Abstract
α7-Nicotinic acetylcholine receptor (α7nAChR) agonists confer protection against a wide variety of cytotoxic insults and suppress oxidative stress and apoptosis in various cell systems, including hepatocytes. We recently demonstrated that nicotine, when combined with a high-fat diet (HFD), triggers oxidative stress, activates hepatocyte apoptosis, and exacerbates HFD-induced hepatic steatosis in male mice. This study evaluates whether PNU-282987 (PNU), a specific α7nAChR agonist, is effective in preventing nicotine plus HFD–induced hepatic steatosis. Adult C57BL6 male mice were fed a normal chow diet or HFD with 60% of calories derived from fat and received twice-daily intraperitoneal injections of 0.75 mg/kg body weight (BW) of nicotine, PNU (0.26 mg/kg BW), PNU plus nicotine, or saline for 10 weeks. PNU treatment was effective in attenuating nicotine plus HFD–induced increase in hepatic triglyceride levels, hepatocyte apoptosis, and hepatic steatosis. The preventive effects of PNU on nicotine plus HFD–induced hepatic steatosis were mediated by suppression of oxidative stress and activation of adenosine 5′-monophosphate-activated protein kinase (AMPK) together with inhibition of its downstream target sterol regulatory element binding protein 1c (SREBP1c), fatty acid synthase (FAS), and acetyl-coenzyme A-carboxylase (ACC). We conclude that the α7nAChR agonist PNU protects against nicotine plus HFD–induced hepatic steatosis in obese mice. PNU appears to work at various steps of signaling pathways involving suppression of oxidative stress, activation of AMPK, and inhibition of SREBP1c, FAS, and ACC. α7nAChR agonists may be an effective therapeutic strategy for ameliorating fatty liver disease, especially in obese smokers.
Pharmacological activation of α7nAChR by PNU-282987, through activation of AMPK and suppression of oxidative stress, protects against nicotine plus high-fat diet–induced hepatic steatosis in obese mice.
Cigarette smoking is the leading preventable cause of death and disability worldwide (1). Smoking is a major risk factor for cardiovascular disease, chronic obstructive pulmonary disease, lung cancer, and nonalcoholic fatty liver disease (NAFLD) (2–6). Importantly, the health risk associated with smoking is further exaggerated by obesity (7, 8).
Nicotine acts on high-affinity nicotinic acetylcholine receptors (nAChRs), such as α4/β2 receptor, and the low-affinity receptors such as α7, centrally and peripherally (9). In rodents, nicotine blocks high-fat diet (HFD)–induced weight gain that was completely blocked by mecamylamine, a nonselective nAChR antagonist, but only partially blocked by the α4/β2 nAChR partial agonist/antagonist varenicline (10). Nicotine, when given with an HFD, leads to hepatic and muscle steatosis that is thought to be due, at least in part, to increased abdominal fat lipolysis (11–13), although the nAChRs involved in this response are not characterized.
Hepatic α3/β4 nAChR has been implicated in chronic nicotine exposure–induced improvement of glycemia and insulin sensitivity (14). Chronic exposure of nicotine, through the α7-nicotinic acetylcholine receptor (α7nAChR)–mediated pathway, improves glucose homeostasis and insulin sensitivity in genetically obese mice and mice with diet-induced obesity (15). Treatment with PNU-282987 (PNU), a specific α7nAChR agonist, is also found to be protective against a wide variety of cytotoxic insults and suppresses oxidative stress and apoptosis in various cell systems, including hepatocytes (16–19). Indeed, α7nAChRs are present in the liver (14, 16, 20) and the α7nAChR-mediated pathway plays a major role in mitigating hepatic ischemia-reperfusion injury (21, 22). TC-7020, an α7nAChR agonist, reduced weight gain and improved metabolic disorders in db/db mice (23).
In the current study, we used PNU and a commonly used model of diet-induced obesity (24–26) to elucidate the role of α7nAChR agonism in nicotine-induced weight loss and hepatic steatosis in mice on an HFD. Given that oxidative stress and inactivation of adenosine 5′-monophosphate–activated protein kinase (AMPK), coupled with hepatocellular apoptosis, play a pivotal role in the pathogenesis of hepatic steatosis (27–29), we also examined the effect of α7nAChR agonism on these parameters.
Materials and Methods
Animals
Male C57BL/6 mice weighing 22 to 24 g (Taconic Farms, Germantown, NY) were used in all experiments. Mice were housed under controlled temperature (22oC) and photoperiod (12-hours light and 12-hours dark cycle) with free access to water and food. Mice were fed either a normal chow diet (NCD) with 5% fat (8.5 KJ/g; laboratory rodent diet #5001, Laboratory Diet, Richmond, IN) or HFD with 60% of calories derived from fat. HFD consisted of 26.2% protein, 26.3% carbohydrate, and 34.9% fat (21.9 KJ/g; D12492, Research Diets, New Brunswick, NJ) for 10 weeks. Groups of five to six mice on either diet received twice daily intraperitoneal (IP) injections of nicotine [0.75 mg/kg body weight (BW)], PNU (0.26 mg/kg BW), PNU plus nicotine (PNU was given 15 minutes before nicotine was administered), or saline for 10 weeks. The rationale for using twice-daily IP administration of nicotine (0.75 mg/kg BW) was based on the results of our previous studies that demonstrated this dose led to levels of nicotine that, when combined with an HFD, triggered greater oxidative stress, activated hepatocellular apoptosis, amplified HFD-induced hepatic steatosis (11, 28), and caused intramyocellular lipid accumulation and intramyofibrillar mitochondrial abnormalities in the skeletal muscle (13).
The rationale for using twice-daily IP injections of PNU (0.26 mg/kg BW) was based on the results of a previous study (30) that showed chronic treatment with PNU at the daily dosage of 0.52 mg/kg BW per day) enhanced insulin sensitivity, as judged by reduction in homeostasis model assessment of insulin resistance, glucose tolerance test, and insulin tolerance test, in mice fed an NCD. Additional support for using this dose of PNU was based on the results of our pilot study, which showed that long-term treatment with twice daily IP injections of PNU at this dose was able to prevent nicotine plus HFD-induced hepatic steatosis in male mice (31).
The rationale for using male C57BL/6 mice is that these male mice, when fed an HFD deriving 60% of calories from fat, develop visceral adiposity, hyperglycemia, insulin and leptin resistance, as well as hepatic steatosis, and are a commonly used model of diet-induced obesity (22–24). Moreover, female C57BL6/J mice fed a similar HFD are less sensitive and develop much milder type 2 diabetes than do their male counterparts (32).
Mice were weighed weekly. The amount of food consumed per mouse was determined daily. Food intake was measured per cage with two to three mice per cage to avoid the stress of individual housing (24) and was calculated per mouse (10). The cumulative caloric intake was calculated in each group as described previously (10). Mice were fasted overnight before being euthanized with a lethal injection of sodium pentobarbital (200 mg/kg BW). Livers were removed. Portions of liver were placed in RNAlater and used for gene expression analysis by quantitative real-time polymerase chain reaction (qPCR) or were snap frozen in liquid N2 and stored frozen for subsequent measurements of triglyceride levels and changes in protein expression by western blotting. The remaining portions of liver were either fixed in 2.5% glutaraldehyde for high-resolution light and electron microscopy or 4% paraformaldehyde for routine histological and immunohistochemical or immunofluorescence studies. Animal handling and experimentation were in accordance with the recommendation of the American Veterinary Medical Association and were approved by the Charles R. Drew University School of Medicine and Science Institutional Animal Care and Use Committee.
Hepatic triglyceride levels
Hepatic triglyceride levels were measured by using Abcam’s triglyceride quantitation kit according to manufacturer’s protocol (Cambridge, MA).
Assessment of apoptosis
In situ detection of cells with DNA strand breaks was performed in paraformaldehyde-fixed, paraffin-embedded liver sections by the terminal deoxynucleotidyl transferase (TdT)–mediated deoxy-UTP nick end labeling (TUNEL) technique (11, 12, 33) using an ApopTag-peroxidase kit (Chemicon International, San Francisco, CA). Negative and positive controls were carried out for all assays. For negative controls, tissue sections were processed in an identical manner except the TdT enzyme was substituted by the same volume of distilled water. Mammary tissue sections from rat pups 3 to 5 days after weaning (Charles River Laboratories, Worcester, MA) in which apoptosis is well characterized (34), were used as positive controls. Enumeration of TUNEL-positive nuclei was carried out in liver sections using a microscope with a ×40 objective and a pair of ×10 eyepieces (Scientific Instruments, Buffalo, NY). Methyl green was used as a counterstain to detect nonapoptotic nuclei. A square grid fitted within one eyepiece provided a reference of 62,500 μm2. The rate of hepatocellular apoptosis was expressed as the percentage of the TUNEL-positive apoptotic nuclei per total nuclei (apoptotic plus nonapoptotic) present within the reference area (11, 12, 33).
Liver pathology
Liver pathology was evaluated using conventional histological analysis on hematoxylin and eosin–stained sections. Additional evaluation of pathology was achieved by high-resolution light microscopy using glutaraldehyde-fixed, osmium tetroxide postfixed, epoxy-embedded, and toluidine blue–stained sections and electron microscopy (11, 33) Accumulation of intracellular fat was quantified by computerized densitometry using the ImagePro Plus software coupled to an Olympus BHS microscope equipped with a VCC video camera (11, 33). For electron microscopic studies, thin sections from selected tissue blocks were sectioned with an LKB ultramichrotome, stained with uranyl acetate, and examined with a Hitachi 600 electron microscope (Hitachi, Indianapolis, IN).
qPCR
Hepatic RNA from mice who were fed an HFD and treated with saline or nicotine in the absence or presence of PNU was extracted with TRIzol Reagent (Invitrogen) using a Pyrex homogenizer. Total RNA was then treated with DNase I (Ambion) for 30 minutes at 37°C and purified with RNeasy mini kit (Qiagen). The purity of RNA was determined by a 260/280 ratio using NanoDrop 2000 (Thermo Fisher Scientific); RNA with >1.9 ratio was considered highly purified. RNA integrity was measured at the University of California, Los Angeles, genomic core using 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA integrity values ranged from 7.5 to 9.2. Complementary DNA (cDNA) was prepared using high-quality RNA to a cDNA kit (Applied Biosystems). cDNA was diluted 1:10, and 3 to 4 μL of the diluted cDNA was used for qPCR. qPCR was done using the Step One Plus Real-Time PCR System (Life Technologies) with a Sybr Green PCR Master Mix (Applied Biosystems). All reactions were analyzed in triplicate and four to five mice from each group were tested. Data were normalized to 18S ribosomal RNA transcripts using the 2∆∆Ct method for relative quantitation of gene expression. Primer sequences were as follows:
mSCD1-F-5′-TGCCCCTGCGGATCTT-3′ (NM_009127.4)
mSCD1-R-5′-GCCCATTCGTACACGTCATT-3′ (NM_009127.4)
mSREBP1-F-5′-TGACCCGGCTATTCCGTGA-3′(NM_011480)
mSREBP1-R-5′-CTGGGCTGAGCAATACAGTTC-3′ (NM_011480)
mFAS-F-5′- CATGACCTCGTGATGAACGTGT-3
mFAS-R-5′- CGGGTGAGGACGTTTACAAAG-3′
mCD36-F-5′-GGAACTGTGGGCTCATTGC-3
mCD36-R-5′-CATGAGAATGCCTCCAAACAC-3′
mLXR-F-5′-GCCTCAATGCCTGATGTTTC-3
mLXR-R-5′-CTGCATCTTGAGGTTCTGTCTTC-3′
mSIRT1-F-5′-ATGACGCTGTGGCAGATTTT-3′
mSIRT1-R-5′-CCGCAAGGCGAGCATAGA-3′
m18SR-F-5′-GTAACCCGTTGAACCCCATT-3′
m18SR-R-5′- CCATCCAATCGGTAGTAGCG-3′
Western blotting
Western blotting was performed using hepatic lysates as described previously (11, 13, 33). In brief, proteins (50 to 80 μg) were separated on a 4% to 12% sodium dodecyl sulfate–polyacrylamide gel with 2-(N-morpholine) ethane sulfonic acid buffer (Invitrogen, Carlsbad, CA) at 200V. The gel was transferred onto an immuno-blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) and kept overnight at 4°C. Membranes were blocked in blocking solution (0.3% Tween 20 in Tris-buffered saline and 10% nonfat dry milk) for 1 hour at room temperature, then probed using a mouse monoclonal 4-hydoxynonenal (4-HNE) or rabbit polyclonal silent information regulator 1 (SIRT1), phospho-adenosine-5-monophosphate–activated protein kinase (p-AMPK), total AMPK, phospho-acetyl-COA-carboxylase (p-ACC), and total ACC antibodies (Table 1) for 1 hour at room temperature or overnight at 4°C with constant shaking. Following three 10-minute washes in Tris-buffered saline plus Tween buffer, membranes were then incubated in anti-mouse or anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories) at a 1:2000 dilution. All antibodies were diluted in blocking buffer. For immunodetection, membranes were washed three times in Tris-buffered saline plus Tween wash buffer, incubated with enhanced chemiluminescence solutions per the manufacturer's specifications (Amersham Biosciences), and exposed to Hyper film enhanced chemiluminescence. Band intensities were determined using Quantity One software from Bio-Rad.
Table 1.
Antibodies Used in This Study
| Target Protein | Antibody | Manufacturer, Catalog No. | RRID | Species Raised in; Polyclonal or Monoclonal | Dilution |
|---|---|---|---|---|---|
| Phospho-AMP activated kinase | p-AMPK α1/2(Thr-172) | Santa Cruz Biotechnology, sc-33524 | AB_2169714 | Rabbit; polyclonal | 1:1500 |
| Total AMPK | AMPKα | Rabbit; monoclonal | 1:2000 | ||
| Phospho-acetyl CoA carboxylase | p-ACC (S79) | Cell Signaling Technology, 2532S | AB_330331 | Rabbit; monoclonal | 1:2000 |
| Total acetyl CoA carboxylase | ACC | Rabbit; monoclonal | 1:2500 | ||
| Sirt1 | Anti-Sirt1 | Cell Signaling Technology, 661S | AB_330337 | Rabbit; polyclonal | 1:2000 |
| 4-HNE | Anti–4-HNE | Mouse; monoclonal | 1:200 | ||
| GAPDH | MSX GAPDH | Cell Signaling Technology, 3662S | AB_2219400 | Mouse; monoclonal | 1:8000 |
| AChRa7 | AchRa7 | Millipore, 07-131 | AB_10067921 | Rabbit; polyclonal | 1:500 |
| OXIS International, 24325 | AB_2716829 | ||||
| Millipore, MAB374 | AB_2107445 | ||||
| Santa Cruz Biotechnology, sc-5544 | AB_2229517 | ||||
Abbreviation: RRID, Research Resource Identifier.
Statistical analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel, San Rafael, CA). Data are presented as mean ± standard error of the mean. We used one-way analysis of variance to compare group differences. If overall analysis of variance revealed significant differences, post hoc (pairwise) comparisons were performed using the Tukey or Tukey-Kramer multiple comparison test. Differences were considered significant at P < 0.05.
Results
BW, cumulative food intake, hepatic triglyceride levels, and hepatic steatosis
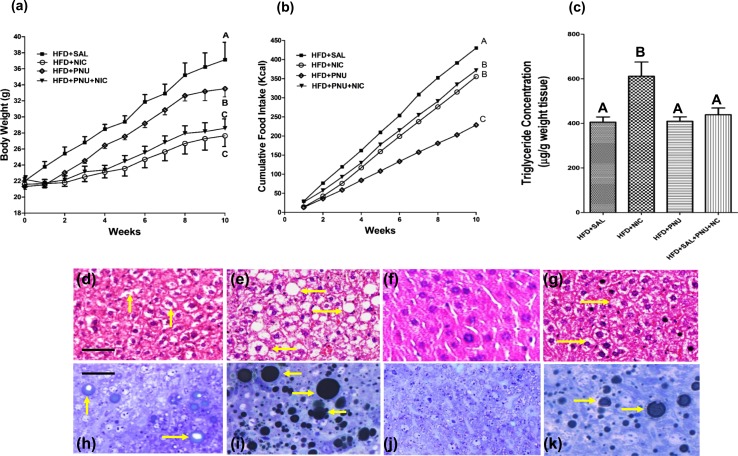
Nicotine-treated mice fed an HFD had significantly lower BW than did vehicle-treated HFD-fed mice [Fig. 1(a)]. By 10 weeks of combined treatment with nicotine and HFD, mean BW was reduced by 27% relative to mice fed with HFD alone. PNU-treated mice fed an HFD also had significantly lower BW than did vehicle-treated mice fed an HFD [Fig. 1(a)]. The effect of PNU on BW reduction in HFD-fed mice, however, was less compared with the reduction observed with mice receiving nicotine treatment [Fig. 1(a)]. There was no difference in BW between PNU-treated and PNU plus nicotine–treated mice fed an HFD [Fig. 1(a)]. As shown in Fig. 1(b), mice fed an HFD consumed more calories than did other experimental groups. The cumulative caloric intake by HFD-fed mice over the 10 weeks was significantly reduced by nicotine treatment [Fig. 1(b)]. PNU treatment alone led to a marked decrease in the cumulative caloric intake in HFD-fed mice even when compared with nicotine-treated mice fed an HFD [Fig. 1(b)]. There was no difference in cumulative caloric intake between PNU-treated and PNU plus nicotine–treated mice fed an HFD [Fig. 1(b)]. Compared with vehicle-treated, PNU-treated, or nicotine plus PNU–treated mice fed an HFD, nicotine-treated mice fed an HFD had significantly higher hepatic triglyceride levels [Fig. 1(c)].
Figure 1.
(a) Body weight measured over 10 weeks in mice fed an HFD and treated with nicotine in the absence or presence of PNU. Nicotine and PNU (albeit less when compared with nicotine) significantly reduced HFD-induced weight gain. (b) Average food intake in HFD mice treated with nicotine in the absence or presence of PNU. Compared with nicotine, PNU treatment alone led to a marked decrease in cumulative food intake in HFD-fed mice. (c) Hepatic triglyceride levels in various treatment groups. Values are given as mean ± standard error of five to six mice per group. Means with unlike superscripts are significantly different from each other (P < 0.05). (d–g) Representative hematoxylin and eosin–stained liver sections show that compared with a mouse fed an HFD, where (d) a modest increase in lipid accumulation (arrow) is detected, (e) combined treatment with nicotine and an HFD caused a marked increase in lipid accumulation in the liver. (f) PNU suppressed HFD induced hepatic lipid accumulation. (g) PNU treatment effectively suppressed nicotine plus HFD–induced hepatic steatosis. (h–k) Representative light microscopic images of glutaraldehyde-fixed, osmium tetroxide postfixed, epoxy-embedded, and toluidine blue–stained liver sections from different treatment groups show that nicotine plus an HFD causes a striking increase in lipid accumulation of varying sizes (arrow) in (i) hepatocytes compared with (h) those from mice fed only an HFD. (j) PNU treatment decreased fat accumulation compared to HFD alone in (h). (k) PNU treatment effectively prevented HFD plus nicotine–induced exacerbation of hepatic steatosis. (d–k) Scale bar = 25 μm. Data are representative of five mice in each group. NIC, nicotine; SAL, saline.
Examination of liver sections stained with hematoxylin and eosin revealed that, compared with mice fed an HFD plus vehicle, where a modest increase in lipid accumulation was detected [Fig. 1(d)], combined treatment with HFD and nicotine led to a marked increase in lipid accumulation in the liver [Fig. 1(e)]. PNU treatment effectively prevented HFD- [Fig. 1(f)] or nicotine plus HFD–induced hepatic steatosis [Fig. 1(g)]. These results were further confirmed by high-resolution light microscopy of glutaraldehyde-fixed, osmium tetroxide postfixed, epoxy-embedded, and toluidine blue–stained liver sections [Fig. 1(h)–1k)]. Glutaraldehyde fixation followed by osmium postfixation allows retention of fat that normally would have been washed out during tissue processing and can be easily seen by toluidine blue staining. Nicotine plus HFD caused a striking increase in larger lipid droplets [Fig. 1(i)] compared with those from mice fed HFD and saline [Fig. 1(h)]. PNU treatment effectively prevented HFD- [Fig. 1(j)] and nicotine plus HFD–induced hepatic steatosis [Fig. 1(k)]. In fact, PNU treatment fully attenuated HFD plus nicotine–induced fat accumulation [Fig. 1(k)] to levels seen in mice fed an HFD and saline [Fig. 1(k)].
Quantitative image analysis further revealed a substantial increase in intracellular lipid content after combined treatment with HFD and nicotine (215 ± 25 μm2) compared with mice fed an HFD and saline (32 ± 3.2 μm2). Treatment with PNU significantly attenuated HFD plus nicotine–induced intracellular fat accumulation (35 ± 3.3 μm2) to levels almost identical to those seen in mice fed an HFD and saline (32 ± 3.2 μm2). Treatment with PNU also substantially attenuated intracellular fat accumulation triggered by HFD alone (18 ± 2.3 μm2).
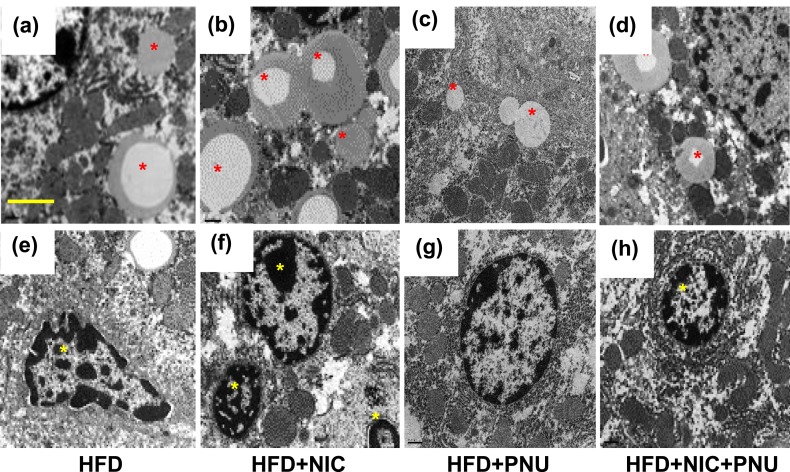
We used transmission electron microscopy to further substantiate the light microscopy findings [Fig. 2(a)–2(h)]. Hepatocytes from mice fed an HFD plus saline exhibited a modest lipid accumulation [Fig. 2(a)]. Addition of nicotine to the HFD led to a striking increase in lipid accumulation of varying sizes in hepatocytes, along with a decrease in the amount of cellular organelles [Fig. 2(b)]. PNU treatment effectively prevented such HFD- [Fig. 2(c)] or nicotine plus HFD–induced hepatic steatosis [Fig. 2(d)]. A distinct increase in the amount of hepatocyte apoptosis, characterized by nuclear condensation and fragmentation, was also noted after combined treatment with HFD and nicotine [Fig. 2(f)] compared with mice fed an HFD [Fig. 2(e)]. PNU treatment effectively prevented such nicotine plus HFD–induced hepatocyte apoptosis [Fig. 2(h)].
Figure 2.
Compared with (a) mice fed only an HFD, nicotine plus HFD caused a striking increase in lipid accumulation (asterisk) of varying sizes in (b) hepatocytes. (c–d) Treatment with PNU decreased (c) HFD and (d) nicotine plus HFD-induced intracellular lipid accumulation compared with that of (a) HFD alone. Compared with (e) mice fed only an HFD, a distinct increase in the amount of hepatocyte apoptosis, characterized by nuclear condensation and fragmentation, was noted after (f) combined treatment with HFD and nicotine; (g–h) the apoptosis can be prevented effectively by PNU treatment. (a–h) Scale bar = 1 μm. Data are representative of four mice in each group. NIC, nicotine; SAL, saline.
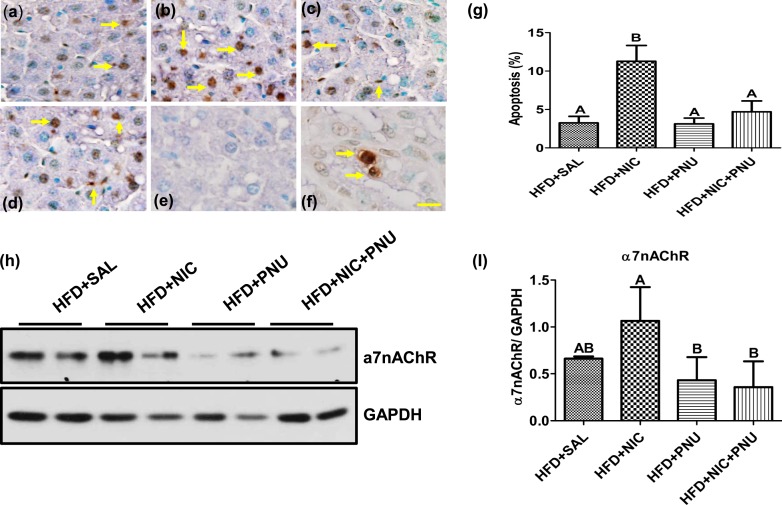
We next analyzed the occurrence of hepatocellular apoptosis by TUNEL. As demonstrated in Fig. 3(a)–3(g), HFD alone led to a modest increase in the incidence of apoptosis. Combined with nicotine, the HFD induced a further substantial increase in the incidence of hepatocellular apoptosis. The incidence of hepatocellular apoptosis was similar between the HFD and HFD plus PNU groups. Notably, treatment with PNU significantly attenuated HFD plus nicotine–induced hepatocellular apoptosis to levels similar to that seen in mice given an HFD and saline. There was no staining in liver sections from the nicotine plus HFD group [Fig. 3(e)] when TdT enzyme was substituted by the same volume of distilled water (i.e., negative control). In the positive-control mammary tissue sections, an intense immunoreactivity was detected in cells undergoing apoptosis [Fig. 3(f)]. Nicotine treatment in the absence or presence of PNU did not alter the incidence of hepatocellular apoptosis in NCD-fed mice (data not shown).
Figure 3.
(a–f) In situ detection of hepatocellular apoptosis by TUNEL. (a) Although HFD alone led to a modest increase in the incidence of apoptosis (yellow arrow), (b) HFD plus nicotine induced a further substantial increase in the incidence of hepatocellular apoptosis. The incidence of hepatocellular apoptosis was similar between the (a) HFD and (c) HFD plus PNU groups. (d) Notably, treatment with PNU significantly attenuated HFD plus nicotine–induced hepatocellular apoptosis to levels almost identical to that seen in (a) mice fed an HFD and saline. (e) A liver section from the nicotine plus HFD group incubated without TdT enzyme (negative control) shows no immunostaining, whereas (f) a mammary tissue section from a rat pup (positive control) shows specific immunoreactivity. (a–f) Scale bar = 25 μm. Data are representative of five mice in each group. (g) Quantitation of hepatocellular apoptosis. Apoptotic rate is expressed as the percentage of TUNEL-positive nuclei per total nuclei (i.e., apoptotic plus nonapoptotic nuclei) counted in a unit reference area. Values are given as mean ± standard error of the mean (SEM; n = 5). Means with unlike superscripts are significantly different. Note the marked increase in the incidence of apoptosis in the HFD plus nicotine group. PNU treatment fully attenuated nicotine plus HFD–induced hepatocyte apoptosis. (h) Western blot analysis shows higher expression of hepatic α7nAChR expression in the combined treatment group with nicotine and HFD than in the HFD-alone group. PNU treatment effectively attenuated nicotine plus HFD–induced increase in α7nAChR expression to levels seen in mice fed only an HFD. Data are representative of six mice in each group. (i) Quantitation of band intensities. Data are normalized to GAPDH. Values are given as mean ± SEM. Means with unlike superscripts are significantly different (P < 0.05). NIC, nicotine; SAL, saline.
We also used immunoblot analysis to detect α7nAChR expression in the liver [Fig. 3(h) and 3(i)]. Hepatic α7nAChR expression was higher in the nicotine and HFD group. PNU treatment effectively prevented nicotine plus HFD–induced increase in α7nAChR expression to levels seen in mice fed an HFD alone. Hepatic expression of α7nAChR was similar in vehicle-treated, PNU-treated, or nicotine plus PNU–treated mice fed an HFD.
PNU prevented nicotine plus HFD-induced hepatic steatosis through stimulation of SIRT1 and APMK signaling together with suppression of oxidative stress
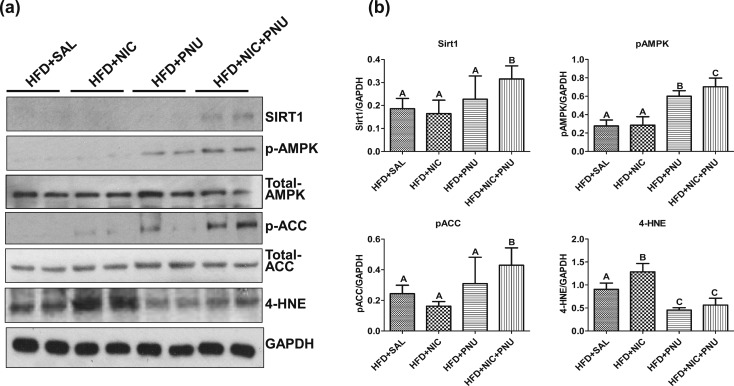
AMPK, a central regulator of cellular energy homeostasis, plays an important role in fatty acid metabolism through its ability to regulate key fatty acid biosynthetic pathways (35, 36). To investigate whether mitigation of nicotine plus HFD–induced hepatic steatosis by PNU was associated with activation of AMPK, we performed western blot analysis of p-AMPK and total AMPK in hepatic lysates [Fig. 4(a)]. As expected from our previous studies (11), the HFD, in the absence or presence of nicotine, led to a striking decrease in hepatic p-AMPK levels [Fig. 4(a)]. Consistent with the finding of inactivation of AMPK in HFD and HFD plus nicotine–treated groups, little or no p-ACC was detected in hepatic lysates in these groups [Fig. 4(a)]. Treatment with PNU significantly attenuated both HFD- and HFD plus nicotine–induced decrease in p-AMPK levels [Fig. 4(a)]. PNU alone failed to significantly attenuate HFD-induced decrease in p-ACC levels but restored p-ACC levels in the combined treatment group [Fig. 4(a)]. There were no significant differences in total AMPK or total ACC levels among various groups.
Figure 4.
(a) Western blot analysis shows that mice fed an HFD in the presence or absence of nicotine had low hepatic SIRT1, p-AMPK, and p-ACC levels, whereas nicotine-treated mice fed an HFD had increased hepatic 4-HNE levels. Treatment with PNU significantly restored HFD plus nicotine-induced decrease in hepatic SIRT1, p-AMPK and p-ACC levels and prevents increase in 4-HNE levels. Data are representative of six mice in each group. (b) Quantitation of band intensities. Data are normalized to GAPDH. Values are given as mean ± standard error of the mean. Means with unlike superscripts are significantly different (P < 0.05). NIC, nicotine; SAL, saline.
Given that SIRT1, a nicotinamide adenine dinucleotide–dependent histone deacetylase, plays an important role in protection against hepatic steatosis under various insults (37–39), we next examined the contribution SIRT1 in PNU-mediated protection of nicotine plus HFD–induced hepatic steatosis. Western blot analysis revealed that mice fed the HFD in the absence or presence of nicotine exhibited little or no hepatic SIRT1 expression [Fig. 4(a)]. Although PNU treatment did not alter HFD-induced decrease in SIRT1 levels, it significantly attenuated nicotine plus HFD–induced decrease in hepatic SIRT1 expression [Fig. 4(a)].
Next, we investigated whether amelioration of nicotine plus HFD–induced hepatic steatosis was associated with suppression of oxidative stress. We performed western blot analysis of 4-HNE [Fig. 4(a)], a biomarker of oxidative stress (40, 41). Nicotine-treated mice fed an HFD had significantly higher levels of hepatic 4-HNE relative to mice fed HFD alone. Treatment with PNU significantly attenuated HFD- and HFD plus nicotine–induced increase in hepatic 4-HNE levels. These findings were further corroborated by densitometric analysis of band intensities [Fig. 4(b)]. No substantial changes in p-AMPK, SIRT1, or 4-HNE levels were noted among various treatment groups in mice fed the NCD (data not shown).
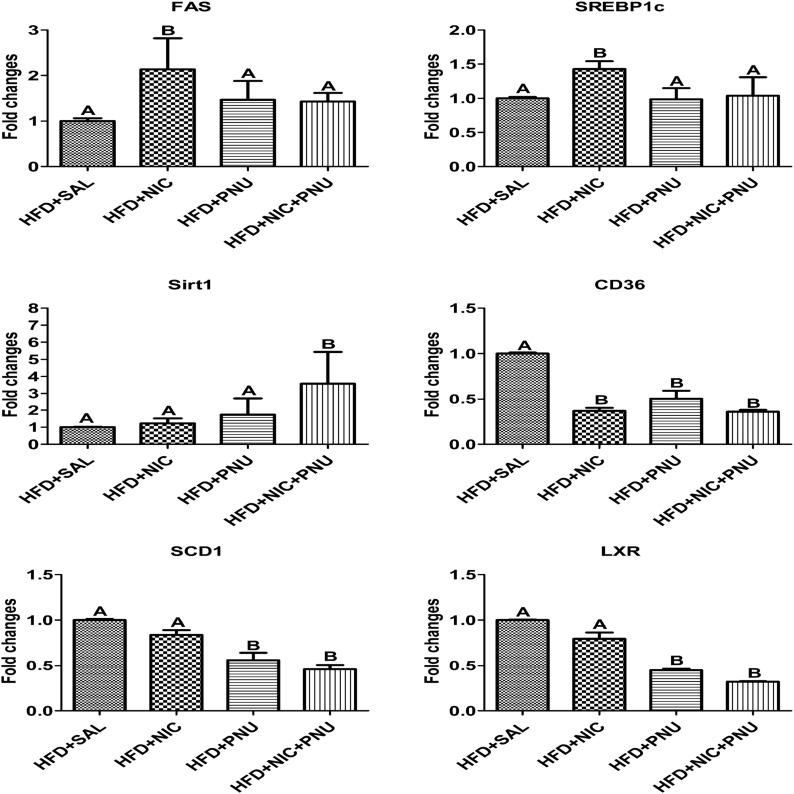
To gain additional insights into the potential mechanisms of PNU-mediated mitigation of nicotine plus HFD–induced hepatic steatosis, we measured the expression of key genes related to hepatic lipid metabolism by qPCR. Consistent with the finding of inactivation of AMPK in HFD plus nicotine–treated groups, we found substantial upregulation of SREBP1c and fatty acid synthase (FAS) (Fig. 5), indicating increased hepatic lipogenesis. Treatment with PNU significantly attenuated nicotine plus HFD–induced upregulation of SREBP1c and FAS to levels seen in HFD plus saline or HFD plus PNU groups (Fig. 5). Consistent with our western blot data, PNU treatment also upregulated hepatic SIRT1 gene expression in nicotine-treated mice fed an HFD. Intriguingly, nicotine treatment resulted in a striking downregulation of CD36 expression in mice fed an HFD. PNU treatment in the absence or presence of nicotine had no effect on nicotine plus HFD–induced changes in CD36 expression. No major changes in the hepatic expression of SCD1 and LXR expression were seen between HFD and HFD plus nicotine–treated groups. Treatment with PNU significantly attenuated both HFD- and HFD plus nicotine–induced upregulation of SCD1 and LXR gene expression (Fig. 5).
Figure 5.
qPCR results show upregulation of SREBP1c and FAS in the HFD plus nicotine group compared with the HFD-only group. Treatment with PNU fully prevented nicotine plus HFD–induced upregulation of SREBP1c and FAS. PNU treatment also upregulated hepatic SIRT1 gene expression in nicotine-treated mice fed an HFD. No substantial changes in the hepatic expression of SCD1 and LXR were noted between the HFD and HFD plus nicotine–treated groups. Treatment with PNU, however, significantly attenuated both HFD- and HFD plus nicotine–induced upregulation of SCD and LXR gene expression. Nicotine treatment also significantly attenuated HFD-induced upregulation of CD36. CD36 expression was similar among the HFD plus nicotine, HFD plus PNU, and HFD plus nicotine plus PNU groups. Values are given as mean ± standard error of the mean of four to five animals per group. Means with unlike superscripts are significantly different (P < 0.05). NIC, nicotine; SAL, saline.
Discussion
In recent studies using the model of diet-induced obesity in C57BL6J mice, we demonstrated that nicotine, when combined with an HFD, triggered greater oxidative stress, activated hepatocellular apoptosis, and exacerbated HFD-induced hepatic steatosis (11, 12). In the current study, we evaluated the protective role of PNU, a α7nAChR agonist, on nicotine plus HFD–induced hepatic steatosis. We are intrigued by the observation that, indeed, PNU significantly blocked HFD-induced weight gain and protected nicotine plus HFD–induced hepatic steatosis in obese mice. The preventive effects of PNU on nicotine plus HFD–induced hepatic steatosis are mediated by suppression of oxidative stress and activation of SIRT1 and AMPK signaling, together with inhibition of its downstream targets, including sterol regulatory element binding protein 1c (SREBP1c), ACC, and FAS.
The results of the current study confirm and extend those of our previous studies by demonstrating that nicotine blocks the HFD-induced weight gain in mice (10, 11, 13). This study is unique, however, in showing that PNU alone can reduce HFD-induced weight gain, albeit less pronounced when compared with nicotine, suggesting an important role of the α7nAChR in the weight-reducing effect of nicotine. Indeed, the α7nAChR plays an important role in central and peripheral mechanisms regulating food intake (9). Consistent with a role of α7nAChR in food intake, we found that PNU treatment alone led to a marked decrease in the cumulative food intake in HFD-fed mice. This notion is supported by another line of evidence showing that TC-7020, an α7nAChR agonist, reduced weight gain and food intake in a mouse model of diabetes (23). Thus, a potential mechanism by which PNU can reduce BW in HFD-fed mice is through appetite suppression, although this needs to be demonstrated by either showing intracerebroventricular injection of PNU suppresses food intake or that a peripheral-acting α7nAChR antagonist such as hexamethonium (42) does not block the effect of α7nAChR agonist on food intake.
Oxidative stress coupled with hepatocyte apoptosis plays a pivotal role in the pathogenesis of NAFLD (27, 29, 43). Our study showed that nicotine, when combined with an HFD, generated greater oxidative stress, as evidenced by an increase in hepatic 4-HNE levels, than did an HFD alone. Oxidative stress has also been implicated in apoptotic signaling in various cell types, including hepatocytes (27, 29, 44). Thus, it is likely that generation of severe oxidative stress could trigger hepatocyte apoptosis and aggravate hepatic steatosis in the combined treatment group through the formation of reactive and biologically active lipid peroxidation products such as 4-HNE (27, 29, 43, 45). This is consistent with our previous data showing that HFD and nicotine can generate oxidative stress in the liver and heart ventricle, but only when they are combined can they cause greater oxidative stress and trigger hepatocyte and cardiomyocyte apoptosis (11, 28). It is noteworthy that despite a substantial increase in oxidative stress, there was little or no change in the incidence of hepatocyte apoptosis in mice fed an HFD alone, suggesting that the oxidative stress generated by HFD alone is not severe enough to trigger cellular apoptosis. This is consistent with earlier reports indicating that cellular responses to oxidative stress vary depending on the cell type, the levels of stress achieved, and the duration of exposure (46). However, we cannot rule out the possibility that in addition to high levels of oxidative stress, other factors may have also contributed to increased hepatocyte apoptosis triggered by the combined treatment with nicotine and HFD.
Notably, PNU treatment suppressed nicotine plus HFD–induced increase in hepatic oxidative stress and hepatocellular apoptosis. Indeed, we detected α7nAChR expression in the liver and found the highest amounts of the receptor in the nicotine plus HFD group. This is consistent with previous reports showing that α7nAChRs are present in the liver (14, 16, 20). PNU treatment significantly prevented nicotine plus HFD–induced increase in α7nAChR expression to levels seen in mice fed an HFD alone. The mechanisms by which PNU prevents nicotine plus HFD–induced increase in α7nAChR expression remain unknown but could be mediated through desensitization of α7nAChR after chronic exposure of the agonist (47).
Evidence also exists that the α7nAChR-mediated pathway plays a major role in mitigating hepatic ischemia-reperfusion injury (21, 22). Furthermore, the α7nAChR agonist PNU is protective against a wide variety of cytotoxic insults and suppresses oxidative stress and apoptosis in various cell systems, including hepatocytes (16–19, 21, 48, 49). The underlying mechanisms of the protective effects of PNU in mitigating nicotine plus HFD–induced oxidative stress and hepatocellular apoptosis could include induction of heme oxygenase 1 via nuclear factor erythroid-2-related factor 2 (18, 22, 33). It also remains possible that PNU can attenuate hepatic steatosis by reducing circulating free fatty acid via reduction of abdominal lipolysis (11), and improving glucose homeostasis and insulin sensitivity through activation of the α7nAchR-mediated cholinergic anti-inflammatory pathway (15). Other studies have found that chronic treatment of PNU at the same dose levels we used substantially enhanced insulin sensitivity, as judged by reduction in the homeostasis model assessment of insulin resistance, glucose tolerance test, and insulin tolerance test in mice fed an NCD (30). Available evidence also suggests that PNU administration attenuates the central insulin action–mediated hepatic interleukin-6 and signal transducer and activator of transcription factors 3 in the liver of mice fed an HFD, suggesting that the cholinergic pathway can play an important role in central insulin action–mediated hepatic response in diet-induced obese mice (50).
One potential limitation of our study is that, at present, we are unable to answer whether PNU would have detrimental effects if used at a 1.5 mg/kg BW per day dosage or if nicotine would have beneficial effects if used at a 0.52 mg/kg BW per day dosage. Indeed, the low dosages (1.5 mg/kg BW per day) of nicotine we used in this study induced adipose tissue lipolysis and hepatic steatosis in mice (11). In contrast, other studies showed that high dosages of nicotine (4 mg/kg BW per day) improved obesity, hepatic steatosis, and endoplasmic reticulum stress in diet-induced obese male rats (51). To address the discrepancy between mice and rats, Wu et al. (20) further studied the metabolic effects of both low dosages (0.8 mg/kg BW per day equal to 1.5 mg/kg BW per day in mice) and high dosages of nicotine (4 mg/kg BW per day) in rats. Intriguingly, they found that the low dosage of nicotine, resulting in a serum concentrations of nicotine that is similar to the clinically relevant concentrations found in habitual smokers, induced insulin resistance, mild loss of BW, and hepatic steatosis. In striking contrast, the high dosage of nicotine, resulting in much higher serum nicotine concentrations than found in habitual smokers, induced severe loss of BW, improved insulin sensitivity, and prevented hepatic steatosis in male rats (20). Although these observations suggest the effects of nicotine could vary depending on dosages, it remains unresolved whether lower doses of nicotine (i.e., <1.5 mg/kg BW per day) will have detrimental effects on health. Likewise, one could also argue that the beneficial effects of PNU that are so obvious at lower daily dosages (i.e., 0.52 mg/kg BW) ameliorating nicotine plus HFD–induced hepatic steatosis might not be so obvious if used at higher (i.e., 1.5 mg/kg BW) daily dosages. In this context, it is pertinent to note that pretreatment of PNU at a dose of 1 mg/kg BW did protect the liver after ischemia reperfusion injury in mice (19). Daily dosages of PNU (i.e., 0.3 mg/kg BW and 1.0 mg/kg BW) further inhibited muscular degeneration in mdx dystrophic mice with a greater efficacy at higher dosages (52). PNU at a slightly higher daily dosage (i.e., 2.4 mg/kg BW) significantly promoted wound healing in a streptozotocin-induced diabetic mouse model (53). Available evidence also suggests that administration of PNU at a much higher dosage (i.e., 12 mg/kg BW) did confer neuroprotection in a mouse model of intracerebral hemorrhage (48). Thus, given that PNU exerts its beneficial effects at various doses levels in protecting organ damage under diverse experimental conditions, it is unlikely that PNU would have detrimental effects if used at higher daily dosages such as 1.5 mg/kg BW. However, this clearly merits further investigation.
Together, these results demonstrate a key role of the α7nAchR agonist in amelioration of nicotine plus HFD–induced hepatic steatosis. Studies are needed to understand which nAchR(s) is involved in nicotine plus HFD–induced hepatic steatosis and to what extent it is mediated by increased adipose tissue lipolysis, resulting in excess free fatty acid delivery and a direct effect of nicotine in the liver.
AMPK is a central regulator of lipid homeostasis and mediates suppression of lipogenic gene expression such as ACC and FAS through inhibition of SREBP1c (36, 54). ACC is the rate-determining enzyme for the synthesis malonyl-CoA, a critical substrate for fatty acid biosynthesis and a potent inhibitor of fatty acid oxidation (36). AMPK can also phosphorylate and inactivate ACC, leading to inhibition of de novo fatty acid and cholesterol synthesis (36). Consistent with a pivotal role for AMPK in lipid homeostasis, we herein show that the additive effects of nicotine and an HFD on the severity of hepatic steatosis were associated with complete inhibition of AMPK. The net effect of AMPK inactivation was decreased phosphorylation and activation of ACC, leading to increased lipogenesis in the liver. qPCR data also showed a substantial upregulation of SREBP1c and FAS, indicating increased hepatic lipogenesis, in the nicotine plus HFD combined treatment group. These results are consistent with those of our earlier reports (11, 55) and those of others (20, 56) linking inhibition of AMPK with NAFLD. Treatment with PNU restored nicotine plus HFD–induced decrease in hepatic p-AMPK and p-ACC levels and prevented upregulation of SREBP1c and FAS. Furthermore, mice fed HFD for 16 weeks also developed hepatic steatosis in association with inhibition of AMPK coupled with activation of ACC that was attenuated by betaine, a naturally occurring metabolite of choline and an essential biochemical component of the methionine-homocysteine cycle (57). Taken together, these results indicate that PNU reduced nicotine plus HFD–induced hepatic steatosis by improving AMPK signaling and, in turn, by inactivating ACC and suppressing lipogenic gene expression such as SREBP1c and FAS. At present, we are unable to determine the possible mechanisms by which PNU restores hepatic AMPK activity in nicotine-treated mice fed an HFD and this clearly merits further investigation.
Of further interest, activation of AMPK can also suppress oxidative stress and inhibit apoptosis in various cell systems (58–60). Thus, it is possible that the protective effect of PNU on nicotine plus HFD–induced hepatocyte apoptosis also could be mediated by activation of AMPK. Given the critical role of AMPK in modulating an ever-expanding array of biological pathways, the findings of this study further underscore the potential ability of α7nAChR agonist to mediate a variety of signal transduction pathways in regulating cell fate.
SIRT1 plays an important role in protection against hepatic steatosis under various insults (37–39). Consistent with this, we also found that PNU treatment stimulated SIRT1 in the combined treatment group. This is further supported by another line of evidence showing that hepatic overexpression of SIRT1 attenuates hepatic steatosis in mice fed an HFD or after fasting (39), whereas deletion of SIRT1 exacerbates HFD-induced hepatic steatosis (38).
We found that PNU treatment suppressed HFD plus nicotine–induced upregulation of SCD1 and LXR gene expression in the liver, suggesting that both SCD1 and LXR play an important role in attenuating nicotine plus HFD–induced hepatic steatosis. This is consistent with previous data showing SCD1 deficiency prevented hepatic steatosis observed in several mouse models, including mice fed high-carbohydrate diets and HFD and ob/ob mice (61, 62). Likewise, downregulation of LXR is involved with suppression of high-cholesterol diet–induced hepatic steatosis though suppression of lipogenic genes such as SREBP1c, SCD1, and FAS (63). Indeed, we found PNU-mediated amelioration of nicotine plus HFD–induced hepatic steatosis is associated with downregulation of these lipogenic genes.
Nicotine treatment resulted in a striking downregulation of CD36, the fatty acid translocase, in mice fed an HFD. PNU treatment in the absence or presence of nicotine had no effect on nicotine plus HFD–induced decrease in CD36 expression. The upregulation of CD36 in the liver is associated with increased steatosis in patients with NAFLD (64, 65), and CD36−/− mice are resistant to alcohol- and high carbohydrate–induced hepatic steatosis (66). These observations raise the question of why mice fed an HFD with substantially increased CD36 levels do not develop hepatic steatosis. Alternatively, despite a substantial downregulation of CD36 in the combined treatment, why do these mice develop hepatic steatosis? One possibility is that decreased CD36 expression in the nicotine plus HFD group could reflect a compensatory mechanism aimed at decreasing fatty acid uptake to compensate for HFD-induced increased hepatic lipogenesis. Evidence also exists that hepatic overexpression CD36 attenuates HFD-induced hepatic steatosis (67). Thus, one could speculate that upregulation of CD36 in the mice fed an HFD might be beneficial in protecting liver under lipid overload and metabolic stress. Thus, the regulation of hepatic lipid homeostasis by CD36 is far more complex and could vary depending on the nature and severity of stress.
In summary (Fig. 6), we have provided insights into the molecular mechanisms by which PNU attenuates nicotine plus an HFD-induced hepatic steatosis and hepatocellular apoptosis. The preventive effects of PNU on nicotine plus HFD–induced hepatic steatosis appear to be mediated by suppression of oxidative stress, activation of SIRT1 and AMPK signaling, together with inhibition of its downstream targets, including SREBP1c, ACC, and FAS. The clinical implication of this study is that α7nAChR agonists may be an effective therapeutic strategy for the amelioration of NAFLD and adverse metabolic sequelae, especially in obese smokers.
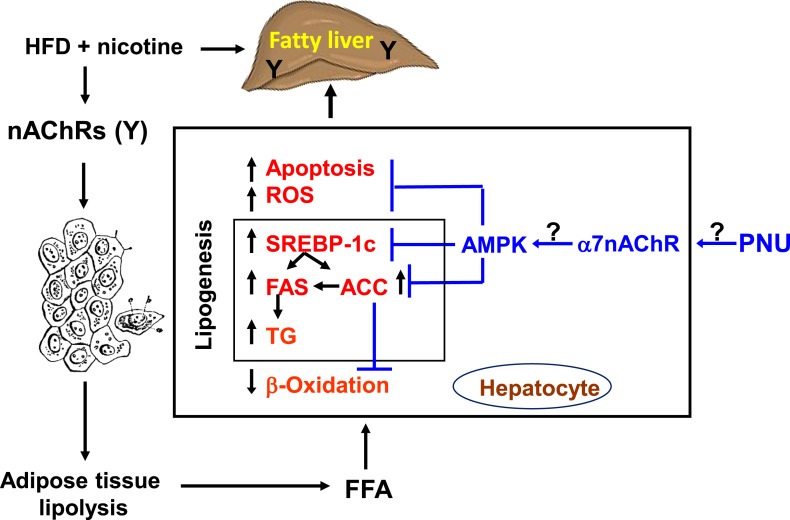
Figure 6.
Model illustrating how PNU attenuates nicotine plus HFD–induced hepatic steatosis. Nicotine plus an HFD promotes abdominal lipolysis, resulting in FFA release from adipose tissue into the circulation, thereby contributing to the buildup of lipids as TG in the liver (11). In addition, nicotine plus an HFD could promote hepatic lipogenesis through inactivation of AMPK and activation of its downstream target ACC, leading to the development of hepatic steatosis. Inactivation of AMPK can also stimulate lipogenesis through upregulation of key genes in the lipogenic pathway such as FAS and ACC by activating the transcription factor sSREBP-1c. Intrahepatic lipid accumulation can also trigger hepatocellular apoptosis through generation of oxidative stress. AMPK inactivation could further sensitize liver cells to nicotine plus HFD–induced apoptosis. PNU seems to protect nicotine plus HFD–induced hepatic steatosis through suppression of oxidative stress and activation of AMPK signaling, together with inhibition of its downstream target, including SREBP1c, ACC, and FAS. FFA, free fatty acid; ROS, reactive oxygen species; TG, triglyceride.
Acknowledgments
We thank the Technology Core of the AXIS Grant 2U54MD007598 from the National Institutes of Health for tissue preparation and hematoxylin and eosin staining.
Financial Support: This work was supported by Diversity-Promoting Institution Drug Abuse Research Program Grant R24DA017298 (to A.P.S.-H.) and Accelerating Excellence in Translational Science Grant 2U54MD007598 from the National Institutes of Health, National Institute on Minority Health and Health Disparities.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 4-HNE
- 4-hydoxynonenal
- ACC
- acetyl-coenzyme A-carboxylase
- AMPK
- adenosine 5′-monophosphate–activated protein kinase
- BW
- body weight
- cDNA
- complementary DNA
- FAS
- fatty acid synthase
- HFD
- high-fat diet
- IP
- intraperitoneal
- nAChR
- nicotinic acetylcholine receptor
- NAFLD
- nonalcoholic fatty liver disease
- NCD
- normal chow diet
- p-ACC
- phospho-acetyl-coenzyme A-carboxylase
- p-AMPK
- phospho-adenosine 5′-monophosphate–activated protein kinase
- PNU
- PNU-282987
- qPCR
- quantitative real-time polymerase chain reaction
- SIRT1
- silent information regulator 1
- SREBP1c
- sterol regulatory element binding protein 1c
- TdT
- terminal deoxynucleotidyl transferase
- TUNEL
- terminal deoxynucleotidyl transferase–mediated deoxy-UTP nick end labeling
- α7nAChR
- α7-nicotinic acetylcholine receptor.
References
- 1.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54(1):113–129. [DOI] [PubMed] [Google Scholar]
- 3.Zaher C, Halbert R, Dubois R, George D, Nonikov D. Smoking-related diseases: the importance of COPD. Int J Tuberc Lung Dis. 2004;8(12):1423–1428. [PubMed] [Google Scholar]
- 4.Hudson NL, Mannino DM. Tobacco use: a chronic illness? J Community Health. 2010;35(5):549–553. [DOI] [PubMed] [Google Scholar]
- 5.Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T, Oketani M, Ido A, Tsubouchi H. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46(6):769–778. [DOI] [PubMed] [Google Scholar]
- 6.Zein CO, Unalp A, Colvin R, Liu YC, McCullough AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54(4):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. [DOI] [PubMed] [Google Scholar]
- 8.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 9.McFadden KL, Cornier MA, Tregellas JR. The role of alpha-7 nicotinic receptors in food intake behaviors. Front Psychol. 2014;5:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangubat M, Lutfy K, Lee ML, Pulido L, Stout D, Davis R, Shin CS, Shahbazian M, Seasholtz S, Sinha-Hikim A, Sinha-Hikim I, O’Dell LE, Lyzlov A, Liu Y, Friedman TC. Effect of nicotine on body composition in mice. J Endocrinol. 2012;212(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman TC, Sinha-Hikim I, Parveen M, Najjar SM, Liu Y, Mangubat M, Shin CS, Lyzlov A, Ivey R, Shaheen M, French SW, Sinha-Hikim AP. Additive effects of nicotine and high-fat diet on hepatic steatosis in male mice. Endocrinology. 2012;153(12):5809–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivey R, Desai M, Green K, Sinha-Hikim I, Friedman TC, Sinha-Hikim AP. Additive effects of nicotine and high-fat diet on hepatocellular apoptosis in mice: involvement of caspase 2 and inducible nitric oxide synthase-mediated intrinsic pathway signaling. Horm Metab Res. 2014;46(8):568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha-Hikim I, Friedman TC, Shin CS, Lee D, Ivey R, Sinha-Hikim AP. Nicotine in combination with a high-fat diet causes intramyocellular mitochondrial abnormalities in male mice. Endocrinology. 2014;155(3):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vu CU, Siddiqui JA, Wadensweiler P, Gayen JR, Avolio E, Bandyopadhyay GK, Biswas N, Chi NW, O’Connor DT, Mahata SK. Nicotinic acetylcholine receptors in glucose homeostasis: the acute hyperglycemic and chronic insulin-sensitive effects of nicotine suggest dual opposing roles of the receptors in male mice. Endocrinology. 2014;155(10):3793–3805. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152(3):836–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Chernyshov V, Kryukova E, Tsetlin V, Komisarenko S, Skok M. Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria. PLoS One. 2012;7(2):e31361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via alpha7 nicotinic acetylcholine receptor. Gastroenterology. 2008;134(7):2122–2131. [DOI] [PubMed] [Google Scholar]
- 18.Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, Soares MP, López MG. The microglial α7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19(11):1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. α7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011;42(12):3530–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, Lu Q, Ouyang C, Xie Z, Zhao Z, Zhuo X, Viollet B, Foretz M, Wu J, Yuan Z, Zou MH. Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med. 2015;21(4):373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Chen Z, Pan Q, Fu S, Lin F, Ren H, Han H, Billiar TR, Sun F, Li Q. The protective effect of PNU-282987, a selective α7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor κB activation in mice. Shock. 2013;39(2):197–203. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Kang JW, Lee SM. Activation of the cholinergic anti-inflammatory pathway by nicotine attenuates hepatic ischemia/reperfusion injury via heme oxygenase-1 induction. Eur J Pharmacol. 2013;707(1-3):61–70. [DOI] [PubMed] [Google Scholar]
- 23.Marrero MB, Lucas R, Salet C, Hauser TA, Mazurov A, Lippiello PM, Bencherif M. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332(1):173–180. [DOI] [PubMed] [Google Scholar]
- 24.Behan JW, Avramis VI, Yun JP, Louie SG, Mittelman SD. Diet-induced obesity alters vincristine pharmacokinetics in blood and tissues of mice. Pharmacol Res. 2010;61:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81(2):243–248. [DOI] [PubMed] [Google Scholar]
- 26.de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nosé V, Puder M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism. 2010;59(8):1092–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44(7):1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha-Hikim I, Friedman TC, Falz M, Chalfant V, Hasan MK, Espinoza-Derout J, Lee DL, Sims C, Tran P, Mahata SK, Sinha-Hikim AP. Nicotine plus a high-fat diet triggers cardiomyocyte apoptosis. Cell Tissue Res. 2017;368(1):159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophy Acta. 2010;1801(3):299–310. [DOI] [PubMed] [Google Scholar]
- 30.Xu TY, Guo LL, Wang P, Song J, Le YY, Viollet B, Miao CY. Chronic exposure to nicotine enhances insulin sensitivity through α7 nicotinic acetylcholine receptor-STAT3 pathway. PLoS One. 2012;7(12):e51217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasan MK, Friedman TC, Espinoza -Derout J, Sims C, Lee DL, Sinha-Hikim I, Sinha Hikim AP. Additive effects of nicotine and high-fat diet on hepatic steatosis and hepatocellular apoptosis: role of alpha-7-nicotinic acetylcholine receptors. In: Program of the 99th Annual Meeting of the Endocrine Society; April 1–4, 2017; Orlando, FL. [Google Scholar]
- 32.Gallou-Kabani C, Vigé A, Gross MS, Rabès JP, Boileau C, Larue-Achagiotis C, Tomé D, Jais JP, Junien C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring). 2007;15(8):1996–2005. [DOI] [PubMed] [Google Scholar]
- 33.Sinha-Hikim I, Shen R, Nzenwa I, Gelfand R, Mahata SK, Sinha-Hikim AP. Minocycline suppresses oxidative stress and attenuates fetal cardiac myocyte apoptosis triggered by in utero cocaine exposure. Apoptosis. 2011;16(6):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strange R, Friis RR, Bemis LT, Geske FJ. Programmed cell death during mammary gland involution. Methods Cell Biol. 1995;46:355–368. [DOI] [PubMed] [Google Scholar]
- 35.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416. [DOI] [PubMed] [Google Scholar]
- 37.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298(3):G364–G374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu F, Gao Z, Zhang J, Rivera CA, Yin J, Weng J, Ye J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology. 2010;151(6):2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L, Zang M. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146(2):539–549.e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30(6):620–650. [DOI] [PubMed] [Google Scholar]
- 41.Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163(6):2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280(3):1117–1136. [PubMed] [Google Scholar]
- 43.Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007; 31(1, Suppl)S61–S66. [DOI] [PubMed] [Google Scholar]
- 44.Serviddio G, Bellanti F, Sastre J, Vendemiale G, Altomare E. Targeting mitochondria: a new promising approach for the treatment of liver diseases. Curr Med Chem. 2010;17(22):2325–2337. [DOI] [PubMed] [Google Scholar]
- 45.Kathirvel E, Chen P, Morgan K, French SW, Morgan TR. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J Gastroenterol Hepatol. 2010;25(6):1136–1143. [DOI] [PubMed] [Google Scholar]
- 46.Videla LA. Cytoprotective and suicidal signaling in oxidative stress. Biol Res. 2010;43(3):363–369. [PubMed] [Google Scholar]
- 47.Kalappa BI, Uteshev VV. The dual effect of PNU-120596 on α7 nicotinic acetylcholine receptor channels. Eur J Pharmacol. 2013;718(1-3):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krafft PR, Altay O, Rolland WB, Duris K, Lekic T, Tang J, Zhang JH. α7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3β inhibition in a mouse model of intracerebral hemorrhage. Stroke. 2012;43(3):844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro E, Buendia I, Parada E, León R, Jansen-Duerr P, Pircher H, Egea J, Lopez MG. Alpha7 nicotinic receptor activation protects against oxidative stress via heme-oxygenase I induction. Biochem Pharmacol. 2015;97(4):473–481. [DOI] [PubMed] [Google Scholar]
- 50.Kan H, Kimura Y, Hyogo H, Fukuhara T, Fujino H, Naeshiro N, Honda Y, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Ochi H, Arihiro K, Chayama K. Non-invasive assessment of liver steatosis in non-alcoholic fatty liver disease. Hepatol Res. 2014;44(14):E420–E427. [DOI] [PubMed] [Google Scholar]
- 51.Seoane-Collazo P, Martínez de Morentin PB, Fernø J, Diéguez C, Nogueiras R, López M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155(5):1679–1689. [DOI] [PubMed] [Google Scholar]
- 52.Leite PE, Gandía L, de Pascual R, Nanclares C, Colmena I, Santos WC, Lagrota-Candido J, Quirico-Santos T. Selective activation of α7 nicotinic acetylcholine receptor (nAChRα7) inhibits muscular degeneration in mdx dystrophic mice. Brain Res. 2014;1573:27–36. [DOI] [PubMed] [Google Scholar]
- 53.Dong MW, Li M, Chen J, Fu TT, Lin KZ, Ye GH, Han JG, Feng XP, Li XB, Yu LS, Fan YY. Activation of α7nAChR promotes diabetic wound healing by suppressing AGE-induced TNF-α production. Inflammation. 2016;39(2):687–699. [DOI] [PubMed] [Google Scholar]
- 54.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha-Hikim I, Sinha-Hikim AP, Shen R, Kim HJ, French SW, Vaziri ND, Crum AC, Rajavashisth TB, Norris KC. A novel cystine based antioxidant attenuates oxidative stress and hepatic steatosis in diet-induced obese mice [published correction appears in Exp Mol Pathol. 2012;93(2):282] Exp Mol Pathol. 2011;91(1):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan H, Shyy JY, Martins-Green M. Second-hand smoke stimulates lipid accumulation in the liver by modulating AMPK and SREBP-1. J Hepatol. 2009;51(3):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kathirvel E, Morgan K, Nandgiri G, Sandoval BC, Caudill MA, Bottiglieri T, French SW, Morgan TR. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: a potential mechanism for hepatoprotection by betaine. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1068–G1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, Dong L, Gao F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19(6):946–957. [DOI] [PubMed] [Google Scholar]
- 59.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia F, Wu C, Chen Z, Lu G.. AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Cardiovasc Drugs Ther. 2011;25(1):21–29. [DOI] [PubMed] [Google Scholar]
- 61.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM. Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem. 2004;279(33):35017–35024. [DOI] [PubMed] [Google Scholar]
- 62.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116(6):1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li M, Meng X, Xu J, Huang X, Li H, Li G, Wang S, Man Y, Tang W, Li J. GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway. Sci Rep. 2016;6(1):25237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. [DOI] [PubMed] [Google Scholar]
- 65.Sheedfar F, Sung MM, Aparicio-Vergara M, Kloosterhuis NJ, Miquilena-Colina ME, Vargas-Castrillón J, Febbraio M, Jacobs RL, de Bruin A, Vinciguerra M, García-Monzón C, Hofker MH, Dyck JR, Koonen DP. Increased hepatic CD36 expression with age is associated with enhanced susceptibility to nonalcoholic fatty liver disease. Aging (Albany NY). 2014;6(4):281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clugston RD, Yuen JJ, Hu Y, Abumrad NA, Berk PD, Goldberg IJ, Blaner WS, Huang LS. CD36-deficient mice are resistant to alcohol- and high-carbohydrate-induced hepatic steatosis. J Lipid Res. 2014;55(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garbacz WG, Lu P, Miller TM, Poloyac SM, Eyre NS, Mayrhofer G, Xu M, Ren S, Xie W. Hepatic overexpression of CD36 improves glycogen homeostasis and attenuates high-fat diet-induced hepatic steatosis and insulin resistance. Mol Cell Biol. 2016;36(21):2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]