Abstract
Fetal alcohol spectrum disorder (FASD), the result of fetal alcohol exposure (FAE), affects 2–11% of children worldwide, with no effective treatments. Hippocampus-based learning and memory deficits are key symptoms of FASD. Our previous studies show hypothyroxinemia and hyperglycemia of the alcohol-consuming pregnant rat, which likely affects fetal neurodevelopment. We administered vehicle, thyroxine (T4) or metformin to neonatal rats post-FAE and rats were tested in the hippocampus dependent contextual fear-conditioning paradigm in adulthood. Both T4 and metformin alleviated contextual fear memory deficit induced by FAE, and reversed the hippocampal expression changes in the thyroid hormone-inactivating enzyme, deiodinase-III (Dio3) and insulin-like growth factor 2 (Igf2), genes that are known to modulate memory processes. Neonatal T4 restored maternal allelic expressions of the imprinted Dio3 and Igf2 in the adult male hippocampus, while metformin restored FAE-caused changes in Igf2 expression only. The decreased hippocampal expression of DNA methyltransferase 1 (Dnmt1), that maintains the imprinting of Dio3 and Igf2 during development, was normalized by both treatments. Administering Dnmt1 inhibitor to control neonates resulted in FAE-like deficits in fear memory and hippocampal allele-specific expression of Igf2, which were reversed by metformin. We propose that neonatal administration of T4 and metformin post-FAE affect memory via elevating Dnmt1 and consequently normalizing hippocampal Dio3 and Igf2 expressions in the adult offspring. The present results indicate that T4 and metformin, administered during the neonatal period that is equivalent to the third trimester of human pregnancy, are potential treatments for FASD and conceivably for other neurodevelopmental disorders with cognitive deficits.
Introduction
Despite efforts in awareness and prevention, one in ten pregnant women still reports alcohol consumption.1 As a result, fetal alcohol spectrum disorder (FASD) affects 2–11% of children worldwide, with increasing prevalence and presumably even more unreported and undiagnosed cases.2 Despite its significance, there are still no validated biological treatments for FASD.3, 4 Some preclinical studies suggest that choline administration is beneficial,5, 6 while others found it without effect for FASD.7 Current treatments, such as stimulants, antidepressants, neuroleptics, and anti-anxiety drugs, alleviate those FASD symptoms common to many psychiatric disorders but are not specific for FASD.8 Therefore, specific treatments are needed to prevent or reverse fetal alcohol-induced defects.
Hippocampal development is impaired in human FASD,9 consequently some of the most debilitating effects of FASD are on hippocampus-based learning and memory10 that is mirrored in animal models of fetal alcohol exposure (FAE).11, 12 The cause of this cognitive vulnerability is not yet known, but one possible mechanism is via abnormal thyroid hormone levels during development of the alcohol-exposed fetus.13, 14 Excessive alcohol consumption decreases thyroxine (T4) levels,15–17 and alcohol use during pregnancy has been reported with significant changes in thyroid function of neonates.18, 19 Preclinical studies demonstrate that maternal alcohol consumption during pregnancy interferes with thyroid hormone availability or function.11, 13, 14, 20–22 Furthermore, clinical or subclinical hypothyroidism of the mother negatively affects neuropsychological development of the child,23, 24 and experimental hypothyroidism in developing rats results in impaired learning.12, 21
Sufficient levels of thyroid hormones are essential for normal brain development and fetus is dependent on maternal T4 prior to the adequate functioning of its own thyroid glands.25 Maternal T4 reaching the fetal brain is being deiodinated to the biologically active form of thyroid hormone (triiodothyronine, T3) in the glia and then transported to the neuron.26 In the neuron, right amount of T3 can regulate the transcription of thyroid hormone-dependent genes. Thus, ethanol-induced maternal hypothyroxinemia can limit the availability of T3 to the fetal brain and affect the regulation of neurodevelopmental genes.
Alternatively, even if necessary amount of T3 reaches the fetal neurons, it can be excessively metabolized by elevated levels of thyroid hormone-inactivating enzyme, deiodinase-III (Dio3) to its inactive form.27 Indeed, increased hippocampal Dio3 expression leads to decreased local T3 levels with subsequent changes in target gene transcription.27 Administration of T4 during gestation normalizes the increased transcript levels of Dio3 in the hippocampus of in utero ethanol-exposed adult offspring.28 Therefore, administering T4 to the ethanol-consuming dams can be effective via reversing the maternal hypothyroxinemia14 and/or by reducing the expression of Dio3 in the fetal and subsequently the adult hippocampus. Both mechanisms can result in alleviation of FAE-caused hippocampus-based cognitive deficits of the adult offspring, as observed.11, 21, 28
Abnormal thyroid function is often concomitant with glucose metabolic dysfunction.29 Both the ethanol-consuming dams and their adult offspring are indeed hyperglycemic without any changes in their insulin levels.30, 31 This phenomenon suggests insulin resistance, namely an increase in release of insulin from the pancreas is required to maintain normal plasma glucose levels. Since insulin-pathway genes, including insulin-like growth factor 2 (Igf2), are associated with hippocampus-based learning and memory processes,32–35 peripheral and central insulin resistance may also contribute to FAE-induced learning and memory deficits.36 Decreased hippocampal levels of Igf2 is detrimental to cognition.37 Given that FAE leads to decreased Igf2 expression during development,38, 39 normalizing Igf2 expression could reverse FAE- induced cognitive deficits.40 Metformin, the most widely used insulin-sensitizing drug, is known to affect Igf2 expression,41 provide neuroprotection against ethanol-induced neurodegeneration,42 enhance short-term memory43 and spatial memory formation.44 Thus, metformin is a logical choice to explore as a potential treatment to reverse FAE-induced memory deficits.
Both Dio3 and Igf2 are imprinted genes, known to be preferentially expressed from the paternal allele in the placenta.45, 46 However, both of these genes show a preferential maternal expression in the adult hippocampus.12, 47 The reason for this “switch” from paternal to maternal expression is not known, but imprinting in general is regulated by differential DNA methylation of the maternal and paternal allele.48 Because DNA methylation is altered by FAE 39, 49–51, the FAE-engendered changes in allelic expression of Dio321 and Igf238 likely occur via modifications of DNA methylation in these imprinted loci.52 Methylation maintenance during development by the DNA methyltransferase 1 (Dnmt1)48 and expression regulation by the enhancer-blocker CCCTC-binding factor (Ctcf) binding sites are highly analogous in the Dio3 and Igf2 imprinted regions.53 If FAE affects DNA methylation and imprinting processes of these genes similarly, the potential beneficial effects of neonatal T4 or metformin treatment could be via reversing the effects of FAE on these imprinted genes and their common regulators.
The primary objective of this study was to identify the effects of neonatal administration of T4 or metformin, respectively, on adult FAE offspring with the goal of finding potential treatment for FAE-induced cognitive deficits. The desirable time frame of treatment is the period post-FAE, and in a neurodevelopmental stage that is equivalent to the third trimester of human pregnancy. Successful treatment at this period increases our study’s translational value, since some women reduce alcohol consumption towards the end of pregnancy,54 neurodevelopment can still be affected, and administration of treatments to the women is more feasible than treating the newborns. The secondary objective was to determine whether neonatal treatment with T4 or metformin would normalize hippocampal expressions of Dio3 and Igf2. Therefore, we administered T4 and metformin to neonatal rats during postnatal days (PD) 1–10 after the maternal consumption of alcohol ceased, when the offspring have already been exposed to the detrimental effects of alcohol. Both male and female offspring were investigated, as sex differences in the effects of FAE have been reported both in human 55 and animal studies. 56, 57 We hypothesized that altered maternal thyroid and glucose functions by alcohol consumption generates an adverse intrauterine environment for the development of the offspring. We further hypothesized that these adverse effects can be reversed by neonatal T4 and metformin administration within a specific developmental window.
Materials and Methods
Please see Supplemental Materials and Methods for a more detailed description of the methods.
Animals
All animal procedures were approved by the Northwestern University Animal Care and Use Committee. All rats were housed in controlled environment and received water ad libitum. For the ex vivo and in vivo treatment studies, maternal diets and animal procedures were performed as described previously.58 Briefly, adult female Sprague-Dawley (S) rats (Harlan, Indianapolis, IN, USA) were mated with adult Brown Norway (B) males (Charles River, Wilmington, MA, USA). We chose to study the S by B (SB) offspring because of their vulnerability to FAE.12, 27 This cross also allows studying allele-specific expression of imprinted genes. Pregnant females received control (C, ad libitum standard lab chow) or pair-fed (PF) and ethanol (E) liquid diets (Lieber-DeCarli ‘82; Bio-Serv. Frenchtown, NJ, USA) during gestation days (G) 8–21. The E diet contained 5% ethanol (w/v, 35% ethanol-derived calories). PF dams received the same amount of isocaloric liquid diet as the paired E dams. Regular laboratory chow was provided ad libitum to all pregnant rats on G21 and their offspring.
Neonates from each prenatal diet group (C, PF and E) and each litter received T4 (0.05µg/gr/day) (Sigma, St. Louis, MO, USA)59 or metformin (200µg/gr/day) (Sigma, St. Louis, MO, USA),60 or distilled water as vehicle by intraperitoneal injection in a volume of 10µl/gr for 10 days, PD1–10. Two different cohorts of animals were used in the T4 and metformin studies with their own matched vehicle littermates. The two sets of vehicle cohorts were statistically the same and therefore combined throughout the study.
The thyroxin dose administered to neonates restored the attenuated thyroid stimulating hormone (TSH) levels in the adult FAE offspring without altering TSH levels of the control animals (Supplemental Figure 1). The dose of metformin has been chosen from published data,60 and the human equivalent of this dose is 2400 mg/75kg/per day,61 which is within the recommended dose range.
In two additional studies, PD1 pups of C animals received vehicle, 5-aza-2’-deoxycytidine (5-Aza; 1µg/gr/day) (Sigma, St. Louis, MO, USA)62 or 5-Aza and metformin (5-Aza+Met; 1µg/gr/day and 200µg/gr/day, respectively) by intraperitoneal injection for 10 days.
The number of animals in each experimental condition is provided in the figure legends.
Primary hippocampal culture
Primary hippocampal cultures were prepared from embryonic day 18 fetuses of dams on C, PF, or E diets (n=3 dams/diet) as described previously.58 Hippocampal neurons were plated at a density of 8×105 cells/60mm dish and cultured for 10 days. Metformin (final concentration of 0.4mM in sterile water) was administered every 48h.63 All cultures were incubated at 37°C with 5% CO2 and processed for RNA isolation.
Behavioral Testing
Context Dependent Fear Conditioning (FC)
1–2 male and female adult offspring per litter were tested as described previously.21 Rats were placed into the automated FC apparatus (TSE, Bad Homburg, Germany) for 3min followed by three mild shocks (0.8mA, 1sec each, 60sec between each shock). 24h later, the animals were placed into the same chamber for 3min without shock exposure, and their freezing behavior was measured. Rats that did not respond to the initial shock were excluded from the study. Two weeks later, adult offspring were sacrificed between 10:00–12:00h.
RNA Isolation and Quantitative Real-Time PCR (qPCR)
Whole hippocampi were dissected as described64 and collected directly into RNAlater reagent (Ambion, Austin, TX, USA). RNA was isolated by using Direct-zol™ RNA MiniPrep (Zymo Research, Orange, CA, USA) and reverse transcription was performed using SuperScript® VILO™ Master Mix (Invitrogen, Carlsbad, CA, USA). qPCR was conducted as described previously58 with 5ng cDNA, specific primer pairs (Supplemental Table 1) and SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) using the ABI 7900HT cycler.
Pyrosequencing
We used the identified SNPs in the Dio3 exon12 and in the 3′untranslated region of Igf247 between S and B strains to track allele-specific expression. PCR was conducted using a forward primer and biotinylated reverse primer flanking the SNPs. Purification of biotinylated PCR products and pyrosequencing were performed by EpigenDx, Inc. using a sequencing forward primer.
Statistical analysis
Ex vivo data were analyzed by two-way ANOVA (prenatal diet and ex vivo treatment). All in vivo data containing prenatal diet were analyzed by three-way ANOVA (prenatal diet, neonatal drug treatment and sex). Significant ANOVA results were followed by Bonferroni post-hoc tests corrected for multiple comparisons. In the 5-Aza studies, data were analyzed by two-way ANOVA (neonatal drug treatment and sex). Since there were no differences in sex effects, male and female data of the 5-Aza studies were combined and analyzed by unpaired two-tailed Student’s t-test. All statistical analyses were performed using Systat (Chicago, IL, USA) and GraphPad Prism 7 (La Jolla, CA, USA) programs.
Results
Please see Table 1 for the summary of the different neonatal treatments’ effects on the measured consequences of FAE.
Table 1.
Summary of changes as a result of neonatal treatments
| Allelic Expression | Total Expression | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weaning Weight | Adult Weight | Fear Memory | Dio3 | Igf2 | Dio3 | Igf2 | Dnmt1 | Ctcf | ||||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| FAE+T4 | ↔ | ↔ | ↔ | ↑ | ↑ | ↑ | ↑ | NC | ↑ | NC | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↔ | ↔ |
| FAE+Metformin | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↔ | NC | ↑ | NC | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
| 5-Aza+Metformi n | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↔ | ↔ | ↑ | NC | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
↔ Did not differ from FAE
↓ or ↑ Reversed by treatment
NCT No FAE-related changes
Neonatal T4 treatment restores FAE-induced hippocampal fear memory deficit and Dio3 expression
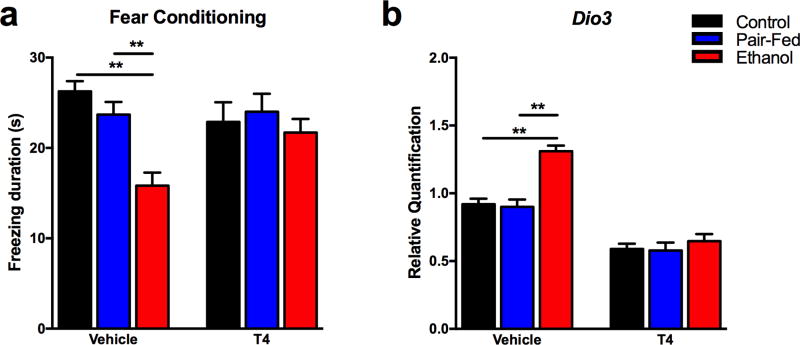
Fetal alcohol exposure resulted in a fear memory deficit in male and female adults, measured as decreased freezing duration in the contextual fear conditioning test (F(2,110)=5.75, p<0.01, sex*diet, F(2,110)=2.78, NS) (Figure 1a). Neonatal T4 treatment, administered after FAE, alleviated this deficit without affecting the memory of C and PF offspring (diet*drug, F(2,110)=3.53, p<0.05).
Figure 1. Hippocampal contextual fear memory deficit and increased Dio3 expression in adult FAE offspring are alleviated by neonatal T4 treatment.
a) Memory deficit was measured as decreased time (in seconds) spent freezing in the second day of contextual FC test. Male and female neonates of dams on different diet from gestation day 8–21 received either T4 (0.05 µg/gr/day) or vehicle treatment between postnatal days 1 and 10, and were tested in adulthood (N = 16–24/prenatal diet/neonatal treatment; sex combined). b) Hippocampal transcript levels of Dio3 were measured by qPCR . N = 8–15/prenatal diet/neonatal treatment. Data represented as mean ± SEM. *p<0.05, **p<0.01, Bonferroni post-hoc test within neonatal treatments.
Expression of hippocampal Dio3 was increased in the hippocampus of male and female E offspring (diet, F(2,57)=10.89, p<0.01) (Figure 1b). Neonatal T4 administration reduced Dio3 expression at large, even more so in the E offspring to the levels of T4-treated controls (drug, F(1,57)=117.37, p<0.01; diet*drug, F(2,57)=4.92, p<0.01).
Body weights of all groups of animals at weaning and in adulthood are shown in Supplemental Tables 2 and 3.
Metformin affects FAE-induced gene expression changes in primary hippocampal culture
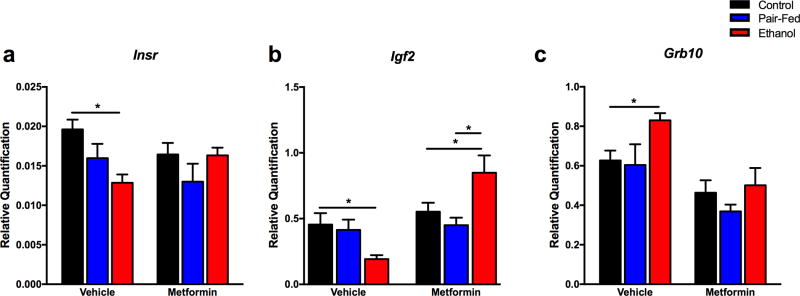
Since metformin has never been employed in FAE studies, its efficacy was evaluated first in our ex vivo primary culture model.53 Metformin is known to affect insulin pathway genes and its effect on the FAE-induced changes of these genes were examined.58 The ex vivo model consisted of our routine FAE ethanol administration in vivo and subsequent evaluation of gene expression after 10 days of metformin or vehicle treatment of the fetal hippocampal culture.58 Among the previously validated insulin pathway genes, FAE effects persisted after 10 days in culture for transcript levels of insulin receptor (Insr) (F(2,33)=3.74, p<0.05), Igf2 (F(2,35)=7.07, p<0.01) and growth factor receptor bound protein 10 (Grb10) (F(2,37)=3.58, p<0.05) (Figure 2a–c). Metformin treatment reversed the FAE-induced decrease in the expression of Insr and Igf2 (Insr: diet*drug, F(2,33)=3.08, p=0.05; Igf2: drug F(1,35)=16.5, p<0.01; diet*drug F(2,35)=9.33, p<0.01) (Figure 2a,b). Metformin also normalized the FAE-induced increase in Grb10 levels (drug, F(1,37)=19.76, p<0.01) (Figure 2c).
Figure 2. Administration of metformin reverses FAE-induced changes in gene expression in the ex vivo primary hippocampal culture.
Metformin (0.4mM, every 48 h, for 10 days) was administered into primary hippocampal culture obtained from fetuses of the three prenatal diet groups (males and females combined). Transcript levels of a) Insr, N = 5–9/prenatal diet/ treatment, b) Igf2, N = 6–8/prenatal diet/treatment, and c) Grb10, N = 6–9/prenatal diet/treatment. Details are as described in Figure 1.
Effects of neonatal metformin treatment on FAE-induced fear memory deficit and hippocampal Igf2 expression
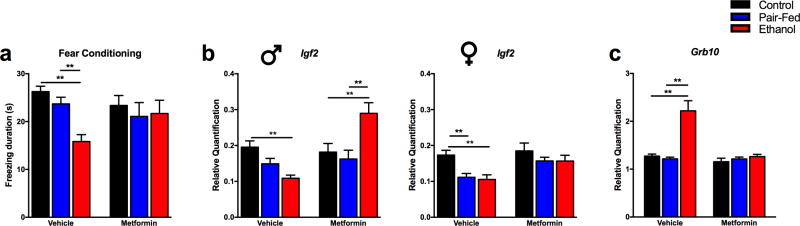
Neonatal metformin treatment tended to alleviate the FAE-induced fear memory deficit in both male and female adult offspring (diet, F(2,93)=6.37, p<0.01; sex*diet, F(2,93)=1.09, NS; drug, F(1,93)=3.56, p=0.06) (Figure 3a).
Figure 3. Neonatal metformin treatment reverses FAE-induced fear memory deficit and hippocampal Igf2 and Grb10 expression in adult offspring.
a) Metformin administration (200 µg/gr/day, from postnatal day 1 to 10) reversed the fear memory deficit of adult Ethanol offspring. N = 11–24/prenatal diet/neonatal treatment; sex combined. Transcript levels of b) Igf2, male (N = 4–11/prenatal diet/neonatal treatment) and female (N = 5–8/prenatal diet/neonatal treatment), c) Grb10 (N = 8–13/ prenatal diet/neonatal treatment, sex combined) in the hippocampus of adult offspring. Details are as described in Figure 1.
Hippocampal transcript levels of Insr were not different between the prenatal diet groups (data not shown). However, metformin not only reversed the FAE-induced decrease, but enhanced the expression of Igf2 in the adult male hippocampus (diet, F(2,71)=5.04, p<0.01; sex, F(1,71)=10.41, p<0.01; drug, F(1,71)=22.14, p<0.01; diet*drug, F(2,71)=11.32, p<0.01; sex*diet*drug, F(2,71)=6.36, p<0.01). In females, the FAE- and PF-induced decrease in Igf2 expression were normalized, but not enhanced by metformin (Figure 3b). Hippocampal Grb10 transcript levels of E male and female adult offspring were normalized by neonatal metformin (diet, F(2,55)=9.78, p<0.01; sex, F (1,55)=2.15, NS; drug, F (1,55)=16.81, p<0.01; diet*drug, F(2,55)=7.63, p<0.01) (Figure 3c).
Body weights of all groups of animals at weaning and in adulthood are shown in Supplemental Tables 4 and 5.
Treatment-specificity in hippocampal allele-specific and total expression of Dio3 and Igf2
Determining the parental contribution to the expression of the imprinted Dio3 and Igf2 genes was possible due to SNPs previously identified between the Sprague Dawley (S) and the Brown Norway (B) strains.12, 47 We were not able to track allele specific expression for Grb10 because there are no SNPs between the S and B cDNAs.
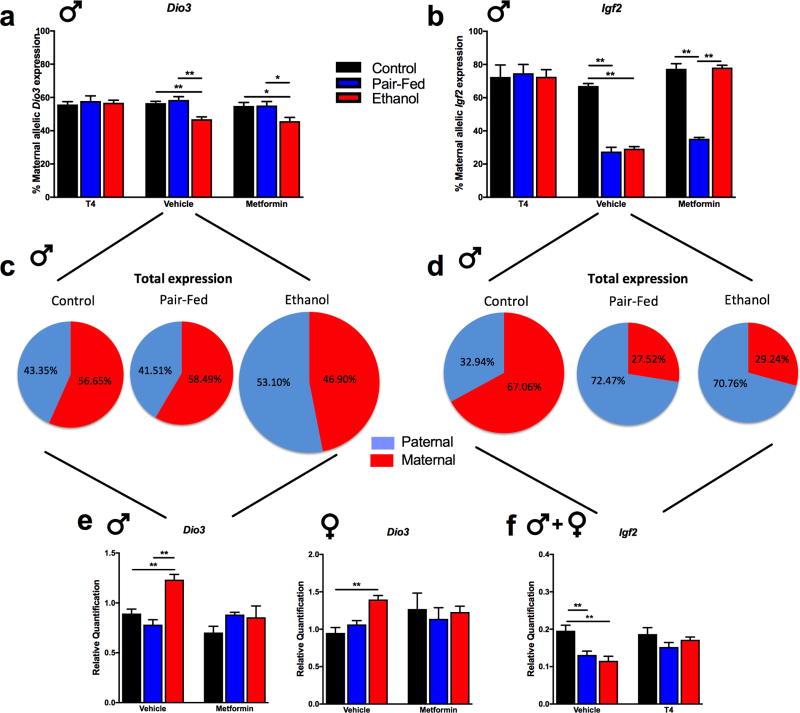
Allele-specific expression of hippocampal Dio3 was affected by FAE and neonatal T4 or metformin treatments in a sex- and treatment-specific manner. Decreased maternal allelic expression of hippocampal Dio3 tended to be normalized by T4 in the adult FAE male (diet, F(2,65)=4.07, p<0.05; drug, F(1,65)=3.08, p=0.08; diet*sex*drug, F(2,65)=2.63, p=0.08) (Figure 4a). In contrast, the decreased maternal allelic expression of hippocampal Dio3 was not normalized by metformin in the adult FAE male hippocampus (Figure 4a). There were no effects of FAE, T4 or metformin on Dio3 expression in the females (Supplemental Figure 2a).
Figure 4. Changes in allele-specific and total expression of hippocampal Dio3 and Igf2 are sex- and treatment-dependent.
Allele-specific expression in adult male hippocampus was determined by pyrosequencing using the already verified SNPs a) Dio3 (N=4–10/prenatal diet/neonatal treatment) and b) Igf2 (N=3–11/prenatal diet/neonatal treatment). Schematic representation of the maternal and the paternal allelic contributions to the total hippocampal expression of c) Dio3 and d) Igf2 (drawn not to the scale). e) Effects of neonatal metformin treatment on FAE-altered transcript levels of hippocampal Dio3 in adult offspring (N=4–8/sex/prenatal diet/neonatal treatment). f) Total transcript levels of Igf2 in the hippocampus of adult offspring in response to FAE and neonatal T4 treatment (N = 8–20/prenatal diet/neonatal treatment, sex combined). Details are as described in Figure 1.
Allelic expression of hippocampal Igf2 also showed sex differences in response to FAE and was affected by both treatments. Maternal contribution to Igf2 expression was significantly lower in the adult PF and FAE male offspring compared to controls (diet, F(2,69)=23.76, p<0.01; sex*diet, F(2,69)=49.40, p<0.01) (Figure 4b). Both T4 and metformin treatment reversed the FAE-induced decrease (T4: drug, F(1,67)=133.68, p<0.01; sex*drug, F(1,67)=4.85, p<0.05; metformin: drug, F(1,69)=32.44, p<0.01; sex*drug, F(1,69)=30.45, p<0.01). However, only the neonatal T4 treatment restored the decrease in maternal allelic Igf2 expression in the adult male PF hippocampus (Figure 4b). There were no effects of FAE or treatments on Igf2 expression in the females (Supplemental Figure 2b).
To interpret the parental dosage effects (allelic expression) in the light of total expression, a schematic representation of the maternal and the paternal allelic contributions to the total hippocampal expression of Dio3 and Igf2 is shown in Figure 4c and d, respectively (drawn not to scale).
The effects of T4 and metformin on the FAE-induced changes in the hippocampal total expression of Dio3 and Igf2 has been shown on Figures 1b and 3b, respectively. Should an overlapping mechanism be responsible for these treatment effects, T4 and metformin would also alter FAE-induced changes in Igf2 and Dio3, respectively. Indeed, the FAE-induced increase in the total expression of Dio3 was normalized by metformin in the hippocampus of adult male, while the correction by metformin was not complete in the female (sex, F(1,62)=27.53, p<0.01; diet*drug, F(2,62)=4.32, p<0.05; sex*drug, F(1,62)=9.61, p<0.01; diet*sex*drug, F(2,62)=3.00, p=0.05; E vehicle vs. E metformin p<0.01) (Figure 4e). Decreased transcript levels of Igf2 in the E and PF hippocampus were restored to control levels by neonatal T4 (drug, F(1,63)=9.20, p<0.01; diet*drug, F(2,63)=3.42, p<0.05) in both sexes equally (Figure 4f). These latter effects parallel those of T4 on Dio3 expression (see Figure 1b).
Dnmt1 as potential mediator of FAE effects
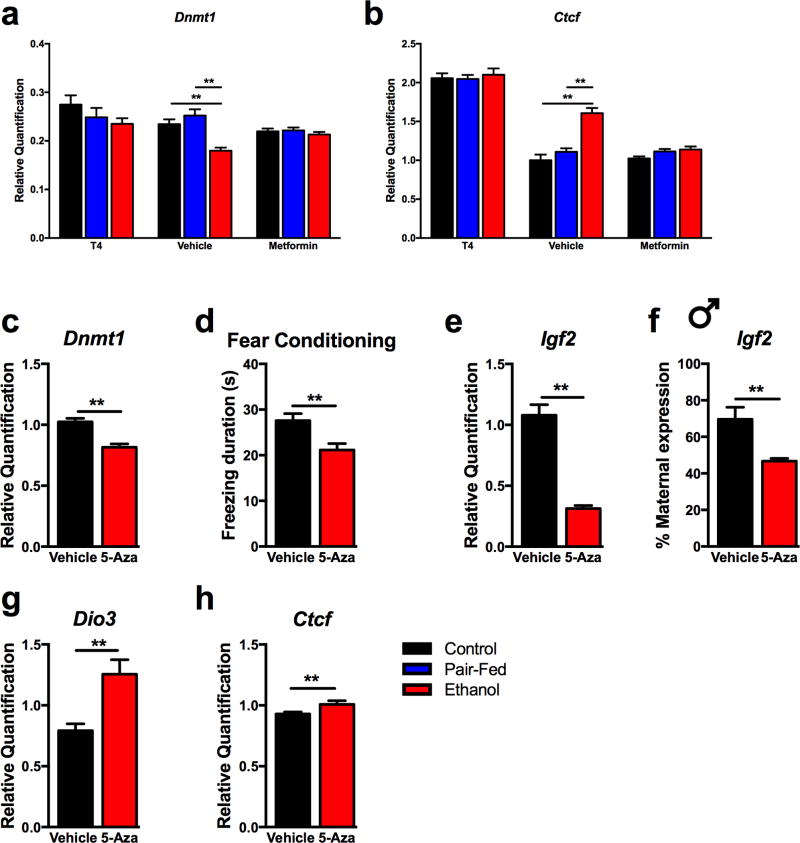
Dnmt1 is involved in learning and memory processes, 65 known to be affected by FAE66 and it is the primary regulator of DNA methylation maintenance relevant to allele-specific imprinting processes.48 Transcript levels of Dnmt1 were decreased in the adult hippocampus by FAE (diet, F(2,69)=15.21, p<0.01) and normalized by both treatments with no sex effects (T4: drug, F(1,63)=13.22, p<0.01; diet*drug, F(2,63)=3.13, p<0.05; metformin: diet*drug, F(2,69)=9.37, p<0.01) (Figure 5a). Ctcf, one of the main regulator of allele-specific Igf2 expression,67 showed increased hippocampal expression after FAE (diet, F(2,52)=20.92, p<0.01). Neonatal T4 did not reverse this effect, but rather it increased Ctcf expression equally in all prenatal treatment groups (T4: drug, F(1,45)=197.39, p<0.01; diet*drug, F(2,45)=31.49, p<0.01) (Figure 5b). In contrast, neonatal metformin reversed the FAE-induced increase in Ctcf expression (metformin: drug, F(1,52)=4.35, p<0.05; diet*drug, F(2,52)=5.54, p<0.01) (Figure 5b).
Figure 5. The role of Dnmt1 in FAE-induced deficits.
a) Hippocampal Dnmt1 expression was affected by FAE and alleviated by neonatal treatments (N = 7–20/prenatal diet/neonatal treatment, sex combined). b) Treatment-specific changes in adult hippocampal Ctcf expression (sex combined T4: N = 9–10/prenatal diet/neonatal treatment; Metformin: N = 7–13/prenatal diet/neonatal treatment). Data is normalized to Vehicle Control animals. c-h) Administration of Dnmt1 inhibitor 5-aza-2’-deoxycytidine (5-Aza, 1 µg/gr/day, from postnatal day 1 to 10) to control neonates mimicked the effects of FAE in fear memory deficit and hippocampal gene expression changes in the adult offspring. N=13–14/neonatal treatment, sex combined, except f) N=4–5 males/neonatal treatment. Details are as described in Figure 1.
As a proof of concept for the role of Dnmt1 in FAE-induced memory deficit, 5-Aza, a Dnmt inhibitor that preferentially inhibits Dnmt166, 68 was administered to control neonates between postnatal days 1 and 10. As expected, 5-Aza treatment reduced Dnmt1 expression in the adult hippocampus (drug, F(1,25)=24.46, p<0.01) (Figure 5c). Neonatal 5-Aza treatment mimicked the effects of FAE on fear memory in both male and female adults (drug, F(1,25)=7.19, p<0.05) (Figure 5d). Total and maternal allelic expression of hippocampal Igf2 were decreased by 5-Aza, the latter with a male-specificity similar to the FAE effects (allelic expression in males, F(1,13)=12.59, p<0.01; total expression in males and females, F(1,24)=72.26, p<0.01) (Figure 5e–f). No effect of 5-Aza treatment has been seen in the allelic expression of Igf2 in the female hippocampus, and in Dio3 allelic expression in either sex (Supplemental Figures 3 and 4). However, total hippocampal expression of both Dio3 and Ctcf were increased by the 5-Aza treatment, again confirming the similarity to FAE effects (Dio3, F(1,24)=61.02, p<0.01; Ctcf, F(1,25)=4.52, p<0.05) (Figure 5g–h). Metformin administered together with 5-Aza eliminated all of the 5-Aza effects (Supplemental Figure 5).
Body weights at weaning and in adulthood are shown in Supplemental Table 6.
Discussion
The major finding of the present study reveals the therapeutic potential of neonatal T4 and metformin treatments, administered after fetal alcohol exposure, for reversing hippocampus-dependent cognitive deficits in adult offspring. The mechanism by which these treatments are effective is via restoring the FAE-induced decrease in hippocampal Dnmt1 expression. Indeed, both FAE and direct inhibition of Dnmt1 caused a memory deficit, while reversing the decreased Dnmt1 expression by neonatal T4 and metformin treatments normalized fear memory. These treatments also restored FAE-induced changes in hippocampal total expression of Dio3 and Igf2, two imprinted genes known to affect hippocampal memory processes. However, only the male hippocampus showed allelic expression changes of Dio3 and Igf2 post-FAE, of which only Igf2 were normalized by both of these treatments.
Dnmt1 is the major maintenance DNA methyltransferase that preserves DNA methylation patterns.48 Recently, Dnmt1 has been associated with the etiology of autism,69 neurodegenerative diseases, and other CNS disorders.65 Decreased transcript levels of Dnmt1 impairs learning and memory processes.70, 71 Correspondingly, administering the Dnmt-inhibitor 5-Aza impairs the formation of contextual fear memory.72 Our results are in agreement with these findings since both FAE and neonatal 5-Aza administration decreased hippocampal expression of Dnmt1, in parallel with contextual fear memory. Conversely, the FAE-induced deficits in Dnmt1 expression and memory were both reversed by neonatal T4 and metformin treatments. Similarly, choline deficiency and supplementation modulate Dnmt1 expression in parallel with learning and memory performance.73, 74 The effect of choline on Dnmt1 is relevant to this study, because preclinical studies suggest that choline administration is also beneficial for FAE-induced learning and memory deficits.5, 6
Dio3 and Igf2 are two imprinted genes with allele-specific methylation patterns conserved by Dnmt1.48 Dnmt1 expression is inversely correlated with the expression of Dio375 and linearly with Igf2.76 These patterns are confirmed in this study, where after FAE adult hippocampus showed increased Dio3 and decreased Igf2 expression concurrently with decreased Dnmt1 expression. Treatments with T4 and metformin reversed these effects and also normalized fear memory. Indeed, decreasing hippocampal levels of Dio3 and increasing that of Igf2 are known to improve memory.21, 28, 77 As the FAE-induced deficit in Dnmt1 expression is also normalized by the treatments, and Dnmt1 is the major regulator of imprinting maintenance, the possible involvement of other imprinted genes in reversing the memory deficit after FAE still exist.
Sex specificity of the FAE effects and of the treatments was also observed in the allelic-expression of Dio3 and Igf2 in the hippocampus, since neither FAE nor the treatments altered allelic expression of these genes in adult females. This sex specific effect of FAE on the allelic expression of two imprinted genes with different imprinting regulators could be caused either by sex differences in the imprinting processes or by peripubertal estrogen-induced compensatory mechanisms. Other developmental studies have shown sex-specific consequences of in utero treatments in the expression of Dio378 and male-specific FAE effects have also been observed for Dio3.12, 28 Interestingly, structural sex differences have also been found in human FASD subjects, although the small sample size in that study precluded to identify sex differences in memory performance.10 Nevertheless, smaller size of anterior hippocampus is found in FASD males, but not females, compared to controls. It appears that in general males demonstrate greater in utero vulnerability.79 FASD also shows higher prevalence in boys,55 confirming to this general finding. Male-specific vulnerability may relate to the sequence of sex-specific timing of re-methylation and imprint establishment and the period of alcohol exposure. As a result, sex differences in the effect of FAE on allele-specific expression of imprinted genes could occur.
The sex-specific effects that emerge after puberty could be the consequence of estrogen-induced compensatory regulation of Dio3 and Igf2 expression80, 81 or the sex-specificity of their epigenetic regulation during this period.82, 83 The divergence of the total expression from the sex-specific allelic expression further suggests hormonal regulation of either the allele-specific or the total expression of Dio3 and Igf2. Interestingly, opposite to the current findings, female-specificity in the total expression of H19, a long noncoding RNA reciprocally imprinted with Igf2, has been found without sex differences in allelic expression.84 Since estrogen responsive elements are present in both the Dio3 and Igf2 imprinted regions (Supplementary Figure 6), estrogen-induced increases in maternal allelic expressions of these genes could compensate for the FAE effects in the female hippocampus.
Limitations of the current study include the timing of the treatments, although we have changed it from maternal21 to neonatal T4 administration in order to reverse the detrimental effects post-FAE. Ideally, the time frame of treatment should be expanded all the way to puberty, as it is argued that neurodevelopmental changes still occur during puberty.83 Furthermore, different alcohol exposure regimens and species could be investigated for determining the general efficacy of these treatments. These future explorations will provide further confirmation for the translational value of T4 and metformin in the treatment of FASD. Another interesting finding, although not a limitation, in this study is the pair-feeding effects on the hippocampal expression of Igf2. Recently described consequences of restricted feeding indicate major changes in adult hippocampal total and allele-specific expression of Igf2 similarly to that found in the present study for pair-feeding.47 The significance that metformin reversed the allele-specific expression changes caused by FAE, but not by pair-feeding, suggests that FAE effects are reversible (at least in this time-frame), while those of PF are not. This latter suggestion is in agreement with the conclusions of the Dutch famine study,85 where altered methylation of the IGF2 imprinting control region and IGF2 expression persist way into adulthood.
In the current study, both neonatal T4 and metformin administration mitigated FAE-induced hippocampal fear memory deficits, even when administered after the termination of in utero ethanol exposure. The convergent effect of neonatal T4 and metformin treatments in reversing this memory deficit in parallel with increasing hippocampal Dnmt1 expression suggests methylation maintenance as a common mechanism of these treatments. As neonatal treatments restored hippocampal Dio3 and Igf2 expressions in addition to those of Dnmt1, it is possible that both of these treatments affect memory via elevating Dnmt1 and consequently Dio3 and Igf2 expressions in the in utero ethanol exposed adult offspring. These results are the first to show that post-FAE T4 or metformin are potential therapeutic agents for FASD when administered in a time-frame equivalent to the third trimester in human pregnancy. The translational value of this study is further increased by the fact that T4 and metformin are commonly and safely used in pregnant women and, therefore, they could be explored in clinical trials for the treatment of FASD.
Supplementary Material
Acknowledgments
We thank Sarah Chung, Wendy Luo, Kathryn M. Harper, Jeanie K. Meckes and Laura J. Sittig for their contributions to this manuscript.
This work was supported by NIH AA017978 to EER.
Footnotes
Competing Financial Interests
All authors reported no financial interests or potential conflicts of interest.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Green PP, McKnight-Eily LR, Tan CH, Mejia R, Denny CH. Vital Signs: Alcohol-Exposed Pregnancies--United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2016;65:91–97. doi: 10.15585/mmwr.mm6504a6. [DOI] [PubMed] [Google Scholar]
- 2.Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res. 2016;40:18–32. doi: 10.1111/acer.12939. [DOI] [PubMed] [Google Scholar]
- 3.Murawski NJ, Moore EM, Thomas JD, Riley EP. Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies. Alcohol Res. 2015;37:97–108. [PMC free article] [PubMed] [Google Scholar]
- 4.Petrenko CL, Alto ME. Interventions in fetal alcohol spectrum disorders: An international perspective. Eur J Med Genet. 2016 doi: 10.1016/j.ejmg.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas JD, Idrus NM, Monk BR, Dominguez HD. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 2010;88:827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birch SM, Lenox MW, Kornegay JN, Paniagua B, Styner MA, Goodlett CR, et al. Maternal choline supplementation in a sheep model of first trimester binge alcohol fails to protect against brain volume reductions in peripubertal lambs. Alcohol. 2016;55:1–8. doi: 10.1016/j.alcohol.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosenbocus S, Chahal R. A review of executive function deficits and pharmacological management in children and adolescents. J Can Acad Child Adolesc Psychiatry. 2012;21:223–229. [PMC free article] [PubMed] [Google Scholar]
- 9.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- 10.Dudek J, Skocic J, Sheard E, Rovet J. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J Int Neuropsychol Soc. 2014;20:181–191. doi: 10.1017/S1355617713001343. [DOI] [PubMed] [Google Scholar]
- 11.Wilcoxon JS, Kuo AG, Disterhoft JF, Redei EE. Behavioral deficits associated with fetal alcohol exposure are reversed by prenatal thyroid hormone treatment: a role for maternal thyroid hormone deficiency in FAE. Mol Psychiatry. 2005;10:961–971. doi: 10.1038/sj.mp.4001694. [DOI] [PubMed] [Google Scholar]
- 12.Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB J. 2011;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott HC, Sun GY, Zoeller RT. Prenatal ethanol exposure selectively reduces the mRNA encoding alpha-1 thyroid hormone receptor in fetal rat brain. Alcohol Clin Exp Res. 1998;22:2111–2117. [PubMed] [Google Scholar]
- 14.Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]
- 15.Geurts J, Demeester-Mirkine N, Glinoer D, Prigogine T, Fernandez-Deville M, Corvilain J. Alterations in circulating thyroid hormones and thyroxine binding globulin in chronic alcoholism. Clin Endocrinol (Oxf) 1981;14:113–118. doi: 10.1111/j.1365-2265.1981.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 16.Heinz A, Bauer M, Kuhn S, Kruger F, Graf KJ, Rommelspacher H, et al. Long-term observation of the hypothalamic-pituitary-thyroid (HPT) axis in alcohol-dependent patients. Acta Psychiatr Scand. 1996;93:470–476. doi: 10.1111/j.1600-0447.1996.tb10679.x. [DOI] [PubMed] [Google Scholar]
- 17.Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–1381. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 18.Herbstman J, Apelberg BJ, Witter FR, Panny S, Goldman LR. Maternal, infant, and delivery factors associated with neonatal thyroid hormone status. Thyroid. 2008;18:67–76. doi: 10.1089/thy.2007.0180. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez JT, Hoffman L, Weavil S, Cvejin S, Prange AJ., Jr The effect of drug exposure on thyroid hormone levels of newborns. Biochem Med Metab Biol. 1992;48:255–262. doi: 10.1016/0885-4505(92)90072-7. [DOI] [PubMed] [Google Scholar]
- 20.Shukla PK, Sittig LJ, Andrus BM, Schaffer DJ, Batra KK, Redei EE. Prenatal thyroxine treatment disparately affects peripheral and amygdala thyroid hormone levels. Psychoneuroendocrinology. 2010;35:791–797. doi: 10.1016/j.psyneuen.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tunc-Ozcan E, Harper KM, Graf EN, Redei EE. Thyroxine administration prevents matrilineal intergenerational consequences of in utero ethanol exposure in rats. Horm Behav. 2016;82:1–10. doi: 10.1016/j.yhbeh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottesfeld Z, Silverman PB. Developmental delays associated with prenatal alcohol exposure are reversed by thyroid hormone treatment. Neurosci. Lett. 1990;109:42–47. doi: 10.1016/0304-3940(90)90535-h. [DOI] [PubMed] [Google Scholar]
- 23.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations experimental findings. J. Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PN, Okosieme OE, Murphy R, Hales C, Chiusano E, Maina A, et al. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: data from the Controlled Antenatal Thyroid Study. J Clin Endocrinol Metab. 2014;99:4291–4298. doi: 10.1210/jc.2014-1901. [DOI] [PubMed] [Google Scholar]
- 25.Heindel JJ, Zoeller RT. Thyroid hormone and brain development: translating molecular mechanisms to population risk. Thyroid. 2003;13:1001–1004. doi: 10.1089/105072503770867165. [DOI] [PubMed] [Google Scholar]
- 26.Freitas BC, Gereben B, Castillo M, Kallo I, Zeold A, Egri P, et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest. 2010;120:2206–2217. doi: 10.1172/JCI41977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011;16:786–787. doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tunc-Ozcan E, Ullmann TM, Shukla PK, Redei EE. Low-dose thyroxine attenuates autism-associated adverse effects of fetal alcohol in male offspring’s social behavior and hippocampal gene expression. Alcohol Clin Exp Res. 2013;37:1986–1995. doi: 10.1111/acer.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64:1130–1139. doi: 10.1111/j.1742-1241.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 30.Harper KM, Tunc-Ozcan E, Graf EN, Redei EE. Intergenerational effects of prenatal ethanol on glucose tolerance and insulin response. Physiol Genomics. 2014;46:159–168. doi: 10.1152/physiolgenomics.00181.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L, Liu Z, Gong J, Zhang L, Wang L, Magdalou J, et al. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol Appl Pharmacol. 2014;274:263–273. doi: 10.1016/j.taap.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 32.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 34.Shahmoradi A, Radyushkin K, Rossner MJ. Enhanced memory consolidation in mice lacking the circadian modulators Sharp1 and −2 caused by elevated Igf2 signaling in the cortex. Proc Natl Acad Sci U S A. 2015;112:E3582–3589. doi: 10.1073/pnas.1423989112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmeisser MJ, Baumann B, Johannsen S, Vindedal GF, Jensen V, Hvalby OC, et al. IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci. 2012;32:5688–5703. doi: 10.1523/JNEUROSCI.0111-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol. 2010;17:e390–404. [PMC free article] [PubMed] [Google Scholar]
- 37.Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haycock PC, Ramsay M. Exposure of mouse embryos to ethanol during preimplantation development: effect on DNA methylation in the h19 imprinting control region. Biol Reprod. 2009;81:618–627. doi: 10.1095/biolreprod.108.074682. [DOI] [PubMed] [Google Scholar]
- 39.Downing C, Johnson TE, Larson C, Leakey TI, Siegfried RN, Rafferty TM, et al. Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure: effects of a methyl-supplemented diet. Alcohol. 2011;45:65–71. doi: 10.1016/j.alcohol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livingstone C, Borai A. Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin Endocrinol (Oxf) 2014;80:773–781. doi: 10.1111/cen.12446. [DOI] [PubMed] [Google Scholar]
- 41.Smieszek A, Czyrek A, Basinska K, Trynda J, Skaradzinska A, Siudzinska A, et al. Effect of Metformin on Viability, Morphology, and Ultrastructure of Mouse Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells and Balb/3T3 Embryonic Fibroblast Cell Line. Biomed Res Int. 2015;2015:769402. doi: 10.1155/2015/769402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira WH, Nunes AK, Franca ME, Santos LA, Los DB, Rocha SW, et al. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016;1644:149–160. doi: 10.1016/j.brainres.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 46.Tsai CE, Lin SP, Ito M, Takagi N, Takada S, Ferguson-Smith AC. Genomic imprinting contributes to thyroid hormone metabolism in the mouse embryo. Curr Biol. 2002;12:1221–1226. doi: 10.1016/s0960-9822(02)00951-x. [DOI] [PubMed] [Google Scholar]
- 47.Harper KM, Tunc-Ozcan E, Graf EN, Herzing LB, Redei EE. Intergenerational and parent of origin effects of maternal calorie restriction on Igf2 expression in the adult rat hippocampus. Psychoneuroendocrinology. 2014;45:187–191. doi: 10.1016/j.psyneuen.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards CA, Ferguson-Smith AC. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol. 2007;19:281–289. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Garro AJ, McBeth DL, Lima V, Lieber CS. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcoholism, clinical experimental research. 1991;15:395–398. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 50.Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod. 2009;81:607–617. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- 51.Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in beta-endorphin-producing POMC neurons of the hypothalamus. Alcohol Clin Exp Res. 2013;37:1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 53.Wylie AA, Murphy SK, Orton TC, Jirtle RL. Novel imprinted DLK1/GTL2 domain on human chromosome 14 contains motifs that mimic those implicated in IGF2/H19 regulation. Genome Res. 2000;10:1711–1718. doi: 10.1101/gr.161600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, et al. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13:274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thanh NX, Jonsson E, Salmon A, Sebastianski M. Incidence and prevalence of fetal alcohol spectrum disorder by sex and age group in Alberta, Canada. J Popul Ther Clin Pharmacol. 2014;21:e395–404. [PubMed] [Google Scholar]
- 56.Varlinskaya EI, Mooney SM. Acute exposure to ethanol on gestational day 15 affects social motivation of female offspring. Behav Brain Res. 2014;261:106–109. doi: 10.1016/j.bbr.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcohol Clin Exp Res. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tunc-Ozcan E, Ferreira AB, Redei EE. Modeling Fetal Alcohol Spectrum Disorder: Validating an Ex Vivo Primary Hippocampal Cell Culture System. Alcohol Clin Exp Res. 2016;40:1273–1282. doi: 10.1111/acer.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu RB, Chaichanwatanakul K, Lin CH, Lebenthal E, Lee PC. Thyroxine effect on exocrine pancreatic development in rats. Am J Physiol. 1988;254:G315–321. doi: 10.1152/ajpgi.1988.254.3.G315. [DOI] [PubMed] [Google Scholar]
- 60.Quaile MP, Melich DH, Jordan HL, Nold JB, Chism JP, Polli JW, et al. Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol. 2010;243:340–347. doi: 10.1016/j.taap.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z, Marcucci G, Byrd JC, Grever M, Xiao J, Chan KK. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2’-deoxycytidine) by a new liquid chromatography/tandem mass spectrometry quantification method. Rapid Commun Mass Spectrom. 2006;20:1117–1126. doi: 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- 63.Gupta A, Bisht B, Dey CS. Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology. 2011;60:910–920. doi: 10.1016/j.neuropharm.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 64.Mehta-Raghavan NS, Wert SL, Morley C, Graf EN, Redei EE. Nature and nurture: environmental influences on a genetic rat model of depression. Transl Psychiatry. 2016;6:e770. doi: 10.1038/tp.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baets J, Duan X, Wu Y, Smith G, Seeley WW, Mademan I, et al. Defects of mutant DNMT1 are linked to a spectrum of neurological disorders. Brain. 2015;138:845–861. doi: 10.1093/brain/awv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou FC, Chen Y, Love A. Cellular DNA methylation program during neurulation and its alteration by alcohol exposure. Birth Defects Res A Clin Mol Teratol. 2011;91:703–715. doi: 10.1002/bdra.20820. [DOI] [PubMed] [Google Scholar]
- 67.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 68.Ghoshal K, Datta J, Majumder S, Bai S, Kutay H, Motiwala T, et al. 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Mol Cell Biol. 2005;25:4727–4741. doi: 10.1128/MCB.25.11.4727-4741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Ciernia AV, LaSalle J. The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat Rev Neurosci. 2016;17:411–423. doi: 10.1038/nrn.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L, van Groen T, Kadish I, Li Y, Wang D, James SR, et al. Insufficient DNA methylation affects healthy aging and promotes age-related health problems. Clin Epigenetics. 2011;2:349–360. doi: 10.1007/s13148-011-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blusztajn JK, Mellott TJ. Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem. 2012;12:82–94. doi: 10.2174/187152412800792706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad Sci U S A. 2013;110:16651–16656. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loughery JE, Dunne PD, O’Neill KM, Meehan RR, McDaid JR, Walsh CP. DNMT1 deficiency triggers mismatch repair defects in human cells through depletion of repair protein levels in a process involving the DNA damage response. Hum Mol Genet. 2011;20:3241–3255. doi: 10.1093/hmg/ddr236. [DOI] [PubMed] [Google Scholar]
- 77.Stern SA, Kohtz AS, Pollonini G, Alberini CM. Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacology. 2014;39:2179–2190. doi: 10.1038/npp.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barua S, Kuizon S, Brown WT, Junaid MA. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. J Mol Neurosci. 2016;58:277–286. doi: 10.1007/s12031-015-0673-8. [DOI] [PubMed] [Google Scholar]
- 79.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takeo C, Ikeda K, Horie-Inoue K, Inoue S. Identification of Igf2, Igfbp2 and Enpp2 as estrogen-responsive genes in rat hippocampus. Endocr J. 2009;56:113–120. doi: 10.1507/endocrj.k08e-220. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. doi: 10.1089/thy.2005.15.865. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy MM, Nugent BM. At the frontier of epigenetics of brain sex differences. Front Behav Neurosci. 2015;9:221. doi: 10.3389/fnbeh.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison KE, Rodgers AB, Morgan CP, Bale TL. Epigenetic mechanisms in pubertal brain maturation. Neuroscience. 2014;264:17–24. doi: 10.1016/j.neuroscience.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reinius B, Kanduri C. Elevated expression of H19 and Igf2 in the female mouse eye. PLoS One. 2013;8:e56611. doi: 10.1371/journal.pone.0056611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.