Significance
Gay men have, on average, a greater number of older brothers than do heterosexual men, a well-known finding within sexual science. This finding has been termed the fraternal birth order effect. Strong scientific interest in sexual orientation exists because it is a fundamental human characteristic, and because its origins are often the focal point of considerable social controversy. Our study is a major advance in understanding the origins of sexual orientation in men by providing support for a theorized but previously unexamined biological mechanism—a maternal immune response to a protein important in male fetal brain development—and by beginning to explain one of the most reliable correlates of male homosexuality: older brothers.
Keywords: sexual orientation, homosexuality, fraternal birth order, NLGN4Y, maternal immune hypothesis
Abstract
We conducted a direct test of an immunological explanation of the finding that gay men have a greater number of older brothers than do heterosexual men. This explanation posits that some mothers develop antibodies against a Y-linked protein important in male brain development, and that this effect becomes increasingly likely with each male gestation, altering brain structures underlying sexual orientation in their later-born sons. Immune assays targeting two Y-linked proteins important in brain development—protocadherin 11 Y-linked (PCDH11Y) and neuroligin 4 Y-linked (NLGN4Y; isoforms 1 and 2)—were developed. Plasma from mothers of sons, about half of whom had a gay son, along with additional controls (women with no sons, men) was analyzed for male protein-specific antibodies. Results indicated women had significantly higher anti-NLGN4Y levels than men. In addition, after statistically controlling for number of pregnancies, mothers of gay sons, particularly those with older brothers, had significantly higher anti-NLGN4Y levels than did the control samples of women, including mothers of heterosexual sons. The results suggest an association between a maternal immune response to NLGN4Y and subsequent sexual orientation in male offspring.
A fraternal birth order (FBO) effect exists in the sexual orientation of men, but not of women, with older brothers (and no other sibling characteristic) increasing the odds of male homosexuality (1–3). Although sexual orientation is likely determined by multiple (potentially independent) factors and FBO is associated with only a proportion of men’s sexual orientation (4–6), the effect has been confirmed many times, including by independent investigators and in non-Western samples (2, 3, 7–10). FBO is independent of potential confounds, such as maternal age (1, 11), and likely operates during prenatal life and not childhood (11). Evidence in favor of a maternal immune hypothesis underlying FBO includes the finding that fetal material enters maternal circulation at parturition (12), as exemplified by male microchimerism occurring in mothers of sons (13, 14) and by hemolytic disease of the newborn (15). There is also evidence that maternal immune products, including antibodies, can enter the fetal compartment and pass the blood/brain barrier of the developing fetal brain (16–20). Finally, an incremental maternal immune response occurs to a Y-linked protein (SMCY/H-Y) in relation to prior male fetuses (21), a phenomenon that, at times, may underlie widespread alteration of fetal development and induce miscarriage (22).
Unlike SMCY, two Y-linked proteins—protocadherin 11 Y-linked (PCDH11Y) and neuroligin 4 Y-linked (NLGN4Y)—have special relevance as candidates to underlie FBO and sexual orientation (2, 3) because they are represented in the fetal brain and feature primarily extracellular structures (23–25), which allows these proteins to be accessible to circulating antibodies. Thus, antibodies could bind to these proteins and alter their role in typical sexual differentiation of the brain (without necessarily resulting in death of the affected cells or inducing miscarriage). Both PCDH11Y and NLGN4Y are part of families of cell adhesion molecules thought to play an essential role in specific cell–cell interactions in brain development (23).
In our study, blood samples and reproductive histories were collected from 54 mothers of gay sons (23 of whom had previously given birth to a heterosexual son) and a control sample of 72 mothers of heterosexual sons, along with additional controls (16 women with no sons and 12 men). We developed in-house ELISAs and used the plasma from participants to examine evidence of the existence of maternal antibodies to these proteins.
Results
We examined antibody concentrations to each of these Y-linked proteins (anti-PCDH11Y, anti-NLGN4Y isoform 1, anti-NLGN4Y isoform 2) separately, but also used a z-scored combination of anti-NLGN4Y isoforms 1 and 2 (combined anti-NLGN4Y). Such a composite could provide a more reliable estimate of response to common epitopes than either assay alone and thus might be a better index of humoral response to NLGN4Y. Due to the skewed nature of the assay data (Fig. S1), our main analyses employed nonparametric statistics, which use rank-orders of data (26).
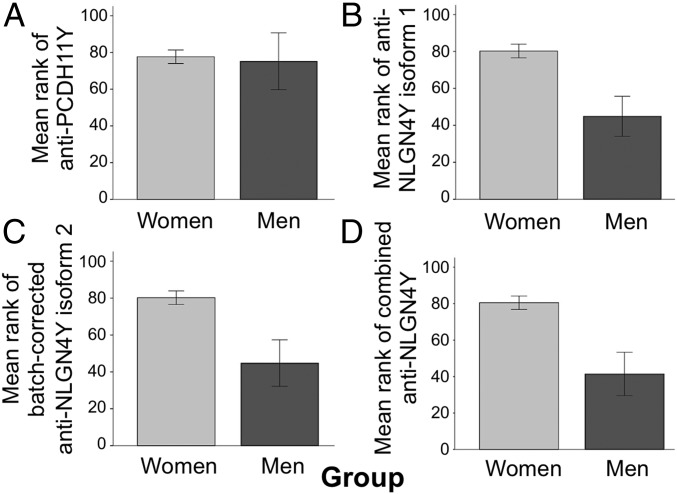
First, in a preliminary test, we expected women’s immune reactivity to exceed men’s on the assumption that, upon exposure, these male-specific substances should be perceived by the women’s immune system as foreign to them. Using two-tailed Mann–Whitney U tests (n = 154), this sex difference was supported for all three anti-NLGN4Y measures (isoform 1: P = 0.007; isoform 2: P = 0.007; combined NLGN4Y: P = 0.003), but not for PCDH11Y (P = 0.855) (Fig. 1 and Table S1), a result that may partly reflect low antibody responses across all participants for PCDH11Y relative to NLGN4Y (Fig. S1).
Fig. 1.
Mean rank of antibody concentrations (from lowest, 1, to highest, 154) for PCDH11Y and NLGN4Y by group (women vs. men; n = 154). Error bars represent SEM. (A) Mean rank of antibody concentrations for PCDH11Y by group (Mann–Whitney U = 824.50, P = 0.855, r = −0.02, two-tailed). (B) Mean rank of antibody concentrations for NLGN4Y isoform 1 by group (Mann–Whitney U = 461, P = 0.007, r = −0.21, two-tailed). (C) Mean rank of antibody concentrations for NLGN4Y isoform 2 by group and corrected for batch effects (Mann–Whitney U = 460, P = 0.007, r = −0.21, two-tailed). (D) Mean rank of antibody concentrations for combined NLGN4Y by group (Mann–Whitney U = 420, P = 0.003, r = −0.23, two-tailed).
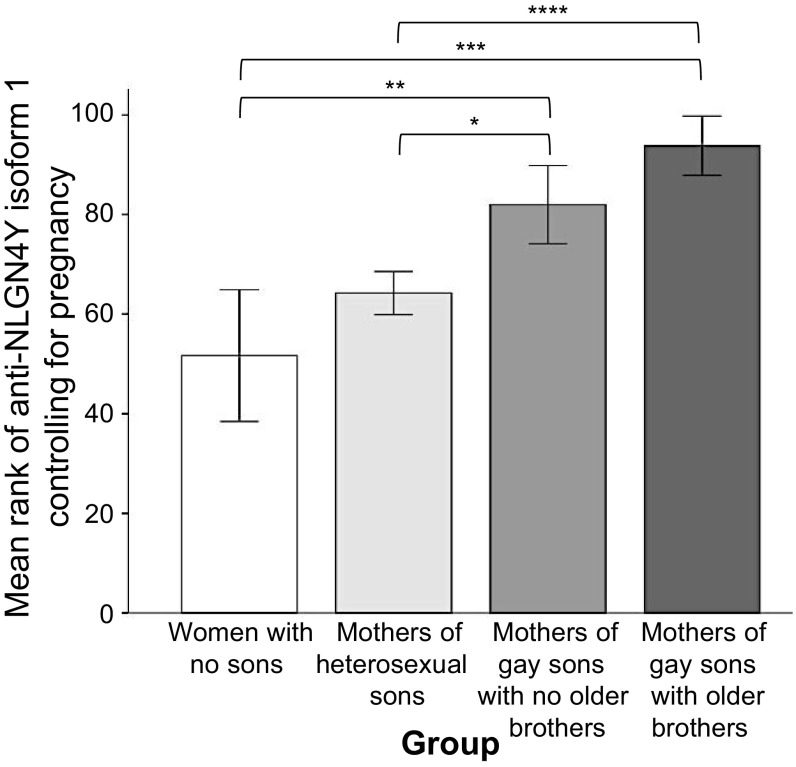
Given that the expected sex difference was confirmed for NLGN4Y, we then examined anti-NLGN4Y levels among women (n = 142) using an omnibus, two-tailed, Jonckheere–Terpstra test for ordered alternatives. In line with the maternal immune hypothesis, the predicted ordering for antibody concentrations to anti-NLGN4Y was as follows: women with no sons < mothers of heterosexual sons < mothers of gay sons with no older brothers < mothers of gay sons with older brothers. Mothers of gay sons with older brothers were predicted to have the highest concentration of antibodies, but we also predicted mothers of gay sons with no older brothers to have higher concentrations than mothers of heterosexual sons and women with no sons, on the assumption that the former could be immunized on a first male pregnancy or, for example, include a subset of mothers immunized by miscarried and possibly undetected male fetuses. In this analysis, we also controlled for total number of pregnancies (live births and miscarriages) because this reproductive variable differed among the groups and was related to anti-NLGN4Y levels (Materials and Methods and Tables S4 and S5). The expected ordering of groups was confirmed for anti-NLGN4Y isoform 1 (P = 0.000096) (Fig. 2), anti-NLGN4Y isoform 2 (P = 0.030), and combined anti-NLGN4Y (P = 0.0012). The largest of these effects, using statistical effect size estimates appropriate to the nonparametric statistics we employ here, would be designated as medium (e.g., rs approximately = 0.30) (Fig. 2).
Fig. 2.
Mean rank of antibody concentrations (from lowest, 1, to highest, 142) for NLGN4Y isoform 1 by group controlling for pregnancy (n = 142). Omnibus standardized test statistic from the Jonckheere–Terpstra test = 3.90, P = 0.000096, two-tailed, r = 0.33. Pairwise comparisons: ****P = 0.00035, r = 0.35; ***P = 0.008, r = 0.39; **P = 0.021, r = 0.30; *P = 0.024, r = 0.19. All Ps for pair-wise comparisons are one-tailed. Error bars represent SEM.
We conducted follow-up, pairwise comparisons on the NLGN4Y results. The P values of these comparisons were one-tailed, in keeping with the directional nature of the Jonckheere–Terpstra test. As expected, mothers of gay sons with older brothers, relative to mothers of heterosexual sons, had higher antibody levels on all three NLGN4Y variables (anti-isoform 1, P = 0.00035; anti-isoform 2, P = 0.011; combined anti-NLGN4Y, P = 0.001). Mothers of gay sons with older brothers, relative to women with no sons, generally had higher antibody levels on all three NLGN4Y variables (anti-isoform 1, P = 0.008; anti-isoform 2, P = 0.09; combined anti-NLGN4Y, P = 0.023). Mothers of gay sons with no older brothers, relative to heterosexual sons, had higher antibody levels on anti-NLGN4Y isoform 1 (P = 0.024) and combined anti-NLGN4Y (P = 0.045). Mothers of gay sons with no older brothers, relative to women with no sons, had higher antibody levels on anti-NLGN4Y isoform 1 (P = 0.021). Mothers of gay sons with older brothers had, relative to mothers of gay sons with no older brothers, higher levels of anti-NLGN4Y isoform 2 (P = 0.049) and combined anti-NLGN4Y (P = 0.047). See Table S2 for details related to the comparisons.
Discussion
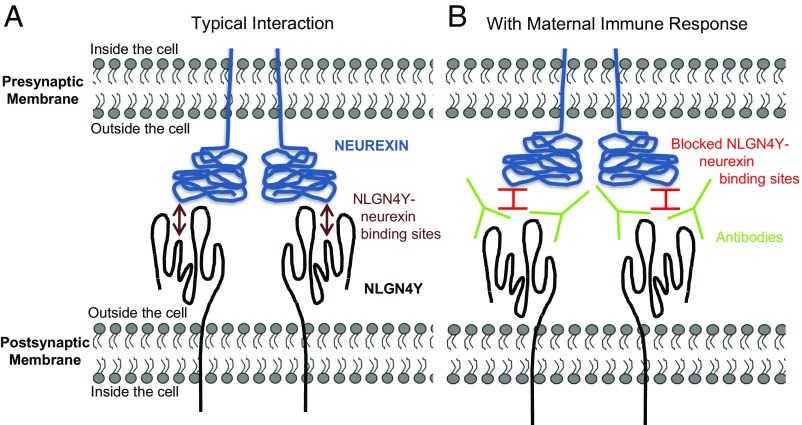
NLGN4Y is presumed to play a role in male fetal brain development (i.e., synaptic functioning) (23, 25), and is primarily extracellular in its expression, making it potentially accessible to functional modulation by maternal antibodies upon their entry into the fetal compartment and upon their crossing the developing blood/brain barrier of a male fetus. Such maternal immunological interactions are hypothesized to divert sexual differentiation of the male fetal brain, with antibodies binding to, and altering, male-specific cell-surface molecules, thereby altering their usual roles in the masculinization of sex dimorphic brain structures. The strongest effects occurred for NLGN4Y isoform 1, an unsurprising finding given that it is the larger of the two NLGN4Y isoforms and thus likely to have more epitopes. However, both common and unique epitopes on the two isoforms may be relevant to the present findings, because some analyses yielded slightly greater effects for the combined isoforms than for either isoform alone. It is not certain how NLGN4Y might operate at the cellular level on the neuropsychology of men’s sexual orientation, but the interaction of NLGN4Y with its binding partners, the neurexins, in the formation of synapses (27) (Fig. 3) may influence relative sexual/romantic attraction to others of a particular sex. The X-linked homolog, NLGN4X, might also play a role in the formation of sexual/romantic attractions, and antibodies raised by NLGN4Y might alter sexual brain development by cross-reacting with fetal NLGN4X, given its similarity to NLGN4Y. Notably, variations in both NLGN4X and NLGN4Y are implicated in sex-linked, neurological functioning associated with forming social connections to others: autism (25, 28).
Fig. 3.
Representation of interaction between the extracellular region of NLGN4Y and neurexin. (A) Typical interaction (27); (B) hypothesized alteration to the typical interaction, caused by antibody binding with NLGN4Y.
Antibodies, along with short-lived antibody-secreting plasma cells produced at initial antigen exposure, often disappear relatively rapidly, with a half-life of 2–3 wk (29). However, long-lived plasma cells replenish antibodies in serum and may exist in bone marrow for an extended period, replenishing the antibody supply, even in the absence of antigen reexposure. Furthermore, memory B cells exist for many years (presumably even beyond long-lived plasma cells), but in order for memory B cells to differentiate into antibody secreting (short- and long-lived) plasma cells, they need re-exposure to the antigen (29). Thus, our main findings, that elevated maternal antibodies to NLGN4Y are detected many years after a last pregnancy and birth of a son, may occur because one or both of the following circumstances exist: (i) long-lived plasma cells replenish the supply of antibodies to NLGN4Y even in the absence of antigen re-exposure and/or (ii) memory B cells are restimulated by the antigen (e.g., via male microchimerism) long after the original exposure occurred.
Elevated antibody levels observed in the mothers of gay sons in our study are probably only a relative index of the physiological levels that existed years earlier during the hypothesized events affecting male fetal development. Thus, between-groups differences detected now (e.g., mothers of gay sons with older brothers vs. mothers of heterosexual sons) are likely not the same magnitude as the between-groups differences that existed during the relevant pregnancies.
Some evidence of an elevated antibody response to NLGN4Y was observed in some women with no sons. Notably, women without known male pregnancies may nonetheless have immune responses to male-specific proteins (30), potentially reflecting (undetected) miscarriages of male fetuses, or even possible immunization from exposure to biological material (e.g., semen) during intercourse (31). Indeed, significant male microchimerism (>20%) has been detected in women without any known male pregnancy (30), making possible an immune response to male-specific proteins that remains detectable long after initial exposure. Caution is warranted in interpreting our finding, however, given our small sample of women with no sons.
Our results begin to explain one of the most reliable correlates of sexual orientation in men (i.e., FBO) and provide evidence of a specific biological mechanism underlying men’s sexual orientation: a maternal immune response to a Y-linked protein important in male fetal brain development. A maternal immune mechanism does not exclude other factors (e.g., prenatal hormones, genetics) advanced to explain sexual orientation (32, 33). Indeed, although most of our key effects were of a notable statistical magnitude (i.e., medium in effect size), it is also clear that only a portion of variation in men’s sexual orientation is accounted for by these effects. Sexual orientation is clearly a complex phenomenon with likely many factors influencing it.
Materials and Methods
Participants.
To recruit participants, advertisements were placed on a Canadian university campus, in Kijiji, in local LGBT (lesbian/gay/bisexual/transgender) magazines and radio stations, and in local newspapers. Participants were also recruited through local Pride festivals, along with local chapters of PFLAG (Parents and Friends of Lesbians and Gays). Six mothers were recruited through a child and adolescent gender identity service at the Centre for Addiction and Mental Health (CAMH) in Toronto. Brock University and CAMH research ethics boards approved this study.
Of the 159 participants who were recruited, 59 were mothers of heterosexual son(s) only (could have daughters, but sons were heterosexual only), 48 were mothers of at least one gay son (but could have heterosexual sons and/or daughters), 16 were women with no sons (11 with daughters only, 5 with no known pregnancies), 6 were mothers of at least one son diagnosed with gender dysphoria (but could have additional sons and/or daughters), 13 were mothers of sons who had an unconfirmed sexual orientation because the sons were too young to know their sexual orientation (could have daughters as well), 3 were mothers of at least one adult transsexual child, and 2 were mothers of at least one bisexual son. Finally, 12 of the participants were men.
The mothers of at least one gender-dysphoric son were reclassified as mothers of at least one gay son because most of these gender-dysphoric sons were likely to be gay men as adults (34, 35). The mothers of sons who had an unconfirmed sexual orientation were reclassified as mothers of heterosexual sons only, because the vast majority of these children were likely to be heterosexual as adults (36, 37). The three mothers of (adult) transsexuals and the two mothers of bisexual sons were not assigned to any other groups because their offspring’s sexual orientation could not be classified with any degree of certainty into gay or heterosexual categories. Thus, the final groups of participants in the analyses were: 54 mothers of at least one gay son (23 born after at least one older brother and 31 who did not have any older brothers), 72 mothers of at least one heterosexual son (and with no gay sons), 16 women with no sons, and 12 men. Although the three mothers of transsexuals and two mothers of bisexual sons were not assigned to any of the study groups, their data were used for some analyses (e.g., data preprocessing). We reran our main analyses filtering out the mothers of at least one son with gender dysphoria and the mothers of sons who had an unconfirmed sexual orientation (n = 19). Although power was decreased due to a loss of participants, the size and direction of between group differences were similar to that obtained with these participants included. Thus, these excluded participants are not driving the significant results in the full sample.
Participants ranged in age from 18 to 80 years. Participant age, in years, was calculated as the date of examination minus their reported date of birth. The sample sizes and mean ages of the five study groups are presented in Table S3. One male subject neglected to report his age, so the mean age for that group is based on 11 cases. A one-way ANOVA showed that age differences between the five groups were statistically significant: F(4, 148) = 17.09, P < 0.0001, n = 153. The main driver of this result was the large difference between the groups of mothers of sons and the groups of men and women with no sons, some of whom were young adults without children.
Measures.
Outcomes of each pregnancy were identified by mothers as a miscarriage/abortion, stillbirth, or live birth. Mothers reported the biological sex of each fetus (i.e., male, female, or unknown). Mothers reported on the sexual orientation (or gender identity) of each child (i.e., heterosexual, gay, lesbian, bisexual, transsexual, or unknown); however, mothers of gender-dysphoric boys did not report on the sexual orientation of their son. Instead the sexual orientation of gender-dysphoric boys was, as mentioned, classified as gay, based on research that indicates such boys are most likely to be gay men as adults (34, 35). For a list of all pregnancy-related questions completed by the mother, see Skorska et al. (38).
Blood Sample Collection and Processing.
Participants were met at their homes or on campus by a research assistant and a phlebotomist. First, participants completed a consent form and a questionnaire on demographics (e.g., age) and, if female, on their pregnancy history. A 10-mL sample of whole blood was drawn from the participants using sodium heparin coated vacutainers (BD Biosciences). Participants were then debriefed and compensated financially.
The whole-blood sample was processed in our laboratory after extraction, typically within 3–4 h. For the purpose of this study, we extracted the plasma using the Ficoll method. Whole blood was mixed with an equal volume of sterile PBS solution and overlaid drop-by-drop on 10 mL of Ficoll in a 50-mL tube. The tube and its contents were centrifuged without braking for 30 min at 600 × g at room temperature. After centrifugation, the upper plasma layer was carefully extracted into 2-mL aliquots and stored at −80 °C or in liquid nitrogen until ELISAs were conducted.
Recombinant Protein Generation.
Recombinant proteins were generated through custom orders: PCDH11Y (Genscript) and NLGN4Y (Creative Biomart). Briefly, constructs were generated for PCDH11Y (NM_032971.1), NLGN4Y transcript variant 1 (NM_014893.4; extracellular domain: 44–676 aa), and transcript variant 2 (NM_001164238.1; extracellular domain: 29–206 aa). HEK293 cells were used for transfection of the constructs and purification of recombinant proteins. The two NLGN4Y isoforms have overlapping and nonoverlapping amino acid sequences (Fig. S2) and likely have both common and unique epitopes.
ELISA.
Protein coating concentrations and plasma dilutions were optimized in our laboratory to obtain the best signal/background ratio. Standard ELISA procedures were carried out. Briefly, 96-well ELISA microplates were coated with the relevant recombinant proteins: 0.125 μg per well of PCDH11Y or 0.125 μg per well of NLGN4Y isoform 1 or 2. Plasma from each participant was titrated and detected with a secondary anti-human IgG antibody labeled with horseradish peroxidase (Sigma). Finally, a substrate chromogen (TMB Substrate; item #7004, Cell Signaling Technology) was added to reveal the immune reaction and optical density was measured using a Biotek ELx800 plate reader. A standard curve was generated for each ELISA plate to normalize the ELISA signal: anti-PCDH11Y antibody (Sigma) and anti-NLGN4Y antibody (Sigma) were titrated for the generation of the standard curve (Fig. S3). Of note, participants from each group (see, for example, Table S3) were represented on each ELISA plate to minimize batch effects, and the individual who performed the ELISA procedure was blind to the group assigned to each participant on each plate.
Data Preprocessing.
Assays were run in six batches. Using one-way ANOVA on observed concentrations, no batch effect was detected for anti-PCDH11Y, F(5, 153) = 0.84, P = 0.523, n = 159, or for anti-NLGN4Y isoform 1, F(5, 153) = 1.76, P = 0.124, n = 159; however, a batch effect was found for anti-NLGN4Y isoform 2, F(5, 153) = 4.66, P = 0.001, n = 159. Because relative, rather than absolute antibody concentrations were of primary interest, we removed the effect of batch from our measure of anti-NLGN4Y isoform 2 by saving the standardized residuals (n = 159). We used these z-scored anti-NLGN4Y isoform 2 concentrations (corrected for batch) in all analyses in the main text and Supporting Information.
As mentioned in the text, in addition to using anti-NLGN4Y isoform 1 and batch-corrected anti-NLGN4Y isoform 2 separately, we also used a z-scored combination (combined anti-NLGN4Y). The Spearman correlation between anti-NLGN4Y isoform 1 and batch-corrected anti-NLGN4Y isoform 2 (ρ = 0.41, P < 0.0001, n = 159) further supported a combination of their assay results into a composite measure. To produce the combined anti-NLGN4Y measure, we standardized the scores for anti-NLGN4Y isoform 1 and then added the (uncorrected) z-scores for isoform 1 to the (corrected) z-scores for isoform 2 (n = 159).
To check whether participants’ age at examination would need to be controlled for, we conducted Spearman’s correlations of age with anti-PCDH11Y, anti-NLGN4Y isoform 1, batch-corrected anti-NLGN4Y isoform 2, and combined anti-NLGN4Y. The correlations were small, ranging from 0.024 to 0.129, and none was statistically significant (n = 153). We also examined years from last pregnancy in all mothers. Across all mothers (n = 137), the correlations with antibody level were similar in magnitude to those of age at examination, ranging between 0.010 and 0.109, and none was significant. Age at examination and years from last pregnancy were therefore not examined in remaining analyses.
To check whether total number of pregnancies (live births plus miscarried fetuses) would need to be controlled for, we conducted Spearman’s correlations of pregnancy with anti-PCDH11Y, anti-NLGN4Y isoform 1, batch-corrected anti-NLGN4Y isoform 2, and combined anti-NLGN4Y within all women (n = 142) and within mothers only (n = 137). As shown in Table S4, the correlations were negative in direction and were generally significant for the three anti-NLGN4Y measures, ranging from −0.153 to −0.229. These results suggested that pregnancy per se might have a tolerogenic effect (39) with regard to NLGN4Y. A one-way ANOVA also showed that differences in number of pregnancies between the four groups were statistically significant, F(3, 138) = 7.10, P = 0.0002, n = 142 (Table S5). Thus, in the key analysis of group comparisons among women for anti-NLGN4Y (see above), we controlled for total number of pregnancies. To do this, regression analyses were conducted with each of the antibody variables as criterion variables and number of pregnancies as the predictor. The standardized residual was saved and the standardized residual was used in the analysis in lieu of the antibody variable.
Supplementary Material
Acknowledgments
We thank K. Arnell, J. Bramley, K. Fallis, I. Gabrie, C. Hafer, L. Jamieson, K. Kilyk, K. Labanowicz, K. Lee, D. Mahoney, D. Molnar, S. Mazzuocco, S. Norgaard, K. Ross, K. Walczyk, and N. Wickramasuriya. This research was supported by Natural Sciences and Engineering Research Council of Canada Grants 334-737-007-2010 (to A.F.B. and R.B.) and 334-737-007-2016 (to A.F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.M.B. is a guest editor invited by the Editorial Board.
See Commentary on page 234.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705895114/-/DCSupplemental.
References
- 1.Blanchard R, Bogaert AF. Homosexuality in men and number of older brothers. Am J Psychiatry. 1996;153:27–31. doi: 10.1176/ajp.153.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard R. Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. J Theor Biol. 2004;230:173–187. doi: 10.1016/j.jtbi.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert AF, Skorska M. Sexual orientation, fraternal birth order, and the maternal immune hypothesis: A review. Front Neuroendocrinol. 2011;32:247–254. doi: 10.1016/j.yfrne.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Cantor JM, Blanchard R, Paterson AD, Bogaert AF. How many gay men owe their sexual orientation to fraternal birth order? Arch Sex Behav. 2002;31:63–71. doi: 10.1023/a:1014031201935. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard R, Bogaert AF. Proportion of homosexual men who owe their sexual orientation to fraternal birth order: An estimate based on two national probability samples. Am J Hum Biol. 2004;16:151–157. doi: 10.1002/ajhb.20006. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert AF. The prevalence of male homosexuality: The effect of fraternal birth order and variations in family size. J Theor Biol. 2004;230:33–37. doi: 10.1016/j.jtbi.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard R. Fraternal birth order, family size, and male homosexuality: Meta-analysis of studies spanning 25 years. Arch Sex Behav. June 12, 2017 doi: 10.1007/s10508-017-1007-4. [DOI] [PubMed] [Google Scholar]
- 8.VanderLaan DP, Vasey PL. Male sexual orientation in independent Samoa: Evidence for fraternal birth order and maternal fecundity effects. Arch Sex Behav. 2011;40:495–503. doi: 10.1007/s10508-009-9576-5. [DOI] [PubMed] [Google Scholar]
- 9.Green R. Birth order and ratio of brothers to sisters in transsexuals. Psychol Med. 2000;30:789–795. doi: 10.1017/s0033291799001932. [DOI] [PubMed] [Google Scholar]
- 10.Rahman Q. The association between the fraternal birth order effect in male homosexuality and other markers of human sexual orientation. Biol Lett. 2005;1:393–395. doi: 10.1098/rsbl.2005.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogaert AF. Biological versus nonbiological older brothers and men’s sexual orientation. Proc Natl Acad Sci USA. 2006;103:10771–10774. doi: 10.1073/pnas.0511152103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rijnink EC, et al. Tissue microchimerism is increased during pregnancy: A human autopsy study. Mol Hum Reprod. 2015;21:857–864. doi: 10.1093/molehr/gav047. [DOI] [PubMed] [Google Scholar]
- 14.Chan WFN, et al. Male microchimerism in the human female brain. PLoS One. 2012;7:e45592. doi: 10.1371/journal.pone.0045592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams MM, Marks JS, Gustafson J, Oakley GP., Jr Rh hemolytic disease of the newborn: Using incidence observations to evaluate the use of RH immune globulin. Am J Public Health. 1981;71:1031–1035. doi: 10.2105/ajph.71.9.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brimberg L, et al. Antibodies as mediators of brain pathology. Trends Immunol. 2015;36:709–724. doi: 10.1016/j.it.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowal C, Athanassiou A, Chen H, Diamond B. Maternal antibodies and developing blood-brain barrier. Immunol Res. 2015;63:18–25. doi: 10.1007/s12026-015-8714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson L, Polizzi A, Morriss-Kay G, Vincent A. Plasma from human mothers of fetuses with severe arthrogryposis multiplex congenita causes deformities in mice. J Clin Invest. 1999;103:1031–1038. doi: 10.1172/JCI5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, et al. Maternal lupus and congenital cortical impairment. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piper KP, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen HS. Secondary recurrent miscarriage and H-Y immunity. Hum Reprod Update. 2011;17:558–574. doi: 10.1093/humupd/dmr005. [DOI] [PubMed] [Google Scholar]
- 23.Skaletsky H, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 24.Blanco P, Sargent CA, Boucher CA, Mitchell M, Affara NA. Conservation of PCDHX in mammals; expression of human X/Y genes predominantly in brain. Mamm Genome. 2000;11:906–914. doi: 10.1007/s003350010177. [DOI] [PubMed] [Google Scholar]
- 25.Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Field A. Discovering Statistics Using IBM SPSS Statistics. 4th Ed Sage; London: 2014. [Google Scholar]
- 27.Dean C, Dresbach T. Neuroligins and neurexins: Linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ross JL, Tartaglia N, Merry DE, Dalva M, Zinn AR. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav. 2015;14:137–144. doi: 10.1111/gbb.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Yan Z, et al. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118:899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Dierselhuis MP, et al. HY immune tolerance is common in women without male offspring. PLoS One. 2014;9:e91274. doi: 10.1371/journal.pone.0091274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balthazart J. Minireview: Hormones and human sexual orientation. Endocrinology. 2011;152:2937–2947. doi: 10.1210/en.2011-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson G, Rahman Q. Born Gay: The Biology of Sex Orientation. Peter Owen; London: 2005. [Google Scholar]
- 34.Steensma TD, McGuire JK, Kreukels BPC, Beekman AJ, Cohen-Kettenis PT. Factors associated with desistence and persistence of childhood gender dysphoria: A quantitative follow-up study. J Am Acad Child Adolesc Psychiatry. 2013;52:582–590. doi: 10.1016/j.jaac.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Green R. The “Sissy Boy Syndrome” and the Development of Homosexuality. Yale Univ Press; New Haven, CT: 1987. [Google Scholar]
- 36.LeVay S. Gay, Straight, and the Reason Why: The Science of Sexual Orientation. Oxford Univ Press; New York: 2010. [Google Scholar]
- 37.Laumann EO, Gagnon JH, Michael RT, Michaels S. The Social Organization of Sexuality: Sexual Practices in the United States. Univ Chicago Press; Chicago: 1994. [Google Scholar]
- 38.Skorska MN, Blanchard R, VanderLaan DP, Zucker KJ, Bogaert AF. Gay male only-children: Evidence for low birth weight and high maternal miscarriage rates. Arch Sex Behav. 2017;46:205–215. doi: 10.1007/s10508-016-0829-9. [DOI] [PubMed] [Google Scholar]
- 39.Bernsen RM, Nagelkerke NJ, al-Ramadi BK. Does paternal antigen-induced secretion of interleukin-10 by T regulatory cells mediate the birth order effect? Med Hypotheses. 2006;67:740–743. doi: 10.1016/j.mehy.2006.04.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.