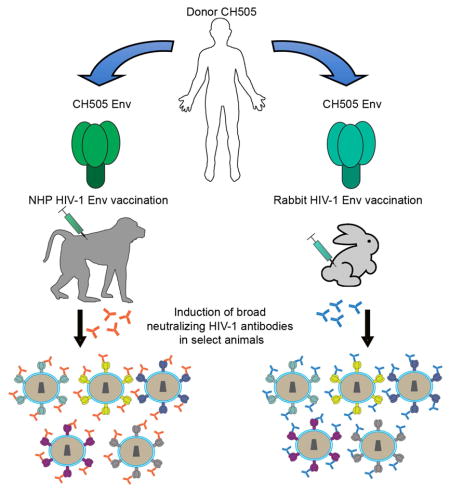
SUMMARY
The events required for the induction of broad neutralizing antibodies (bnAbs) following HIV-1 envelope (Env) vaccination are unknown, and their induction in animal models as proof-of-concept would be critical. Here, we describe the induction of plasma antibodies capable of neutralizing heterologous primary (tier 2) HIV-1 strains in one macaque and two rabbits. Env immunogens were designed to induce CD4 binding site (CD4bs) bnAbs, but surprisingly, the macaque developed V1V2-glycan bnAbs. Env immunization of CD4bs bnAb heavy chain rearrangement (VHDJH) knock-in mice similarly induced V1V2-glycan neutralizing antibodies (nAbs), wherein the human CD4bs VH chains were replaced with mouse rearrangements bearing diversity region (D)-D fusions, creating antibodies with long, tyrosine-rich HCDR3s. Our results show Env vaccination can elicit broad neutralization of tier 2 HIV-1, demonstrate V1V2 glycan bnAbs are more readily induced than CD4bs bnAbs, and define VH replacement and diversity region fusion as potential mechanisms for generating V1V2-glycan bnAb site antibodies.
eTOC
Saunders et al. demonstrate that HIV-1 broadly neutralizing antibodies can be induced by vaccination, albeit only in select animals. Studies in neutralizing antibody variable heavy chain (VH) knock-in mice suggest a mechanism for broadly neutralizing antibody induction that involves heavy chain replacement and fusion of immunoglobulin diversity (D) regions.
INTRODUCTION
HIV-1 bnAbs develop in ~50% of HIV-1 infected individuals after several years of infection (Hraber et al., 2014). There are 5 envelope (Env) conserved sites of vulnerability that are targeted by bnAbs during infection, but antibodies that recognize these sites are unusual and predisposed to negative immune regulation (Bonsignori et al., 2017b; Haynes et al., 2005; Liu et al., 2015; Mascola and Haynes, 2013). Thus, HIV-1 Env glycoprotein vaccination does not result in induction of bnAbs, but rather elicits autologous nAbs and/or heterologous tier 1 neutralizing antibodies (Bradley et al., 2016; Crooks et al., 2015; Sanders et al., 2015).
Studies of antibody-HIV co-evolution in single individuals who made bnAbs after infection have provided insights into how bnAbs develop (Bonsignori et al., 2017a; Bonsignori et al., 2016; Doria-Rose et al., 2014; Gao et al., 2014; Liao et al., 2013a; MacLeod et al., 2016; Simonich et al., 2016). African individual CH505 developed two different CD4bs bnAbs, CH235.12 and CH103 (Bonsignori et al., 2016; Gao et al., 2014; Liao et al., 2013a). The CH505 transmitted founder (TF) virus bound well to the CH103 bnAb unmutated common ancestor (UCA) (Liao et al., 2013a) providing a potential germline-targeting immunogen. If individual animals or humans can be found that made bnAbs after vaccination, insights could be gained regarding bnAb induction mechanisms. Here, we have immunized rabbits and rhesus macaques (RMs) with TF-evolved Env proteins (Liao et al., 2013a). We demonstrate that vaccination with various forms of Env can elicit HIV-1 bnAbs, albeit only in a small subset of animals, and show that VH replacement and diversity region (D)-D fusion events are mechanisms for V1V2-glycan nAb induction in bnAb UCA VH knock-in mice.
RESULTS
Env trimer immunization in rabbits
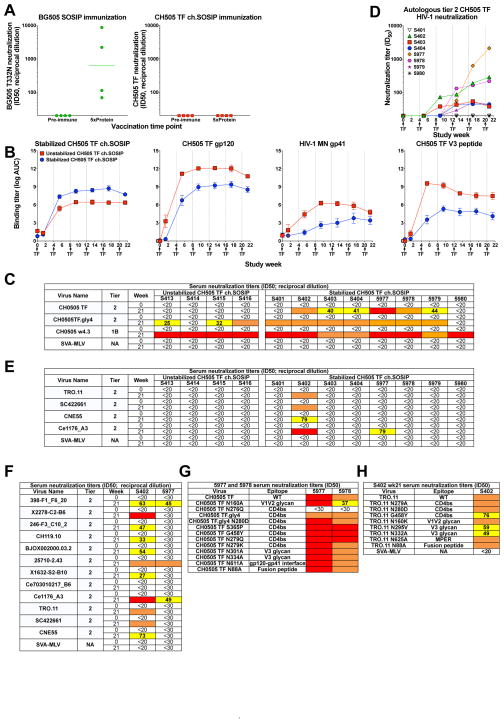
We first sought to induce tier 2 neutralizing antibodies in rabbits. To induce tier 2 nAbs in rabbits, we designed a CH505 TF chimeric (ch) SOSIP with the BG505 gp41, since this design increased trimeric Env formation (Figure S1; Zhou et al., 2017). We immunized rabbits with the CH505 TF ch.SOSIP and compared immunogenicity to BG505 6R.SOSIP.664 (Sanders et al., 2013). Interestingly, BG505-immunized rabbits made serum autologous tier 2 nAbs, while the CH505 TF-immunized rabbits did not (Mann-Whitney test comparing between immunized groups P<0.05; Figure 1).
Figure 1. Vaccination with stabilized CH505 SOSIP trimers elicits broad serum heterologous tier 2 nAbs in rabbits.
(A) Serum autologous tier 2 neutralization titers by BG505 6R.SOSIP.664-immunized (left) and unstabilized CH505 TF ch.SOSIP-immunized (right) rabbits. ID50 titers are shown as reciprocal serum dilution in all panels. Neutralization titers were higher in the BG505 group than the CH505 TF group (Mann-Whitney test P<0.05).
(B) Serum IgG ELISA binding titers to HIV Env antigens for rabbits immunized with unstabilized (red) and stabilized (blue) CH505 ch.SOSIPs. The mean ± s.e.m. of the group is shown (n=4 or 8). Immunizations are indicated by arrows and vertical lines on the x-axis in B and D. CH505 TF V3 peptide (TRPNNKTRTSIRIGPGQAFYATGQVIGDIREAY).
(C) Autologous serum neutralization ID50 titers before vaccination (week 0) and at the end of vaccination (week 21). Neutralization titers are color-coded as white <20; yellow 20–89; orange 90–500: red >500 in C–F. Stabilized trimers elicited higher CH505 TF neutralization titers than unstabilized trimers (Mann-Whitney test P<0.05).
(D) The kinetics of autologous tier 2 neutralization in stabilized CH505 TF ch.SOSIP-immunized rabbits. ID50 is shown for each individual rabbit listed in the legend.
(E) Rabbit Serum neutralization ID50 titers against heterologous tier 2 viruses.
(F) Rabbit S402 and 5977 serum neutralization breadth.
(G) 5977 and 5978 serum neutralization ID50 titers against CH505 TF viruses with mutations in known bnAb epitopes. All mutations reduce bnAb neutralization at the indicated epitope except, CH505 TF gly4 which enhances CD4bs neutralization. N280D and S365P mutations reduce CH235 and CH103 neutralization respectively. N276Q confers resistant to CD4bs bnAb VRC04 neutralization.
(H) S402 serum neutralization ID50 titers against wildtype and mutant forms of TRO.11 viruses. Data shown in each panel of this figure are representative of 2 or 3 independent experiments.
We hypothesized that further stabilization would improve CH505 trimer immunogenicity. We stabilized the CH505 TF ch.SOSIP with E64K and A316W mutations (Figure S1; de Taeye et al., 2015). These mutations stabilized the CH505 TF ch.SOSIP in that they eliminated exposure of epitopes in the V3 loop and coreceptor binding site (Figure S1C). To compare the immunogenicity of unstabilized and stabilized trimers we vaccinated rabbits with both types of CH505 TF ch.SOSIP (Table S1). The stabilized trimer group exhibited 3-fold lower gp120 V3 linear peptide and gp41 antibodies (Figure 1B). Tier 1 neutralization titers were comparable in both groups (Figure 1C). However, autologous tier 2 (difficult-to-neutralize) CH505 TF neutralization was only elicited in rabbits that received the stabilized CH505 TF ch.SOSIP (6 of 8 rabbits) (Mann-Whitney test comparing stabilized versus unstabilized groups P<0.05; Figures 1C and 1D). Deletion of CD4bs shielding glycans (CH505 TF.gly4; Zhou et al., 2017) demonstrated that immunization with the stabilized trimer induced CD4bs antibodies more frequently and to a higher magnitude than unstabilized trimers (Figure 1C).
Neutralization breadth requires the neutralization of heterologous tier 2 viruses. While none of the rabbits immunized with unstabilized CH505 TF ch.SOSIP neutralized heterologous tier 2 viruses (Figure 1E), rabbit S402 and 5977 in the stabilized SOSIP group neutralized tier 2 viruses (Figure 1E and 1F). Serum from S402 neutralized 11 of 12 viruses (deCamp et al., 2014) with ID50 titers that were weak in some cases at 1:27 but reached as high as 1:621 for other tier 2 isolates. Serum from 5977 neutralized three tier 2 viruses at ID50 values ranging from 1:45 to 1:107. The neutralization activity in both rabbits was reproducible in repeat assays. IgG purified from S402 serum neutralized heterologous tier 2 virus TRO.11 (Figure S2A).
To map neutralization determinants we assessed neutralization against a panel of CH505 TF and TRO.11 viruses with mutations in four known bnAb epitopes. Autologous tier 2 neutralization activity in the serum of rabbits 5977 and 5978 was abrogated by an asparagine to glutamine mutation at position 276 within the CD4bs (Figure 1G). The asparagine serves as a N-linked glycan site, which is required by the CD4bs nAb HJ16 and CH31 (Figure S2E; Balla-Jhagjhoorsingh et al., 2013). For rabbit 5978, mutation of the N-linked glycan site N160 in the V2 loop also diminished neutralization activity (Figure 1G). A glycine to tyrosine mutation at position 458 within the CD4bs of TRO.11 reduced S402 serum heterologous neutralization approximately 2-fold (Figure 1H). Additionally, substituting alanine for asparagine at position 332 within the V3 glycan site reduced S402 serum neutralization ID50 titers 3-fold (Figure S3D). The partial reduction of neutralization by N332A may be due to induced antibodies preferring a N334 glycan, since the CH505 TF Env has a N-linked glycosylation site at N334 and not N332.
Env trimer immunizations in rhesus macaques
We have previously shown that cleavage-deficient gp140 (gp140C) TF oligomeric Envs from individuals that did not make high levels of bnAbs were immunogenic and could induce very low levels of nAbs to select tier 2 HIV-1 strains in guinea pigs (Liao et al., 2013b). While gp140C proteins are not mimics of the native trimer (Ringe et al., 2013; Sanders et al., 2013), they have induced tier 2 autologous virus nAbs in macaques (Bradley et al., 2016; Hessell et al., 2016). To determine if a similar Env design using sequences from an individual that developed bnAbs could induce tier 2 heterologous virus neutralization in primates, we immunized RMs with sequential CH505 Env gp140C trimers (Bonsignori et al., 2017b) primed with CH505 TF gp145 DNA delivered by electroporation (Table S1). Four CH505 Envs (TF, week 53, week 78 and week 100) were selected for their ability to bind to sequential stages of the CH103 CD4bs bnAb lineage (Bonsignori et al., 2017b; Liao et al., 2013a). Env-binding antibodies were induced in RMs after a single DNA-electroporation immunization (Figure 2A). Antibody titers continued to increase for 4 weeks and were augmented with a second DNA-electroporation immunization. RMs were subsequently immunized with TF gp140C glycoproteins followed by CH505 week 53, week 78 and week 100 gp140C Envs.
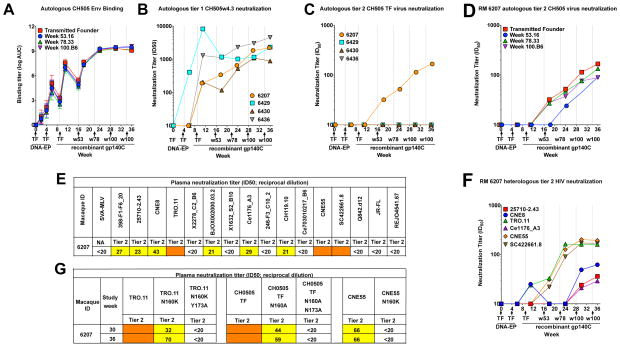
Figure 2. CH505 sequential Env vaccination elicits V1V2-glycan autologous and heterologous tier 2 broadly neutralizing antibody responses in a rhesus macaque.
(A) Rhesus plasma IgG ELISA binding titers to CH505 gp140Cs immunogens indicated in the legend. Each curve represents the mean ± s.e.m. of the four macaques. Immunizations are indicated by arrows and vertical lines on the x-axis in A–D, F.
(B,C) Rhesus plasma neutralization ID50 titers against (B) autologous tier 1 virus CH505 w4.3 and autologous tier 2 virus CH505 TF. ID50 titers are reciprocal plasma dilution for each rhesus macaque shown in the legend.
(D) RM 6207 plasma neutralization of all four tier 2 CH505 viruses included in the sequential vaccination regimen.
(E) RM 6207 week 36 plasma neutralization of nine of sixteen (56%) tier 2 viruses. ID50 titers are the geometric mean of two independent experiments. Neutralization titers are color-coded as white <20; yellow 20–89; orange 90–500: red >500 in E and F.
(F) The kinetics of macaque 6207 plasma neutralization of 6 heterologous tier 2 viruses.
(G) Macaque 6207 week 30 and 36 plasma neutralization of wildtype and V1V2-glycan mutant tier 2 viruses. Data shown in each panel of this figure are representative of 2 to 5 independent experiments.
All four macaques neutralized the autologous tier 1 virus CH505 w4.3 (Figure 2B). However, nAbs of autologous CH505 tier 2 virus were elicited only in RM 6207 after the CH505 week 53 immunization (Figure 2C). RM 6207 plasma neutralized three of four autologous tier 2 viruses after the CH505 week 53 gp140C immunization and neutralized all four sequential autologous viruses (HIV-1 CH505 TF, week 53, week 78, week 100) after CH505 week 78 gp140C immunization (Figure 2D).
RM 6207 plasma two weeks after the final immunization neutralized 9 of 16 (56%) tier 2 heterologous viruses from multiple HIV-1 clades (Figure 2E). Heterologous tier 2 nAbs were detected at multiple timepoints (Figure 2F). Purified immunoglobulin (Ig) from weeks 30 and 36 neutralized TRO.11, confirming the neutralization was mediated by Ig (Figure S3B). We have immunized with the same vaccine regimen a total of 8 macaques-including the four macaques reported here. RM6207 is the only macaque that possessed tier 2 HIV-1 neutralization in its plasma and, coincidentally, was the only macaque with prevaccination Scl-70 autoantibodies (Saunders KO and Haynes BF, unpublished data), suggesting host factors may have a role in bnAb induction.
We next mapped 6207 plasma neutralization activity site. Plasma neutralization was V1V2-glycan targeted as demonstrated by reduced neutralization when Env N160 was mutated to alanine or lysine, and loss of neutralization when N160 and Y/N173 were both mutated to alanine (Figure 2G, Figure S3E; McLellan et al., 2011). The induction of V1V2-glycan bnAbs was unexpected, since the immunization regimen was designed to elicit CD4bs bnAbs. However, recent studies determined that the naive RM B cell repertoire does not include an ortholog of the CH103 human light chain variable (V) λ3-1 gene, thus, restricting elicitation of CH103-like CD4bs bnAb lineages in RMs (Williams et al., 2017).
The development of bnAbs in humans has been associated with high frequencies of blood T follicular helper (Tfh)-like cells (Locci et al., 2013; Moody et al., 2016). CH505 Env antigen-specific Tfh-like cells were higher in blood but not lymph nodes in RM 6207 than in RMs that did not make tier 2 nAbs seven days after the final immunization (data not shown).
Plasma autoantibodies occur more frequently in individuals who develop bnAbs during HIV-1 infection compared to HIV-infected individuals that do not develop bnAbs (Moody et al., 2016). RM 6207 that made bnAbs, had pre- and post-vaccination autoantibodies against the DNA topoisomerase Scl70 (Figure S3F and S3G). In a large group of non-vaccinated RMs, only 3 of 114 (2.6%) were positive for plasma Scl70 antibodies. Among Scl70+ macaques, the Scl70 antibody titer in RM 6207 was the highest by 2.5-fold (Figure S3H).
Induction of V1V2-glycan-targeted nAbs in CH103 UCA “HC only” KI mice
Recently, we reported that in CH103 UCA double knock-In (KI) mice (where the UCA LC rearrangement is co-expressed with its cognate HC rearrangement), expression of UCA HC/LC pairs with CH505 Env-specific reactivity are limited by peripheral immune tolerance, while abundantly produced in the bone marrow (Williams et al., 2017). However, we also found that in macaques, the lack of an ortholog to the human CH103 UCA light chain (LC) rearrangement may also contribute in limiting expression of CH505 Env-binding UCA HC/LC pairs in RMs (Williams et al., 2017). Since the CH505 sequential Env regimen was designed to induce CH103-like CD4bs bnAbs, yet in RMs induced only V1V2-glycan site-targeted bnAbs, here we turned instead to “HC only” (VHDJH) CH103 UCA KI mice (whose knocked-in HCs can only pair with endogenous mouse LCs), to determine if and how an analogous alternate vaccine-induced response to CH505 Env immunization may occur.
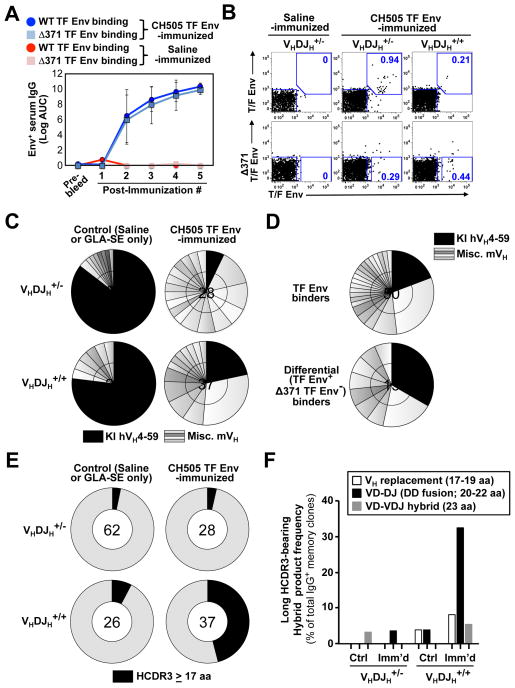
Immunization of CH103 UCA HC only KI mice with CH505 TF gp120 Env resulted in TF Env-binding IgG+ plasma antibody and memory B cell responses that were mostly independent of the isoleucine at amino acid (aa) 371 for Env binding (Figures 3A and B; Lynch et al., 2012), suggesting non-CD4bs CH103 bnAb lineage-specific responses were induced. We recovered TF Env-binding IgG+ single memory B cells from immunized CH103 UCA HC-only mice and analyzed their HC/LC pairs by sequencing (Figures 3C and D). Indeed, only 18% and 26% of Env-specific B cells from TF Env-immunized heterozygous and homozygous CH103 UCA HC only KI mice, respectively, retained the human VH4-59 germline segment (hVH4-59). In contrast, 91% and 80% of B cells from sham (saline)-immunized heterozygous and homozygous CH103 UCA HC only KI mice expressed hVH4-59 respectively, demonstrating efficient HC allelic exclusion of the endogenous allele by the rearranged KI allele, and indicating TF Env vaccination selected memory clones utilizing endogenous mVH segments.
Figure 3. Env-vaccination of CH103 UCA homozygous HC only KI mice elicits high frequencies of memory B-cells with DD fusion-derived long HCDR3s.
(A–B) “Off-target” class-switched Ab/B cell responses in TF Env-immunized CH103 HC only mice. (A) ELISA binding titers (mean log AUC ±s.e.m) of serum IgG from HC only mice either vaccinated with CH505 TF Env gp120s or administered saline (n=5/group; 2 heterozygous VHDJH+/−, 3 homozygous VHDJH+/+). (B) FACS plots of TF Env-reactive IgG+ memory B cells. isolated 10d after the 5th immunizations of HC only heterozygous and homozygous animals. Upper and lower rows show total TF Env binders and differential binders (Δ371Ile TF−, WT TF+; enriched for lineage+ clones), respectively.
(C–F) Vaccine-induced loss of hVH4-59 expression in homozygous HC only memory B cells results from selection for long HCDR3-bearing VDDJ-type hybrid antibodies. (C) Pie charts showing distribution of hVH4-59 segments (black slices) relative to endogenous murine VH families (gray slices) utilized by TF Env-binding IgG+ memory B cells from TF Env-immunized heterozygous (n=2) or homozygous (n=2) animals. For comparison, shown are IgG+ memory B cells in saline-administered heterozygous (n=1) or homozygous (n=2) mice. Number of HC/LC pairs sequenced is shown in the center of the pie chart. (D) Same as shown in (C), but represented instead as total versus differential TF Env-binders, amongst all VHDJH KI animals examined. (E) HCDR3 lengths in memory IgG+ clones from the same animals as in (C–D), with HCDR3s ≥17 aa denoted by black shading. (F) Relative distributions of atypical HC rearrangements, in class-switched memory B cells from HC only KI strains.
In homozygous CH103 UCA HC-only KI mice, endogenous VH-utilization can only be obtained by secondary HC rearrangement events (Figure S4). Therefore, we compared VHDJH rearrangement sequence junctions in sham and TF Env-immunized CH103 UCA KI animals. Strikingly, IgG+ memory B cell clones sorted from TF Env-immunized CH103 UCA homozygous HC-only mice were enriched for two distinct types of de novo hybrid mouse-human secondary HC rearrangement products bearing long (>17 aa) heavy chain complementarity determining region 3 (HCDR3) regions (Figure 3E and 3F): 1) those generated by VH replacement events (producing 17–18 aa HCDR3s; Figure S4), and 2) those resulting from mouse VHD-human VHDJH rearrangements, either which retained the 3′ human hV4-59 footprint (producing 23–24 aa HCDR3s), or “DD fusion-like” products, lacking most or all of the hV4-59 footprint (yielding 20–22 aa HCDR3s) (Figure S4).
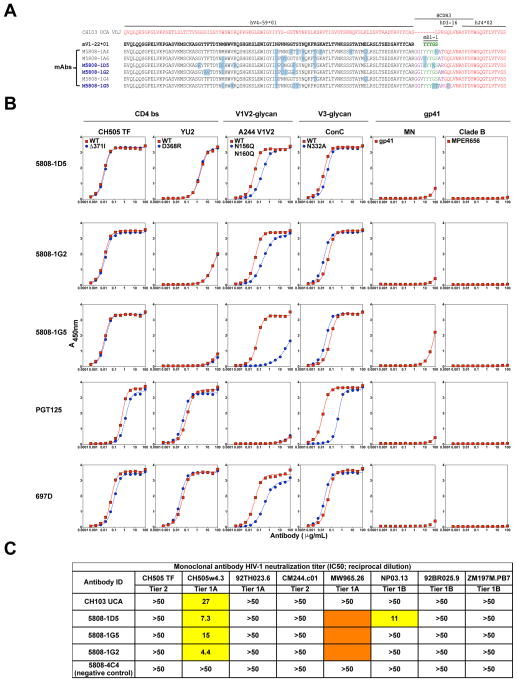
Mouse VHD to human DJH hybrids were the most frequently observed secondary rearrangement in the memory B cell pool (Figure 3F and Table S2). Importantly, similar DD fusion-containing rearrangements can be made in humans, either by a similar mechanism (involving use of an embedded heptamer, followed by footprint removal) or by a different atypical HC mechanism, involving 12/23 rule-violating D-D rearrangements (Figure S4D; Zhang et al., 2004). Given the potential physiologic relevance and high frequencies of DD fusion products induced by TF Env immunization, we generated six recombinant human monoclonal antibodies (mAbs) (Figure 4A) derived from distinct long HCDR3-bearing VDDJ hybrid rearrangements we sorted from the TF Env-specific IgG+ memory B cells (Figures 3E and 3F, Table S2). All mAbs utilized mD1-1 (Figure 4A), one of the longest and most tyrosine-rich mouse D regions as well as mVH1-22, whose closest human ortholog is VH1-8)—a VH family utilized by the V1V2-glycan bnAbs PGT141-PGT145 and PGDM1400 (Table S3; Sok et al., 2014; Walker et al., 2011). Interestingly, none of the mAbs had CD4bs specificity, but instead targeted the V1V2 region and bound better to wt HIV-1 gp120 relative to N156A N160A mutated Env (Figure 4B). Moreover, these V1V2 glycan-directed mAbs also exhibited HIV-1 neutralization activity, reflected by their ability to neutralize Tier 1a and 1b HIV-1 isolates (Figure 4C). Thus, nAbs elicited by CH505 Envs in both macaques and mice have similar Env specificity and map to the V1V2-glycan bnAb binding site. These V1V2-glycan antibodies demonstrate secondary HC rearrangement as a mechanism for redirecting CD4bs antibody lineages to target the V1V2 glycan epitope.
Figure 4. Vaccine-induced nAbs with VDDJ-derived long HCDR3s have N160K-dependent V1V2 glycan specificity.
(A) HC aa alignments of the six mAbs derived from memory B cells of vaccinated HC only homozygous mice. MAb VH aa are colored black if shared with, and blue if different from the germline mVH1-22 segment (bolded black). Red aa are sequence shared with human DH3-16 and JH4 from the knocked-in CH103 UCA VHDJH rearrangement. Green aa indicate mouse D1-1 inserted via VD-VDJ hybrid events. Putative P and/or N-additions are colored purple. Putative P and/or N-additions 3′ of D1-1 eliminate either all or most of the VH4-59 footprint generated by recombination of (mVH1-22)-mD1-1 to cryptic 3′ RSS. MAb names in Blue bolded letters produced sufficient quantities for characterization in (B–C).
(B) MAb reactivities to bnAb CD4bs, V1V2 glycan, V3 glycan, and MPER epitopes. Shown are mAb reactivities to indicated pairs of WT and mutant Envs (y-axis) as a function of mAb concentrations (x-axis) in ELISA.
(C) Tier 1A or 2 HIV-1 neutralization (IC50 neutralization potency values indicated) by the isolated mAbs. The original CH103 UCA mAb, and an additional mAb 5808-4C4 (expressing same Vκ6-14-bearing LCs as the other mAbs, but using knocked-in CH103 UCA HC rearrangements) were used as positive and negative neutralization controls respectively.
DISCUSSION
Here we have demonstrated that immunizations with different forms of HIV-1 CH505 Envs can induce bnAbs in rabbits and macaques, and bnAb lineages can switch specificities after Env vaccination. For these studies, we used rabbits since their serum has a low background level of HIV neutralization in the TZM/bl neutralization assay and thus serve as a useful small animal model for immunogenicity testing of Env candidates. HIV-1 bnAb knock-in mice express bnAb precursors at higher frequencies allowing the immunobiology of bnAb development to be evaluated. In this study, the CH103 VH only knock-in mice were critical for determining that CH505 Env immunization could select for VH replacement and D-D-like fusions events that generate V1V2 glycan-specific antibodies. Rhesus macaques have an antibody repertoire similar to humans, and we suspect, this model more likely represents the response to Env immunogens that will occur in humans.
For V1V2 glycan bnAb responses, long HCDR3 are required for neutralization (McLellan et al., 2011; Pancera et al., 2010). D-D fusions have been implicated in the process of human CH01 V1V2 bnAb lineage development (Bonsignori et al., 2011). Our studies in CH103 UCA CD4bs VHDJH-only KI mice demonstrated that immunization with the CH505 TF Env was able to select for V1V2-targeted B cells with B cell receptors with long HCDR3 regions due to multiple recombination events including D-D fusions.
These findings have implications for HIV-1 vaccine development. First, they suggest that multiple recombinant forms of Env may induce broad neutralizing antibody B cell clonal lineages to mature. Whether having more stabilized, native trimer Env forms will improve the percentage of bnAb responders remains to be determined. That macaque RM 6207 was the only macaque that had pre-vaccination autoantibodies, suggested less stringent immune tolerance controls (Haynes and Verkoczy, 2014).
Second, in rabbits, we observed that 6/8 animals developed autologous tier 2 nAbs and two animals developed heterologous nAbs with repetitive CH505 TF Env immunization. Whether use of sequential Env immunizations versus repetitive TF Env immunizations will be of use in development of a higher frequency of bnAbs will be tested against the human B cell repertoire in humans in the upcoming HIV Vaccine Trials Network study, HVTN 115.
Third, we show two examples (RM 6207 and CH103 VHDJH-only mice) of immunizations with CD4bs-targeted immunogens with diversion of the Ab response targeted to the V1V2-glycan bnAb site. Thus, V1V2-glycan bnAbs may be easier to induce than CH103-like CD4bs bnAbs since CH103 as well as other CD4bs bnAbs are subject to immune tolerance controls (McGuire et al., 2016; Williams et al., 2017).
Fourth, our studies in CH103 UCA VHDJH KI mice also raise the possibility that when holes in the primary antibody repertoire exist, B cell responses targeting conserved neutralizing epitopes can develop by alternate B cell receptor maturation pathways. In RMs the lack of a LC that can pair with CH103 HC VH4-59 was serendipitous, whereas in the homozygous CH103 UCA HC KI mouse model the LC repertoire hole was by design, and revealed DD fusions as a solution for making long HCDR3 antibodies that can engage the Env immunogen. The homozygous HC only KI system has bnAb precursors that are particularly amenable to secondary HC events via recombination with their 3′ cryptic recombination signal sequence (RSS). Such unconventional/secondary RAG-mediated IgH rearrangements have been described in the 3H9 anti-DNA autoantibody knock-in mouse model (Chen et al., 1995), and are most likely generated at the pro-B cell stage (Davila et al., 2007). Antibodies with very long HCDR3s (>28 aa) are normally infrequent in pre-immune human primary B cell receptor repertoires, but those with HCDR3s between 20 and 28 aa in length (and relatively less negatively charged) i.e. like those of PG9, CH01 and the V1V2 bnAb-like clones isolated in the KI model here, are not as rare (Briney et al., 2012). It remains to be determined if RMs or humans also use VH replacement and/or D-D fusions to generate heterologous V1V2-glycan bnAb responses. For example, CH505 TF Env may serve as an early priming immunogen for eliciting long HCDR3-bearing V1V2-glycan bnAb precursor B cell clones, prior to boosting with V1V2-directed HIV immunogens. Zhang and Cooper have suggested that HIV-1 bnAbs could indeed arise from VH replacement events based on Ig sequence signatures (Zhang et al., 2004; Zhang et al., 2003). In addition to the DD fusion event itself, mAbs from KI mice use VH genes also used by human bnAbs and they have long and tyrosine-heavy HCDR3s that are required by V1V2-glycan bnAbs for accessing glycan-occluded bnAb epitopes (Pejchal et al., 2010).
In summary, we have shown proof-of-concept that bnAbs can be induced in select individual rabbits and primates. Moreover, bnAb induction may occur with Env vaccination in the presence of Ig LC repertoire holes and default to less disfavored neutralizing antibody specificities such as V1V2-glycan bnAbs. The next steps are optimizing Env structures and/or sequential Envs, as well as reducing host constraints on bnAb generation to expand the frequency of bnAb responses in nonhuman primates, and ultimately in humans (Haynes and Burton, 2017).
EXPERIMENTAL PROCEDURES
Animals and Immunizations
Indian-origin rhesus macaques (n=4) were administered a DNA electroporation (DNA-EP) prime-protein boost vaccine (Table S1). New Zealand rabbits were immunized intramuscularly 5 or 6 times with SOSIP protein. CH103 UCA mice were immunized up to seven times with CH505 TF gp120 protein. All animals were cared for in an AAALAC-accredited facility in accordance with NIH guidelines. All animal procedures were IACUC approved prior to performance. See Supplemental experimental procedures for further details.
Flow cytometry single cell sorting of mouse memory B cells
Single IgG+ memory B cells were sorted from total splenocytes. CD4bs-specifc B cells were identified as those that bound wildtype CH505 TF gp120 but not the CD4bs knockout mutant gp120. See Supplemental experimental procedures for further details.
PCR isolation and immunogenetic analysis of mouse HC and LC rearrangements
Mouse endogenous and human knockin immunoglobulin genes were amplified by RT-PCR, sequenced, and cloned into expression vectors. Full details are in the Supplemental experimental procedures.
Direct ELISAs
ELISAs were performed as previously described (Saunders et al., 2017). Binding titers are shown as log area-under-the-curve (AUC). See Supplemental experimental procedures.
TZM-bl neutralization assay
Plasma from all animal models and purified antibodies were tested for HIV-1 neutralization using the TZM-bl assay (Li et al., 2005). Neutralization titers are the dose that inhibits 50% of replication (ID50).
HIV-1 Env Peptide Array
Peptide arrays were performed with 1:50-diluted serum samples as previously described (Shen et al., 2017). See Supplemental experimental procedures for further details.
Autoantigen ELISA
Autoantibodies were measured by the FDA-approved AtheNA Multi-Lyte® ANA II Test Kit from Zeus Scientific, Inc. per the manufacturer’s instructions.
Activation-induced marker (AIM) assay
The AIM assay was performed with rhesus macaque PBMC and lymph node cell suspensions as previously described (Havenar-Daughton et al., 2016). See Supplemental experimental procedures.
Design of CH505 chimeric, stabilized Env trimer immunogens
CH505 SOSIPs were designed as chimeras and stabilized with I203C and A433C (Zhou et al., 2017) or E64K and A316W mutations (de Taeye et al., 2015). See also Supplemental experimental procedures and Figure S1 for further details.
HIV-1 Envelope production and purification
HIV-1 gp120 and gp140 proteins were produced as previously described (Saunders et al., 2017). SOSIP trimers were produced in 293F cells and purified with PGT145 affinity and gel filtration chromatography. See Supplemental experimental procedures.
Negative stain electron microscopy and single particle 2D class averaging
Proteins were diluted in HMK100 buffer and stained with 2% uranyl acetate. Images were obtained with a Philips 420 electron microscope at 49000x magnification. The EMAN2 program was used to perform class averaging of the single particle images (Tang et al., 2007).
Antibody-SOSIP biolayer interferometry
Biolayer interferometry was performed as previously described (Alam et al., 2017).
Statistical analyses
Neutralization titers between immunization groups were compared using two-tailed Mann-Whitney tests (alpha level = 0.05). Tests were performed with GraphPad Prism v7.0.
Supplementary Material
Highlights.
HIV-1 vaccine-induced broadly neutralizing antibodies targeted the V1V2 glycan site.
V1V2 glycan antibodies arose when host factors limit CD4 binding site antibodies.
V-region secondary rearrangements were a mechanism for generating V1V2 glycan antibodies.
Acknowledgments
We acknowledge technical assistance from Giovanna Hernandez, Erika Dunford, Esther Lee, Rachel Reed, Kedamawit Tilahun, Andrew Foulger, and Aja Sanzone, Callie Vivian, Stormi Chadwick, Maggie Barr, Lawrence Armand, Kara Anasti, James Alin, Qing Zhou, and the Duke Human Vaccine Institute Flow Cytometry core. We thank Beatrice Hahn and Persephone Borrow for insightful comments about the manuscript. This work was supported by NIAID extramural project grants R01-AI120801 (K.O.S.), R01AI087202 and R01AI118571 (L.K.V.) and NIH, NIAID, Division of AIDS UM1 grant AI100645 for the Center for HIV/AIDS Vaccine Immunology-Immunogen Discovery (CHAVI-ID; to B.F.H.).
Footnotes
DECLARATION OF INTERESTS
B.F.H. and K.O.S. have patent applications on Envs used in this study; A.S. has patents on Hiltonol®.
AUTHOR CONTRIBUTIONS
Experimental Design; KOS, LKV, DCM, MC, AS, SZ, GZ, YL, BFH; Investigation and assays; KOS, CJ, JZ, HC, MH, HBV, XS, RS, LS, AN, AE, KX, MGJ, DWC, SZ, GZ, SGR,; Wrote manuscript KOS, LKV, BFH, with editing by all co-authors; Supervision KOS, LKV, XS, AMT, RS, SS, GDT, JRM, MAM, DWC, HPE, PDK, SMA, YL, DCM, BFH; Data analysis KOS, LKV, XS, AMT, GDT, MAM, DWC, SZ, GZ, HPE, PDK, SMA, YL, DCM, BFH; Funding: KOS LKV and BFH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SM, Aussedat B, Vohra Y, Ryan Meyerhoff R, Cale EM, Walkowicz WE, Radakovich NA, Anasti K, Armand L, Parks R, et al. Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Science translational medicine. 2017:9. doi: 10.1126/scitranslmed.aai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla-Jhagjhoorsingh SS, Corti D, Heyndrickx L, Willems E, Vereecken K, Davis D, Vanham G. The N276 glycosylation site is required for HIV-1 neutralization by the CD4 binding site specific HJ16 monoclonal antibody. PloS one. 2013;8:e68863. doi: 10.1371/journal.pone.0068863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. Journal of virology. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, Wiehe K, Alam SM, Aussedat B, Walkowicz WE, Hwang KK, et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Science translational medicine. 2017a:9. doi: 10.1126/scitranslmed.aai7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, Haynes BF. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunological reviews. 2017b;275:145–160. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, Joyce MG, Ozorowski G, Chuang GY, Schramm CA, Wiehe K, et al. Maturation Pathway from Germline to Broad HIV-1 Neutralizer of a CD4-Mimic Antibody. Cell. 2016;165:449–463. doi: 10.1016/j.cell.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T, Fera D, Bhiman J, Eslamizar L, Lu X, Anasti K, Zhang R, Sutherland LL, Scearce RM, Bowman CM, et al. Structural Constraints of Vaccine-Induced Tier-2 Autologous HIV Neutralizing Antibodies Targeting the Receptor-Binding Site. Cell reports. 2016;14:43–54. doi: 10.1016/j.celrep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briney BS, Willis JR, Crowe JE., Jr Human peripheral blood antibodies with long HCDR3s are established primarily at original recombination using a limited subset of germline genes. PloS one. 2012;7:e36750. doi: 10.1371/journal.pone.0036750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- Crooks ET, Tong T, Chakrabarti B, Narayan K, Georgiev IS, Menis S, Huang X, Kulp D, Osawa K, Muranaka J, et al. Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site. PLoS pathogens. 2015;11:e1004932. doi: 10.1371/journal.ppat.1004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila M, Liu F, Cowell LG, Lieberman AE, Heikamp E, Patel A, Kelsoe G. Multiple, conserved cryptic recombination signals in VH gene segments: detection of cleavage products only in pro B cells. The Journal of experimental medicine. 2007;204:3195–3208. doi: 10.1084/jem.20071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien JP, van den Kerkhof TL, Burger JA, Pritchard LK, Pugach P, Yasmeen A, et al. Immunogenicity of Stabilized HIV-1 Envelope Trimers with Reduced Exposure of Non-neutralizing Epitopes. Cell. 2015;163:1702–1715. doi: 10.1016/j.cell.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, et al. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. Journal of virology. 2014;88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, Lu X, Cai F, Hwang KK, Song H, Zhou T, et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, Kasturi SP, Dan JM, Bothwell M, Sanders RW, et al. Cytokine-Independent Detection of Antigen-Specific Germinal Center T Follicular Helper Cells in Immunized Nonhuman Primates Using a Live Cell Activation-Induced Marker Technique. Journal of immunology. 2016;197:994–1002. doi: 10.4049/jimmunol.1600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Burton DR. Developing an HIV vaccine. Science. 2017;355:1129–1130. doi: 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Verkoczy L. AIDS/HIV. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, Pandey S, Sutton WF, Burwitz BJ, Gray M, et al. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. Journal of immunology. 2016;196:3064–3078. doi: 10.4049/jimmunol.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. Aids. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. Journal of virology. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013a;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Tsao CY, Alam SM, Muldoon M, Vandergrift N, Ma BJ, Lu X, Sutherland LL, Scearce RM, Bowman C, et al. Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1. Journal of virology. 2013b;87:4185–4201. doi: 10.1128/JVI.02297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, et al. Polyreactivity and autoreactivity among HIV-1 antibodies. Journal of virology. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. Human circulating PD-1+CXCR3−CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Tran L, Louder MK, Schmidt SD, Cohen M, Dersimonian R, Euler Z, Gray ES, Abdool Karim S, Kirchherr J, et al. The development of CD4 binding site antibodies during HIV-1 infection. Journal of virology. 2012;86:7588–7595. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod DT, Choi NM, Briney B, Garces F, Ver LS, Landais E, Murrell B, Wrin T, Kilembe W, Liang CH, et al. Early Antibody Lineage Diversification and Independent Limb Maturation Lead to Broad HIV-1 Neutralization Targeting the Env High-Mannose Patch. Immunity. 2016;44:1215–1226. doi: 10.1016/j.immuni.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunological reviews. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Gray MD, Dosenovic P, Gitlin AD, Freund NT, Petersen J, Correnti C, Johnsen W, Kegel R, Stuart AB, et al. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nature communications. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MA, Pedroza-Pacheco I, Vandergrift NA, Chui C, Lloyd KE, Parks R, Soderberg KA, Ogbe AT, Cohen MS, Liao HX, et al. Immune perturbations in HIV-1–infected individuals who make broadly neutralizing antibodies. Science Immunology. 2016 doi: 10.1126/sciimmunol.aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancera M, McLellan JS, Wu X, Zhu J, Changela A, Schmidt SD, Yang Y, Zhou T, Phogat S, Mascola JR, et al. Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1. Journal of virology. 2010;84:8098–8110. doi: 10.1128/JVI.00966-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Walker LM, Stanfield RL, Phogat SK, Koff WC, Poignard P, Burton DR, Wilson IA. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe RP, Sanders RW, Yasmeen A, Kim HJ, Lee JH, Cupo A, Korzun J, Derking R, van Montfort T, Julien JP, et al. Cleavage strongly influences whether soluble HIV-1 envelope glycoprotein trimers adopt a native-like conformation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18256–18261. doi: 10.1073/pnas.1314351110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, et al. A next-generation cleaved, soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS pathogens. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, et al. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KO, Nicely NI, Wiehe K, Bonsignori M, Meyerhoff RR, Parks R, Walkowicz WE, Aussedat B, Wu NR, Cai F, et al. Vaccine Elicitation of High Mannose-Dependent Neutralizing Antibodies against the V3-Glycan Broadly Neutralizing Epitope in Nonhuman Primates. Cell reports. 2017;18:2175–2188. doi: 10.1016/j.celrep.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Basu R, Sawant S, Beaumont D, Kwa SF, LaBranche C, Seaton KE, Yates NL, Montefiori DC, Ferrari G, et al. HIV-1 gp120 Protein and MVAgp140 Boost Immunogens Increase immunogenicity of a DNA/MVA HIV-1 Vaccine. Journal of virology. 2017 doi: 10.1128/JVI.01077-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonich CA, Williams KL, Verkerke HP, Williams JA, Nduati R, Lee KK, Overbaugh J. HIV-1 Neutralizing Antibodies with Limited Hypermutation from an Infant. Cell. 2016;166:77–87. doi: 10.1016/j.cell.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17624–17629. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. EMAN2: an extensible image processing suite for electron microscopy. Journal of structural biology. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WB, Zhang J, Jiang C, Nicely NI, Fera D, Luo K, Moody MA, Liao HX, Alam SM, Kepler TB, et al. Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations. Nature communications. 2017;8:1732. doi: 10.1038/s41467-017-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Burrows PD, Cooper MD. The molecular basis and biological significance of VH replacement. Immunological reviews. 2004;197:231–242. doi: 10.1111/j.0105-2896.2004.0107.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zemlin M, Wang YH, Munfus D, Huye LE, Findley HW, Bridges SL, Roth DB, Burrows PD, Cooper MD. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- Zhou T, Doria-Rose NA, Cheng C, Stewart-Jones GBE, Chuang GY, Chambers M, Druz A, Geng H, McKee K, Kwon YD, et al. Quantification of the Impact of the HIV-1-Glycan Shield on Antibody Elicitation. Cell reports. 2017;19:719–732. doi: 10.1016/j.celrep.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.