Abstract
Neutrophils are essential effector cells of the innate immune system that have recently been recognized as thyroid hormone (TH) target cells. Cellular TH bioavailability is regulated by the deiodinase enzymes, which can activate or inactivate TH. We have previously shown that the TH inactivating enzyme type 3 deiodinase (D3) is present in neutrophils. Furthermore, D3 knockout (D3KO) mice show impaired bacterial killing upon infection. We hypothesized that D3 plays a role in neutrophil function during infection by actively regulating local TH availability. We measured TH concentrations in cerebrospinal fluid (CSF) from patients with bacterial meningitis and controls. Bacterial meningitis resulted in marked changes in CSF TH levels, characterized by a strong increase of thyroxine and reverse-triiodothyronine concentrations. This altered TH profile was consistent with elevated D3 activity in infiltrating neutrophils at the site of infection. D3 knockdown in zebrafish embryos with pneumococcal meningitis resulted in increased mortality and reduced neutrophil infiltration during infection. Finally, stimulated neutrophils from female D3KO mice exhibited impaired NADPH-oxidase activity, an important component of the neutrophil bacterial killing machinery. These consistent findings across experimental models strongly support a critical role for reduced intracellular TH concentrations in neutrophil function during infection, for which the TH inactivating enzyme D3 appears essential.
The thyroid hormone inactivating type 3 deiodinase (D3) is important for neutrophil function as demonstrated in vivo during bacterial infection and ex vivo in female D3KO neutrophils.
Neutrophils are crucial for the host defense against invading pathogens. These highly specialized innate immune cells are capable of phagocytosing and killing bacteria and other microorganisms (1). Neutrophil function is of fundamental importance for survival, as illustrated by the detrimental clinical consequences of an impaired neutrophil bacterial killing machinery (2). Innate immune cells, including neutrophils, have recently been recognized as novel thyroid hormone (TH) target cells (3).
TH is essential for growth and development (4). The thyroid gland secretes TH into the circulation, mainly in the form of the prohormone thyroxine (T4). T4 requires conversion to the active hormone triiodothyronine (T3) to exert its biological activity (4). The conversion of TH at the cellular and tissue level is largely regulated by the deiodinase enzymes (5). This family of enzymes can either activate or inactive TH within the cell, thus controlling intracellular TH bioavailability. The TH inactivating enzyme type 3 deiodinase (D3) converts T4 and T3 to their inactive metabolites [reverse T3 (rT3) and 3,3′-diiodothyronine (T2), respectively] (5). Due to its high expression in infiltrating murine and human neutrophils, D3 is thought to play an important role in their function (3, 6, 7). In addition, mice that lack D3 demonstrate impaired bacterial clearance following infection (8). These data suggest that D3 is important for bacterial killing, a hallmark of neutrophil function.
We hypothesized that D3 plays a role in neutrophil function during bacterial infection by actively regulating local TH bioavailability. Here we show that bacterial meningitis results in an altered TH profile at the site of infection, consistent with elevated D3 activity in infiltrating neutrophils. In addition, a lack of D3 results in impaired survival during bacterial meningitis in a zebrafish model. Finally, neutrophils derived from D3 knockout (D3KO) mice display functional abnormalities compared with wild-type (WT) cells. Our data suggest that neutrophil function during infection requires strict control of TH availability, for which D3 appears essential.
Materials and Methods
Study approval
All animal and human experiments were conducted in compliance with institutional and (inter)national guidelines and regulations. In the case of the Dutch Bacterial Meningitis Cohort, written informed consent was obtained from all participating patients or their legally authorized representatives prior to study inclusion (9).
Human subjects
Cerebrospinal fluid (CSF) samples were derived from the Dutch Bacterial Meningitis Cohort, which includes adults with community-acquired bacterial meningitis with positive CSF cultures. Samples were obtained from the initial diagnostic lumbar puncture. These patients were identified by the Netherlands Reference Laboratory for Bacterial Meningitis as described in detail previously (10). The study protocol was approved by the Academic Medical Center Medical Ethical Committee, and written informed consent was obtained from all participating patients or their legally authorized representatives prior to study inclusion. Control samples were derived from residual CSF obtained for diagnostic purposes from patients with thunderclap headache in whom a lumbar puncture was performed to rule out subarachnoid hemorrhage. Control CSF samples were used anonymously, in accordance with the Dutch Code of Conduct for the Secondary Use of Human Tissue (version 2011) issued by the Federation of Dutch Medical Scientific Societies. The full code of conduct is available online (https://www.federa.org/sites/default/files/images/print_version_code_of_conduct_english.pdf).
Thyroid hormone measurements in CSF
Thyroid hormone concentrations in CSF were measured using liquid chromatography–tandem mass spectrometry, as described previously (11, 12), with some minor modifications detailed below. Samples were deproteinized by adding 100 µL acetonitrile to 30 µL CSF together with 5 µL 13C6-labeled internal standards. Following centrifugation, supernatants were transferred to glass tubes and dried using a SpeedVac. The residue was resuspended in 50 µL 0.1% NH4OH, and TH concentrations were measured on an Acquity UPLC–Xevo TQ-S tandem mass spectrometer system (Waters) as previously described for tissue (11, 12). The detection limit of our assay was ±0.1 nmol/L. Samples were measured in consecutive runs, and the same control samples were included in all runs to detect interassay variation. Outcome was defined based on the Glasgow Outcome Scale (GOS) (13). Scoring is as follows: 1 = death, 2 = vegetative state, 3 = severe disability, 4 = moderate disability, and 5 = mild or no disability. Unfavorable and favorable outcomes were defined as a GOS of 1 to 4 and a GOS of 5, respectively.
Zebrafish embryo care and deiodinase knockdown
Zebrafish handling, embryo care, and microinjections were performed as described previously (14). Embryos from the transparent casper zebrafish line [Tg(mitfaw2/w2;roya9/a9)] and the Tg(mpx:GFP)i114 line with green fluorescent protein–labeled neutrophils were used. An antisense oligonucleotide morpholino (MO) knockdown approach was used to transiently block D3 expression as described previously (15). A standard control morpholino (SCMO) was used as a control. All MOs were purchased from Gene Tools. MO sequences were derived from Heijlen et al. (15) [D3 morpholino (D3MO) against dio3b: 5′-CTGCGGAGCCCTGCAGCATCTCCAT-3′; SCMO: 5′-CCTCTTACCTCAGTTACAATTTATA-3′]. The MO solutions were prepared in sterile distilled water and 0.5% (w/v) phenol red solution (Sigma-Aldrich; P0290) to aid visualization of the injection process. The optimal concentration of 0.4 mM and injection volume of 2 nL were used as previously described (15). The volume was injected into the yolk of one- to four-cell stage embryos, resulting in a delivery of ∼6.6 ng MO per embryo. Embryos were raised at 28°C in E3 medium (5.0 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl·2H2O, 0.33 mM MgCl2·6H2O) supplemented with 0.3 mg/L methylene blue. The specificity of the D3MO used here has been previously demonstrated by rescue experiments using human D3 messenger RNA (15) and resulted in a decrease in D3 activity levels of 96%. Zebrafish were handled in compliance with the local animal welfare regulations and maintained according to standard protocols (zfin.org). All protocols adhered to the international guidelines specified by the European Council Directive 86/609/EEC and were conducted in compliance with institutional and national guidelines and regulations.
Bacterial strains and culture
Streptococcus pneumoniae D39 serotype 2 WT strain and a red fluorescent S. pneumoniae D39 HlpA-mCherry mutant strain were used (16, 17). The bacterial culture method and injection process has been described in detail previously (14). Briefly, bacteria were grown overnight on Columbia agar plates supplemented with 5% defibrinated sheep blood in a humidified atmosphere with 5% CO2. Chloramphenicol (4.5 µg/mL) was added to agar plates for selection of the HlpA-mCherry mutant strain. Following overnight culture, bacteria were grown to mid-log phase in Todd Hewitt broth with yeast extract, harvested by centrifugation, washed, and suspended in sterile 0.5% (w/v) phenol red solution (Sigma-Aldrich) to aid visualization of the injection process. The number of colony-forming units per injection was determined by quantitative plating of the injection volume.
Infection of zebrafish embryos
D3MO and SCMO knockdown casper zebrafish embryos were infected with 500 colony-forming units of WT S. pneumoniae D39 in the hindbrain ventricle as previously described, resulting in pneumococcal meningitis (14). The mortality rate was determined by monitoring live and dead embryos at fixed time points between 24 and 96 hours postinfection. The experiment was repeated three times independently (total n = 60 embryos per group).
Zebrafish embryo imaging and analysis
D3MO and SCMO knockdown Tg(mpx:GFP)i114 zebrafish embryos expressing green fluorescent neutrophils were injected with red fluorescent S. pneumoniae D39 HlpA-mCherry mutant and harvested at fixed time points between 1 and 5 hours postinfection. Total neutrophil count was measured in D3MO and SCMO knockdown Tg(mpx:GFP)i114 zebrafish embryos at 2 days postfertilization. Embryos were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS). For optimal imaging, embryos were embedded in 1% low-melting-point agarose dissolved in PBS in an open uncoated 8-well microscopy μ-Slide (http://ibidi.com). Confocal images were generated with a Leica TCS SP8 Confocal Microscope and analyzed using Leica Application Suite X software and ImageJ.
D3KO mice care and procedures
D3KO mice in a CD-1 background harboring a previously described mutation were used (18). As D3KO mice are subfertile (18), D3KO animals were generated using heterozygous breeders. WT littermates were used as controls. Both female and male WT and D3KO mice were used. Animals were housed under 12-hour light/12-hour dark cycles and had ad libitum access to water and regular chow. Adult mice (3 to 5 months old) were killed using CO2 asphyxiation. Bone marrow was isolated immediately from femurs and tibias and processed for flow cytometry staining or neutrophil isolation. Procedures were approved by the Institution of Animal Care and Use Committee at Maine Medical Center Research Institute.
Neutrophil isolation and culture
Neutrophils were isolated from whole bone marrow using the mouse neutrophil isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions. Following isolation, neutrophils were washed in PBS (room temperature), resuspended in HEPES medium (19), supplemented with 1 mg/mL glucose, and immediately used for further experiments. Neutrophil purity was assessed using flow cytometry staining for the highly specific murine neutrophil marker Ly6G (20) (clone 1A8; BD Biosciences) and was always at least 90%.
Flow cytometric analysis of whole bone marrow
Whole bone marrow was stained using a panel of fluorescently labeled antibodies (listed in Table 1). All samples were incubated with mouse FC block (BD Biosciences) prior to staining, and relevant isotype control antibodies were used to control for background staining. Bone marrow cell populations from both D3KO and WT mice were quantified using a MACSQuant flow cytometer (Miltenyi Biotec), and data were analyzed using FlowJo software (version 10; FlowJo, LLC). Flow cytometry gating of whole bone marrow is detailed in Supplemental Fig. 1 (620KB, pdf) . Cells that expressed CD19, CD335, or CD3e were excluded from analysis [markers for B cells (21), natural killer cells (22), and T cells (23), respectively]. The following populations were identified: early hematopoietic blast cells CD117+/CD11b−, neutrophil precursors CD117+/CD11b+, monocyte precursors Ly-6G−/Ly-6C+/CD11blo, monocyte Ly-6G−/Ly-6C+/CD11bhi, and neutrophil Ly-6G+/Ly-6C−/CD11bhi.
Table 1.
Flow Cytometry Antibodies
| Protein Target | Fluorescent Conjugate | Species Raised in; Monoclonal or Polyclonal | Clone | Catalog No. | Manufacturer |
|---|---|---|---|---|---|
| Ly-6G | PerCP-Cy5.5 | Rat IgG2A; monoclonal | 1A8 | 560602 | BD Biosciences |
| Ly-6C | PE | Rat IgG2c; monoclonal | HK1.4 | 12-5932 | eBioscience |
| CD117 (cKit) | APC | Rat IgG2b; monoclonal | 2B8 | 17-1171 | eBioscience |
| CD11b | APC-Cy7 | Rat IgG2b; monoclonal | M1/70 | 557657 | BD Biosciences |
| CD19 | PE-Cy7 | Rat IgG2A; monoclonal | 1D3 | 25-0193 | eBioscience |
| CD335 | PE-Cy7 | Rat IgG2A; monoclonal | 29A1.4 | 25-3351 | eBioscience |
| CD3e | PE-Cy7 | Armenian hamster; monoclonal | 145-2C11 | 25-0031 | eBioscience |
| CD16/CD32 (FC block) | Rat IgG2A; monoclonal | 93 | 14-0161 | eBioscience |
Analysis of neutrophil counts in whole blood
After euthanasia, blood was extracted from the cava vein of D3KO and WT mice, and 40 µL of each sample was collected in EDTA microtainer tubes. Samples were placed on ice and used no later than 4 hours after extraction. When ready for analysis, samples were mixed 10 times by inversion and analyzed automatically using a ProCyte DX Hematology Analyzer (IDEXX Laboratories).
Neutrophil apoptosis
Freshly isolated neutrophils (2 × 105/mL) were incubated under end-over-end rotation at 37°C. Samples were harvested at fixed timepoints and stained for Annexin V and propidium iodide using the Annexin V apoptosis detection kit (eBioscience). Samples were run on a MACSQuant flow cytometer (Miltenyi Biotec), and data were analyzed using FlowJo software (version 10; FloJo, LLC).
Neutrophil phagocytosis
Zymosan bioparticles fluorescently labeled with the pH-sensitive fluorophore pHrodo green (Molecular Probes) were opsonized using opsonizing reagent (Molecular Probes) according to the manufacturer’s instructions. Zymosan is a yeast particle and phagocytic stimulus. Neutrophils were incubated with opsonized pHrodo-conjugated zymosan [multiplicity of infection (MOI) = 5] at 37°C or on ice (as a control) for 1 hour. Fluorescence was quantified using a MACSQuant flow cytometer (Miltenyi Biotec) and FlowJo software (version 10; FlowJo, LLC). Flow cytometry gating of phagocytosing neutrophils is detailed in Supplemental Fig. 2 (620KB, pdf) . Neutrophils in the FITC+ gate were considered phagocytosing neutrophils.
Neutrophil NAPDH oxidase activity
Extracellular H2O2 production was determined as a measure for NADPH oxidase activity using the Amplex Red Hydrogen Peroxide Kit (Molecular Probes). Neutrophils (2 × 104) were incubated with phorbol 12-myristate 13-acetate (PMA; 100 ng/mL) in the presence of Amplex Red (50 µM) and horseradish peroxidase (0.1 U/mL). Fluorescence (excitation 535 nm; emission 595 nm) was measured at 2-minute intervals for 30 minutes using a Gen5 2.09 microplate reader (BioTek).
Statistics
Statistical tests used are described in detail in the relevant figure legends. P values <0.05 were considered statistically significant. Tests were performed using GraphPad Prism 7 (GraphPad Software) or SPSS version 23 (SPSS, Inc.).
Results
Bacterial meningitis alters TH concentrations in the CSF
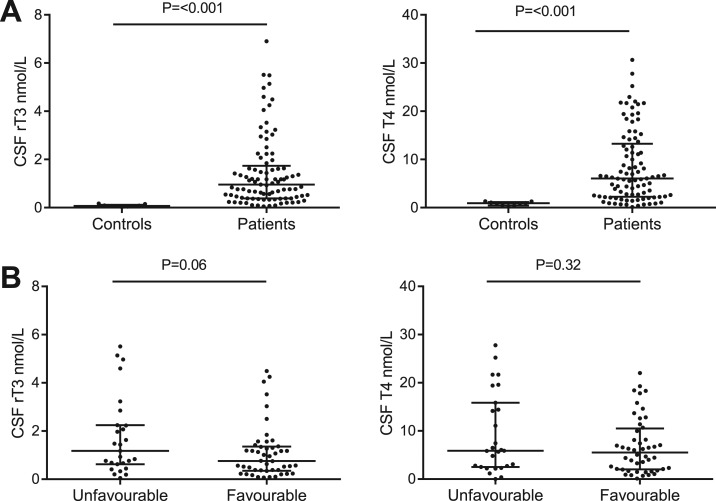
To study whether infiltrating neutrophils can alter TH concentrations at the site of infection, we measured TH concentrations in CSF from the diagnostic lumbar puncture of patients with bacterial meningitis (n = 95) vs controls. CSF samples from patients with benign thunderclap headache (n = 9) who had undergone a diagnostic lumbar puncture to rule out subarachnoid hemorrhage were used as a control. Bacterial meningitis resulted in profound changes in CSF TH concentrations. Both T4 and rT3 were strongly elevated in CSF from bacterial meningitis patients compared with controls (Fig. 1A). T3, 3,5-T2 and 3,3′-T2 were undetectable in all samples (detection limit ±0.1 nmol/L). In addition, we analyzed whether CSF TH concentrations were correlated with clinical outcome. No statistically significant differences in CSF TH concentrations were found among patients with bacterial meningitis patients who had an unfavorable or a favorable outcome, as defined on the GOS (Fig. 1B).
Figure 1.
Bacterial meningitis changes thyroid hormone profile in CSF. (A) TH concentrations measured using liquid chromatography–tandem mass spectrometry in CSF samples from patients with bacterial meningitis (n = 93) and controls with benign thunderclap headache (n = 9). T3 and T2 were undetectable in all samples (<±0.1 nmol/L). (B) Comparison of CSF T4 and rT3 levels in CSF from patients with bacterial meningitis who had an unfavorable outcome (n = 27) and patients with a favorable outcome (n = 48). No outcome data were available for 18 patients. Data are presented as median ± IQR. P values for Mann-Whitney U test are indicated.
The median value of CSF T4 was 6.04 nmol/L [interquartile range (IQR), 2.31 to 12.81 nmol/L] in patients with bacterial meningitis, significantly higher than the 0.92 nmol/L (IQR, 0.44 to 1.16 nmol/L) found in CSF from controls. CSF rT3 concentrations were also higher during bacterial meningitis (median, 1.01 nmol/L; IQR, 0.38 to 1.84 nmol/L) compared with controls (median, 0.07 nmol/L; IQR, 0.055 to 0.12 nmol/L).
D3 knockdown impairs zebrafish embryo survival and neutrophil migration during bacterial meningitis
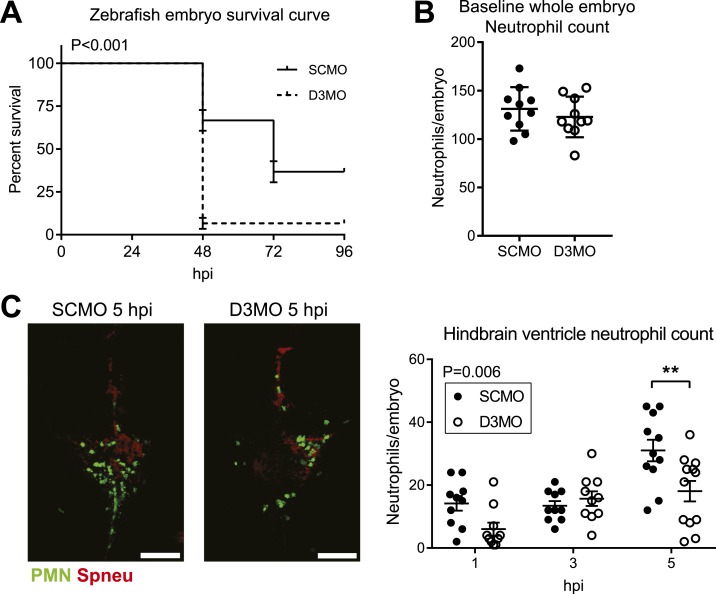
As infiltrating neutrophils appear to affect TH concentrations at the site of infection, we next assessed whether cellular TH bioavailability could also affect neutrophil function in vivo. We combined a validated model for knockdown of D3 in zebrafish embryos using MO oligonucleotide gene knockdown technology (15) with an established zebrafish embryo model for pneumococcal meningitis (14) and determined the effect of modulating TH bioavailability during bacterial meningitis. Following treatment with a D3MO or an SCMO, zebrafish embryos were injected with S. pneumoniae D39, serotype 2, in the hindbrain ventricle. This injection leads to meningitis (14). In the D3MO zebrafish embryos, survival of pneumococcal meningitis was significantly decreased (Fig. 2A). In addition, although the whole-body neutrophil count at baseline did not differ between the groups (Fig. 2B), the amount of infiltrating neutrophils at the site of infection was lower in D3MO zebrafish embryos compared with controls (Fig. 2C).
Figure 2.
Effect of D3 knockdown during pneumococcal meningitis in zebrafish embryos. (A) Survival curve for zebrafish embryos treated with SCMO (n = 60) or D3MO (n = 60) and infected with approximately 500 colony-forming units of WT S. pneumoniae. Pooled data from three independent experiments are shown. Error bars represent standard error. P value for log-rank (Mantel-Cox) test is indicated. hpi, hours postinfection. (B) Quantification of whole-body baseline neutrophils in uninfected D3MO and SCMO Tg(mpx:GFP) zebrafish embryos with fluorescent neutrophils from confocal microscopy images. (C) Confocal microscopy images of S. pneumoniae D39 HlpA-mCherry–infected D3MO and SCMO Tg(mpx:GFP) zebrafish embryos with fluorescent neutrophils (green) and pneumococci (red). Scale bar = 100 µm. Quantification of neutrophils in the hindbrain ventricle from confocal microscopy images (n = 10 to 12 embryos per group). Data represent mean ± standard error of the mean. P value for two-way analysis of variance is indicated. Post hoc analysis (Bonferroni) P value: **P < 0.01. PMN, polymorphonuclear leukocytes (neutrophils).
D3KO neutrophils exhibit impaired NADPH oxidase activity
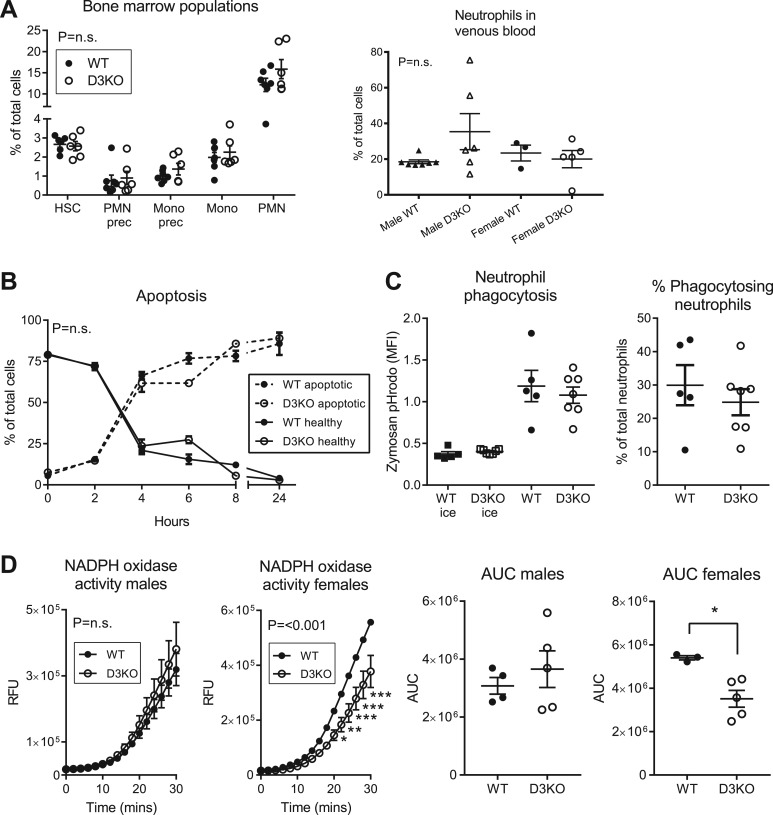
To determine whether D3 does indeed play a functional role in neutrophils, we assessed neutrophil function in D3KO mice and their WT littermates. Impaired neutrophil function could be due to reduced availability of neutrophils caused by a defect in neutrophil production by the bone marrow. Therefore, we studied the hematopoietic populations present in the bone marrow of D3KO and WT mice. There were no differences in relative amounts of bone marrow neutrophils between D3KO and WT mice or in amounts of other cell types of the hematopoietic lineage (Fig. 3A). The percentage of circulating neutrophils in venous blood was also unchanged in D3KO mice (Fig. 3A).
Figure 3.
Neutrophil function in D3KO mice. (A) Hematopoietic bone marrow populations in D3KO mice (n = 6) and WT littermates (n = 7) quantified using flow cytometry. Percentage of total cells is shown for early hematopoietic blast cells, neutrophil precursors (PMN prec), monocyte precursors (mono prec), monocytes (mono), and neutrophils (PMN). See Supplemental Fig. 1 (620KB, pdf) for gating. Neutrophils were quantified in venous blood derived from the cava vein of D3KO (n = 6 male, n = 5 female) and WT (n = 7 male, n = 3 female) mice. (B) Ex vivo survival of neutrophils incubated at 37°C. Samples were stained for Annexin V and propidium iodide (PI). Percentage of healthy cells (Annexin V−/PI−) and apoptotic cells (Annexin V+) are shown (n = 3 to 4 mice per genotype per time point). (C) Neutrophils (D3KO, n = 7; WT, n = 5) were incubated with zymosan fluorescently labeled with pHrodo green (MOI = 5) for 1 hour at 37°C or on ice. pHrodo+ cells (see Supplemental Fig. 2 (620KB, pdf) for gating) were considered phagocytosing neutrophils. MFI, median fluorescence intensity. (D) D3KO and WT neutrophils were incubated with PMA. Fluorescence, indicating H2O2 release, was measured. The effect of D3KO on NADPH oxidase activity was present only in cells from female mice (D3KO, n = 5; WT, n = 3) and was not observed in cells from male mice (D3KO, n = 5; WT, n = 4). Area under the curve (AUC) was analyzed using the unpaired two-tailed Student t test (*P < 0.05). RFU, relative fluorescent unit. (A–D) All data represent mean ± standard error of the mean. The n values indicate number of animals used. Assays were performed in triplicate. Experiments were repeated independently two to four times. P values for two-way analysis of variance are indicated. Post hoc analysis (Bonferroni) P values: *P < 0.05, **P < 0.01, ***P < 0.001. PMN, polymorphonuclear leukocytes.
To determine whether D3 directly affects neutrophil function, we isolated neutrophils from the bone marrow of D3KO and WT mice and assessed neutrophil survival, phagocytosis of zymosan particles, and NADPH oxidase activity ex vivo. Neutrophil survival and phagocytosis were both unchanged in D3KO mice (Fig. 3B and 3C). In contrast, NADPH oxidase activity, as measured by the cells’ ability to produce H2O2 upon stimulation, was impaired in D3KO neutrophils (Fig. 3D). Interestingly, the reduction in NADPH oxidase activity was due to the effect in female animals (Fig. 3D). Following this finding, we reanalyzed the hematopoietic bone marrow and neutrophil phagocytosis data, taking into account the sex of the animals. No difference was found between D3KO and WT animals when only comparing animals of the same sex. Neutrophil survival could not be reanalyzed as there were insufficient female samples to analyze the data separately for both sexes.
Discussion
There is increasing evidence that innate immune cells are important TH target cells (3). Neutrophils are known to contain the TH inactivating enzyme D3 (6, 7, 24). As mice that lack D3 exhibit impaired bacterial killing capacity (8), D3 was hypothesized to play an important role in these cells. To our knowledge, the current study is the first to demonstrate a functional role for D3 in neutrophils. In addition, our data suggest that D3, and thus adequate regulation of intracellular TH levels, plays a crucial role in neutrophil function during infection in vivo.
Bacterial meningitis results in profound changes in CSF TH concentrations. Bacterial meningitis is a severe infectious disease of the central nervous system that is associated with substantial morbidity and mortality (9, 25) and characterized by high neutrophil infiltration into the CSF (26). The most common causative pathogen of bacterial meningitis is S. pneumoniae (70% of cases) (9).
Patients with bacterial meningitis had significantly higher levels of T4 in their CSF. As T4 is not generated locally but produced by the thyroid exclusively, the T4 observed in the CSF during bacterial meningitis must originate from the systemic circulation. This could be due to increased permeability of the blood-brain barrier, which is commonly observed during bacterial meningitis (26). Furthermore, T4 is known to concentrate at a localized site of infection, as seen in a pulmonary abscess (27). During illness, serum TH levels undergo profound changes characterized by decreased serum T3 and T4 concentrations and increased rT3 concentrations (28). This is known as nonthyroidal illness syndrome (NTIS) and is correlated with illness severity and outcome in a wide range of diseases (28). During NTIS, serum T4 concentrations can drop as low as 40 to 45 nmol/L (29) (reference range in our laboratory: 70 to 150 nmol/L). The concentration of CSF T4 is approximately 2.5% of serum T4 in healthy euthyroid humans (30). Assuming that all CSF T4 is derived from the circulation, this would mean that during bacterial meningitis, at least 15% of the serum T4 concentration leaks to the CSF compartment.
Higher rT3 concentrations were also observed in the CSF of patients with bacterial meningitis compared with controls. Even though serum rT3 concentrations are expected to increase during bacterial meningitis, the concentrations we find in the CSF are too high to be explained by leakage from the circulation. Serum rT3 values can increase up to approximately 2 nmol/L during NTIS (29, 31) (reference range in our laboratory: 0.11 to 0.44 nmol/L). The median CSF rT3 concentration found in patients with bacterial meningitis is thus ∼50% of the serum rT3 concentrations found during NTIS. As this is much higher than the 15% of serum T4 found in the CSF, this cannot be explained by leakage from the circulation but is most likely the result of conversion of T4 by D3 within the CSF. A study in nonhuman primates demonstrated that the mean increase in CSF rT3 and CSF T4 after intravenous injection of the respective hormones was similar or even slightly lower for rT3, indicating that the transport from the serum to the CSF compartment is normally comparable for T4 and rT3 (32). Neutrophils contain substantial amounts of D3 (7), and bacterial meningitis is characterized by large-scale neutrophil infiltration into the CSF (26). Therefore, we conclude that changes in the TH profile of CSF during bacterial meningitis are very likely explained by leakage of T4 from the circulation due to increased blood-brain barrier permeability followed by local inactivation of T4 to rT3 by D3 in infiltrating neutrophils. These results suggest that infiltrating neutrophils are capable of altering TH concentrations at the site of infection.
D3 knockdown in zebrafish embryos results in impaired survival during pneumococcal meningitis as well as in reduced numbers of neutrophils at the site of infection. These results suggest that D3 is essential for the immune response against bacterial infection in vivo. This is in accordance with previous data in D3KO mice showing increased bacterial load after pulmonary infection with S. pneumoniae compared with WT mice (8). The effects we observed in zebrafish are likely to be caused by impaired neutrophil function due to changes in intracellular TH concentrations in these important TH target cells. Although baseline whole-body neutrophil count is not affected by D3 knockdown, D3MO zebrafish exhibit less infiltrating neutrophils at the site of infection. Mobilization of neutrophils is regulated by these cells’ ability to sense inflammatory mediators that guide them toward the site of infection in a process known as chemotaxis (33). A possible mechanism could be via rT3, which initiates actin polymerization in astrocytes (34). Therefore, a lack of D3 could impair neutrophil actin polymerization by decreasing rT3 concentrations, thereby reducing cellular mobility. It should be noted that no data are available on the proposed effects of rT3 on neutrophil mobility, and therefore this mechanism remains speculative. As D3 knockdown in zebrafish is known to affect development, we cannot exclude that the changes in survival are (partially) due to defects at the whole organism level due to the lack of D3 (15). However, the effects of D3MO on zebrafish survival are much less severe than the differences observed here (15).
Neutrophils can also secrete these inflammatory mediators, thereby recruiting more cells from the circulation. Elevated T3 due to deficient D3 function could lead to abnormal production of chemokines needed for neutrophil recruitment.
Neutrophils have a variety of killing mechanisms at their disposal. Two of the main neutrophil antimicrobial mechanisms are phagocytosis followed by the release of reactive oxygen species and antimicrobial proteins into the phagosome (1). Reactive oxygen species are produced by NADPH oxidase within the cell upon contact with a pathogen (1). Impaired neutrophil NAPDH oxidase activity in humans causes a syndrome known as chronic granulomatous disease, which is characterized by recurrent severe bacterial infections due to impaired neutrophil killing (2). Primary murine D3KO neutrophils from female mice exhibit impaired NADPH oxidase activity. Interestingly, the reduction in NADPH oxidase activity was not observed in male animals. Sex differences in the immune response have been broadly described in general, with the female immune system exhibiting a greater inflammatory response than the male immune system (35). This is confirmed by the increased NADPH oxidase response observed in neutrophils from female vs male WT animals. Following this finding in D3KO mice, we reanalyzed the CSF TH concentrations from patients with bacterial meningitis but found no effect of sex in a multivariate analysis, although the small number of patients precludes firm conclusions on sex differences. Impaired NADPH oxidase activity in neutrophils from D3KO mice could explain the impaired bacterial killing found in these animals (8). Interestingly, this initial study was performed in only female mice, suggesting that D3 may be relevant only to murine neutrophil function in vivo in female animals. This is speculative, however, as no studies are available on bacterial killing or response to infection in male D3KO mice. The observation that D3KO mice have unchanged hematopoietic bone marrow populations and circulating neutrophil counts compared with WT mice suggests that D3 is not required for neutrophil generation in the bone marrow. Whether TH directly affects NADPH oxidase activity is currently unknown (36, 37). No data available show a direct effect of TH on Nox2, the NADPH oxidase relevant for neutrophil microbial killing (37).
In summary, bacterial meningitis results in altered TH concentrations at the site of infection. These changes in CSF TH profile are consistent with elevated D3 activity in infiltrating neutrophils. In addition, lack of D3 in zebrafish leads to decreased neutrophil recruitment and increased mortality during bacterial meningitis in vivo and in mice to impaired neutrophil function in vitro, as evidenced by decreased NADPH oxidase activity. These consistent findings across experimental models suggest that neutrophil function during infection requires strict control of TH availability for which D3 appears essential.
Acknowledgments
The authors thank Prof. Jan-Willem Veening (University of Lausanne) for the S. pneumoniae–mCherry strain.
Financial Support: A.H.v.d.S. is supported by the Academisch Medisch Centrum (AMC) Graduate School PhD Scholarship and the AMC Foundation Young Talent Fund. A.H. is supported by grant DK095908 from the National Institute of Diabetes, Digestive and Kidney Disease. M.C.B. is supported by an NWO-Veni grant 2012 (916.13.078) from the Netherlands Organization for Health Research and Development (ZonMw). D.v.d.B. is supported by grants from the Netherlands Organization for Health Research and Development [ZonMw; NWO-Vidi grant 2010 (016.116.358)] and the European Research Council (ERC Starting Grant 281156). This work used the Flow Cytometry Core Facility at Maine Medical Center Research Institute, which is supported by COBRE grant P30GM103465 (D. Wojchowski, principal investigator), from the National Institute of General Medical Sciences.
Author Contributions: A.H.v.d.S., K.K.J., M.C.B., E.F., D.v.d.B., and A.B. designed experiments. V.M.D. provided expert advice and reagents for zebrafish morpholino experiments. A.H.v.d.S., K.K.J., A.K., and H.C.v.B. performed experiments. M.T.A., A.H., C.M.J.E.V.-G., M.C.B., E.F., D.v.d.B., and A.B. supervised experiments. A.H.v.d.S., K.K.J., H.C.B., and M.C.B. analyzed data. A.H.v.d.S., K.K.J., M.C.B., E.F., D.v.d.B., and A.B. wrote the manuscript. All authors corrected the manuscript and approved the final version.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Fluorescent Conjugate | Antigen Sequence (if Known) | Name of Antibody/Clone | Catalog No. | Manufacturer | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|---|---|
| Ly-6G | PerCP-Cy5.5 | 1A8 | 560602 | BD Biosciences | Rat IgG2A; monoclonal | AB_1727563 | ||
| Ly6C | PE | HK1.4 | 12-5932 | eBioscience | Rat IgG2c; monoclonal | AB_10804510 | ||
| CD117 (cKit) | APC | 2B8 | 17-1171 | eBioscience | Rat IgG2b; monoclonal | AB_469429 | ||
| CD11b | APC-Cy7 | M1/70 | 557657 | BD Biosciences | Rat IgG2b; monoclonal | AB_396772 | ||
| CD19 | PE-Cy7 | 1D3 | 25-0193 | eBioscience | Rat IgG2A; monoclonal | AB_657664 | ||
| CD335 | PE-Cy7 | 29A1.4 | 25-3351 | eBioscience | Rat IgG2A; monoclonal | AB_2573441 | ||
| CD3e | PE-Cy7 | 145-2C11 | 25-0031 | eBioscience | Armenian hamster; monoclonal | AB_469571 | ||
| CD16/CD32 (FC block) | 93 | 14-0161 | eBioscience | Rat IgG2A; monoclonal | AB_467132 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- CSF
- cerebrospinal fluid
- D3
- type 3 deiodinase
- D3KO
- D3 knockout
- D3MO
- D3 morpholino
- GOS
- Glasgow Outcome Scale
- IQR
- interquartile range
- MO
- morpholino
- MOI
- multiplicity of infection
- NTIS
- nonthyroidal illness syndrome
- PBS
- phosphate-buffered saline
- PMA
- phorbol 12-myristate 13-acetate
- rT3
- reverse triiodothyronine
- SCMO
- standard control morpholino
- T2
- 3,3′-diiodothyronine
- T3
- triiodothyronine
- T4
- thyroxine
- TH
- thyroid hormone
- WT
- wild-type.
References
- 1.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 2.Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol Biol. 2014;1124:501–515. [DOI] [PubMed] [Google Scholar]
- 3.van der Spek AH, Fliers E, Boelen A. Thyroid hormone metabolism in innate immune cells. J Endocrinol. 2016;232(2):R67–R81. [DOI] [PubMed] [Google Scholar]
- 4.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29(7):898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boelen A, Boorsma J, Kwakkel J, Wieland CW, Renckens R, Visser TJ, Fliers E, Wiersinga WM. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid. 2008;18:1095–1103 [DOI] [PubMed] [Google Scholar]
- 7.van der Spek AH, Bloise FF, Tigchelaar W, Dentice M, Salvatore D, van der Wel NN, Fliers E, Boelen A. The thyroid hormone inactivating enzyme type 3 deiodinase is present in bactericidal granules and the cytoplasm of human neutrophils. Endocrinology. 2016;157(8):3293–3305. [DOI] [PubMed] [Google Scholar]
- 8.Boelen A, Kwakkel J, Wieland CW, St Germain DL, Fliers E, Hernandez A. Impaired bacterial clearance in type 3 deiodinase-deficient mice infected with Streptococcus pneumoniae. Endocrinology. 2009;150(4):1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijlsma MW, Brouwer MC, Kasanmoentalib ES, Kloek AT, Lucas MJ, Tanck MW, van der Ende A, van de Beek D. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339–347. [DOI] [PubMed] [Google Scholar]
- 10.Brouwer MC, Heckenberg SG, de Gans J, Spanjaard L, Reitsma JB, van de Beek D. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology. 2010;75(17):1533–1539. [DOI] [PubMed] [Google Scholar]
- 11.Ackermans MT, Kettelarij-Haas Y, Boelen A, Endert E. Determination of thyroid hormones and their metabolites in tissue using SPE UPLC-tandem MS. Biomed Chromatogr. 2011;26(4):485–490. [DOI] [PubMed] [Google Scholar]
- 12.de Vries EM, Eggels L, van Beeren HC, Ackermans MT, Kalsbeek A, Fliers E, Boelen A. Fasting-induced changes in hepatic thyroid hormone metabolism in male rats are independent of autonomic nervous input to the liver. Endocrinology. 2014;155(12):5033–5041. [DOI] [PubMed] [Google Scholar]
- 13.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1(7968):1031–1034. [DOI] [PubMed] [Google Scholar]
- 14.Jim KK, Engelen-Lee J, van der Sar AM, Bitter W, Brouwer MC, van der Ende A, Veening JW, van de Beek D, Vandenbroucke-Grauls CM. Infection of zebrafish embryos with live fluorescent Streptococcus pneumoniae as a real-time pneumococcal meningitis model. J Neuroinflammation. 2016;13(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijlen M, Houbrechts AM, Bagci E, Van Herck SL, Kersseboom S, Esguerra CV, Blust R, Visser TJ, Knapen D, Darras VM. Knockdown of type 3 iodothyronine deiodinase severely perturbs both embryonic and early larval development in zebrafish. Endocrinology. 2014;155(4):1547–1559. [DOI] [PubMed] [Google Scholar]
- 16.Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type Iii. J Exp Med. 1944;79(2):137–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beilharz K, van Raaphorst R, Kjos M, Veening JW. Red fluorescent proteins for gene expression and protein localization studies in Streptococcus pneumoniae and efficient transformation with DNA assembled via the Gibson assembly method. Appl Environ Microbiol. 2015;81(20):7244–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75(4):604–611. [DOI] [PubMed] [Google Scholar]
- 20.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2007;83(1):64–70. [DOI] [PubMed] [Google Scholar]
- 21.Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28(5):609–619. [DOI] [PubMed] [Google Scholar]
- 22.Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, Crawford HC, Zong WX. DNA alkylating therapy induces tumor regression through an HMGB1-mediated activation of innate immunity. J Immunol. 2011;186(6):3517–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leo O, Foo M, Sachs DH, Samelson LE, Bluestone JA. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci USA. 1987;84(5):1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boelen A, Kwakkel J, Alkemade A, Renckens R, Kaptein E, Kuiper G, Wiersinga WM, Visser TJ. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology. 2005;146(12):5128–5134. [DOI] [PubMed] [Google Scholar]
- 25.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849–1859. [DOI] [PubMed] [Google Scholar]
- 26.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adelberg HM, Siemsen JK, Jung RC, Nicoloff JT. Scintigraphic detection of pulmonary bacterial infections with labeled thyroid hormones and pertechnetate. Radiology. 1971;99(1):141–146. [DOI] [PubMed] [Google Scholar]
- 28.Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88(7):3202–3211. [DOI] [PubMed] [Google Scholar]
- 30.Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973;37(3):415–422. [DOI] [PubMed] [Google Scholar]
- 31.Boelen A, Platvoet-Ter Schiphorst MC, Wiersinga WM. Association between serum interleukin-6 and serum 3,5,3′-triiodothyronine in nonthyroidal illness. J Clin Endocrinol Metab. 1993;77(6):1695–1699. [DOI] [PubMed] [Google Scholar]
- 32.Chernow B, Burman KD, Johnson DL, McGuire RA, O’Brian JT, Wartofsky L, Georges LP. T3 may be a better agent than T4 in the critically ill hypothyroid patient: evaluation of transport across the blood-brain barrier in a primate model. Crit Care Med. 1983;11(2):99–104. [DOI] [PubMed] [Google Scholar]
- 33.Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol. 2008;18(11):R485–R494. [DOI] [PubMed] [Google Scholar]
- 34.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho DP, Dupuy C. Thyroid hormone biosynthesis and release. Mol Cell Endocrinol. 2017;458:6–15. [DOI] [PubMed] [Google Scholar]
- 37.Segal AW. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol. 2008;40(4):604–618. [DOI] [PMC free article] [PubMed] [Google Scholar]