Clinical Significance
The annual costs of spinal conditions related to intervertebral disc (IVD) degeneration exceed $190 billion in the US.(1) In industrialized countries, low back pain is extremely common, with a prevalence of 60–90%.(2) Despite this prevalence and soaring cost, there is no specific treatment that restores the physiological function of the degenerate IVD. Thus, developing new treatment strategies to repair the degenerating IVD is vital.
Current treatments for disc-related pain include surgical and non-surgical approaches,(3) and often result in incomplete symptomatic relief. A key limitation of current treatments for disc degeneration is that they do not maintain or restore native tissue structure and mechanical function. Therefore, there is a pressing need for new therapies to treat disc degeneration that retain and/or restore disc structure and mechanical function by directly addressing the underlying causes and mechanisms.
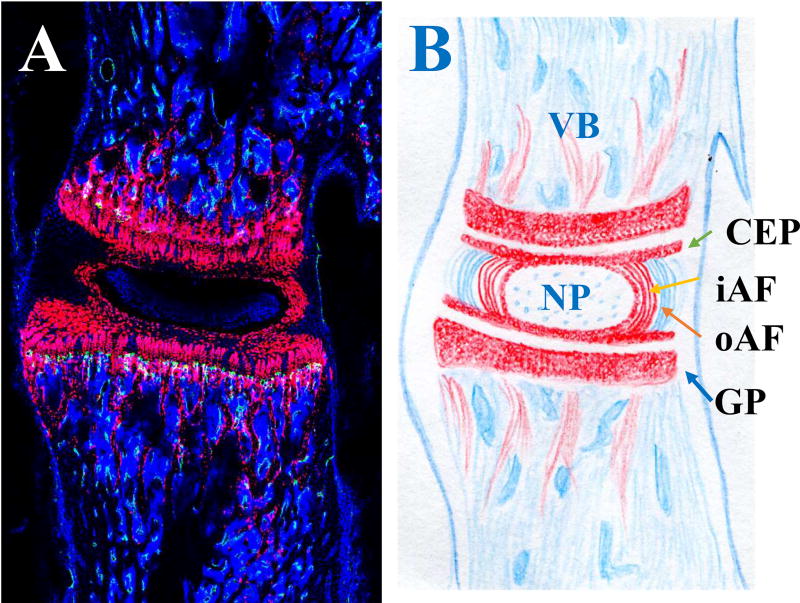
The IVD is an elegant structure, with a gelatinous inner core (the nucleus pulposus (NP)) that functions as a shock absorber, converting axial loads into radial forces. The concentric outer rings (annulus fibrosus (AF)) enclose the inner core (Figure 1). The elegance and complexity of the IVD structure is illustrated in Figure 1. Specifically, tamoxifen was used at postnatal day 6 to induce the expression of Cre-recombinase driven by the type II collagen promoter (Col2CreER). The lumbar spine was examined at postnatal day 28 (Figure 1A). The motion segment consists of an IVD with the adjacent vertebral bodies (VB) (Figure 1B). Cell nuclei, stained with DAPI, are shown in blue (Figure 1A). Inner AF cells express type II collagen (col2) and have been highlighted by red fluorescent protein variant (tdTomato; Figure 1A). The cartilaginous endplate (CEP), growth plate (GP) and cancellous bone adjacent to the GP also express col2, and thus also expressed tdTomato (shown in red in Figure 1A&B).
Figure 1. Mouse lumbar intervertebral disc (IVD).
A. Sagittal section of a Col2CreER;R26- tdTomato mouse IVD. B: schematic drawing of the vertebral body (VB)-IVD-VB motion segment. Red: type II collagen expressing cells; Blue: cell nuclei stained with DAPI. NP: nucleus pulposus; CEP: cartilaginous endplate; iAF: inner annulus fibrosus (AF); oAF: outer AF; GP: growth plate.
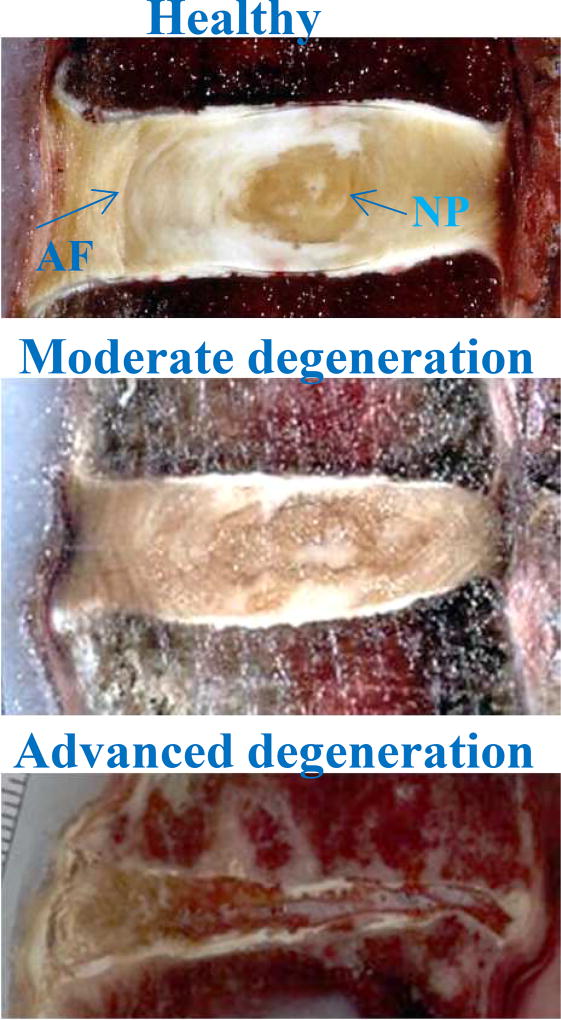
The IVD progressively degenerates with age in humans (Figure 2), and strategies to repair the IVD depend on stage of degeneration.(4, 5) Cell therapy and cell-based gene therapy aim to address moderate IVD degeneration (Figure 2). In early disc degeneration, an attractive strategy is to encourage resident progenitor cells to proliferate. At this stage, various protein factors such as growth factors might be effective since resident cells may respond to the stimuli and produce additional extracellular matrix. However, the number and functional capacity of endogenous stem cells tend to become reduced with aging and degeneration.(6) Therefore, in moderate stages of degeneration, when fewer viable cells remain in the diseased IVD, cell or gene therapy is likely to be required to repopulate the disc and provide additional trophic factors. In advanced stages of degeneration when both cell and extracellular matrix loss is severe, tissue-engineering approaches may be needed.(7, 8)
Figure 2. Gross morphology illustrating progressive human disc degeneration.
NP: nucleus pulposus; AF: annulus fibrosus.
The quality of life for people with chronic pain is often irreversibly affected due to rewiring of brain circuitry,(9) supporting the notion that early treatments will result in better outcomes. The ideal therapy for moderate IVD degeneration would: 1) be minimally invasive; 2) attenuate local inflammation; and 3) restore tissue structure and biomechanical function. In order to achieve success, transplantation of cells into the degenerate IVD must overcome several hurdles. First, transplanted cells must survive in the harsh IVD environment that is low in nutrients, oxygen and pH, exhibits elevated inflammatory cytokine expression, and experiences fluctuations in mechanical stress. Secondly, to achieve therapeutic efficacy, cells must remain viable and in place, produce extracellular matrix (ECM) rich in proteoglycans and type II collagen, or secrete trophic factors that stimulate resident cells to do so.
Patient selection
Most people with IVD degeneration do not have back pain,(10) and thus do not require any intervention. For patients with intractable back pain due to internal disc disruption, cell therapy may help to repair the structure and modulate inflammation, thus reducing pain. When selecting which disc is most symptomatic, the best tool available may be discography, with patient response of severe concordant pain. Discography is a valuable tool since positive findings during discography correlate with levels of cytokines/chemokines in the tissue, thus providing a pathophysiological basis for discogenic back pain.(11, 12) Discography, however, may cause further IVD degeneration,(13) due to needle puncture damage and the toxicity of injected anesthetics and contrast media.(14–16) Based on these observations, only patients with severe axial back pain that is suspected to be due to degenerative disc disease are clinical candidates for discography. Patients with severe back pain confirmed to be related to IVD degeneration should undergo cell therapy. Therefore, after patient-confirmed severe concordant pain with provocation, cells could be injected during the same procedure to avoid puncturing the IVD multiple times.
Patients undergoing partial discectomy are another group of individuals who could benefit from cell therapy, since disc degeneration accelerates after partial removal of the disc.(17) Cells could be injected during surgery, after removal of the disc fragment(s) impinging on the nerve roots. The above indications for cell therapy require that cells are ready to use before the procedure.
Cell Sources and Types
Autologous and allogeneic cells have been used in clinical trials, but xenogeneic cells have only been used in animal studies. Autologous cells are ideal, due to concerns over disease transmission and immune responses. Autologous mesenchymal stromal cells can be harvested from bone marrow or adipose tissue. The main limitation is that most patients with back pain are middle aged, and their stem cells have limited expansion potential. A small clinical study (10 patients) examining the possible efficacy of hematopoietic stem cells in disc repair did not show any treatment effect.(18) A more recent study using bone marrow concentrate cells did show pain reduction.(19) The EuroDISC study is the largest (112 patients) prospective multicenter randomized controlled trial comparing patients who had discectomy with or without subsequent treatment with expanded autologous IVD cells; this treatment led to moderate success in preserving the disc structure.(20, 21) The limitation of this study is that patients underwent two procedures, and the cells expanded may have included fibroblasts and inflammatory cells.
Allogeneic cells could be isolated from umbilical cord blood,(22) umbilical tissue,(23) or articular surface.(24, 25) Allogeneic cells from younger donors have higher expansion potential than most autologous cells. There is less ethical concern using these cells than with embryonic stem cells. Only one clinical trial using allogeneic young articular chondrocyte transplantation has been completed. This trial recruited 15 patients and showed promising results.(26)
Xenogeneic cells have only been tested in animal models; most of these studies used various human cells to repair injured animal IVDs.(17)
The main cells used in animal studies include stem cells, IVD cells and articular chondrocytes of autologous, allogeneic and xenogeneic sources (Table 1). Thirty-seven of the animal studies reviewed utilized stem cells. Among these, 10 studies used autologous stem cells,(27–36) 15 studies used allogeneic cells,(25, 37–50) and 12 used xenogeneic cells (23, 51–61) (Table 1). Studies involving allogeneic and xenogeneic cells have shown good survival of these cells in the IVD, confirming that the disc niche is a relatively immunologically privileged site. In the intact IVD, there is limited blood supply to the outer 1/3 of the posterior annulus fibrosus.(62) With injury and degeneration, there is nerve and blood vessel ingrowth,(63) possibly allowing immune cells to migrate into the diseased tissues. In fact, we have observed macrophages in both the injured IVD, and injured IVD injected with allogeneic articular chondrocytes, but did not find significant differences in macrophage infiltration between the two groups.(24) The infiltration of macrophages did not appear to result in elimination of allogeneic or xenogeneic cells implanted into the disc space.
Table 1.
Cell Sources and Types Used in Animal Models.
| Cell Source | Autologous | Allogeneic | Xenogeneic | Total Studies |
|---|---|---|---|---|
| Stem Cells | 10 | 15 | 12 | 37 |
| Intervertebral Disc Cells | 6 | 5 | 2 | 13 |
| Articular Chondrocytes | 0 | 2 | 1 | 3 |
Differentiated cells used in animal studies include IVD cells and articular chondrocytes. Among the 13 animal studies using IVD cells to repair the degenerating IVD, 6 used autologous cells.(64–69) Five studies used allogeneic cells,(70–74) and 2 used xenogeneic cells.(51, 54) Among the studies using articular chondrocytes, 2 studies used allogeneic cells,(24, 25) and one study used xenogeneic chondrocytes (54) to repair the injured IVD. All studies reported some improvement of the disc structure, while allogeneic articular chondrocyte transplantation was reported to attenuate local inflammation.(24)
Mesenchymal stem cells (MSCs) from various sources (e.g., bone marrow, fat,(28) umbilical cord blood,(22) Wharton's jelly,(23, 75) olfactory stem cells(42)) or induced pluripotent stem cells (76) have also been investigated for repairing the degenerate IVD. While readily available, MSCs may suffer from overt cell loss when implanted in the undifferentiated state, due to inability to survive in the harsh, nutrient-poor environment.(77) Induced pluripotent stem cells from autologous or allogeneic sources are very attractive.(78, 79) There are concerns over teratoma formation in the disc space, a consideration that needs further examination.
There are only limited direct comparisons between the outcomes of IVDs treated with stem cells, differentiated disc cells or articular chondrocytes; the differentiated cells seem to be superior in producing more cartilage-like matrix.(25, 51) Further work is needed to directly compare the survival of undifferentiated stem cells, stem cells pre-conditioned to the IVD environment with biomechanical stress and hypoxia, and differentiated cells. Likewise, direct comparison of the cells’ ability to attenuate local inflammation, and to improve disc structure and biomechanical function is needed.
Scaffolds
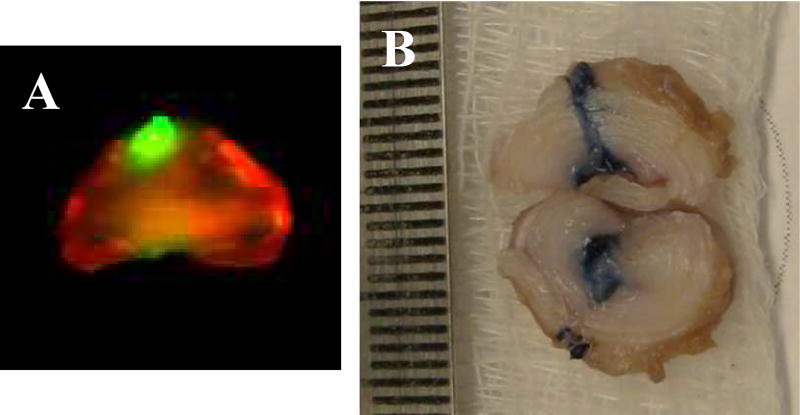
There are concerns over bone spur formation due to injury to the IVD during cell injection or leakage of the cells.(45) We have observed non-calcified cartilaginous protrusion(s) that contain injected articular chondrocytes at the needle insertion site (Figure 3). Using a scaffold seems to reduce cell leakage and osteophyte formation.(31) Among the 50 studies reviewed here, 25 used some form of scaffold. Of these, fibrin gel has been reported to reduce cell leakage.(71) Use of collagen microspheres has been shown to reduce osteophyte formation (which may be consequent on cell leakage).(31) Our preliminary data have shown that young allogeneic articular chondrocytes (AC) injected into the center of the injured rabbit IVD survive and reduce host inflammation, but also can leak at the injection site (Figure 3). To reduce leakage and support cell growth, our group has developed hyaluronic acid (HA)-based hydrogels that preserve the chondrocytic phenotype and growth;(80–83) these materials solidify at body temperature and are thus ideal for injection-based therapies. We have also shown that hyaluronic acid hydrogels promote NP cell phenotype stability.(84) We have further tested a tripleinterpenetrating-network (TIN) hydrogel that enhances biomechanical properties of the repaired IVD and supports cell delivery.(85) Other natural and synthetic scaffold materials, including laminin,(86) pig bone gelatin and cartilage extracellular matrix,(87) collagen,(88) composite of collagen with alginate,(89) and carboxymethylcellulose (90) have been tested for engineering IVD by seeding with cells in vitro, followed by implantation into the disc space. All the above scaffolds could be modified into injectable form and used in minimally invasive cell therapy. Thus, these hydrogels should be assessed as scaffolds to prevent this leakage, and to enhance biomechanical properties of the repaired IVD in the future.
Figure 3. Rabbit intervertebral disc injected with chondrocytes labeled with infrared dye and transduced with adenovirus expressing β-galactosidase.
A: infrared scan; B: X-gal stain. Ruler in right panel is 1mm/space.
Animal models
A critical step towards the clinical translation of new therapies for IVD degeneration is testing in an appropriate in vivo model. Disc degeneration is a complex process involving both mechanical and biochemical factors. When considering the appropriate animal model for disc degeneration studies, the choice of species represents a balance between size, morphology, mechanical properties, nutrient diffusion, repair potential and logistical concerns. Rabbits are the most frequently used among the studies reviewed, likely reflecting the cost and size of the IVDs (Table 2). Larger animals such as the sheep, goat or pig have bigger IVDs, with shapes more similar to that of humans. However, the costs would be higher and therefore are more appropriate for definitive pre-clinical studies. Among the 50 animal studies reviewed here, 28 studies used rabbits, 7 used rats, 5 used dogs, 6 pigs, and there were 1 goat, 2 sheep and 1 mouse study (Table 2). Sheep and goat have IVDs resembling those of humans in size and absence of notochordal cells,(91) but only 3 studies out of 50 used these models. Although the authors agree that a large animal model is critical given the risk of implant ejection and subsequent catastrophic injury, large animal studies are not consistently conducted before clinical trials. However, it is our belief that study in a large animal without persisting notochord (i.e., goat or sheep) should be considered before clinical trials.
Table 2.
Animal Models.
| Animal Models |
Mouse | Rat | Rabbit | Pig | Dog | Goat | Sheep |
|---|---|---|---|---|---|---|---|
| Number of Studies | 1 | 7 | 28 | 6 | 5 | 1 | 2 |
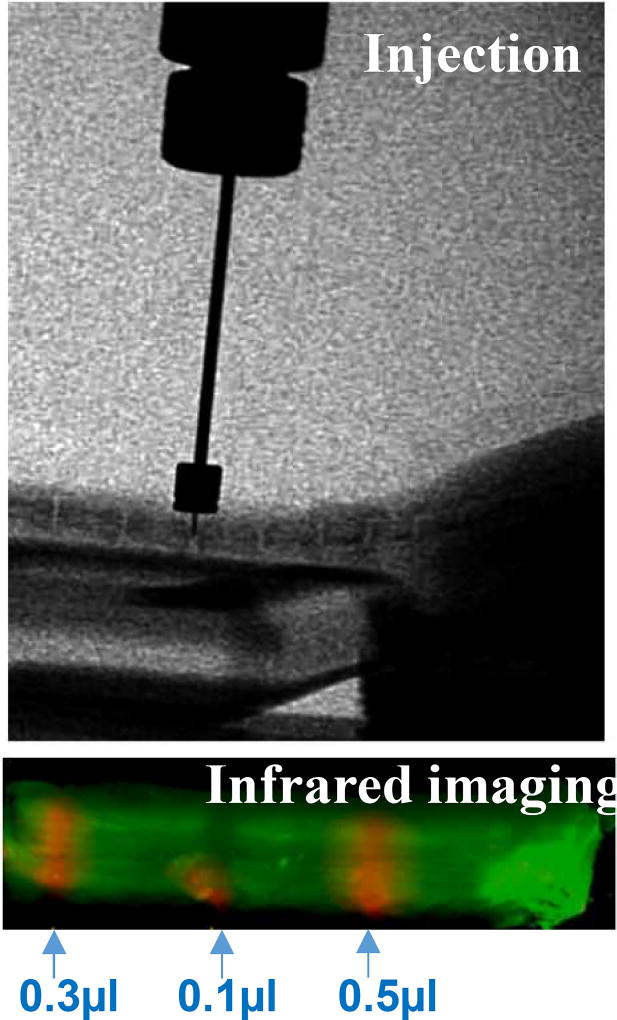
The mouse model has the advantage of opportunities for genetic manipulations (as illustrated in Figure 1), and is less costly than using larger animals. Challenges of working with the mouse IVD mainly reflect its small size: surgical precision is crucial, and the amount of tissue for molecular and biochemical assays is limited. Our group and others have developed the mouse injury model.(92, 93) In addition, we have developed microinjection methods, and use cell tracing methods to confirm precision injection (Figure 4). Various volumes of protein labeled with infrared (IR) dye have been injected into the degenerating IVD in the mouse tail. Tail IVD degeneration has been induced with a needle puncture. It is worth noting that injecting into the intact IVD is exceedingly difficult, due to the positive pressure within the disc. Further refinement of the mouse model is our priority and an important future direction.
Figure 4. Protein labeled with infrared (IR) dye was injected into the degenerating mouse tail IVD.
Role of inflammation in IVD degeneration and back pain
IVD degeneration is a slowly progressing cascade mediated in part by inflammation.(94) Inflammatory stimulation directly alters the mechanobiology of NP cells (95) and inhibits cell extracellular matrix (ECM) production.(96, 97) Inflammation in the IVD tissues is also increasingly recognized to be associated with back pain.(11, 12, 98–100) More recently, serum biomarkers have been reported that vary with diagnosis.(101, 101, 101, 101, 102, 102) In particular, serum levels of IL-6 were significantly higher in subjects with LBP compared with control subjects.(101, 102) Novel treatments targeting inflammation are being pursued.(103, 104) The high levels of proinflammatory mediators found in disc tissue from patients undergoing fusion for discogenic back pain suggest that production of proinflammatory mediators within the IVD may be a major factor in the genesis of a painful lumbar disc.(11, 12, 98, 99) These findings strongly suggest that in addition to the commonly used histology and extracellular matrix composition, inflammatory markers may be used as outcome measures in response to cell therapy. However, among the 50 studies, only 2 examined local inflammation.(24, 41) All the clinical trials used patient symptoms as outcome measures. Future work should focus on identification of inflammatory mediators as outcome measures.
Benchmark for success
None of the above animal studies or clinical trials completely restored IVD structure. Although mechanical function changes in degenerative discs have been well documented,(105) whether cell therapy can restore biomechanical function has not been previously determined. In light of the fact that patients primarily seek medical care for back pain, attenuating local inflammation should be a priority amongst benchmarks for success. The ideal therapy should also be minimally invasive, and concurrent with other procedures such as discography or discectomy. Restoration of tissue structure and biomechanical function are important, and preservation of spinal motion is also desirable.
Acknowledgments
Yejia Zhang, MD, PhD has been supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, 1K08 HD049598). Harvey E. Smith has been supported by a Department of Veterans Affairs Career Development Award (CDA; VA RR&D IK2 RX001476-01). This work is supported, in part, by research grants from the Department of Veterans Affairs (VA RR&D I01 RX001321 and VA1I21RX001896).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have read the journal’s policy on disclosures of potential conflicts of interest, and have none to declare. All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors.
References
- 1.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH task force on research standards for chronic low back pain. Spine (Phila Pa 1976) 2014 Jun 15;39(14):1128–43. doi: 10.1097/BRS.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 2.Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976) 1999 Sep 1;24(17):1813–9. doi: 10.1097/00007632-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Moss I, An HS, Shen F, Li Z, Andersson GBJ, Zhang Y. The Nonsurgical Treatment of Back Pain. In: Shapiro Irving M, Risbud Makarand V., editors. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. Springer-Verlag Wien; 2014. pp. 250–9. [Google Scholar]
- 4.Zhang Y, Chee A, Thonar EJ, An HS. Intervertebral disk repair by protein, gene, or cell injection: a framework for rehabilitation-focused biologics in the spine. PM R. 2011 Jun;3(6 Suppl 1):S88–94. doi: 10.1016/j.pmrj.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Maidhof R, Alipui DO, Rafiuddin A, Levine M, Grande DA, Chahine NO. Emerging trends in biological therapy for intervertebral disc degeneration. Discov Med. 2012 Dec;14(79):401–11. [PubMed] [Google Scholar]
- 6.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowles RD, Gebhard HH, Hartl R, Bonassar LJ. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13106–11. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JT, Kim DH, Milby AH, Pfeifer CG, Smith LJ, Elliott DM, et al. In vivo performance of an acellular disc-like angle ply structure (DAPS) for total disc replacement in a small animal model. J Orthop Res. 2016 May 26; doi: 10.1002/jor.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fields HL. Neuroscience. More pain; less gain. Science. 2014 Aug 1;345(6196):513–4. doi: 10.1126/science.1258477. [DOI] [PubMed] [Google Scholar]
- 10.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990 Mar;72(3):403–8. [PubMed] [Google Scholar]
- 11.Zhang Y, Chee A, Shi P, Adams SL, Markova DZ, Anderson DG, et al. Intervertebral Disc Cells Produce Interleukins Found in Patients with Back Pain. Am J Phys Med Rehabil. 2015 Oct 22; doi: 10.1097/PHM.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kepler CK, Markova DZ, Dibra F, Yadla S, Vaccaro AR, Risbud MV, et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1beta in painful human intervertebral discs. Spine (Phila Pa 1976) 2013 May 15;38(11):873–80. doi: 10.1097/BRS.0b013e318285ae08. [DOI] [PubMed] [Google Scholar]
- 13.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976) 2009 Oct 1;34(21):2338–45. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 14.Chee AV, Ren J, Lenart BA, Chen EY, Zhang Y, An HS. Cytotoxicity of local anesthetics and nonionic contrast agents on bovine intervertebral disc cells cultured in a three-dimensional culture system. Spine J. 2014 Mar 1;14(3):491–8. doi: 10.1016/j.spinee.2013.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Sowa G, Vo N, Vadala G, O'Connell S, Studer R, et al. Effect of bupivacaine on intervertebral disc cell viability. Spine J. 2010 Feb;10(2):159–66. doi: 10.1016/j.spinee.2009.08.445. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Vo NV, Sowa GA, Hartman RA, Ngo K, Choe SR, et al. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J. 2011 Feb;11(2):139–46. doi: 10.1016/j.spinee.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015 Apr;11(4):243–56. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 18.Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006 Feb;15(1):136–7. doi: 10.1089/scd.2006.15.136. [DOI] [PubMed] [Google Scholar]
- 19.Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015 Jan;33(1):146–56. doi: 10.1002/stem.1845. [DOI] [PubMed] [Google Scholar]
- 20.Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006 Aug 15;(Suppl 3):S397–405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007 Feb;24(1):5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Anderson DG, Markova D, An HS, Chee A, Enomoto-Iwamoto M, Markov V, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013 May;92(5):420–9. doi: 10.1097/PHM.0b013e31825f148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leckie SK, Sowa GA, Bechara BP, Hartman RA, Coelho JP, Witt WT, et al. Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013 Mar;13(3):263–72. doi: 10.1016/j.spinee.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Chee A, Shi P, Wang R, Moss I, Chen EY, et al. Allogeneic Articular Chondrocyte Transplantation Downregulates Interleukin 8 Gene Expression in the Degenerating Rabbit Intervertebral Disk In Vivo. Am J Phys Med Rehabil. 2015 Jul;94(7):530–8. doi: 10.1097/PHM.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acosta FL, Jr, Metz L, Adkisson HD, Liu J, Carruthers-Liebenberg E, Milliman C, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011 Dec;17(23–24):3045–55. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coric D, Pettine K, Sumich A, Boltes MO. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine. 2013 Jan;18(1):85–95. doi: 10.3171/2012.10.SPINE12512. [DOI] [PubMed] [Google Scholar]
- 27.Bendtsen M, Bunger CE, Zou X, Foldager C, Jorgensen HS. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. Spine (Phila Pa 1976) 2011 Mar 15;36(6):E373–9. doi: 10.1097/BRS.0b013e3181dce34c. [DOI] [PubMed] [Google Scholar]
- 28.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: experiments in a canine model. Spine (Phila Pa 1976) 2009 Oct 1;34(21):2297–304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 29.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008 May;26(5):589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 30.Ho G, Leung VY, Cheung KM, Chan D. Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res. 2008;49(1):15–21. doi: 10.1080/03008200701818595. [DOI] [PubMed] [Google Scholar]
- 31.Li YY, Diao HJ, Chik TK, Chow CT, An XM, Leung V, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A. 2014 May 20;(9–10):1379–91. doi: 10.1089/ten.tea.2013.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003 Sep;24(20):3531–41. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 33.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005 Nov 1;30(21):2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 34.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006 Jan;27(3):335–45. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010 Oct;28(10):1267–75. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]
- 36.Omlor GW, Bertram H, Kleinschmidt K, Fischer J, Brohm K, Guehring T, et al. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur Spine J. 2010 Apr;19(4):601–12. doi: 10.1007/s00586-009-1255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barczewska M, Wojtkiewicz J, Habich A, Janowski M, Adamiak Z, Holak P, et al. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS One. 2013 Sep 13;8(9):e74658. doi: 10.1371/journal.pone.0074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai F, Wu XT, Xie XH, Wang F, Hong X, Zhuang SY, et al. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. Int Orthop. 2015 Jan;39(1):149–59. doi: 10.1007/s00264-014-2481-0. [DOI] [PubMed] [Google Scholar]
- 39.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004 Mar;32(3):430–4. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 40.Hee HT, Ismail HD, Lim CT, Goh JC, Wong HK. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc. J Bone Joint Surg Br. 2010 May;92(5):726–36. doi: 10.1302/0301-620X.92B5.23015. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12(6):R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay-Sim A. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009 Jul;9(7):585–94. doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008 Nov-Dec;8(6):888–96. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Subhan RA, Puvanan K, Murali MR, Raghavendran HR, Shani S, Abdullah BJ, et al. Fluoroscopy assisted minimally invasive transplantation of allogenic mesenchymal stromal cells embedded in HyStem reduces the progression of nucleus pulposus degeneration in the damaged ntervertebral [corrected] disc: a preliminary study in rabbits. Scientific World Journal. 2014;2014:818502. doi: 10.1155/2014/818502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vadala G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012 May;6(5):348–55. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Zhou Y, Huang B, Liu LT, Liu MH, Wang J, et al. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A. 2014 Mar;20(5–6):908–20. doi: 10.1089/ten.TEA.2012.0703. [DOI] [PubMed] [Google Scholar]
- 47.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010 Sep;10(9):802–10. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Yi Z, Guanjun T, Lin C, Zifeng P. Effects of Transplantation of hTIMP1-Expressing Bone Marrow Mesenchymal Stem Cells on the Extracellular Matrix of Degenerative Intervertebral Discs in an in vivo Rabbit Model. Spine (Phila Pa 1976) 2014 Apr 8; doi: 10.1097/BRS.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Drapeau S, Howard SA, Thonar EJ, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976) 2011 Mar 1;36(5):372–7. doi: 10.1097/BRS.0b013e3181d10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005 Jan;(430):219–26. doi: 10.1097/01.blo.0000146534.31120.cf. (430) [DOI] [PubMed] [Google Scholar]
- 51.Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010 Dec;10(12):1089–97. doi: 10.1016/j.spinee.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun HJ, Kim YS, Kim BK, Kim EH, Kim JH, Do BR, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012 Sep-Oct;78(3–4):364–71. doi: 10.1016/j.wneu.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh P, Moore R, Vernon-Roberts B, Goldschlager T, Pascoe D, Zannettino A, et al. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012 May;16(5):479–88. doi: 10.3171/2012.1.SPINE11852. [DOI] [PubMed] [Google Scholar]
- 54.Henriksson HB, Hagman M, Horn M, Lindahl A, Brisby H. Investigation of different cell types and gel carriers for cell-based intervertebral disc therapy, in vitro and in vivo studies. J Tissue Eng Regen Med. 2012 Oct;6(9):738–47. doi: 10.1002/term.480. [DOI] [PubMed] [Google Scholar]
- 55.Henriksson HB, Svanvik T, Jonsson M, Hagman M, Horn M, Lindahl A, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009 Jan 15;34(2):141–8. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 56.Jeong JH, Jin ES, Min JK, Jeon SR, Park CS, Kim HS, et al. Human mesenchymal stem cells implantation into the degenerated coccygeal disc of the rat. Cytotechnology. 2009;59(1):55–64. doi: 10.1007/s10616-009-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeong JH, Lee JH, Jin ES, Min JK, Jeon SR, Choi KH. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir (Wien) 2010 Oct;152(10):1771–7. doi: 10.1007/s00701-010-0698-2. [DOI] [PubMed] [Google Scholar]
- 58.Prologo JD, Pirasteh A, Tenley N, Yuan L, Corn D, Hart D, et al. Percutaneous image-guided delivery for the transplantation of mesenchymal stem cells in the setting of degenerated intervertebral discs. J Vasc Interv Radiol. 2012 Aug;23(8):1084–1088. e6. doi: 10.1016/j.jvir.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 59.Sheikh H, Zakharian K, De La Torre RP, Facek C, Vasquez A, Chaudhry GR, et al. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009 Mar;10(3):265–72. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 60.Tam V, Rogers I, Chan D, Leung VY, Cheung KM. A comparison of intravenous and intradiscal delivery of multipotential stem cells on the healing of injured intervertebral disk. J Orthop Res. 2014 Jun;32(6):819–25. doi: 10.1002/jor.22605. [DOI] [PubMed] [Google Scholar]
- 61.Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009 Mar;27(3):374–9. doi: 10.1002/jor.20567. [DOI] [PubMed] [Google Scholar]
- 62.Palmgren T, Gronblad M, Virri J, Kaapa E, Karaharju E. An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976) 1999 Oct 15;24(20):2075–9. doi: 10.1097/00007632-199910150-00002. [DOI] [PubMed] [Google Scholar]
- 63.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997 Jul 19;350(9072):178–81. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 64.Benz K, Stippich C, Fischer L, Mohl K, Weber K, Lang J, et al. Intervertebral disc cell- and hydrogel- supported and spontaneous intervertebral disc repair in nucleotomized sheep. Eur Spine J. 2012 Sep;21(9):1758–68. doi: 10.1007/s00586-012-2443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976) 2003 Dec 1;28(23):2609–20. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 66.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine (Phila Pa 1976) 2002 Aug 1;27(15):1626–33. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura K, Mochida J. Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine (Phila Pa 1976) 1998 Jul 15;23(14):1531–8. doi: 10.1097/00007632-199807150-00006. discussion 1539. [DOI] [PubMed] [Google Scholar]
- 68.Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000 Nov;18(6):988–97. doi: 10.1002/jor.1100180620. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe K, Mochida J, Nomura T, Okuma M, Sakabe K, Seiki K. Effect of reinsertion of activated nucleus pulposus on disc degeneration: an experimental study on various types of collagen in degenerative discs. Connect Tissue Res. 2003;44(2):104–8. [PubMed] [Google Scholar]
- 70.Nomura T, Mochida J, Okuma M, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin Orthop Relat Res. 2001 Aug;(389):94–101. doi: 10.1097/00003086-200108000-00015. (389) [DOI] [PubMed] [Google Scholar]
- 71.Bertram H, Kroeber M, Wang H, Unglaub F, Guehring T, Carstens C, et al. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem Biophys Res Commun. 2005 Jun 17;331(4):1185–92. doi: 10.1016/j.bbrc.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 72.Huang B, Zhuang Y, Li CQ, Liu LT, Zhou Y. Regeneration of the intervertebral disc with nucleus pulposus cell-seeded collagen II/hyaluronan/chondroitin-6-sulfate tri-copolymer constructs in a rabbit disc degeneration model. Spine (Phila Pa 1976) 2011 Dec 15;36(26):2252–9. doi: 10.1097/BRS.0b013e318209fd85. [DOI] [PubMed] [Google Scholar]
- 73.Ruan DK, Xin H, Zhang C, Wang C, Xu C, Li C, et al. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng Part A. 2010 Jul;16(7):2381–9. doi: 10.1089/ten.TEA.2009.0770. [DOI] [PubMed] [Google Scholar]
- 74.Sato M, Asazuma T, Ishihara M, Ishihara M, Kikuchi T, Kikuchi M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue- engineering method. Spine (Phila Pa 1976) 2003 Mar 15;28(6):548–53. doi: 10.1097/01.BRS.0000049909.09102.60. [DOI] [PubMed] [Google Scholar]
- 75.Chon BH, Lee EJ, Jing L, Setton LA, Chen J. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res Ther. 2013 Oct 2;4(5):120. doi: 10.1186/scrt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS One. 2013 Sep 25;8(9):e75548. doi: 10.1371/journal.pone.0075548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007 Jun;13(6):1325–31. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y, Rahaman MN, Bal BS. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS One. 2014 Jul 23;9(7):e100885. doi: 10.1371/journal.pone.0100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y, Fu S, Rahaman MN, Mao JJ, Bal BS. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A. 2015;103(3):1053–9. doi: 10.1002/jbm.a.35243. [DOI] [PubMed] [Google Scholar]
- 80.Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10117–22. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011 Sep;32(27):6425–34. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012 Aug;8(8):3027–34. doi: 10.1016/j.actbio.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA, Mauck RL. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed Mater. 2012 Apr;7(2) doi: 10.1088/1748-6041/7/2/024110. 024110,6041/7/2/024110. Epub 2012 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim DH, Martin JT, Elliott DM, Smith LJ, Mauck RL. Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomater. 2015 Jan 15;12:21–9. doi: 10.1016/j.actbio.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith LJ, Gorth DJ, Showalter BL, Chiaro JA, Beattie EE, Elliott DM, et al. In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng Part A. 2014 Jul;20(13–14):1841–9. doi: 10.1089/ten.tea.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francisco AT, Hwang PY, Jeong CG, Jing L, Chen J, Setton LA. Photocrosslinkable laminin- functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014 Mar;10(3):1102–11. doi: 10.1016/j.actbio.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu B, Xu H, Wu Y, Li X, Zhang Y, Ma X, et al. Intervertebral Disc Tissue Engineering with Natural Extracellular Matrix-Derived Biphasic Composite Scaffolds. PLoS One. 2015 Apr 20;10(4):e0124774. doi: 10.1371/journal.pone.0124774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008 Feb;29(4):438–47. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Guillaume O, Naqvi SM, Lennon K, Buckley CT. Enhancing cell migration in shape- memory alginate- collagen composite scaffolds: In vitro and ex vivo assessment for intervertebral disc repair. J Biomater Appl. 2015 Apr;29(9):1230–46. doi: 10.1177/0885328214557905. [DOI] [PubMed] [Google Scholar]
- 90.Reza AT, Nicoll SB. Characterization of novel photocrosslinked carboxymethylcellulose hydrogels for encapsulation of nucleus pulposus cells. Acta Biomater. 2010 Jan;6(1):179–86. doi: 10.1016/j.actbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. Biomed Res Int. 2016;2016:5952165. doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013 Aug;31(8):1276–82. doi: 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Injury-induced sequential transformation of notochordal nucleus pulposus to chondrogenic and fibrocartilaginous phenotype in the mouse. J Pathol. 2009 May;218(1):113–21. doi: 10.1002/path.2519. [DOI] [PubMed] [Google Scholar]
- 94.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009 Jan;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 95.Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One. 2014 Jun 17;9(6):e99621. doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1- MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 2008 Feb 15;33(4):356–65. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, An HS, Toofanfard M, Li Z, Andersson GB, Thonar EJ. Low-dose interleukin-1 partially counteracts osteogenic protein-1-induced proteoglycan synthesis by adult bovine intervertebral disk cells. Am J Phys Med Rehabil. 2005 May;84(5):322–9. doi: 10.1097/01.phm.0000159972.85053.7e. [DOI] [PubMed] [Google Scholar]
- 98.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Spontaneous production of monocyte chemoattractant protein-1 and interleukin-8 by the human lumbar intervertebral disc. Spine (Phila Pa 1976) 2002 Jul 1;27(13):1402–7. doi: 10.1097/00007632-200207010-00006. [DOI] [PubMed] [Google Scholar]
- 99.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002 Mar;84(2):196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 100.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014 Jan;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weber KT, Satoh S, Alipui DO, Virojanapa J, Levine M, Sison C, et al. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol Res. 2015 Dec;63(1–3):170–80. doi: 10.1007/s12026-015-8709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016 Jan 7;18 doi: 10.1186/s13075-015-0887-8. 3,015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Q, Jin L, Shen FH, Balian G, Li XJ. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells--a potential novel treatment for intervertebral disc degeneration. Spine J. 2013 Nov;13(11):1571–80. doi: 10.1016/j.spinee.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Q, Jin L, Mahon BH, Chordia MD, Shen FH, Li X. Novel treatment of neuroinflammation against low back pain by soluble fullerol nanoparticles. Spine (Phila Pa 1976) 2013 Aug 1;38(17):1443–51. doi: 10.1097/BRS.0b013e31828fc6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inoue N, Espinoza Orias AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011 Oct;42(4):487–99. vii. doi: 10.1016/j.ocl.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]