Abstract
Sodium deficit poses a life-threatening challenge to body fluid homeostasis and generates a sodium appetite - the behavioral drive to ingest sodium. Dr. Randall R. Sakai greatly contributed to our understanding of the hormonal responses to negative sodium balance and to the central processing of these signals. Reactivity to the taste of sodium solutions and the motivation to seek and consume sodium changes dramatically with body fluid balance. Here, we review studies that collectively suggest that sodium deficit recruits the mesolimbic system to play a role in the behavioral expression of sodium appetite. The recruitment of the mesolimbic system likely contributes to intense sodium seeking and reinforces sodium consumption observed in deficient animals. Some of the hormones that are released in response to sodium deficit act directly on both dopamine and nucleus accumbens elements. Moreover, the taste of sodium in sodium deficient rats evokes a pattern of dopamine and nucleus accumbens activity that is similar to responses to rewarding stimuli. A very different pattern of activity is observed in non-deficient rats. Given the well-characterized endocrine response to sodium deficit and its central action, sodium appetite becomes an ideal model for understanding the role of mesolimbic signaling in reward, reinforcement and the generation of motivated behavior.
Keywords: Dopamine, Motivation, Reward, Nucleus accumbens, Sodium appetite, Homeostasis
Randall Sakai was my first academic colleague. I say this because he had no cause for a stake in my development as a scientist. He was not my boss nor was he an official academic mentor of mine. I met Randall just after starting a position as a laboratory technician in the lab of Dr. Harvey Grill at the University of Pennsylvania. I was 22 years old and knew nothing of the world of academia. Randall, with no self-interest what-so-ever, gleefully took it upon himself to help me feel comfortable in my new surroundings. He also educated me – teaching me what grants were all about and how students progress through academia. He took me fishing and taught me about sushi, the work of Alexander Calder and countless other aspects of life in which the typical 22-year-old fresh out of college is deficient. As I grew as a scientist, Randall challenged me and helped me to hone my arguments. He took delight in introducing me to leaders in the field of ingestive behavior. He danced at my wedding, cared about my children and was a champion of my career. I was not alone in these experiences and I know that many are grateful for Randall’s influence. I will always remember and be most grateful for Randall’s sharp wit, keen eye for data, its implications, explanatory power and limits, but just as much for his enjoyment of life, revelry in the success of others and genuine friendship.
[~MFR]
1. Sodium appetite as motivated behavior
The survival of living beings is dependent on the monitoring of internal body state and compensatory homeostatic regulation. In the age of the obesity epidemic, much attention is rightly focused on the neural and endocrine systems that drive feeding upon caloric deprivation and terminate feeding upon satiety. Understanding how these processes become altered to permit obesity is necessary to reverse the pervasiveness of obesity and its comorbidities. Chronic dysregulation of body fluid homeostasis also has associated disease states. Congestive heart failure greatly contributes to heart disease, the leading cause of death in the United States [1,2]. Furthermore, challenges to body fluid homeostasis pose dire threats to survival including dehydration and hyponatremia – certainly more immediately than caloric deficit [3]. Thus, in both diseased and healthy individuals, body fluid homeostasis remains paramount for survival.
Body fluid homeostasis requires tight regulation of both body fluid volume and composition – primarily through the consumption of water and sodium but also through excretory mechanisms. Dr. Randall R. Sakai dedicated a substantial portion of his career to understanding the hormonal signals that arise from negative sodium balance and how those signals interact with the central nervous system to drive sodium intake – a phenomenon known as sodium appetite. Randall, together with his student Eric Krause, drafted a beautiful review of sodium appetite as an homage to the revolutionary work of Curt Richter, who first described sodium appetite in 1936 [4,5]. Randall and his co-authors over the years have focused on the hormonal responses to sodium depletion and the integration of these signals by central circuits to generate compensatory physiological processes and behaviors. Randall greatly contributed to our understanding of how mineralocorticoids and glucocorticoids interact in the service of sodium appetite [6–8]. This brief review is not meant to extensively cover the sensing of sodium deficit. For this, the reader is strongly encouraged to seek out Randall’s work as well as reviews [4,9–11] and books [12,13] written by others. Instead, we aim to honor the work of Randall Sakai by briefly reviewing studies that investigate the drastic changes in motivation that are characteristic of sodium appetite and the neurobiological underpinnings of the drive to consume sodium in a sodium deficient state.
Sodium deprivation and depletion produce a powerful, specific and innate appetite for sodium which promotes appetitive and consummatory behaviors directed towards sodium-containing substances (e.g. sodium chloride, NaCl). In a laboratory setting, sodium deplete animals preferentially ingest solutions containing the sodium ion and no prior experience with sodium-containing solutions is required for this to occur [14,15]. The sodium ion of a NaCl solution is detected by taste mechanisms [16–19] which allow for the immediate discrimination of salt solutions and selective consumption of only those which satisfy the need state of the animal. Interference with sodium taste transduction mechanisms (through blockade of lingual sodium channels or transection of cranial nerves which relay gustatory information to the brain) essentially eliminates the expression of a sodium-selective appetite [16,20]. The taste of sodium, therefore, drives the intake behavior that is necessary to replenish internal sodium deficit.
The value of the taste of sodium is highly dynamic. Hypertonic, concentrated sodium solutions are avoided by sodium replete animals [15]. However, in a sodium deplete state, the taste of concentrated NaCl becomes transformed to one that is appetitive, palatable [21] and reinforcing [15,22]. In this way, the value of sodium solutions changes dramatically with physiological state, making sodium appetite an ideal model for exploring the central systems that influence value assignment, goal-directed behavior and reinforcement.
The reinforcing nature of sodium for sodium deplete rats was noted in several early studies and has been replicated many times over. Consumption of NaCl [14] and average lick rates for a hyperton-ic NaCl solution [15] greatly increase with sodium depletion. This increase in intake behavior is not a consequence of a reflexive response but is instead indicative of voluntary motivated behavior. Indeed, sodium deplete rats will make operant responses for access to sodium [22–24], will increase running speed down a runway to obtain a sodium reward [25] and will display approach behavior towards cues associated with sodium availability [26,27]. Insight into the neural machinery required for these motivational processes was gained through work with chronic supracollicular decerebrate (CD) rats that had a severed connection between the forebrain and caudal brainstem [28]. Similarly to intact rats, CD rats concentrate their urine after hypertonic sodium injection; demonstrating some intact responses to challenges of body fluid homeostasis. However, CDs, unlike intact rats, fail to increase both intake of hypertonic sodium and appetitive responses to the taste of hypertonic sodium following depletion [29]. These studies have demonstrated that the organization and reinforcement of behaviors necessary to acquire sodium under deplete conditions require involvement of higher order fore-brain signaling processes.
The neurotransmitter dopamine and its release in forebrain target regions (e.g. nucleus accumbens [NAc]; dorsal striatum) were first proposed to be involved in reinforcement processes after the seminal studies of Roy Wise and colleagues [30,31]. However, the precise role of the neurotransmitter in motivated behavior is the subject of continued debate. Dopamine has been hypothesized to serve as a ‘teaching signal’ that can drive the formation of associations between environmental stimuli and positive outcomes [32, 33]. This seems to fit well with suggested cellular roles for dopamine in long-term potentiation and depression in regions like the NAc and striatum [34,35]. Mesolimbic dopamine is also important for enhancing the salience of, and directing behavior towards, reward-associated cues (incentive salience; [36]). Dopamine is also critical for more effortful responses required to obtain rewards [37]. While there are some aspects of these psychological constructs that are at odds with one another, the prevailing opinion is that dopamine plays a key role in assigning value to relevant stimuli and appropriately directing behavior towards those stimuli [38].
2. The generation of sodium appetite primes the mesolimbic system
Challenges to homeostasis prime dopamine circuits for responses to stimuli that satisfy the need state of the animal. Like the behavioral responses to homeostatic need, the dopamine system itself has been shown to be gated by physiological state. Metabolic state (e.g. hunger and satiety) and the hormonal proxies of metabolic state (e.g. ghrelin, insulin, leptin, GLP-1, amylin) bidirectionally regulate mesolimbic dopamine signaling. For example, food restriction increases the firing rate and burst firing of midbrain dopamine neurons [39]. In addition, in response to food, burst firing of midbrain dopamine neurons and striatal dopamine release are increased in food restricted animals [40–42]. Hormones that serve as proxies for hunger also increase dopamine neural activity [43], dopamine release [44] and the dopamine response to food [42] and food cues [45]. Conversely, satiety or hormonal manipulations inducing satiety decrease VTA neuron firing rate [46,47] and dopamine levels in the NAc [48–50].
Sodium appetite is also sufficient to induce changes in the mesolimbic system that could contribute to enhanced dopamine signaling when an animal encounters sodium or environmental cues that predict access to sodium. For example, sodium depletion alone decreases the rate of dopamine reuptake by the dopamine transporter (DAT; [51,52]). The hormone aldosterone, a mineralocorticoid, is critical for the expression of a sodium appetite [6]. Administration of aldosterone to NAc tissue, taken from rats in positive sodium balance (e.g. sodium replete), is sufficient to recapitulate the ability of sodium depletion to decrease the rate of dopamine reuptake [51]. A decrease in DAT binding was also observed in sodium replete rats treated with the mineralocorticoid deoxycorticosterone acetate (DOCA) [53]. Reduction in DAT number or function would result in increased extracellular dopamine exposure time and diffusion upon release [54,55]. In turn, extracellular dopamine would be increased over longer periods of time; an effect that has been associated with generating motivation and the vigor of goal-directed behavior [56]. Systemic delivery of mineralocorticoids alone is sufficient to increase phosphorylated tyrosine hydroxylase, suggesting that the hormonal response to sodium deprivation increases dopamine production and possibly signaling [57]. It is important to note that sodium depletion causes additional changes within the mesolimbic system such as the regulation of NAc neuropeptide production [58]. Together, these data indicate that sodium depletion and the hormones released in response to sodium deficit act either indirectly or directly on the mesolimbic system to modulate signaling with the potential for heightened dopamine signaling being a key feature.
3. The taste of sodium differentially drives mesolimbic activity based on physiological state
The sham drinking paradigm was developed to study ingestion in the absence of post-ingestive feedback signaling. Here, appetitive and consummatory behaviors directed at the taste of a stimulus can be isolated and carefully studied. Shamfed rats reduce consumption of palatable liquid sucrose following dopamine receptor antagonism [59]. We found that similar manipulations of the mesolimbic system reduce sham drinking of sodium solutions in sodium deplete rats [60]. This study, together with one showing a reduction in sodium appetite in sham drinking rats by interference with taste transduction mechanisms (e.g. peripheral taste nerve transection; [61]), suggests that the taste of the sodium ion differentially drives dopamine signaling based on physiological state. Brief access to hypertonic sodium following sodium depletion is associated with an increase in the ratio of dopamine metabolites relative to dopamine [62] - indirect evidence of dopamine signaling and support of this hypothesis.
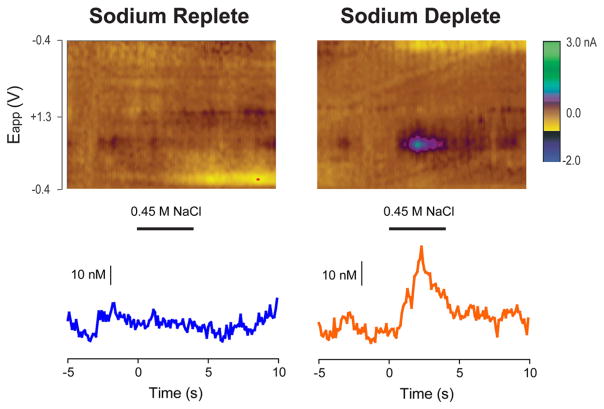
Recent work in our lab lends direct support for this hypothesis. We used fast-scan cyclic voltammetry to sample dopamine concentration changes in the NAc in ‘real-time’ (10 samples/s; [63]). Periodic intra-oral infusions of hypertonic NaCl were made in both sodium replete and deplete rats. Thus, dopamine measurements were commensurate with the sensory experience of ingestion. On individual trials, intra-oral NaCl evoked a high concentration increase in NAc dopamine, within 100 s of milliseconds of infusion onset, but only in sodium deplete rats [27; see also Fig. 1]. Consistent with the idea that state-dependent dopamine signaling is driven by the taste of sodium, we found that lingual blockade of amiloride-sensitive sodium channels eliminated the NaCl-evoked NAc phasic dopamine response in deplete rats [27].
Fig. 1.
Intra-oral infusion of 0.45 M NaCl evokes increases in NAc core dopamine release in only sodium deplete animals. (Top) Single-trial colorplots depict changes in current (color) as a function of applied electrical potential [Eapp (V); y-axis] and time (s; x-axis). Dopamine [identified by its oxidation peak (~0.6 V, green feature)] was evoked during the 4-s intra-oral infusion period (beginning at 0-s and indicated by the horizontal bar below) in sodium deplete (right) but not sodium replete (left) rats. (Bottom) Dopamine concentration changes in response to 0.45 M NaCl in sodium replete (blue) and sodium deplete (orange) rats. Concentration changes were extracted from the colorplots above. Current from the oxidation of dopamine was converted to concentration using post-recording calibration of the electrodes used during data collection.
Similar to dopamine release patterns, the responses of NAc output neurons (medium spiny neurons [MSNs]) to the taste of NaCl are modulated by sodium appetite. Electrophysiological recordings of MSN activity during intra-oral infusions show that appetitive stimuli (e.g. sucrose, saccharin) evoke decreases in the firing rate of the majority of taste-responsive neurons [64,65]. Aversive stimuli (e.g. quinine, sucrose after devaluation with lithium chloride in a conditioned taste aversion paradigm), on the other hand, evoke the opposite pattern – increases in the firing rate of the majority of taste-responsive neurons [64,66]. Interestingly, hypertonic NaCl evokes an aversive-like pattern of NAc activity in replete rats. However, after sodium depletion, the same solution evokes an appetitive-like pattern [67]. It remains unclear the degree to which differential dopamine signaling to NaCl in replete versus deplete conditions accounts for the different patterns of NAc neural activity. Since the NAc receives input from other sodium-appetite associated regions of the brain (e.g. the amygdala, [8,68–70]), it is possible that multiple mechanisms are responsible for differential NAc activity evoked by the taste of sodium in replete versus deplete rats. Still, state-dependent phasic dopamine release in the NAc likely contributes to the strong motivational drive to seek out sodium and reinforces consumption upon encountering a sodium source as detected by the gustatory system.
4. Potential mechanisms
Sodium balance gates the ability of the taste of sodium to drive dopamine release and appetitive encoding by NAc neurons. Multiple mechanisms are likely permissive and essential for the differential mesolimbic responses seen in replete and deplete rats. First, gustatory signals evoked by the sodium ion in the intra-oral cavity are altered by sodium depletion. NaCl responses of the chorda tympani nerve, a branch of the seventh cranial nerve that carries gustatory information from taste buds to the brainstem [71,72], and second order taste neurons in the nucleus of the solitary tract (NTS) [73] are modulated by sodium depletion [74]. Thus, drive from the gustatory system is different when sodium is tasted in a deplete versus replete state. Second, as detailed above, sodium depletion and mineralocorticoids that are increased in response to depletion can act directly on the mesolimbic system to alter responsivity [51, 53,75]. Third, it is possible that sodium depletion increases NAc dopamine neurotransmission by increasing the excitability of dopamine neuron cell bodies in the ventral tegmental area (VTA) through afferent pathways. While empirical evidence has yet to emerge in support of this third possibility, the VTA does receive direct and indirect input from numerous sodium appetite regulatory centers of the brain [76].
The need for sodium is sensed by a population of neurons in the NTS. These neurons are activated by sodium depletion [77] and are sensitive to aldosterone and other indicators of sodium deficiency [78]. These hindbrain “HSD2” neurons, named for their expression of the glucocorticoid-inactivating enzyme 11-β-hydroxysteroid dehydrogenase type 2, are well positioned to integrate hormonal and neural signals communicating body fluid homeostasis. HSD2 neurons are located around a blood-brain barrier-deficient zone of the NTS allowing for access to hormonal signals relaying sodium need (i.e. aldosterone) [79]. These same neurons also detect the ingestion of sodium through direct sensory input from the gut via the vagus nerve [80] or indirectly through projections from the area postrema (AP), a circumventricular organ that monitors changes in plasma hormones [81]. While direct projections from HSD2 neurons to the VTA have not yet been shown, it is plausible that HSD2 neurons monitor body fluid homeostasis and tune mesolimbic dopamine signaling through several multisynaptic connections to the VTA.
Both NTS HSD2 [77] and AP neurons [82] project to two visceral relay centers: the pre-locus coeruleus nucleus (pre-LC) and the inner division of the external lateral parabrachial subnucleus (PBel-inner). All pre-LC and PBel-inner neurons that become c-fos activated with sodium depletion constitutively express a transcription factor called Forkhead box protein 2 (FoxP2) [83]. These FoxP2 cells project directly to the VTA as well as various other midbrain and forebrain sites which potentially influence mesolimbic circuitry [76]. It is likely that the VTA uses these inputs to evaluate a sodium taste stimulus based on physiological state and responds with the generation of an appropriate dopamine response (Fig. 2). Interestingly, lesions of the parabrachial nucleus eliminate sodium appetite [84,85], pointing to this structure as a logical site to explore for passing critical taste/state information to the VTA. Future studies will be needed to determine the essential gating mechanisms by which physiological state alters the mesolimbic response to the taste of hypertonic sodium solutions.
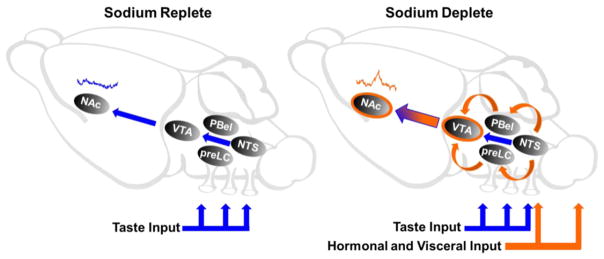
Fig. 2.
Theoretical circuit-level diagram depicting VTA inputs that drive dopamine responses in the NAc under sodium replete and deplete conditions. (Left) Under sodium replete conditions, the taste of NaCl fails to evoke an increase in NAc dopamine (blue concentration trace). The NTS represents a hypothetical path for gustatory information to influence NAc dopamine signaling. Although direct projections from the NTS to the VTA have been reported, it remains unclear if these inputs convey gustatory information. Blue arrows denote a potential route for gustatory information from the oral cavity to influence the VTA. (Right) Following sodium depletion, taste input (blue arrows) converges on the VTA with hormonal and visceral input (orange arrows) to drive increases in NAc dopamine to the taste of NaCl (orange concentration trace). Hormonal and visceral input is transmitted to the NTS through both circulation and vagal input. The NTS carries signals communicating sodium need to the pre-LC and PBel, both of which send direct projections to the VTA (orange arrows). In addition, circulating hormones may act directly on the VTA or NAc (orange circles) to gate dopamine release in response to NaCl.
5. What is the role of the mesolimbic system in sodium appetite?
While dopamine antagonists suppress the sham drinking of NaCl in sodium deplete rats, neither systemic [60] nor intra-NAc [58] dopamine antagonism has an effect on the expression of sodium appetite in rats with intact post-ingestive feedback. These results are supported by a handful of studies which demonstrate that dopamine is not essential for sodium appetite [36,86,87]. Instead, dopamine may serve to facilitate ingestive behavior through its neuromodulatory actions on MSNs. For example, dopamine binding to the D2 receptor is thought to inhibit the activity of D2-expressing MSNs [35]. In turn, NAc inhibitory responses to taste are correlated with licking and appetitive behavior ([64,67,88] but also see [70]).
While dopamine release may facilitate NAc encoding that promotes consumption, it may also play a role in plastic changes to NAc circuits. NAc dopamine has long been thought to play a role in reinforcement, with phasic dopamine responses serving as a teaching signal [32,33]. The phasic dopamine responses evoked by hypertonic NaCl under sodium deplete conditions [27, Fig. 1] are similar to those observed in response to a sucrose reward in hungry rats [89]. Similarly to phasic dopamine responses evoked by other rewards, phasic dopamine comes to be evoked by cues predictive of hypertonic NaCl but only when rats are trained and tested in a sodium deplete state [27]. This learned response can serve to incentivize approach and consumption under the right physiological and environmental conditions [26].
Randal Sakai found that sodium intake is enhanced with multiple sodium depletions [90]. This sensitization of sodium appetite parallels the increased behavioral response to psychostimulants after repeated injections. We found that multiple sodium depletion episodes induce morphological changes [75] that are similar to those observed following sensitization to psychomotor stimulants (e.g. cocaine, amphetamine; [91,92]). Sodium depletion also cross-sensitizes responses to psychostimulants [75,93] – suggesting overlapping physiological underpinnings of sodium- and drug-directed behaviors under particular conditions. Interestingly, it has been suggested that after multiple depletion episodes, the consumption of sodium under deplete conditions no longer causes the release of dopamine, as dopamine metabolite/dopamine ratios do not increase to sodium after multiple depletions [62]. These results suggest that after the initial sodium deprivation, dopamine signaling may play less of a role in the behavioral and neuroendocrine responses to deprivation. It is therefore possible that the initial dopamine-dependent reinforcement of the taste of NaCl under deplete conditions is sufficient to induce plasticity within the NAc that underlies subsequent seeking and consumption upon reencountering NaCl in a deficient state.
Randall’s work is among seminal studies which identify the hormonal response to sodium deficiency and its actions on central circuits which drive compensatory behavior. Collectively, this work presents a relatively well-described series of biological events to explain how motivated behavior arises from homeostatic challenge. The involvement of the mesolimbic system in body fluid homeostasis is certain, although the nature of its involvement remains unclear. The same is true in the case of energy balance, where the mesolimbic system is modulated by the same peptides and hormones which regulate food intake through actions on hypothalamic and hindbrain sites [94–99]. However, compared to food deprivation, the physiological responses to sodium depletion are more thoroughly characterized. In using sodium appetite to understand the role of the mesolimbic system, general insight into the central responses to homeostatic challenge can be determined. In so doing, future work can speak to a therapeutic intervention at the level of the mesolimbic system for over-consumption of salt, sugar and calories that might protect against cardiovascular disease and obesity.
HIGHLIGHTS.
Sodium appetite primes neural circuits critical for motivated behavior
Taste and state are integrated to differentially drive mesolimbic activity.
Dopamine signaling is likely tuned by multisynaptic hindbrain to VTA circuits.
Footnotes
This work was supported by grant number DA025634 (MFR), University of Illinois at Chicago Dean’s Scholar Fellowship (SMF).
References
- 1.Skorecki KL, Brenner BM. Body fluid homeostasis in congestive heart failure and cirrhosis with ascites. Am J Med. 1982;72:323–338. doi: 10.1016/0002-9343(82)90824-5. http://dx.doi.org/10.1016/0002-9343(82)90824-5. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics—2015 update. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. http://dx.doi.org/10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Harring TR, Deal NS, Kuo DC. Disorders of sodium and water balance. Emerg Med Clin North Am. 2014;32:379–401. doi: 10.1016/j.emc.2014.01.001. http://dx.doi.org/10.1016/j.emc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Krause EG, Sakai RR. Richter and sodium appetite: from adrenalectomy to molecular biology. Appetite. 2007;49:353–367. doi: 10.1016/j.appet.2007.01.015. http://dx.doi.org/10.1016/j.appet.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter C. Increased salt appetite in adrenalectomized rats. Am J Phys. 1936;115:155–161. [Google Scholar]
- 6.Sakai RR, Nicolaïdis S, Epstein AN. Salt appetite is suppressed by interference with angiotensin II and aldosterone. Am J Phys. 1986;251:R762–R768. doi: 10.1152/ajpregu.1986.251.4.R762. http://www.ncbi.nlm.nih.gov/pubmed/3532826. [DOI] [PubMed] [Google Scholar]
- 7.Sakai RR, Epstein AN. Dependence of adrenalectomy-induced sodium appetite on the action of angiotensin II in the brain of the rat. Behav Neurosci. 1990;104:167–176. doi: 10.1037/0735-7044.104.1.167. http://www.ncbi.nlm.nih.gov/pubmed/2317275. [DOI] [PubMed] [Google Scholar]
- 8.Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57:1337–1345. doi: 10.1046/j.1523-1755.2000.00972.x. http://dx.doi.org/10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 9.Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:177–209. doi: 10.1113/expphysiol.2007.039891. http://dx.doi.org/10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- 10.Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Ren Physiol. 2009;297:F559–F576. doi: 10.1152/ajprenal.90399.2008. http://dx.doi.org/10.1152/ajprenal.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley SW, Johnson AK. The biopsychology of salt hunger and sodium deficiency. Pflugers Arch - Eur J Physiol. 2015;467:445–456. doi: 10.1007/s00424-014-1676-y. http://dx.doi.org/10.1007/s00424-014-1676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denton D. Hunger for Salt: An Anthropological Physiological and Medical Analysis. Springer London; London: 1982. [Google Scholar]
- 13.Schulkin J. Sodium Hunger: The Search for a Salty Taste. 1. Cambridge University Press; New York: 1992. [Google Scholar]
- 14.Nachman M. Taste preferences for sodium salts by adrenalectomized rats. J Comp Physiol Psychol. 1962;55:1124–1129. doi: 10.1037/h0041348. http://www.ncbi.nlm.nih.gov/pubmed/13937025. [DOI] [PubMed] [Google Scholar]
- 15.Handal PJ. Immediate acceptance of sodium salts by sodium deficient rats. Psychon Sci. 1965;3:315–316. http://dx.doi.org/10.3758/BF03343156. [Google Scholar]
- 16.Spector AC, Guagliardo NA, John SJS. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996;16:8115–8122. doi: 10.1523/JNEUROSCI.16-24-08115.1996. http://www.ncbi.nlm.nih.gov/pubmed/8987836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roitman MF, Bernstein IL. Amiloride-sensitive sodium signals and salt appetite: multiple gustatory pathways. Am J Phys. 1999;276:R1732–R1738. doi: 10.1152/ajpregu.1999.276.6.R1732. http://www.ncbi.nlm.nih.gov/pubmed/10362754. [DOI] [PubMed] [Google Scholar]
- 18.Geran LC, Spector AC. Anion size does not compromise sodium recognition by rats after acute sodium depletion. Behav Neurosci. 2004;118:178–183. doi: 10.1037/0735-7044.118.1.178. http://dx.doi.org/10.1037/0735-7044.118.1.178. [DOI] [PubMed] [Google Scholar]
- 19.Roper SD. The taste of table salt. Pflugers Arch - Eur J Physiol. 2015;467:457–463. doi: 10.1007/s00424-014-1683-z. http://dx.doi.org/10.1007/s00424-014-1683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Phys. 1992;263:R169–R176. doi: 10.1152/ajpregu.1992.263.1.R169. http://www.ncbi.nlm.nih.gov/pubmed/1636783. [DOI] [PubMed] [Google Scholar]
- 21.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–660. doi: 10.1037//0735-7044.98.4.652. http://www.ncbi.nlm.nih.gov/pubmed/6540589. [DOI] [PubMed] [Google Scholar]
- 22.Quartermain D, Miller NE, Wolf G. Role of experience in relationship between deficiency and rate of bar pressing for salt. J Comp Physiol Psychol. 1967;63:417–420. doi: 10.1037/h0024611. http://dx.doi.org/10.1037/h0024611. [DOI] [PubMed] [Google Scholar]
- 23.Krieckhaus EE, Wolf G. Acquisition of sodium by rats: interaction of innate mechanisms and latent learning. J Comp Physiol Psychol. 1968;65:197–201. doi: 10.1037/h0025547. http://www.ncbi.nlm.nih.gov/pubmed/5668303. [DOI] [PubMed] [Google Scholar]
- 24.Clark JJ, Bernstein IL. Sensitization of salt appetite is associated with increased “wanting”; but not “liking” of a salt reward in the sodium-deplete rat. Behav Neurosci. 2006;120:206–210. doi: 10.1037/0735-7044.120.1.206. http://dx.doi.org/10.1037/0735-7044.120.1.206. [DOI] [PubMed] [Google Scholar]
- 25.Schulkin J, Arnell P, Stellar E. Running to the taste of salt in mineralocorticoid-treated rats. Horm Behav. 1985;19:413–425. doi: 10.1016/0018-506x(85)90038-8. http://www.ncbi.nlm.nih.gov/pubmed/4085995. [DOI] [PubMed] [Google Scholar]
- 26.Robinson MJF, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Curr Biol. 2013;23:282–289. doi: 10.1016/j.cub.2013.01.016. http://dx.doi.org/10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cone JJ, Fortin SM, McHenry JA, Stuber GD, McCutcheon JE, Roitman MF. Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proc Natl Acad Sci U S A. 2016;113:1943–1948. doi: 10.1073/pnas.1519643113. http://dx.doi.org/10.1073/pnas.1519643113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. http://www.ncbi.nlm.nih.gov/pubmed/630410. [DOI] [PubMed] [Google Scholar]
- 29.Grill HJ, Schulkin J, Flynn FW. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci. 1986;100:536–543. doi: 10.1037//0735-7044.100.4.536. http://www.ncbi.nlm.nih.gov/pubmed/3741604. [DOI] [PubMed] [Google Scholar]
- 30.Wise RA, Spindler J, de Wit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. http://dx.doi.org/10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 31.Wise RA. Dopamine learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. http://dx.doi.org/10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. http://www.ncbi.nlm.nih.gov/pubmed/9054347. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. http://dx.doi.org/10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond Ser B Biol Sci. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. http://dx.doi.org/10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surmeier DJ, Carrillo-Reid L, Bargas J. Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience. 2011;198:3–18. doi: 10.1016/j.neuroscience.2011.08.051. http://dx.doi.org/10.1016/j.neuroscience.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. http://www.ncbi.nlm.nih.gov/pubmed/9858756. [DOI] [PubMed] [Google Scholar]
- 37.Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N, Correa M. Mesolimbic dopamine and the regulation of motivated behavior. Curr Top Behav Neurosci. 2016;27:231–257. doi: 10.1007/7854_2015_383. http://dx.doi.org/10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- 38.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–126. doi: 10.1038/nn.4173. http://dx.doi.org/10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinelli M, Rudick CN, Hu X-T, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. http://www.ncbi.nlm.nih.gov/pubmed/16613555. [DOI] [PubMed] [Google Scholar]
- 40.Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, Paladini CA, Beckstead MJ. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J Neurosci. 2013;33:13861–13872. doi: 10.1523/JNEUROSCI.5099-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.5099-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. http://www.ncbi.nlm.nih.gov/pubmed/7623143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. http://dx.doi.org/10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abizaid A, Liu Z-W, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao X-B, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. http://dx.doi.org/10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. http://dx.doi.org/10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 45.Cone JJ, Roitman JD, Roitman MF. Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 2015;133:844. doi: 10.1111/jnc.13080. http://dx.doi.org/10.1111/jnc.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu Z-W, Gao X-B, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. http://dx.doi.org/10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Labouèbe G, Liu S, Dias C, Zou H, Wong JCY, Karunakaran S, Clee SM, Phillips AG, Boutrel B, Borgland SL. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci. 2013;16:300–308. doi: 10.1038/nn.3321. http://dx.doi.org/10.1038/nn.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krügel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–187. doi: 10.1016/j.ejphar.2003.09.047. http://www.ncbi.nlm.nih.gov/pubmed/14660021. [DOI] [PubMed] [Google Scholar]
- 49.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013;8:e77284. doi: 10.1371/journal.pone.0077284. http://dx.doi.org/10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith MEA, Carr KD, Rice ME. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. http://dx.doi.org/10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roitman MF, Patterson TA, Sakai RR, Bernstein IL, Figlewicz DP. Sodium depletion and aldosterone decrease dopamine transporter activity in nucleus accumbens but not striatum. Am J Phys. 1999;276:R1339–R1345. doi: 10.1152/ajpregu.1999.276.5.R1339. http://dx.doi.org/10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- 52.Figlewicz DP, Patterson TA, Zavosh A, Brot MD, Roitman M, Szot P. Neurotransmitter target for endocrine regulation. Horm Metab Res. 1999;31:335–339. doi: 10.1055/s-2007-978749. http://dx.doi.org/10.1055/s-2007-978749. [DOI] [PubMed] [Google Scholar]
- 53.Lucas LR, Pompei P, McEwen BS. Salt appetite in salt-replete rats: involvement of mesolimbic structures in deoxycorticosterone-induced salt craving behavior. Neuroendocrinology. 2000;71:386–395. doi: 10.1159/000054559. 54559. [DOI] [PubMed] [Google Scholar]
- 54.Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. http://dx.doi.org/10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 2016;6:123–148. doi: 10.1016/j.baga.2016.02.001. http://dx.doi.org/10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niv Y. Cost, benefit, tonic, phasic: what do response rates tell us about dopamine and motivation? Ann N Y Acad Sci. 2007;1104:357–376. doi: 10.1196/annals.1390.018. http://dx.doi.org/10.1196/annals.1390.018. [DOI] [PubMed] [Google Scholar]
- 57.Grafe LA, Flanagan-Cato LM. Differential effects of mineralocorticoid and angiotensin II on incentive and mesolimbic activity. Horm Behav. 2016;79:28–36. doi: 10.1016/j.yhbeh.2015.12.002. http://dx.doi.org/10.1016/j.yhbeh.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas LR, Grillo CA, McEwen BS. Salt appetite in sodium-depleted or sodium-replete conditions: possible role of opioid receptors. Neuroendocrinology. 2007;85:139–147. doi: 10.1159/000102536. http://dx.doi.org/10.1159/000102536. [DOI] [PubMed] [Google Scholar]
- 59.Geary N, Smith GP. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav. 1985;22:787–790. doi: 10.1016/0091-3057(85)90528-3. http://www.ncbi.nlm.nih.gov/pubmed/2989944. [DOI] [PubMed] [Google Scholar]
- 60.Roitman MF, Schafe GE, Thiele TE, Bernstein IL. Dopamine and sodium appetite: antagonists suppress sham drinking of NaCl solutions in the rat. Behav Neurosci. 1997;111:606–611. doi: 10.1037//0735-7044.111.3.606. http://www.ncbi.nlm.nih.gov/pubmed/9189275. [DOI] [PubMed] [Google Scholar]
- 61.Frankmann SP, Sollars SI, Bernstein IL. Sodium appetite in the sham-drinking rat after chorda tympani nerve transection. Am J Phys. 1996;271:R339–R345. doi: 10.1152/ajpregu.1996.271.2.R339. http://www.ncbi.nlm.nih.gov/pubmed/8770132. [DOI] [PubMed] [Google Scholar]
- 62.Frankmann SP, Broder L, Dokko JH, Smith GP. Differential changes in central monoaminergic metabolism during first and multiple sodium depletions in rats. Pharmacol Biochem Behav. 1994;47:617–624. doi: 10.1016/0091-3057(94)90167-8. http://www.ncbi.nlm.nih.gov/pubmed/8208782. [DOI] [PubMed] [Google Scholar]
- 63.Fortin SM, Cone JJ, Ng-Evans S, McCutcheon JE, Roitman MF. Sampling phasic dopamine signaling with fast-scan cyclic voltammetry in awake, behaving rats. Curr Protoc Neurosci. 2015;70:7.25.1–7.25.20. doi: 10.1002/0471142301.ns0725s70. http://dx.doi.org/10.1002/0471142301.ns0725s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. http://dx.doi.org/10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 65.Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. http://dx.doi.org/10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 66.Roitman MF, Wheeler RA, Tiesinga PHE, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem. 2010;17:539–546. doi: 10.1101/lm.1869710. http://dx.doi.org/10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loriaux AL, Roitman JD, Roitman MF. Nucleus accumbens shell, but not core, tracks motivational value of salt. J Neurophysiol. 2011;106:1537–1544. doi: 10.1152/jn.00153.2011. http://dx.doi.org/10.1152/jn.00153.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seeley RJ, Galaverna O, Schulkin J, Epstein AN, Grill HJ. Lesions of the central nucleus of the amygdala. II: Effects on intraoral NaCl intake. Behav Brain Res. 1993;59:19–25. doi: 10.1016/0166-4328(93)90147-i. http://www.ncbi.nlm.nih.gov/pubmed/8155286. [DOI] [PubMed] [Google Scholar]
- 69.Johnson AK, de Olmos J, Pastuskovas CV, Zardetto-Smith AM, Vivas L. The extended amygdala and salt appetite. Ann N Y Acad Sci. 1999;877:258–280. doi: 10.1111/j.1749-6632.1999.tb09272.x. http://www.ncbi.nlm.nih.gov/pubmed/10415654. [DOI] [PubMed] [Google Scholar]
- 70.Tandon S, Simon SA, Nicolelis MAL. Appetitive changes during salt deprivation are paralleled by widespread neuronal adaptations in nucleus accumbens, lateral hypothalamus, and central amygdala. J Neurophysiol. 2012;108:1089–1105. doi: 10.1152/jn.00236.2012. http://dx.doi.org/10.1152/jn.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Contreras RJ. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977;121:373–378. doi: 10.1016/0006-8993(77)90162-7. http://www.ncbi.nlm.nih.gov/pubmed/832170. [DOI] [PubMed] [Google Scholar]
- 72.Contreras RJ, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. http://www.ncbi.nlm.nih.gov/pubmed/458420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobs KM, Mark GP, Scott TR. Taste responses in the nucleus tractus solitarius of sodium-deprived rats. J Physiol. 1988;406:393–410. doi: 10.1113/jphysiol.1988.sp017387. http://www.ncbi.nlm.nih.gov/pubmed/3254418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho YK, Smith ME, Norgren R. Low-dose furosemide modulates taste responses in the nucleus of the solitary tract of the rat. Am J Phys Regul Integr Comp Phys. 2004;287 doi: 10.1152/ajpregu.00090.2004. [DOI] [PubMed] [Google Scholar]
- 75.Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22:RC225. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. 20026416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin J-W, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat. 2011;42:1–23. doi: 10.1016/j.jchemneu.2011.05.003. http://dx.doi.org/10.1016/j.jchemneu.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary: efferent projections. J Comp Neurol. 2006;498:223–250. http://www.ncbi.nlm.nih.gov/pubmed/16933386. [PubMed] [Google Scholar]
- 78.Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci. 2006;26:411–417. doi: 10.1523/JNEUROSCI.3115-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.3115-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Phys. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. http://www.ncbi.nlm.nih.gov/pubmed/2260724. [DOI] [PubMed] [Google Scholar]
- 80.Shin J-W, Loewy AD. Gastric afferents project to the aldosterone-sensitive HSD2 neurons of the NTS. Brain Res. 2009;1301:34–43. doi: 10.1016/j.brainres.2009.08.098. http://dx.doi.org/10.1016/j.brainres.2009.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius. Neuroscience. 2006;141:1995–2005. doi: 10.1016/j.neuroscience.2006.05.059. http://dx.doi.org/10.1016/j.neuroscience.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 82.Stein MK, Loewy AD. Area postrema projects to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei: brainstem sites implicated in sodium appetite regulation. Brain Res. 2010;1359:116–127. doi: 10.1016/j.brainres.2010.08.085. http://dx.doi.org/10.1016/j.brainres.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geerling JC, Stein MK, Miller RL, Shin J-W, Gray PA, Loewy AD. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res. 2011;1375:19–27. doi: 10.1016/j.brainres.2010.11.028. http://dx.doi.org/10.1016/j.brainres.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci. 1991;105:944–954. doi: 10.1037//0735-7044.105.6.944. http://dx.doi.org/10.1037/0735-7044.105.6.944. [DOI] [PubMed] [Google Scholar]
- 85.Grigson PS, Colechio EM, Power ML, Schulkin J, Norgren R. Parabrachial lesions in rats disrupt sodium appetite induced by furosemide but not by calcium deprivation. Physiol Behav. 2015;140:172–179. doi: 10.1016/j.physbeh.2014.11.070. http://dx.doi.org/10.1016/j.physbeh.2014.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf G. Hypothalamic regulation of sodium intake: relations to preoptic and teg-mental function. Am J Phys. 1967;213:1433–1438. doi: 10.1152/ajplegacy.1967.213.6.1433. http://www.ncbi.nlm.nih.gov/pubmed/4864715. [DOI] [PubMed] [Google Scholar]
- 87.Shibata R, Kameishi M, Kondoh T, Torii K. Bilateral dopaminergic lesions in the ventral tegmental area of rats influence sucrose intake, but not umami and amino acid intake. Physiol Behav. 2009;96:667–674. doi: 10.1016/j.physbeh.2009.01.002. http://dx.doi.org/10.1016/j.physbeh.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. http://dx.doi.org/10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- 90.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–731. doi: 10.1037//0735-7044.101.5.724. http://www.ncbi.nlm.nih.gov/pubmed/3675851. [DOI] [PubMed] [Google Scholar]
- 91.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. http://www.ncbi.nlm.nih.gov/pubmed/9334421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. http://www.ncbi.nlm.nih.gov/pubmed/10215912. [DOI] [PubMed] [Google Scholar]
- 93.Clark JJ, Bernstein IL. Reciprocal cross-sensitization between amphetamine and salt appetite. Pharmacol Biochem Behav. 2004;78:691–698. doi: 10.1016/j.pbb.2004.05.002. http://dx.doi.org/10.1016/j.pbb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 94.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. http://dx.doi.org/10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 95.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Phys Regul Integr Comp Phys. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. http://www.ncbi.nlm.nih.gov/pubmed/12776726. [DOI] [PubMed] [Google Scholar]
- 96.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. http://dx.doi.org/10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. http://dx.doi.org/10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. http://dx.doi.org/10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ. Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology. 2014;39:605–613. doi: 10.1038/npp.2013.235. http://dx.doi.org/10.1038/npp.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]