in subjects with depressive tendencies compared with nondepressed subjects. Moreover, since Yang et al.9 demonstrated that depressed patients presented stronger activations in the neutral networks associated with inhibitory control function, it was hypothesized that inhibitory control resources might be inefficiently recruited in subjects with depressive tendencies when they are required to forget negative words. Accordingly, it was expected that negative words may elicit larger cue-evoked N2 amplitudes for TBF items in subjects with depressive tendencies compared to nondepressed subjects. Furthermore, this study examined the potential influence of individual differences (measured by several self-reported scales) on the performance of DF. According to previous studies using emotional material, the performance of DF might be affected by individual differences in depression8,29,30, rumination9,31, trait anxiety30,32, and the behavioral activation/inhibition system52. Consequently, this study included measures of individual differences in depression, ruminative tendencies, trait anxiety, and the activation/inhibition system (see detailed information in Methods).
Methods
Participants
Considering that psychiatric medications may affect behavioral and/or ERP results53,54, this study examined the impaired directed forgetting in individuals with depressive tendencies rather than in depressed patients. Furthermore, because previous studies have demonstrated a correlation between anxiety and deficits of directed forgetting32,55,56, this study only recruited participants with a moderate level of trait anxiety, i.e., the directed forgetting was compared between the individuals with and without depressive tendencies, and both the groups had a moderate level of anxiety.
In a mental health screening of Shenzhen University, all the freshman students (n = 6000) completed the Beck Depression Inventory Second Edition (BDI-II57) and the Trait form of Spielberger's State-Trait Anxiety Inventory (STAI-T58,59). In this sample, students with STAI-T scores within the middle 50% (from 25% to 75%) of the distribution were considered as subjects with a moderate level of trait anxiety60,61. Among the individuals with a moderate level of trait anxiety, students with BDI-II scores ≤ 13 were labeled as individuals without depressive tendencies, whereas students with BDI-II scores > 13 were labeled as individuals with depressive tendencies. According to Beck et al.57, a BDI-II score ≤ 13 indicates minimal depression, and a BDI-II score > 13 indicates mild (14–19), moderate (20–28), or severe depression (29–63). Among the students who met all these criteria, we randomly invited 60 individuals to participate the current study (30 individuals with and 30 individuals without depressive tendencies). As shown in Table 1, no significant difference was found between the two groups with respect to age, sex, handedness and STAI-T scores.
Table 1.
Demographic data of the two groups. Descriptive data are presented as mean ± standard error.
| Characteristics | Without depressive tendencies (n = 30) | With depressive tendencies (n = 30) | Statistics |
|---|---|---|---|
| Mean age, y | 18.3 ± 0.1 | 18.5 ± 0.1 | t(58) = −1.40, p = 0.167 |
| Sex, male/female | 16/14 | 15/15 | |
| Handedness, right/left | 30/0 | 30/0 | |
| BDI-II | 4.0 ± 0.4 | 19.8 ± 1.1 | t(58) = −13.2, p < 0.001 |
| STAI-T | 41.9 ± 1.1 | 44.8 ± 1.1 | t(58) = −1.84, p = 0.072 |
| RRS | 36.2 ± 1.1 | 56.0 ± 2.0 | t(58) = −8.76, p < 0.001 |
| BIS | 19.7 ± 0.6 | 24.0 ± 0.7 | t(58) = −4.48, p < 0.001 |
| BAS | 42.0 ± 0.8 | 42.3 ± 1.3 | t(58) = −1.96, p = 0.846 |
BDI-II, Beck Depression Inventory (Second Edition).
STAI-T, the Trait form of Spielberger's State-Trait Anxiety Inventory.
RRS, the Rumination Response Scale.
BIS/BAS, the Behavioral Inhibition System and Behavioral Activation System Scale.
Exclusion criteria for both groups were 1) any Axis I and II disorders according to the Diagnostic and Statistical Manual (DSM-IV)62; 2) seizure disorder; 3) history of head injury with possible neurological sequelae, and 4) substance abuse or dependence in the past six months.
Participants were told about the equipment used in the experiment and their tasks. Written informed consent was obtained prior to the experiment. The experimental protocol was approved by the Ethics Committee of Shenzhen University and this study was performed strictly in accordance with the approved guidelines.
Self-reported measures
The BDI-II is a widely used self-reported measure of depressive symptoms57. It consists of 21 items that assess the level of depressive symptoms in the past two weeks. The BDI-II scores from 0 to 63; a high score indicates a high level of depressive tendency.
The STAI-T is developed to evaluate a relatively enduring tendency of anxiety58,59. It contains 20 items and scores from 20 to 80; a high score indicates a high level of trait anxiety.
The Ruminative Response Scale (RRS)63–65 is used to assess how participants tend to respond to sad feelings and symptoms of dysphoria. It contains 22 items and scores from 22 to 88; a high score indicates a high level of ruminative tendency.
The Behavioral Inhibition System and Behavioral Activation System Scale (BIS/BAS) is designed to assess individual differences in the sensitivity of two motivational systems, i.e., a behavioral approach system and a behavioral avoidance system66. The BIS sub-scale contains 7 items (scores from 7 to 28) and the BAS contains 13 items (scores from 13 to 52). A high score on BIS or BAS indicates a larger tendency to regulate aversive or appetitive motives so as to move away from unpleasant, or to move toward desired, events and stimuli.
Experimental design and stimuli
A design with two (instruction: remember vs. forget) × two (valence of material: neutral vs. negative) × two (group: with depressive tendencies vs. without depressive tendencies) factors was used in this study.
This study used 640 words (320 negative and 320 neutral ones) from Chinese Affective Words System67, with equal number of words between adjectives and nouns. Each word consisted of two Chinese characters. The material was assessed for its familiarity, valence and arousal on a 9-point scale with a large sample of Chinese participants in a previous survey. The negative and neutral words had a median level of arousal (negative = 5.00 ± 0.80, neutral = 4.43 ± 0.64; t(638) = 10.0, p < 0.001), and the two categories differed significantly in valence (negative = 3.52 ± 0.75, neutral = 5.76 ± 0.50; t(638) = −44.4, p < 0.001). No difference was found in the familiarity between negative and neutral words (negative = 5.32 ± 0.59, neutral = 5.33 ± 0.58; t(638) = −0.17, p = 0.868).
Considering that the DF paradigm consisted of two phases (study and recognition test)13, the 640 words were randomly divided into two subsets, with equal number of words between neutral and negative conditions and between nouns and adjectives. One subset (320 words) was used in the study phase while the whole 640 words were used in the recognition test (i.e., 320 new and 320 old words). The valence (negative: t(318) = −0.96, p = 0.336; neutral: t(318) = −0.85, p = 0.397), arousal (negative: t(318) = 0.39, p = 0.694; neutral: t(318) = 0.32, p = 0.748), and familiarity (negative: t(318) = 1.28, p = 0.201; neutral: t(318) = −0.20, p = 0.840) of negative and neutral words were counterbalanced between the two subsets. Furthermore, the valence (negative: t(158) = −1.36, p = 0.176; neutral: t(158) = −1.25, p = 0.213), arousal (negative: t(158) = 1.29, p = 0.199; neutral: t(158) = 1.14, p = 0.255), and familiarity (negative: t(158) = −0.56, p = 0.579; neutral: t(158) = −0.55, p = 0.580) of negative and neutral words were counterbalanced between the “to be remembered” and “to be forgotten” conditions. The allocation of the words to each experimental condition was not changed during the experiment, while the presentation order of words within one condition was randomized across subjects.
Procedure
Before the DF task, participants were required to complete the four questionnaires (BDI-II, STAI-T, RRS and BIS/BAS).
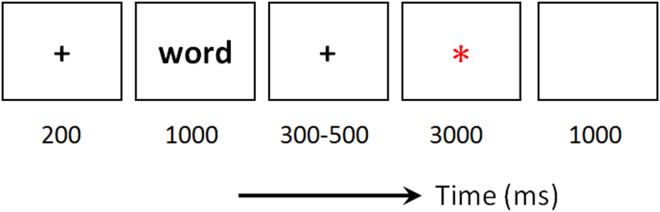
In the study phase, the 320 words were sequentially presented and each word appeared only once. The phase was comprised of four blocks (80 words in each block), separated by self-paced rest periods. As shown in Fig. 1, the word was presented for 1 sec and the instructive cue was presented for 3 sec. The cue was a green or a red asterisk, requiring participants to forget or remember the previously presented word (forget vs. remember = 50% vs. 50%). The assignment of colors to “forget” and “remember” instructions was counterbalanced across participants.
Figure 1.
Illustration of one directed forgetting trial. Participants were required to forget or remember the previously presented word in response to the red or green asterisk. The assignment of colors to “forget” and “remember” tasks was counterbalanced across participants.
In the phase of memory recognition test, the old/new discrimination task was performed. The 320 studied words were mixed with another 320 new words. The words were sequentially presented and each word appeared only once. Participants were required to discriminate as quickly and as accurately as possible whether the word was presented in the study phase, irrespective of the “forget/remember” instructions. They responded by pressing the “F” or “J” button on a computer keyboard with their left or right index finger. The assignment of keys to old and new words was counterbalanced across participants. The inter-trial interval was 2000 ms. The phase was comprised of four blocks (160 words in each block), separated by self-paced rest periods.
A task-irrelevant task (mathematical ability test, 10 min) was filled between the study phase and the recognition phase (see also other studies17,20,23,34).
EEG recording and analysis
Brain electrical activity was recorded referentially against left mastoid and off-line re-referenced to the average of the left and right mastoids, using a 64-channel amplifier with a sampling frequency of 250 Hz (Brain Products, Gilching, Germany). Electroencephalography (EEG) data were recorded during the study phase, with electrode impedances kept below 5 kΩ. Ocular artifacts were removed from EEGs using a regression procedure implemented in NeuroScan software (Scan 4.3). An on-line analogue filter (0.01–100 Hz) and a notch filter (50 Hz) continuously worked during the recording.
The recorded EEG data were off-line filtered (0.01–30 Hz) and segmented beginning 200 ms prior to the onset of stimulus and lasting for 1200 ms. Trials contaminated with large artifacts (peak-to-peak deflection exceeded ±200 μV) were excluded from further analyses. As a result, 74 ± 6.3 trials (mean ± std), 76 ± 4.9 trials, 75 ± 5.0 trials, and 74 ± 6.1 trials per subject were left in the group without depressive tendencies for the “remember-negative”, “remember-neutral”, “forget-negative”, and “forget-neutral” conditions. Meanwhile, 74 ± 7.1 trials, 73 ± 7.6 trials, 74 ± 6.2 trials, and 75 ± 5.8 trials per subject were left in the group with depressive tendencies for the four conditions (there were a total of 80 trials in each condition). All epochs were baseline-corrected with respect to the mean voltage over the 200 ms preceding the onset of stimulus, followed by averaging in association with experimental conditions.
This study focused on the ERPs elicited by words and instructive cues. Although ERP data were recorded from all the 64 electrode sites, we analyzed only the sites at which the component of interest was large and the waveform showed a representative pattern. Time windows for mean amplitude calculation were centered at the peak latencies of ERP components in grand-mean waveforms, with a shorter window length for early components and a longer length for late components.
For the word-evoked ERP, we analyzed average amplitudes of occipital P1, frontal P2 and parietal LPP components. The P1 amplitude was calculated as the average amplitude at the electrode sites of O1 and O2 between 80 to 120 ms after the onset of words. The P2 amplitude was calculated as the average amplitude at the electrode sites of F1, F2 and Fz between 220 to 270 ms post stimulus. The LPP amplitude was calculated as the average amplitude at the electrode sites of P1, P2 and Pz between 500 to 900 ms post stimulus.
For the cue-evoked ERP, we analyzed average amplitudes of occipital P1, frontal N2 and parietal P3 components. In particular, the P1 amplitude was calculated as the average amplitude at the electrode sites of O1 and O2 between 115 to 155 ms after the onset of cues. The N2 amplitude was calculated as the average amplitude at the electrode sites of F1, F2 and Fz between 240 to 290 ms post stimulus. The P3 amplitude was calculated as the average amplitude at the electrode sites of CP1, CP2 and CPz between 260 to 460 ms post stimulus.
It should be stated that the analysis of the word-evoked P1 and P2, and cue-evoked P1 was a post-hoc decision based on our data, because these components have not been reported in previous DF studies.
Statistics
Descriptive data were presented as mean ± standard error, unless otherwise mentioned. The significance level was set at 0.05.
Repeated-measures ANOVA was performed on behavioral and ERP measurements, with instruction (remember vs. forget) and the emotion category of words (negative vs. neutral) as within-subject factors, and group (individuals with depressive tendencies vs. individuals without depressive tendencies) as the between-subject factor. Significant interactions were analyzed using simple effects model. Bonferroni correction was used for multiple comparisons.
Two-tailed Pearson’s r correlation was performed between the five self-reported measures and behavioral/ERP data. Correction for multiple comparisons was based on Holm’s stepwise method. To test the independency of one correlation, partial correlation was used to test correlation between a given self-reported measure and behavioral/ERP data while controlling for the other four self-reported measures.
Results
For the sake of brevity, this section only reports the most important results. Please refer to the supplementary material for the other significant findings.
Behavioral data
Recognition rate
The main effect of instruction was significant (F(1,58) = 22.6, p < 0.001, ). The words in the remember-cue condition were recognized with a higher rate (83.0 ± 1.3%) than the words in the forget-cue condition (76.5 ± 0.9%).
The main effect of emotion was significant (F(1,58) = 5.64, p = 0.021, ). Negative words were recognized with a higher rate (80.6 ± 0.9%) compared to neutral words (78.9 ± 1.0%).
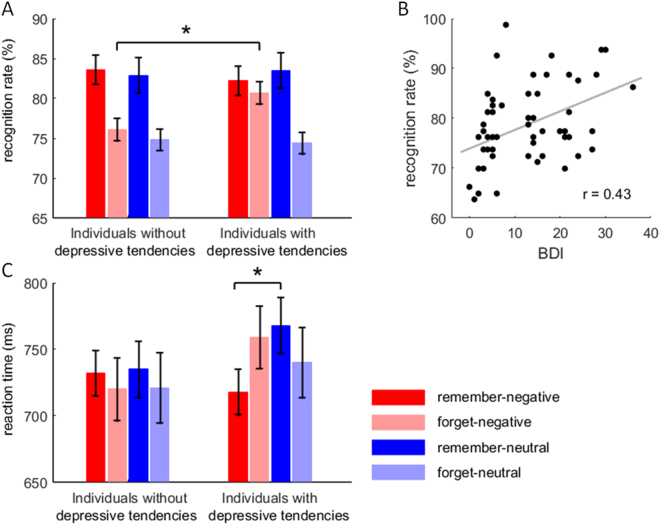
The interaction of instruction by emotion by group was significant (F(1,58) = 6.38, p = 0.014, ; Fig. 2A). Compared with subjects without depressive tendencies (76.1 ± 1.4%), subjects with depressive tendencies had a larger recognition rate (80.7 ± 1.4%) when negative words were required to be forgotten (F(1,58) = 5.48, p = 0.023). However, this group difference did not achieve significant level in any of the other conditions (F(1,58) < 1; individuals without depressive tendencies vs. with depressive tendencies: remember negative words = 83.6 ± 1.8 vs. 82.2 ± 1.8%, remember neutral words = 82.9 ± 2.2 vs. 83.5 ± 2.2%, forget neutral words = 74.8 ± 1.3 vs. 74.4 ± 1.3%).
Figure 2.
Behavioral results. (A) The recognition rate in different conditions. (B) Significant correlation between the BDI sore and the recognition rate in the forget-negative-word condition. (C) The reaction time in the recognition test. Bars represent ± standard error of the mean.
Reaction time (RT)
The interaction of instruction by emotion by group was significant (F(1,58) = 6.58, p = 0.013, = 0.102; Fig. 2C). For the words followed by the remember-cue, subjects with depressive tendencies had a shorter RT when they recognized negative words (718 ± 17.1 ms) than neutral words (768 ± 21.0 ms; F(1,58) = 13.6, p = 0.001). However, this emotion effect did not achieve significant level in any of the other conditions (F(1,58) < 1.42, p > 0.238; negative vs. neutral: remember in subjects without depressive tendencies = 732 ± 17.1 vs. 735 ± 21.0 ms, forget in subjects without depressive tendencies = 720 ± 23.5 vs. 721 ± 26.4 ms, forget in subjects with depressive tendencies = 759 ± 23.5 vs. 740 ± 26.4 ms).
ERPs
Word-occipital P1
No significant effect was found between conditions.
Word-frontal P2
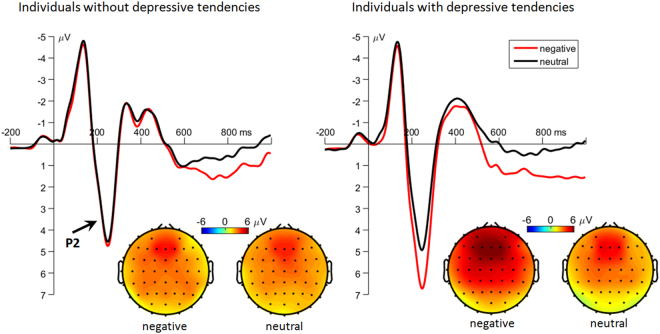
The main effect of emotion was significant (F(1,58) = 7.52, p = 0.008, = 0.115). Negative words evoked larger P2 amplitudes (5.13 ± 0.46 μV) than neutral words (4.21 ± 0.45 μV) did.
The interaction of emotion by group was significant (F(1,58) = 5.08, p = 0.028, = 0.081; Fig. 3). Compared with subjects without depressive tendencies (4.09 ± 0.65 μV), subjects with depressive tendencies had larger P2 amplitudes (6.18 ± 0.65 μV) for negative words (F(1,58) = 5.14, p = 0.027). However, this group difference did not achieve significant level for neutral words (F(1,58) < 1; subjects without depressive tendencies = 3.92 ± 0.64 μV, subjects with depressive tendencies = 4.50 ± 0.64 μV).
Figure 3.
The grand-mean ERP waveforms and topographies of the word-evoked frontal P2 component. Waveforms were calculated by averaging the data at the electrodes of Fz, F1 and F2. Topographies were calculated by averaging the data within a time window of 220 to 270 ms after the onset of words.
Word-parietal LPP
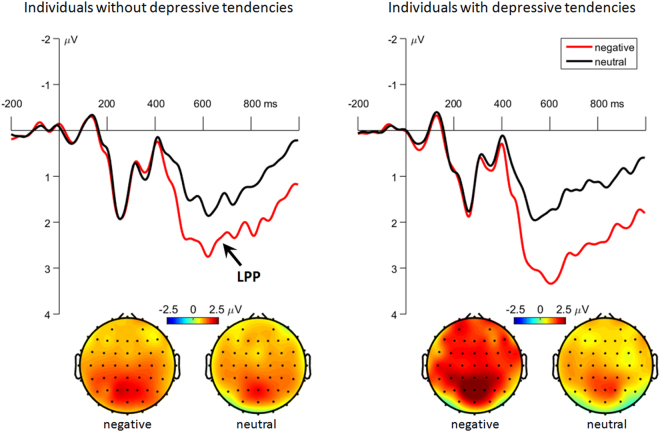
The main effect of emotion was significant (F(1,58) = 44.4, p < 0.001, = 0.434). Negative words evoked larger LPP amplitudes (2.35 ± 0.16 μV) than neutral words (1.31 ± 0.17 μV) did.
The interaction of emotion by group was significant (F(1,58) = 4.61, p = 0.036, = 0.074; Fig. 4). Compared with subjects without depressive tendencies (2.01 ± 0.23 μV), subjects with depressive tendencies had larger LPP amplitudes (2.68 ± 0.23 μV) for negative words (F(1,58) = 4.16, p = 0.046). However, this group difference did not achieve significant level for neutral words (F(1,58) < 1; subjects without depressive tendencies = 1.31 ± 0.25 μV, subjects with depressive tendencies = 1.31 ± 0.25 μV).
Figure 4.
The grand-mean ERP waveforms and topographies of the word-evoked parietal LPP component. Waveforms were calculated by averaging the data at the electrodes of Pz, P1 and P2. Topographies were calculated by averaging the data within a time window of 500 to 900 ms after the onset of words.
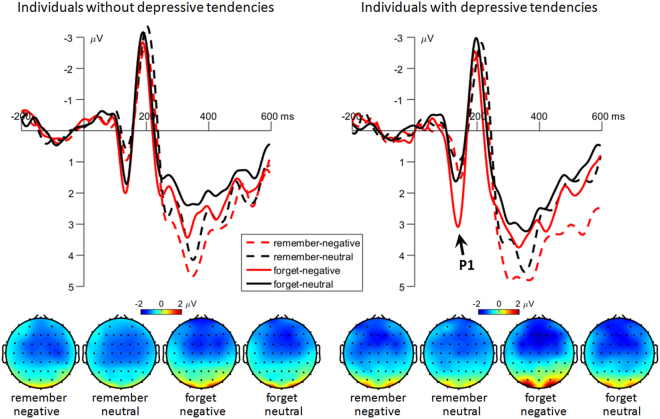
Cue-occipital P1
The main effect of instruction was significant (F(1,58) = 44.4, p < 0.001, 0.434). The forget-cue evoked larger P1 amplitudes (1.38 ± 0.14 μV) than the remember-cue (0.44 ± 0.14 μV) did.
The main effect of emotion was significant (F(1,58) = 11.5, p = 0.001, = 0.165). The P1 amplitude was larger in negative-word trials (1.16 ± 0.14 μV) than in neutral-word trials (0.66 ± 0.14 μV).
The interaction of instruction by emotion by group was significant (F(1,58) = 6.01, p = 0.017, = 0.094; Fig. 5). Compared with subjects without depressive tendencies (1.21 ± 0.24 μV), subjects with depressive tendencies had larger P1 amplitudes (2.30 ± 0.24 μV) in the condition of forgetting previously presented negative words (F(1,58) = 10.2, p = 0.002). However, this group difference did not achieve significant level in any of the other conditions (F(1,58) = 0.55 to 1.74, p = 0.192 to 0.461; individuals without vs. with depressive tendencies: remember previous negative words = 0.32 ± 0.26 vs. 0.80 ± 0.26 μV, remember previous neutral words = 0.12 ± 0.23 vs. 0.51 ± 0.23 μV, forget previous neutral words = 1.14 ± 0.23 vs. 0.89 ± 0.23 μV).
Figure 5.
The grand-mean ERP waveforms and topographies of the cue-evoked occipital P1 component. Waveforms were calculated by averaging the data at the electrodes of O1 and O2. Topographies were calculated by averaging the data within a time window of 115 to 155 ms after the onset of cues.
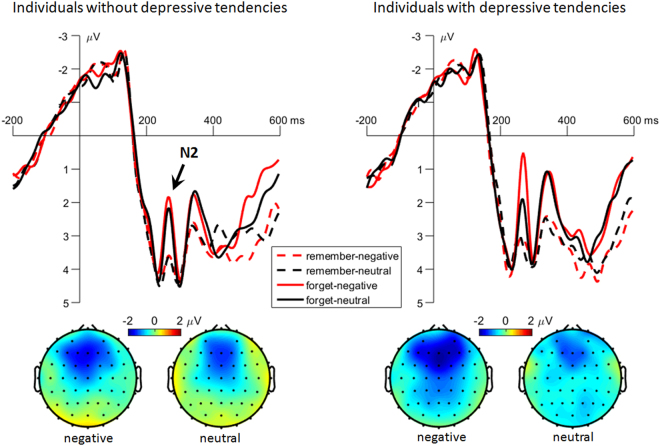
Cue-frontalN2
The main effect of instruction was significant (F(1,58) = 43.8, p < 0.001, 0.430). The forget-cue evoked larger negative-going amplitudes (2.60 ± 0.17 μV) than the remember-cue (3.78 ± 0.17 μV) did.
The main effect of emotion was significant (F(1,58) = 10.1, p = 0.002, 0.148). The N2 amplitude was more negative in negative-word trials (2.97 ± 0.17 μV) than in neutral-word trials (3.41 ± 0.16 μV).
The interaction of instruction by emotion by group was significant (F(1,58) = 4.44, p = 0.039, 0.071; Fig. 6). Compared with subjects without depressive tendencies (2.90 ± 0.27 μV), subjects with depressive tendencies had larger negative-going N2 amplitudes (1.41 ± 0.27 μV) in the condition of forgetting previous negative words (F(1,58) = 15.4, p < 0.001). However, this group difference did not achieve significant level in any of the other conditions (F(1,58) < 1; individuals without vs. with depressive tendencies: remember previous negative words = 3.92 ± 0.29 vs. 3.66 ± 0.29 μV, remember previous neutral words = 3.92 ± 0.27 vs. 3.64 ± 0.27 μV, forget previous neutral words = 3.18 ± 0.30 vs. 2.91 ± 0.30 μV).
Figure 6.
The grand-mean ERP waveforms and topographies of the cue-evoked frontal N2 component. Waveforms were calculated by averaging the data at the electrodes of Fz, F1 and F2. Topographies were calculated by averaging the data within a time window of 240 to 290 ms after the onset of cues. To display negative-going amplitudes for the N2, the topographies were based on the difference amplitudes between forget and remember conditions.
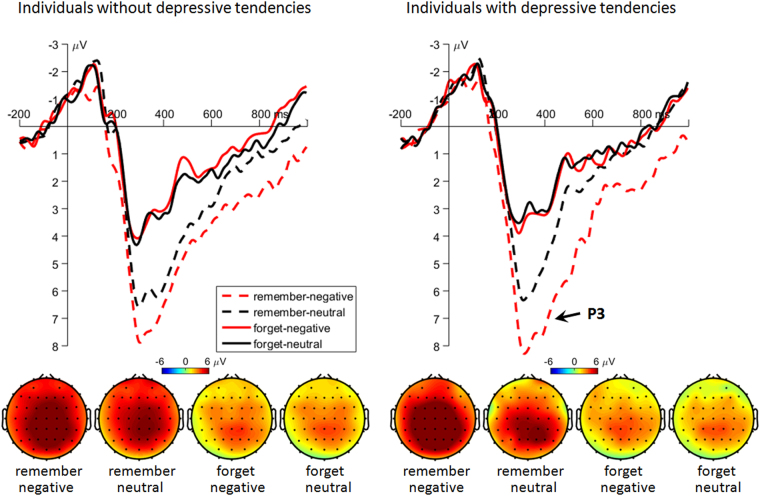
Cue-parietal P3
The main effect of instruction was significant (F(1,58) = 198, p < 0.001, 0.774; Fig. 7). The remember-cue evoked larger P3 amplitudes (5.96 ± 0.21 μV) than the forget-cue (3.19 ± 0.21 μV) did.
Figure 7.
The grand-mean ERP waveforms and topographies of the cue-evoked parietal P3 component. Waveforms were calculated by averaging the data at the electrodes of CPz, CP1 and CP2. Topographies were calculated by averaging the data within a time window of 260 to 460 ms after the onset of cues.
The main effect of emotion was significant (F(1,58) = 19.4, p < 0.001, 0.251). The P3 amplitude was larger in negative-word trials (4.99 ± 0.20 μV) than in neutral-word trials (4.16 ± 0.22 μV).
Correlation
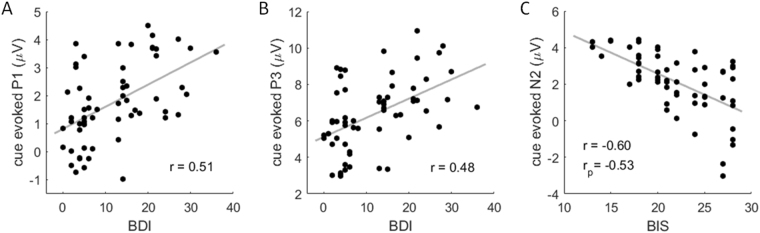
According to the results reported above, correlations were performed between the five self-reported scores (BDI, STAI-T, RRS, BIS and BAS) and the six behavioral/ERP indices (i.e., the recognition rate in the forget-negative-word condition, the RT in the remember-negative-word condition, the word-evoked P2 and LPP in the negative condition, and the cue-evoked P1 and N2 in the forget-negative-word condition). In total, we performed 30 (5 × 6) correlations.
Results showed four significant correlations after correction for multiple comparisons. The BDI score was correlated with the recognition rate (r = 0.431, p < 0.001, corrected p = 0.016; Fig. 2B), the amplitudes of cue-evoked P1 (r = 0.512, p < 0.001, corrected p < 0.001), and cue-evoked P3 (r = 0.485, p < 0.001, corrected p = 0.002) (Fig. 8A and B). The BIS score was correlated with the amplitude of cue-evoked N2 (r = −0.604, p < 0.001, corrected p < 0.001) (Fig. 8C) (Note: Since the N2 is a negative-going component, the negative correlation means that higher BIS scores were associated with larger N2 amplitudes).
Figure 8.
Significant correlations between self-reported measures and ERP data. (A) Correlation between the BDI sore and the cue-evoked P1 amplitude in the forget-negative-word condition. (B) Correlation between the BDI sore and the cue-evoked P3 amplitude in the remember-negative-word condition. (C) Correlation between the BIS sore and the cue-evoked N2 amplitude in the forget-negative-word condition. Note that the N2 is a negative-going component, so the correlation reveals that larger BIS scores were correlated with larger N2 amplitudes. (D) Correlation between the RRS sore and the cue-evoked P3 amplitude in the remember-negative-word condition.
After controlling for the other four self-reported measures, only one significant correlation was detected. The BIS score was correlated with the amplitude of cue-evoked N2 (rp = −0.527, p < 0.001, corrected p < 0.001).
In addition, it was found that the three self-reported measures were pairwise correlated (BDI and RRS: r = 0.808, p < 0.001; BDI and BIS: r = 0.539, p < 0.001; BIS and RRS: r = 0.580, p < 0.001).
Discussion
In the current study, the ability of intentional forgetting of negative and neutral materials in individuals with depressive tendencies was examiend. It was demonstrated that this population had difficulties in suppressing the memory encoding of negative items, while the suppression of memory encoding of neutral words was relatively intact. The behavioral results suggest that the deficit of memory inhibition is specifically pronounced for negative stimuli, which is consistent with previous studies8,9,31,32.
The ERP data revealed abnormal word-evoked (P2 and LPP) and cue-evoked (P1 and N2) components for negative words in participants with depressive tendencies. In contrast to the hypothesis, negative words did not elicit larger cue-evoked P3 amplitudes for TBR items in subjects with depressive tendencies, compared to the control group. However, this result was consistent with the obtained recognition rate (subjects with depressive tendencies did not remember more negative words than nondepressed subjects), and other studies9,31,32.
Based on these results, we propose that two mechanisms may contribute to the failure of forgetting negative material in individuals with depressive tendencies.
Mechanism 1: Inefficient selective attention to and suppression of negative material
The most important finding of this study was the N2 interactive effect; the “forget” instructive cue elicited larger N2 for negative words in subjects with, compared to subjects without, depressive tendencies. The frontal N2 is generally identified as an ERP marker of inhibitory control19,23,37,68. Accordingly, the “forget” cue evoked an obvious N2 while the “remember” cue did not. The data also revealed a main effect of emotion; larger N2 amplitudes were associated with negative words than with neutral words. This emotional effect is consistent with previous DF findings in healthy people, demonstrating that individuals usually recruit more inhibitory resources (indicated by larger N2 amplitudes) to suppress negative memories23 because forgetting negative stimuli is more laborious and less efficient16,20,33,69. In line with this interpretation, the N2 interaction of the current study suggests that subjects with depressive tendencies recruit even more inhibitory control resources to reduce the long-term memory of negative stimuli than nondepressed subjects, but this compensatory effort does not make up for their inefficiency in intentional forgetting. This is consistent with what was reported by Yang et al., who found that forgetting negative items, compared to neutral items, caused enhanced activation in the inferior and superior frontal gyrus in depressed patients9. Furthermore, the proposed association between the “compensatory effort” and the enhanced N2 was confirmed by the correlation between the BIS score and the N2 amplitude: a larger N2 in the forget-negative-word condition was associated with a larger motivation (or effort) to keep away from negative stimuli. This correlation was observed in both depressed and nondepressed individuals.
Another interesting finding was that the cue-evoked P1 displayed exactly the same pattern as the cue-evoked N2: the instruction of forgetting negative words evoked larger P1 amplitudes in subjects with depressive tendencies, than that in subjects without depressive tendencies. The occipital P1 is an early ERP component associated with feature-based selective attention45 and it is sensitive to task demands70 and arousal levels46. The P1 result indicates that the “forget” cue following negative words attracted more early attention in individuals with depressive tendencies, compared to individuals without depressive tendencies. It is possible that the enhanced P1 in depressed individuals reflected a heightened unconscious amplification of the instruction of “forget the previous negative word” so as to facilitate the task71,72. Alternatively, the “forget-negative-word” cue evoked a higher level of arousal in subjects with depressive tendencies. Similar with this finding, previous studies demonstrated that depressed subjects have larger P1 amplitudes compared with nondepressed subjects, especially following the presentation of negative material73–77. However, since this is the first report of this P1 finding in the DF literature, further study is needed to verify the speculation.
Altogether, individuals with depressive tendencies, on the one hand, could not forget negative words successfully, and on the other hand, allocated more cognitive resources than nondepressed ones when suppressing negative material. Therefore, the present study proposes that the selective attention system (P1) and the inhibitory control system (N2) function in an inefficient way in individuals with depressive tendencies (or mild depression).
Mechanism 2: Excessive preliminary processing of negative material
Beyond what the previous studies reported on the abnormal neural activation in the implementation of the remember/forget cue8,9, this study also provided evidence for the excessive preliminary processing of negative words in individuals with depressive tendencies: during the encoding procedure, negative words elicited larger P2 and LPP in subjects with depressive tendencies, compared to subjects without depressive tendencies.
The discovery of this LPP interactive effect is in accordance with the hypothesis of this study. The LPP has been widely considered to reflect sustained attentional allocation and continuous processing of emotional stimuli73,78–82. The enhanced LPP for negative compared to neutral items suggests that negative items were processed more extensively than neutral items, which is consistent with previous DF studies20,23,33,34. In line with this interpretation, the even larger LPP for negative words in subjects with depressive tendencies indicates a more intensive encoding of negative material.
An unexpected finding was that the frontal P2 was larger in response to negative than to neutral words in participants with depressive tendencies. According to previous literature, P2 is associated with the motivational salience of a stimulus and usually reflects motivation-related attentional allocation48–50. Many emotional studies found larger P2 amplitudes in response to negative than to neutral and positive stimuli83–87, indicating an attentional bias towards negative material. Furthermore, Bernat et al. proposed that the P2 plays a key role in determining which emotional stimuli become conscious ones, since the time window of 200 to 300 ms is on the boundary of nonconscious and conscious processing88. In this study, nondepressed subjects did not display differential P2 amplitudes between negative and neutral conditions, while subjects with depressive tendencies elicited larger P2 when viewing negative words. This result suggests that negative items may have more motivational significance to individuals with depressive tendencies, who exhibit excessive negative biases in many aspects of the cognitive processes1,2.
Taken together, the enhanced word-evoked P2 and LPP indicate an excessive preliminary processing of negative items in individuals with depressive tendencies, which may result in the subsequent failure of directed forgetting of negative material20,23. Although this excessive processing of negative words did not lead to higher recognition rate for negative TBR items in these individuals, a significantly shorter reaction time was observed in this group for negative compared to neutral TBR items.
Nondepressed individuals are able to suppress negative material
This study also found that, nondepressed participants could successfully forget both neutral and negative materials.
Previous DF studies in healthy individuals resulted in conflicting conclusions on the issue of emotional bias. Some proposed that participants failed to forget negative material20,33,69, while others argued that both neutral and negative material could be intentionally forgotten16,23,34. The inhomogeneity of experimental samples may be one of the reasons for the inconsistent results. After controlling for individual differences such as depression and trait anxiety, this study provided evidences which support the latter argument. Also, it was indicated that the BDI score was positively correlated with the recognition rate of negative TBF items. This result prompts the consideration of the level of depression as a variable when examining the emotional DF effect in a given population.
Limitations
Two limitations should be considered for an appropriate interpretation of the current result. First, two possible mechanisms that may contribute to the failure of forgetting negative material in individuals with depressive tendencies were proposed. However, since the results indicated that the individuals with depressive tendencies also excessively processed negative material (reflected by enhanced word-evoked P2 and LPP), the recruitment of additional inhibitory resources in the implementation of the forget cue (reflected by enhanced cue-evoked N2) may be a compensation mechanism for this excessive processing, rather than due to the inefficiency of memory suppression of negative material. Thus, further studies using other paradigms are required to clarify this issue. Second, this study considered undergraduate students who scored > 13 in BDI-II as individuals with depressive tendencies. Thus the participants in the depressive tendency group were mildly depressed individuals. Further study is necessary to test the generalizability of the current conclusion to clinically depressed patients. Furthermore, because anxiety and depressive symptoms are highly comorbid, this study only selected participants with a moderate level of trait anxiety.
Conclusion
The current study showed that individuals with depressive tendencies experienced difficulty in forgetting negative material. Individuals with depressive tendencies displayed enhanced word-evoked P2 and LPP for negative items and enhanced cue-evoked P1 and N2 for negative TBF items, compared to individuals without depressive tendencies. Consequently, we proposed two mechanisms during memory encoding that may contribute to the failure to forget negative material in mild depression. The two proposed mechanisms, which may influence the intentional forgetting in a negatively valenced context, are the inefficient early selective attention and memory suppression (reflected by cue-evoked P1 and N2) and the excessive preliminary processing (reflected by word-evoked P2 and LPP).
Electronic supplementary material
Acknowledgements
This study was funded by the National Natural Science Foundation of China (31571120), the National Key Basic Research Program of China (973 Program, 2014CB744600), the Training Program for Excellent Young College Faculty in Guangdong (YQ2015143), Shenzhen Basic Research Project (JCYJ20170302143246158), and the Project for Young Faculty of Humanities and Social Sciences in Shenzhen University (17QNFC43).
Author Contributions
Conceived and designed the experiments: D.Z. and D.J.; performed the experiments: H.X.; analyzed the data: D.Z.; wrote the manuscript: D.Z., H.X. and D.J.; provided lab equipment for running the study: D.Z.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hui Xie and Donghong Jiang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19570-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beck AT, Bredemeier K. A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science. 2016;4:596–619. doi: 10.1177/2167702616628523. [DOI] [Google Scholar]
- 2.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 3.Clak, D. A. & Beck, A. T. Scientific foundations of cognitive theory and therapy of depression. John Wiley & Sons: New York (1999).
- 4.Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 6.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman MG, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11:85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, et al. Abnormal brain activation during directed forgetting of negative memory in depressed patients. J Affect Disord. 2016;190:880–888. doi: 10.1016/j.jad.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Xie H, Liu Y, Luo Y. Neural correlates underlying impaired memory facilitation and suppression of negative material in depression. Sci Rep. 2016;6:37556. doi: 10.1038/srep37556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joormann J. Cognitive inhibition and emotion regulation in depression. Curr Dir Psychol Sci. 2010;19:161–166. doi: 10.1177/0963721410370293. [DOI] [Google Scholar]
- 12.Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Trends Cogn Sci. 2014;18:279–292. doi: 10.1016/j.tics.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjork, R. A. Retrieval inhibition as an adaptive mechanism in human memory. In Varieties of Memory & Consciousness (eds Roediger, H. L. & Craik, F. I. M.), pp. 309–330 Erlbaum: New Jersey (1989).
- 14.Basden BH, Basden DR, Gargano GJ. Directed forgetting in implicit and explicit memory tests: A comparison of methods. J Exp Psychol Learn Mem Cogn. 1993;19:603–616. doi: 10.1037/0278-7393.19.3.603. [DOI] [Google Scholar]
- 15.Bastin C, et al. The neural substrates of memory suppression: a FMRI exploration of directed forgetting. PloS One. 2012;7:e29905. doi: 10.1371/journal.pone.0029905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowicka A, Marchewka A, Jednoróg K, Tacikowski P, Brechmann A. Forgetting of emotional information is hard: an fMRI study of directed forgetting. Cereb Cortex. 2010;21:539–549. doi: 10.1093/cercor/bhq117. [DOI] [PubMed] [Google Scholar]
- 17.Rizio AA, Dennis NA. The neural correlates of cognitive control: successful remembering and intentional forgetting. J Cogn Neurosci. 2013;25:297–312. doi: 10.1162/jocn_a_00310. [DOI] [PubMed] [Google Scholar]
- 18.Wylie GR, Foxe JJ, Taylor TL. Forgetting as an active process: An fMRI investigation of item-method–directed forgetting. Cereb Cortex. 2008;18:670–682. doi: 10.1093/cercor/bhm101. [DOI] [PubMed] [Google Scholar]
- 19.Cheng SK, Liu IC, Lee JR, Hung DL, Tzeng OJL. Intentional forgetting might be more effortful than remembering: An ERP study of item-method directed forgetting. Biol Psychol. 2012;89:283–292. doi: 10.1016/j.biopsycho.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Hauswald A, Schulz H, Iordanov T, Kissler J. ERP dynamics underlying successful directed forgetting of neutral but not negative pictures. Soc Cogn Affect Neurosci. 2011;6:450–459. doi: 10.1093/scan/nsq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh LT, Hung DL, Tzeng OJL, Lee JR, Cheng SK. An event-related potential investigation of the processing of Remember/Forget cues and item encoding in item-method directed forgetting. Brain Res. 2009;1250:190–201. doi: 10.1016/j.brainres.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 22.van Hooff JC, Ford RM. Remember to forget: ERP evidence for inhibition in an item-method directed forgetting paradigm. Brain Res. 2011;1392:80–92. doi: 10.1016/j.brainres.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, et al. Different neural substrates underlying directed forgetting for negative and neutral images: An event-related potential study. Brain Res. 2012;1441:53–63. doi: 10.1016/j.brainres.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 24.Zacks RT, Radvansky G, Hasher L. Studies of directed forgetting in older adults. J Exp Psychol Learn Mem Cogn. 1996;22:143–156. doi: 10.1037/0278-7393.22.1.143. [DOI] [PubMed] [Google Scholar]
- 25.Bjork RA. Positive forgetting: The noninterference of items intentionally forgotten. Journal of Verbal Learning and Verbal Behavior. 1970;9:255–268. doi: 10.1016/S0022-5371(70)80059-7. [DOI] [Google Scholar]
- 26.Fawcett JM, Taylor TL. Forgetting is effortful: Evidence from reaction time probes in an item-method directed forgetting task. Mem Cognit. 2008;36:1168–1181. doi: 10.3758/MC.36.6.1168. [DOI] [PubMed] [Google Scholar]
- 27.Lee YS, Lee HM. Divided attention facilitates intentional forgetting: Evidence from item-method directed forgetting. Conscious Cogn. 2011;20:618–626. doi: 10.1016/j.concog.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS. Cognitive load hypothesis of item-method directed forgetting. Q J Exp Psychol (Hove) 2012;65:1110–1122. doi: 10.1080/17470218.2011.644303. [DOI] [PubMed] [Google Scholar]
- 29.Cottencin O, et al. Directed forgetting in depression. J Int Neuropsychol Soc. 2008;14:895–899. doi: 10.1017/S1355617708081186. [DOI] [PubMed] [Google Scholar]
- 30.Hauswald A, Kissler J. Directed forgetting of complex pictures in an item method paradigm. Memory. 2008;16:797–809. doi: 10.1080/09658210802169087. [DOI] [PubMed] [Google Scholar]
- 31.Joormann J, Tran TB. Rumination and intentional forgetting of emotional material. Cogn Emot. 2009;23:1233–1246. doi: 10.1080/02699930802416735. [DOI] [Google Scholar]
- 32.Power MJ, Dalgleish T, Claudio V, Tata P, Kentish J. The directed forgetting task: application to emotionally valent material. J Affect Disord. 2000;57:147–157. doi: 10.1016/S0165-0327(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 33.Bailey K, Chapman P. When can we choose to forget? An ERP study into item-method directed forgetting of emotional words. Brain Cogn. 2012;78:133–147. doi: 10.1016/j.bandc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Brandt KR, Nielsen MK, Holmes A. Forgetting emotional and neutral words: An ERP study. Brain Res. 2013;1501:21–31. doi: 10.1016/j.brainres.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, et al. Suppression of aversive memories associates with changes in early and late stages of neurocognitive processing. Neuropsychologia. 2012;50:2839–2848. doi: 10.1016/j.neuropsychologia.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Donkers FC, Van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-W. [DOI] [PubMed] [Google Scholar]
- 38.Mecklinger A, Parra M, Waldhauser GT. ERP correlates of intentional forgetting. Brain Res. 2009;1255:132–147. doi: 10.1016/j.brainres.2008.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Nowicka A, Jednoróg K, Wypych M, Marchewka A. Reversed old/new effect for intentionally forgotten words: an ERP study of directed forgetting. Int J Psychophysiol. 2009;71:97–102. doi: 10.1016/j.ijpsycho.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Paz-Caballero MD, Menor J, Jiménez JM. Predictive validity of event-related potentials (ERPs) in relation to the directed forgetting effects. Clin Neurophysiol. 2004;115:369–377. doi: 10.1016/j.clinph.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Herbert C, Junghofer M, Kissler J. Event related potentials to emotional adjectives during reading. Psychophysiology. 2008;45:487–498. doi: 10.1111/j.1469-8986.2007.00638.x. [DOI] [PubMed] [Google Scholar]
- 42.Wauthia E, Rossignol M. Emotional processing and attention control impairments in children with anxiety: an integrative review of event-related potentials findings. Front Psychol. 2016;7:562. doi: 10.3389/fpsyg.2016.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nat Neurosci. 2009;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]
- 46.Vogel EK, Luck S. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. doi: 10.1111/1469-8986.3720190. [DOI] [PubMed] [Google Scholar]
- 47.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 48.Potts GF, Liotti M, Tucker DM, Posner MI. Frontal and inferior temporal cortical activity in visual target detection: Evidence from high spatially sampled event-related potentials. Brain Topogr. 1996;9:3–14. doi: 10.1007/BF01191637. [DOI] [Google Scholar]
- 49.Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Riis JL, et al. Compensatory neural activity distinguishes different patterns of normal cognitive aging. NeuroImage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rütgen M, Seidel EM, Riečanský I, Lamm C. Reduction of empathy for pain by placebo analgesia suggests functional equivalence of empathy and first-hand emotion experience. J Neurosci. 2015;35:8938–8947. doi: 10.1523/JNEUROSCI.3936-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delaney PF, Goldman JA, King JS, Nelson-Gray RO. Mental toughness, reinforcement sensitivity theory, and the five-factor model: personality and directed forgetting. Pers Individ Dif. 2015;83:180–184. doi: 10.1016/j.paid.2015.04.020. [DOI] [Google Scholar]
- 53.Needham BL, et al. Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry. 2015;20:520–528. doi: 10.1038/mp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisz JR, et al. Testing standard and modular designs for psychotherapy treating depression, anxiety, and conduct problems in youth: A randomized effectiveness trial. Arch Gen Psychiatry. 2012;69:274–282. doi: 10.1001/archgenpsychiatry.2011.147. [DOI] [PubMed] [Google Scholar]
- 55.Liang CW, Hsu WY, Hung FC, Wang WT, Lin CH. Absence of a positive bias in social anxiety: The application of a directed forgetting paradigm. J Behav Ther Exp Psychiatry. 2011;42:204–210. doi: 10.1016/j.jbtep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 56.McNally RJ. Memory and anxiety disorders. Philos Trans R Soc Lond B Biol Sci. 1997;352:1755–1759. doi: 10.1098/rstb.1997.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck, A. T., Steer, R. A. & Brown, G. K. Beck Depression Inventory-Second Edition Manual. The Psychological Corporation: San Antonio, TX (1996).
- 58.Shek DT. The Chinese version of the State‐Trait Anxiety Inventory: Its relationship to different measures of psychological well‐being. J Clin Psychol. 1993;49:349–358. doi: 10.1002/1097-4679(199305)49:3<349::AID-JCLP2270490308>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 59.Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R. & Jacobs, G.A. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press: Palo Alto (1983).
- 60.Gu R, Ge Y, Jiang Y, Luo YJ. Anxiety and outcome evaluation: the good, the bad and the ambiguous. Biol Psychol. 2010;85:200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, et al. The temporal course of the influence of anxiety on fairness considerations. Psychophysiology. 2014;51:834–842. doi: 10.1111/psyp.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM) Washington: American psychiatric association; 1994. pp. 143–147. [Google Scholar]
- 63.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumaticstress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. J Pers Soc Psychol. 1991;61:115–121. doi: 10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- 64.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the durationof episodes of depressed mood. J Abnorm Psychol. 1993;102:20–28. doi: 10.1037/0021-843X.102.1.20. [DOI] [PubMed] [Google Scholar]
- 65.Nolen-Hoeksema S, Parker LE, Larson J. Ruminative coping with depressedmood following loss. J Pers Soc Psychol. 1994;67:92–104. doi: 10.1037/0022-3514.67.1.92. [DOI] [PubMed] [Google Scholar]
- 66.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- 67.Wang YN, Zhou LM, Luo YJ. The pilot establishment and evaluation of Chinese affective words system. Chinese Mental Health Journal. 2008;22:608–612. [Google Scholar]
- 68.Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from event-related potentials. J Cogn Neurosci. 2007;19:2071–2081. doi: 10.1162/jocn.2007.19.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Payne BK, Corrigan E. Emotional constraints on intentional forgetting. J Exp Soc Psychol. 2007;43:780–786. doi: 10.1016/j.jesp.2006.07.005. [DOI] [Google Scholar]
- 70.Taylor MJ. Non-spatial attentional effects on P1. Clin Neurophysiol. 2002;113:1903–1908. doi: 10.1016/S1388-2457(02)00309-7. [DOI] [PubMed] [Google Scholar]
- 71.Koivisto M, Revonsuo A. Event-related brain potential correlates of visual awareness. Neurosci Biobehav Rev. 2010;34:922–934. doi: 10.1016/j.neubiorev.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Railo H, Koivisto M, Revonsuo A. Tracking the processes behind conscious perception: a review of event-related potential correlates of visual consciousness. Conscious Cogn. 2011;20:972–983. doi: 10.1016/j.concog.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 73.Auerbach RP, Stanton CH, Proudfit GH, Pizzagalli DA. Self-referential processing in depressed adolescents: A high-density event-related potential study. J Abnorm Psychol. 2015;124:233–245. doi: 10.1037/abn0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai Q, Feng Z, Koster EH. Deficient distracter inhibition and enhanced facilitation for emotional stimuli in depression: an ERP study. Int J Psychophysiol. 2011;79:249–258. doi: 10.1016/j.ijpsycho.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 75.Dai Q, Wei J, Shu X, Feng Z. Negativity bias for sad faces in depression: An event-related potential study. Clin Neurophysiol. 2016;127:3552–3560. doi: 10.1016/j.clinph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D, He Z, Chen Y, Wei Z. Deficits of unconscious emotional processing in patients with major depression: An ERP study. J Affect Disord. 2016;199:13–20. doi: 10.1016/j.jad.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Q, et al. Early perceptual anomaly of negative facial expression in depression: An event-related potential study. Clin Neurophysiol. 2015;45:435–443. doi: 10.1016/j.neucli.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 78.Cuthbert BN, et al. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 79.Hajcak, G., Weinberg, A., MacNamara, A. & Foti, D. ERPs and the study of emotion. In Handbook of event-related potential components (eds Luck, S. & Kappenman, E.), pp. 441–474 Oxford University Press: New York (2012).
- 80.Johnston VS, Miller DR, Burleson MH. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23:684–694. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 81.Lewis KL, Taubitz LE, Duke MW, Steuer EL, Larson CL. State rumination enhances elaborative processing of negative material as evidenced by the late positive potential. Emotion. 2015;15:687–693. doi: 10.1037/emo0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. J Cogn Neurosci. 2011;23:2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- 83.Carretié L, Hinojosa JA, Mercado F, Tapia M. Cortical response to subjectively unconscious danger. Neuroimage. 2005;24:615–623. doi: 10.1016/j.neuroimage.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. Int J Psychophysiol. 2001;41:75–85. doi: 10.1016/S0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 85.Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neurosci Lett. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 86.Huang YX, Luo YJ. Attention shortage resistance of negative stimuli in an implicit emotional task. Neurosci Lett. 2007;412:134–138. doi: 10.1016/j.neulet.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 87.Olofsson JK, Polich J. Affective visual event-related potentials: arousal, repetition, and time-on-task. Biol Psychol. 2007;75:101–108. doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. Int J Psychophysiol. 2001;42:11–34. doi: 10.1016/S0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.