SUMMARY
Interactions between genetic and epigenetic effects shape brain function, behavior, and the risk for mental illness. Random X inactivation and genomic imprinting are epigenetic allelic effects that are well known to influence genetic architecture and disease risk. Less is known about the nature, prevalence, and conservation of other potential epigenetic allelic effects in vivo in the mouse and primate brain. Here we devise genomics, in situ hybridization, and mouse genetics strategies to uncover diverse allelic effects in the brain that are not caused by imprinting or genetic variation. We found allelic effects that are developmental stage and cell type specific, that are prevalent in the neonatal brain, and that cause mosaics of monoallelic brain cells that differentially express wild-type and mutant alleles for heterozygous mutations. Finally, we show that diverse non-genetic allelic effects that impact mental illness risk genes exist in the macaque and human brain. Our findings have potential implications for mammalian brain genetics.
In Brief
Huang and Ferris et al. uncover diverse forms of non-genetic allelic effects in vivo in the mouse and primate brain that can interact with heterozygous mutations to generate mosaics of brain cells that differentially express mutant versus wild-type alleles.
INTRODUCTION
Recent genomic studies of neuropsychiatric disorders created a wealth of data on the genetics of these disorders (Gratten et al., 2014; McCarroll et al., 2014). Less is known about how epigenetic mechanisms interface with genetic mutations to cause brain dysfunction. Studies of genomic imprinting and random X inactivation demonstrated that epigenetic effects impacting a single allele can profoundly influence genetic architecture, phenotypes, and disease susceptibility (Deng et al., 2014a; Peters, 2014). Genomic imprinting effects are relatively enriched in the brain, but they impact the expression of fewer than 200 autosomal genes in the mouse and human (Babak et al., 2015; Bonthuis et al., 2015; Perez et al., 2015). Thus, the mechanisms controlling gene expression for most autosomal genes are thought to regulate both alleles equally. However, since genetic risk factors for mental illness are frequently heterozygous in affected individuals—meaning only one allele is mutated—the discovery of other epigenetic allelic effects in vivo that influence the expression of wild-type (WT) versus mutant (MT) alleles could improve our understanding of brain genetics.
Autosomal, epigenetic allele-specific expression (ASE) effects other than imprinting have been described (Chess, 2016). In vivo, antigen receptors, olfactory receptors (ORs), and clustered protocadherins exhibit monoallelic expression. From in vitro studies, random monoallelic effects have also been observed for many autosomal genes in human and mouse lymphoblastoid cell lines (Gimelbrant et al., 2007; Zwemer et al., 2012), neural stem cell lines (Jeffries et al., 2012), and embryonic stem cell (ESC) lines (Eckersley-Maslin et al., 2014; Gendrel et al., 2014). Further, studies of human ESCs showed that ASE and allele-specific chromatin structures are widespread (Dixon et al., 2015). However, these studies focused on cell lines, which can exhibit epigenetic instability that impacts allelic expression (Mekhoubad et al., 2012; Nazor et al., 2012; Stadtfeld et al., 2012).
Studies of transcription at the single-cell level also uncovered autosomal ASE effects (Borel et al., 2015; Deng et al., 2014b; Marinov et al., 2014; Raj and van Oudenaarden, 2008), though it is unclear which effects are due to transcriptional noise and which are bona fide in vivo ASE effects. A recent single-cell transcriptome analysis of clonally derived mouse fibroblasts and human T cells concluded that clonal, random monoallelic effects similar to X inactivation are rare on the autosomes (Reinius et al., 2016); this challenges previous studies of random monoallelic effects in cell lines. Overall, a better understanding of the nature, diversity, prevalence, and conservation of epigenetic ASE effects in vivo is needed.
ASE effects in vivo in the mouse (Crowley et al., 2015; Pinter et al., 2015) and in different human tissues (Leung et al., 2015; Roadmap Epigenomics Consortium et al., 2015) have been largely attributed to genetic variation in cis-regulatory regions; this can cause allelic differences in chromatin states and gene expression (Heinz et al., 2013; Kasowski et al., 2013; Kilpinen et al., 2013). Currently, in vivo approaches to detect epigenetic random monoallelic effects are limited to an indirect chromatin signature derived from cell lines (Nag et al., 2013; Savova et al., 2016). Thus, beyond a few select cases, we know little about the nature and prevalence of non-genetic ASE effects in vivo.
Here, we introduce a genomics strategy and statistical framework to perform genome-wide screens for diverse forms of non-genetic allelic expression effects in vivo in the mouse and primate brain. The approach is designed to detect imprinting, random monoallelic expression and other possible allelic effects. We apply our methodology in the mouse to investigate whether non-genetic ASE effects are especially prevalent for specific developmental stages, brain regions, and tissue types and whether they impact the cellular expression of heterozygous mutations in vivo. By further screening for allelic effects in the macaque brain, we investigate the conservation of non-genetic allelic effects between mice and primates and determine whether these effects impact genes linked to mental illness in the macaque and human brain. Our study provides evidence for diverse non-genetic allelic effects in the mammalian brain that are not due to imprinting but can shape genetic architecture at the cellular level and arise in a developmental-stage and cell-specific manner.
RESULTS
An In Vivo Genome-wide Approach to Detect Diverse Non-genetic ASE Effects
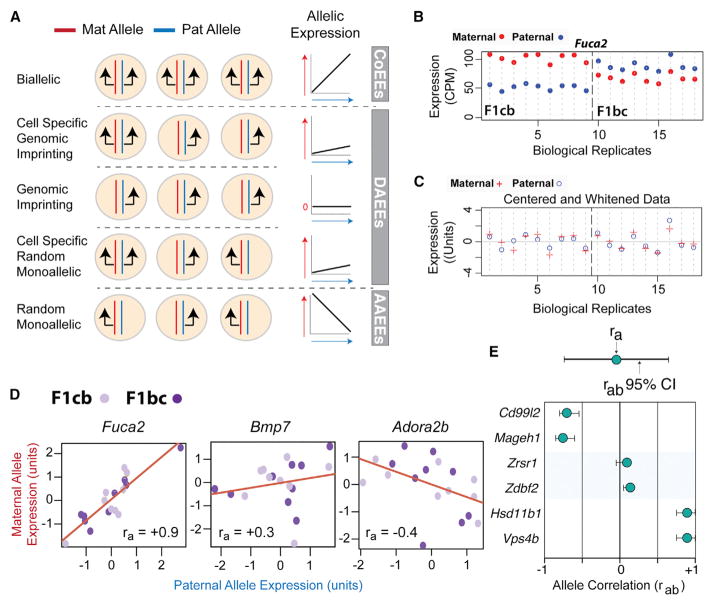
To screen for diverse ASEs in vivo, we developed a genomics approach that tests the assumption that most genes express their maternal and paternal alleles equally (allele co-expression). The approach is based, in part, on our previous study of genomic imprinting (Bonthuis et al., 2015) in which RNA-seq is performed on tissue from CastEiJ (Cast) × C57BL6/J (B6) F1 hybrid offspring derived from initial (F1cb, Cast mother × B6 father) and reciprocal (F1bc, Cast father × B6 mother) crosses. Here, instead of defining imprinting effects, we test for allele co-expression by examining the correlation of maternal and paternal allele expression levels across many RNA-seq biological replicates from different F1cb and F1bc hybrid mice. The approach potentially detects: (1) allele co-expression effects (CoEEs), whereby maternal and paternal allele expression is strongly correlated; (2) differential allele expression effects (DAEEs), whereby the maternal and paternal alleles are weakly correlated or not correlated; and (3) antagonistic allele expression effects (AAEEs), for which increased expression of one allele is associated with decreased expression of the other (Figure 1A). At the cellular level, allele CoEEs are expected to involve biallelic expression, DAEEs could arise due to canonical genomic imprinting or other diverse allelic effects, and AAEEs are expected to involve random monoallelic effects for which maternal allele-expressing cells arise at the expense of paternal allele-expressing cells and vice versa (Figure 1A). F1 hybrid mice are derived from isogenic lines, eliminating allelic effects due to genetic variation. Further, by focusing on gene expression at the tissue level, transcriptional noise and bursting in individual cells is averaged across all cells in the tissue sample, eliminating this cellular noise (Piras and Selvarajoo, 2015).
Figure 1. A Genomics and Statistical Approach to Screen for Diverse ASE Effects In Vivo.
(A) Schematic of the expected relationship between different allelic effects at the cellular level and maternal and paternal allele-correlated expression patterns at the tissue level. Black arrows indicate the expressed alleles in individual cells. Linear regression line schematics depict the relative expression patterns of the two alleles across replicates (allelic expression), with the red arrow indicating increasing maternal allele expression and the blue arrow indicating increasing paternal allele expression. The slope of the regression line depicts the co-expression relationship between the two alleles. Biallelic expression promotes allele CoEEs, genomic imprinting promotes DAEEs, cell-type-specific genomic imprinting or random monoallelic expression promotes partial DAEEs, and random monoallelic expression in all cells promotes AAEEs (see text).
(B and C) Example of maternal (red dot) and paternal (blue dot) allele expression patterns across RNA-seq biological replicates (n = 18) for Fuca2 in the DRN of adult female F1cb and F1bc mice (B). Note the correlated expression of the two alleles despite complex cross and parent-of-origin effects. After centering the F1cb and F1bc RNA-seq data, the variance in the expression levels of the maternal (red plus) and paternal (blue circle) alleles reveals correlated allelic expression patterns (ra = +0.9) (C).
(D) RNA-seq analysis of maternal and paternal allele expression correlation (ra) reveals genes with co-expressed alleles (Fuca2), genes with DAEEs (Bmp7), and genes with AAEEs (Adora2b).
(E) Schematic of the presentation of the observed ra and estimated rab 95% CI for individual genes. Below are examples of random monoallelic X-linked genes with AAEEs (cd99l2, Mageh1), canonical imprinted genes with DAEEs (Zrsr1, Zdbf2), and autosomal genes with allele CoEEs (Hsd11b1, Vps4b).
For our screen, we initially focused on the dorsal raphe nucleus (DRN), which is the largest serotonergic nucleus innervating the forebrain and which regulates a variety of brain functions implicated in mental illness (Soiza-Reilly and Commons, 2014). We collected RNA-seq data for several F1cb (n = 9) and F1bc (n = 9) biological replicates for the adult female DRN and tallied maternal and paternal allele expression levels (Bonthuis et al., 2015). Females are used so that random mono-allelic X-linked genes can be used as an internal control (see below). To analyze allele co-expression patterns across replicates, we center and whiten the allele expression data for the F1cb and F1bc crosses (see STAR Methods) and then compute the Pearson correlation for maternal and paternal allele expression levels across all 18 replicates for each gene (Figures 1B and 1C). We refer to the observed Pearson correlation between the two alleles as the “ra” value (Figure 1C). Our approach revealed autosomal genes with putative allele CoEEs (Fuca2), DAEEs (Bmp7), or AAEEs (Adora2b) (Figure 1D). However, low ra values indicating DAEEs or AAEEs could simply be caused by noise in the data. To address this problem, we devised an empirical Bayesian approach to estimate the 95% confidence interval (CI) for the ground-truth biological ra value (rab) for any expressed gene (Figure 1E; also see STAR Methods and Figure S1). With our approach, we defined the rab 95% CI for each gene expressed in the adult female DRN and observed high-confidence AAEEs for random, monoallelic X-linked genes (Figure 1E; Cd99l2, Mageh1), DAEEs for known canonical imprinted genes (Figure 1E; Zrsr1, Zdbf2), and allele CoEEs for some autosomal genes (Figure 1E; Hsd11b1, Vps4b). Below, we use this general methodology to uncover diverse, non-genetic ASE effects in vivo in the mouse and primate.
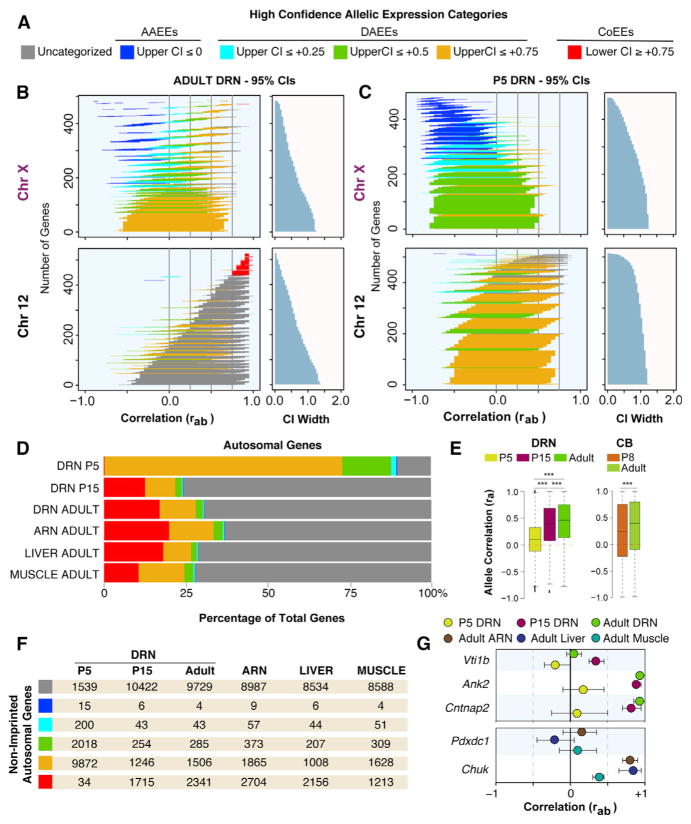
Diverse Allelic Effects Exist In Vivo in the Mouse, and ASE Effects Predominate in the Neonatal Brain
We screened for non-genetic allelic effects in the mouse to uncover the diversity of allelic effects that exist in vivo, to discover the identity of the impacted genes, and to test whether ASEs are enriched in particular stages of brain development, brain regions, or tissue types. From the adult female DRN data, we defined genes with high-confidence AAEEs as those with an upper-bounded rab 95% CI ≤ 0, indicating a negative allele correlation (Figure 2A). To define criteria for high-confidence DAEEs and CoEEs, we examined the data for random monoallelic X-linked genes. We found that 94% of the 517 X-linked genes expressed in the adult female DRN have an upper-bounded 95% CI ≤ +0.75 (Figure 2B), indicating a threshold to detect random monoallelic effects across the different expression level and variance conditions in the data (see STAR Methods). Only one X-linked gene, Ddx3x, has a lower-bounded rab 95% CI ≥ +0.75, and this gene is known to escape X inactivation (Yang et al., 2010; Figure S4D). Therefore, we defined high-confidence allele CoEEs as genes with a lower-bounded rab 95% CI ≥ +0.75, indicating a robust positive correlation, and defined DAEEs as genes with an upper-bounded rab 95% CI ≤ +0.75, like X-linked genes (Figure 2A).
Figure 2. In Vivo Screen for ASE Effects in the Mouse Brain at Different Ages and Across Different Adult Tissues Reveals Genes with AAEEs, DAEEs, and Allele CoEEs and an Enrichment for DAEEs in the Neonatal Brain.
(A) Criteria for categorizing genes according to AAEEs, DAEEs, and allele CoEEs; CI represents rab 95% confidence interval.
(B) Plots of the rab 95% CIs for all chromosome (chr)X and chr12 genes expressed in the adult female DRN reveal high-confidence AAEEs, subcategories of DAEEs, and high-confidence allele CoEEs. Gene CIs are colorized according to the appropriate category (A), and uncategorized genes are indicated in gray. The width of the CIs are presented in the right graph. Most X-linked genes have an upper-bounded 95% CI less than or equal to +0.75, indicating a threshold for ASE effects. Few autosomal genes achieve this strict threshold (see chr12 data).
(C) Plots of the rab 95% CIs for all chrX and chr12 genes expressed in the P5 female DRN reveal a substantial shift toward increased DAEEs among autosomal genes.
(D) The percentage of all expressed autosomal genes with different allelic effects in the P5, P15, and adult female DRN as well as in the adult female ARN, muscle, and liver. DAEEs are prevalent in the P5 DRN, and allele CoEEs are more prevalent in juvenile and adult tissues.
(E) Boxplots of the ra values for all autosomal genes analyzed in the P5, P15, and adult DRN and in the P8 and adult cerebellum (CB). A significant genome-wide developmental change in ASE effects occurs, involving increased DAEEs at P5 and P8 and increased allele co-expression at P15 and in adults. DRN, one-way ANOVA, and Tukey’s HSD post-test were used; CB, two-tailed t test. ***p < 0.001.
(F) Number of non-imprinted, autosomal genes in each allelic expression category (see A) for each age and tissue type.
(G) Examples of genes with DAEEs at all ages in the DRN (Vti1b) or age-specific DAEEs in the DRN (Ank2, Cntnap2); examples of genes that exhibit DAEEs across different tissue types (Pdxdc1) or in a tissue-specific manner (Chuk).
We further defined three subcategories of DAEEs. The first category includes genes with an upper-bounded 95% CI ≤+0.75 (Figures 2A and 2B). The second and third categories capture more robust DAEEs for genes with an upper-bounded CI ≤ +0.5 and CI ≤ +0.25, respectively (Figures 2A and 2B). Notably, 100% of the canonical imprinted genes in the adult mouse DRN (Bonthuis et al., 2015) meet our criteria for high-confidence DAEEs or AAEEs. Further, six known monoallelic protocadherin genes (Hirano et al., 2012) have DAEEs, including Pcdhβ12, Pcdhβ3, Pcdhβ15, Pcdhβ10, Pcdhβ6, and Pcdhβ20, but none of the expressed biallelic Pcdh genes have DAEEs (Table S1). Genes with AAEEs or DAEEs are not enriched among genes with strong Cast or B6 allele expression biases (Figures S2A–S2C). This result supports the non-genetic nature of the allelic effects and is expected because Cast versus B6 allelic differences are the same in each of the F1 hybrid offspring and are not an expected source of DAEEs or AAEEs. Genes with AAEEs or DAEEs do not have reduced expression levels or fewer SNPs relative to other autosomal genes (Figures S2D–S2F); however, genes with allele CoEEs exhibit higher expression levels (Figure S2E). Overall, our screen has excellent sensitivity and specificity for the detection of diverse and robust non-genetic allelic effects in vivo.
In the adult female DRN, we found that 70% of the 13,909 autosomal genes analyzed do not meet criteria for high-confidence allelic effects (Figure 2B, e.g., chromosome [chr]12, gray CIs). Most autosomal genes have rab 95% CIs with an upper bound near +1.0 (Figure 2B, chr12, gray and red CIs), which reflects the expected trend toward allele CoEEs. Intriguingly however, our screen uncovered four autosomal genes with high-confidence AAEEs (0.02%), 1,834 genes with DAEEs (13%), and 2,341 genes (16%) with allele CoEEs. Therefore, we found 4,179 autosomal genes that are distinguished by their unique allelic effects in the adult DRN.
To compare ASEs at different stages of brain development, we performed our screen in the DRN of post-natal day (P)5 neonates and P15 juveniles and contrasted the results to adults. Remarkably, 88% of autosomal genes in the P5 DRN exhibit high-confidence DAEEs (Figure 2C) compared to only 11% in the P15 DRN and 13% in the adult DRN (Figure 2B), revealing a profound increase in DAEEs in neonates (Figure 2D). The number of genes with high-confidence AAEEs did not change substantially between P5 (0.2%), P15 (0.1%), and adult (0.08%) offspring (Figures 2D and 2F). However, the number of genes with allele CoEEs increased by over ~50-fold in P15 juveniles and adults compared to P5 neonates (Figures 2D and 2F). For example, Vti1b exhibits DAEEs in the P5, P15, and adult DRN, while Ank2 and Cntnap2 exhibit DAEEs at P5 but allele CoEEs at P15 and in adults (Figure 2G). To independently validate this surprising developmental change, we analyzed independently generated F1cb and F1bc RNA-seq datasets for the female P8 and P60 cerebellum (Perez et al., 2015). Boxplots of the observed ra values for autosomal genes confirmed a statistically significant age effect involving a genome-wide shift from lower ra values in neonates to higher ra values in older mice in both the DRN and cerebellum (Figure 2E). Therefore, independent studies of different brain regions show a developmental shift from DAEEs in the neonatal brain to increased allele CoEEs in the adult brain.
Genomic imprinting effects are enriched in the brain (Babak et al., 2015; Bonthuis et al., 2015; Perez et al., 2015), and therefore we tested whether the brain is generally enriched for non-genetic ASE effects. We compared allelic effects in the adult female DRN and arcuate nucleus (ARN) of the hypothalamus to liver (endoderm-derived) and skeletal muscle (mesoderm-derived). The results uncovered autosomal genes with high-confidence AAEEs, DAEEs, and allele CoEEs in each brain region (DRN and ARN) and tissue type (brain, liver, and muscle) (Figures 2D and 2F). Very few autosomal genes exhibit AAEEs in any tissue (or age) (Figure 2F). We found that ~17% of autosomal genes in the ARN and muscle exhibit DAEEs compared to 11% in liver and 7% in the DRN, indicating modest brain region and tissue differences. Genes with high-confidence DAEEs in all tissues, such as Pdxdc1, were identified, and so were genes with putative tissue differences in allelic effects, such as Chuk, which has DAEEs in the liver but not in the muscle or ARN (Figure 2G). Overall, an enrichment for non-genetic ASE effects in the brain was not observed, and diverse allelic effects are present in all major tissue types in the mouse (Table S1).
We sought to define the subset of autosomal genes with ASE effects not due to imprinting. Previously, we found that less than ~2% of genes exhibit imprinting in the adult female DRN, ARN, liver, and muscle (Bonthuis et al., 2015). Here, we also profiled imprinting effects in the P5 and P15 female DRN (Table S1). With these imprinting datasets, we determined that only ~6.9% and ~1.5% of all autosomal genes with AAEEs and DAEEs, respectively, are imprinted (Figure S3A and Table S1). Therefore, most of the ASE effects detected in our screen are unrelated to imprinting. We found that these allelic effects are highly reproducible by pyrosequencing (see supplemental data and Figures S3B–S3D). Finally, we unexpectedly discovered developmental changes to the allelic expression of X-linked genes in the female brain; these results are also presented in a supplemental data section (supplemental data and Figure S4). Below, we investigate the cellular nature of autosomal allele CoEEs, DAEEs, and AAEEs in the brain.
Allele CoEEs, DAEEs, and AAEEs Involve Differences in the Prevalence of Biallelic versus Monoallelic Brain Cells In Vivo
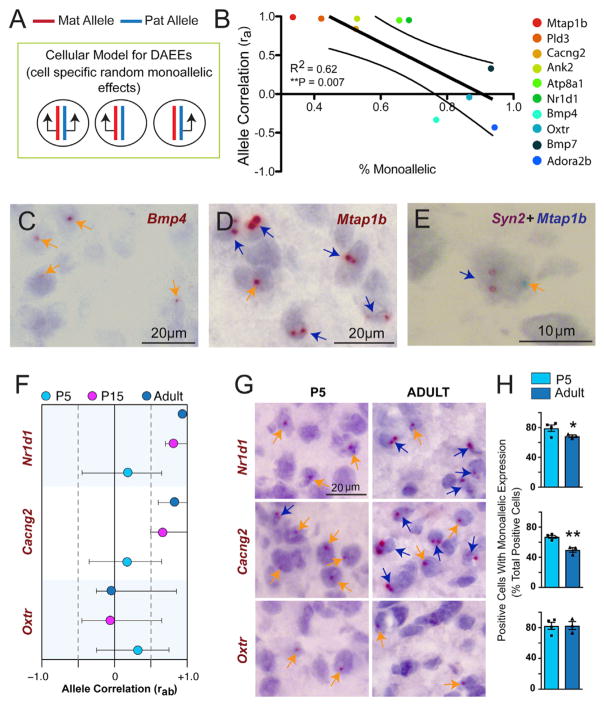
We hypothesized that DAEEs are related to random monoallelic expression in subpopulations of cells (Figure 3A). To discover the cellular nature of AAEEs, DAEEs and allele CoEEs, we resolved allelic expression at the cellular level in mouse brain tissue sections by designing RNAscope probes to rapidly processed introns in the nascent RNA of target genes (Bonthuis et al., 2015). With this approach, we quantified putative monoallelic and biallelic cells in the female DRN and ventral periaqueductal gray region in isogenic B6 adult females for ten randomly selected genes with ra values ranging from +0.99 to −0.4. Our results reveal that genes with DAEEs or AAEEs exhibit monoallelic expression in more brain cells than genes with allele CoEEs (Figure 3B). Indeed, a linear regression analysis found that a lower ra value is associated with a significant increase in the percentage of monoallelic brain cells (Figure 3B). For example, Bmp4 has a low ra value of −0.33 and exhibits monoallelic expression in 76% of positive cells and biallelic expression in 24% of positive cells (Figures 3B and 3C). In contrast, Mtap1b has an ra value of +0.99 and exhibits biallelic expression in 66% of positive cells (Figures 3B and 3D).
Figure 3. Genes with DAEEs Exhibit Increased Monoallelic Expression at the Cellular Level in the Brain Compared to Genes with Co-expressed Alleles.
(A) Schematic hypothesis indicates that non-imprinted, autosomal genes with DAEEs may exhibit random monoallelic effects in some cells and biallelic expression in others in the brain.
(B) For ten randomly selected, non-imprinted autosomal genes with different ra values, allele-specific nascent RNA in situ hybridization analysis was performed in tissue sections of the DRN and in the ventral periaqueductal gray region of the midbrain from adult female B6 mice. The percentage of monoallelic cells from the total number of positive cells (x axis) is plotted as a function of the ra value (y axis) for each gene. Linear regression reveals a significant negative correlation, indicating that genes with lower ra values are associated with more monoallelic brain cells. Approximately 30% of monoallelic cells are false due to partial nuclei from cryosectioning, and therefore the x axis begins at 30%.
(C and D) Examples of allelic, nascent RNA in situ hybridization staining in the adult female B6 DRN and ventral periaqueductal gray region. Genes with low ra values, such as Bmp4 (C), predominantly exhibit monoallelic expression at the cellular level (orange arrows), while genes with high ra values, such as Mtap1b (D), predominantly exhibit biallelic expression (blue arrows).
(E) Co-labeling of Mtap1b (blue staining) with the biallelic neuron marker gene Syn2 (brown staining) reveals subpopulations of neurons with putative monoallelic Mtap1b and biallelic Syn2 expression.
(F–H) Plots of the ra values and rab 95% confidence intervals for two genes that shift from DAEEs at P5 to allele co-expression in the adult DRN (Nr1d1 and Cacng2) and one gene that exhibits DAEEs at P5 and P15 and a trend toward DAEEs in adults (Oxtr) (F). Examples of monoallelic and biallelic cells in the P5 versus adult female B6 DRN and ventral periaqueductal gray region (G). More monoallelic cells are observed in the P5 than the adult for Nr1d1 and Cacng2, but not for Oxtr. Cell counts reveal significantly more monoallelic than biallelic cells in P5 neonates compared to adults for Nr1d1 and Cacng2, but no difference for Oxtr, consistent with rab data for these genes (H). N = 4, Student’s t test, *p < 0.05, **p < 0.01.
We previously found that ~25% of apparent monoallelic cells are potentially due to cryosectioning artifacts (Bonthuis et al., 2015). To determine whether the monoallelic cells observed for genes with high ra values are artifacts, we co-labeled with the biallelic internal control gene and neuron marker Syn2 (Bonthuis et al., 2015). Co-labeling for Mtap1b+Syn2 in the DRN revealed subpopulations of neurons in the midbrain that exhibit biallelic Syn2 expression but monoallelic expression for Mtap1b (Figure 3E), suggesting that not all of the monoallelic cells for these genes are artifacts. Indeed, some genes with high ra values, such as Atp8a1, have relatively high numbers of apparent monoallelic cells (Figure 3B). We expect that these apparent monoallelic effects are due to transcriptional bursting. Bursting effects are averaged across cells in the tissue in our RNA-seq data, revealing the allele CoEEs at the mRNA and tissue level.
Nr1d1 and Cacng2 are two autism-linked genes that exhibit DAEEs in the P5 DRN but increased allele co-expression in the P15 and adult brain (Figure 3F). For these genes, we found a significant decrease in the number of monoallelic cells and an increase in the number of biallelic cells in adults compared to P5 offspring (Figures 3G and 3H). As a control, Oxtr exhibits similar DAEEs at all ages tested (Figure 3F) and no significant change in the prevalence of monoallelic versus biallelic cells in the P5 versus adult DRN (Figures 3G and 3H). Overall, we conclude that AAEEs and DAEEs involve more monoallelic expression at the cellular level, while allele CoEEs involve more biallelic expression.
DAEEs Shape Genetic Architecture at the Cellular Level In Vivo
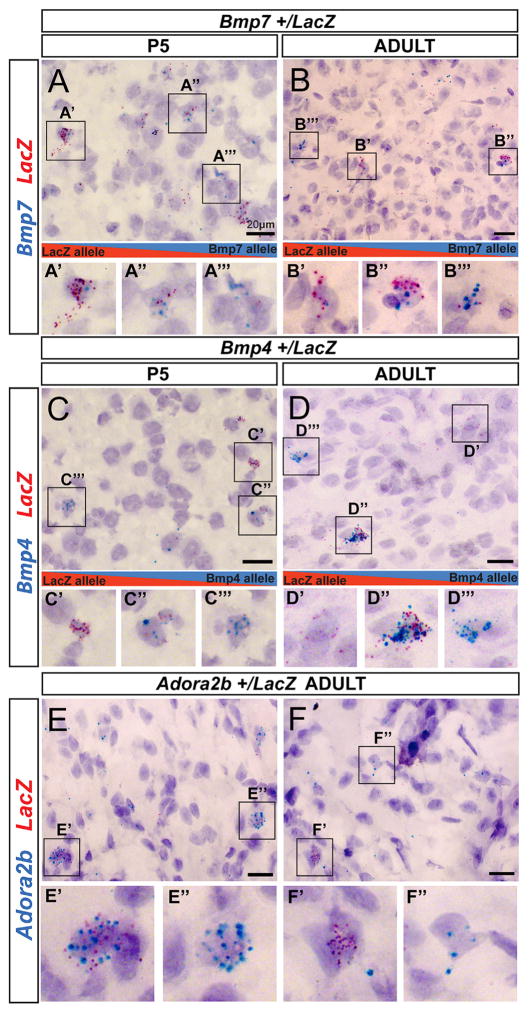
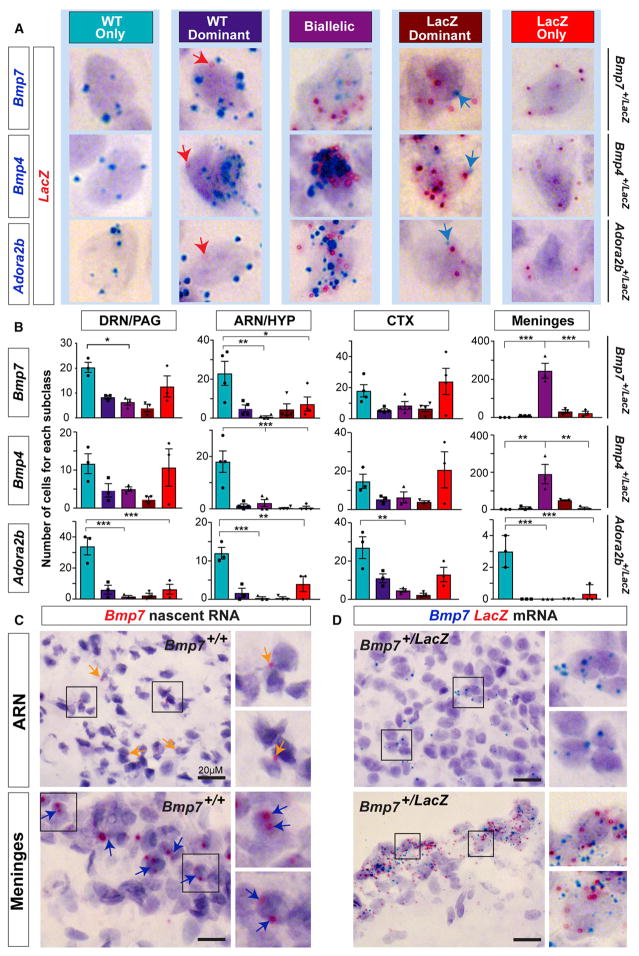
We next tested the hypothesis that AAEEs and DAEEs cause mosaics of monoallelic mutant (monoallelic-MT) and monoallelic wild-type (monoallelic-WT) allele-expressing cells for heterozygous mutations in the brain. We obtained heterozygous knockout-LacZ reporter mice on a B6 background for three genes with DAEEs in the P5 and adult brain, including Bmp7, Bmp4, and Adora2b (Figure 3B and Table S1). To detect the cellular expression of WT versus MT alleles in each heterozygous line, we designed RNAscope in situ hybridization probes to detect mRNA from the WT alleles and a probe to detect the LacZ mRNA from the MT allele. RNAscope probes are semi-quantitative, and each staining dot corresponds to a single transcript. By co-labeling with probes targeting the WT and MT alleles in the heterozygous mice, we compared the relative expression of the two alleles at the cellular level in the brain.
We first analyzed midbrain tissue sections in P5 and adult Bmp7+/LacZ mice by co-labeling with probes targeting Bmp7 and LacZ mRNA, and we uncovered subpopulations of cells that preferentially express the MT (LacZ-expressing) allele (Figures 4A′ and 4B′), both alleles (Figures 4A″ and 4B″), or the WT allele ((Figures 4A‴ and 4B‴). The probe targeting LacZ mRNA yielded very little non-specific staining in Bmp7+/+ brain tissue (Figure S5). Further, the probe targeting WT Bmp7 mRNA did not exhibit non-specific staining in Bmp7LacZ/LacZ homozygous brain tissue (Figure S5) and robustly labeled the choroid plexus in E14.5 Bmp7+/+ embryos (Figure S5), which highly express Bmp7 (Segklia et al., 2012). Thus, our approach accurately detects expression of MT and WT alleles and reveals that DAEEs can shape genetic architecture at the cellular level in the brain. A similar analysis of MT and WT allelic expression in Bmp4+/LacZ (Figures 4C and 4D) and Adora2b+/LacZ heterozygous mice (Figures 4E and 4F) also uncovered brain cells that preferentially express either the MT or WT allele as well as biallelic cells, indicating that the result generalizes to other genes with DAEEs.
Figure 4. DAEEs Cause Mosaics of Brain Cells that Differentially Express Mutant versus Wild-Type Alleles for Inherited Heterozygous Mutations.
(A and B) An analysis of the cellular expression of mutant (MT) versus wild-type (WT) alleles in Bmp7+/LacZ knockout-reporter heterozygous mice at P5 (A) and in adults (B) in the DRN and periaqueductal gray region of the midbrain. Semi-quantitative RNAscope in situ hybridization probes targeting the mRNA from the MT LacZ allele (red) versus the WT Bmp7 allele (blue) reveal sub-populations of brain cells that preferentially express the MT allele (A′ and B′) and the WT allele (A‴ and B‴) and biallelic cells that express both alleles (A″ and B″).
(C–F) The cellular expression of MT versus WT alleles in Bmp4+/LacZ knockout-reporter heterozygous mice at P5 (C) and in adults (D) and in adult Adora2b+/LacZ mice (E and F). Subpopulations of cells that preferentially express the MT allele (C′, D′, and F′) are present in the brain for each mouse line, as are cells that preferentially express the WT allele (C‴, D‴, E″, and F″) and biallelic cells (C″, D″, and E′).
In our analysis, we observed five general cell classes, including biallelic cells, cells with a MT or WT allele bias, and monoallelic cells that only express the MT or the WT allele (Figure 5A). We counted the relative numbers of these five cell classes in the adult female DRN/PAG, the arcuate nucleus (ARN) region of the hypothalamus, the cortex (CTX), and in the meninges. In the Bmp7+/LacZ, Bmp4+/LacZ, and Adora2b+/LacZ mice, we found that positive cells in the DRN/PAG, ARN/HYP, and CTX are predominantly monoallelic for either the WT or MT allele; a subpopulation of cells exhibit allele expression biases; and a smaller subpopulation exhibit biallelic expression (Figure 5B). We further found that the number of WT- versus LacZ-allele-expressing cells in the DRN/PAG and CTX is not significantly different for Bmp7 and Bmp4 (Figure 5B); however, a significant bias to monoallelically express the WT allele was observed in the ARN/HYP for both genes (Figure 5B), revealing a difference in the genetic architecture between the brain regions. For Adora2b, a significant bias to express the WT allele was observed in the DRN/PAG, ARN/HYP, and meninges, and a trend exists in the CTX (Figure 5B). Thus, the relative prevalence of monoallelic-MT and monoallelic-WT brain cells can be gene, cell, and brain region specific.
Figure 5. DAEEs Involve Brain-Region-Specific and Cell-Type-Specific Allele-Silencing Effects that Shape Genetic Architecture in the Brain.
(A) RNAscope in situ hybridization labeling for Bmp7, Bmp4, or Adora2b (blue staining) and LacZ (red staining) mRNA in the brain reveals subclasses of positive cells according to the relative allelic expression of the WT or MT LacZ allele in Bmp7+/LacZ, Bmp4+/LacZ, and Adora2b+/LacZ adult female mice.
(B) Numbers of monoallelic (WT or MT LacZ allele), dominant-allele biased (WT or MT LacZ allele), and biallelic cells in the DRN-PAG, ARN-HYP, CTX, and meninges in Bmp7+/LacZ, Bmp4+/LacZ, and Adora2b+/LacZ knockin-knockout adult mice. One-way ANOVA with Tukey’s HSD post-test was used; *p < 0.05, **p < 0.01, ***p < 0.001.
(C and D) Monoallelic (orange arrows) and biallelic (blue arrows) Bmp7+ cells identified by nascent RNA in situ in the ARN-HYP and in meningial cells in B6 adult female Bmp7+/+ mice (C). Monoallelic and biallelic cells are also detected in the ARN/HYP and meninges, respectively, by mRNA in situ hybridization for Bmp7 (blue) and LacZ (red) in adult Bmp7+/LacZ mice (D).
In contrast to the cells analyzed in other brain regions, the majority of Bmp7+ and Bmp4+ meningeal cells are biallelic (Figure 5B). We investigated these apparent cell-type-specific allelic effects with two independent approaches. First, Bmp7+ cells in the ARN region exhibit monoallelic expression by nascent RNA in situ hybridization in Bmp7+/+ mice (Figure 5C) and by mRNA in situ hybridization in Bmp7+/LacZ mice (Figure 5D). In contrast, in meningeal cells, nascent RNA in situ revealed predominantly biallelic Bmp7 expression (Figure 5C), and most meningeal cells expressed both the WT Bmp7 and MT LacZ mRNA in Bmp7+/LacZ mice (Figure 5D). Therefore, two independent methods indicate that DAEEs can be regulated in a cell-type-specific manner in the brain.
Notably, major changes to the proportion of monoallelic-MT and monoallelic-WT cells in reciprocal heterozygotes were not observed, ruling out imprinting as a plausible mechanism (Figures S6A–S6C). In addition, we tested whether ectopic expression of the LacZ transgene could be occurring by comparing the numbers of WT-allele-expressing cells in B6 control mice to the total numbers of monoallelic-MT, monoallelic-WT, and biallelic cells in the heterozygotes. No significant difference was observed (Figures S6E and S6F), indicating that the LacZ allele is not ectopically labeling an additional cell population. Overall, DAEEs can shape genetic architecture at the cellular level in the brain.
Non-genetic DAEEs Exist in the Primate Brain
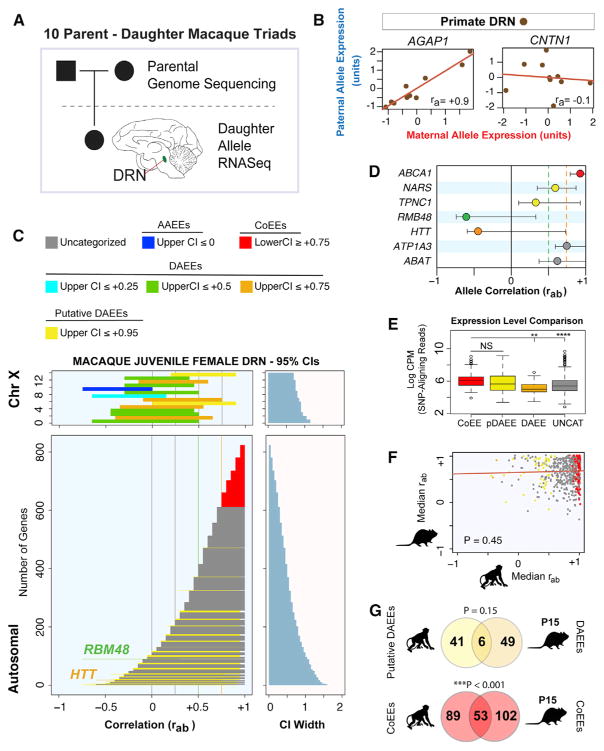
Our screen uncovered diverse non-genetic allelic effects in the mouse brain, but we do not know whether similar effects exist in the primate brain. Therefore, we analyzed allele co-expression in the DRN region of cynomolgus macaques (Macaca fascicularis), which are a well-established primate model for humans with a highly polymorphic genome (Higashino et al., 2012; Yan et al., 2011). For ten mother-father-daughter triads, we identified polymorphic sites that can discriminate maternal and paternal alleles in the daughters by performing whole-genome sequencing on DNA extracted from blood samples from the parents for each triad (Figure 6A). Next, to measure allelic expression in the daughters, we performed whole-transcriptome profiling on DRN RNA extracted from juveniles (3 to 20 months of age) (Figure 6A and STAR Methods).
Figure 6. Identification of Autosomal DAEEs and Allele CoEEs in the Primate Brain.
(A) Schematic of the strategy to profile allele co-expression in the DRN region of juvenile female cynomolgus macaques. For ten parent-offspring trios, we performed whole-genome sequencing of the parents and transcriptome sequencing of RNA extracted from the DRN of the daughters. SNPs that distinguish maternal from paternal alleles in the daughters are determined from the parental genomes and RNA-seq datasets.
(B) By analyzing ra values for the 838 genes with maternal and paternal allele RNA-seq data in all ten daughters, we defined genes with allele CoEEs (AGAP1) and potential DAEEs (CNTN1) in the juvenile female macaque DRN.
(C) Plots of the non-genetic rab 95% CIs for 17 X-linked genes and 821 autosomal genes, colorized according to the categories of high-confidence allelic effects indicated in the legend. Most X-linked genes exhibit AAEEs or DAEEs. Most autosomal genes exhibit allele CoEEs (red) or do not exhibit sufficiently robust allelic effects to be categorized with high confidence (gray); however, high-confidence DAEEs were discovered for RBM48 and HTT. In addition, more modest putative DAEEs were observed for several autosomal genes (see main text).
(D) The ra values and non-genetic rab 95% CIs for examples of primate genes with allele CoEEs (ABCA1), putative DAEEs (NARS, TPNC1), high-confidence DAEEs (RBM48, HTT), and uncategorized genes (ATP1A3, ABAT).
(E) Boxplots comparing the primate DRN expression level of autosomal and X-linked genes with allele CoEEs, putative DAEEs (pDAEEs), DAEEs, and uncategorized (UNCAT) genes. A significant main effect of gene class was observed (one way ANOVA, p < 0.0001), and a Tukey’s HSD post-test revealed no difference between genes with CoEEs versus putative DAEEs, but genes with CoEEs are expressed at a higher level than genes with DAEEs or uncategorized genes. **p < 0.01, ***p < 0.001. Expression level is based on SNP-aligning reads only. CPM represents counts per million reads.
(F) A comparison of the median rab values extracted from the rab 95% CIs for autosomal genes examined for allelic effects in the macaque compared to the values in the mouse for the orthologs. A Spearman rank partial-correlation analysis controlling for expression levels reveals no significant conservation for allelic effects at the gene level between the two species (p = 0.45).
(G) Venn diagram indicates that six genes with putative DAEEs in the primate also have DAEEs in the P15 mouse DRN, while 53 genes with allele CoEEs in the primate also have CoEEs in the mouse. The overlap for CoEEs between the species is greater than expected by chance, indicating some conservation of these effects (hypergeometric test p value shown).
We are able to analyze allele co-expression for 821 autosomal genes and 17 X-linked genes because they have allele-discriminating SNPs in all ten daughters (Table S4). For these 838 genes, we calculated the ra statistic and uncovered autosomal genes with putative allele CoEEs, such as AGAP1, or DAEEs, such as CNTN1 (Figure 6B). However, unlike inbred mice, apparent DAEEs could arise in primates due to genetic variation in regulatory elements. Therefore, we modified our statistical framework to estimate the impact of genetic variation, biological variation, and technical noise in our primate data, and we defined the rab 95% CI for the ground-truth, non-genetic allelic effects exhibited by each gene (see STAR Methods).
We found that the rab 95% CIs for X-linked genes have reduced values compared to autosomal genes; this is consistent with random monoallelic effects (Figure 6C). Most X-linked genes (88%) have an upper-bounded rab 95% CI less than or equal to +0.75, indicating a threshold for high-confidence primate DAEEs. We found that two autosomal genes (0.2% of autosomal genes tested) meet this threshold: RBM48 (RNA binding motif protein 48) and HTT (Huntingtin) (Figures 6C and 6D). RBM48 is a protein-coding gene with largely unknown functions, while HTT is mutated in Huntington’s disease (HD). HD is an autosomal-dominant disorder, and epigenetic allelic effects for HTT could potentially have important implications. We did not identify significant imprinting effects for any of the 838 genes (5% FDR threshold), indicating that the DAEEs are not due to genomic imprinting. Therefore, DAEEs are a conserved phenomenon between mice and primates. In addition, we identified 213 autosomal genes with high-confidence allele CoEEs (Figure 6C). By considering our results as a random sampling of the 13,030 annotated autosomal genes expressed in the juvenile macaque DRN, we estimate a genome-wide total of 26 and 3,387 genes with high-confidence DAEEs and CoEEs, respectively.
In our analysis of the primate data, we identified 68 genes with an upper-bounded rab 95% CI ≤ +0.95 (Figure 6C, yellow CIs). These genes do not meet the criteria for high-confidence DAEEs; however, since 92% of autosomal genes have an upper-bounded CI equal to +1 (Figure 6C), an upper bound of less than +0.95 suggests at least partial non-genetic DAEEs. In support of this interpretation, we observed that two X-linked genes also have an upper bound of less than +0.95 (Figure 6C). We call these putative DAEEs to distinguish them from the more robust DAEEs detailed above. For example, NARS and TPNC1 exhibit putative DAEEs, which are clearly distinct from both the allele CoEEs exhibited by ABCA1 and the uncategorized allelic effects for ATP1A3 and ABAT that involve an upper rab CI equal to 1.0+, like most genes (Figure 6D). Genes with putative DAEEs do not exhibit reduced expression levels compared to genes with allele CoEEs or uncategorized genes (Figure 6E), indicating that their allelic effects are a distinguishing feature. We estimate a genome-wide total of 1,042 autosomal genes (8%) with putative DAEEs in the primate DRN, and we investigate these in detail below.
To test whether allelic effects are conserved at the gene level between primates and mice, we identified non-imprinted, autosomal genes with allelic expression data and orthologs in both species. We derived the median rab value for each gene from the P15 mouse DRN and juvenile primate DRN rab 95% CI modeling data. A partial correlation analysis comparing these median rab values between the two species after controlling for gene expression levels did not uncover a statistically significant correlation (Figure 6F). Indeed, neither RBM48 nor HTT exhibit DAEEs in the mouse, and only six genes with putative DAEEs in the primate exhibit DAEEs in the mouse (Figure 6G). We did find a statistically significant overlap between the two species for the genes with allele CoEEs (Figure 6G). In summary, DAEEs exist in the primate brain, but largely impact different genes than in the mouse.
Putative DAEEs Impact Genes Linked to Mental Illness in the Macaque and Human Brain
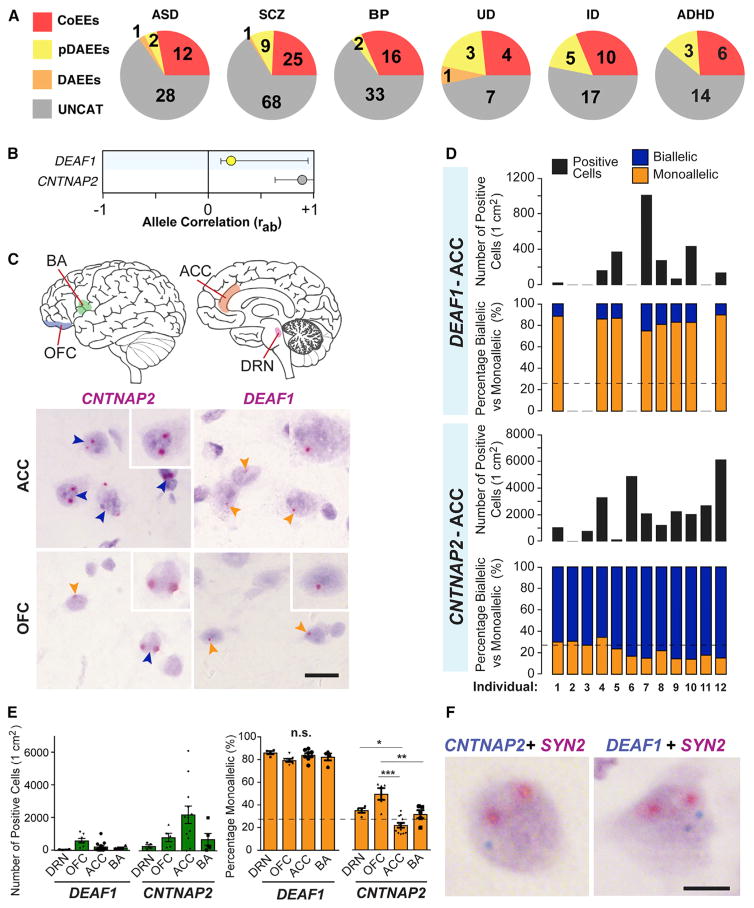
Having determined that DAEEs exist in the primate DRN, we set out to test whether genes that are risk factors for mental illness are impacted. Using the DisGeNet Database (Bauer-Mehren et al., 2010), we identified risk genes for six different major forms of mental illness, including autistic disorders (ASD), schizophrenia (SCZ), bipolar disorder (BP), unipolar depression (UD), intellectual disability (ID), and attention deficit hyperactivity disorder (ADHD). Among the 821 autosomal genes analyzed in the primate DRN, we found high-confidence DAEEs or putative DAEEs for 7% of the 33 ASD risk genes analyzed, 4% of the 51 BP risk genes, 15% of the 33 ID risk genes, 10% of the 103 SCZ risk genes, 26% of the 15 UD risk genes, and 13% of the 23 ADHD risk genes (Figure 7A). We also identified subsets of risk genes with allele CoEEs (Figure 7A). Overall, putative DAEEs impact some mental illness risk genes in the primate brain.
Figure 7. DAEEs Impact Genes Linked to Mental Illness in the Macaque and Human Brain.
(A) Pie charts of the numbers of mental illness genes found with high-confidence CoEEs, DAEEs, or putative DAEEs in the macaque DRN. Shown are results for genes linked to autism spectrum disorder (ASD), schizophrenia (SCZ), bipolar disorder (BP), intellectual disability (ID), unipolar depression (UD), and attention deficit hyperactivity disorder (ADHD).
(B) The primate DRN ra values and non-genetic rab 95% CIs for the autosomal autism genes CNTNAP2 (uncategorized) and DEAF1 (putative DAEEs).
(C) Nascent RNA in situ hybridization in four human brain regions was performed for CNTNAP2 (allele CoEEs in macaque and mouse) and DEAF1 (DAEEs in macaque) to determine the proportion of biallelic versus monoallelic cells. Examples of allele expression staining for each gene are shown for the ACC and OFC. CNTNAP2 exhibits biallelic expression (dark blue) in most cells in the human ACC, while DEAF1 exhibits monoallelic expression (orange arrows). In the OFC, CNTNAP2 is predominantly biallelic, but monoallelic cells are observed, and DEAF1 is predominantly monoallelic. OFC represents orbitofrontal CTX; BA represents Broca’s Area; ACC represents anterior cingulate cortex.
(D) Analysis of the number of CNTNAP2+ and DEAF1+ cells in 1 cm2 cryosections of the ACC for 12 adult control females and the proportion of biallelic versus monoallelic cells. The results reveal that the total number of Cntnap2+ and Deaf1+ cells per cm2 is highly variable between individuals, but the relative proportion of biallelic versus monoallelic brain cells is highly reproducible and consistent between individuals for each gene. DEAF1 exhibits monoallelic expression in ~80% of positive cells, while CNTNAP2 exhibits biallelic expression in ~70%–85% of positive cells. Dashed line indicates the percentage of cells with potential monoallelic expression due to cryosectioning artifacts.
(E) Comparison of the number of positive cells per cm2 and the percentage of positive cells that are monoallelic between four different human brain regions for CNTNAP2 and DEAF1 (N = 5–12 cases per region). CNTNAP2+ cells are most prevalent in the ACC, while DEAF1+ cells are most prevalent in the OFC. The percentage of CNTNAP2+ cells that are monoallelic in the OFC is significantly greater than in the ACC or BA (one-way ANOVA with Tukey’s HSD post-test). Significantly more monoallelic CNTNAP2+ cells were also observed in the DRN than in the ACC. Brain region differences in the percentage of monoallelic DEAF1+ cells were not observed. *p < 0.05, **p < 0.01, ***p < 0.0001.
(F) Double-allele-specific in situ hybridization labeling for CNTNAP2 or DEAF1 (blue staining) and the neuron marker control gene SYN2 (red staining) revealed subpopulations of monoallelic CNTNAP2+ neurons that exhibit biallelic SYN2 expression in the human brain. In addition, subpopulations of biallelic DEAF1+ neurons were also identified in the human brain.
We sought to test whether putative DAEEs are associated with an increased prevalence of monoallelic brain cells and simultaneously to determine whether the results from our macaque study are relevant to the human brain. We focused on two genes, DEAF1 and CNTNAP2. DEAF1 exhibits putative DAEEs in the macaque DRN (Figure 7B) and is linked to autism, language impairment, and intellectual disability in humans (Rajab et al., 2015; Vulto-van Silfhout et al., 2014). CNTNAP2 exhibits a trend toward allele CoEEs in the macaque DRN (Figure 7B), exhibits high-confidence allele CoEEs in the mouse DRN (Figure 2G), and is also linked to autism, language impairment, and intellectual disability in humans (Alarcón et al., 2008; Arking et al., 2008). Using nascent RNA in situ hybridization, we contrasted the allelic expression of these two autism genes at the cellular level in post-mortem human brain tissue for four brain regions from control adult females aged 49 to 68 years. The brain regions we studied are implicated in ASDs (Amaral et al., 2008) and include the DRN (n = 5), orbitofrontal cortex (OFC) (n = 5), anterior cingulate cortex (ACC) (n = 12), and Broca’s Area (BA) (n = 5) (Figure 7C).
We first tested whether allelic effects are consistent or variable between different individuals by counting the number of monoallelic versus biallelic CNTNAP2+ and DEAF1+ cells in 1 cm2 sections of the ACC for twelve unrelated females. The results reveal that the total number of positive cells per cm2 is highly variable between individuals, but hundreds to thousands of positive cells could be analyzed for allelic expression in each individual, with the exception of four brains that did not have DEAF1+ cells in the ACC (Figure 7D). Positive controls confirmed that the RNA was intact for each sample (not shown). We found that ~80% of DEAF1+ cells in the ACC are monoallelic, while only ~13%–30% of CNTNAP2+ cells are monoallelic, and the majority are biallelic in each individual (Figures 7C and 7D). Therefore, for at least some cases, genes with putative DAEEs are associated with more monoallelic brain cells than genes that do not exhibit these effects, and the effects are reproducible between individuals, indicating that genetic variation in regulatory elements is not causing the effects.
We extended our analysis to include different brain regions, including the DRN, OFC, and BA, and found that DEAF1 exhibits monoallelic expression in ~80% of cells in each brain region (Figure 7E). However, for CNTNAP2, we found a statistically significant main effect of brain region (p < 0.0001, one-way ANOVA). We learned that the proportion of monoallelic CNTNAP2+ cells is greater in the OFC than in the ACC and BA and is greater in the DRN than in the ACC (Figure 7E). By performing co-labeling between CNTNAP2 and the biallelic neuron marker gene SYN2, we found neuronal nuclei with biallelic SYN2 expression and monoallelic CNTNAP2 expression (Figure 7F), indicating potential monoallelic CNTNAP2+ neurons. In addition, we identified a small subpopulation of biallelic DEAF1+ neurons (Figure 7F). Overall, our results uncover non-genetic allelic effects in the primate brain that can impact genes linked to mental illness and differ between cells and brain regions.
DISCUSSION
Epigenetic ASE effects, such as random X inactivation and genomic imprinting, can profoundly influence the genetic architecture of human disease and brain disorders, though the spectrum of non-genetic ASE effects that exist in vivo has been unclear. Here, we devised a genomics and statistical framework to perform in vivo screening for diverse non-genetic allelic effects in the mouse and primate brain. By screening for allelic effects at different developmental stages and in different brain regions and tissue types in the mouse, we uncovered hundreds of autosomal genes with high-confidence DAEEs or AAEEs that are not due to genomic imprinting or genetic variation and are reproducible by pyrosequencing. Remarkably, DAEEs are revealed to be highly prevalent in the neonatal brain, while allele CoEEs are more prevalent at later developmental stages. At the cellular level, genes with DAEEs or AAEEs are associated with more monoallelic brain cells, while genes with allele CoEEs are associated with more biallelic brain cells. We determined that DAEEs interact with inherited heterozygous variants to cause mosaics of monoallelic-MT, monoallelic-WT, and biallelic brain cells in vivo. These effects can be cell type specific, since meningeal cells exhibit biallelic expression for Bmp4 and Bmp7, while other brain cell populations are predominantly monoallelic. Finally, from a genomics study of macaque parent-offspring triads and a histological study of human brain tissue sections, we provide evidence that non-genetic autosomal DAEEs that are not due to imprinting exist in the primate brain, impact genes linked to mental illness, and are associated with reproducible, monoallelic expression effects at the cellular level. Our findings have potential implications for brain genetics, as discussed below.
Potential Mechanisms Regulating AAEEs, DAEEs, and Allele CoEEs
The mechanisms underlying most of the different allelic effects we uncovered are currently unclear. Moreover, the relationship between the AAEEs and DAEEs defined in vivo in our study and the autosomal random monoallelic effects that have been described in cell lines is also unclear (Eckersley-Maslin et al., 2014; Gendrel et al., 2014; Gimelbrant et al., 2007; Jeffries et al., 2013; Zwemer et al., 2012). Some random monoallelic genes identified in mouse neuronal progenitor cell lines (Gendrel et al., 2014) have significantly reduced ra values in our mouse brain data and may exhibit ASE effects in vivo in the post-natal mouse DRN, but many do not (Table S3). More striking is that we show DAEEs to be highly prevalent in the developing neonatal brain, while allele CoEEs are more prevalent at later developmental stages, and that random monoallelic effects in embryonic stem cell lines were previously shown to increase in frequency following differentiation into neural progenitors (Eckersley-Maslin et al., 2014; Gendrel et al., 2014). Thus, our in vivo findings and previous in vitro studies both indicate that developmental gene expression programs involve complex epigenetic, allelic effects that might shape the impact of genetic variants on cell differentiation and survival during brain development.
We found that very few autosomal genes exhibit high-confidence AAEEs in vivo; however, AAEEs are relatively prevalent for X-linked genes, as expected for random X inactivation. Our data, therefore, indicate that autosomal, random monoallelic effects that are similar to X inactivation are very rare in vivo. These findings are potentially consistent with a recent study using single-cell RNA-seq; it concluded that the clonal random monoallelic effects observed in some cell lines are rare in primary fibroblasts and T cells (Reinius et al., 2016). In the future, in vivo lineage-tracing studies could more directly investigate the clonal relationships between cells with AAEEs, DAEEs, or allele CoEEs for specific genes in the brain.
The mechanisms regulating random monoallelic effects in cell lines are resistant to perturbations of DNA methylation and some histone modifications (Eckersley-Maslin et al., 2014; Gendrel et al., 2014). We found similar results for some of the DAEEs uncovered in our study (W.-C.H. and C.G., unpublished data). Nuclear architecture and chromatin looping may play important roles in regulating DAEEs versus allele CoEEs. CTCF and cohesin-mediated chromatin looping is required for genomic imprinting (Prickett et al., 2013, and references therein) and for the monoallelic expression of clustered protocadherins (Guo et al., 2012; Hirayama et al., 2012; Monahan et al., 2012). Additionally, allelic competition for enhancers contributes to the monoallelic expression of ORs (Markenscoff-Papadimitriou et al., 2014) and is also a plausible mechanism for DAEEs in our data. Overall, while genome architecture is cell type specific and probabilistic in nature (Cavalli and Misteli, 2013), little is known about allele architecture in specific brain cell populations. Advances in our ability to model sources of variance in RNA-seq data may further enhance the detection of non-genetic allelic effects using our approach, and new studies are needed to examine the temporal stability and mechanisms regulating the different allelic effects we uncovered.
The Functional Significance of Diverse Non-genetic Allelic Effects in the Brain
The functional significance of AAEEs, DAEEs, and CoEEs can be contemplated in the context of brain disorders and disease risk or as a biological mechanism of in vivo gene regulation. With respect to the former, mental illnesses are phenotypically variable and involve heterozygous variants and multiple genes; often, the same gene is implicated in different disorders (Gratten et al., 2014; McCarroll et al., 2014). Complex interactions between cellular non-genetic ASE effects and heterozygous genetic variants might be an important factor in shaping mental illness. Future studies are needed to determine whether the prevalence, identity, and location of monoallelic-MT cells in the brain can shape behavioral phenotypes in heterozygous mutant animals. Further, if environmental factors can change ASE effects, then complex interactions between environmental effects, allele genotype, and ASE effects may also have phenotypic consequences.
The potential in vivo functions of AAEEs, DAEEs, and CoEEs are unclear. Monoallelic expression is thought to come at an evolutionary cost to offspring fitness, since diploidy masks the effects of recessive mutations (Otto and Goldstein, 1992). We found that DRN autosomal genes with developmentally stable DAEEs are significantly enriched for DNA repair functions (data not shown). Genes with AAEEs or DAEEs are not expressed at lower levels than other autosomal genes and are not likely to play important roles in regulating gene dosage. In contrast, genes with allele CoEEs have elevated expression, indicating that promoting coordinated allelic expression could increase gene levels. The increased prevalence of DAEEs in the neonatal mouse DRN and cerebellum corresponds well with the peak of cell death in the developing brain (Ahern et al., 2013) and might increase genetic diversity for cell selection during periods of cell pruning. Other various functional roles for autosomal monoallelic effects have also been proposed (Chess, 2012), and in some cases, the phenomenon may simply reflect underlying genome architecture. Finally, our study uncovered significantly increased allele co-expression for X-linked genes in the juvenile and adult female brain compared to the neonatal brain or adult liver and muscle. Previous studies indicated a special role for X-linked genes in regulating brain function and cognition (Nguyen and Disteche, 2006, and references therein), and future studies that uncover the cellular basis of the developmental changes to X-linked allele co-expression in the female brain may reveal important new aspects of gene regulation on the X chromosome.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Samples | ||
| Primate brain and blood samples | Wake Forest School of Medicine | NA |
| Human brain tissue | Dallas Brain Collection and NIH Neurobiobank | NA |
| Critical Commercial Assays | ||
| RNAscope 2.5 HD Detection Reagent - RED | Advanced Cell Diagnostics | Cat#322360 |
| RNAscope 2-plex Detection Reagents | Advanced Cell Diagnostics | Cat#320701 |
| PyroMark Gold Q24 Reagents | QIAGEN | Cat#970802 |
| RNeasy Micro Kit | QIAGEN | Cat#74004 |
| Deposited Data | ||
| Raw and Analyzed data | This paper | GEO: GSE93788 |
| Raw data of RNA-seq on mouse ARN, DRN, liver and muscle | Bonthuis et al. (2015) | GEO: GSE70484 |
| Allele count table for P8 and P60 cerebellum comparison | Perez et al. (2015) | GEO: GSE67556 |
| Published random monoallelic autosomal gene lists | Gendrel et al. (2014), Eckersley-Maslin et al. (2014), Zwemer et al. (2012), Reinius et al. (2016), Savova et al. (2016) | Sources listed in Table S3 |
| Mouse reference genome (NCBI37/mm9) | Genome Reference Consortium | https://www.ncbi.nlm.nih.gov/grc/mouse |
| Experimental Models: Organisms/Strains | ||
| Mouse: Bmp7LacZ/+/Bmp4 LacZ/+ | Godin et al., 1998; Lawson et al., 1999 | N/A |
| Mouse: B6N(Cg)-Adora2b < tm1.1(KOMP)V1cg >/J | The Jackson Laboratory | #018592 |
| Mouse: C57BL/6J | The Jackson Laboratory | #000664 |
| Mouse: CAST/EiJ | The Jackson Laboratory | #000928 |
| Sequence-Based Reagents | ||
| Primers for pyrosequencing | This paper | Table S4 |
| Ecoli-LacZ-C2 | Advanced Cell Diagnostics | Cat#313451-C2 |
| Hs-CNTNAP2-intron | Advanced Cell Diagnostics | Cat#435841 |
| Hs-DEAF1-intron | Advanced Cell Diagnostics | Cat#435851 |
| Mm-Adora2b | Advanced Cell Diagnostics | Cat#445281 |
| Mm-Adora2b-Intron | Advanced Cell Diagnostics | Cat#435861 |
| Mm-Ank2-Intron4 | Advanced Cell Diagnostics | Cat#423941 |
| Mm-Atp8a1-Intron16 | Advanced Cell Diagnostics | Cat#423951 |
| Mm-Bmp4 | Advanced Cell Diagnostics | Cat#401301 |
| Mm-Bmp4-Intron3 | Advanced Cell Diagnostics | Cat#449861 |
| Mm-Bmp7 | Advanced Cell Diagnostics | Cat#407901 |
| Mm-Bmp7-Intron5 | Advanced Cell Diagnostics | Cat#444521 |
| Mm-Cacng2-Intron1 | Advanced Cell Diagnostics | Cat#478221 |
| Mm-Mtap1b-Intron2 | Advanced Cell Diagnostics | Cat#439861 |
| Mm-Nr1d1-Intron1 | Advanced Cell Diagnostics | Cat#478231 |
| Mm-Oxtr-Intron2 | Advanced Cell Diagnostics | Cat#478241 |
| Mm-Pld3-Intron9 | Advanced Cell Diagnostics | Cat#423961 |
| Mm-Syn2-Intron-C2 | Advanced Cell Diagnostics | Cat#436631-C2 |
| Software and Algorithms | ||
| R | R Core Team (2016) | https://www.r-project.org/ |
| ImageJ | Schneider et al. (2012) | https://imagej.nih.gov/ij/ |
| PyroMark Q24 Software | QIAGEN | v2.0.6.20 |
| PyroMark Assay Design SW | QIAGEN | v2.0 |
| Prism | GraphPad | http://www.graphpad.com |
| Novoalign | Novocraft | http://www.novocraft.com/ |
| EdgeR | Robinson et al. (2010) | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Christopher Gregg (chris.gregg@neuro.utah.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Lines and Breeding
All mouse experiments were performed in accordance with protocols approved by the University of Utah Institutional Animal Care and Use Committee. B6, Cast and Adora2b+/LacZ mice were obtained from the Jackson Laboratory. Bmp7+/LacZ and Bmp4+/LacZ mice have been previously described (Godin et al., 1998; Lawson et al., 1999). P0 is the day of birth. Adult mice in our study are 8–12 weeks of age. Female mice are analyzed in all cases, so that random X inactivation serves as an internal control. Mice were maintained on 12 hr light/dark cycle and given water and food ad libitum (Harlan Tecklad rodent diet 2920X; Madison, WI.). Cage bedding was Paperchip bedding (Shepherd Specialty Paper) and animals were housed with sex-matched littermates (2–5 mice per cage) and weaned at P21.
Macaque Brain Tissue
All primate experiments were performed in accordance with protocols approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee. DRN brain tissue was obtained from juvenile cynomolgus macaque females for RNA-seq analysis by micro-dissection from frozen brain tissue slabs. DRN micro-dissections included the ventral PAG region. Blood samples were obtained from the parents for each daughter for DNA extraction and genome sequencing. Detailed information for each sample is provided in Table S4.
Human Brain Tissue
Human adult female fresh frozen brain tissue samples for target regions were obtained from control cases in the Dallas Brain Collection and the NIH Neurobiobank. Reported RIN values (RNA Integrity Number) for analyzed brains was > 7. Neuropathic analysis was normal for each case. Age ranged from 53 to 83 years. Tissue obtained from the NIH Neurobiobank came from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Heathcare Center, Los Angeles, CA 90073, which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society and the Department of Veterans Affairs. Post-mortem human brain tissue obtained from the UT Neuropsychiatry Research Program (Dallas Brain Collection-DBC) at UT Southwestern Medical Center was collected after consent from the deceased/donor’s legal next of kin (NOK) was received, along with permission to access medical records and to conduct a direct telephone interview with the NOK or a primary caregiver. All clinical and medical information obtained for each donor is reviewed by a research panel, composed of at least three research and/or clinical psychiatrists and psychologists, after which a consensus diagnoses for each case is made using DSM-IV criteria. Blood and vitreous toxicology screens for drugs of abuse, alcohol, and prescription drugs, are conducted for each donor subject when possible. Collection of post-mortem human brain tissue is approved by the University of Texas Southwestern Medical Center Institutional Review Board [IRB#102010-053].
METHOD DETAILS
Mouse and Primate Analysis of DAEEs
RNA-seq in reciprocal F1 Cast ×B6 hybrid mice was performed as detailed previously (Bonthuis et al., 2015). For the macaque DRN, RNA was extracted using the QIAGEN RNeasy Kit and whole transcriptome sequencing was performed using the RiboZero TruSeq Stranded Total RNA Library Prep Kit (Illumina). Mouse and primate DRN micro-dissections included the ventral PAG region. Whole genome sequencing of the parents was performed using the TruSeq DNA PCR-free Library Prep Kit (Illumina). Primate transcriptome and genome sequencing involved performing 100bp paired-end read sequencing on a HiSeq 2500 (Illumina). Data analysis was performed using GATK and custom software as detailed below. Metrics for all genomics experiments are presented in Table S4. The number of biological replicates for each mouse tissue RNA-seq experiment are: P5 female DRN (n = 14 (7 replicates per F1cb/F1bc cross)), P15 female DRN (n = 14 (7 replicates per F1cb/F1bc cross)), adult female DRN (n = 18 (9 replicates per F1cb/F1bc cross)), arcuate nucleus (ARN) of the hypothalamus (n = 18 (9 replicates per F1cb/F1bc cross)), adult female liver (endoderm-derived, n = 16 (8 per F1cb/F1bc cross)) and adult female skeletal muscle (mesoderm-derived, n = 16 (8 per F1cb/F1bc cross)), P8 cerebellum (n = 12 (6 per F1cb/F1bc cross)), and P60 cerebellum (n = 12 (6 per F1cb/F1bc cross)).
Analysis of Non-genetic Allelic Effects by RNA-Seq in the Mouse
The DRN (dorsal raphe nucleus) was micro-dissected from P5, P15 and adult female F1i and F1r hybrid mice, as described previously (Bonthuis et al., 2015). The DRN dissections include portions of the ventral periaqueductal gray. RNA was extracted using the RNAeasy Micro Kit (QIAGEN). RNA was pooled from 4–10 offspring from at least three different litters to provide ~3 ug of total RNA for each biological replicate. Samples were prepared by the University of Utah High-Throughput Genomics core facility with Illumina TruSeq RNA Sample Prep v2 with oligo dT selection for HiSeq 59 Cycle Single-Read Sequencing v4 and sequenced on an Illumina HiSeq 2500 (Illumina). For adults, 9 F1i and 9 F1r biological replicates were performed, for P15 and P5, 7 F1i and 7 F1r biological replicates were performed. Datasets for the adult female ARN, liver and muscle have been previously described (Bonthuis et al., 2015).
To examine allele co-expression in mice, Cast and B6 allele read counts were normalized to counts per million (CPM) using EdgeR (Robinson et al., 2010). Normalization factors were calculated for the sum of both alleles and then applied to the alleles counts separately. The mouse cerebellum allelic RNA-seq dataset was obtained from Perez et al. (2015)’s published dataset (GEO: GSE67556). To remove cross effects in our allele expression data, the allele expression data was centered for each gene by subtracting the mean Cast and B6 allele expression level from the actual Cast and B6 allele expression data, respectively, for each cross. Centered values were whitened by dividing by the standard deviation of parental allele expression. After centering and whitening (Figure 1B), Pearson correlation coefficients (ra values) were computed for maternal and paternal allele co-expression for each gene.
Genes differ in terms of expression level and biological variance characteristics, and the allele expression correlation values (ra values) for genes with low expression and little variance will be more susceptible to the effects of technical noise than genes with high expression and high biological variance. To address this problem, we devised an empirical Bayesian approach to estimate the 95% confidence interval (CIs) for the ground-truth, biological ra value for each gene (rab). For each dataset, we categorized genes according to their expression level characteristics by defining 100 different expression level quantiles in our RNA-seq data (Figure S1A). Next, we categorized genes according to their variance characteristics by calculating the biological coefficient of variance (BCV) from the RNA-seq replicates for each gene based on the SNP aligning reads using the edgeR statistical package (Robinson et al., 2010). From the BCV for each gene in the data, we defined 20 quantiles of genes based on the BCV values for each dataset (Figure S1B). Therefore, for each age or tissue type RNA-seq dataset, we defined a total of 2000 different expression level × BCV conditions (100 × 20) that represent the different expression characteristics of the genes.
For each of these 2000 different conditions, we modeled the impact of biological variance (gamma distribution) and RNA-seq technical noise (Poisson distribution) on ground-truth rab values ranging from −1 to +1 (in 0.05 increments). In our approach, we simulated maternal and paternal reads for each of the RNA-seq replicates for each rab value based on the gamma distribution with mean expression and BCV characteristics that are appropriate for the expression level × BCV condition to be modeled. We then simulated the effects of RNA-seq technical noise by performing Poisson resampling of the initially simulated reads. These two steps are iterated 10,000 times, which generates a distribution of observed ra values for each one of the ground-truth rab values modeled (from −1 to +1 in 0.05 increments). We perform this entire modeling procedure for all 2000 expression × BCV conditions in the data. Our approach is based on studies that showed that technical variance in RNA-seq data is accurately modeled by the Poisson distribution (Bullard et al., 2010; Marioni et al., 2008; Oberg et al., 2012), and that combined biological + technical variance can be modeled using the negative binomial distribution (Anders and Huber, 2010; Oberg et al., 2012; Robinson et al., 2010). Similar to our approach, the negative binomial is a gamma-Poisson mixture distribution.
The goal of our modeling is to be able to define the 95% CI for the ground-truth rab statistic for any observed ra value in our RNA-seq data. In order to achieve this goal, we tallied the number of times each ra value was observed from the modeling results for each ground-truth rab. These results were assembled into frequency tables in which the x axis is the ground-truth rab value and the y axis is the observed ra values derived from the modeling. One table was assembled for each of the 2000 expression × BCV conditions. Thus, for any observed ra value in our RNA-seq data, we look up the table with the corresponding expression × BCV characteristics of the gene, identify the row for the observed ra value and extract the data for the entire row, thereby providing the frequency distribution of ground-truth rab values from which we define the rab 95% CI for the gene.
In the final step of our procedure, we weight the frequency distributions in the tables based on an empirically determined prior probability distribution. We expect that most X-linked genes will exhibit low rab values due to random X inactivation in the female brain, and most autosomal genes will exhibit relatively high rab values due to biallelic expression. Indeed, the observed ra values in our RNA-seq results are consistent with this expectation, since the distribution for autosomal genes peaks near +1.0, while the distribution for X-linked genes peaks near 0, or for some datasets peak at a negative ra value (Figure S1C). To reflect this prior knowledge, we re-weight the modeled data tables according to a Beta distribution, ranging from −1 to +1 (in 0.05 bins), with parameters derived from the distribution of observed ra values for autosomal or X-linked genes (Figure S1D). This prior has little impact on most of the data, but changes the CIs for autosomal and X-linked genes with the lowest expression and variance characteristics by shifting the CIs toward the overall trend in the data (Figure S1D). We found that we cannot reliably model genes with a mean SNP-aligning read expression level of 0.2 counts per million (CPM) or less, and removed these genes. In addition, in the mouse studies, genes that monoallelically express Cast or B6 alleles due to genetic effects were filtered from the datasets.
Analysis of Non-genetic Allelic Effects by RNA-Seq in the Macaque
For the macaque study, RNA was extracted from micro-dissected fresh frozen brain tissue sections of the macaque DRN and ventral periaqueductal gray region using the QIAGEN RNeasy Kit. Whole transcriptome sequencing was performed using the RiboZero TruSeq Stranded Total RNA Library Prep Kit (Illumina). For parental genome analysis, whole genome sequencing of the parents was performed on DNA extracted from blood samples using the TruSeq DNA PCR-free Library Prep Kit (Illumina). Metrics for all genomics experiments are presented in Table S5. Primate transcriptome and genome sequencing involved performing 100bp paired-end read sequencing on a HiSeq 2500 (Illumina). Data analysis was performed using GATK and custom software as detailed below.
The genomes of the parents in the Crab-eating macaque (Macaca fascicularis) study were sequenced and aligned to the macFas5 genome using Novoalign (Novocraft Technologies). These parental genomes were genotyped using the GATK Unified Genotyper. The resulting variant calls were used to identify SNPs that are potentially heterozygous in the offspring. This information was incorporated into individualized transcriptome indices in which IUPAC codes indicated the ambiguity of SNPs that might be heterozygous in the offspring allowing for an unbiased daughter transcriptome alignment, which was also performed with Novoalign. Daughter RNA-seq reads containing heterozygous SNPs were counted as maternal or paternal reads using a custom pysam-based python script that accounts for the parental genotypes and the daughter genotype apparent in the transcriptome alignment. Maternal and Paternal distinguishing reads for 838 genes with distinguishing SNPs in all 10 daughters were summed for each gene and CPM normalized using EdgeR (Robinson et al., 2010). The allele expression levels were centered by subtracting the mean expression level. Pearson’s correlation values were calculated across the 10 daughter macaques and confidence intervals for each gene were estimated based on gamma-Poisson resampling of the data as detailed above for the mouse data.
Estimation of the Effects of Genetic Variation on AAEEs, DAEEs, and Allele CoEEs in the Primate Brain
To estimate the impact of genetic variation in cis-regulatory regions for the primate data, we performed the following analysis. To begin, we sought to estimate the proportion of genes with genetic effects on allelic expression in each daughter. Our approach takes advantage of data in F1cb and F1bc F1 hybrid mice, for which we know the identity of the genes with genetic allelic effects. In these mice, the genes are impacted in the same manner in each individual, since the animals are derived from crosses of inbred lines and there is very little genetic variation. Previously, we identified all genes in F1cb and F1bc mice with statistically significant Cast versus B6 allele expression biases in the female mouse DRN (Bonthuis et al., 2015). We determined that 68% of all expressed genes (10,421 genes in total) in the F1cb and F1bc mouse DRN have a significant strain genetic effect (Cast or B6 allele bias). We sought to express this effect as a function of the amount of genetic variation in putative functional non-coding regulatory regions in the genome, in order to use this ratio to estimate the proportion of genes impacted by genetic effects in each primate daughter. Promoter regions are non-coding regulatory elements that are often defined as the region 2kbp upstream of a transcriptional start site (He et al., 2014) and can be defined in both mice and macaques. In contrast, enhancers are not well annotated in cynomolgus macaques; therefore, for the purposes of estimating genetic variation in noncoding regulatory elements in each species, we focused on promoter regions in the mouse and macaque genome, defined as the sequence 2 kpb upstream of a transcriptional start site. We then analyzed genetic variation within these regions in F1cb/F1bc mice and in each macaque daughter.
For the mice, Cast versus B6 genome sequence variants were obtained from the Sanger Mouse Genome Project vcf files. From these files, we identified all Cast/B6 variant sites and intersected the sites with the bedfile of mouse promoter regions. The analysis revealed a variant rate of 8.11 variants per kb in putative regulatory elements (VRreg) for the Cast versus B6 genome contrast. We can therefore express the percentage of genes with genetic allelic effects (PGE) in the mouse as a function of the VRreg,
With this data for the mouse, we can use the maternal and paternal genome sequences for each primate daughter to determine VRreg and then estimate the PGE for each primate daughter. Our approach assumes that species/individuals with more genetic variation in promoter regions throughout the genome will have more genes with genetic allelic effects as compared to species/individuals with less genetic variation. Based on this assumption, and our above analysis of Cast × B6 F1 hybrid mice, we estimated the percentage of genes that are expected to have significant genetic allelic effects in each of the macaque daughters, as follows. We used the genome sequence data we generated for each of the macaque mothers and fathers to compute the VRreg for each daughter (Table S5). From this value and the mouse data, we estimate the percentage of genes with genetic allelic expression effects (PGE) in each of the ten daughters as follows:
Next, we estimate the probability that a single gene will have a genetic effect (PGE) in each daughter (Table S5). Future studies that map various regulatory elements in the mouse and macaque genomes may be able to refine this estimation, which is currently based on promoter regions only.
Having estimated the proportion of genes with genetic allelic effects in each daughter, we next sought to estimate the magnitude of the genetic allele expression bias exhibited by each impacted primate gene. This estimation is also derived from the mouse data. As noted above, we have identified genes in F1cb and F1bc mice with significant Cast or B6 allele expression biases due to cis-regulatory genetic differences between Cast and B6 mice (Bonthuis et al., 2015) (FDR 5%, data presented in Table S6). In addition, we determined the fold Cast or B6 allele expression bias due to genetic effects for each significantly impacted gene (Table S6, see log2 fold allele bias column). We use these results as a representation of the distribution of the allelic effect sizes expected in the primate due to genetic variation. Thus, by working from mouse data for which the allelic effects are well controlled and easily interpreted, we are able to estimate both the probability of a genetic effect for a given gene in each primate daughter and the magnitude of the resulting allelic expression bias. As detailed below, we introduce these parameters into our empirical Bayesian modeling procedure to estimate the impact of genetic variation on our confidence on the underlying non-genetic rab value for each gene in our primate data.
Similar to our modeling approach to define the rab 95% CI for the mouse, our statistical modeling for the primate involves first seeding reads for a non-genetic, ground-truth allele correlation, rab, which is performed based on the gamma distribution to simulate biological variance. The reads are seeded with a mean and variance determined from the expression level and BCV computed from the primate gene expression data. Reads are seeded for ten replicates representing each of the ten primate daughters. Next, we introduce genetic effects into the simulated data. For each of the ten daughters, we randomly assign each daughter a score of 1 (genetic effect) or 0 (non-genetic effect) using a binomial distribution weighted according to the estimated probability of a gene having a genetic effect in that daughter (Table S5). If the daughter draws a genetic effect, one allele is randomly selected and multiplied by a fold allele expression bias drawn at random from the distribution of Cast versus B6 allele expression biases in the mouse, thereby introducing the estimated genetic effect into the data. This procedure is performed for each of the ten primate daughters. Once the genetic effects have been introduced, Poisson resampling is performed to model the effects of RNA-seq technical variance and the observed ra value is calculated from the data. This entire procedure is iterated 10,000 times for each gene, generating a distribution of observed ra values that reflects the effects of biological variation, genetic variation and technical noise in the data. This modeling procedure is repeated for all ground-truth, non-genetic effect rab values from −1 to +1 in 0.05 increments. The method provides a look up table of observed ra values (y axis) and corresponding ground-truth, non-genetic rab values (x axis). For the primate, since we only have 838 genes to model, rather than working from expression level and estimated BCV quantiles as was done in the mouse, we generate one lookup table for every gene based on the mean expression level and BCV for that gene. With these tables, we define the 95% CI for the ground-truth, non-genetic rab for each gene in the primate.
Examination of Genomic Imprinting in the Mouse and Primate Brain
Genomic imprinting analysis was performed using the generalized linear modeling function in edgeR to test for a main effect of the parental origin of the allele (mice: (read count ~strain-of-origin + parent-of-origin); primate: (allele read count ~parent-of-origin)), as previously described (Bonthuis et al., 2015). P values were adjusted for multiple testing using the BH method. For mice, the false discovery rate (FDR) threshold for imprinting effects was set to 1%. For the primate datasets, we did not identify any genes with significant imprinting effects at either the 1% or the 5% FDR thresholds. Metrics for all genomics datasets are in Table S4.
Examination of the Conservation of Non-genetic Allelic Effects between Mice and Primates at the Gene Level
For each gene, we estimate the ground-truth, non-genetic rab 95% CI for allelic effects and from our modeling tables for CI estimation we are able to define the median rab value for each gene. The median rab value is the best single value to represent the underlying non-genetic allelic effect for a gene. Therefore, for primate genes analyzed for allelic effects we compare the juvenile primate median rab value to the juvenile mouse median rab value for the mouse ortholog of the gene. If the effects are well conserved between the two species, we expect the median rab values to be correlated after differences in expression level have been controlled for. To do this comparison, we calculated the partial correlation coefficient for the non-parametric Spearman Rank statistic using the pcor.test function in R.
Nascent RNA and mRNA In Situ Hybridization Analysis
Nascent RNA in situ hybridization analysis was performed using RNAscope probes and HD 2.5 staining kit (Advanced Cell Diagnostics) as previously described (Bonthuis et al., 2015). RNAscope in situ hybridization for mRNA was performed using the RNAscope 2-plex kit according to the manufacturers instructions. The manufacturers positive control probe was used to evaluate RNA integrity in human brain tissue samples and samples with poor staining were not analyzed.
Mouse Tissue Processing
Wild-type or mutant adult female mice were perfused by 4% paraformaldehyde (PFA) and then fixed in 4% PFA overnight at 4°C. The brain was immersed in 10% sucrose at 4°C for 24 hr. This step was repeated with 20% and 30% sucrose. The brain was embedded in Optimal Cutting Temperature compound (OCT) and stored at −80°C. ARN and DRN 14 μm sections were collected using a cryostat. RNA signal was detected following the RNAscope Chromogenic Assay Sample Preparation for Fixed Frozen Tissue (Part 1) technical note and RNAscope 2-Plex or HD Kit (Part 2). E14.5 brains were dissected from the pregnant mouse and then fixed in 4% PFA overnight at 4°C. The steps of sucrose dehydration, cryosection, and staining are described above. P5 brain tissues were embedded directly after dissection and 14 μm sections were collected by cryosection. The staining was performed following the RNAscope Chromogenic Assay Sample Preparation for Fresh Frozen Tissue (Part 1) and following the instructions for the RNAscope 2-Plex Kit (Part 2). Tissue sections were imaged with a Zeiss ApoTome2 microscope using a 40x oil objective. Three images were taken per section from at least three sections per animal for nascent RNA detection. Four images were taken per section from two sections per animal for wild-type/LacZ allele detection in heterozygous mice. Cells were counted using Cell Counter function in ImageJ software. To categorize cell types expressing the WT (wild-type) and/or LacZ alleles in the brain tissue of heterozygous knockin-knockout mice, cells were classified according to five categories: (1) WT only: Cells only expressing the WT allele; (2) WT dominant cells: Cells dominantly expressing the WT allele with a least one LacZ mRNA staining dot; (3) Biallelic: Cells expressing relatively equal levels of the WT and LacZ alleles; (4) LacZ dominant cells: Cells dominantly expressing the LacZ allele with a least one WT mRNA dot; (5) LacZ only: Cells only expressing the LacZ allele. The number of cells for each class was summed from all of the images taken for a single animal. This summed number of cells per animal is presented in bar graph figures in Figures 3, 5, and S6.
Human Tissue Processing
Fresh frozen human brain tissue blocks from the DRN/vPAG (dorsal raphe nucleus/ventral periaqueductal gray) region, BA (Broca’s Area), OFC (orbitofrontal cortex) and ACC (anterior cingulate cortex) were embedded in OCT compound and cryosectioned at 14 microns thickness across a series of 12 slides, with two sections per slide. A 1 cm2 region of tissue was traced out for each sample using a pap pen and stained using RNAscope in situ hybridization probes, as described above. The number of monoallelic versus biallelic cells for each gene analyzed was quantified per 1 cm2 section for each individual and brain region.
Pyrosequencing
Allele-specific pyrosequencing was performed as previously detailed (Bonthuis et al., 2015). Briefly, we identified SNP sites that discriminate Cast and B6 alleles and amplified ~100–200 base pairs surrounding the SNP site. The sequencing primer was designed around the SNP site and the ratio of Cast versus B6 base calls was determined in each sample according to the Pyromark software. Amplification primers and sequencing primers are provided in Table S4. Pyrosequencing was performed and analyzed using the PyroMark Q24 system and software (QIAGEN) in the University of Utah DNA Sequencing Core Laboratory. We performed 5–8 biological replicates for each F1cb/F1bc cross for each gene and calculated the Cast:B6 allele expression ratio for each replicate. Using a ratiometric statistic for correlation (Abelin et al., 2014), we measured the dispersion of Cast:B6 allele expression ratios across the F1cb/F1bc replicates samples by computing the coefficient of variation (CV). The ratiometric statistic was computed as follows:
where
AEP is the expression level of the paternal allele,
AEM is the expression level of the maternal allele,
And abs is the absolute value.
The linear regression analysis comparing the RNA-seq ra values to the PyroSEQ ΔCV values for the validated genes was performed using Prism software.
QUANTIFICATION AND STATISTICAL ANALYSIS
All n’s in the study represent biological replicates involving different animals. Error bars represent standard error of the mean (SEM). Statistical analyses for the genomics are provided in detail above and appropriate sample sizes were estimated by statistical modeling using the procedures detailed above. RNA-seq experiments for F1cb and F1bc mouse samples were carried out with a randomized, blocked design on the flowcell to prevent batch effects causing systematic differences between F1cb and F1bc samples. Statistical analysis for cell counts involved a t test or a one-way ANOVA with a Tukey’s HSD post-test, as indicated in the figure legends. Quantification was carried out in ImageJ (Schneider et al., 2012), as detailed in the Mouse and Human Tissue Processing subsections under METHOD DETAILS. To evaluate RNA quality in mouse and human tissue samples, an internal positive control probe that is provided with the ACDBio RNAScope kit was run for every staining reaction and samples that failed to show consistent labeling across the tissue for the internal control probe were not included in the analysis. Blind quantifications were performed for cell counts comparing monoallelic and biallelic cells in brain tissue sections from animals at different ages (Figure 3). A power analysis for the cell quantification experiments was not performed.
DATA AND SOFTWARE AVAILABILITY
The BAM files for the adult mouse DRN, ARN, liver and muscle were published previously (Bonthuis et al., 2015). The data for the cerebellum was published previously (Perez et al., 2015). The P5 and P15 mouse DRN RNA-seq BAM files and macaque genome and RNA-seq BAM files have been deposited to the GEO database. The accession number for the data reported in this paper is GEO: GSE93788.
Supplementary Material
Highlights.
In vivo genome-wide screen uncovers diverse non-genetic allelic effects
Non-genetic allelic effects are prevalent in the neonatal mouse brain
Allelic effects cause mosaics of mutant and wild-type cells for heterozygous mutations
Allelic effects exist in the primate brain and impact genes linked to mental illness
Acknowledgments
We thank Drs. Suzi Mansour, Jared Rutter, and Brad Cairns and members of the Gregg lab for reviewing earlier versions of the manuscript, and we thank Drs. Li Zhang and Matthew Jorgensen for primate tissue collection. This work has been supported by NIH 1R01MH109577-01 (C.G.), a Simons Foundation Autism Research Initiative Explorer Award (C.G.), a University of Utah Seed Grant (C.G.), and NIH P40RR021380 (J.D.W.). C.G. is a New York Stem Cell Foundation Robertson Investigator. This research was supported by the New York Stem Cell Foundation.
Footnotes
Supplemental Information includes six figures, six tables, and supplemental text and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2017.01.033.
A video abstract is available at http://dx.doi.org/10.1016/j.neuron.2017.01.033#mmc9.
AUTHOR CONTRIBUTIONS
Conceptualization, W.-C.H., E.F., and C.G.; Methodology, W.-C.H., E.F., T.C., K.M.B., and C.G.; Investigation, W.-C.H., E.F., T.C., C.S.H., J.D.W., K.G., J.L.C., K.M.B., and C.G.; Software, E.F., W.-C.H., K.M.B., and C.G.; Formal Analysis, W.-C.H., E.F., T.C., and C.G.; Transgenic Mice, W.-C.H. and J.L.C.; Primate Tissue and Analysis, E.F., W.-C.H., J.D.W., C.S.H., and C.G.; Human Tissue Analysis, T.C., K.G., C.T., and C.G.; Data Visualization, W.-C.H., E.F., C.S.H., and C.G.; Writing, C.G., W.-C.H., and E.F.; Supervision, J.D.W., C.T., and C.G.; Funding, J.D.W. and C.G.
References
- Abelin ACT, Marinov GK, Williams BA, McCue K, Wold BJ. A ratiometric-based measure of gene co-expression. BMC Bioinformatics. 2014;15:331. doi: 10.1186/1471-2105-15-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Krug S, Carr AV, Murray EK, Fitzpatrick E, Bengston L, McCutcheon J, De Vries GJ, Forger NG. Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: effects of age and sex. J Comp Neurol. 2013;521:2551–2569. doi: 10.1002/cne.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babak T, DeVeale B, Tsang EK, Zhou Y, Li X, Smith KS, Kukurba KR, Zhang R, Li JB, van der Kooy D, et al. Genetic conflict reflected in tissue-specific maps of genomic imprinting in human and mouse. Nat Genet. 2015;47:544–549. doi: 10.1038/ng.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer-Mehren A, Rautschka M, Sanz F, Furlong LI. DisGeNET: a Cytoscape plugin to visualize, integrate, search and analyze gene-disease networks. Bioinformatics. 2010;26:2924–2926. doi: 10.1093/bioinformatics/btq538. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Huang WC, Stacher Hörndli CN, Ferris E, Cheng T, Gregg C. Noncanonical genomic imprinting effects in offspring. Cell Rep. 2015;12:979–991. doi: 10.1016/j.celrep.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Borel C, Ferreira PG, Santoni F, Delaneau O, Fort A, Popadin KY, Garieri M, Falconnet E, Ribaux P, Guipponi M, et al. Biased allelic expression in human primary fibroblast single cells. Am J Hum Genet. 2015;96:70–80. doi: 10.1016/j.ajhg.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard JH, Purdom E, Hansen KD, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics. 2010;11:94. doi: 10.1186/1471-2105-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G, Misteli T. Functional implications of genome topology. Nat Struct Mol Biol. 2013;20:290–299. doi: 10.1038/nsmb.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A. Mechanisms and consequences of widespread random monoallelic expression. Nat Rev Genet. 2012;13:421–428. doi: 10.1038/nrg3239. [DOI] [PubMed] [Google Scholar]
- Chess A. Monoallelic gene expression in mammals. Annu Rev Genet. 2016;50:317–327. doi: 10.1146/annurev-genet-120215-035120. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Zhabotynsky V, Sun W, Huang S, Pakatci IK, Kim Y, Wang JR, Morgan AP, Calaway JD, Aylor DL, et al. Analyses of allele-specific gene expression in highly divergent mouse crosses identifies pervasive allelic imbalance. Nat Genet. 2015;47:353–360. doi: 10.1038/ng.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Berletch JB, Nguyen DK, Disteche CM. X chromosome regulation: diverse patterns in development, tissues and disease. Nat Rev Genet. 2014a;15:367–378. doi: 10.1038/nrg3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Ramsköld D, Reinius B, Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014b;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Jung I, Selvaraj S, Shen Y, Antosiewicz-Bourget JE, Lee AY, Ye Z, Kim A, Rajagopal N, Xie W, et al. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersley-Maslin MA, Thybert D, Bergmann JH, Marioni JC, Flicek P, Spector DL. Random monoallelic gene expression increases upon embryonic stem cell differentiation. Dev Cell. 2014;28:351–365. doi: 10.1016/j.devcel.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel AV, Attia M, Chen CJ, Diabangouaya P, Servant N, Barillot E, Heard E. Developmental dynamics and disease potential of random monoallelic gene expression. Dev Cell. 2014;28:366–380. doi: 10.1016/j.devcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Gimelbrant A, Hutchinson JN, Thompson BR, Chess A. Widespread monoallelic expression on human autosomes. Science. 2007;318:1136–1140. doi: 10.1126/science.1148910. [DOI] [PubMed] [Google Scholar]
- Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–3482. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Keller MC, Visscher PM. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci. 2014;17:782–790. doi: 10.1038/nn.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Monahan K, Wu H, Gertz J, Varley KE, Li W, Myers RM, Maniatis T, Wu Q. CTCF/cohesin-mediated DNA looping is required for protocadherin α promoter choice. Proc Natl Acad Sci USA. 2012;109:21081–21086. doi: 10.1073/pnas.1219280110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chen C, Teng L, Tan K. Global view of enhancer-promoter interactome in human cells. Proc Natl Acad Sci USA. 2014;111:E2191–E2199. doi: 10.1073/pnas.1320308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Romanoski CE, Benner C, Allison KA, Kaikkonen MU, Orozco LD, Glass CK. Effect of natural genetic variation on enhancer selection and function. Nature. 2013;503:487–492. doi: 10.1038/nature12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino A, Sakate R, Kameoka Y, Takahashi I, Hirata M, Tanuma R, Masui T, Yasutomi Y, Osada N. Whole-genome sequencing and analysis of the Malaysian cynomolgus macaque (Macaca fascicularis) genome. Genome Biol. 2012;13:R58. doi: 10.1186/gb-2012-13-7-r58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K, Kaneko R, Izawa T, Kawaguchi M, Kitsukawa T, Yagi T. Single-neuron diversity generated by Protocadherin-β cluster in mouse central and peripheral nervous systems. Front Mol Neurosci. 2012;5:90. doi: 10.3389/fnmol.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Tarusawa E, Yoshimura Y, Galjart N, Yagi T. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons. Cell Rep. 2012;2:345–357. doi: 10.1016/j.celrep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Jeffries AR, Perfect LW, Ledderose J, Schalkwyk LC, Bray NJ, Mill J, Price J. Stochastic choice of allelic expression in human neural stem cells. Stem Cells. 2012;30:1938–1947. doi: 10.1002/stem.1155. [DOI] [PubMed] [Google Scholar]
- Jeffries AR, Collier DA, Vassos E, Curran S, Ogilvie CM, Price J. Random or stochastic monoallelic expressed genes are enriched for neurodevelopmental disorder candidate genes. PLoS ONE. 2013;8:e85093. doi: 10.1371/journal.pone.0085093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasowski M, Kyriazopoulou-Panagiotopoulou S, Grubert F, Zaugg JB, Kundaje A, Liu Y, Boyle AP, Zhang QC, Zakharia F, Spacek DV, et al. Extensive variation in chromatin states across humans. Science. 2013;342:750–752. doi: 10.1126/science.1242510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpinen H, Waszak SM, Gschwind AR, Raghav SK, Witwicki RM, Orioli A, Migliavacca E, Wiederkehr M, Gutierrez-Arcelus M, Panousis NI, et al. Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science. 2013;342:744–747. doi: 10.1126/science.1242463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Jung I, Rajagopal N, Schmitt A, Selvaraj S, Lee AY, Yen CA, Lin S, Lin Y, Qiu Y, et al. Integrative analysis of haplotype-resolved epigenomes across human tissues. Nature. 2015;518:350–354. doi: 10.1038/nature14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov GK, Williams BA, McCue K, Schroth GP, Gertz J, Myers RM, Wold BJ. From single-cell to cell-pool transcriptomes: stochasticity in gene expression and RNA splicing. Genome Res. 2014;24:496–510. doi: 10.1101/gr.161034.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008;18:1509–1517. doi: 10.1101/gr.079558.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenscoff-Papadimitriou E, Allen WE, Colquitt BM, Goh T, Murphy KK, Monahan K, Mosley CP, Ahituv N, Lomvardas S. Enhancer interaction networks as a means for singular olfactory receptor expression. Cell. 2014;159:543–557. doi: 10.1016/j.cell.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Feng G, Hyman SE. Genome-scale neurogenetics: methodology and meaning. Nat Neurosci. 2014;17:756–763. doi: 10.1038/nn.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekhoubad S, Bock C, de Boer AS, Kiskinis E, Meissner A, Eggan K. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell. 2012;10:595–609. doi: 10.1016/j.stem.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K, Rudnick ND, Kehayova PD, Pauli F, Newberry KM, Myers RM, Maniatis T. Role of CCCTC binding factor (CTCF) and cohesin in the generation of single-cell diversity of protocadherin-α gene expression. Proc Natl Acad Sci USA. 2012;109:9125–9130. doi: 10.1073/pnas.1205074109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag A, Savova V, Fung HL, Miron A, Yuan GC, Zhang K, Gimelbrant AA. Chromatin signature of widespread monoallelic expression. eLife. 2013;2:e01256. doi: 10.7554/eLife.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazor KL, Altun G, Lynch C, Tran H, Harness JV, Slavin I, Garitaonandia I, Müller FJ, Wang YC, Boscolo FS, et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell. 2012;10:620–634. doi: 10.1016/j.stem.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. High expression of the mammalian X chromosome in brain. Brain Res. 2006;1126:46–49. doi: 10.1016/j.brainres.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Oberg AL, Bot BM, Grill DE, Poland GA, Therneau TM. Technical and biological variance structure in mRNA-Seq data: life in the real world. BMC Genomics. 2012;13:304. doi: 10.1186/1471-2164-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto SP, Goldstein DB. Recombination and the evolution of diploidy. Genetics. 1992;131:745–751. doi: 10.1093/genetics/131.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JD, Rubinstein ND, Fernandez DE, Santoro SW, Needleman LA, Ho-Shing O, Choi JJ, Zirlinger M, Chen SK, Liu JS, Dulac C. Quantitative and functional interrogation of parent-of-origin allelic expression biases in the brain. eLife. 2015;4:e07860. doi: 10.7554/eLife.07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. The role of genomic imprinting in biology and disease: an expanding view. Nat Rev Genet. 2014;15:517–530. doi: 10.1038/nrg3766. [DOI] [PubMed] [Google Scholar]
- Pinter SF, Colognori D, Beliveau BJ, Sadreyev RI, Payer B, Yildirim E, Wu CT, Lee JT. Allelic imbalance is a prevalent and tissue-specific feature of the mouse transcriptome. Genetics. 2015;200:537–549. doi: 10.1534/genetics.115.176263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras V, Selvarajoo K. The reduction of gene expression variability from single cells to populations follows simple statistical laws. Genomics. 2015;105:137–144. doi: 10.1016/j.ygeno.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Prickett AR, Barkas N, McCole RB, Hughes S, Amante SM, Schulz R, Oakey RJ. Genome-wide and parental allele-specific analysis of CTCF and cohesin DNA binding in mouse brain reveals a tissue-specific binding pattern and an association with imprinted differentially methylated regions. Genome Res. 2013;23:1624–1635. doi: 10.1101/gr.150136.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajab A, Schuelke M, Gill E, Zwirner A, Seifert F, Morales Gonzalez S, Knierim E. Recessive DEAF1 mutation associates with autism, intellectual disability, basal ganglia dysfunction and epilepsy. J Med Genet. 2015;52:607–611. doi: 10.1136/jmedgenet-2015-103083. [DOI] [PubMed] [Google Scholar]
- Reinius B, Mold JE, Ramsköld D, Deng Q, Johnsson P, Michaëlsson J, Frisén J, Sandberg R. Analysis of allelic expression patterns in clonal somatic cells by single-cell RNA-seq. Nat Genet. 2016;48:1430–1435. doi: 10.1038/ng.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savova V, Chun S, Sohail M, McCole RB, Witwicki R, Gai L, Lenz TL, Wu CT, Sunyaev SR, Gimelbrant AA. Genes with monoallelic expression contribute disproportionately to genetic diversity in humans. Nat Genet. 2016;48:231–237. doi: 10.1038/ng.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segklia A, Seuntjens E, Elkouris M, Tsalavos S, Stappers E, Mitsiadis TA, Huylebroeck D, Remboutsika E, Graf D. Bmp7 regulates the survival, proliferation, and neurogenic properties of neural progenitor cells during corticogenesis in the mouse. PLoS ONE. 2012;7:e34088. doi: 10.1371/journal.pone.0034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza-Reilly M, Commons KG. Unraveling the architecture of the dorsal raphe synaptic neuropil using high-resolution neuroanatomy. Front Neural Circuits. 2014;8:105. doi: 10.3389/fncir.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SSK, Kim SY, Bestor TH, Shioda T, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44:398–405. S1–S2. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulto-van Silfhout AT, Rajamanickam S, Jensik PJ, Vergult S, de Rocker N, Newhall KJ, Raghavan R, Reardon SN, Jarrett K, McIntyre T, et al. Mutations affecting the SAND domain of DEAF1 cause intellectual disability with severe speech impairment and behavioral problems. Am J Hum Genet. 2014;94:649–661. doi: 10.1016/j.ajhg.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Zhang G, Fang X, Zhang Y, Li C, Ling F, Cooper DN, Li Q, Li Y, van Gool AJ, et al. Genome sequencing and comparison of two nonhuman primate animal models, the cynomolgus and Chinese rhesus macaques. Nat Biotechnol. 2011;29:1019–1023. doi: 10.1038/nbt.1992. [DOI] [PubMed] [Google Scholar]
- Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwemer LM, Zak A, Thompson BR, Kirby A, Daly MJ, Chess A, Gimelbrant AA. Autosomal monoallelic expression in the mouse. Genome Biol. 2012;13:R10. doi: 10.1186/gb-2012-13-2-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.