Abstract
Placental growth factor (PGF) is member of the vascular endothelial growth factor (VEGF) family of angiogenesis regulators. VEGFA is an established regulator of ovulation and formation of the corpus luteum. To determine whether PGF also mediates aspects of ovulation and luteinization, macaques received gonadotropins to stimulate multiple follicular development. Ovarian biopsies and whole ovaries were collected before (0 hours) and up to 36 hours after human chorionic gonadotropin (hCG) administration to span the ovulatory interval. PGF and VEGFA were expressed by both granulosa cells and theca cells. In follicular fluid, PGF and VEGFA levels were lowest before hCG. PGF levels remained low until 36 hours after hCG administration, when PGF increased sevenfold to reach peak levels. Follicular fluid VEGFA increased threefold to reach peak levels at 12 hours after hCG, then dropped to intermediate levels. To explore the roles of PGF and VEGFA in ovulation, luteinization, and follicular angiogenesis in vivo, antibodies were injected into the follicular fluid of naturally developed monkey follicles; ovariectomy was performed 48 hours after hCG, with ovulation expected about 40 hours after hCG. Intrafollicular injection of control immunoglobulin G resulted in no retained oocytes, follicle rupture, and structural luteinization, including granulosa cell hypertrophy and capillary formation in the granulosa cell layer. PGF antibody injection resulted in oocyte retention, abnormal rupture, and incomplete luteinization, with limited and disorganized angiogenesis. Injection of a VEGFA antibody resulted in oocyte retention and very limited follicle rupture or structural luteinization. These studies demonstrate that PGF, in addition to VEGFA, is required for ovulation, luteinization, and follicular angiogenesis in primates.
Placental growth factor is produced within the primate ovulatory follicle and is necessary for oocyte release, follicle rupture, luteinization, and new vessel formation within the ovulatory follicle.
Ovulation is a series of events arising from changes within the dominant follicle. Angiogenesis is a crucial step in this process. Angiogenic factors, such as vascular endothelial growth factor (VEGF) A (VEGFA) regulate new capillary formation within the ovulatory follicle. In vivo administration of antibodies directed against VEGF or VEGF receptors (VEGFRs) or truncated or soluble VEGFRs can prevent ovulation in primates (1–8). These antiangiogenic agents can also prevent maturation of the cumulus-oocyte complex and structural luteinization as the ovulatory follicle transitions to become the corpus luteum (3, 6, 8–10), further compromising ovarian function and fertility.
The midcycle gonadotropin surge increases production of VEGFA by granulosa and theca cells of ovulatory follicles (11–18). Vascular growth factors such as VEGFA are believed to form a gradient to stimulate new capillary growth. The high concentration of growth factors in the granulosa and theca cells likely attracts vascular endothelial cells from the surrounding stromal vessels to form new capillaries, which extend into the luteinizing granulosa cell layer (10, 19). Consistent with this concept, new endothelial cell networks are first observed in the granulosa cell layer of human and monkey follicles 24 hours after the luteinizing hormone (LH) surge; follicular angiogenesis is well under way at the time of ovulation (19, 20).
VEGF family members involved in angiogenesis include VEGFA, VEGFB, VEGFC, VEGFD, and placental growth factor (PGF) (21). VEGF family members interact with receptors on endothelial cells to stimulate angiogenesis. Activation of VEGFR1 [fms-related tyrosine kinase 1 (FLT1)] increases endothelial cell proliferation and vascular permeability (22, 23). Activation of VEGFR2 [kinase insert domain receptor (KDR)] increases endothelial cell proliferation and migration as well as capillary formation and vascular permeability (24, 25). Dimerization between FLT1 and KDR as well as interactions between VEGFRs and coreceptors such as neuropilins add complexity to this response system (26). Whereas VEGFA can activate FLT1 and KDR, PGF and VEGFB activate only FLT1 (21). VEGFC and VEGFD are thought to act primarily via VEGFR3 [fms-related tyrosine kinase 4 (FLT4)] on lymphatic endothelial cells to stimulate lymphangiogenesis, although actions at vascular endothelial cells have been reported (27).
PGF is known primarily for its organizational role in embryonic and placental vessel formation (28). Our laboratory has previously shown that PGF treatment increases ovarian microvascular endothelial cell proliferation and capillary sprout formation in vitro (20). However, PGF synthesis and actions within the ovulatory follicle in vivo have not been reported. Previous studies using agents directed against VEGFRs to block ovulation were intended to block VEGFA action, but many of these reagents also bind to and neutralize PGF (1–3, 5–8). In the current study, we show that PGF is a vascular growth factor produced within the ovulatory follicle in response to the ovulatory gonadotropin surge. PGF neutralization in primate ovulatory follicles compromises oocyte release, follicle rupture, and follicular angiogenesis, demonstrating that PGF is necessary for both ovulation and development of the corpus luteum.
Materials and Methods
Animals
Whole ovaries were obtained from adult female cynomolgus macaques (Macaca fascicularis) at Eastern Virginia Medical School (Norfolk, VA). All animal protocols were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Eastern Virginia Medical School Animal Care and Use Committee. Animal husbandry was performed as described previously (29). Briefly, adult females with regular menstrual cycles were routinely observed for menstruation; the first day of menstruation marked day 1 of the cycle. Blood samples were obtained with chemical restraint (ketamine, 5 to 10 mg/kg body weight) as needed by femoral venipuncture, and serum was stored at −20°C. Serum estradiol and progesterone levels were determined by using the Immulite 1000 immunoassay system (Siemens Medical Diagnostics Solutions, Rockville, MD). Aseptic surgeries were performed by laparotomy in a dedicated surgical suite under isoflurane anesthesia. Postoperative analgesia was accomplished with buprenorphine and a nonsteroidal anti-inflammatory drug (ketoprofen or meloxicam).
Ovarian stimulation
An ovarian stimulation model was used to obtain ovaries with multiple ovulatory follicles (30). Beginning within 3 days of initiation of menstruation, monkeys received 90 IU of recombinant human follicle-stimulating hormone (FSH; Merck & Co., Kenilworth, NJ) for 6 to 8 days, followed by 2 to 3 days of 90 IU of FSH plus 60 IU of recombinant human LH (Serono Reproductive Biology Institute, Rockland, MA) to stimulate the growth of multiple follicles. Animals also received a gonadotropin-releasing hormone antagonist [either 0.5 mg/kg Antide (Serono) or 30 µg/kg Ganirelix (Merck)] daily to prevent an endogenous ovulatory LH surge. Follicular development was monitored by ultrasonography and rising serum estradiol. During aseptic surgery, aspiration of follicles >4 mm was performed 12 hours, 24 hours, or 36 hours after administration of 1000 IU of recombinant human chorionic gonadotropin (hCG; Serono). To inhibit follicular prostaglandin production during the periovulatory interval, some animals were treated as described above; these animals also received the prostaglandin G/H synthase and cyclooxygenase (PTGS2) inhibitor celecoxib (32 mg orally every 12 hours; Pfizer, New York, NY) beginning with hCG administration and continuing until surgery (29).
Controlled ovulation with follicle injection
A model of controlled ovulation with follicle injection model was used to introduce an antibody into the ovulatory follicle (31). Beginning on days 5 to 7 of the menstrual cycle, animals were monitored for rising serum estradiol to indicate development of a large preovulatory follicle. Animals then received a gonadotropin-releasing hormone antagonist (acyline, 60 µg/kg; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, MD) to prevent endogenous LH surge and 60 IU of FSH and 60 IU of LH for 2 days to maintain healthy development of the follicle. On the third day, intrafollicular injection of antibodies against PGF (catalog no. 250825; Abbiotec, San Diego, CA; n = 5), VEGFA (catalog no. 251901; Abbiotec; n = 3), or control immunoglobulin G (IgG) antibody (catalog no. 254513; Abbiotec; n = 3) was performed during aseptic surgery; an estimated 10 µg of antibody protein was delivered to each follicle at injection. Immediately after surgery, 1000 IU hCG (Serono) was administered to initiate ovulatory events. Ovariectomy was performed 48 hours after follicle injection and hCG, with ovulation anticipated at about 40 hours. For one PGF antibody–injected ovary, the ovary was damaged at ovariectomy; this ovary was not used for assessment of rupture site area or oocyte retention.
Tissue preparation
Monkey granulosa cells and oocytes were pelleted from the follicular aspirates by centrifugation at 250g. The supernatant (follicular fluid) was removed and stored at −80°C. After oocyte removal, a granulosa cell–enriched population of the remaining cells was obtained by Percoll gradient centrifugation (29). Granulosa cells were used immediately for cell culture or were frozen in liquid nitrogen and stored at −80 C. Viability of granulosa cell–enriched preparations was assessed by trypan blue exclusion and averaged 80%. Whole ovaries were bisected such that at least two ovulatory follicles >4 mm in diameter were present on each piece. Pieces were fixed in 10% formalin and embedded in paraffin.
Real-time reverse transcriptase polymerase chain reaction
Levels of messenger RNA (mRNA) for PGF, total VEGFA, VEGFA165, and VEGFA121 were assessed by quantitative polymerase chain reaction (PCR) using a Roche Lightcycler (Roche Diagnostics, Atlanta, GA). Total RNA was obtained from granulosa cells, treated with deoxyribonuclease, and reverse transcribed as previously described (32). PCR was performed by using the FastStart DNA Master SYBR Green I kit (Roche) following manufacturer’s instructions. Primers were designed according to human or monkey sequences and spanned an intron to prevent undetected amplification of genomic DNA. PCR products were sequenced (Genewiz, South Plainfield, NJ) to confirm amplicon identity (Supplemental Table 1 (22.6KB, docx) ). All data are expressed as the ratio of mRNA of interest to β-actin (ACTB) mRNA for each sample, where a value of 1.0 indicates the same number of copies of mRNA of interest and copies of ACTB in a given mRNA sample.
Histology
Whole ovaries were fixed in 10% formalin for 24 hours and embedded in paraffin, oriented such that sections included the follicle apex and follicle wall opposite the apex at the maximal follicle diameter to ensure optimal view of the follicle apex (31). Ovaries were serially sectioned at 5 μm, with each section retained in order. Every fifth section was deparaffinized in xylene baths, rehydrated through a series of ethanol washes, stained with 30% hematoxylin and 60% eosin (Sigma-Aldrich, St. Louis, MO), and coverslipped with Permount mounting medium (Fisher Scientific, Suwanee, GA). Stained sections were imaged by using an Olympus microscope with a DP70 digital camera system and associated software (Olympus, Melville, NY). Whole follicle images were assembled from multiple microscopic images of a single tissue section by using Image Composite Editor (Microsoft Corp., Redmond, WA).
At least two independent observers performed histologic evaluation of sections from each ovary (31). Evaluation of sections included identification of the oocyte and (if present) condition of the cumulus (tight/expanded), as well as presence/absence of a rupture site. The size of each rupture site was quantified by measuring the width on the section with the largest rupture site, counting the number of 5-μm sections where the rupture site was present, and using these measurements to calculate the area of an oval. Luteinization of the mural granulosa cell layer was assessed by using a tissue section, which included the maximal diameter of the follicle and the rupture site, if rupture occurred. The granulosa cell layer immediately opposite the apex or thinnest portion of the remaining follicle wall was assessed. The thickness of the granulosa cell layer was measured as distance from the granulosa cell basement membrane to antrum, with at least four replicate measurements made for each tissue section evaluated.
Apoptosis was assessed with the DeadEnd fluorescence terminal deoxynucleotidyl transferase–mediated (2′-deoxyuridine, 5′-triphosphate) nick end labeling (TUNEL) system (Promega, Madison, WI) following manufacturer’s instructions for paraffin-embedded tissues. Granulosa cells of a small antral follicle with structural evidence of atresia were used as a positive control; granulosa cells of a healthy ovulatory follicle 36 hours after hCG administration were used as a negative control. For every tissue examined, an adjacent section was stained with omission of the terminal deoxynucleotidyl transferase, which also served as a negative control. Mounting media contained 4′,6-diamidino-2-phenylindole as a nuclear counterstain.
Western blot
Granulosa cell lysate preparation and Western blot were performed essentially as previously described (19). Briefly, granulosa cell lysates were loaded onto a 4% to 12% gradient gel (Fisher Scientific). Proteins were transferred to a polyvinylidene fluoride membrane (Immobilon; Millipore, Billerica, MA) and probed by using antibodies against PGF (0.005 mg/mL; Abbiotec) and VEGFA (0.002 mg/mL; Abbiotec). Membranes were incubated with anti-rabbit AP-conjugated secondary antibody (Applied Biosystems, Invitrogen, Carlsbad, CA) and protein bands visualized with Tropix CDP-Star according to the manufacturer’s instructions (Applied Biosystems, Invitrogen).
Enzyme-linked immunosorbent assay for PGF and VEGFA
Granulosa cell lysates (as prepared as for Western blotting) and follicular fluid samples were assessed by using PGF (Thermo Scientific, Hudson, NH) and VEGFA (R&D Systems, Minneapolis, MN) enzyme-linked immunosorbent assays (ELISAs) and analyzed according to kit instructions. Granulosa cell lysate PGF and VEGFA levels were normalized to the protein content of lysates, as determined by the bicinchoninic acid method (Sigma-Aldrich). Intra-assay coefficient of variation (CV) and interassay CV for the PGF ELISA were 3.4% and 14.9%, respectively. Intra-assay and interassay CVs for the VEGFA ELISA were 1.5% and 5.3%, respectively.
Immunohistochemistry
Immunostaining was performed by using paraffin-embedded ovaries sectioned at 5 µm essentially as previously described (19). Briefly, tissue sections were heated, deparaffinized, exposed to antigen retrieval with either sodium citrate (for detection of VEGFA and CYP17A1) or Tris-EDTA (for detection of PGF). When staining for von Willebrand factor (vWF), antigen retrieval was not performed. All tissues were blocked with 5% nonimmune serum in PBS containing 0.1% Triton X-100. Slides were incubated overnight with primary antibody against PGF (0.02 mg/mL; Abbiotec), VEGFA (0.005 mg/mL; Abbiotec), CYP17A1 (1:2000 dilution, polyclonal antibody supplied by Dr. Michael Waterman, Vanderbilt University School of Medicine), or vWF (0.005 mg/mL; catalog no. A0082; Dako, Carpinteria, CA) and color-developed by using a rabbit Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Slides were counterstained in hematoxylin, dehydrated, and permanently coverslipped. All images were obtained by using an Olympus BX41 microscope fitted with a DP70 digital camera and associated software. For all experiments, omission of the primary antibody served as a negative control.
Assessment of luteinization and angiogenesis
Granulosa cell layer thickness was assessed as previously described (31). Briefly, an ovarian section stained with hematoxylin and eosin was selected, which included the maximal diameter of the follicle and the rupture site (if rupture occurred) or thinnest portion of the remaining follicle wall (if rupture did not occur). The granulosa cell layer immediately opposite the apex or thinnest portion of the remaining follicle wall was assessed. The distance from granulosa cell basement membrane to antral edge of granulosa cells was measured, with a minimum of eight replicate measurements made for each ovarian tissue.
Endothelial cell invasion into granulosa cell layer was assessed by using ovarian tissues immunostained for the endothelial cell protein vWF as described above. Endothelial cell invasion into the granulosa cell layer was assessed by using a tissue section, which included the maximal diameter of the follicle and the rupture site or thinnest portion of the remaining follicle wall. The granulosa cell layer immediately opposite the apex or thinnest portion of the remaining follicle wall was assessed. The distance from the granulosa cell basement membrane to the vWF+ cell closest to the follicle antrum was determined, with at least five replicate measurements made for each tissue section evaluated.
Three-dimensional (3D) modeling of capillary sprouting from stromal vessels was performed as previously described (19). Briefly, five adjacent 5-µm ovarian sections were immunostained for vWF as described above. vWF+ cells from these adjacent sections were traced into WinSurf software (WinSurf Technology, Hertfordshire, UK) and reconstructed following the manufacturer’s instructions.
Endothelial cell proliferation was assessed in vitro by using proliferating populations of ovarian microvascular endothelial cells obtained from monkey ovulatory follicles (19). PGF and VEGFA (both from R&D Systems) were preincubated 24 hours at 4°C with either the PGF antibody or VEGFA antibody used for follicle injection, with the antibody in fourfold molar excess. Endothelial cells were cultured on chamber slides overnight in basal media (Lonza, Fisher Scientific). The next day, cells were treated with PGF or VEGFA [each at 5 ng/mL (20)], either alone or preincubated with one of the two antibodies as described above. After 24 hours of treatment in vitro, cells were fixed in 10% formalin for Ki67 detection and quantification of proliferating cells as previously described (19).
Data analysis
Data were assessed for heterogeneity of variance by Bartlett test. Data were log transformed when the Bartlett test yielded P < 0.05; log-transformed data were subjected to the Bartlett test to confirm that P > 0.05. All data sets were assessed by t test or analysis of variance (ANOVA) (without or with repeated measures) as indicated in the figure legends. ANOVA was followed by the Duncan multiple range test when P < 0.05. Statistical analyses were performed by using StatPak software, version 4.12 (Northwest Analytical, Portland, OR). Significance was assumed at P < 0.05. Data are expressed as mean ± standard error of the mean.
Results
Ovulatory follicles produce PGF in response to the gonadotropin surge
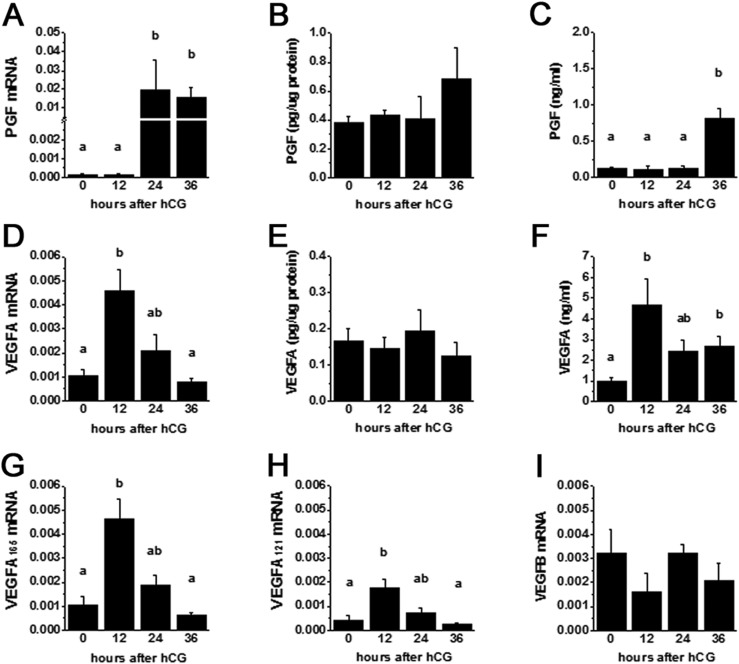
To examine PGF synthesis and accumulation in the ovulatory follicle, granulosa cells were obtained from monkeys experiencing ovarian stimulation before (0 hours) or 12 to 36 hours after administration of an ovulatory dose of hCG to span the ovulatory interval in primates. Total granulosa cell RNA was assessed for PGF mRNA content. PGF mRNA was low at 0 hours and 12 hours after hCG (Fig. 1A). At 24 hours after hCG, PGF mRNA levels increased 100-fold and remained high at 36 hours after hCG.
Figure 1.
Granulosa cell expression of VEGF family members. Granulosa cells obtained from monkey ovulatory follicles after ovarian stimulation in the absence of hCG (0 hours) and 12, 24, or 36 hours after hCG were assessed by quantitative PCR for (A) PGF mRNA, (D) total VEGFA mRNA, (G) VEGFA165 mRNA, (H) VEGFA121 mRNA, and (I) VEGFB mRNA. mRNA levels are expressed relative to ACTB mRNA in each sample. Granulosa cell concentration of protein for (B) PGF and (E) VEGFA were determined by ELISA and normalized to total protein for each sample. Follicular fluid obtained from monkeys by follicle aspiration before and after hCG was assessed for concentration of protein for (C) PGF and (F) VEGFA and normalized to total protein for each sample. For each panel, data were assessed by ANOVA and Duncan post hoc test. Data are expressed as mean ± standard error of the mean. Groups with no common letters are different, P < 0.05. n = 3 to 6 samples per group.
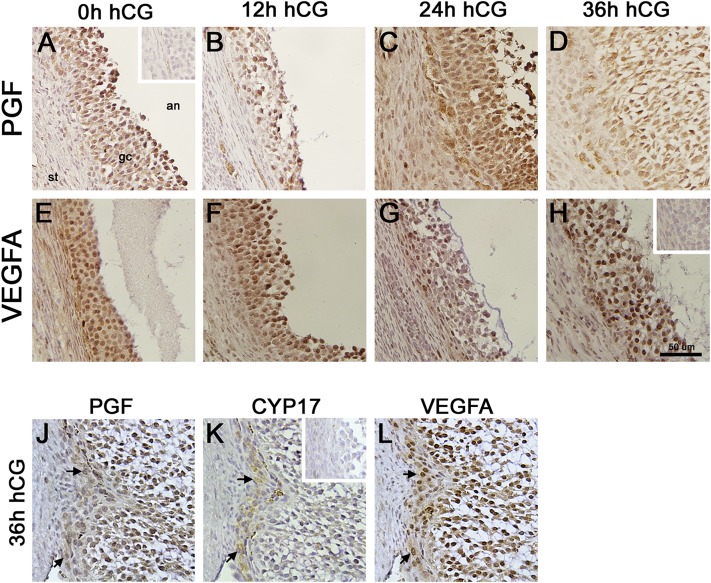
To determine whether changes in PGF mRNA were accompanied by similar changes in PGF protein, ovaries from monkeys experiencing ovarian stimulation were used for immunohistochemical detection of PGF (Fig. 2A–2D). PGF was detected in granulosa cells at all time points examined. Western blotting confirmed antibody detection of proteins of appropriate size (Supplemental Fig. 1 (3.8MB, pdf) ). PGF concentration in granulosa cell lysates was unchanged across the ovulatory interval (Fig. 1B). In contrast, follicular fluid levels of PGF were dynamic, with low levels measured 0 hours to 24 hours after hCG and sevenfold higher levels measured at 36 hours after hCG (Fig. 1C).
Figure 2.
PGF and VEGFA immunodetection in monkey ovulatory follicles. Monkey ovaries obtained after ovarian stimulation in (A and E) the absence of hCG (0 hours) and (B and F) 12, (C and G) 24, or (D and H) 36 hours after hCG were used for immunodetection of (A–D) PGF or (E–H) VEGFA as indicated by presence of brown precipitate. Additional serial sections from an ovary obtained after ovarian stimulation and 36 hours hCG were used for immunodetection of (J) PGF, (K) the theca cell enzyme CYP17, and (L) VEGFA to confirm colocalization of PGF and VEGFA with CYP17 (arrows). Tissue sections were counterstained with hematoxylin (blue). Insets show absence of brown stain was confirmed when primary antibodies were omitted for (A) PGF, (H) VEGFA, and (K) CYP17. All images are at same magnification and use bar in panel H (50 µm). All images are oriented as in panel A, with stroma (st) in lower left, granulosa cells (gc) central, and antrum (an) in upper right. Images are representative of n = 3 to 4 monkeys per group.
Ovulatory follicles produce VEGFA in response to the gonadotropin surge
To confirm dynamic regulation of VEGFA in granulosa cells of cynomolgus monkey ovulatory follicles, mRNA for total VEGFA as well as VEGFA isoforms were quantified by quantitative PCR. Total VEGFA mRNA levels were low at 0 hours, rose fourfold to peak levels by 12 hours after hCG, then declined to low levels by 36 hours after hCG (Fig. 1D). Levels of mRNA for VEGFA165 and VEGFA121 followed a similar pattern (Fig. 1G and 1H). Copy numbers for VEGFA165 and VEGFA121 indicate that these isoforms are the predominant VEGFA isoforms in the monkey follicle. Primers specific for the antiangiogenic VEGFA165b did not amplify product in monkey granulosa cells.
Immunocytochemical detection of VEGFA protein in monkey ovaries showed detection of VEGFA protein in monkey granulosa cells throughout the ovulatory interval (Fig. 2E–2H). Western blotting confirmed antibody detection of proteins of appropriate size (Supplemental Fig. 1 (3.8MB, pdf) ). VEGFA concentration in granulosa cell lysates was unchanged across the ovulatory interval (Fig. 1E). Follicular fluid levels of VEGFA were low at 0 hours, rose fourfold to peak 12 hours after hCG, and remained somewhat elevated for the rest of the ovulatory interval (Fig. 1F).
PGF and VEGFA are present in theca cells of ovulatory follicles
Immunodetection of PGF and VEGFA in stroma immediately adjacent to granulosa cells indicated that theca cells may also express PGF and VEGFA. Adjacent sections from monkey ovaries were immunostained for PGF, the theca cell–specific steroidogenic enzyme CYP17A1, and VEGFA (Fig. 2J–2L). CYP17A1 staining was present in cells also staining for PGF and VEGFA, indicating that theca cells of primate follicles express these VEGF family members.
Ovulatory follicles produce other VEGF family members
VEGFB, VEGFC, and VEGFD are also able to bind to and activate VEGF receptors. VEGFB mRNA was detected in monkey granulosa cells (Fig. 1I). VEGFB mRNA levels did not change across the ovulatory interval. Granulosa cell content of VEGFC and VEGFD mRNAs also did not change across the ovulatory interval in monkey granulosa cells as previously reported (27).
VEGF family member protein levels are not regulated by follicular prostaglandins
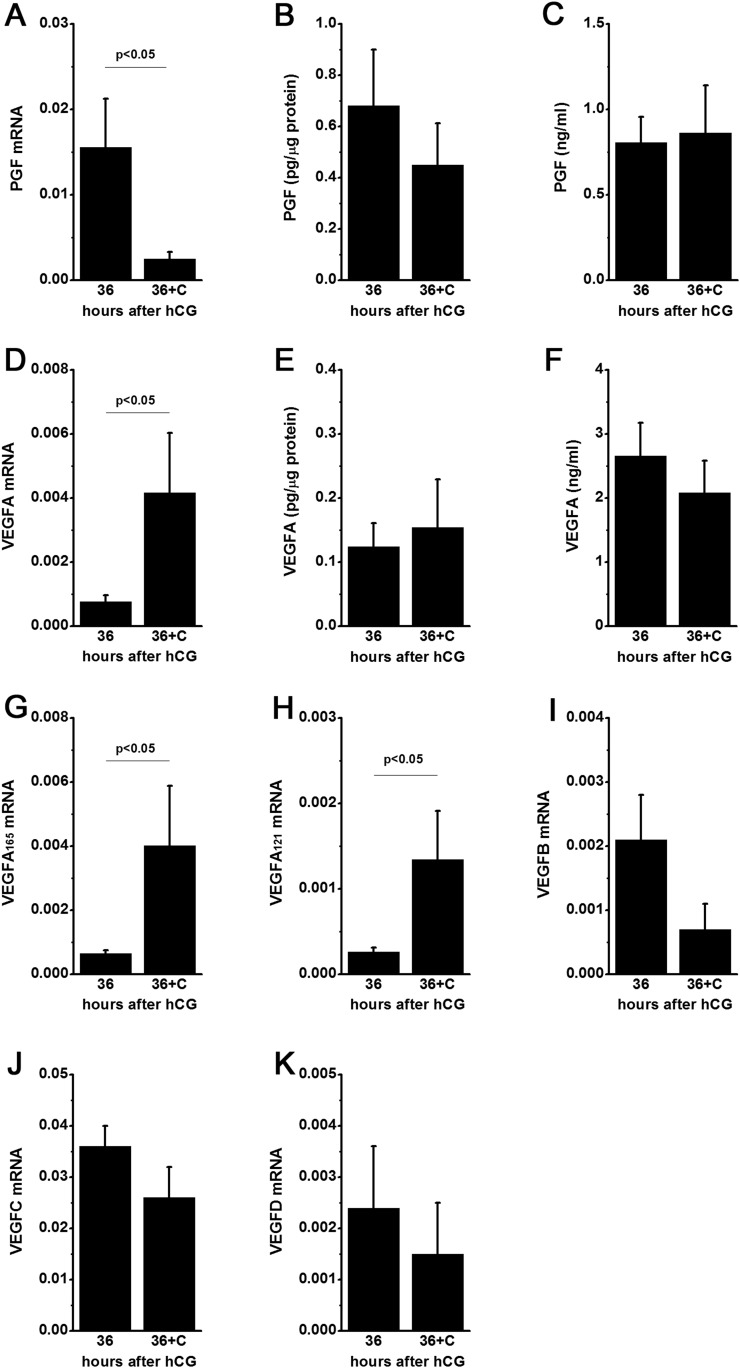
Changing levels of PGF and VEGFA mRNA in granulosa cells and proteins in follicular fluid correlate with increased follicular levels of prostaglandin E2, a key mediator of ovulation (33). To determine whether follicular prostaglandins regulate VEGF family members, additional monkeys experienced ovarian stimulation and also received the PTGS2-selective inhibitor celecoxib to block follicular prostaglandin synthesis (29) (Fig. 3). Celecoxib treatment reduced PGF mRNA levels in granulosa cells collected 36 hours after hCG. However, PGF protein concentrations in granulosa cell lysates and follicular fluid were not altered by celecoxib treatment. Whereas celecoxib treatment increased mRNA levels for total VEGFA, VEGFA165, and VEGFA121 at 36 hours after hCG, celecoxib treatment did not alter protein levels for VEGFA in granulosa cell lysates or follicular fluid. Celecoxib treatment did not alter mRNA levels of VEGFB, VEGFC, or VEGFD in monkey granulosa cells.
Figure 3.
Inhibition of follicular prostaglandin synthesis with celecoxib does not alter PGF or VEGFA protein levels in monkey ovulatory follicles obtained 36 hours after hCG treatment. Celecoxib (36+C) reduced granulosa cell mRNA for (A) PGF but did not alter PGF protein in (B) granulosa cell lysates or (C) follicular fluid. Celecoxib increased granulosa cell mRNA for (D) total VEGFA, (G) VEGFA165, and (H) VEGFA121 but did not alter VEGFA protein in (E) granulosa cell lysates or (F) follicular fluid. Granulosa cell mRNA for (I) VEGFB, (J) VEGFC, and (K) VEGFD was not altered by celecoxib treatment. Data are expressed as mean ± standard error of the mean. Within each panel, groups different by unpaired t test where indicated, P < 0.05. n = 3 to 6 monkeys/group. Data for VEGFC mRNA 36 hours after hCG and VEGFD mRNA 36 hours after hCG were previously published (27) and are used here by permission.
PGF and VEGFA are required for ovulation, luteinization, and follicular angiogenesis
To evaluate the effects of PGF and VEGFA on ovulation, luteinization, and follicular angiogenesis, dominant follicles of monkey ovaries were injected with an antibody to PGF or VEGFA; injection of additional follicles with isotype-matched IgG served as a control. An ovulatory dose of hCG was administered immediately after follicle injection; ovaries were photographed in situ and then removed 48 hours after hCG, with ovulation expected about 40 hours after hCG in untreated ovaries. Serum estradiol and progesterone levels were not different between treatment groups before or after follicle injection (Supplemental Fig. 2 (3.8MB, pdf) ).
To confirm the health of injected follicles, TUNEL was performed. Follicles injected with control IgG, PGF antibody, or VEGFA antibody showed no evidence of apoptosis at the time of ovary removal (Supplemental Fig. 3 (3.8MB, pdf) ). To confirm that each antibody used for follicle injection selectively neutralized PGF or VEGFA, each growth factor was preincubated with the PGF antibody or the VEGFA antibody; these growth factors and preincubated growth factors were used to stimulate proliferation of monkey ovarian microvascular endothelial cells in vitro (Supplemental Fig. 3 (3.8MB, pdf) ). PGF and VEGFA each stimulated proliferation as previously reported (20). PGF preincubated with PGF antibody did not stimulate proliferation. Similarly, VEGFA preincubated with VEGFA antibody did not stimulate proliferation. In contrast, PGF preincubated with VEGFA antibody and VEGFA preincubated with PGF antibody yielded proliferation rates similar to those of PGF and VEGFA in the absence of antibody incubation, confirming the specificity of these antibodies for their target growth factors.
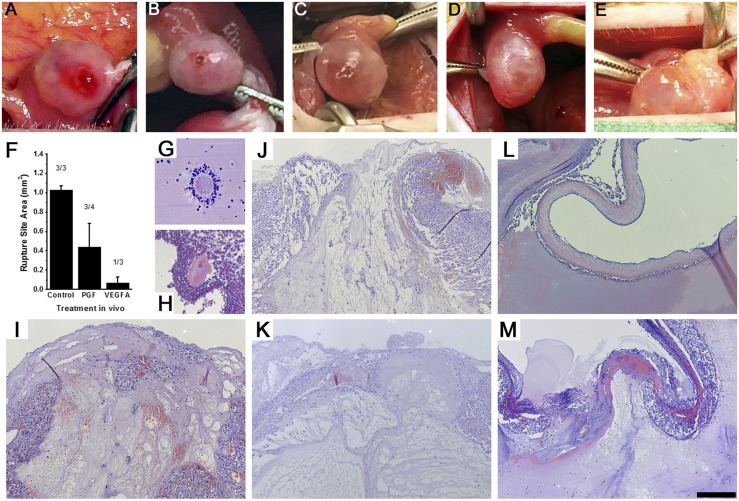
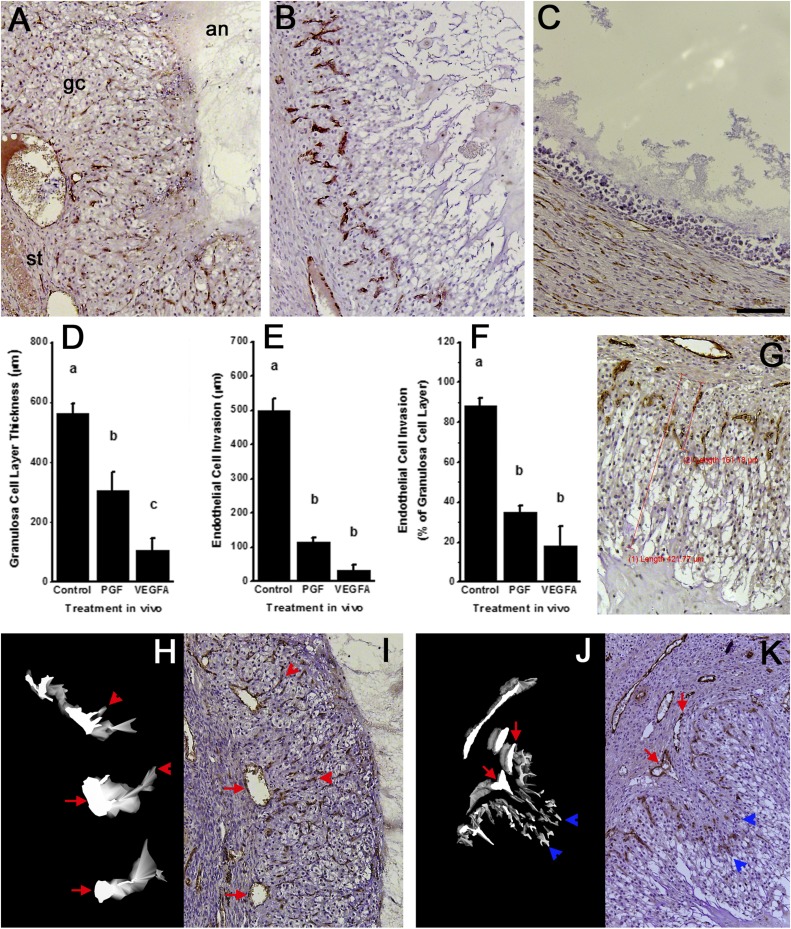
Control IgG-injected follicles displayed structural changes characteristic of normal ovulation and luteinization. At the time of surgical removal, each control IgG-injected follicle possessed a bloody and protruding ovulatory stigmata (Fig. 4A). Upon histological exam, no oocytes were identified in these follicles, and each had a single, large rupture site (Table 1; Fig. 4I; Supplemental Fig. 4 (3.8MB, pdf) ). Control IgG-injected follicles were well luteinized. The granulosa cell layer was thickened, with hypertrophied granulosa cells (Fig. 5A; Supplemental Fig. 4 (3.8MB, pdf) ). Endothelial cells were consistently present throughout the granulosa cell layer, reaching from the stroma to approach the follicle antrum. 3D modeling of endothelial cells confirmed that capillaries forming in the granulosa cell layer branch from small vessels that connect to larger stromal vessels surrounding the follicle (Fig. 5H and 5I). All characteristics of control IgG-injected follicles were consistent with evaluation of vehicle-injected monkey follicles in a previous report (31).
Figure 4.
Follicle rupture and retained oocytes after intrafollicular injection of antibodies against PGF or VEGFA. (A–E) Ovulatory stigmata and (I–M) histological views of rupture sites are shown after injection with (A and I) control IgG antibody, (B, C, J, and K) PGF antibody, or (D, E, L, and M) VEGFA antibody. (F) Graph shows area of rupture site (mean ± standard error of the mean) for each treatment group. Absence of rupture was set equal to 0 mm2. Number of ruptured follicles/total follicles is given above each bar. Examples of oocytes found trapped in follicles after injection with (G) PGF antibody or (H) VEGFA antibody are also shown. All histological images of rupture sites are at the same magnification; scale bar in panel M = 200 µm. Each oocyte is shown at approximately maximal diameter of about 100 µm.
Table 1.
Oocyte Retention and Follicle Rupture
| Variable | Control IgG | PGF | VEGFA |
|---|---|---|---|
| Oocyte retained | 0/3 | 2/4 | 3/3 |
| Rupture site present | 3/3 | 3/4 | 1/3 |
Figure 5.
Structural luteinization and angiogenesis in follicles after intrafollicular injection of antibodies against PGF or VEGFA. Top panels show histology of follicle wall after injection with (A) control IgG antibody, (B) PGF antibody, or (C) VEGFA antibody. All images are in same orientation, with stroma (st) lower left, granulosa cells (gc) central, and antrum (an) upper right as indicated in panel A. Endothelial cells are identified as vWF+ cells (brown); nuclei are stained blue. Graphs show morphometric analysis of (D) granulosa cell layer thickness and (E) endothelial cell invasion into the granulosa cell layer as distance from granulosa cell basement membrane to outermost edge of granulosa or endothelial cells, (G) as indicated by red lines on the accompanying image. (F) The ratio of endothelial cell invasion to granulosa cell layer thickness is also shown. For each graph, data are shown as mean ± standard error of the mean and were assessed by ANOVA and Duncan post hoc test, groups with no common superscripts are different, P < 0.05. n = 3 to 5 ovaries per group. All histological images are at the same magnification; scale bar in panel (C) = 100 µm. (H and J) 3D modeling of endothelial cells (white on black background) is shown alongside (I and K) representative histological images of vWF immunostained ovarian tissues after injection with (H and I) control IgG antibody or (J and K) PGF antibody used for 3D modeling of endothelial cells. Red arrows indicate stromal vessels, red arrowheads indicate capillary-like structures that connect to a stromal vessel, and blue arrowheads indicate endothelial cells within the granulosa cell layer that lack connection to a stromal vessel. Images from control IgG antibody show four small vessels within the granulosa cell layer; all four small vessels connect to stromal vessel indicated with the red arrow in adjacent tissue sections not used for 3D model construction.
PGF neutralization compromises ovulation
Follicles injected with the PGF antibody did not experience normal follicle rupture. Oocytes were identified in 50% of PGF antibody–injected follicles (Table 1). These oocytes were found in the follicle antrum either lacking cumulus or surrounded by expanded cumulus (Fig. 4G). Most PGF antibody–injected follicles (75%) experienced follicle rupture (Table 1). One follicle possessed a thickened and reddened apex with a very small rupture site (Fig. 4C). Other PGF antibody–injected follicles showed evidence of follicle rupture, including one with a small round hole above the follicle, another with a slit along one entire side of the follicle, and one with clear clotted material protruding from the apex (Fig. 4D and not shown). One PGF antibody–injected follicle was unruptured at ovariectomy (not shown). In no case was the rupture site consistent with the appearance of normal ovulatory stigmata, as observed in control IgG antibody–injected follicles. Overall, PGF antibody–injected follicles had smaller rupture sites when compared with control IgG-injected follicles (Fig. 4F, 4J, and 4K).
Luteinization was also compromised in PGF antibody–injected follicles. Follicles in this treatment group had thinner granulosa cell layers when compared with control IgG–injected follicles (Fig. 5B and 5D). Poor endothelial cell invasion into the granulosa cell layer was also noted (Fig. 5B and 5E). In contrast with control IgG-injected follicles, endothelial cell invasion into the granulosa cell layer was minimal, reaching only 35% ± 3% of the distance from granulosa cell basement membrane to antrum (Fig. 5F). 3D modeling of endothelial cells showed that endothelial cell branching from stromal vessels was very limited. The few endothelial cells present within the granulosa cell layer were not continuous with other endothelial cells and appeared to be migrating solo toward the follicle antrum (Fig. 5J and 5K). Interestingly, two of five follicles injected with the PGF antibody showed uneven luteinization (Supplemental Fig. 4 (3.8MB, pdf) ). One side of the follicle displayed granulosa cell hypertrophy and endothelial cell invasion somewhat similar to control IgG-injected follicles, whereas the other side of the follicle showed little evidence of granulosa cell hypertrophy or endothelial cell invasion.
VEGFA neutralization compromises ovulation
VEGFA antibody–injected follicles either failed to rupture (Fig. 4E and 4L) or possessed a very small rupture site (Fig. 4D and 4M), resulting in an average rupture site size that was smaller than measured in control IgG- or PGF antibody–injected follicles (Fig. 4F; Table 1). Unruptured follicles had very thin apexes (Fig. 4L). Retained oocytes were identified in all VEGFA antibody–injected follicles (Table 1). Two oocytes were located in unexpanded cumulus and were in contact with the mural granulosa cell layer (Fig. 4H); the remaining oocyte was located in the follicle antrum surrounded by expanded cumulus (not shown).
Follicles injected with VEGFA antibody showed very limited luteinization. Granulosa cell hypertrophy was minimal or absent (Fig. 5C; Supplemental Fig. 4 (3.8MB, pdf) ), resulting in a thin granulosa cell layer when compared with follicles injected with control IgG or PGF antibody (Fig. 5D). VEGFA antibody–injected follicles were very large and fluid-filled in vivo but collapsed upon fixation and dehydration (Supplemental Fig. 4 (3.8MB, pdf) ). Endothelial cells were rarely observed in the granulosa cell layer of VEGFA antibody–injected follicles and, when present, penetrated very short distances beyond the granulosa cell basement membrane (Fig. 5E and 5F). Because endothelial cells were rarely observed in the granulosa cell layer, 3D modeling of endothelial cells was not attempted.
Discussion
Regulation of ovulatory angiogenesis by vascular growth factors is an essential component of normal ovarian function, including follicle development to secondary follicle stage, antral formation, development to preovulatory size, ovulation, luteal formation, and proper luteal function (34–36; present study). PGF and VEGFA also regulate vascular permeability; both of these VEGF family members have been implicated in plasma extravasation during luteal formation (23, 37). In the absence of properly regulated angiogenesis, disorders of ovulation and the corpus luteum can manifest, including anovulatory infertility and ovarian hyperstimulation syndrome [reviewed by Fraser (38)].
PGF produced within the primate ovulatory follicle is an important mediator of the ovulatory cascade. This report shows dynamic regulation of PGF mRNA and protein in the ovulatory follicle. Follicular levels of mRNA and protein increased after the ovulatory gonadotropin surge, with granulosa cells and theca cells likely both contributing to the elevated follicular fluid levels of PGF detected 36 hours after hCG administration, or just before ovulation. Administration of hCG triggered an increase in granulosa cell PGF mRNA and protein, consistent with previous reports of PGF in follicular fluid of women undergoing fertility treatments (12, 39). The delay between hCG administration and elevated PGF mRNA in granulosa cells suggests factors in addition to the ovulatory gonadotropin surge may regulate PGF production. The ovulatory increase in follicular prostaglandins is unlikely to be involved in regulation of follicular PGF. Although hypoxia is the typical stimulus to increase PGF mRNA, analysis of the PGF gene did not reveal a hypoxia-inducible factor 1α (HIF1A) regulatory site (28). However, transcription factors, such as nuclear factor of activated T cells and nuclear factor-κB (NFκB) have been proposed to explain the sensitivity of PGF expression to hypoxic conditions (28). In retinal pigment epithelium, PGF expression is increased in response to hyperosmolarity as well as hypoxia (40). In addition, PGF was increased by aldosterone and hypoglycemia in a placental cell line (41). Identification of the key regulators of PGF protein specifically in follicular granulosa cells will require additional study.
PGF neutralization by intrafollicular injection of PGF antibody resulted in severely compromised ovulation and luteinization. Oocytes were retained in 50% of follicles, evidence of ovulation failure. PGF antibody–injected ovaries showed a variety of significant abnormalities of follicle rupture, including complete failure of rupture. This study demonstrates a critical role for PGF in oocyte release and follicle rupture. Structural luteinization was reduced, as assessed by limited granulosa cell layer hypertrophy. PGF antibody–injected follicles also showed incomplete follicular angiogenesis, with reduced endothelial cell invasion into the granulosa cell layer. 3D modeling showed that many endothelial cells were not part of capillary-like structures that connect to stromal vessels. Our findings are similar to those of Carmeliet et al. (23) who found fewer and smaller-diameter vessels formed in the corpora lutea of mice lacking PGF expression when compared with wild-type controls. PGF clearly plays an essential role in angiogenesis of the granulosa cell layer as the ovulatory follicle transforms into the corpus luteum. Overall, our in vivo studies demonstrate that PGF is essential for ovulation and early structural luteinization of the primate follicle.
The current study also confirms a key role for VEGFA in ovulatory events. VEGFA was present in both granulosa cells and theca cells, as previously reported for females in a wide variety of species, including monkeys and humans (11, 13, 14–16). Granulosa cell VEGFA mRNA increased rapidly after the LH surge, then fell to moderate levels for the remainder of the ovulatory interval. In contrast to PGF, increased levels of VEGFA were measured in follicular fluid soon after the ovulatory gonadotropin stimulus, similar to previous reports in monkeys and women (11, 17). This rapid increase in follicular VEGFA mRNA and protein is consistent with the concept that gonadotropin rapidly activates the HIF1A pathway as a primary stimulus for VEGFA expression (42). Despite altering VEGFA mRNA levels, follicular prostaglandins are probably not important regulators of VEGFA protein in primate follicles.
Our studies provide details regarding the essential role for VEGFA in ovulation, luteinization, and angiogenesis in vivo. Follicular injection of a VEGFA antibody resulted in a small rupture site or complete absence of follicle rupture, with retained oocytes identified in all injected follicles. Granulosa cell hypertrophy was minimal; the granulosa cell layer was compact and appeared similar to the granulosa cell layer of preovulatory follicles before the ovulatory gonadotropin stimulus (19). TUNEL results indicate that VEGFA antibody–injected follicles were healthy, without evidence of apoptosis. Follicular angiogenesis was minimal. Vascular endothelial cells were present primarily in the follicular stroma of VEGFA antibody–injected follicles, with very limited migration into the granulosa cell layer. Most published reports on VEGFA action in the primate ovary focus on follicle development to the antral stage or after formation of the corpus luteum (10). In the few studies designed to determine the role of VEGFA specifically in ovulation, ovulation failure after administration of the soluble VEGF receptor FLT1 or a hybrid VEGF “trap” molecule (5, 6) was interpreted to demonstrate a critical role for VEGFA in ovulation. However, these reagents can neutralize both PGF and VEGFA. The current study used an antibody to selectively neutralize VEGFA, clearly demonstrating the essential actions of VEGFA in primate ovulation and luteinization.
Endothelial cell networks observed in the primate ovulatory follicle support the concept that new capillaries form by branching angiogenesis (19; present study). Vascular growth factors, like those produced by granulosa cells and theca cells, provide the stimulus for new capillary formation. Vascular endothelial cells in existing vessels respond and migrate toward the source of these stimuli, leading growth of a new capillary. Endothelial cells adjacent to a migrating cell proliferate to become the stalk of the new capillary. Migratory endothelial cells are believed to respond to growth factors primarily via KDR, whereas endothelial cells forming the stalk respond primarily via FLT1 (26).
PGF and VEGFA action in the monkey ovulatory follicle in vivo, as determined from our antibody-injected follicles, is consistent with these roles for FLT1 and KDR in follicular angiogenesis in vivo. When PGF was neutralized, VEGFA remained available to stimulate angiogenesis. In PGF antibody–injected follicles, some endothelial cells migrated into the granulosa cell layer. However, endothelial cells did not penetrate into the granulosa cell layer as far as was seen in control IgG-injected follicles, and our 3D reconstructions showed that capillary-like stalks did not form behind migrating cells. This profile is consistent with stimulation of KDR (by available VEGFA) and limited activity at FLT1 due to PGF neutralization. Our in vivo observations are similar to our in vitro demonstration that PGF, a potent ligand for FLT1, effectively stimulated endothelial cell proliferation and formation of longer capillary stalks (20). In contrast, VEGFA antibody injection resulted in very few endothelial cells present in the granulosa cell layer. This is consistent with the key role for KDR activation in initiating endothelial cell migration, the first step in capillary spout formation (20). Although PGF is probably available to interact with FLT1 during antibody neutralization of VEGFA, PGF action at FLT1 is thought to stimulate endothelial cell proliferation and capillary stalk formation after migration is underway. Overall, these data indicate that FLT1 and KDR play distinct roles as new capillaries form in the ovulatory follicle and support the hypothesis that PGF and VEGFA each play unique and complementary roles in follicular angiogenesis in vivo.
In our in vivo studies, neither PGF nor VEGFA alone could recapitulate normal follicular angiogenesis or ovulatory function. PGF is a ligand only for FLT1 (43), consistent with the limited ability of PGF to stimulate angiogenesis. VEGFA can act at both FLT1 and KDR (43), but VEGFA alone did not stimulate ovulatory angiogenesis in vivo, similar to that seen in control IgG-injected ovaries. PGF is reported to have higher affinity for FLT1 when compared with VEGFA (44), so PGF may be a more potent ligand than VEGFA in vivo to promote angiogenic events via FLT1. Although most in vitro studies determine receptor function using homodimers of VEGF family members, heterodimers of PGF and VEGFA have also been reported in vivo (45). PGF and VEGFA heterodimers activate FLT1/KDR heterodimeric receptors, which may have additional functions in angiogenesis (46).
The temporal patterns of PGF and VEGFA accumulation in the follicle may reflect their differing roles in ovulatory angiogenesis. In the current study, PGF and VEGFA did not accumulate in granulosa cells. This is consistent with the concept that PGF and VEGFA proteins are not stored within granulosa cells but released rapidly after synthesis is complete. VEGFA levels in follicular fluid peaked early in the ovulatory interval, whereas PGF levels peaked just before ovulation. This temporal pattern is consistent with the concept that endothelial cell migration (stimulated efficiently via KDR and VEGFA) occurs before capillary stalk formation (stimulated efficiently via FLT1 and PGF) (47). Despite this attractive and simple hypothesis, local regulation of vascular growth factors within ovarian tissues is complex. Binding to extracellular matrix, glycosylation, and other factors modulates availability of VEGF family members (43). The most abundant forms of VEGFA in the monkey follicle, VEGFA165 and VEGFA121, lack the heparin-binding domain and may not be sequestered in stroma. Interactions between PGF and stroma have not been reported. Theca cells, as well as granulosa cells, produce both PGF and VEGFA (48–50; present study). Theca cells are located very near the stromal vessels. These stromal vessels are the source of vascular endothelial cells for ovulatory angiogenesis, so theca cell production of PGF and VEGFA may be critical for initiation of ovulatory angiogenesis. In short, complex dynamics probably control availability of PGF and VEGFA to interact with endothelial cell VEGFRs. However, additional studies will be needed to demonstrate that changing follicular levels of PGF and VEGFA are required for follicular angiogenesis or ovulation.
Angiogenesis of the follicle is critical, not just for ovulation but also for development and function of the corpus luteum. In the current study, antibody-injected ovaries were removed on the equivalent of luteal day 2 to optimally assess oocyte release, follicle rupture, luteinization, and early events in follicular angiogenesis. Mature luteal function cannot be assessed in these studies. Serum progesterone levels at the time of ovary removal did not differ between treatment groups, but lower mean serum progesterone levels after PGF or VEGFA neutralization suggest that luteal function may be somewhat compromised after neutralization of PGF or VEGFA within the follicle. Additional studies would be needed permit full assessment of luteal function in the absence of PGF or VEGFA.
Acknowledgments
The authors thank Priyanka Aytoda, Genevieve Campbell, and Angela Pham for technical assistance. Recombinant human FSH and Ganirelix were generously provided by Merck & Co. Serono Reproductive Biology Institute kindly provided recombinant human LH and Antide. The Eunice Kennedy Shriver National Institutes of Child Health and Human Development provided Acyline.
Financial Support: This study was supported by The Eunice Kennedy Shriver National Institutes of Health and Human Development (Grant P01 HD071875 to D.M.D.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| vWF | — | vWF | Dako, A0082 | Rabbit; polyclonal | 0.005 mg/mL | AB_2315602 |
| VEGFA | — | VEGFA | Abbiotec, 251901 | Rabbit; polyclonal | 0.002–0.005 mg/mL | AB_10635879 |
| PGF | — | PGF | Abbiotec, 250825 | Rabbit; polyclonal | 0.02–0.005 mg/mL | AB_2161050 |
| None | — | Control rabbit IgG | Abbiotec, 254513 | Rabbit; polyclonal | None | AB_2715565 |
| CYP17A1 | — | CYP17A1 | Dr. Michael Waterman, Vanderbilt University School of Medicine | Rabbit; polyclonal | 1:2000 | AB_2715566 |
| Ki67 | — | Ki67 | Dako, M7240, clone MIB-1 | Mouse; monoclonal | 0.00035 mg/mL | AB_2142367 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- 3D
- three-dimensional
- ACTB
- β-actin
- ANOVA
- analysis of variance
- CV
- coefficient of variation
- ELISA
- enzyme-linked immunosorbent assay
- FLT1
- fms-related tyrosine kinase 1
- FLT4
- fms-related tyrosine kinase 4
- FSH
- follicle-stimulating hormone
- hCG
- human chorionic gonadotropin
- HIF1A
- hypoxia-inducible factor 1α
- IgG
- immunoglobulin G
- KDR
- kinase insert domain receptor
- LH
- luteinizing hormone
- mRNA
- messenger RNA
- NFκB
- nuclear factor-κB
- PCR
- polymerase chain reaction
- PGF
- placental growth factor
- PTGS2
- prostaglandin G/H synthase and cyclooxygenase
- TUNEL
- terminal deoxynucleotidyl transferase–mediated (2′-deoxyuridine, 5′-triphosphate) nick end labeling
- VEGF
- vascular endothelial growth factor
- VEGFA
- vascular endothelial growth factor A
- VEGFR
- vascular endothelial growth factor receptor
- vWF
- von Willebrand factor.
References
- 1.Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141(3):995–1000. [DOI] [PubMed] [Google Scholar]
- 2.Wulff C, Wilson H, Rudge JS, Wiegand SJ, Lunn SF, Fraser HM. Luteal angiogenesis: prevention and intervention by treatment with vascular endothelial growth factor trap(A40). J Clin Endocrinol Metab. 2001;86(7):3377–3386. [DOI] [PubMed] [Google Scholar]
- 3.Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor Trap(A40)). Endocrinology. 2001;142(7):3244–3254. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab. 2001;86(2):768–772. [DOI] [PubMed] [Google Scholar]
- 5.Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143(7):2797–2807. [DOI] [PubMed] [Google Scholar]
- 6.Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67(4):1305–1312. [DOI] [PubMed] [Google Scholar]
- 7.Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71(4):239–248. [DOI] [PubMed] [Google Scholar]
- 8.Hazzard TM, Rohan RM, Molskness TA, Fanton JW, D’Amato RJ, Stouffer RL. Injection of antiangiogenic agents into the macaque preovulatory follicle: disruption of corpus luteum development and function. Endocrine. 2002;17(3):199–206. [DOI] [PubMed] [Google Scholar]
- 9.Einspanier R, Schönfelder M, Müller K, Stojkovic M, Kosmann M, Wolf E, Schams D. Expression of the vascular endothelial growth factor and its receptors and effects of VEGF during in vitro maturation of bovine cumulus-oocyte complexes (COC). Mol Reprod Dev. 2002;62(1):29–36. [DOI] [PubMed] [Google Scholar]
- 10.Fraser HM, Duncan WC. Vascular morphogenesis in the primate ovary. Angiogenesis. 2005;8(2):101–116. [DOI] [PubMed] [Google Scholar]
- 11.Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5(12):1115–1121. [DOI] [PubMed] [Google Scholar]
- 12.Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Regulation of vascular endothelial growth factor-A and its soluble receptor sFlt-1 by luteinizing hormone in vivo: implication for ovarian follicle angiogenesis. Fertil Steril. 2008;89(4):922–926. [DOI] [PubMed] [Google Scholar]
- 13.Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Høst T, Borup R, Grøndahl ML. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29(5):997–1010. [DOI] [PubMed] [Google Scholar]
- 14.Mauro A, Martelli A, Berardinelli P, Russo V, Bernabò N, Di Giacinto O, Mattioli M, Barboni B. Effect of antiprogesterone RU486 on VEGF expression and blood vessel remodeling on ovarian follicles before ovulation. PLoS One. 2014;9(4):e95910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury MWH, Scaramuzzi RJ, Wheeler-Jones CPD, Khalid M. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology. 2010;73(7):856–872. [DOI] [PubMed] [Google Scholar]
- 16.Miyabayashi K, Shimizu T, Kawauchi C, Sasada H, Sato E. Changes of mRNA expression of vascular endothelial growth factor, angiopoietins and their receptors during the periovulatory period in eCG/hCG-treated immature female rats. J Exp Zoolog A Comp Exp Biol. 2005;303(7):590–597. [DOI] [PubMed] [Google Scholar]
- 17.Baskind NE, Orsi NM, Sharma V. Follicular-phase ovarian follicular fluid and plasma cytokine profiling of natural cycle in vitro fertilization patients. Fertil Steril. 2014;102(2):410–418. [DOI] [PubMed] [Google Scholar]
- 18.Christenson LK, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab. 1997;82(7):2135–2142. [DOI] [PubMed] [Google Scholar]
- 19.Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod. 2015;92(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trau HA, Brännström M, Curry TEJ Jr, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod. 2016;31(2):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araújo VR, Duarte AB, Bruno JB, Pinho Lopes CA, de Figueiredo JR. Importance of vascular endothelial growth factor (VEGF) in ovarian physiology of mammals. Zygote. 2013;21(3):295–304. [DOI] [PubMed] [Google Scholar]
- 22.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7):a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. [DOI] [PubMed] [Google Scholar]
- 24.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255(5047):989–991. [DOI] [PubMed] [Google Scholar]
- 25.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39(4-5):225–237. [DOI] [PubMed] [Google Scholar]
- 26.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SO, Trau HA, Duffy DM. Vascular endothelial growth factors C and D may promote angiogenesis in the primate ovulatory follicle. Biol Reprod. 2017;96(2):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seachord CL, VandeVoort CA, Duffy DM. Adipose differentiation-related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod. 2005;72(6):1305–1314. [DOI] [PubMed] [Google Scholar]
- 30.Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: implications for control of primate ovulation by prostaglandin E2. J Clin Endocrinol Metab. 2005;90(2):1021–1027. [DOI] [PubMed] [Google Scholar]
- 31.Kim SO, Harris SM, Duffy DM. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology. 2014;155(4):1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy DM, Seachord CL, Dozier BL. Microsomal prostaglandin E synthase-1 (mPGES-1) is the primary form of PGES expressed by the primate periovulatory follicle. Hum Reprod. 2005;20(6):1485–1492. [DOI] [PubMed] [Google Scholar]
- 33.Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21(5):652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFee RM, Rozell TG, Cupp AS. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 2012;349(3):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter MG, Robinson RS, Mann GE, Webb R. Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci. 2004;82-83:461–477. [DOI] [PubMed] [Google Scholar]
- 36.Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. 2004;82-83:127–140. [DOI] [PubMed] [Google Scholar]
- 37.Herr D, Bekes I, Wulff C. Regulation of endothelial permeability in the primate corpora lutea: implications for ovarian hyperstimulation syndrome. Reproduction. 2015;149(2):R71–R79. [DOI] [PubMed] [Google Scholar]
- 38.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tal R, Seifer DB, Grazi RV, Malter HE. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: correlation with ovarian stimulation. Reprod Biol Endocrinol. 2014;12(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollborn M, Reichmuth K, Prager P, Wiedemann P, Bringmann A, Kohen L. Osmotic induction of placental growth factor in retinal pigment epithelial cells in vitro: contribution of NFAT5 activity. Mol Biol Rep. 2016;43(8):803–814. [DOI] [PubMed] [Google Scholar]
- 41.Eisele N, Albrecht C, Mistry HD, Dick B, Baumann M, Surbek D, Currie G, Delles C, Mohaupt MG, Escher G, Gennari-Moser C. Placental expression of the angiogenic placental growth factor is stimulated by both aldosterone and simulated starvation. Placenta. 2016;40:18–24. [DOI] [PubMed] [Google Scholar]
- 42.Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12(1):297–307. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. [DOI] [PubMed] [Google Scholar]
- 44.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269(41):25646–25654. [PubMed] [Google Scholar]
- 45.Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98(11):2507–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, Miller MR, Newby DE, Gu Y, Barleon B, Weich H, Ahmed A. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:972. [DOI] [PubMed] [Google Scholar]
- 47.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–953. [DOI] [PubMed] [Google Scholar]
- 48.Koos RD. Increased expression of vascular endothelial growth/permeability factor in the rat ovary following an ovulatory gonadotropin stimulus: potential roles in follicle rupture. Biol Reprod. 1995;52(6):1426–1435. [DOI] [PubMed] [Google Scholar]
- 49.Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and localisation of vascular endothelial growth factor and basic fibroblast growth factor during the final growth of bovine ovarian follicles. J Endocrinol. 2000;167(3):371–382. [DOI] [PubMed] [Google Scholar]
- 50.Abir R, Ao A, Zhang XY, Garor R, Nitke S, Fisch B. Vascular endothelial growth factor A and its two receptors in human preantral follicles from fetuses, girls, and women. Fertil Steril. 2010;93(7):2337–2347. [DOI] [PubMed] [Google Scholar]