Abstract
Constitutively activated STAT1 and elevated IFN-γ are both characteristic of T cell large granular lymphocytic leukemia (T-LGLL), a rare incurable leukemia with clonal expansion of cytotoxic T cells due to defective apoptosis. Interferon gamma (IFN-γ) is an inflammatory cytokine that correlates with worse progression and symptomology in multiple autoimmune diseases and cancers. In canonical IFN-γ-STAT1 signaling, IFN-γ activates STAT1, a transcription factor, via phosphorylation of tyrosine residue 701 (p-STAT1). p-STAT1 then promotes transcription of IFN-γ, creating a positive feedback loop. We previously found that calcitriol treatment of the TL-1 cell line, a model of T-LGLL, significantly decreased IFN-γ secretion and p-STAT1 while increasing the vitamin D receptor (VDR) protein. Here we further explore these observations. Using TL-1 cells, IFN-γ decreased starting at 4 h following calcitriol treatment, with a reduction in the intracellular and secreted protein levels as well as the mRNA content. A similar reduction in IFN-γ transcript levels was observed in primary T-LGLL patient peripheral blood mononuclear cells (PBMCs). p-STAT1 inhibition followed a similar temporal pattern and VDR upregulation inversely correlated with IFN-γ levels. Using EB1089 and 25(OH)D3, which have high or low affinity for VDR, respectively, we found that the decrease in IFN-γ correlated with the ability of EB1089, but not 25(OH)D3, to upregulate VDR. However, both compounds inhibited p-STAT1; thus the reduction of p-STAT1 is not solely responsible for IFN-γ inhibition. Conversely, cells treated with VDR siRNA exhibited decreased basal IFN-γ production upon VDR knockdown in a dose-dependent manner. Calcitriol treatment upregulated VDR and decreased IFN-γ regardless of initial VDR knockdown efficiency, strengthening the connection between VDR upregulation and IFN-γ reduction. Our findings suggest multiple opportunities to further explore the clinical relevance of the vitamin D pathway and the potential role for vitamin D supplementation in T-LGLL.
Keywords: vitamin D, STAT1, IFN-gamma, cytokines, transcription factors, large granular lymphocytic leukemia, vitamin D receptor, type II interferon
1. Introduction
T cell large granular lymphocytic leukemia (T-LGLL) is a rare incurable leukemia characterized by clonal expansion of CD8+ cytotoxic T cells resembling CD45RA+ terminal effector memory cells [1–4]. T-LGLL often co-occurs with inflammatory autoimmune diseases including rheumatoid arthritis [1]. T-LGLL is hypothesized to result from chronic antigen stimulation, leading to a constitutively activated subset of T cells with defective apoptosis [1]. Current treatment options utilize single agent immunosuppressants, such as methotrexate, cyclophosphamide, and cyclosporine A for management of symptoms [1]. Despite good clinical responses, T-LGLL is not cured by such an approach.
Current research efforts aim to develop treatments that target highly activated pathways in T-LGLL. One such pathway is the Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway [5]. T-LGLL cells have constitutively activated STAT1 and STAT3 [6] and T-LGLL patients have significantly elevated serum interferon gamma (IFN-γ) levels [7]. In canonical IFN-γ-STAT1 signaling, IFN-γ binds to the IFN-γ receptor [8–10]. The receptor then interacts with JAK1 and JAK2, causing phosphorylation of the receptor by the JAKs [8–10]. This allows recruitment and activation of STAT1 through phosphorylation at tyrosine residue 701 (Y701) [9, 11, 12]. Phosphorylated STAT1 monomers then dimerize and move into the nucleus to transcribe STAT1 gene targets, including IFN-γ [8, 13]. Thus, IFN-γ activates STAT1 [10, 12, 14], which in turn promotes further production of IFN-γ [15–17] and creates a positive feedback loop.
IFN-γ, a type II interferon, plays an important role in proper immune function yet it also contributes to progression and worse symptomology in multiple cancers and autoimmune diseases [18–24]. Excessive IFN-γ production also negatively impacts normal peripheral blood mononuclear cells (PBMCs) by inhibiting proliferation, reducing antigen presentation, and inducing apoptosis [25–29]. In aplastic anemia, a disease that may co-occur with T-LGLL [30], IFN-γ is directly involved in the disease development through inhibition of myeloid progenitors and lineage differentiation [24]. Therefore, the ability to turn off the production of IFN-γ and STAT1 activation is a therapeutic focus in several diseases, including T-LGLL.
Current JAK-STAT targeting agents exhibit considerable side effects and limited potency in clinical development for other diseases [31]. However, vitamin D, a steroid, has also been shown to act as a JAK-STAT pathway inhibitor in several cancers and autoimmune diseases with relatively few side effects [32, 33]. Calcitriol, the hormonally active form of vitamin D, exerts effects through binding to the vitamin D receptor (VDR). This binding increases VDR protein levels and induces a VDR conformational change allowing VDR to modulate transcription of genes [34, 35]. Although vitamin D is easily obtained through fatty foods and ultraviolet radiation exposure, deficiency is rather common [36] and is linked to the development and severity of certain diseases [37]. Vitamin D supplementation often alleviates symptoms or inhibits pro-tumorigenic pathways [32, 33, 38–40]
Recently, we found that calcitriol treatment significantly decreased IFN-γ output of TL-1, a patient-derived cell line model of T-LGLL [41], as well as p-STAT1 levels in TL-1 cells and primary T-LGLL patient samples [42]. VDR was also significantly increased in TL-1 cells and primary T-LGLL cells following calcitriol treatment, with T-LGLL patient PBMCs exhibiting varying degrees of VDR protein levels [32]. We hypothesized that calcitriol decreased p-STAT1 levels, which caused decreased IFN-γ transcription. This, in turn, would decrease IFN-γ protein secretion and IFN-γ receptor activation as well as the associated STAT1 phosphorylation, essentially breaking the positive feedback loop. To explore this proposed mechanism in the current study, we utilized genetic and pharmacological manipulation of the vitamin D pathway in the TL-1 cell line. We then confirmed our findings in primary PBMCs isolated from patients with T-LGLL. We found that although IFN-γ and p-STAT1 were decreased in the same temporal manner, the proteins were regulated independently of each other, with IFN-γ reduction requiring VDR upregulation. Furthermore, basal VDR levels positively correlated with IFN-γ while calcitriol treatment reduced IFN-γ regardless of basal VDR levels. Taken together, these findings suggest that vitamin D supplementation or targeting VDR may serve as new therapeutic options for reducing IFN-γ production and STAT1 activation in T-LGLL.
2. Material and methods
2. 1. Human subjects
Primary patient PBMCs were isolated from confirmed T-LGLL patients. Criteria for inclusion encompassed expanded population of circulating T-LGL cells, immunophenotyping of cell surface markers, and T-LGL clonality [4]. Patients were excluded from this study on the basis of current immunosuppressant treatment or previous autoimmune disease diagnosis. Information regarding gender, age, T-LGLL cell purity at the time of diagnosis, and T cell receptor type can be found in Table 1. All specimens analyzed in this study were obtained from human subjects consented to the LGL Leukemia Registry at the University of Virginia (IRB-HSR#17000 “Large Granular Lymphocyte Leukemia Registry”) and studied under the University of Virginia IRB #17070 “Pathogenesis of Large Granular Lymphocyte Leukemia.” STAT3 mutational profiling was performed as previously described [42].
Table 1. T-LGLL Patient Clinical Data Summary.
Fresh PBMCs were obtained from five T-LGLL patients for use in qPCR studies. Inclusion criteria are described in the materials and methods section. Relevant clinical information is included in this table.
| Patient # | Sex | Age | TCR | CD3+CD8+ purity (%) | ANC | STAT3 Mutation Status | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | F | 68 | αβ | 30* | 2459 | Y640F | None |
| 2 | M | 73 | αβ | 81 | 1800 | WT | None |
| 3 | F | 29 | 77% αβ, 7% γδ | 48 | 460 | WT | None |
| 4 | M | 52 | αβ | 93 | 1200 | Y640F | None, previously on methotrexate/cyclophosphamide |
| 5 | F | 69 | αβ | 42 | 2500 | WT | None, previously on cyclosporine/methotrexate/Neupogen |
CD3+CD8+CD7− instead of CD3+CD8+
2.2. Reagents
Calcitriol (1,25-(OH)2 vitamin D3) was purchased from Cayman Chemical (Cat #71820) and used at 100 nM unless otherwise noted. Radioimmunoprecipitation assay buffer (RIPA) (Cat #R0278), protease and phosphatase inhibitor cocktails (Cat #P8340, Cat #P5726), and cycloheximide (Cat #01810) were purchased from Sigma Aldrich. FBS was purchased from Seradigm (Cat# 97068-085). IL-2 was purchased from Miltenyi Biotec (Cat #130-097-743). Clarity enhanced chemiluminescence (ECL) reagent (Cat #170-5061) and PVDF membrane and filter paper (Cat #170-4274), were purchased from BioRad. RPMI 1640 (Cat #10-00-CV) and Pierce bicinchoninic acid (BCA) protein assay kit (Cat #PI23225) were purchased from ThermoFisher Scientific. Polyacrylamide gels (4–12%; Cat #NW04125BOX) were purchased from Life Technologies. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethozyphenyl)-2-(4-sulfonphenyl)-2H-tetrazolium (MTS) Cell Proliferation Colorimetric Assay Kit was purchased from BioVision (Cat #K300-2500). EB1089 was purchased from R&D Systems (Cat #3993). 25(OH)D3 was purchased from Cayman Chemical (Cat #9000683). ON-TARGETplus Pooled Human VDR siRNA (Cat #L-003448-00-0005) and ON-TARGETplus Control Non-Targeting Pool siRNA (Cat #D-001810-01-05) were purchased from Dharmacon.
2.3. Primary cells
PBMCs from T-LGLL patients were isolated by Ficoll-hypaque gradient separation as described previously [43]. Cell viability was determined by Trypan blue exclusion assay. Cells were maintained in RPMI 1640 supplemented with 10% FBS at 37°C, 5% CO2 at a density of 1 million cells/mL for 24 h with or without calcitriol. Protein was harvested using RIPA lysis buffer with protease and phosphatase inhibitor cocktails as described in the western blot section.
2.4. Cell culture
Media for all experiments was RPMI 1640 supplemented with 10% FBS. The TL-1 cell line, a model of T-LGLL, was previously derived from a T-LGLL patient by our laboratory and immortalized with the retroviral HTLV-2 tax gene [41]. This cell line was authenticated using short tandem repeat DNA profiling of both cell line and patient DNA (Genetica DNA laboratories). TL-1 cell medium was supplemented with IL-2 (Miltenyi Biotec, Cat #130-097-743) at 200U/mL and maintained at 37°C, 5% CO2. Cells were plated at a density of 1 million cells/mL for all experiments, unless indicated. Calcitriol, 25(OH)D3 and EB1089 were dissolved in 100% ethanol with the final percentage of ethanol in the media less than 0.1%. Cells were treated with varying doses of calcitriol, 25(OH)D3, and EB1089, then harvested, lysed, and protein extracted at the designated time points between 0 to 24 h.
2.5. Protein half-life determination
TL-1 cells were pre-treated with calcitriol or ethanol for 1 h prior to creating time 0 lysates or adding cycloheximide (10 μg/mL). Cells were then lysed to obtain protein at 0.25, 0.5, 1, 2, 4, and 6 h following cycloheximide treatment. Viability was assessed using MTS at 6 h after cycloheximide treatment and was unchanged (data not shown).
2.6. VDR knockdown
VDR siRNA knockdown was performed using Invitrogen Neon Transfection System 100 μL Kit (Cat #MPK10096). TL-1 cells were plated at 2.5 million cells/mL and treated with 50 nM, 100 nM, or 200 nM VDR siRNA or 50 nM scramble siRNA for 48 h. After 48 h, protein was harvested from a subset of TL-1 cells from each condition to assess knockdown status and viability. The MTS Cell Proliferation Colorimetric Assay Kit was used to assess cell viability after 24 h of calcitriol treatment. The reaction was incubated at 37°C, 5% CO2 for 1 h, and formazan product was detected on a plate reader (Cytation3 Imaging Reader) at 492 nm. Data were normalized to the scramble treatment. All conditions were done in quadruplicate. The remaining TL-1 cells from each condition were then counted and re-plated at 1 million cells/mL in fresh media and IL-2, in addition to calcitriol or ethanol vehicle for 24 h. Supernatant was harvested for cytokine analysis by Luminex (Millipore Sigma, Cat #HCYTOMAG-60K-06) and cells were lysed to obtain protein for western blotting.
2.7. RNA extraction and quantitative PCR
TL-1 cells were treated with calcitriol or vehicle control for 24 h. After 24 h, cells were harvested and lysed with Invitrogen TRIzol Reagent (Cat #15596018) at 350 μl per 4 million cells and stored at −80°C. RNA was isolated using Zymo Research Direct-zol MiniPrep kit (Cat #R2050) and quantified using Invitrogen Qubit RNA Broad Range Assay kit (Cat #Q10210) and Invitrogen Qubit 2.0 Fluorometer (Cat # Q32866). Clontech RNA to cDNA EcoDry Premix (Double Primed) (Cat #639548) was used to reverse transcribe the extracted RNA. BioRad iTaq Universal SYBR Green Supermix (Cat #1725121) and PrimePCR SYBR Green Assay primers were used for all qPCR reactions: VDR (Cat #10025636, qHsaCID0023190), STAT1 (Cat #10025636, qHsaCED0043612), IFNG (Cat #10025636, qHsaCID0017614), UBC (Cat #10025636, qHsaCED0023867), CYP24A1 (Cat # 10025636, qHsaCID0007605), and B2M (Cat #10025636, qHsaCID0015347). Each sample was loaded in triplicate for each primer and each condition had three independent biological replicates. Cycle number and annealing temperature were followed according to the Prime PCR protocol. Results were normalized to vehicle control.
2.8. Western blot
Treated cells were washed with PBS then lysed in RIPA buffer with protease and phosphatase inhibitors. Protein content was quantified using the BCA assay. Proteins were electrophoresed on a 4–12% polyacrylamide gel and then transferred to a PVDF membrane and blocked with primary antibody overnight at 4°C according to manufacturer recommendations. Cell Signaling Technology primary antibodies used in these studies were: Vitamin D3 Receptor (Cat #12550), STAT1 (Cat #9175), Phospho-STAT1 (Y701) (Cat #7649), IFN-γ XP (Cat #8455), and β-actin (Cat #3700). After the membrane was washed, it was incubated with secondary antibody (anti-rabbit IgG-HRP linked #7074 or anti-mouse IgG-HRP linked #7076) for 1 h then treated with ECL substrate. Images were captured with a BioRad ChemiDoc MP instrument and analyzed using Image Lab software (BioRad).
2.9. Cytokine analysis
After treatment with calcitriol, 25(OH)D3, or EB1089, cells were pelleted, and aliquots of the media were collected and stored at −80°C prior to analysis by the UVA Flow Cytometry Core using the Luminex MAGPIX bead-based multiplex analyzer. All conditions were done in triplicate.
2.10. Statistical analysis
Data were analyzed using GraphPad Prism software version 7. An unpaired 2-tailed Student’s t-test was utilized and P-values of <0.05 were considered significant.
3. Results
3.1. Calcitriol treatment of T-LGLL cells reduces IFN-γ at the protein and mRNA levels
We previously found that calcitriol significantly reduced IFN-γ levels in conditioned media collected 24 h after calcitriol treatment [42]; thus we aimed to better understand the mechanism for IFN-γ reduction. Such inhibition could conceivably occur through changes in transcription, translation, or secretion. A time course was carried out to determine the kinetics of IFN-γ protein reduction. TL-1 cells were treated with calcitriol or vehicle control and protein was harvested over the next 24 h. Immunoblot results were normalized to percent change from vehicle control to better visualize the calcitriol-induced changes. Intracellular IFN-γ protein levels were consistently decreased from 4 h until 24 h (Figure 1A, B). The reduction of secreted IFN-γ levels was detected at our earliest measured time point, 6 h, and further decreased until 24 h (Figure 1B). This suggested that IFN-γ synthesis was inhibited first, prompting us to investigate whether this originates at the transcript level. TL-1 cells were treated with calcitriol or vehicle control for 4 or 24 h, followed by qPCR detection of IFN-γ gene expression. Calcitriol treatment reduced IFN-γ mRNA levels by 52% and 40% at 4 h and 24 h (Figure 1C), thereby demonstrating that calcitriol suppresses IFN-γ transcripts. This finding was recapitulated in PBMCs from T-LGLL patients treated with calcitriol for 24 h (Figure 1C).
Figure 1. IFN-γ is reduced on the mRNA and protein levels following calcitriol treatment.
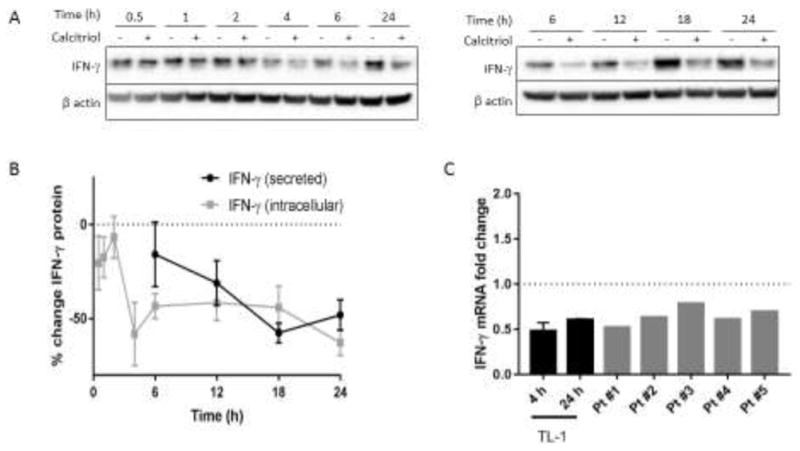
A. Western blot analysis was performed on TL-1 lysates harvested at time points following calcitriol or vehicle treatment. Representative western blots are shown, with β actin used as a loading control. B. IFN-γ band intensity from part A was normalized to β actin and then expressed relative to the ethanol control to illustrate changes in protein levels as a result of calcitriol treatment (n=3–7, +/− SEM). Supernatant was collected from cells treated as in A and sent for Luminex analysis of secreted IFN-γ (n=3, +/− SEM). C. TL-1 cells were treated with calcitriol for 4 or 24 h and IFN-γ transcript levels were assayed by qPCR. Primary T-LGLL patient PBMCs were treated with calcitriol for 24 h. Results were normalized to housekeeping gene UBC and then to the ethanol control (for TL-1, n=3, +/− SEM).
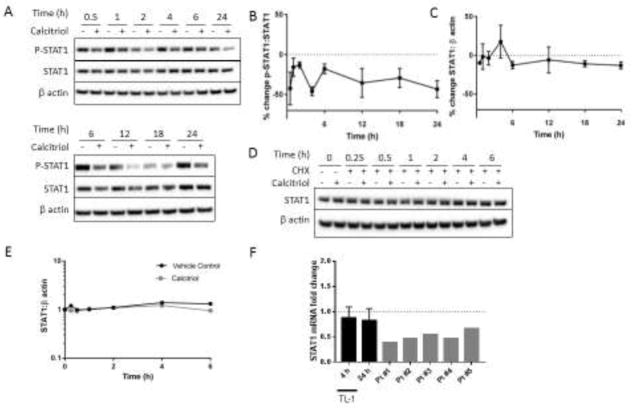
3.2. Calcitriol-mediated p-STAT1 reduction correlates with IFN-γ
We hypothesized that IFN-γ inhibition was due to a decrease in p-STAT1 transcriptional activity as we previously found that calcitriol reduces p-STAT1 levels at 24 h [42]. To address this, we investigated the temporal reduction of p-STAT1 in TL-1 cells treated with calcitriol and compared this to the IFN-γ reduction. Calcitriol reduced p-STAT1:STAT1 in a similar trend as IFN-γ reduction in TL-1 (Figure 2A, 2B). Total STAT1 protein levels were relatively unaltered over this timeframe (Figure 2A, 2C) and STAT1 protein half-life was not affected by calcitriol (Figure 2D, 2E). STAT1 transcripts were decreased by less than 20% after calcitriol treatment for 4 or 24 h in the TL-1 cell line but decreased by roughly 50% on average in PBMCs from T-LGLL patients (Figure 2F), potentially suggesting that STAT1 is more sensitive to reduction by calcitriol in T-LGLL PBMCs. Taken together, these data indicate that p-STAT1 content is suppressed upon calcitriol treatment, but changes in STAT1 protein levels were not the drivers for the decrease in p-STAT1 in the TL-1 cell line.
Figure 2. p-STAT1 reduction correlates with IFN-γ inhibition and is independent of total STAT1 protein levels.
A. Western blot analysis was performed on TL-1 protein lysates harvested at time points following calcitriol or vehicle treatment. Representative western blots are shown, with β actin used as a loading control. B. p-STAT1 (Y701) or C. STAT1 results were normalized to total STAT1 or β actin, respectively, and then further normalized to the vehicle control to illustrate changes in protein levels as a result of calcitriol treatment (n=3–7, +/− SEM). D. TL-1 cells were pretreated with calcitriol or ethanol for 1 h and then treated with cycloheximide (10 μg/mL) to inhibit protein synthesis. Lysates were prepared and western blot analysis was performed at the indicated time points for the designated proteins. E. Quantification of D, normalizing STAT1 to β actin. F. TL-1 cells were treated with calcitriol for 4 or 24 h and STAT1 transcript levels were assayed by qPCR. Primary T-LGLL patient PBMCs were treated with calcitriol for 24 h. Results were normalized to the housekeeping gene UBC and then to the ethanol control (for TL-1, n=3, +/− SEM).
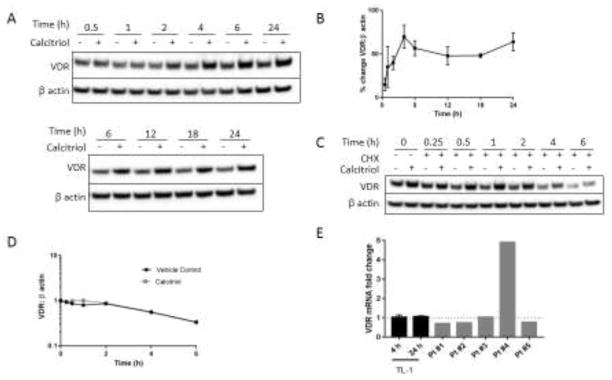
3.3. VDR protein upregulation follows calcitriol treatment in a similar temporal manner as IFN-γ and p-STAT1 reduction
Our previous work showed 24 h calcitriol treatment upregulates VDR in normal donor activated CD8+ T cells, TL-1 cells, and T-LGLL cells, but not normal donor naïve T cells [42]. In this study, we wanted to determine the timing of VDR upregulation and how it relates to p-STAT1 and IFN-γ in TL-1 cells. VDR protein levels gradually increased following calcitriol treatment until they reached a maximum at 4 h and maintained this level for 24 h after treatment (Figure 3A, 3B). The initial increase in VDR protein level was not due to extension of VDR half-life (Figure 3C, 3D). VDR transcript levels were relatively unchanged at 4 and 24 h following calcitriol treatment of TL-1 cells and nearly all primary T-LGLL PBMCs followed the same trend at 24 h (Figure 3E). Taken together with the inability of VDR protein levels to be increased when protein synthesis was blocked (Figure 3C, Figure 3D), VDR upregulation is most likely due to changes in translational regulation. In summary, increased VDR proteins levels showed temporal correlation with the reduction in IFN-γ and p-STAT1, leading us to next determine whether this upregulation is necessary for the decreased IFN-γ and p-STAT1 levels.
Figure 3. VDR protein levels increase following calcitriol treatment.
A. Western blot analysis was performed on TL-1 protein lysates harvested at time points following treatment with calcitriol or vehicle control and probed for the VDR. Representative western blots are shown. B. Quantification of western blots from A normalized to β actin (n=3–7, +/− SEM). C. TL-1 cells were pretreated with calcitriol or ethanol for 1 h and then treated with cycloheximide (10 μg/mL) to inhibit protein synthesis. Lysates were prepared at the indicated time points and western blot analysis was performed. D. Quantification of C, normalizing VDR to β actin. E. TL-1 cells were treated with calcitriol for 4 or 24 h and VDR transcript levels were assayed by qPCR. Primary T-LGLL patient PBMCs were treated with calcitriol for 24 h. Results were normalized to the housekeeping gene UBC and then to the ethanol control (for TL-1, n=3, +/− SEM).
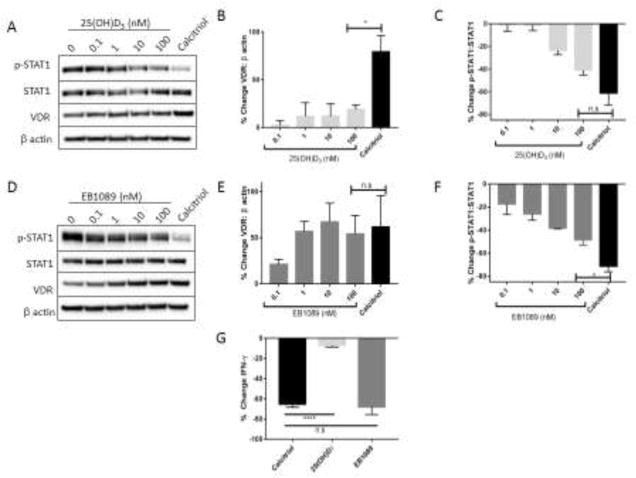
3.4. Upregulation of VDR is correlated with the reduction in IFN-γ levels but not p-STAT1 content
To determine whether VDR upregulation is necessary to suppress IFN-γ and p-STAT1 levels, we utilized EB1089, a potent calcitriol analog, and 25(OH)D3, the inactive circulating form of vitamin D. EB1089 strongly engages the VDR and induces a conformational change that promotes transcription of VDR targets [44] while 25(OH)D3 weakly interacts with the VDR [45]. Treatment with 25(OH)D3 did not upregulate VDR protein (Figure 4A, 4B) but decreased p-STAT1 (Figure 4A, Figure 4C) in a manner comparable to calcitriol. CYP24A1 mRNA levels, which serve as a readout of VDR transcriptional activity, support the conclusion that 25(OH)D3 exhibits limited engagement of VDR and that TL-1 cells have very minimal capacity to convert this analogue to the active form at 24 h (Supplementary Figure 1A) [46]. Conversely, EB1089 increased VDR (Figure 4D, 4E) and also decreased p-STAT1 (Figure 4D, 4F), as previously observed with calcitriol. Both EB1089 and calcitriol significantly reduced IFN-γ production on the protein and mRNA transcript level compared to 25(OH)D3 (P<0.0001) (Figure 4G, Supplementary Figure 1B). Therefore, VDR upregulation is correlated with the decreases in IFN-γ but not p-STAT1, indicating that IFN-γ and p-STAT1 are not regulated by the same mechanism following calcitriol treatment.
Figure 4. VDR upregulation is necessary for reduction in IFN-γ but not p-STAT1 after treatment with vitamin D or analogs.
TL-1 cells were treated with increasing dosages of the inactive form of vitamin D, 25(OH)D3 (A–C), or the high affinity calcitriol analog EB1089 (D–F), as well as vehicle control or calcitriol for comparison. A, D. Protein lysates were prepared and western blot analysis was performed (n=3, +/− SEM). Representative western blots are shown. Protein levels were normalized to β actin for VDR (B, E) or STAT1 for p-STAT1 (C, F), and then further normalized to the vehicle control to illustrate changes as a result of calcitriol treatment. G. Conditioned media was collected from the 100 nM calcitriol, 25(OH)D3, and EB1089 samples after 24 h and submitted for Luminex cytokine analysis (n.s=not significant, *p<0.05, ****p<0.001).
3.5. VDR levels correlate with IFN-γ production
We previously observed that T-LGLL patient PBMCs have varying amounts of VDR protein [47]; therefore, we investigated the relationship between VDR protein levels and IFN-γ production. Using varying dosages of VDR siRNA, we determined that TL-1 cell viability was not decreased upon greater than 80% knockdown of VDR (Figure 5A, 5B). VDR was upregulated in all groups upon calcitriol treatment (Figure 5C, 5D), showing that calcitriol upregulates VDR regardless of baseline VDR levels. VDR protein levels correlated with VDR mRNA levels following VDR knockdown, and VDR mRNA was not stabilized by calcitriol treatment of cells with VDR knockdown (Supplementary Figure 2). In the absence of supplemented calcitriol, VDR levels and IFN-γ levels exhibited a clear trend, with IFN-γ production being higher in TL-1 cells with higher basal VDR levels and decreasing IFN-γ as knockdown efficacy increased (Figure 5D, 5E). Calcitriol reduced secreted IFN-γ regardless of initial knockdown status, bringing the amount of IFN-γ to relatively the same level (Figure 5D, 5E). In the absence of calcitriol, this relationship between VDR levels and cytokine production was unique for IFN-γ as basal VDR levels did not correlate with IL-10 (Figure 5F).
Figure 5. VDR levels correlate with IFN-γ production.
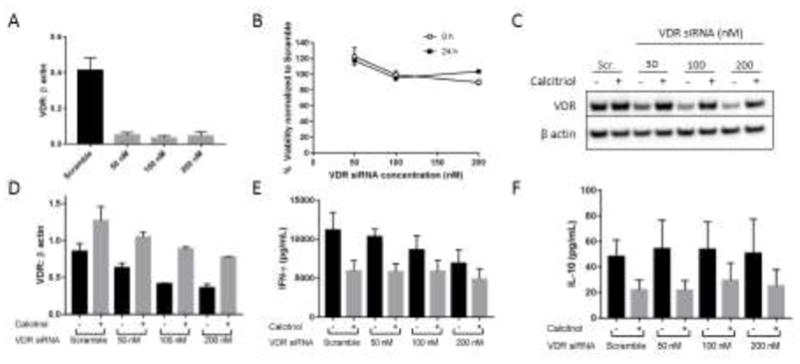
TL-1 cells were treated with VDR or scrambled siRNA for 48 h. Cells were then harvested for western blot analysis of knockdown efficiency (A) and plated for 24 h viability assay to assess survival with knockdown (B). An additional aliquot was re-plated with calcitriol or ethanol for 24 h, followed by protein harvest (C–E). C. Representative western blot of VDR knockdown status 24 h following calcitriol or ethanol treatment. D. Quantification of VDR protein levels in C, normalized to β actin (n=3, +/− SEM). E–F. Conditioned media was subjected to cytokine analysis to quantify IFN-γ (E) or IL-10 (F) production following calcitriol treatment and VDR knockdown (n=3, +/− SEM).
4. Discussion
In this study, we demonstrated that calcitriol treatment of TL-1 cells and T-LGLL patient PBMCs rapidly suppressed IFN-γ mRNA and protein levels, and inhibition was maintained for at least 24 h (Figure 1, Figure 6). In TL-1 cells, p-STAT1 was inhibited in a similar temporal manner and was not due to reduced STAT1 protein (Figure 2) or the reduction in IFN-γ (Figure 4). We also found that the kinetics of VDR upregulation were similar to the reduction in IFN-γ and p-STAT1 and were not due to extension of VDR protein half-life or increase in VDR transcript levels (Figure 3). This is contrary to previous studies that implicated VDR stabilization and protection from proteasome-mediated degradation as the mode of VDR increase following calcitriol treatment [48, 49]. Rather, our data showed that in TL-1 cells, VDR upregulation was dependent on protein synthesis, as cycloheximide blocked the upregulation of VDR and calcitriol did not increase VDR transcript levels (Figure 3).
Figure 6. Model of calcitriol-mediated reduction in p-STAT1 and IFN-γ in T-LGLL.
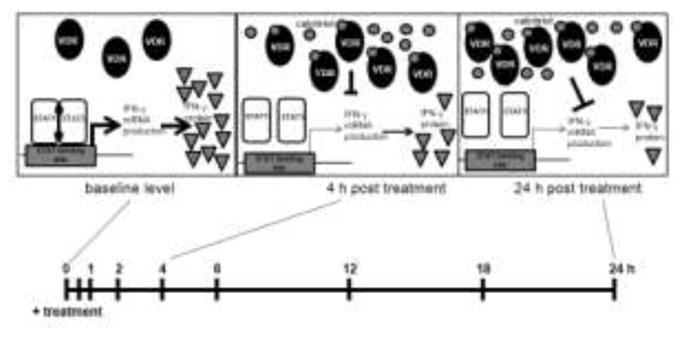
In TL-1 cells supplemented with IL-2, there is a detectable level of VDR and high baseline STAT1 Y701 phosphorylation and IFN-γ production. Within 4 h of calcitriol treatment of TL-1, p-STAT1 and IFN-γ are reduced while VDR is increased. This effect is sustained at 24 h and the final outcome is decreased IFN-γ production that correlates with increased VDR protein. p-STAT1 levels are not dependent on the VDR increase and do not correlate with IFN-γ reduction, showing that p-STAT1 and IFN-γ decreases are mediated by different mechanisms.
Treatment of the TL-1 cell line with either EB1089 or 25(OH)D3 established that VDR upregulation is necessary for calcitriol effects on IFN-γ but not p-STAT1 (Figure 4). Reduced IFN-γ transcript levels (Figure 1) were observed in the same timeframe as the upregulation of VDR (Figure 3). This suggests that VDR transcriptionally regulates IFN-γ as demonstrated in Jurkat T cells where VDR transcriptionally inhibits IFN-γ through binding to the IFN-γ promoter [50]. Upregulation of VDR is required for the transcriptional and cytokine-related effects of calcitriol in normal B cells and activated T cells [51, 52]. As p-STAT1 was reduced without a change in total protein levels and regardless of VDR upregulation in TL-1 (Figure 4), the mechanism behind the p-STAT1 decrease is most likely not transcriptional. Ongoing studies aim to elucidate the role of kinases or phosphatases in modulating the activation state of STAT1.
Current research efforts in T-LGLL focus on inhibiting STAT signaling. However, based on our findings (Figure 4), the use of STAT inhibitors may not reduce IFN-γ. This is particularly relevant for T-LGLL patients with co-occurring IFN-γ-mediated diseases including aplastic anemia [24, 30] and lupus erythematosus (SLE) [53]. Therefore, patients with co-occurring T-LGLL and aplastic anemia or SLE could benefit from a therapy, such as calcitriol, that specifically targets IFN-γ production. As there appears to be a portion of IFN-γ production that is independent of calcitriol regulation (Figure 5), combinatorial treatments of calcitriol and T-LGLL treatments such as cyclosporine A, an inhibitor of IFN-γ [54–61], could be considered.
Many hematological malignancies highly express VDR [47, 62, 63], suggesting a functional role for VDR in cancer cells. To explore the importance of VDR in T-LGLL, we utilized VDR siRNA to modulate VDR levels in order to replicate the varying levels found in patient cells [47]. These experiments present three major findings (Figure 5). First, in the absence of supplemented calcitriol, VDR levels correlated with IFN-γ production. Second, T-LGLL patient cells experience a calcitriol-induced reduction in IFN-γ regardless of VDR levels. Third, there is an unidentified mechanism for IFN-γ production that cannot be reduced by calcitriol. Additional work is needed to evaluate any disturbances in vitamin D metabolism or signaling within the T-LGLL population as well as the potential clinical relevance of our findings.
In summary, this study increases our understanding of the mechanism behind calcitriol-mediated decreases in IFN-γ in T-LGLL. Calcitriol suppresses IFN-γ transcription within 4 h, which correlates with VDR upregulation. In the absence of supplemented calcitriol, VDR levels correlate with IFN-γ production. Further studies are required to determine the relationship between IFN-γ and disease state. Future clinical studies are required to evaluate the ability of calcitriol supplementation to reduce IFN-γ production in T-LGLL patients. Thus, the vitamin D pathway offers multiple opportunities for clinical utility in T-LGLL.
Supplementary Material
Highlights.
IFN-γ levels and p-STAT1 are reduced by calcitriol within 4 h in TL-1 cell line.
IFN-γ is transcriptionally suppressed by calcitriol and requires VDR upregulation.
Reduction in p-STAT1 by calcitriol does not require VDR upregulation.
Calcitriol inhibits IFN-γ and p-STAT1 through independent mechanisms.
In the absence of calcitriol, VDR levels correlate with IFN-γ production.
Calcitriol reduces IFN-γ to a similar amount regardless of basal VDR levels.
Acknowledgments
Funding: This work was funded by the National Cancer Institute of the National Institute of Health under award number R01CA098472, the Bess Family Charitable Fund (to TPL), the LGL Leukemia Foundation (to TPL), the Immunology Training Grants T32AI007496 (to PMK), the UVA Wagner Fellowship (to PMK), and the University of Virginia Cancer Center National Cancer Institute P30-CA044579-23. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Alex Wendling and Matt Schmachtenberg for support with blood processing to obtain PBMCs and Holly Davis and Andrea Hines for LGLL Registry support. We also thank Shubha Dighe for technical guidance with siRNA, Dr. Su-Fern Tan for guidance on cycloheximide experiments, and Kabir Ahluwalia for technical support. We thank Mike Solga in the UVA Flow Cytometry Core for performing Luminex Assays. We wish to acknowledge and extend a special thanks to the T-LGLL patient who inspired the original project and to all the patients enrolled in our registry for their continued support.
Abbreviations
- IFN-γ
interferon gamma
- IL
interleukin
- JAK
Janus kinase
- T-LGL
T cell large granular lymphocyte
- T-LGLL
T cell large granular lymphocyte leukemia
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PBMC
peripheral blood mononuclear cell
- STAT
signal transducer and activator of transcription
- VDR
vitamin D receptor
Footnotes
Contributions: PMK participated in the research design, conducted experiments, performed data analysis, and wrote the manuscript. KCO participated in the research design, conducted experiments, and contributed to the writing of the manuscript. TLO participated in the research design and conducted experiments. CEH conducted experiments and contributed to the writing of the manuscript. KNC conducted experiments. DJF and TPL participated in research design and contributed to the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamy T, Loughran TP., Jr How I treat LGL leukemia. Blood. 2011;117:2764–2774. doi: 10.1182/blood-2010-07-296962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leblanc F, Zhang D, Liu X, Loughran TP. Large granular lymphocyte leukemia: from dysregulated pathways to therapeutic targets. Future Oncol. 2012;8:787–801. doi: 10.2217/fon.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinway SN, LeBlanc F, Loughran TP., Jr The pathogenesis and treatment of large granular lymphocyte leukemia. Blood Rev. 2014;28:87–94. doi: 10.1016/j.blre.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamy T, Moignet A, Loughran TP., Jr LGL leukemia: from pathogenesis to treatment. Blood. 2017;129:1082–1094. doi: 10.1182/blood-2016-08-692590. [DOI] [PubMed] [Google Scholar]
- 5.Rajala HL, Porkka K, Maciejewski JP, Loughran TP, Jr, Mustjoki S. Uncovering the pathogenesis of large granular lymphocytic leukemia-novel STAT3 and STAT5b mutations. Ann Med. 2014;46:114–122. doi: 10.3109/07853890.2014.882105. [DOI] [PubMed] [Google Scholar]
- 6.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP., Jr Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loughran TP, Jr, Zickl L, Olson TL, Wang V, Zhang D, Rajala HL, Hasanali Z, Bennett JM, Lazarus HM, Litzow MR, Evens AM, Mustjoki S, Tallman MS. Immunosuppressive therapy of LGL leukemia: prospective multicenter phase II study by the Eastern Cooperative Oncology Group (E5998) Leukemia. 2015;29:886–894. doi: 10.1038/leu.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi MR, Merlino G. The two faces of interferon-gamma in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 10.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 11.Shuai K, Horvath CM, Huang LH, Qureshi SA, Cowburn D, Darnell JE., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 12.Shuai K, Ziemiecki A, Wilks AF, Harpur AG, Sadowski HB, Gilman MZ, Darnell JE. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 13.Abroun S, Saki N, Ahmadvand M, Asghari F, Salari F, Rahim F. STATs: An Old Story, Yet Mesmerizing. Cell J. 2015;17:395–411. doi: 10.22074/cellj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girdlestone J, Wing M. Autocrine activation by interferon-gamma of STAT factors following T cell activation. Eur J Immunol. 1996;26:704–709. doi: 10.1002/eji.1830260329. [DOI] [PubMed] [Google Scholar]
- 15.Kang HB, Ahn KS, Oh SR, Kim JW. Genkwadaphnin induces IFN-gamma via PKD1/NF-kappaB/STAT1 dependent pathway in NK-92 cells. PLoS One. 2014;9:e115146. doi: 10.1371/journal.pone.0115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebler J, Wirtz S, Klein S, Protschka M, Blessing M, Galle PR, Neurath MF. A key pathogenic role for the STAT1/T-bet signaling pathway in T-cell-mediated liver inflammation. Hepatology. 2003;38:1573–1580. doi: 10.1016/j.hep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brocker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41:562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- 20.Podhorecka M, Dmoszynska A, Rolinski J. Intracellular IFN-gamma expression by CD3+/CD8+ cell subset in B-CLL patients correlates with stage of the disease. Eur J Haematol. 2004;73:29–35. doi: 10.1111/j.1600-0609.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- 21.Solerte SB, Cravello L, Ferrari E, Fioravanti M. Overproduction of IFN-gamma and TNF-alpha from natural killer (NK) cells is associated with abnormal NK reactivity and cognitive derangement in Alzheimer’s disease. Ann N Y Acad Sci. 2000;917:331–340. doi: 10.1111/j.1749-6632.2000.tb05399.x. [DOI] [PubMed] [Google Scholar]
- 22.Schurch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-gamma. J Exp Med. 2013;210:605–621. doi: 10.1084/jem.20121229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pokryszko-Dragan A, Frydecka I, Kosmaczewska A, Ciszak L, Bilinska M, Gruszka E, Podemski R, Frydecka D. Stimulated peripheral production of interferon-gamma is related to fatigue and depression in multiple sclerosis. Clin Neurol Neurosurg. 2012;114:1153–1158. doi: 10.1016/j.clineuro.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 24.Lin FC, Karwan M, Saleh B, Hodge DL, Chan T, Boelte KC, Keller JR, Young HA. IFN-gamma causes aplastic anemia by altering hematopoietic stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699–3708. doi: 10.1182/blood-2014-01-549527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvy BA, Riley RL. IFN-gamma abrogates IL-7-dependent proliferation in pre-B cells, coinciding with onset of apoptosis. Immunology. 1994;81:381–388. [PMC free article] [PubMed] [Google Scholar]
- 26.Sammicheli S, Dang VP, Ruffin N, Pham HT, Lantto R, Vivar N, Chiodi F, Rethi B. IL-7 promotes CD95-induced apoptosis in B cells via the IFN-gamma/STAT1 pathway. PLoS One. 2011;6:e28629. doi: 10.1371/journal.pone.0028629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshikawa H, Nakajima Y, Tasaka K. IFN-gamma induces the apoptosis of WEHI 279 and normal pre-B cell lines by expressing direct inhibitor of apoptosis protein binding protein with low pI. J Immunol. 2001;167:2487–2495. doi: 10.4049/jimmunol.167.5.2487. [DOI] [PubMed] [Google Scholar]
- 28.O’Neil D, Swanton C, Jones A, Medd PG, Rayment N, Chain B. IFN-gamma down-regulates MHC expression and antigen processing in a human B cell line. J Immunol. 1999;162:791–798. [PubMed] [Google Scholar]
- 29.de Bruin AM, Demirel O, Hooibrink B, Brandts CH, Nolte MA. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121:3578–3585. doi: 10.1182/blood-2012-05-432906. [DOI] [PubMed] [Google Scholar]
- 30.Tzankov A, Medinger M. Aplastic anemia: possible associations with lymphoproliferative neoplasms. Int J Lab Hematol. 2014;36:382–387. doi: 10.1111/ijlh.12224. [DOI] [PubMed] [Google Scholar]
- 31.Furqan M, Akinleye A, Mukhi N, Mittal V, Chen Y, Liu D. STAT inhibitors for cancer therapy. J Hematol Oncol. 2013;6:90. doi: 10.1186/1756-8722-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulling PM, Olson KC, Olson TL, Feith DJ, Loughran TP., Jr Vitamin D in hematological disorders and malignancies. Eur J Haematol. 2017;98:187–197. doi: 10.1111/ejh.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY, Chen WC, Chen MF. 1alpha,25-Dihydroxyvitamin D3 Inhibits Esophageal Squamous Cell Carcinoma Progression by Reducing IL6 Signaling. Mol Cancer Ther. 2015;14:1365–1375. doi: 10.1158/1535-7163.MCT-14-0952. [DOI] [PubMed] [Google Scholar]
- 34.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinol Metab Clin North Am. 2010;39:255–269. doi: 10.1016/j.ecl.2010.02.007. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trump DL, Deeb KK, Johnson CS. Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 2010;16:1–9. doi: 10.1097/PPO.0b013e3181c51ee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritterhouse LL, Lu R, Shah HB, Robertson JM, Fife DA, Maecker HT, Du H, Fathman CG, Chakravarty EF, Scofield RH, Kamen DL, Guthridge JM, James JA. Vitamin d deficiency in a multiethnic healthy control cohort and altered immune response in vitamin D deficient European-American healthy controls. PLoS One. 2014;9:e94500. doi: 10.1371/journal.pone.0094500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HJ, Muindi JR, Tan W, Hu Q, Wang D, Liu S, Wilding GE, Ford LA, Sait SN, Block AW, Adjei AA, Barcos M, Griffiths EA, Thompson JE, Wang ES, Johnson CS, Trump DL, Wetzler M. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer. 2014;120:521–529. doi: 10.1002/cncr.28368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Li H, Xie H, Fu M, Guo B, Ding Y, Li W, Yu H. 25-Hydroxyvitamin D3 attenuates experimental periodontitis through downregulation of TLR4 and JAK1/STAT3 signaling in diabetic mice. J Steroid Biochem Mol Biol. 2013;135:43–50. doi: 10.1016/j.jsbmb.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 40.Yu W, Ge M, Lu S, Shi J, Feng S, Li X, Zhang J, Wang M, Huang J, Shao Y, Huang Z, Zhang J, Nie N, Zheng Y. Decreased expression of vitamin D receptor may contribute to the hyperimmune status of patients with acquired aplastic anemia. Eur J Haematol. 2016;96:507–516. doi: 10.1111/ejh.12628. [DOI] [PubMed] [Google Scholar]
- 41.Ren T, Yang J, Broeg K, Liu X, Loughran TP, Jr, Cheng H. Developing an in vitro model of T cell type of large granular lymphocyte leukemia. Leuk Res. 2013;37:1737–1743. doi: 10.1016/j.leukres.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson KC, Kulling PM, Olson TL, Tan SF, Rainbow RJ, Feith DJ, Loughran TP., Jr Vitamin D decreases STAT phosphorylation and inflammatory cytokine output in T-LGL leukemia. Cancer Biol Ther. 2017;18:290–303. doi: 10.1080/15384047.2016.1235669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamy T, Liu JH, Landowski TH, Dalton WS, Loughran TP., Jr Dysregulation of CD95/CD95 ligand-apoptotic pathway in CD3(+) large granular lymphocyte leukemia. Blood. 1998;92:4771–4777. [PubMed] [Google Scholar]
- 44.Quack M, Carlberg C. Selective recognition of vitamin D receptor conformations mediates promoter selectivity of vitamin D analogs. Mol Pharmacol. 1999;55:1077–1087. doi: 10.1124/mol.55.6.1077. [DOI] [PubMed] [Google Scholar]
- 45.Wan LY, Zhang YQ, Chen MD, Du YQ, Liu CB, Wu JF. Relationship between Structure and Conformational Change of the Vitamin D Receptor Ligand Binding Domain in 1alpha,25-Dihydroxyvitamin D3 Signaling. Molecules. 2015;20:20473–20486. doi: 10.3390/molecules201119713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, Kaur S, Raza K, Sansom DM. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–5164. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson KC, Kulling PM, Olson TL, Tan S-F, Rainbow RJ, Feith DJ, Loughran TP. Vitamin D decreases STAT phosphorylation and inflammatory cytokine output in T-LGL Leukemia. Cancer Biology & Therapy. 2016 doi: 10.1080/15384047.2016.1235669. 00-00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kongsbak M, von Essen MR, Boding L, Levring TB, Schjerling P, Lauritsen JP, Woetmann A, Odum N, Bonefeld CM, Geisler C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS One. 2014;9:e96695. doi: 10.1371/journal.pone.0096695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, Fisher GJ, Voorhees JJ. 1,25-Dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol. 1999;13:1686–1694. doi: 10.1210/mend.13.10.0362. [DOI] [PubMed] [Google Scholar]
- 50.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Morgan JW, Morgan DM, Lasky SR, Ford D, Kouttab N, Maizel AL. Requirements for induction of vitamin D-mediated gene regulation in normal human B lymphocytes. J Immunol. 1996;157:2900–2908. [PubMed] [Google Scholar]
- 52.Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, Mathieu C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 53.Welcher AA, Boedigheimer M, Kivitz AJ, Amoura Z, Buyon J, Rudinskaya A, Latinis K, Chiu K, Oliner KS, Damore MA, Arnold GE, Sohn W, Chirmule N, Goyal L, Banfield C, Chung JB. Blockade of interferon-gamma normalizes interferon-regulated gene expression and serum CXCL10 levels in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2015;67:2713–2722. doi: 10.1002/art.39248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi T, Momoi Y, Iwasaki T. Cyclosporine A inhibits the mRNA expressions of IL-2, IL-4 and IFN-gamma, but not TNF-alpha, in canine mononuclear cells. J Vet Med Sci. 2007;69:887–892. doi: 10.1292/jvms.69.887. [DOI] [PubMed] [Google Scholar]
- 55.Fellman CL, Archer TM, Stokes JV, Wills RW, Lunsford KV, Mackin AJ. Effects of oral cyclosporine on canine T-cell expression of IL-2 and IFN-gamma across a 12-h dosing interval. J Vet Pharmacol Ther. 2016;39:237–244. doi: 10.1111/jvp.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Archer TM, Fellman CL, Stokes JV, Pinchuk LM, Lunsford KV, Pruett SB, Langston VC, Mackin AJ. Pharmacodynamic monitoring of canine T-cell cytokine responses to oral cyclosporine. J Vet Intern Med. 2011;25:1391–1397. doi: 10.1111/j.1939-1676.2011.00797.x. [DOI] [PubMed] [Google Scholar]
- 57.Fellman CL, Stokes JV, Archer TM, Pinchuk LM, Lunsford KV, Mackin AJ. Cyclosporine A affects the in vitro expression of T cell activation-related molecules and cytokines in dogs. Vet Immunol Immunopathol. 2011;140:175–180. doi: 10.1016/j.vetimm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Kuga K, Nishifuji K, Iwasaki T. Cyclosporine A inhibits transcription of cytokine genes and decreases the frequencies of IL-2 producing cells in feline mononuclear cells. J Vet Med Sci. 2008;70:1011–1016. doi: 10.1292/jvms.70.1011. [DOI] [PubMed] [Google Scholar]
- 59.Andersson J, Nagy S, Groth CG, Andersson U. Effects of FK506 and cyclosporin A on cytokine production studied in vitro at a single-cell level. Immunology. 1992;75:136–142. [PMC free article] [PubMed] [Google Scholar]
- 60.Oray M, Toker E. Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea. 2013;32:1149–1154. doi: 10.1097/ICO.0b013e31828ffdf8. [DOI] [PubMed] [Google Scholar]
- 61.Ahn JK, Seo JM, Yu J, Oh FS, Chung H, Yu HG. Down-regulation of IFN-gamma-producing CD56+ T cells after combined low-dose cyclosporine/prednisone treatment in patients with Behcet’s uveitis. Invest Ophthalmol Vis Sci. 2005;46:2458–2464. doi: 10.1167/iovs.04-0792. [DOI] [PubMed] [Google Scholar]
- 62.Renne C, Benz AH, Hansmann ML. Vitamin D3 receptor is highly expressed in Hodgkin’s lymphoma. BMC Cancer. 2012;12:215. doi: 10.1186/1471-2407-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mrotzek C, Felcht M, Sommer A, Schrader A, Klemke CD, Herling M, Schlaak M, Fabri M. Vitamin D controls apoptosis and proliferation of cutaneous T-cell lymphoma cells. Exp Dermatol. 2015;24:798–800. doi: 10.1111/exd.12746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.